Abstract
COP9 constitutive photomorphogenic homolog subunit 5 (COPS5), also known as Jab1 or CSN5, has been implicated in a wide variety of cellular and developmental processes. By analyzing male germ cell–specific COPS5-deficient mice, we have demonstrated previously that COPS5 is essential to maintain male germ survival and acrosome biogenesis. To further determine the role of Cops5 in peritubular myoid cells, a smooth muscle lineage surrounding seminiferous tubules, we herein derived mice conditionally deficient for the Cops5 gene in smooth muscle cells using transgenic Myh11-Cre mice. Although these conditional Cops5-deficient mice were born at the expected Mendelian ratio and appeared to be normal within the first week after birth, the homozygous mice started to show growth retardation after 1 week. These mice also exhibited a variety of developmental and reproductive disorders, including failure of development of reproductive organs in both males and females, spermatogenesis defects, and impaired skeletal development and immune functions. Furthermore, conditional Cops5-deficient mice revealed dramatic impairment of the endocrine system associated with testicular functions, including a marked reduction in serum levels of gonadotropins (follicle-stimulating hormone, luteinizing hormone), testosterone, insulin-like growth factor 1, and glucose, but not vasopressin. All homozygous mice died before age 67 days in the study. Collectively, our results provide novel evidence that Cops5 in smooth muscle lineage plays an essential role in postnatal development and reproductive functions.
Keywords: COPS5, smooth muscle cells, endocrine system, hormone homeostasis, reproduction, skeletal development, immune functions
COP9 constitutive photomorphogenic homolog subunit 5 (COPS5), also known as Jab1 and CSN5, is implicated in various cellular and developmental processes (1). As the fifth component of the constitutive photomorphogenic-9 signalosome, COPS5 is located mainly in the nucleus and likely acts as the catalytic center that mediates the cleavage of Nedd8 from cullin subunits of Skp1-cullin 1-F-box (SCF)-type E3 ligase complexes (2). Removal of Nedd8 from cullins decreases the ubiquitin ligase activity of SCF-type complexes such as SCF, Cockayne syndrome group A (CSA), or DNA-binding protein 2 (DDB2), modifying the post-translational stability of numerous proteins (3). Moreover, free COPS5 is present in both the cytoplasm and the nucleus, suggesting diverse biological activities, which is consistent with the association of COPS5 with a variety of diseases such as cancer (4), chronic mountain sickness (5), vitiligo (6), atherosclerosis (foam cell formation) (7), cardiac hypertrophy (8), amyloidopathies (9), chronic rhinosinusitis (10), and nasal polyposis (10). Of note, COPS5 has been directly linked to impaired spermatogenesis and infertility. Mice deficient for COPS5 specifically in male germ cells showed dramatically reduced sperm numbers and were infertile (11). However, it is not clear whether COPS5 also plays a role in somatic cells of the testis, such as Leydig cells, Sertoli cells, and peritubular myoid (PTM) cells.
Smooth muscle cells (SMCs) are a critical component of multiple tissues. PTM cells, a type of SMC, are the somatic cells surrounding seminiferous tubules in the testis. In mice, PTM cells may arise from cells within the developing gonad, or alternatively from a layer of cells surrounding the outside of the gonad. In either case, they become recognizable at 13.5 days postconception (12). Unlike other myoid cells, PTM cells are induced to show a smooth muscle characteristic, such as the expression of smooth muscle actin, mainly by androgens and follicle-stimulating hormone (FSH). This suggests a crucial role for Leydig and Sertoli cells in the differentiation of PTM (13). Developing in close proximity to other somatic cells and germ cells from early embryonic stages, PTM cells are intimately involved in testes development and spermatogenesis. The interplay between PTM cells and surrounding somatic counterparts in the adult testis is intrinsic to overall testicular function and is involved in the development of productional disorders including azoosperm and infertility. Indeed, a knockout (KO) of androgen receptors in PTM cells significantly reduced Leydig cell markers, including steroidogenic factor 1, insulin-like growth factors (IGFs), and IGF-3, in a certain population of Leydig cells (14). Sertoli cell function was also impaired in this KO model, suggesting a paracrine interaction between PTM cells and other testicular cells. Given the crucial role of Sertoli cells in spermatogenesis, providing the structural and functional environment for spermatogonial stem cells (SSCs), PTM cells might also have an indirect influence on SSCs. Moreover, separated only by a basal lamina from SSCs, PTM cells may directly contribute to the SSC niche by secreting factors, such as glial cell line–derived neurotrophic factor (15). Furthermore, since PTM cells contribute to periodical contraction and relaxation of seminiferous tubules, disruption of their function is a strategy for human male contraception (16). Testicular tubular fibrosis, which involves alteration of PTM cell morphology and function (ie, paracrine and contractile abilities), is associated with male infertility (17). These observations provide a compelling incentive to understand the mechanism driving the activities of PTM cells and their effects on testicular functions.
Given that few studies have been conducted to investigate the functions of Cops5 in somatic cells of the testis, we set out to investigate the role of Cops5 in PTM cells. Using mice conditionally deficient for the Cops5 gene in SMCs, including PTM cells, we found that Cops5 plays a crucial role in organogenesis, including in the reproductive system, during early development and hormone homeostasis. Surprisingly, the deletion of Cops5 in SMCs caused significant growth retardation, skeletal and immunological defects, and early death. Analysis of gross anatomy identified significant alternations in organ coefficients of the brain, spleen, stomach, intestine, and reproductive organs. Radiological imaging also revealed scoliosis in both male and female Cops5 KO mice, accompanied by reduced expression of collagen (COL) and endomucin (EMCN). Detailed biochemical analysis revealed a significantly altered serum level of hormones in Cops5 KO mice, suggesting profound changes in endocrine homeostasis. These include reduced gonadotropins (FSH, luteinizing hormone [LH]), testosterone, and a concomitant decrease in IGF-1, but not vasopressin. Serum glucose level was also significantly reduced in the conditional KO (cKO) mice. In summary, we show here that the absence of Cops5 in SMCs leads to impairment in both postnatal development and spermatogenesis, which has been shown to contribute to general development and defective reproductive function.
Materials and Methods
Ethics Statement
All animal experiments were performed in compliance with the Guidelines for the Care and Use of Animals, as adopted by the National Institutes of Health. The animal protocols were approved by Wayne State University Institutional Animal Care with the Program Advisory Committee (protocol number: 18-02-0534).
Housing Conditions
All animals were housed in an animal room that was maintained at 23 ± 1 °C and 40 ± 30% relative humidity with air ventilation at a speed of 13 times per hour and a 12-hour light–dark cycle. Rodent diet standard chow (radiation sterilized) and drinking water (tap water, via automatic water supply system) were provided at libitum.
Generation of Conditional Cops5 Knockout Mice
To generate conditional Cops5 KOs (hereafter referred to as Cops5 cKO), males carrying the Myh11-Cre transgene were crossed for 2 generations with females in which Cops5 is floxed. Specifically, 3- to 4-month-old Cops5flox/flox females (kindly provided by Dr. Ruggero Pardi, Università Vita) were crossed with 3- to 4-month-old males that express Cre recombinase under the control of the Myh11 promoter (Jackson Laboratory, Bar Harbor, ME, Stock number: 007742). The male of the resulting Cops5flox/+;Myh11-Cre mice were crossed back with Cops5flox/flox females at the age of 3 to 4 months. Thus, the offspring had the following genotypes: (1) Myh11-Cre+;Cops5flox/flox, (2) Myh11-Cre+;Cops5flox/+, (3) Myh11-Cre−;Cops5flox/flox, and (4) Myh11-Cre−;Cops5flox/+. The first were considered to be homozygous cKO mice, and the remaining male littermates were used as the controls. The representative genotyping result is shown elsewhere (Fig. S1 (18)). All mice were on a C57 background.
Genotyping
For genotyping, mouse toe biopsies were taken at the age of 1 week, and genomic DNA was prepared as polymerase chain reaction (PCR) templates. All primer sequences are available elsewhere (Table S1 (18)). PCR with Cops5-for/Cops5-rev primer pair resulted in a 273-bp wild-type product and a 397-bp floxed Cops5 product; PCR with Cre-for/Cre-rev primer pair resulted in a 111-bp Cre product in mice with the Myh11-Cre allele (Fig. S1 (18)). A standard PCR program, 95 °C for 5 minutes, 35 cycles of 95 °C for 45 seconds, 58 to 60 °C for 30 seconds, 72 °C for 1 minute, followed by 72 °C for 10 minutes, was carried out.
