Abstract
OBJECTIVE
To analyze the current status of clinical trial registration of Traditional Chinese Medicine (TCM) for the treatment of neurological diseases.
METHODS
Interventional clinical trials of TCM treatment for ischemic stroke, hemorrhagic stroke, vascular cognitive impairment, tension-type headache before September 22, 2020 on the platform of Chinese Clinical Trial Registry (ChiCTR), and ClinicalTrials.gov were searched. Two researchers independently selected the literature and extracted data.
RESULTS
A total of 180 interventional clinical trials were included for analysis. Out of 180 trials, 127 were from ChiCTR and 53 from ClinicalTrials.gov. The countries primary sponsoring the included trials were China (176, 97.8%), and the common categories of primary sponsors were hospital (131, 72.8%). Among the study design, the largest proportion of allocation was randomized (172, 95.6%), interventional model assignment was parallel (163, 90.6%), masking was double blind 49 (27.2%), and the sample size was ≤ 400 (144, 80.0%). The trials were most carried out at a single center (102, 56.7%). Among the included studies, 112 (62.2%) registered on ChiCTR attached the ethical approval documents. In terms of trial stages, 50 (27.7%) studies were in phase Ⅳ. The mostly used intervention was Chinese herbal medicines (99, 55%), acupuncture (68, 37.8%) was the second. By searching the registration number on China National Knowledge Infrastructure Database and PubMed, 38 (21.1%) registered trials were published, including 25 protocol studies and 14 research results with one (NCT02275949) published both the protocol and the results.
CONCLUSIONS
Irregular and inadequate reporting, untimely update and publication, insufficient information on traditional medicine unique characteristics, and lack of international collaborations are the problems existing in the interventional clinical registration trials of traditional medicine treatment on neurological diseases. More efforts need to be made from the above aspects to standardize and improve the registration of traditional medicine trials.
Keywords: medicine, Chinese traditional, ischemic stroke, cognitive dysfunction, tension-type headache, hemorrhagic stroke, Chinese Clinical Trial Registry, ClinicalTrials.gov, critical appraisal
1. INTRODUCTION
Neurological disorders were the leading cause of the disability-adjusted life-years (DALYs) (276 million) and second leading cause of deaths (9.0 million) in 2016, globally.1 Among the contributors of neurological DALYs, stroke, including ischemic stroke and hemorrhagic stroke ranked first.1 In China, the burden of stroke is particularly high, where it remains the leading cause of disability and death in adults.2,3 Vascular cognitive impairment and dementia (VCI and VD) is the second leading cause of dementia, causing around 15% of cases.4,5 A national cross-sectional study showed that the overall prevalence of vascular dementia was 1.6%, representing 3920 thousand individuals with vascular dementia among Chinese adults aged 60 years or older.6 Tension-type headache (TTH) is one of the most common headache type and the most common health complaint among children and adults,7,8 negatively affecting the ability to participate in various activities in school, sport, social and home setting, especially when the headache becomes chronic and more frequent.9,10
Above all, ischemic stroke, hemorrhagic and VCI are major and disabling diseases; meanwhile, TTH is common and recurrent. All of the four neurological diseases have become a heavy health and economic burden, not only in China but around the world.
Traditional Chinese Medicine (TCM) has a long history, and continues to play an important role in clinical practice for the prevention and treatment of stroke, VCI and TTH.11-13 The application of stroke TCM clinical pathways, i.e. treating stroke with TCM therapy alone or in combination with other treatment measures, can significantly reduce length of stay, and the cost of hospitalization were lower than the control group, regardless of the possible limitations.14 The advantages of TCM in the treatment of VCI are reflected in its ability to improve clinical symptoms, prevent aggravation and reduce risk factors.15-17 Acupuncture is often used to treat TTH and has been recommended by the European Federation of Neurological Societies as a complementary treatment option for TTH.18,19
With the development of evidence-based medicine, the standardized registration and research methods of TCM clinical trials have received increasing attention. Registration is of great significance to the improvement of the quality of TCM clinical trials and is an important part of the transparency of clinical trials.20, 21 The number of TCM registered trials has increased rapidly and the top two registries were the Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov, as two members of World Health Organization International Clinical Trials Registration Platform.22
This study was aimed to explore the current status of clinical trial registration of TCM interventions for four neurological diseases from the ChiCTR, and Clinical-Trials.gov, taking ischemic stroke, hemorrhagic stroke, VCI, and TTH as examples.
