Abstract
OBJECTIVE
To investigate the protective efficacy of Bushen Culuan decoction 补肾促卵方, BCD) on ovarian follicle and follicular granulosa cells in mice with premature ovarian insufficiency (POI) induced by tripterygium wilfordii polyglycoside, and to study the potential mechanism underlying the action.
METHODS
Eighty female Balb/c mice were randomly divided into 4 groups (n = 20 each): blank group, model group, Bushen Culuan decoction intervening group (BCD group) and estradiol valerate intervening group (EV group). In the first 14 model establishing d, mice in model group, BCD group and EV group were under Tripterygium wilfordii polyglycoside (TWP) gavage to establish POI models. In the 14-day therapeutic stage, mice in BCD group were taken BCD 18.35 mg·kg-1·d-1, mice in EV group were taken EV solution 0.15 mg·kg-1·d-1, while mice in blank group and model group were taken normal saline. When the mice accomplished therapy, whole blood was collected for serum hormone including follicle stimulating hormone (FSH), luteal hormone (LH), estradiol (E2), anti-mullerian hormone (AMH) levels and vascular endothelial growth factor (VEGF), bone morphogenetic protein-7 (BMP-7) measurement. Ovarian tissues were harvested for morphologic observation, follicle counting, ovarian follicular graulosa cell apoptosis test and testing BMP-7 and caspase-3 expressions.
RESULTS
The body weights of the mice kept growing stably in the process expect in TWP intervening stage. Compared with model group, BCD group had significantly higher ovarian index, serum E2, AMH, VEGF, BMP-7 levels and significantly lower FSH level (P < 0.05). Meanwhile the VEGF level in BCD group was higher than in EV group (P < 0.05). Compared with model group, the histopathological damage and GCs apoptosis were mitigated; developing follicle counting, BMP-7 expression were up-regulated, and caspase-3 expression was down-regulated in BCD groups (P < 0.05).
CONCLUSION
BCD treatment could attenuate pathological process in POI ovaries, suppress GC apoptosis, probably through promoting BMP-7 expression and following inhibiting caspase-3 activation.
Keywords: primary ovarian insufficiency, tripterygium, granulosa cells, apoptosis, Bushen Culuan decoction
1. INTRODUCTION
Premature ovarian insufficiency (POI) is a female clinical syndrome caused by loss of ovarian activity before 40 years old. The characteristics of POI is menstrual disturbance (amenorrhea or oligomenorrhea) with high gonadotrophin levels (FSH > 25 U/L) and low estradiol level.1 As the standard treatment of POI, hormone replacement therapy (HRT) can relief the symptom caused by low estradiol level such as hot flash, vaginal discomfort and dyspareunia. HRT with early initiation can also protect cardiovascular system and prevent the loss of bone mineral density.2 However, There is still controversy of HRT management on the risk of hormone dependent cancer such as ovarian cancer and breast cancer occurrence.3,4 Multiple causes can induce POI, such as genetic defects, ovarian structural damage, hormone and its receptor defects and autoimmune disorder etc. Apoptosis of ovarian follicular granulosa cells (GCs) is involved in all of those conditions, so the prevention of GCs apoptosis can be a potential therapeutic method in treating POI.5-7
Bone morphogenetic protein-7 (BMP-7), a member of Transforming growth factor-β has been proved has strong anti-apoptosis capacity of GCs by suppressing caspase activity.8 The expression of BMP-7 in follicle will make the follicle develop into a mature follicle; on the contrary, the lack of BMP-7 will leading an ending of an atretic follicle.9
Chinese herbal prescription Bushen Culuan decoction (补肾促卵方, BCD) has been used for treating ovulatory dysfunction diseases which POI is included.10 BCD has been proved in clinical study that it could elevate E2 level, promote ovarian development, ovulation and pregnancy in treatment of infertility caused by POI.11 It also has been confirmed that BCD could promote ovulation and corpus luteum formation in young rats.12 The current study aimed to explore whether BCD can protect the follicle structure and ovarian function by preventing GCs apoptosis through up-regulating BMP-7 in POI Balb/c mouse.
2. MATERIALS AND METHODS
2.1. Animals
A total of 105 specific pathogen free (SPF) 8-week old Balb/c female mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. [certification No. SCXK (Jing) 2012-0001, Beijng, China]. The mice were divided into 5 per cage and housed in animal facility under SPF condition in Xiyuan Hospital, China Academy of Chinese Medical Science with standard rodent chow and fresh distilled water. The temperature was maintained at (22 ± 2) ℃ with a relative humidity of 45%-70% and a 12-h light/dark cycle. All experiments were conducted according to international guidelines on animal research and ethics.