Sperm Morphology and Parameter Assessment
Animals were sacrificed by CO2 inhalation followed by cervical dislocation. The cauda epididymis was cut and minced in a 6-cm culture dish containing 1 mL of phosphate-buffered saline (PBS), and incubated for 15 minutes at 37 °C to allow for the release of sperm. Then, sperm were collected by removal of the tissue and fixed in fresh 4% (w/v) formaldehyde solution for 15 minutes. Sperm morphology was assessed by a BX51 Olympus microscope (Olympus Corp., Melville, NY, and Center Valley, PA) equipped with a ProgRes C14 camera (Jenoptik Laser). Sperm motility was observed using an inverted microscope (Tokyo, Japan) equipped with a 10× objective. Movies were recorded at 15 frames/second with a SANYO (Osaka, Japan) color charge-coupled device, high-resolution camera (vcc-3972), and Pinnacle Studio HD (version 14.0) software. For each sperm sample, 10 fields were analyzed. Individual spermatozoa were tracked using ImageJ and the plug-in MTrackJ. Sperm motility was calculated as the curvilinear velocity, which is equivalent to the curvilinear distance traveled by each spermatozoon in 1 second (curvilinear velocity = curvilinear distance/t).
Histological Analysis
For histological analysis, tissues were excised and fixed with 4% paraformaldehyde in PBS, pH 7.4, for at least 24 hours at 4 °C. The fixed tissues were subsequently processed for conventional paraffin embedment, and 5-μm-thick sections were cut and stained with hematoxylin and eosin with standard histochemical protocols. The sections were examined with a BX51 Olympus microscope (Olympus Corp.), and photographs were captured with a ProgRes C14 camera (Jenoptik Laser).
Microcomputed Tomography Evaluation
The harvested bones were fixed overnight in 4% paraformaldehyde/PBS (v/v) at 4 °C. After fixation, samples were dehydrated with serial ethanol (25%, 50%, 75%, and 100%) and stored at −20 °C until scanning. The specimens were scanned with a microcomputed tomography system (μCT100 Scanco Medical, Bassersdorf, Switzerland). Scan settings were voxel size 10 μm, medium resolution, energy 70 kV, intensity 114 μA, 0.5 mm AL filter, and integration time 500 μs.
Immunofluorescence
For immunofluorescence, freshly dissected samples were fixed overnight in 4% paraformaldehyde/PBS at 4 °C and processed for sections. Sections were blocked in 5% goat serum in PBS (v/v; blocking solution), followed by overnight incubation at 4 °C with primary antibodies diluted in blocking solution: mouse anticollagen I (Abcam, Cat# ab34710, Lot# GR3264194; 1:100, RRID:AB_731684; https://antibodyregistry.org/search?q=AB_731684), rabbit anticollagen II (Abcam, Cat# ab34712, Lot# n284007; 1:100, RRID: AB_731688; https://antibodyregistry.org/search?q=AB_731688), rat antiendomucin antibody (Santa Cruz, Cat# sc-65495, Lot# F2618, 1 μg/mL, RRID:AB_2100037; https://antibodyregistry.org/search?q=AB_2100037), mouse antialpha smooth muscle actin antibody (Abcam, Cat# ab21027, Lot# GR3428459-3; 0.1 μg/mL, RRID:AB_1951138; https://antibodyregistry.org/search?q=AB_1951138). Thereafter, sections were washed in PBS and incubated with secondary fluorescent antibody Alexa Fluor–conjugated secondary antibodies (red fluorescence, Alexa Fluor 594 donkey antirat IgG (H + L), Life Technologies Corporation, Cat# A-21209, Lot# 1547508, RRID:AB_2535795, https://antibodyregistry.org/search?q=A-21209; green fluorescence, donkey antimouse IgG (H + L) Alexa Fluor Plus 488, Invitrogen, Cat# A-32766, Lot# 1F271737 RRID:AB_2536183, https://antibodyregistry.org/search?q=A-32766; orange fluorescence, Alexa Fluor 647 donkey antirabbit IgG (H + L), Invitrogen, Cat# A-31573, Lot# 2544598 RRID:AB_2536183, https://antibodyregistry.org/search?q=A-31573) at 1:250 dilution with blocking solution at room temperature for 1 hour. Sections were then washed and mounted with Prolong gold antifade mounting medium with 4-,6-diamidino-2-phenylindole (DAPI; Invitrogen) on glass slides. Fluorescence images were captured using a Zeiss LSM 700 fluorescence microscope with a built-in Nikon DS-Qi1Mc digital camera and a DS-Fi1 digital camera, acquired using Nikon NIS Elements 3.2 imaging software (Nikon Instruments Inc., Melville, NY).
Measurement of Serum Hormone Levels
Serum testosterone, FSH, and LH were measured at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (U54-HD28934). IGF-1 was measured at the Aging Research Biomarker Core, USC Leonard Davis School of Gerontology. Serum Vasopressin was measured at Endocrine Technologies Core at Oregon National Primate Research Center.
Serum Glucose Level
Random serum glucose levels were measured at sacrifice using a glucose monitor (glucometer; Bayer, Indianapolis, IN).
Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM) of 4 to 6 mice from each group. Statistical analysis was carried out using Student's test (t-test) for comparison between 2 groups, with P < .05 as the threshold for a statistically significant difference.
Results
Homozygous Cops5 cKO Mice Exhibited Significant Growth Retardation and Early Death
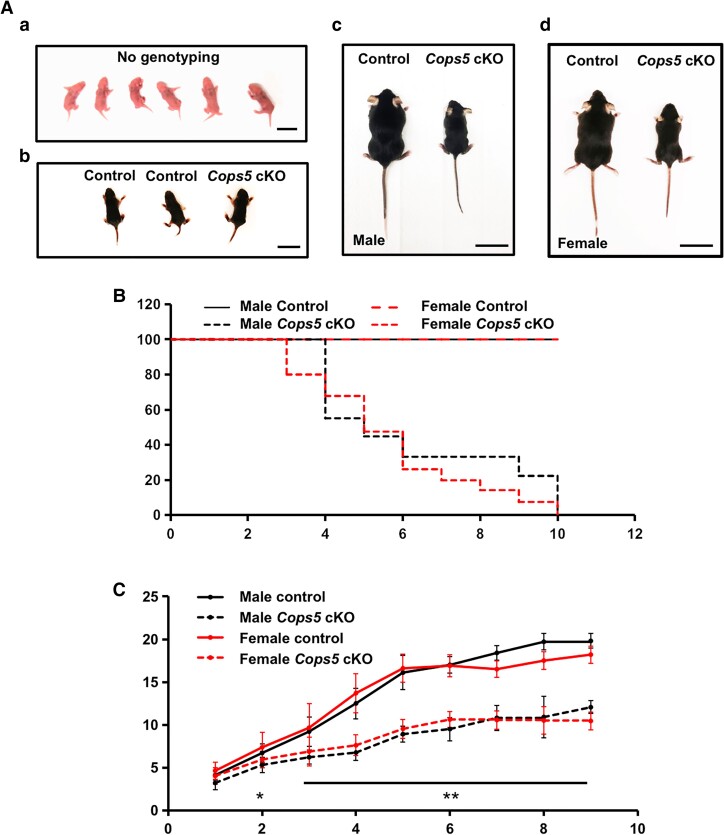
All homozygous Cops5 cKO mice were grossly normal up to 1 week of age. From the second week, both the Cops5 cKO males and females started to show reduced size (Fig. 1A), and began to die. In this study, no Cops5 cKO mice survived more than 67 days after birth. In contrast, no control mice died during the study (Fig. 1B). Before the Cops5 cKO mice died, all showed signs of severe dehydration. The body weight of the control and Cops5 cKO mice were recorded weekly. There was no significant difference between controls and cKO mice in the first week after birth in either males or females. However, from the second week of age, the body weight of the Cops5 cKO mice was significantly lower than that of controls, and the difference increased with age (Fig. 1C).
Figure 1.
