Abstract
Background
Alpha-fetoprotein-producing gastric cancer (AFPGC) is a rare type of aggressive gastric cancer (GC) with a dismal prognosis. We present a patient with AFPGC who achieved long-term survival through a multidisciplinary approach.
Case presentation
A 67-year-old man with advanced GC was referred to our hospital for systemic chemotherapy. He was diagnosed with cStage IVB AFPGC. During 2nd-line treatment, we could not control bleeding from the GC itself. After complete resection, during chemotherapy, portal venous tumor thrombi (PVTTs) and liver metastases were identified. With nivolumab followed by irinotecan, the PVTTs and liver metastases disappeared. Without immunotherapy and chemotherapy for 23 months, the patient has survived for 48 months so far with no recurrence of GC.
Conclusion
Long-term survival with AFPGC can be accomplished by using several different approaches, such as surgery, immunotherapy, and chemotherapy.
Keywords: Alpha-fetoprotein, Gastric cancer, Long-term survival, Multidisciplinary therapy
Background
Alpha-fetoprotein (AFP) is an oncofetal protein [1]. In the fetus, AFP is synthesized mainly in the liver and yolk sac and peaks in concentration at 14 weeks of gestation. Afterward, serum AFP decreases gradually over the 1st year of age. Elevated serum AFP levels in adults are used as a clinical biomarker for hepatocellular carcinoma or yolk sac tumors [2, 3]. Most AFP-producing tumors originate from the foregut endoderm, which includes the stomach [4].
AFP-producing gastric cancer (AFPGC) is a rare type of gastric cancer (GC). The reported incidence of GC is 1.3–15% [5]. AFPGC has a poor prognosis and is characterized by higher rates of venous invasion, lymphatic invasion, and metachronous or synchronous liver metastases than other GCs [6]. Here, we report a patient with AFPGC who achieved long-term survival through a multidisciplinary approach.
Case presentation
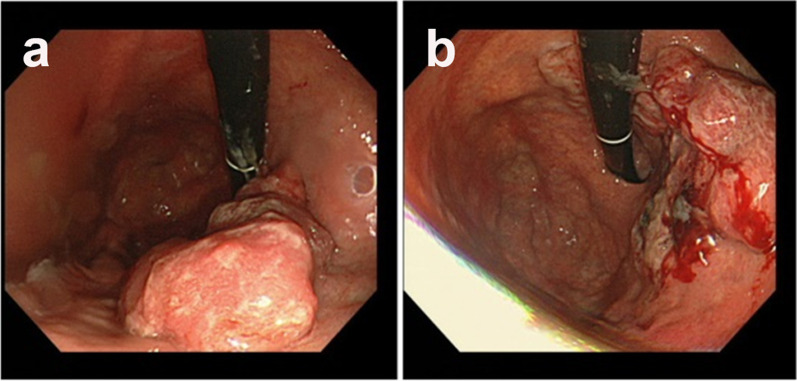
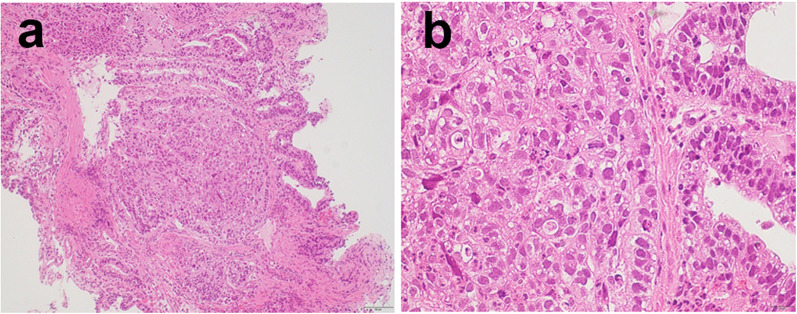
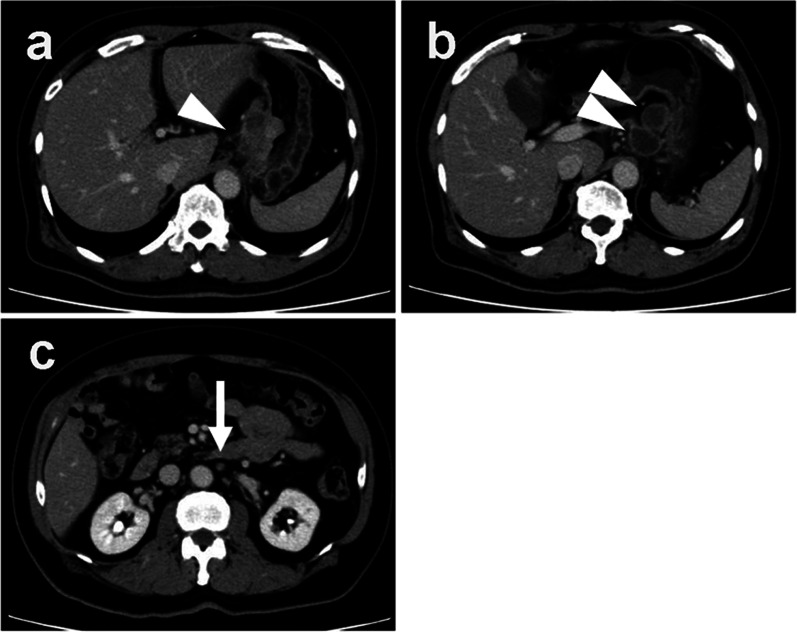
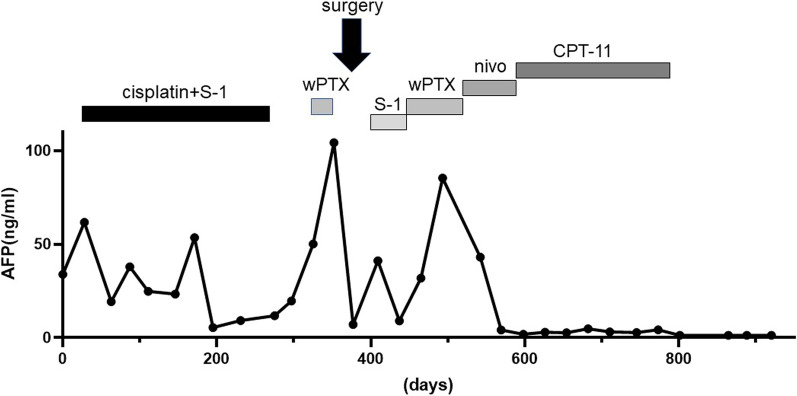