2.2. Drugs preparation and main reagents
BCD was prepared by drug manufacturing department of Xiyuan Hospital. It was composed of Tusizi (Semen Cuscutae, Lot No. 1805012), Yinyanghuo (Herba Epimedii Brevicornus, Lot No. 1803033), Xianmao (Rhizoma Curculiginis, Lot No. 1712011), Chuanxuduan (Radix Dipsaci Asperoidis, Lot No. 1802012), Gouqizi (Fructus Lycii, Lot No. 1804011), Nuzhenzi (Fructus Ligustri Lucidi, Lot No. 1712011), Zelan (Herba Lycopi Hirti, Lot No. 1707011), Puhuang (Pollen Typhae, Lot No. 1804011), Xiangfu (Rhizoma Cyperi, Lot No. 1804011), Chuanshanlong (Rhizoma Dioscoreae Nipponicae, Lot No. 1804011). All herbs are recorded in Pharmacopoeia of the People’s Republic of China (2015) and all dosages are remains in approved safe dose of the pharmacopoeia. The crude herbs were purchased from Baicao Kangshen Pharmaceutical Co., Ltd., Hebei, China. The decoction was concentrated to 1.83 g/mL of the crude herb.14
Estradiol valerate (Progynova®) tablets were purchased from Bayer Phamaceutical Co., Ltd. (German, Lot No.407A). One tablet containing 1 mg of estradiol valerate was pulverized and sifted to powder, then was dissolved in distilled water to make the estradiol valerate concentration into 0.01 mg/mL.
Tripterygium wilfordii polyglycoside (TWP) tablets (Lot No. 20180720) were purchased from Huangshi Feiyun Pharmacy Co., Ltd., Hubei, China, 0.1 g sodium carboxymethylcellulose (CMC) was dissolved in 40mL distilled water, then 0.125 mL Tween-80 was added in the solution. Totally 1600 mg Tripterygium wilfordii polyglycoside tablets (every tablet weighs 80 mg and contains 10 mg TWP) were pulverized and sifted to powder, then added into the solution. The suspension was made to 50 mL constant volume with distilled water and mixed well. The final concentration was 4 mg/mL.
Enzyme-linked immunosorbent assay (ELISA) kits for FSH (Lot No. AGD/SRA-EA03018), LH (Lot No. AGD/SRA-EA02854), E2 (Lot No. AGD/SRA-EA02902), AMH (Lot No. AGD/SRA-EM03137), VEGF (Lot No. AGD/SRA-EA02859), BMP-7 (Lot No. AGD/SRA-EA02768); the secondary antibodies (Lot No. BOS5127) and GAPDH antibody (Lot No. GR-0010) for Western blotting were all purchased from Bossbio Co., Ltd. (Beijing, China). Primary antibodies (BMP-7, Lot No. AB56023; caspase-3, Lot No. AB44976) for Western blotting were purchased from Abcam (Cambridge, MA, USA). In Situ Cell Death Detection Kit, POD was purchased from Roche, Germany.
2.3. Mouse grouping and intervention
Totally 105 Balb/c female mice were adaptively fed for 4 d, then their estrous cycles were observed for 10 d. 80 mice with a regular (5.5 ± 0.5)-day estrous cycle were screened into the study. They were randomly divided into 4 groups of 20 each by using random number table: blank group, model group, Bushen Culuan decoction intervening group (BCD group) and estradiol valerate intervening group (EV group).
Once screened into the study, the mice in model group, BCD group and EV group were induced to establish POI model by gastric gavage with TWP 80 mg·kg-1·d-1 twice a day for 14 d while the mice in BC were intervened by normal saline twice a day for 14 d. From day 15, the mice in BCD were intervened with BCD gavage 18.35 mg·kg-1·d-1 once a day, the mice in EV group were intervened with EV solution gavage 0.15 mg·kg-1·d-1 once a day for 14 d while the mice in blank group and model group being intervened with normal saline for 14 d.
All mice in model group, BCD group and EV group were sacrificed at 9-10 a.m. on day 29. The mice in blank group were sacrificed at 9-10 a.m. on the first day in anestrum phase of an estrous cycle from day 29 through vaginal exfoliated cell observation.