Homozygous Cops5 cKO mice exhibit significant growth retardation and early death. (A) Representative images of 1-day-old (a), 1-week-old (b), and 2-week-old (c, d) Cops5 cKO mice and littermate controls. No differences were observed between the control group and the Cops5 cKO mice when they were 1 week old and younger (a, b). Bar = 1 cm; But, at 2 weeks old, both cKO males (c) and females (d) are smaller than the control littermates. Bar = 4 cm. (B) Kaplan–Meier survival curve of the Cops5 cKO mice and control littermates. The survival rate of mice in the Cops5 cKO mice was significantly lower than that in the control mice in both males and females. (C) Cops5 cKO mice display a remarkable reduction in body weight compared with control mice starting from the second week in both males (n = 6) and females (n = 4). A t-test was used for the evaluation of statistical significance. *P < .05, **P < .001.
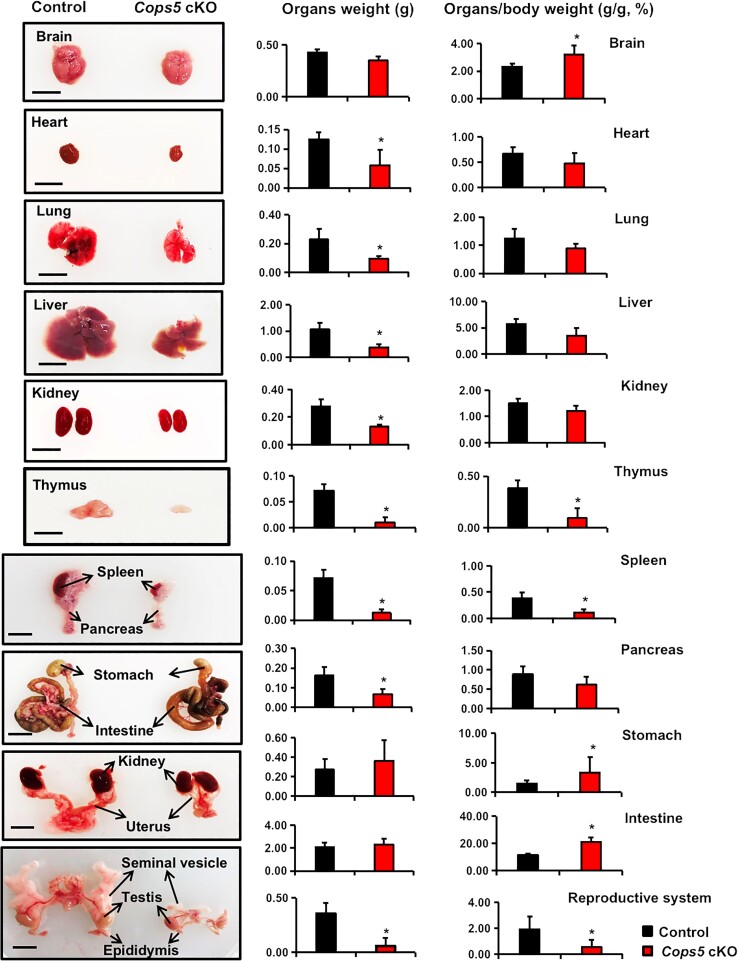
Development of Selective Organs, Especially the Reproductive Organs was Dramatically Affected in the Cops5 cKO Mice
To better understand the cause of early mortality, mice were sacrificed when exhibiting signs of imminent death and major organs were removed. These organs were weighed, and the organ index (organ weight/body weight) was calculated. The gross weights of the brain, stomach, and intestine in Cops5 cKO mice were similar to that of the control littermates. However, the organ indexes of the cKO mice were significantly higher due to reduced body weight. The heart, lung, liver, kidney, and pancreas was smaller in the cKO mice, but the organ indexes were similar when compared with the littermate controls. The thymus, spleen, and reproductive organs were extremely small, and the organ indexes were significantly lower in the cKO mice. Both male and female reproductive tracks, including the seminal vesicles, epididymis, oviduct, and uterus were extremely small in the cKO mice, making it impossible to calculate the organ index of these organs (Fig. 2). To compare the COPS5 protein levels between the control and the Cops5 cKO mice, Western blot was conducted using the lysates from the selective organs collected from these mice. In most organs, there was no difference in COPS5 level. However, the COPS5 level was significantly reduced in the spleen in Cops5 cKO mice (Fig. S2 (18)).
Figure 2.
Gross anatomy of organs from the control and Cops5 cKO mice. Internal organs from both the control and Cops5 cKO mice were collected and weighed, and the organ index (organ/body weight) was calculated. Presented here are representative images from 3-week-old mice along with gross organ weight and organ indexes. The kidney and uterus were from female mice, and the other organs were from male mice.*P < .05 compared with the controls. n = 4, bar = 1 cm.
Fertility was Reduced in Cops5 cKO Mice That Survived to Sexual Maturity
Even though most homozygous mice died before reaching sexual maturity, a fraction of the cKO mice survived to sexual maturation. To examine the fertility of these mice we set up breeding between 42-day-old cKO males with 2-month-old wild-type females. None of the homozygous males showed sexual behavior, such as chasing or approaching the females. The breeding period lasted from 2 weeks to 25 days until the cKO males were euthanized. Vaginal plugs were not observed in any of the wild-type females and none of them became pregnant. In contrast, all wild-type females bred with control males were pregnant and delivered pups (Table 1) (19). Fertility of homozygous females of the same age was also examined by breeding with 2-month-old wild-type males. Although vaginal plugs were observed in these cKO female mice, none of them became pregnant (Table 1) (19).
Table 1.
Fertility, vaginal plugs, and average litter size related to male and female control and Cops5 cKO mice
| Genotype | Male fertility (n = 4) | Vaginal plugs (n = 4) | Average litter size (n = 4) | Female fertility (n = 5) | Vaginal plugs (n = 5) | Average litter size (n = 5) |
|---|---|---|---|---|---|---|
| Control | 4/4 | 4/4 | 9.00 ± 1.60 | 5/5 | 5/5 | 7.80 ± 0.96 |
| Cops5 cKO | 0/4 | 0/4 | 0* | 0/5 | 5/5 | 0* |
To test fertility, 42-day-old males or females were bred with 2-month-old wild-type fertile females and males respectively for at least 2 weeks. Vaginal plugs and litter size were recorded.
*P < .05. Statistically significant differences.
Spermatogenesis Was Abnormal in Cops5 cKO Males That Survived to Sexual Maturation
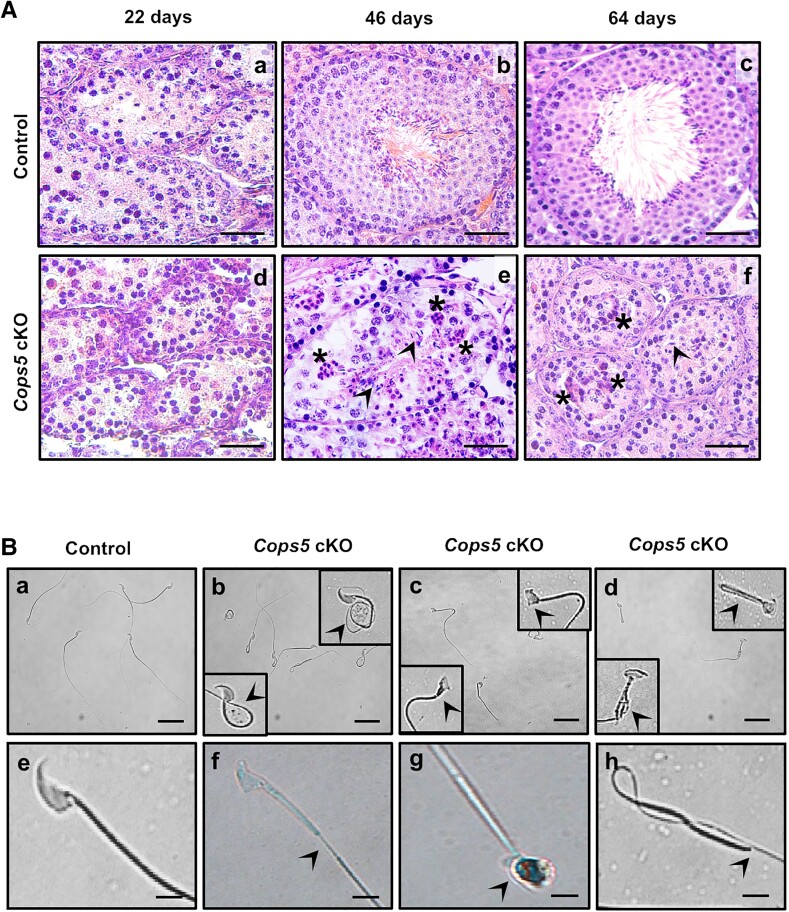
Testis histology of Cops5 cKO males and their wild-type littermates was examined at selected ages. In 22-day-old mice, no difference was observed between wild-type and cKO mice, both displaying a large number of round spermatids (Fig. 3A). However, clear differences were observed when testis histology of 46- and 64-day-old mice was analyzed. Of the cKO mice, 66.7% (n = 12) had no sperm, and 40% (n = 10) showed meiosis arrest. In cKO mice there were fewer elongated spermatids in the seminiferous epithelium, and vacuoles were observed in the epithelium, indicating degeneration and dissolution of germ cells (Fig. 3A; Fig. S3 (18)).