In December 2018, a 67-year-old man was referred to our hospital for systemic chemotherapy. During diabetes follow-up, his anemia progressed, and serum carcinoembryonic antigen (CEA) level became high. By upper gastrointestinal endoscopy, a type III tumor was found stretching from the fundus to the corpus of the stomach (Fig. 1). A biopsy from the stomach showed human epidermal growth factor receptor 2 (HER2)-negative adenocarcinoma (tub1; Fig. 2). Its microsatellite instability (MSI) status was stable. A computed tomography (CT) scan showed a thickened gastric wall with several enlarged lymph nodes along the lesser curvature and a swollen paraaortic lymph node (Fig. 3). The cancer stage was cT4aN3M1, cStage IVB. His serum AFP level was 33.90 ng/ml (normal range < 15 ng/ml). He was diagnosed with AFPGC. He underwent six cycles of 1st-line therapy consisting of cisplatin and S-1, and three months later he presented at the emergency department due to hematemesis. Paclitaxel was administered as a 2nd-line therapy, but the chemotherapy could not control his bleeding from the GC. He underwent total gastrectomy plus D2 + No. 16b1 dissection to control the bleeding. Right after radical surgery in January 2020, a CT scan showed no metastases. Adjuvant chemotherapy (S-1) was administered, and peritonitis carcinoma was suspected. During paclitaxel rechallenge administration as a 3rd-line therapy, serum AFP increased (Fig. 4). Magnetic resonance imaging (MRI) showed PVTTs in segment 6/7 (S6/7) and segment 8 (S8, Fig. 5). We switched the paclitaxel to nivolumab. After the 2nd cycle of nivolumab, serum creatinine kinase increased. There were no symptoms of myasthenia gravis. To avoid immune-related adverse effects, after the 3rd cycle, we changed nivolumab to irinotecan. At that point, a CT scan revealed that liver metastases were not clear as before, but intrahepatic cholangiectasis was present at S6/7 and S8. After irinotecan was initiated, the serum AFP level remained within the normal range, and the liver metastases kept shrinking. Given the long-term effects of nivolumab, we stopped irinotecan at the 4th cycle in February 2021. During follow-up without immunotherapy or chemotherapy, in October 2021 a CT scan revealed that the liver metastases had disappeared and that there were no other recurrent lesions. Positron emission tomography (PET)/CT also showed no recurrence of the tumor. At present (December 2022), the patient has reached 48 months of survival without any recurrence.