The general status including estrous cycle, behaviour, activity, mental status, fur condition, urine and stool condition were observed. All mice were weighed on Day-14 (the 1st adaptive feeding day), Day 1 (the 1st POI model establishing day with TWP intervening), Day 15 (the 1st therapeutic intervening day) and Day 29 (the day after the last therapeutic intervening day).
On sacrificing day, the mice were anaesthetized by a dose of 50 mg/kg injection with 1% pentobarbital sodium, weighed and collected femoral arterial blood samples. The blood was centrifuged twice in a condition of 4 ℃ and 3000 r/min to get serum to test sex hormone, anti-mullerian hormone (AMH), vascular endothelial growth factor (VEFG) and BMP-7 levels. Serum samples No. 1-10 in every group were tested for FSH, LH and E2, and samples No. 11-20 in every group tested for AMH, VEGF and BMP-7. After the mice being sacrificed, the uterus and bilateral ovaries were removed, dissected and weighed on an ice-cold glass plate. All left ovaries were fixed by 4% paraformaldehyde to make pathological sections in order to get follicle count and to measure granulosa cell apoptosis. All right ovaries were transferred by liquid nitrogen and stored in –80 ℃ for Western blot.
2.4. Ovarian morphology and follicle count
Ovarian were embedded in paraffin and cut into 4 μm thick sections. The sections were stained with hematoxylin and eosin stain after de-paraffinization. The histologic sections were observed for ovarian morphology under 40 × magnification and for follicle structure and counting under 200× magnification. The follicle counting of each grade including primordial follicle, preantral follicle, antral follicle, preovulatory follicle, corpus luteum and atretic follicle were recorded. The follicle counting was done by two investigators.
2.5. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technology
Granulosa cell apoptosis of ovarian follicle was detected by TUNEL staining. 4 μm thick ovary sections were deparaffinized, rehydrated, and incubated in 20 μg/mL proteinases K solution for 30 min at room temperature. The slides were rinsed by phosphate-buffered saline (PBS) then incubated with TdT enzyme at 37 ℃ for 1 h in a humidified chamber. After being incubated in converter-POD solution at 37 ℃for 30 min, the slides were detected with stable DAB. The slides pre-treated with 50U/mL DNase I (1500 U/mL in 50 mmol/L Tris-HCl, pH 7.5, 10 mmol/L MgCl2 1 mg/mL, 1 mg/mL bovine serum albumin, BSA) or incubated in reaction buffer without TdT were used as positive and negative control. The slides were finally counterstained by haematoxylin, dehydrated and mounted. Nuclear dark brown staining with light staining cytoplasm can be identified as apoptotic cells. Random five non-overlapped areas of each slide were observed. Apoptotic index was recorded by the ratio of apoptotic cells to all cells.
2.6. Enzyme linked immunosorbent assay examination
Serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), AMH, VEGF, and BMP-7 expression levels were detected by ELISA. All indicator levels were measured by double antibody sandwish method kit. All procedures were processed according to the kit protocol respectively, measured absorbance in the 450 nm wavelength with an enzyme-labeled instrument.
2.7. Western blotting
BMP-7 and caspase-3 expression levels in ovarian tissue were measured by Western blotting. Each sample containing about 100 μL ovarian tissue was added into 300 μL radio immunoprecipitation tissue lysis buffer. The tissue was centrifuged at 12 000 r/min for 30 min at 4 ℃ after being homogenized by sonication. The protein concentration of the resulting supernatant of the solution was measured by a bicinchoninic acid (BCA) protein assay kit. Protein from tissues was then separated by sodium dodecyl sulfate polyacrylamide gel electro-phoresis (SDS-PAGE) with 10% polyacrylamide gel, then transferred to a nitrocellulose membrane. The protein on membrane was blocked with 5% non-fat dry milk for 2 h of room temperature. The membrane was incubated with primary antibodies at a 1∶1000 concentration overnight at 4 ℃. Subsequently, the membrane was rinsed by PBS with 0.05% Tween-20 (PBST) and incubated with secondary antibodies for 1 h at room temperature. The membrane was washed with PBST, and signal to the membrane were detected via chemiluminescence, with GAPDH as the internal reference. The TANON GIS system was used to analyze the BMP-7/GAPDH, caspase-3/GAPDH grayscale signal ratios from the resulting film.