Figure 3.
Abnormal spermatogenesis in Cops5 cKO mice. (A) Histological analysis with testis section stained by hematoxylin and eosin. The control mice showed a normal arrangement of the seminiferous epithelium and spermatozoa in the tubule (a-c) at different ages, whereas the testis from Cops5 cKO mice at either 46 or 64 days of age showed an irregular arrangement of the seminiferous epithelium (e, f). Few germ cells developed to the elongating spermatids (e, f, arrowheads). The asterisks point to a vacuole formed inside the seminiferous tubules, indicating the degeneration of germ cells (e, f). Bar = 25 μm. (B) Representation images of sperm from control (a, e) and Cops5 cKO (b-d, f-h) mice. (a, e) Normal spermatozoa in control mice; (b-d, f-h) multiple abnormal morphologies of spermatozoa (arrowheads) were observed in the Cops5 cKO mice, including coiled tail (b), distorted heads (c), bent and forked tail (d), discontinuous tail (f), round head (g), and absence of head (h) in the Cops5 cKO mice. Bar 50 μm for upper images (a-d), and bar 10 μm for lower images (e-h).
Sperm did develop in some Cops5 cKO mice; however, the number and motility of sperm were dramatically reduced, although some sperm showed normal morphology (Table 2) (19). Analysis of sperm morphology revealed a significantly high occurrence of abnormal sperm in Cops5 cKO mice. The residual sperm from Cops5 cKO mice displayed severe abnormalities, including fully folded tails, amorphous heads, and hairpin loops (Fig. 3B).
Table 2.
Sperm parameter analysis of control and Cops5 cKO mice
| Genotype | Sperm count (106, n = 4) | Percentage of abnormal sperm (%, n = 4) | Percentage of motile sperm (%, n = 4) | Sperm motility (curvilinear velocity, μm/s, n = 4) |
|---|---|---|---|---|
| Control | 13.28 ± 1.91 | 5.25 ± 0.76 | 69.25 ± 3.30 | 72 ± 3.74 |
| Cops5 cKO | 2.91 ± 2.29* | 70.75 ± 10.87* | 16.25 ± 4.86* | 20.5 ± 6.56* |
Sperm were collected from cauda epididymides of the control and Cops5 cKO mice. Sperm count and motility, and percentage of abnormal and motile sperm were calculated.
*P < .05. Statistically significant differences.
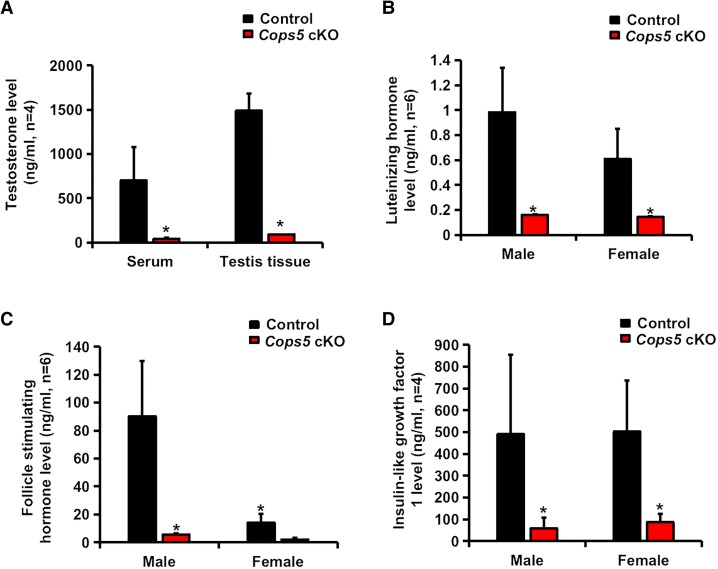
Sex Hormone Levels Were Dramatically Reduced in Cops5 cKO Mice
Hormones related to organ development were measured in sera and tissues. Testosterone level was significantly reduced in both serum and testicular tissues in 66-day-old Cops5 cKO mice relative to littermate controls (Fig. 4A). Moreover, homozygous Cops5 cKO mice of both sexes had significantly lower levels of LH (Fig. 4B), FSH (Fig. 4C), and IGF-1 (Fig. 4D) in serum than littermate controls. These results indicate that deletion of Cops5 in SMCs has a profound effect on hormone levels.
Figure 4.
Hormone levels were dramatically reduced in Cops5 cKO mice. (A) Testosterone level was decreased in both serum and testis tissue in the Cops5 cKO mice compared with the control group at 66 days old. (B-D) Luteinizing hormone (B), follicle-stimulating hormone (C), and insulin-like growth factor 1 (D) in serum were significantly decreased in Cops5 cKO mice. Statistical significance was evaluated by a 2-tailed unpaired Student's t-test (*P < .05).
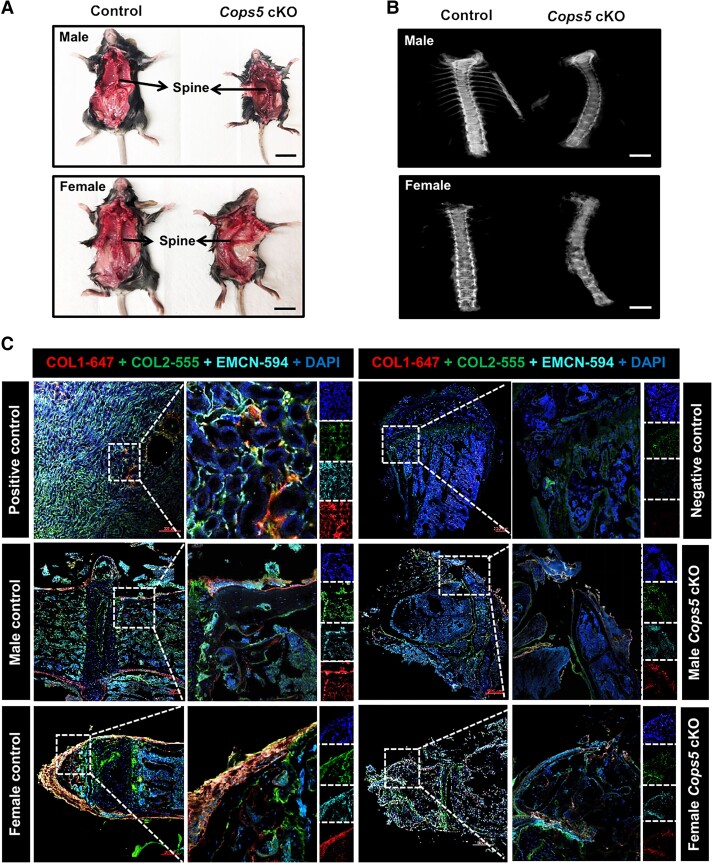
Cops5 cKO Mice Exhibited Impaired Skeletal Development
An intriguing finding in this study was that all Cops5 cKO mice exhibited scoliosis upon visual inspection (Fig. 5A), which was further confirmed by Faxitron (high-resolution x-ray) (Fig. 5B). To seek the causes of scoliosis, we examined the expression of selective proteins, including COL1, COL2, and EMCN, that are known to contribute to skeleton development and myoid tissue function. Indeed, the expression of COL1, COL2, and EMCN was reduced markedly in the spines of Cops5 cKO mice (Fig. 5C).
Figure 5.