Fig. 1.
Endoscopic appearance. a, b Type III tumor at the lesser curvature of the stomach
Fig. 2.
Microscopic findings on the biopsied GC. Hematoxylin and eosin staining. a ×100, b ×400
Fig. 3.
Abdominal CT. a Irregular mass at the lesser curvature of the stomach (arrowhead). b Enlarged lymph nodes at the lesser curvature of the stomach (arrowheads). c Suspected enlarged paraaortic lymph nodes (arrow)
Fig. 4.
Clinical course. As a 2nd therapy, weekly paclitaxel (wPTX) without ramucirumab was administered due to hematemesis. Before surgery, the patient experienced a 2nd hematemesis. wPTX weekly paclitaxel; nivo nivolumab
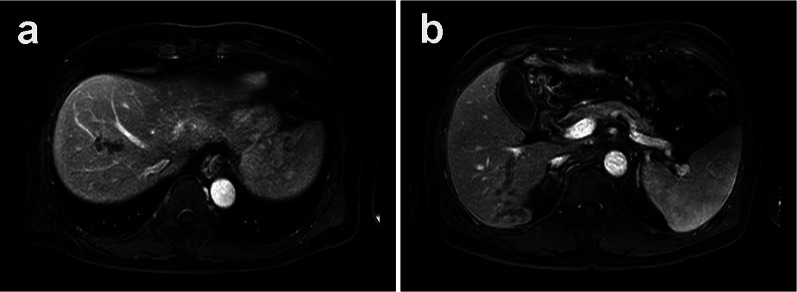
Fig. 5.
Ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI). At the portal venous phase, the portal vein was cut off in the middle. Dilated vessels are observed from the obstruction to peripheral sites, indicating PVTT. a S8, b S6/7
Discussion
The prognosis of AFPGC is poor, however, the prognosis can be improved by a multidisciplinary approach. Recently, several long-surviving AFPGC patients have been reported [6–8]. Those studies highlight the importance of multidisciplinary approach to survival with AFPGC.
To ascertain the length of survival with AFPGC, we searched the literature using the terms “gastric cancer”, “AFP”,”AFPGC”, “prognosis”, and “clinicopathological” in PubMed up to 30 September 2022. We included clinical studies that analyzed overall survival time and 5-year survival rates, written in English, with detailed clinical information available. We excluded studies that (i) examined fewer than 10 cases, (ii) examined only hepatoid histology, and (iii) had unavailable full texts. The incidence of liver metastasis at the time GC was diagnosed and during follow-up after surgery was included. We identified 23 studies of AFPGC that studied clinicopathological features (Table 1) [5, 9–30]. The median overall survival time with AFPGC is 14–72 months, and the 5-year survival rate is 8.3–66.0%. Although the survival period and survival rate vary from study to study, the survival is longer for other GCs than for AFPGC in each study [5, 10, 11, 14, 19–21, 23, 30].
Table 1.