2.8. Statistical analysis
Statistical software SPSS 20.0(IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. IBM Corp., Armonk, NY, USA) was applied to analyse the data. Measurement data were described by mean ± standard deviation ($\bar{x}$ ± s). T-test was used for evaluating the difference between two groups and the data was satisfied with normal distribution. Mann-Whitney test was used for the data not being satisfied with normal distribution. The Kolmogorov-Smirnov test was used to assess normality. A one-way analysis of variance (ANOVA) was used for multiple intergroup comparison. Student-Newman-Keuls (SNK)-q test was applied to compare pairs of groups. The χ2 test was used for enumeration data. A P < 0.05 was considered statistically significant.
3. RESULTS
3.1. General status
The appetite, activity, urine and stool condition were kept normal with glossy fur of all mice from Days-14 to 1. Almost all mice under gastric gavage had mild loose stool during Days 1 to 3 and returned normal shortly afterward. Around one third of the mice with TWP intervening to establish POI model were found curling up or dry fur in TWP intervening stage then returned normal in therapeutic stage.
There is no significant difference of the mice weight between any two groups on Days –14 or 1 (P > 0.05). On Day 15, the mice in blank group were heavier than the mice in EV group (P < 0.05). On Day 29, the mice in blank group were heavier than the mice in EV group and in model group (P < 0.05). The mice in all 4 groups gained weight in observation stage (Days –14 to 1) and therapeutic stage (Days 15 to 29) (P < 0.05). In POI model establishing stage (Days 1 to 15), the mice in EV lost weight (P < 0.05) while in other 3 groups had no significant change (P > 0.05) (Table 1).
Table 1.
Body weight comparison among each group (g, $bar{x}$ ± s)
| Group | n | Day –14 | Day 1 | Day 15 | Day 29 |
|---|---|---|---|---|---|
| Blank | 20 | 18.8±0.7 | 19.3±1.0 | 19.5±0.7a | 19.9±0.7c |
| Model | 20 | 19.0±0.5 | 19.4±0.6 | 19.2±0.5 | 19.4±0.5 |
| BCD | 20 | 19.2±0.6 | 19.5±0.5 | 19.3±0.9 | 19.70±0.7 |
| EV | 20 | 19.1±0.6 | 19.5±0.6 | 19.1±0.3b | 19.5±0.4 |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. aP < 0.05, compared with EV group at the same time; bP < 0.05, compared with Day 1 in the same group; cP < 0.05, compared with model group and EV group at the same time.
3.2. Estrous cycles
All mice screened in the study had regular estrous cycles from days–14 to–1. In POI model establishing stage, the ratio of irregular cycle mice in model group, BCD group and EV group was 95%, 90% and 95%, respectively. The number of normal mice in blank group was significantly more than the other 3 groups (P < 0.05). Until the end of therapeutic stage, the number of abnormal mice in BCD group was significantly less than the number in model group (P < 0.05) (Table 2).
Table 2.
Estrous cycle comparison among each group (χ2)
| Group | n | Days –14-–1 Normal |
Days 1-14 | Days 15-28 | Ratio of irregular cycle (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Prolonged | Stagnate | No cycle | Normal | Prolonged | Stagnate | No cycle | |||||
| Blank | 20 | 20a | 19 | 0 | 1 | 0 | 18 | 1 | 1 | 0 | 10 | |
| Model | 20 | 20 | 1 | 6 | 3 | 10 | 5 | 7 | 5 | 3 | 75 | |
| BCD | 20 | 20 | 2 | 8 | 1 | 9 | 17 | 2 | 0 | 1 | 15b | |
| EV | 20 | 20 | 1 | 5 | 6 | 8 | 17 | 1 | 1 | 1 | 15 | |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. aP < 0.05, compared with the other 3 groups; bP < 0.05, compared with model group.
3.3. Gonad index
Ovarian index = bilateral ovarian wet weight (mg)/ body mass before blood collection (g). Uterine index = uterine wet weight (mg)/ body mass before blood collection (g). The ovarian index of BCD group was significantly higher than that of model group (P < 0.05). All other gonad index had no statistical difference among any other two groups (P > 0.05) (Table 3).
Table 3.
Gonad index comparison among each group ($bar{x}$ ± s)
| Group | n | Ovarian index | Uterine index |
|---|---|---|---|
| Blank | 20 | 0.87±0.35 | 3.42±1.92 |
| Model | 20 | 0.72±0.30 | 2.67±0.71 |
| BCD | 20 | 0.97±0.28a | 3.41±2.47 |
| EV | 20 | 0.84±0.38 | 2.96±1.47 |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. aP < 0.05, compared with model group.