Cops5 cKO mice exhibited scoliosis. (A) Representative images of the Cops5 cKO mice exhibiting scoliosis in both males (upper) and females (lower) and the littermate controls. Bar = 2 cm. (B) Examination of the spinal cord by Faxitron (high-resolution x-ray). Bar = 4 mm. (C) The expression of proteins that are associated with skeleton development was reduced in Cops5 cKO mice, evidenced by reduced density of immunofluorescence staining for COL1, COL2, and EMCN in spine sections.
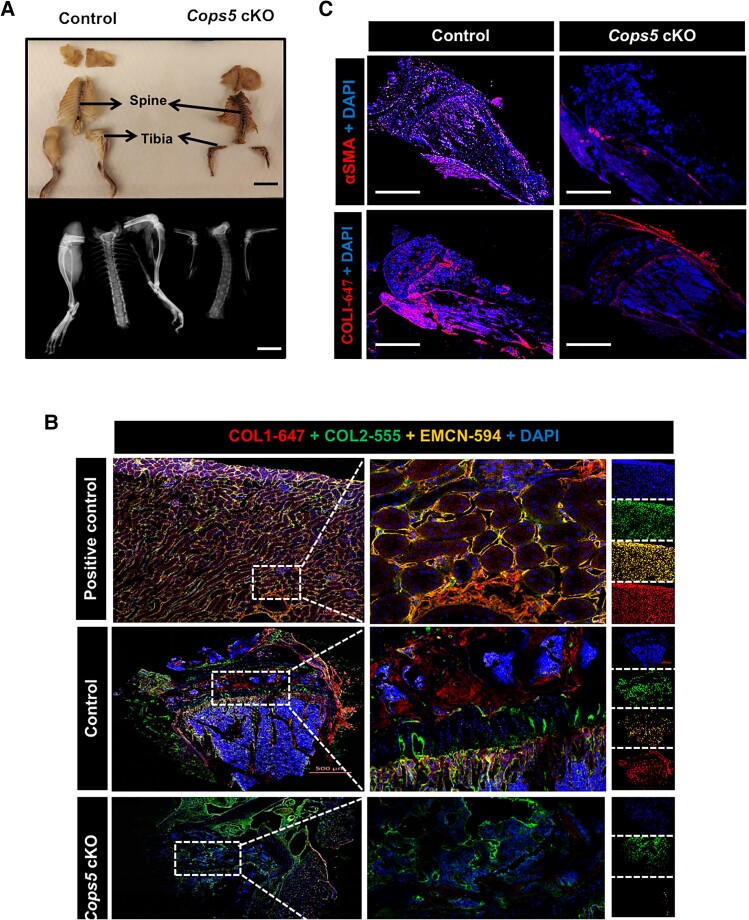
Furthermore, the length and width of the tibia from Cops5 cKO mice were reduced greatly (Fig. 6A). Immunofluorescence staining of the tibial section suggests profound changes in the expression of these bone development–related proteins (Fig. 6B), and concomitantly reduced expression of α-smooth muscle actin (α-SMA) (Fig. 6C). These findings indicate that the effects of impaired skeleton development observed could be partly attributed to the altered expression pattern of bone development–related genes.
Figure 6.
Impaired skeletal development of Cops5 cKO mice. (A) Representative images showing the variation of the length and width of the tibia between the control and Cops5 cKO mice. Bar = 2 cm (upper panel); bar = 8 mm (lower panel). (B) Immunofluorescence images showing reduced expression of EMCN and COL1 in the tibia of Cops5 cKO mice. (C) Immunofluorescence images showing decreased α-SMA and COL1 in the tissues around the tibia of Cops5 cKO mice. Bar = 1000 μm.
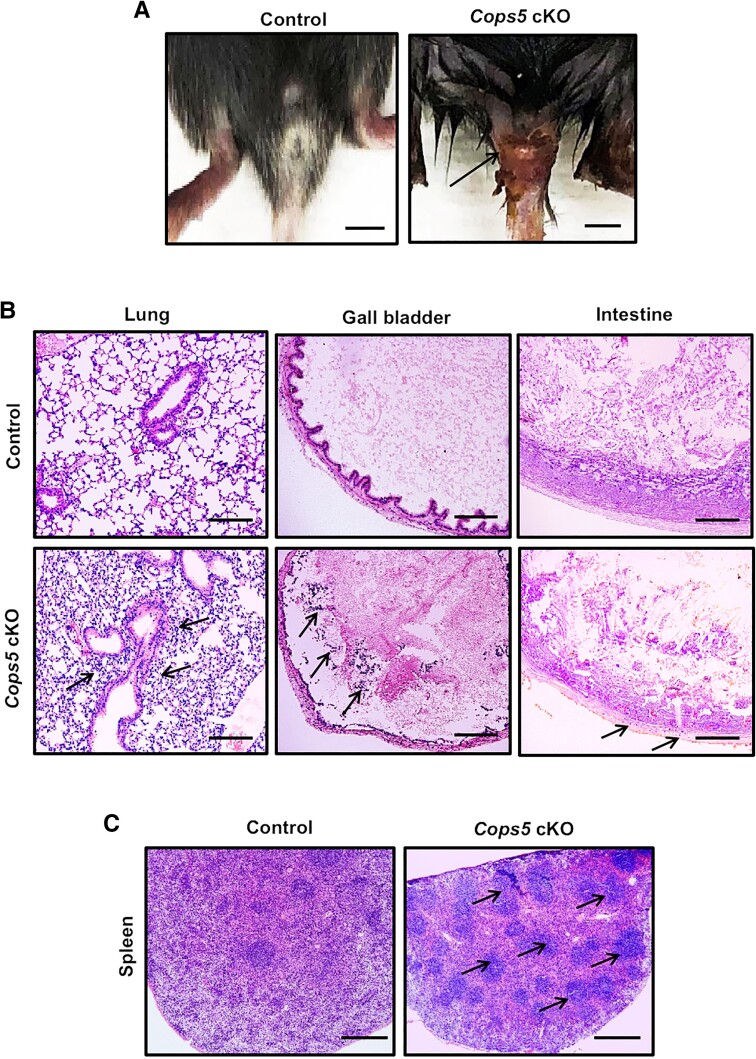
Infection Risk Was Increased in Cops5 cKO Mice
Some cKO mice, particularly those that died early (at around 3-4 weeks old), presented obvious infection at the base of the tail, evidenced by severe subcutaneous redness and swelling (Fig. 7A). Consistent with these signs of genital infection, histopathological analysis showed increased myeloid infiltration (mainly neutrophils) in the lung, gall bladder, and intestines in Cops5 cKO mice (Fig. 7B). Spleen histology revealed an increased number of germinal centers in the spleen of the Cops5 cKO mice, indicating an active immune response (Fig. 7C).
Figure 7.
Evidence of infection in Cops5 cKO mice. (A) Representative images showing genital infection in the Cops5 cKO mice. Bar = 1 cm. (B) Increased myeloid infiltration (mainly neutrophils, arrows) in the lung, gall bladder, and intestines in Cops5 cKO mice. Bar = 100 μm. (C) Increased number of germinal centers (arrows) in the spleen of the Cops5 cKO mice. Bar = 250 μm.