Clinical characteristics of AFPGC
| Publication | Duration | Treatment | Number | OS (months) | 5-year survival rate | Liver metastases | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFP | Non-AFP | AFP | Non-AFP | AFP | Non-AFP | AFP | Non-AFP | ||||
| Chang et al. [9] | 1990 | Nov 1979–Dec 1988 | All | 24 | NA | NA | NA | 8.3% | NA | NA | NA |
| Curative gastrectomy | 8 | NA | NA | NA | 25% | NA | NA | NA | |||
| Palliative surgery | 16 | NA | NA | NA | NA | NA | NA | NA | |||
| Chang et al. [10] | 1992 | Nov 1979 | All | 27 | 478 | NA | NA | 11.6% | 52.8% | 72% | 9.80% |
| Radical operation | NA | NA | NA | NA | 33.3% | 69.5% | NA | NA | |||
| Kono et al. [11] | 2002 | Oct 1983–Dec 1999 | All, surgery | 27 | 945 | NA | NA | 28.4% | 62.0% | 63% | 9% |
| Curative gastrectomy | 15 | 634 | NA | NA | 48.5% | 87.0% | NA | NA | |||
| Adachi et al. [12] | 2003 | June 1982–Mar 2001 | All (includes gastrectomy) | 270 | NA | 14 | NA | 22% | NA | 33% | NA |
| Curative gastrectomy | 136 | NA | 29 | NA | 42% | NA | NA | NA | |||
| Kochi et al. [13] | 2004 | 1989–2002 | StageIV (FLEP chemotherapy) includes curative surgery | 10 | 47 | 15.8 | 10.3 | NA | NA | 60.00% | 23.40% |
| Ishigami et al. [14] | 2006 | 1990–2001 | Curative surgery | 19 | 468 | NA | NA | 31% | 69% | 12% | 2% |
| Liu et al. [15] | 2010 | Jan 1996–Dec 2007 | All, gastrectomy | 104 | 208 | NA | NA | 28% | 38% | 60.60% | NA |
| Inoue et al. [16] | 2010 | Jan 1992–Dec 2001 | All | 53 | NA | NA | NA | 34% | NA | 52.80% | NA |
| Chun and Kwon [5] | 2011 | Feb 2001–Dec 2008 | Curative gastrectomy | 35 | 659 | 72 | NA | 66% | 80% | NA | NA |
| Liu et al. [17] | 2012 | Jan 1996–Dec 2007 | All, surgery | 59 | 208 | NA | NA | 41% | 38% | 49.20% | 11.50% |
| Li et al. [18] | 2013 | Not mentioned | All | 317 | NA | 31.1 | NA | 0–49.8% | NA | 56.7% | 19.80% |
| Hirajima et al. [19] | 2013 | 1997–2011 | Gastrectomy | 23 | 1276 | NA | NA | 50.3% | 76.5% | 43% | 3% |
| Lin et al. [20] | 2014 | June 1988–Dec 2011 | All, surgery | 58 | 1236 | NA | NA | 17.8% | 45.8% | 27.60% | 4.40% |
| 0.0% | |||||||||||
| Chen et al. [21] | 2015 | Jan 2004–Dec 2008 | Gastrectomy | 86 | 1200 | NA | NA | 18.6% | 48.7% | 6.98% | 1.50% |
| Wang et al. [22] | 2015 | Jan 2009–Dec 2012 | Surgery (radical or palliative) | 45 | 589 | 40.3 | NA | NA | NA | 57.80% | 3.74% |
| Reim et al. [23] | 2017 | Jan 2002–August 2007 | All, R0 resection | 97 | 2937 | NA | NA | 57.00% | 79.40% | NA | NA |
| He et al. [24] | 2017 | Jan 2010–May 2016 | All | 82 | NA | NA | NA | NA | NA | 20.70% | NA |
| All | 72 | NA | 42.02 | NA | NA | NA | NA | NA | |||
| Surgery | 60 | NA | 45.43 | NA | NA | NA | NA | NA | |||
| Non-surgery | 12 | NA | 12.85 | NA | NA | NA | NA | NA | |||
| Bozkaya et al. [25] | 2017 | 2009–2015 | All | 53 | 309 | 12.6 | 22.1 | NA | NA | 81.60% | 45.90% |
| Bozkaya et al. [26] | 2017 | 2009–2015 | All, cStageiV | 34 | 135 | 11.3 | 11.4 | NA | NA | 70.60% | 31.90% |
| Modified docetaxel + cisplatin + 5-FU | |||||||||||
| Wang et al. [27] | 2018 | 2006–2016 | No surgery | 105 | NA | 13.9 | NA | NA | NA | 60% | NA |
| Liu et al. [28] | 2020 | Jan 2013–Mar 2016 | Surgery (radical or palliative) | 16 | 123 | 40 | 55 | NA | NA | NA | NA |
| Wang et al. [29] | 2020 | Jan 2007–Oct 2018 | All | 96 | NA | 16.5 | NA | 7.80% | NA | 39.60% | NA |
| Curative surgery ± chemotherapy | 20 | NA | 47 | NA | NA | NA | NA | NA | |||
| Chemotherapy alone palliative therapy | 76 | NA | 13.5 | NA | NA | NA | NA | NA | |||