3.4. Serum indicator level
As shown in Table 4, FSH level was significantly higher in model group compared with all other 3 groups (P < 0.05), while there is no statistical FSH level difference among other 3 groups. All 4 groups had statistically the same LH level in this study. E2 level in blank group was higher than model group and EV group respectively (P < 0.05), at the same time E2 level in BCD group was higher than model group (P < 0.05). BCD group had a higher AMH level compared with blank group and model group (P < 0.05), and a higher VEGF level than the other 3 groups (P < 0.05). BMP-7 level in BCD group was higher than in model group.
Table 4.
Serum hormone comparison among each group ($bar{x}$ ± s)
| Group | n | FSH (IU/L) | LH (ng/mL) | E2 (ng/L) | AMH (ng/mL) | VEGF (ng/L) | BMP-7 (pg/mL) |
|---|---|---|---|---|---|---|---|
| Blank | 10 | 9.26±2.36 | 2.18±0.29 | 65.39±9.74b | 14.98±3.12 | 183.48±29.26 | 1202.88±139.00 |
| Model | 10 | 11.41±2.39a | 2.38±0.77 | 56.32±10.91 | 14.09±2.49 | 180.36±48.23 | 1078.86±213.40 |
| BCD | 10 | 9.89±1.35 | 2.01±0.38 | 64.13±10.72c | 16.95±3.51d | 228.24±48.90e | 1311.59±230.36f |
| EV | 10 | 10.11±1.45 | 2.21±0.60 | 57.99±9.06 | 15.56±3.16 | 163.64±39.58 | 1180.40±216.47 |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: Estradiol2; AMH: anti-Müllerian hormone; VEGF: vascular endothelial growth factor; BMP-7: bone morphogenetic protein-7. aP < 0.05, compared with the other 3 groups; bP < 0.05, compared with model group and EV group; cP < 0.05, compared with model group; dP < 0.05, compared with blank group and model group; eP < 0.05; compared with the other 3 groups; fP < 0.05, compared with model group.
3.5. Ovarian morphology and follicle count
In general, the ovarian follicles in blank group, BCD group and EV group had relatively regular shape with orderly arranged granular cells (GCs). The follicles in blank group had the best situation among all groups. Ovarian stroma swelling and fibrosis were observed in model group with loosely or irregularly arranged GCs of follicles.
The counts of primordial follicles, preantral follicles, antral follicles, and corpus luteum in BCD group were all significantly more than the counts in model group (P < 0.05). The counts of preantral follicles in BCD group was significantly more than the counts in EV group (P < 0.05). The count of atretic follicles in BCD group was significantly less than it in model group (P < 0.05). There was no significant difference in any type of follicle count between blank group and BCD group (P > 0.05) (Figure 1, Table 5).
Figure 1. Ovaries of each group (HE Staining, ×200).
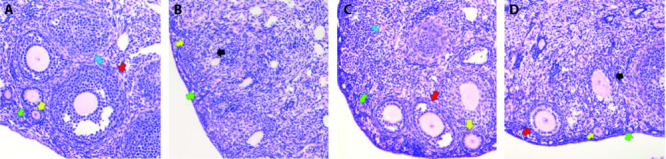
A: blank group; B: model group; C: BCD group; D: EV group. Arrows: apoptotic cells. White arrows: primordial follicles. Yellow arrows: preantral follicles. Red arrows: antral follicles. Blue arrows: corpus luteum. Black arrows: atretic follicles. Blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d.
Table 5.
Unit follicle account comparison among each group ($bar{x}$ ± s)
| Group | n | Primordial follicles | Preantral follicles | Antral follicles | Preovulatory follicles | Corpus luteum | Atretic follicles |
|---|---|---|---|---|---|---|---|
| Blank | 10 | 21.60±7.37 | 17.30±3.30 | 3.30±1.57 | 1.50±0.31 | 3.80±1.55 | 1.60±0.34 |
| Model | 10 | 10.10±3.81 | 11.10±4.31 | 2.00±1.49 | 0.60±0.22 | 2.10±0.99 | 4.70±0.42 |
| BCD | 10 | 21.70±4.19a | 15.20±3.36ab | 3.60±1.65a | 1.30±0.26 | 3.50±1.58a | 1.80±0.47a |
| EV | 10 | 18.80±3.68 | 12.80±3.85 | 2.70±1.16 | 0.90±0.23 | 2.40±1.26 | 2.30±0.60 |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. aP < 0.05, compared with model group; bP < 0.05, compared with EV group.