2. METHODS
2.1. Search strategy
The databases of ChiCTR and ClinicalTrials.gov were used to search for the appropriate registration trials. The search deadline was September 22, 2020. The search strategy was conducted including “(ischemic stroke OR hemorrhagic stroke OR vascular cognitive impairment OR tension type headache)”, and the study type was limited to interventional study. Two researchers (WJJ, FGJ) performed the literature retrieval.
2.2. Inclusion criteria
The inclusion criteria of this study were as follows. Subjects recruited in the trial suffered from ischemic stroke, hemorrhagic stroke, VCI, or TTH. The intervention/treatment was TCM, containing Chinese medicine (decoction, granules, pills, tablets, powder, capsule and so on), Chinese medicine injection (material extraction), acupuncture (electroacupuncture), moxi-bustion, Tai Chi, Baduanjin, massage and so on.
2.3. Exclusion criteria
The trials in which the participants had the following diseases were excluded: stroke without specifying ischemic stroke or hemorrhagic, stroke-related complications, i.e. post-stroke depression, mild cognitive impairment related to early Alzheimer’s disease, TTH combined with other headache types, i.e. migraine. The intervention/treatment excluded other complementary and alternative medicine, such as yoga, manual therapy, massage based on Thai traditional massage and so on.
2.4. Data extraction
Data extraction were independently performed by two researchers (WJJ, FGJ) using a pre-determined form. Disagreements were resolved through negotiation or by LX. The form contains the information of registration number, date of registration, public title, study primary investigator, approval number of ethics committee and date, primary sponsor, study design, phase, actual study start date and completion data, sample size, intervention group measures, control group measures , interventional model, masking, age eligible for study, locations, countries of recruitment, study settings and publications. For the blank data, we would check the detailed description to supplement them. Otherwise, we would fill in Not Reported (N/R).
2.5. Statistical analysis
The categorical variables were expressed in numbers and percentages. All calculations were performed with Microsoft Excel 97-2003 (Micorsoft). The figures appearing in this study were drawn with GraphPad Prism8.0.1 (GraphPad Software).
3. RESULTS
3.1. Search results
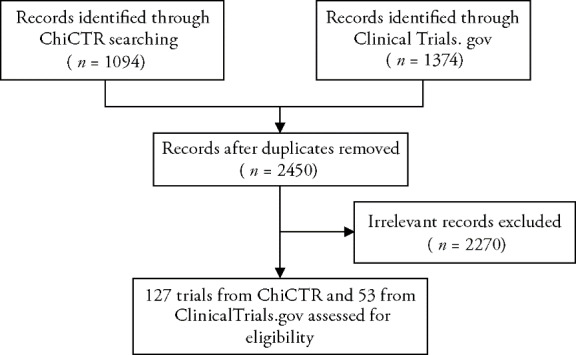
Up to September 22 2020 2468 records were retrieved. After removing irrelevant and duplicate records, a total of 180 trials were eligible and included in the analysis. Out of 180 included trials, 126 (70.0%) were from ChiCTR, and 53 (29.4%) from the ClinicalTrials.gov. The retrieval process is shown in Figure 1.
Figure 1. Flowchart of retrieval of the included registered clinical trials.
3.2. Characteristics of the registered clinical trials
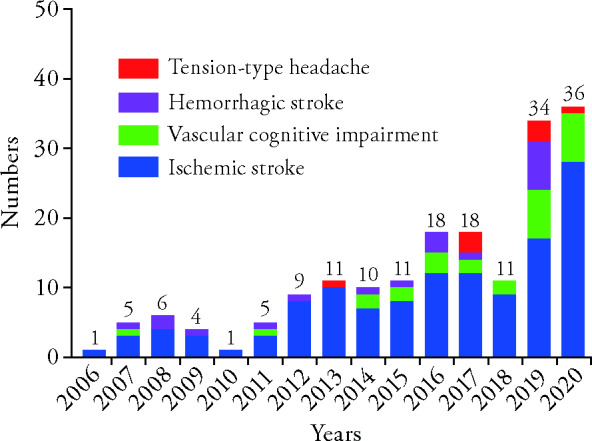
The included registered trials were registered during the period of 2006-2020. The trend was steadily increasing, from 1 in 2006 to 36 in 2020 (Figure 2). The first trial registered on July 13th, 2006 was a randomized controlled trial titled "AISTCM-Outcome Measurement of Acute Ischemic Stroke with Traditional Chinese Medicine" (NCT00351806), and was sponsored by Guangzhou University of Traditional Chinese Medicine. The number of included trials for ischemic stroke is the most (126, 70.0%). The registration for hemorrhagic stroke and VCI began in 2007, and TTH in 2013 (Figure 2).