| Zhan et al. [30] | 2022 | Jan 2008–Dec 2015 | All, R0 resection | 191 | 2127 | NA | NA | 39.79% | 55.00% | 46.60% | 24.90% |
NA not assessed; OS overall survival
Surgical procedures are one of the most important parts to a multidisciplinary approach [9, 21]. The median overall survival with AFPGC after curative surgery is 29–72 months, and the 5-year survival rate is 25.0–66.0%. Moreover, radical surgery and curative-intent surgery extend the survival time compared with palliative surgery [24]. However, if the cancer has metastasized, surgical treatment does not improve the outcome [24]. Surgical procedures contribute to prolonging the survival benefit to AFPGC, but not AFPGC with metastasis [24], indicating that a multidisciplinary approach is necessary to achieve long-term survival.
In the present case, we performed total gastrectomy plus D2 + No. 16b1 dissection to control the bleeding. At that time, the clinical stage was not changed from Stage IV because enlarged No.16b1 was suspected as a metastasized lymph node by a CT scan. The reason why we performed total gastrectomy plus D2 + No. 16b1 dissection is following two reasons; first, enlarged lymph nodes along the lesser curvature and gastric cancer became a lump. Total gastrectomy plus D2 was safer than palliative gastrectomy. Second, total gastrectomy plus D2 was performed with curative intent. Taking account of the effects of pre-operative chemotherapies, we performed No.16b1 dissection to aim at a R0 resection. Pathological studies after the operation showed No.16b1 was not metastasized.
Treatment with immunotherapy and/or chemotherapy also contributes to long-term survival. In the present case, after radical surgery, a CT scan showed liver metastases with PVTTs, which disappeared after the administration of nivolumab followed by irinotecan. The incidence of liver metastases with AFPGC is higher than that with other GCs. From our literature review, the incidence of liver metastasis with AFPGC is 6.98–72%, which is higher than that with other GCs (Table 1). The presence of PVTT in advanced GC is rare, with a prevalence of 1.2% [31], while the incidence of PVTT in AFPGC is as high as 12.4% [27]. The prognosis of PVTT with gastric cancer is dismal, with a median survival of 5.4 months. Liver metastases and PVTT have been found as independent prognostic factors for AFPGC by multivariate analysis [15, 19, 20, 27].
Nivolumab might have had a greater influence on long-term survival than irinotecan in the present case. The efficacies of nivolumab and irinotecan are not different. We compared two studies in which nivolumab (ONO-4538-12, ATTRACTION-2) or irinotecan was used as a third-line or later therapy for advanced GC [32, 33]. There were no differences in the disease control rate between nivolumab (40.0%) and irinotecan (43.2%). The median overall survival time was also not different: 5.26 months [95% confidence interval (CI) 4.60–6.37] for nivolumab and 6.6 months (95% CI 5.9–7.3) for irinotecan. On the other hand, nivolumab has long-lasting effects similar to those of other immune checkpoint inhibitors. In the ATTRACTION-2 study, the Kaplan‒Meier curve for OS had a raised tail, which was caused by an increasing number of long-term survivors [34]. Currently, we are following up with the present patient without continuing any therapy. The disappearance of the metastasized tumor by nivolumab followed by irinotecan and the continuous effects of nivolumab might explain the long-term survival of the present case.