3.6. Ovarian granulosa cell apoptotic index
Plenty of GCs with dark brown nucleus and lightly stained cytoplasm were found apoptotic in the ovaries in model group. Few apoptotic GC was found in the ovaries in normal group. The apoptotic index of the blank group, model group, BCD group and EV group was 9% ± 4%, 76% ± 12%, 21% ± 5% and 32% ± 9%, respectively. There were significant differences between any two groups (P < 0.01) (Figure 2).
Figure 2. Comparison of ovarian granulosa cell apoptosis (TUNEL staining, ×400).
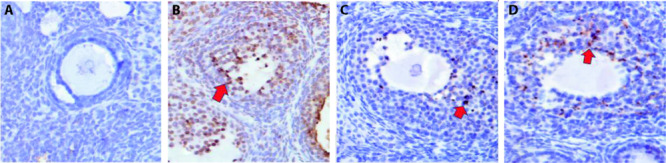
A: blank group; B: model group; C: BCD group; D: EV group. Arrows: apoptotic cells. Blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg kg-1·d-1, 14 d. TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; BCD: Bushen Culuan decoction; EV: estradiol valerate.
3.7. BMP-7 and caspase-3 expressions of ovarian tissues
Compared with model group, the BMP-7 expression level was up-regulated significantly in BCD group, meanwhile the caspase-3 expression level was down-regulated in BCD group. BMP-7 expression level was also significantly higher than it in EV group (P < 0.05). There was no statistical difference of BMP-7 or caspase-3 expression level between blank group and BCD group (P > 0.05) (Figure 3, Table 6).
Figure 3. Comparison of BMP-7 and caspase-3 expressions by Western blot (n = 3).
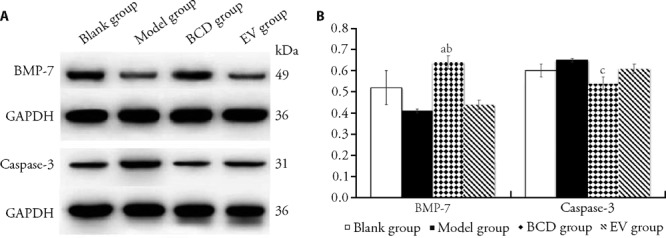
Blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. BMP-7: bone morphogenetic protein-7. aP < 0.05, compared with model group. bP < 0.05, compared with EV group. cP < 0.05, compared with model group.
Table 6.
BMP-7 and caspase-3 expressions among each group ($bar{x}$ ± s)
| Group | n | BMP-7 | Caspase-3 |
|---|---|---|---|
| Blank | 3 | 0.520±0.080 | 0.590±0.030 |
| Model | 3 | 0.410±0.010 | 0.650±0.010 |
| BCD | 3 | 0.640±0.030ab | 0.540±0.040c |
| EV | 3 | 0.440±0.020 | 0.610±0.040 |
Notes: blank group: equal amount of normal saline, 14 d; model group: equal amount of normal saline, 14 d; BCD group: Bushen Culuan decoction, 18.35 g·kg-1·d-1, 14 d; EV group: estradiol valerate intervening group, 0.15 mg·kg-1·d-1, 14 d. BMP-7: bone morphogenetic protein-7; BCD: Bushen Culuan decoction; EV: estradiol valerate. aP < 0.05, compared with model group; bP < 0.05, compared with EV group; cP < 0.05, compared with model group.
4. DISCUSSION
POI is characterized by its high heterogeneity of etiology and multifactorial mechanism. There are more than 50% of the patients cannot find a clear cause which belong to idiopathic POI.14 Since the pathogenesis cannot be imperfectly explained by a single factor or gene, there is no etiological treatment for POI as yet. Traditional Chinese medicine has been reported that has definite effectiveness in treating POI via both Chinese herb therapy15 and acupuncture therapy.16 In preliminary study, we proved 80 mg·kg-1·d-1 TWP gavage twice a day for 14 d can successfully establish a POI mouse model. We also proved the mouse ovarian functions were not recovered 14 d after intervening suspension. So, we used the same way to establish the POI model in this study. In this study, estrous cycle observation and analysis showed that in POI model establishing stage, most mice intervened with TWP had disturbed estrous cycles. The situation was accorded with the estrous cycle changing pattern in preliminary study and manifested successful establishment of POI mouse model.