Figure 2. Numbers of registered clinical trials of each diseases from 2006 to 2020 each year.
As the primary sponsor country, China accounted for 97.8% (176/180), the others are Turkey, Croatia, Brazil, and Israel. The organizations sponsored more than three trials were shown in Figure 3.
Figure 3. Center of the registered clinical trials.
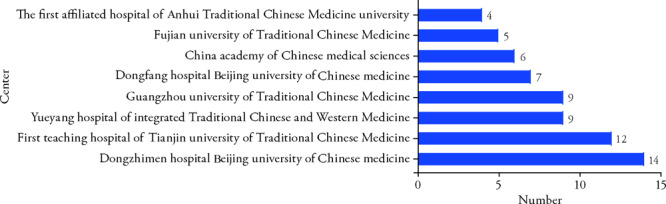
Table 1 shows the characteristics of the included clinical trials. The common primary sponsors were hospital (131, 72.8%) and university (37, 20.6%). There were 102 (56.7%) clinical trials carried out at a single center, 73 (40.5%) were jointly conducted by multiple centers, and 5 (2.8%) trials did not report.
Table 1.
Characteristics of the registered clinical trials
| Item | Detail | Record [n (%)] |
|---|---|---|
| Primary sponsor | Hospital | 131 (72.8) |
| University | 37 (20.6) | |
| Research institute | 6 (3.3) | |
| Government | 3 (1.6) | |
| Individual | 1 (0.6) | |
| Industry | 2 (1.1) | |
| Assignment | Single arm | 4 (2.2) |
| Parallel | 163 (90.6) | |
| Crossover | 3 (1.7) | |
| Othersa | 6 (3.3) | |
| Not reported | 4 (2.2) | |
| Method of allocation | randomized | 172 (95.6) |
| Non-randomized | 4 (2.2) | |
| Not applicable | 4 (2.2) | |
| Masking | Single blind | 21 (11.7) |
| Double blind | 49 (27.2) | |
| Triple blind | 26 (14.4) | |
| Open label | 14 (7.8) | |
| Not reported | 70 (38.9) | |
| Trial participating center | Multi-center | 73 (40.5) |
| Single center | 102 (56.7) | |
| Not reported | 5 (2.8) | |
| Sample size | ≤ 200 | 103 (57.2) |
| 201-400 | 41 (22.8) | |
| 401-1000 | 24 (13.3) | |
| 1000+ | 12 (6.7) | |
| Recruitment status | Completed | 46 (25.6) |
| Recruiting | 49 (27.2) | |
| Not yet recruiting | 76 (42.2) | |
| Unknown | 9 (5.0) | |
| Ethical approval | Yes | 112 (62.2) |
| Unclear | 68 (37.8) | |
| Study phase | 0 | 23 (12.8) |
| Ⅰ | 7 (3.9) | |
| Ⅱ | 12 (6.7) | |
| Ⅲ | 4 (2.2) | |
| Ⅳ | 50 (27.7) | |
| New treatment measure clinical study | 12 (6.7) | |
| Not applicable | 72 (40.0) | |
| Publication | Yes | 38 (21.1) |
| No | 142 (78.9) | |
| Intervention | acupuncture | 68 (37.8) |
| Chinese herbal medicinesb | 99 (55.0) | |
| Othersc | 9 (5.0) | |
| Combinationd | 4 (2.2) |
Notes: aOther assignment consisted of sequential assignment (1 trial), factorial assignment (1 trial), and 4 trials not applicable. bDifferent dosage forms of Chinese herbal medicine were included, such as decoction, granules, pills, tablets, powder, capsule, injection (material extraction), and so on. cOther intervention consisted of Moxibustion treatment (1 trial), massage (2 trials), Baduanjin (2 trials), Taiji (4 trials). dCombination interventions indicated a combination of two or more interventions.