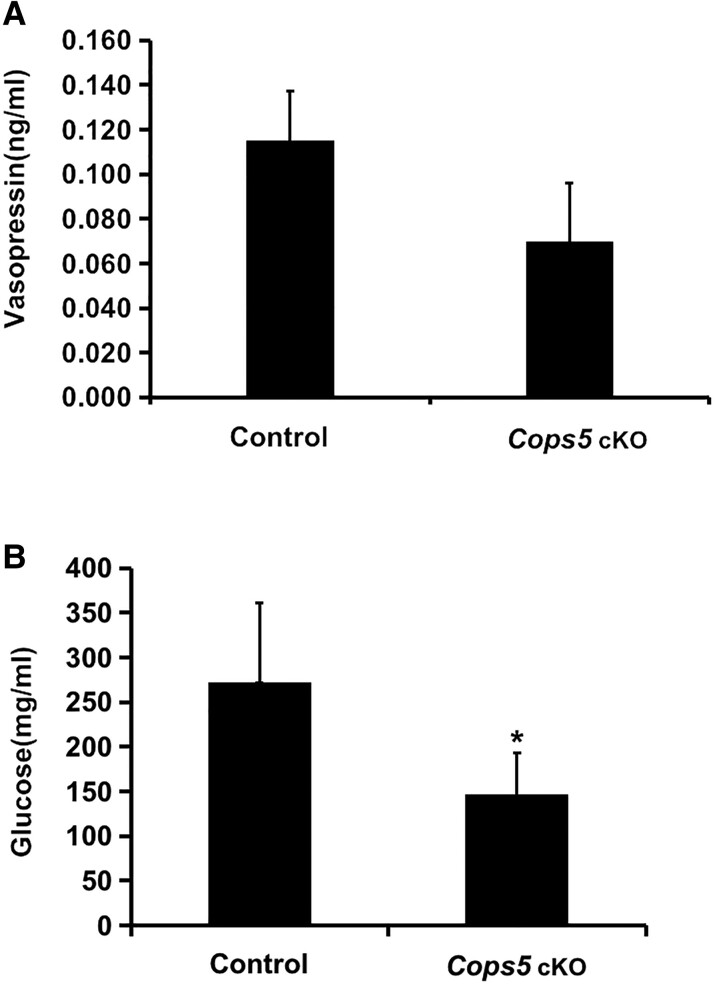
Level of Serum Glucose but not Vasopressin Was Reduced in Cops5 cKO Mice
Cops5 cKO mice showed signs of significant dehydration before death. To examine the potential causes of dehydration, both serum glucose and vasopressin (antidiuretic hormone) levels were analyzed. There were no significant differences in vasopressin levels between Cops5 cKO mice and controls (Fig. 8A). However, the serum glucose level was significantly lower in Cops5 cKO mice (Fig. 8B), suggesting that these mice had an Addison-like condition of adrenal gland underperformance, rather than hyperglycemia, which can induce water loss and dehydration.
Figure 8.
Serum glucose but not vasopressin was affected in Cops5 cKO mice. (A) Serum vasopressin levels (n = 5). (B) Serum glucose levels in control and Cops5 cKO mice (n = 8). Notice that serum glucose but not vasopressin was significantly reduced in Cops5 cKO mice (*P < .05).
Discussion
Cops5 plays a fundamental role in embryo development. Cops5-null embryos died soon after implantation (20). We are interested to know its function in reproduction, particularly in male reproduction. We had previously crossed the floxed Cops5 mice with transgenic Stra8-iCre mice so that the Cops5 gene was disrupted in germ cells only (11). All the homozygous mutant mice survived to adulthood without showing any gross abnormalities. The only phenotype identified was male infertility associated with abnormally developed germ cells; development of other reproductive organs was not affected (11) and the phenotype was totally different from the mutant mice described here. Germ cell development was also affected in this model; however, this might be secondary to other primary defects when the Cops5 gene was disrupted in SMCs in the whole body.
The original plan of our current study was to investigate the role of the Cops5 gene in PTM cells in the testis. Based on the study conducted by Chen and Liu (21), we crossed the floxed Cops5 mice to the Myh11-Cre transgenic mice. The homozygous mutant mice developed more severe phenotypes than expected, which could not be explained by disrupting the Cops5 gene in the PTM cells only.
Even though Myh11-Cre transgenic mice have been previously used to disrupt the Gdnf gene selectively in PTM cells (22), it has been suggested that the Myh11 promoter drives cre recombinase expression in other SMCs in addition to PTM cells (21). The severe and diverse phenotypic effects described here are consistent with the possibility that in the current mouse model, the Cops5 gene was disrupted not only in the PTM cells, but also in SMCs elsewhere in the animal. Therefore, the phenotype described here was due to the disruption of Cops5 in SMCs, but not PTMs. To study the role of Cops5 in PTMs, another transgenic line that drives Cre expression specifically in the PTMs cells should be used in future studies. Interestingly, the COPS5 protein level was not changed in most tissues/organs analyzed when the gene was disrupted in SMCs; 1 possibility is that SMCs are not the dominant cell population in these tissues/organs.
Failure of the development of reproductive organs in the Cops5 cKO mice strongly suggests a functional failure of sex hormone production or action. The observed striking reduction in serum LH, and FSH in both males and females, as well as reduced serum and testicular testosterone in males strongly indicates a failure of hormone production. LH and FSH are important pituitary hormones required for reproductive processes in both males and females (23). LH and FSH are released by the anterior pituitary in response to pulsatile gonadotropin-releasing hormone stimulation by the hypothalamus (24). In females, the combined action of FSH and LH stimulates the growth of ovarian follicles and steroidogenesis with the production of androgens that are then converted to estrogens (25). In males, FSH stimulates the Sertoli cells, resulting in spermatogenesis, and LH causes the interstitial Leydig cells of the testes to produce testosterone (26, 27). Given that our major interest is to study male reproduction and the fact that the ovaries were too small in the Cops5 cKO mice, we did not analyze ovarian histology or estrogen levels in the serum and ovary. In male Cops5 cKO mice, the reproductive phenotype is also characterized by reduced sex hormones. Homozygous males displayed a complete absence of sex behaviors, which can be explained by the extremely low testosterone level in vivo. Due to the small size of the animals and high cost of breeding more mice, as well as technical difficulties, we were not able to collect sufficient serum to measure other potentially relevant hormones, such as those upstream of LH/FSH, mainly gonadotropin-releasing hormone, or downstream of testosterone, including 5-dihydrotestosterone and estrogens (28). Additionally, we were not able to measure the potentially relevant adrenal steroid hormones cortisol and aldosterone. We predict that production of all these hormones would be affected when the Cops5 gene is inactivated in SMCs.
A principle and representative androgen involved in regulating the progression of spermatogenesis in mammals is testosterone. Withdrawal of testosterone is linked to the failure of round spermatids to complete spermiogenesis (29, 30). In most of the Cops5 cKO males surviving to sexual maturation, spermatogenesis was arrested at round spermatids, likely due to the extremely low levels of testosterone.
In addition to the effects of Cops5 cKO on reproductive organs, several other somatic organs were also affected, particularly the spleen, thymus, and skeleton system. Even though most organ weights were significantly lower in the Cops5 cKO mice. Given the smaller size of these mice, the organ indexes were not significantly reduced in most organs except the spleen and thymus. Interestingly, both are immunologic organs. It has been well established that steroid hormones, including glucocorticoids and the sex hormones, estrogen, progesterone, and testosterone, possess immunomodulatory roles that include effects on T and B cell development, lymphoid organ size, lymphocyte cell death, immune function, and susceptibility to autoimmune disease (31, 32). It is possible that the failure of development of the immunologic organs, including spleen and thymus, was caused by the significantly changed steroid hormone levels in the Cops5 cKO mice, which also may have contributed to the increased susceptibility to infection.
SMCs have been linked to the complex process of inflammation. In atherosclerosis, which is a cardiovascular disease with a strong inflammatory component, atherosclerotic lesions are composed of at least 30% vascular SMC-derived cells (33). These pleiotropic SMCs are strongly involved in the initiation of a chronic, nonresolving inflammatory state, including the migration of immune-competent cells (33). In testes from men with impaired spermatogenesis, cytoarchitecture of the tubular wall, which is composed mainly of PTM cells, is frequently remodeled, concomitant with infiltration by macrophages and mast cells (34). The active immune response observed in Cops5 KO mice suggests an essential role of Cops5 in SMCs during innate immune responses. The precise role of COPS5 in SMCs during immune response in tissue warrants future investigation.
Steroid hormones also play a key role in bone development. Thus, scoliosis and reduced bone density reported here might be caused by the defect in steroid hormone production observed in the Cops5 cKO mice. In addition, the extracellular matrix (ECM) influences organogenesis by modulating cell behavior. During bone development, the chondrocytes deposit an ECM comprising type II COL (COL2), and then differentiate into hypertrophic chondrocytes, depositing an ECM comprising type X COL (COL10) (35). This ECM is then partially mineralized, resorbed, and replaced by a matrix comprising predominantly type I COL (COL1) (35). This conserved developmental program involves intimate interaction between ECM and the various cell types in skeletal tissues, where ECM regulates cell adhesion, differentiation, proliferation, and responses to growth factors (36). In the present study, COL1, which accounts for 90% of the total organic component of bone ECM, was significantly decreased in the spine and tibia. Produced by mesenchymal lineage cells such as osteoblasts and fibroblasts, COL1 plays an essential role in organogenesis, osteogenesis, and bone-related diseases (37). Myosin heavy chain (Myh11) is a known marker gene for myofibroblasts (38). The attenuated immunostaining signals of COL1 might suggest that the loss of Cops5 in the cKO mice causes a secretion disorder in skeletal muscle fibroblasts, the main producer of COL1, which in turn interferes with skeleton development. Moreover, vascular invasion is an important cellular event during bone growth. Invading blood vessels convey osteoblasts to form bone on the cartilage template (39). These vasculatures can be observed and analyzed using endothelial markers such as EMCN, a typical membrane glycoprotein that is mainly expressed in skeletal endothelial cells (40‐42). In the present study, decreased EMCN in the spine and tibia might suggest impaired vascular invasion during skeleton development, which might be attributable to both abnormal hormonal homeostasis and ECM formation.