The follicle development depends on hypothalamus-pituitary-ovary axis (HPOA) regulation. The regulatory function can be reflected on serum hormone level changing, so serum hormone levels are the most common indicators to test ovarian function in clinical and experimental studies. Among all serum hormone indicators, FSH and E2 levels are the most commonly used. In this study, compared with model group, BCD group significantly reduced FSH level and elevated E2 level, which was coincided with the result with our clinical study.11 In recent years, AMH are applied in ovarian function and ovarian reserve measurement by its steady serum level in an estrous cycle. A higher AMH level can indicate a larger number of follicles has made the transition from the primordial pool into the growing follicle pool without being controlled by gon-adotropins.17 BCD group had a higher AMH level compared with blank group and model group showed its protective function of ovarian reserve.
The ovarian weight and follicular developing situation directly shows ovarian function, the pathological indicator was regarded as golden standard of ovarian function.18 Since GC apoptosis is one of the initial factors of POI mechanism,5 the apoptotic index can be an important indicator in POI treating measurement.19-21 In our study, ovarian index in BCD group was significantly higher than it in model group; primordial follicle and all developing follicle counts were all significantly more than in model group and atretic follicle count was less than in model group. Moreover, the TUNEL analysis showed a prominent anti-apoptosis capacity of BCD than spontaneous recovery of model group and Estradiol Valerate of EV group. The lower ovarian caspase-3 expression in BCD group also proved the anti-apoptosis capacity of BCD.
In the past few years, BMP-7 has been proved that it plays important parts in different aspects of women reproductive system.22 BMP-7 has strong anti-apoptosis capacity of GCs.8 The anti-apoptosis function is achieved through a mitochondrial apoptosis-independent pathway by up-regulating survivin and xlinked inhibitor of apoptosis protein expressions to inhibit the caspase activity, then suppresses the abruption of CAD-ICAD complex.8 In our study, both serum BMP-7 level and ovarian BMP-7 expression were significantly higher than it in model group, and ovarian BMP-7 expression was even higher than it in EV group. Compared with blank group, BCD group also had higher tendency of serum and ovarian BMP-7 levels without statistical difference. The outcome illustrated BCD regulating function of BMP-7 in POI model.
BMP-7 also be proved can inducing VEGF expression through Smad 5 pathway, then suppress GCs apoptosis and promote follicular vasculature development by cross-talking with BMP-4 and VEGF.24 In our study, the serum VEGF level in BCD group was higher than it in the other 3 group, this result further confirmed the positive correlation among BCD treatment, BMP-7 and VEGF.
In conclusion, our findings suggested that Bushen Culuan decoction could enhance mouse ovarian function to prevent POI by suppressing follicular granulosa cell apoptosis. The result may achieve through up-regulating BMP-7 expression to inhibited caspase-3 activity and elevated VEGF level. This study provided experimental evidence based on the previous clinical study, and partly explained the mechanism of BCD to treat POI. In next step we will locate the cell signaling transducing pathway.
References
- [1]. Chen ZJ, Tian QJ, Qiao J, et al. Expert consensus of premature ovarian insufficiency clinical diagnosis and treatment. Zhong Hua Fu Can Ke Za Zhi 2017;52:577-81. [DOI] [PubMed] [Google Scholar]
- [2]. Webber L, Davies M, Anderson R, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926-37. [DOI] [PubMed] [Google Scholar]
- [3]. Hu YL, Zhang JL, Hou XW, Jin L, Zhang Y. Menopausal hormone therapy and risk of ovarian cancer: a Meta-analysis. Zhong Guo Zhong Liu Xue Za Zhi 2016;22:549-53. [Google Scholar]
- [4]. Zhang JQ, Zhang Q, Meng L. Overview of estrogen / progestin replacement therapy and breast cancer occurrence risk. Zhong Guo Fu You Bao Jian 2015;30:6669-72. [Google Scholar]