Of the 180 trials, 172 (95.6%) were RCTs. In the 172 RCTs, 163 (94.8%) were parallel assignment. About the masking, 49 (27.2%) trials were double blind, followed by 26 (14.4%) triple blind, 21 (11.7%) single blind and 14 (7.8%) open label, 70 (38.9%) trials were not reported. Among the included trials, 112 trials registered on ChiCTR attached the ethical approval documents. About the recruitment status, 46 (25.6%) studies have completed, 49 (27.2%) trials have started to recruit patients; however, 76 (42.2%) trials were not yet recruiting. In terms of trial stages, 50 (27.7%) studies were in the extended validation stage of indicated drugs on the market (phase Ⅳ), 23 (12.8%) trials were exploratory or in the preliminary experimental stage, 7 (3.9%) trials were in phaseⅠ, 12 (6.7%) in phase Ⅱ, 4 (2.2%) trials were in phase Ⅲ, 12 (6.7%) were new treatment measure clinical study. Other studies (72, 40%) belonged to not applicable item. The sample size of 144 studies was less than 400 subjects, which made up 80.0% of the registered studies. The average sample size was 345 and ranged from 19 (NCT04091100) to 6300 (NCT01958957) per trials. The mostly used intervention was Chinese herbal medicines (99, 55%), acupuncture (68, 37.8%) was the second.
By searching the registration number on China National Knowledge Infrastructure Database (CNKI) and PubMed, after removing duplicate publications, 38 registered trials were published, including 25 protocol studies and 14 research results, with one of the registered trials (NCT02275949) published both the protocol and the results.
4. DISCUSSION
In this study, we have included TCM interventional clinical trials registration in the treatment of ischemic stroke, hemorrhagic stroke, VCI, and TTH from the ChiCTR, and ClinicalTrials.gov during 2006-2020. Our results indicate that the number of TMC trial registrations has increased since 2007, the year China joined the WHOICTRP, and ChiCTR became the world's fourth first-level registration institution,23 which is consistent with a previous study.22 After 2011, there was a steadily growing trend, which might be related to the notice issued by the State Administration of Traditional Chinese Medicine of China on the issuance of TCM diagnosis and treatment plans for 8 encephalopathy and psychiatric diseases (including stroke, vascular dementia, and headache).24
In this study, we also found several problems. First, irregular and inadequate reporting. Of 180 clinical trials from the ChiCTR, 9 did not provide the approval number of the ethics committee, and 5 did not provide the date of approval by the ethics committee; the other 54 trials did not provide any ethics information whatsoever. In some trials registered on ChiCTR, the study leader's Chinese name and English name are inconsistent. Similarly, the same primary sponsor institution has different names, which is inconsistent with the official website translation of the institution. The above imperfect details bring difficulties to subsequent retrieval and statistics. Complete and standardized clinical trial registration is the obligation and moral responsibility of researchers, and it is also a responsibility to trial participants. It is not solely for publishing high impact factor articles. ChiCTR is currently the only clinical trial registration center in China established in accordance with the standards of the World Health Organization International Clinical Trial Registration Platform and the Ottawa Working Group.25, 26 It has been in operation for 15 years so far, and many details already mentioned above should be improved. China Evidence-based Center for Traditional Chinese Medicine established in March 2019 provides the possibility to strengthen the training of evidence-based capabilities for scientific researchers and clinical staff, while puts forward higher requirements for the registration center.27
Second, untimely update and publication. Out of 46 trials completing the recruitment, only 15 (32.6%) published study protocol or results. This goes against the purpose of clinical trial registration, which is to make trial data open and transparent.28 And it is difficult to identify whether the article has publication bias and selective reporting.29 On the other hand, it also wasted the public resources. Publication of clinical research data is considered an ethical imperative.30 Furthermore, all clinical trials called for timely registration and pub-lication of all results.31
Third, lack of international collaborations. In this study, the multi-center cooperation ratio is 40.5%; moreover, almost limited to China. Collaboration is the funda-mental principle for modern research. Multi-center cooperation can not only overcome the possible deviations in the understanding of individual research, but also avoid the limitations represented by individual research results. Multi-center and multi-disciplinary extensive collaborative research on the same issue can give full play to academic advantages, which is necessary for the development of medical science.32 The four diseases involved in this study are common, refractory, major, and chronic diseases in the world. International collaboration is necessary to overcome the “same enemy”.33, 34 Further studies by cross-international multi-center cooperation in various populations are required to fill the gaps in the knowledge of mechanism and treatment of neurological diseases.