Failure of sex hormone and glucocorticoid production would not be expected to cause animal death. The fact that all the cKO mice showed severe dehydration before death also suggests a failure of mineralocorticoid (aldosterone) production or action in these mice. The major function of aldosterone is to regulate salt and water balance (43). Abnormally low levels of aldosterone in the cKO mice, especially in combination with low cortisol production, will lead to sodium and water loss and could contribute to the premature death of these animals. Reduced hormonal output from the adrenal context is also consistent with the observation of hypoglycemia in the cKO mice. In humans, dehydration and hypoglycemia are common symptoms of Addison disease, which is caused by damage to the adrenal cortex and reduced hormonal output of adrenal corticosteroids.
Given the growth retardation, we also examined the serum IGF-1 level. IGF-1 is a circulating autocrine/paracrine factor that regulates prenatal and postnatal growth in many tissues (44). IGF-1 is produced primarily by the liver as an endocrine hormone as well as in target tissues in a paracrine/autocrine fashion. Its production is stimulated by growth hormone, which is made in the anterior pituitary gland, released into the bloodstream, and then stimulates the liver to produce IGF-1. IGF-1 then stimulates systemic body growth and has growth-promoting effects on almost every cell in the body, especially skeletal muscle, cartilage, and bone (45). Not surprisingly, the Cops5 cKO mice had a significantly reduced serum IGF-1 level. IGF-1 KO mice rarely survive (44). IGF-1 null mice that do survive have diminished organismal growth (44). For instance, Cops5 cKO mice showed significant growth retardation, early death, and reduced organ weight, which can be attributed to decreased serum IGF-1. Moreover, IGF-1 is one of the major growth factors that direct skeletal muscle development, growth, and regeneration. Specific inactivation of IGF-1 in skeletal muscle leads to 10% to 30% smaller muscles, whereas increasing IGF-1 in muscles causes improves in disease muscle phenotype and function (46). Interestingly, IGF-1 has been implicated in deformities of the spine, such as adolescent idiopathic scoliosis. Single nucleotide polymorphism of the IGF-1 gene was found to be associated with the curve severity of adolescent idiopathic scoliosis, although the underlying mechanisms of the functionality of the polymorphism of the IGF-1 gene are not clear (47). As IGF-1 is synthesized by multiple mesenchymal cell types, further work focusing on the function of Cops5 in IGF-1 production is required to fully understand its role in skeleton development and disease.
It is not clear why the inactivation of Cops5 in SMCs dramatically changed circulatory hormone levels. The hormones are secreted through glandular ducts that are surrounded by a layer of SMCs. One possibility is that the secretary function is disrupted in cKO mice and that gives rise to reduced circulatory hormone levels. It is also possible that the hormone synthesis process was disrupted.
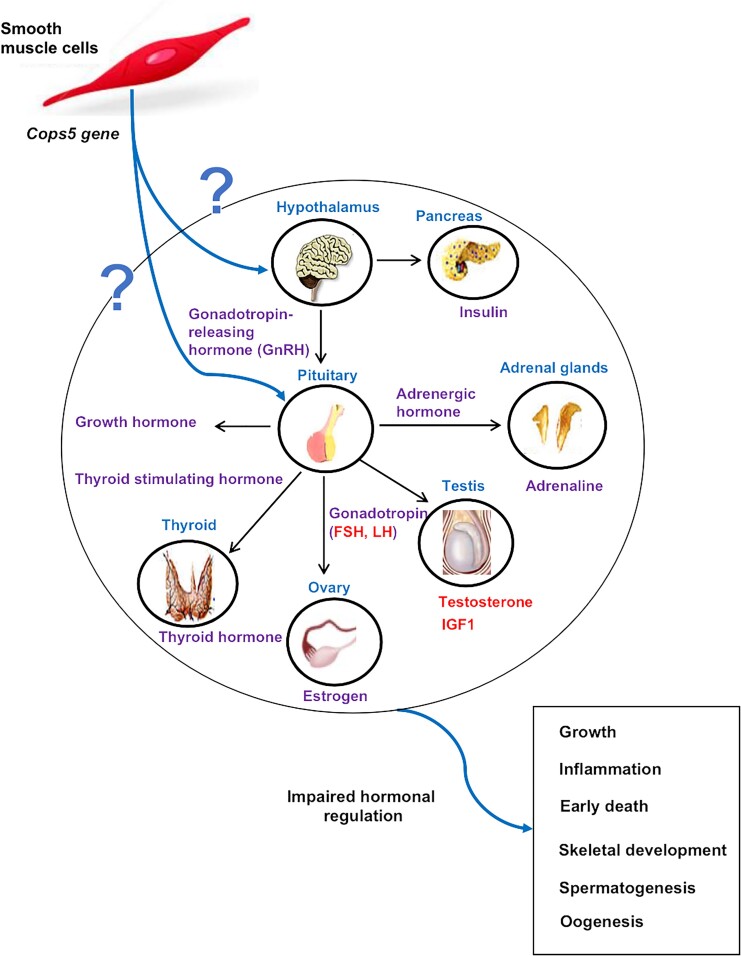
Collectively, our data provide valuable insights into the role of Cops5 in regulating biological functions in postnatal development and survival. The study suggests that loss of Cops5 in SMCs can result in profound changes in organ development and tissue cytoarchitecture, particularly in reproductive and immunologic organs and bone. Impaired steroid hormone production might be the primary cause for these disorders (Fig. 9). However, the mechanisms of how COPS5 controls steroid hormone synthesis/secretion await further investigation.
Figure 9.
Proposed mechanism of inactivation of Cops5 gene in smooth muscle cells causes disruption of reproductive hormone homeostasis and failure of development in mice.
Acknowledgments
Histology was conducted at the Henry-Ford histology core.
Abbreviations
- cKO
conditional knockout
- COPS5
COP9 constitutive photomorphogenic homolog subunit 5
- ECM
extracellular matrix
- EMCN
endomucin
- FSH
follicle-stimulating hormone
- IGF
insulin-like growth factor
- KO
knockout
- LH
luteinizing hormone
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PTM
peritubular myoid
- SMC
smooth muscle cell
- SSC
spermatogonial stem cell
Contributor Information
Qian Huang, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China; Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Yonghong Man, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China.
Wei Li, Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Qi Zhou, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China; Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Shuo Yuan, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China; Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Yi Tian Yap, Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Neha Nayak, Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Ling Zhang, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China.
Shizheng Song, Department of Occupational and Environmental Health, School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei 430065, China.
Joseph Dunbar, Department of Physiology, Wayne State University, Detroit, MI 48210, USA.
Todd Leff, Department of Pathology, Wayne State University, Detroit, MI 48210, USA.
Xu Yang, Arthroplasty Research Laboratory, Hospital for Special Surgery, New York, NY 10021, USA.
Zhibing Zhang, Department of Physiology, Wayne State University, Detroit, MI 48210, USA; Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI 48210, USA.