- [5]. Ye N, Dong XY, Li DH. The progress in apoptotic mechanism of ovarian granulosa cells involved in premature ovarian failure. Shou Du Yi Ke Da Xue Xue Bao 2014;35:379-83. [Google Scholar]
- [6]. Liu LQ, Liu YF, Yang M, et al. Effectiveness of tonifying-kidney and regulating-liver therapy on diminished ovarian reserve: a systematic review and Meta-analysis of randomized controlled trials. J Tradit Chin Med 2020;40:343-54. [DOI] [PubMed] [Google Scholar]
- [7]. Sun YY, Tan Y, Chen SP. Effectiveness of nourishing Yin and tonifying Yang sequential therapy in combination with Climen on diminished ovarian reserve: a retrospective study. J Tradit Chin Med 2020;40:150-6. [PubMed] [Google Scholar]
- [8]. Kayamori T, Kosaka N, Miyamoto A, Takashi S. The differential pathways of bone morphogenetic protein (BMP)-4 and -7 in the suppression of the bovine granulosa cell apoptosis. Mol Cell Biochem 2009;323:161-8. [DOI] [PubMed] [Google Scholar]
- [9]. Zhu GQ, Cui YH, Wang QL, Kang YG, Lü YZ, Wang JG. Bone morphogenetic proteins (BMP) 2, 4, 6 and 7 affect ovarian follicu- lar development through regulation of follicle-stimulating horm- one receptor (FSHR) and luteinizing hormone receptor (LHR) expression in goat granulosa cells. J Cell Biol Genet 2013;3:14-21. [Google Scholar]
- [10]. Ma K, Fu FZ, Jiang K, Sun LH, Cai LX. The effectiveness of Tiaojing Zhuluan decoction treating anovulatory infertility. Zhong Hua Zhong Xi Yi Jie He Za Zhi 1998;18:372-3. [Google Scholar]
- [11]. Ma K, Liu YF, He JQ, Li M, Shan J. A multi-center, randomized, double-blind clinical study on Bushen Huoxue in treatment of ovulatory dysfunction caused infertility. Zhong Guo Zhong Yao Za Zhi 2015;40:2911-5. [PubMed] [Google Scholar]
- [12]. Ma K. The effectiveness of Bushen Culuan decoction on ovulation and corpus luteum function in experimental rats. Fujian Zhong Yi Yao Za Zhi 1997;28:3-4. [Google Scholar]
- [13]. Zhao W, Sun GZ. The medication dosage conversion among different animals. Zhong Guo Xu Mu Shou Yi Za Zhi 2010;5:52-3. [Google Scholar]
- [14]. Zhao SG, Chen ZJ. Molecular mechanisms of major reproductive diseases. Sheng Ming Ke Xue Tong Bao 2017;29:43-51. [Google Scholar]
- [15]. Shang YJ, Chen Y, Lu S. Systemic review and Meta-analysis of Bushen Huoxue Chinese medicine in the treatment of premature ovarian failure. Zhong Yi Za Zhi 2018;59:1295-9. [Google Scholar]
- [16]. Luo X, Li X, Cheng J, et al. Systemic review and Meta-analysis of efficacy of acupuncture in the treatment of premature ovarian failure. Zhong Yi Za Zhi 2016;57:1027-32. [Google Scholar]
- [17]. Visser JA, de Jong FH, Laven JSE, Themmen APN. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 2005;131:1-9. [DOI] [PubMed] [Google Scholar]
- [18]. Yan DW, Zhou L, Sun ZY. Research progress in establishing animal models of premature ovarian failure and its evaluation indexes. Zhong Hua Yao Li Yu Du Li Xue Za Zhi 2015;29:486-92. [Google Scholar]
- [19]. Grasa P, Sheikh S, Krzys N, et al. Dysregulation of follicle development in a mouse model of premature ovarian insufficiency. Reproduction 2016;152:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Luan Y, Edmonds ME, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol 2019;240:243-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Ma M, Chen XY, Li B, Li XT. Melatonin protects premature ovarian insufficiency induced by Tripterygium glycosides: role of SIRT1. Am J Transl Res 2017;9:1580-602. [PMC free article] [PubMed] [Google Scholar]
- [22]. Yuan Y. Research progress of influence of bone morphogenetic protein 7 in women reproductive system and its intervention with Traditional Chinese Medicine. Guangzhou Zhong Yi Yao Da Xue Xue Bao 2019;36:297-302. [Google Scholar]
- [23]. Wang WN, Zhang WF, Li DH, et al. Lichong decoction inhibits micro-angiogenesis by reducing the expressions of hypoxia inducible factor-1α and vascular endothelial growth factor in hysteromyoma mouse model. J Tradit Chin Med 2020;40:928-37. [DOI] [PubMed] [Google Scholar]
- [24]. Shimizu T, Magata F, Abe Y, Miyamoto A. Bone morphogenetic protein 4 (BMP-4) and BMP-7 induce vascular endothelial growth factor expression in bovine granulosa cells. Anim Sci J 2012;83:663-7. [DOI] [PubMed] [Google Scholar]