Fourth, insufficient or absent information on TCM unique characteristics. Just like the previous study, 22 the included registered clinical trials lacked description of TCM characteristics, including lack of connection with TCM theory in terms of background, and few TCM characteristic indicators in the outcomes. TCM is the sum of knowledge, skills and practice based on the theories, beliefs and experiences inherent in various cultures. Without considering TCM unique characteristics, the study may not be able to include suitable participants and could not correctly evaluate the efficacy of TCM. It is necessary to enrich the information on TM details according to the published files, such as CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation and Elaboration.35
There are several limitations in our study. First, it is difficult to cover all neurological diseases in this study. The four common, major and refractory diseases (ischemic stroke, hemorrhagic stroke, VCI, TTH) are represented, which to a certain extent reflects the current registration status of TM intervention trials for neurological diseases. Second, our analysis focused on the limited registering information from ChiCTR, and ClinicalTrials.Gov, instead of the full study protocols.
In conclusion, the characteristics of registered inter-ventional clinical trials of four neurological diseases (ischemic stroke, hemorrhagic stroke, VCI, and TTH) from ChiCTR, and ClinicalTrials.gov shows that the registered trials need regular and adequate reporting, timely update and publication, sufficient information on traditional medicine unique characteristics. Additionally, international collaborations are also important to provide effective treatment, which will therefore be beneficial to the development and dissemination of Traditional Chinese Medicine.
Contributor Information
Yunling ZHANG, Email: yunlingzhang2004@126.com.
Xing LIAO, Email: okfrom2008@hotmail.com.
REFERENCES
- [1]. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019;18:459-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Wu SM, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394-405. [DOI] [PubMed] [Google Scholar]
- [3]. Wang LD, Liu JM, Yang Y, et al. The prevention and treatment of stroke still face huge challenges — brief report on stroke prevention and treatment in China, 2018. Zhong Guo Xun Huan Za Zhi 2019;34:105-19. [Google Scholar]
- [4]. Czakó C, Kovács T, Ungvari Z, et al. Retinal biomarkers for Alzheimer's disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis. GeroScience 2020;42:1499-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. O'Brien JT, Thomas A. Vascular dementia. Lancet 2015;386:1698-706. [DOI] [PubMed] [Google Scholar]
- [6]. Jia LF, Du YF, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 2020;5:e661-71. [DOI] [PubMed] [Google Scholar]
- [7]. Stovner LJ, Nichols e, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:954-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. James SL, Abate D, Abate AH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden Disease Study 2017. Lancet 2018;392:1789-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Wöber-Bingöl Ç, Wöber C, Uluduz D, et al. The global burden of headache in children and adolescents-developing a questionnaire and methodology for a global study. J Headache Pain 2014;15:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018;38:1-211. [DOI] [PubMed] [Google Scholar]
- [11]. Ni XJ, Lin H, Li H, et al. Evidence‐based practice guideline on integrative medicine for stroke 2019. J Evid Based Med 2020;13:137-52. [DOI] [PubMed] [Google Scholar]
- [12]. Zhou XX, Huang JS, Xie M, Wu CH. Expert consensus for TCM preventive treatment of diseases: vascular mild cognitive impairment. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2020;27:1-5. [Google Scholar]
- [13]. Yu SY, Zhang MJ, Zhou JY, Liu RZ, Wan Q, Li YS. Headache care in China. Headache 2014;54:601-9. [DOI] [PubMed] [Google Scholar]
- [14]. Wang B, Chen D, Zhou HE, Shi HX, Xie Q. Influence of clinical pathways used the hospitals of Traditional Chinese Medicine on patients hospitalized with stroke: a systematic review and Meta-analysis. J Tradit Chin Med 2017;37:159-64. [DOI] [PubMed] [Google Scholar]