Conclusion
We report a case of AFPGC with long-term survival after surgery, immunotherapy, and chemotherapy. AFPGC is known to have a very poor prognosis, but long-term survival can be achieved by a multidisciplinary approach.
Acknowledgements
Not applicable.
Abbreviations
- AFP
Alpha-fetoprotein
- AFPGC
Alpha-fetoprotein producing gastric cancer
- CEA
Carcinoembryonic antigen
- CT
Computed tomography
- EOB-MRI
Ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging
- GC
Gastric cancer
- nivo
Nivolumab
- MRI
Magnetic resonance imaging
- MSI
Microsatellite instability
- NA
Not assessed
- OS
Overall survival
- PVTT
Portal venous tumor thrombus
- wPTX
Weekly paclitaxel
Author contributions
TO drafted the manuscript. All authors were involved in the management of the patient. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated during this case report are included in this article.
Declarations
Ethics approval and consent to participate
This study was approved by the research ethics committee of Izumi City General Hospital.
Consent for publication
The patient provided written informed consent for publication of this case report and any accompanying images. A copy of the consent form has been made available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soltani K. Alpha-fetoprotein: a review. J Investig Dermatol. 1979;72:211–213. doi: 10.1111/1523-1747.ep12530749. [DOI] [PubMed] [Google Scholar]
- 2.Tatarinov IuS. Detection of embryo-specific alpha-globulin in the blood sera of patients with primary liver tumour. Vop Med Khim. 1964;10:90–91. [PubMed] [Google Scholar]
- 3.Norgaard-Pedersen B, Albrechtsen R, Teilum G. Serum alpha-foetoprotein as a marker for endodermal sinus tumour (yolk sac tumour) or a vitelline component of “teratocarcinoma”. Acta Pathol Microbiol Scand A. 1975;83:573–589. doi: 10.1111/j.1699-0463.1975.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 4.McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975;35:991–996. [PubMed] [Google Scholar]
- 5.Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer. 2011;11:23–30. doi: 10.5230/jgc.2011.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada M, Tsujimoto H, Ichikura T, Nagata H, Ito N, Nomura S, et al. A case of a long-term survival achieved by surgical treatment and chemotherapy for late recurrence of AFP-producing gastric cancer. Surg Case Rep. 2019;5:106. doi: 10.1186/s40792-019-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Fujiya M, Ijiri M, Takahashi K, Ando K, Nomura Y, et al. A case of alpha-fetoprotein-producing adenocarcinoma of the esophagogastric junction in which long-term survival was achieved by means of individualized multidisciplinary therapy. J Gastrointest Cancer. 2019;50:617–620. doi: 10.1007/s12029-018-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao S, Nakata B, Tendo M, Kuroda K, Hori T, Inaba M, et al. Salvage surgery after chemotherapy with S-1 plus cisplatin for α-fetoprotein-producing gastric cancer with a portal vein tumor thrombus: a case report. BMC Surg. 2015;15:5. doi: 10.1186/1471-2482-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–1485. [PubMed] [Google Scholar]
- 10.Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321–325. [PubMed] [Google Scholar]
- 11.Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–65. doi: 10.1159/000065838. [DOI] [PubMed] [Google Scholar]
- 12.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 13.Kochi M, Fujii M, Kaiga T, Takahashi T, Morishita Y, Kobayashi M, et al. FLEP chemotherapy for alpha-fetoprotein-producing gastric cancer. Oncology. 2004;66:445–449. doi: 10.1159/000079498. [DOI] [PubMed] [Google Scholar]
- 14.Ishigami S, Natsugoe S, Nakashima H, Tokuda K, Nakajo A, Okumura H, et al. Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer. Hepatogastroenterology. 2006;53:338–341. [PubMed] [Google Scholar]
- 15.Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. 2010;97:1056–1061. doi: 10.1002/bjs.7081. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012;106:299–303. doi: 10.1002/jso.23073. [DOI] [PubMed] [Google Scholar]