Funding
This research was supported by the Wayne State University Start-up fund (to Z.Z.), and the National Institute of Health (HD105944 to Z.Z.). Y.T.Y. was supported by an MCI fellowship. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by NIH grant (U54-HD28934); Endocrine Technologies Core (ETC) at Oregon National Primate Research Center (ONPRC) is supported (in part) by NIH grant P51 OD011092 for operation of the Oregon National Primate Research Center.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Pan Y, Yang H, Claret FX. Emerging roles of Jab1/CSN5 in DNA damage response, DNA repair, and cancer. Cancer Biol Ther. 2014;15(3):256‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavadini S, Fischer ES, Bunker RD, et al. Cullin-RING ubiquitin E3 ligase regulation by the COP9 signalosome. Nature. 2016;531(7596):598‐603. [DOI] [PubMed] [Google Scholar]
- 3. Suisse A, Békés M, Huang TT, Treisman JE. The COP9 signalosome inhibits Cullin-RING E3 ubiquitin ligases independently of its deneddylase activity. Fly (Austin). 2018;12(2):118‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan C, Wang D, Liu G, Pan Y. Jab1/Cops5: a promising target for cancer diagnosis and therapy. Int J Clin Oncol. 2021;26(7):1159‐1169. [DOI] [PubMed] [Google Scholar]
- 5. Stobdan T, Akbari A, Azad P, et al. New insights into the genetic basis of Monge's disease and adaptation to high-altitude. Mol Biol Evol. 2017;34(12):3154‐3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malhotra AG, Jha M, Singh S, Pandey KM. Construction of a comprehensive protein-protein interaction map for vitiligo disease to identify key regulatory elements: a systemic approach. Interdiscip Sci. 2018;10(3):500‐514. [DOI] [PubMed] [Google Scholar]
- 7. Asare Y, Ommer M, Azombo FA, et al. Inhibition of atherogenesis by the COP9 signalosome subunit 5 in vivo. Proc Natl Acad Sci U S A. 2017;114(13):E2766‐E2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheng Z, Xu Y, Li F, Wang S, Huang T, Lu P. CSN5 attenuates Ang II-induced cardiac hypertrophy through stabilizing LKB1. Exp Cell Res. 2019;376(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 9. Wang R, Wang H, Carrera I, Xu S, Lakshmana MK. COPS5 protein overexpression increases amyloid plaque burden, decreases spinophilin-immunoreactive puncta, and exacerbates learning and memory deficits in the mouse brain. J Biol Chem. 2015;290(14):9299‐9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cayli S, Eyibilen A, Gurbuzler L, et al. Jab1 expression is associated with TGF-beta1 signaling in chronic rhinosinusitis and nasal polyposis. Acta Histochem. 2012;114(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 11. Huang Q, Liu H, Zeng J, et al. COP9 signalosome complex subunit 5, an IFT20 binding partner, is essential to maintain male germ cell survival and acrosome biogenesis. Biol Reprod. 2020;102(1):233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pelosi E, Koopman P. Development of the Testis. Reference Module in Biomedical Sciences. Elsevier, 2017:4. [Google Scholar]
- 13. Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23(12):4218‐4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welsh M, Moffat L, Belling K, et al. Androgen receptor signalling in peritubular myoid cells is essential for normal differentiation and function of adult Leydig cells. Int J Androl. 2012;35(1):25‐40. [DOI] [PubMed] [Google Scholar]
- 15. Chen LY, Brown PR, Willis WB, Eddy EM. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology. 2014;155(12):4964‐4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang YQ, Batool A, Chen SR, Liu YX. GATA4 Is a negative regulator of contractility in mouse testicular peritubular myoid cells. Reproduction. 2018;156(4):343‐351. [DOI] [PubMed] [Google Scholar]
- 17. Mayer C, Adam M, Glashauser L, et al. Sterile inflammation as a factor in human male infertility: involvement of toll like receptor 2, biglycan and peritubular cells. Sci Rep. 2016;6(1):37128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Q, Man YH, Li W, et al. Supplementary information for: Inactivation of Cops5 in smooth muscle cells causes abnormal reproductive hormone homeostasis and development in mice. Figshare2023. Deposited April 7, 2023. 10.6084/m9.figshare.22575034.v1 [DOI] [PMC free article] [PubMed]
- 19. Huang Q, Man YH, Li W, et al. Data from: Inactivation of Cops5 in smooth muscle cells causes abnormal reproductive hormone homeostasis and development in mice. Figshare2023. Deposited April 7, 2023. 10.6084/m9.figshare.22573402.v1 [DOI] [PMC free article] [PubMed]
- 20. Tomoda K, Yoneda-Kato N, Fukumoto A, Yamanaka S, Kato JY. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J Biol Chem. 2004;279(41):43013‐43018. [DOI] [PubMed] [Google Scholar]
- 21. Chen SR, Liu YX. Myh11-Cre is not limited to peritubular myoid cells and interaction between Sertoli and peritubular myoid cells needs investigation. Proc Natl Acad Sci U S A. 2016;113(17):E2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen LY, Willis WD, Eddy EM. Targeting the Gdnf gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A. 2016;113(7):1829‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans JJ. Modulation of gonadotropin levels by peptides acting at the anterior pituitary gland. Endocr Rev. 1999;20(1):46‐67. [DOI] [PubMed] [Google Scholar]
- 24. Kota AS, Ejaz S. Precocious Puberty. StatPearls, 2022. [PubMed] [Google Scholar]
- 25. Sirois J, Kimmich TL, Fortune JE. Developmental changes in steroidogenesis by equine preovulatory follicles: effects of equine LH, FSH, and CG. Endocrinology. 1990;127(5):2423‐2430. [DOI] [PubMed] [Google Scholar]
- 26. Aldahhan RA, Stanton PG, Ludlow H, de Kretser DM, Hedger MP. Experimental cryptorchidism causes chronic inflammation and a progressive decline in Sertoli cell and Leydig cell function in the adult rat testis. Reprod Sci. 2021;28(10):2916‐2928. [DOI] [PubMed] [Google Scholar]
- 27. Gooren LJ, Kruijver FP. Androgens and male behavior. Mol Cell Endocrinol. 2002;198(1-2):31‐40. [DOI] [PubMed] [Google Scholar]
- 28. Anderson LJ, Migula D, Abay R, et al. Androgens and estrogens predict sexual function after autologous hematopoietic stem cell transplant in men. Andrology. 2022;10(2):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharpe RM. Testosterone and spermatogenesis. J Endocrinol. 1987;113(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 31. Lü FX, Abel K, Ma Z, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krzych U, Strausser HR, Bressler JP, Goldstein AL. Effects of sex hormones on some T and B cell functions, evidenced by differential immune expression between male and female mice and cyclic pattern of immune responsiveness during the estrous cycle in female mice. Am J Reprod Immunol (1980). 1981;1(2):73‐77. [DOI] [PubMed] [Google Scholar]
- 33. Sorokin V, Vickneson K, Kofidis T, et al. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front Immunol. 2020;11:599415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walenta L, Fleck D, Frohlich T, et al. ATP-mediated Events in peritubular cells contribute to Sterile testicular inflammation. Sci Rep. 2018;8(1):1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herlofsen SR, Küchler AM, Melvik JE, Brinchmann JE. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng Part A. 2011;17(7-8):1003‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang W, Liu Y, Zhang H. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021;11(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Yang S, Lovisa S, et al. Type-I collagen produced by distinct fibroblast lineages reveals specific function during embryogenesis and osteogenesis imperfecta. Nat Commun. 2021;12(1):7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie T, Wang Y, Deng N, et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018;22(13):3625‐3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Filipowska J, Tomaszewski KA, Niedźwiedzki L, Walocha JA, Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park-Windhol C, Ng YS, Yang J, Primo V, Saint-Geniez M, D'Amore PA. Endomucin inhibits VEGF-induced endothelial cell migration, growth, and morphogenesis by modulating VEGFR2 signaling. Sci Rep. 2017;7(1):17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ji G, Xu R, Niu Y, et al. Vascular endothelial growth factor pathway promotes osseointegration and CD31hiEMCNhi endothelium expansion in a mouse tibial implant model: an animal study. Bone Joint J. 2019;101–B(7 Suppl C):108‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu R, Yallowitz A, Qin A, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24(6):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horton R. Aldosterone and aldosteronism. Steroids. 2003;68(14):1135‐1138. [DOI] [PubMed] [Google Scholar]
- 44. Powell-Braxton L, Hollingshead P, Warburton C, et al. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12b):2609‐2617. [DOI] [PubMed] [Google Scholar]
- 45. Ahmad SS, Ahmad K, Lee EJ, Lee YH, Choi I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells. 2020;9(8):1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernández AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest. 2002;109(3):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan M, Wang H, Fang H, Zhang C, Gao S, Zou Y. Association between IGF1 gene single nucleotide polymorphism (rs5742612) and adolescent idiopathic scoliosis: a meta-analysis. Eur Spine J. 2017;26(6):1624‐1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.