- [15]. Cai YY, Jiang WM. Research progress of Traditional Chinese Medicine intervention vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2017;42:1837-41. [DOI] [PubMed] [Google Scholar]
- [16]. Hu WY, Han ZY, Ma HP, Lin JF, Chang Z. Systematic evaluation and Meta-analysis of efficacy and safety of Tianzhi granules in treatment of vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2020;45:4766-75. [DOI] [PubMed] [Google Scholar]
- [17]. Gou J, Yang HX, Yu Y, Guo RJ. Systematic review and Meta-analysis of efficacy and safety of compound Congrong Yizhi capsules on vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2020;45:1924-32. [DOI] [PubMed] [Google Scholar]
- [18]. Coeytaux RR, Befus D. Role of acupuncture in the treatment or prevention of migraine, tension-type headache, or chronic headache disorders. Headache 2016;56:1-3. [DOI] [PubMed] [Google Scholar]
- [19]. Bendtsen L, Evers S, Linde M, Mitsikostas DD, Sandrini G, Schoenen J. EFNS guideline on the treatment of tension-type headache — report of an EFNS task force. Eur J Neurol 2010;17:1318-25. [DOI] [PubMed] [Google Scholar]
- [20]. Wu TX, Li YP, Li J, Zhong ZH, Jia WN. Chinese clinical trial registration and publishing system, and explanation. Zhong Guo Xun Zheng Yi Xue Za Zhi 2006;6:395-6. [Google Scholar]
- [21]. WHO. WHO. International Clinical Trials Registry Platform (ICTRP), Trial registration. https://www.who.int/clinical-trials-registry-platform/network/trial-registrationhttps://www.who.int/clinical-trials-registry-platform/network/trial-registration. (Accessed 20th January 2021). [Google Scholar]
- [22]. Zhang X, Tian R, Yang Z, et al. Quality assessment of clinical trial registration with Traditional Chinese Medicine in WHO registries. BMJ Open 2019;9:e025218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. The Central People’s Government of the People’s Republic of China, China and India join the WHO international clinical trial registration platform. http://www.gov.cn/jrzg/2007-07/25/content_697236.htmhttp://www.gov.cn/jrzg/2007-07/25/content_697236.htm (2007, accessed 7th October 2020). [Google Scholar]
- [24]. National Administration of Traditional Chinese Medicine of China, Notice on the issuance of TCM diagnosis and treatment plans for 8 diseases of encephalopathy and psychiatry. http://yzs.satcm.gov.cn/zhengcewenjian/2018-03-24/3203.htmlhttp://yzs.satcm.gov.cn/zhengcewenjian/2018-03-24/3203.html. (21st January 2011, accessed 7th October 2020). [Google Scholar]
- [25]. Wu TX, Li YP, Yao X, Li J. Clinical trial registration: to improve the quality of clinical research in China. Zhong Guo Xun Zheng Yi Xue Za Zhi 2006;3:153-6. [Google Scholar]
- [26]. WHO. WHO International Clinical Trials Registry Platform (ICTRP), About ICTRP. https://www.who.int/ictrp/about/en/https://www.who.int/ictrp/about/en/. (Accessed 7th October 2020). [Google Scholar]
- [27]. Wang YY, Huang LQ. Taking a broad and long-term view to establish China Center for Evidence Based Traditional Chinese Medicine (CCEBTCM). Zhong Guo Xun Zheng Yi Xue Za Zhi 2019;19:1131-7. [Google Scholar]
- [28]. Li YP, Wu TX, Li J, Zhong ZH, Jia WN. Joint statement of establishing Chinese clinical trial registration and publishing system. Zhong Xi Yi Jie He Xue Bao 2006;4:331-2. [PubMed] [Google Scholar]
- [29]. Ross JS, Gross CP, Krumholz HM. Promoting transparency in pharmaceutical industry-sponsored research. Am J Public Health 2012;102:72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Pearn J. Publication: an ethical imperative. BMJ 1995;310:1313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Breil T, Boettcher M, Hoffmann G F, Ries M. Publication status of completed registered studies in pediatric appendicitis: a cross-sectional analysis. BMJ Open 2018;8:e021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Li J, Jin X, He YY, Fang YX, Wei JM. Practical explorations of multi-center clinical research organized by medical scientific periodicals. Bian Ji Xue Bao 2015;1:47-9. [Google Scholar]
- [33]. Fisher M, Amarenco P, Aronowski J, et al. International collaborations are essential for stroke. Stroke 2019;50:2993-4. [DOI] [PubMed] [Google Scholar]
- [34]. Webster TJ. Think international collaborations are safe? Think again. Int J Nanomedicine 2019;14:6933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med 2017;167:112-21. [DOI] [PubMed] [Google Scholar]