- 18.Li XD, Wu CP, Ji M, Wu J, Lu B, Shi HB, et al. Characteristic analysis of α-fetoprotein-producing gastric carcinoma in China. World J Surg Oncol. 2013;11:246. doi: 10.1186/1477-7819-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirajima S, Komatsu S, Ichikawa D, Kubota T, Okamoto K, Shiozaki A, et al. Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol. 2013;19:6055–6061. doi: 10.3748/wjg.v19.i36.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HJ, Hsieh YH, Fang WL, Huang H, Li AF. Clinical manifestations in patients with alpha-fetoprotein-producing gastric cancer. Curr Oncol. 2014;21:e394–e399. doi: 10.3747/co.21.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Qu H, Jian M, Sun G, He Q. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Mark. 2015;30:e387–e393. doi: 10.5301/jbm.5000167. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Li C, Xu Y, Xing Y, Qu L, Guo Y, et al. Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp Pathol. 2015;8:6345–6355. [PMC free article] [PubMed] [Google Scholar]
- 23.Reim D, Choi YS, Yoon HM, Park B, Eom BW, Kook MC, et al. Alpha-fetoprotein is a significant prognostic factor for gastric cancer: results from a propensity score matching analysis after curative resection. Eur J Surg Oncol. 2017;43:1542–1549. doi: 10.1016/j.ejso.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 24.He R, Yang Q, Dong X, Wang Y, Zhang W, Shen L, et al. Clinicopathologic and prognostic characteristics of alpha-fetoprotein-producing gastric cancer. Oncotarget. 2017;8:23817–23830. doi: 10.18632/oncotarget.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozkaya Y, Demirci NS, Kurtipek A, Erdem GU, Ozdemir NY, Zengin N. Clinicopathological and prognostic characteristics in patients with AFP-secreting gastric carcinoma. Mol Clin Oncol. 2017;7:267–274. doi: 10.3892/mco.2017.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozkaya Y, Doğan M, Yazıcı O, Erdem GU, Demirci NS, Zengin N. The efficacy of modified docetaxel-cisplatin-5-fluorouracil regimen as first-line treatment in patients with alpha-fetoprotein producing gastric carcinoma. Bosn J Basic Med Sci. 2017;17:138–143. doi: 10.17305/bjbms.2017.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YK, Shen L, Jiao X, Zhang XT. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterol. 2018;24:266–273. doi: 10.3748/wjg.v24.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Li B, Yan B, Liu L, Jia Y, Wang Y, et al. The clinicopathological features and prognosis of serum AFP positive gastric cancer: a report of 16 cases. Int J Clin Exp Pathol. 2020;13:2439–2446. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Li J, Xu D, Li R, Gong P. Dynamic change in serum alpha-fetoprotein level predicts treatment response and prognosis of alpha-fetoprotein-producing gastric cancer. Medicine (Baltimore) 2020;99:e23326. doi: 10.1097/MD.0000000000023326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Z, Chen B, Yu J, Zheng J, Zeng Y, Sun M, et al. Elevated serum alpha-fetoprotein is a significant prognostic factor for patients with gastric cancer: results based on a large-scale retrospective study. Front Oncol. 2022;12:901061. doi: 10.3389/fonc.2022.901061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eom BW, Lee JH, Lee JS, Kim MJ, Ryu KW, Choi IJ, et al. Survival analysis of gastric cancer patients with tumor thrombus in the portal vein. J Surg Oncol. 2012;105:310–315. doi: 10.1002/jso.22083. [DOI] [PubMed] [Google Scholar]
- 32.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 33.Makiyama A, Arimizu K, Hirano G, Makiyama C, Matsushita Y, Shirakawa T, et al. Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer. 2018;21:464–472. doi: 10.1007/s10120-017-0759-9. [DOI] [PubMed] [Google Scholar]
- 34.Harris SJ, Brown J, Lopez J, Yap TA. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med. 2016;13(2):171–193. doi: 10.20892/j.issn.2095-3941.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this case report are included in this article.