Abstract
Objectives:
To determine performance of C-reactive protein (CRP) in diagnosis of early-onset sepsis (EOS), and to assess patient outcomes with and without routine use of CRP.
Study design:
Retrospective cohort study of infants admitted to two neonatal intensive care units. CRP was routinely used in EOS evaluations during 2009–2014; this period was utilized to determine CRP performance at a cut-off of ≥10 mg/L in diagnosis of culture-confirmed EOS. Routine CRP use was discontinued during 2018–2020; outcomes among infants admitted during this period were compared with those in 2012–2014.
Results:
From 2009–2014, 10,134 infants were admitted; 9,103 (89.8%) had CRP and 7,549 (74.5%) had blood culture obtained within 3 days of birth. CRP obtained ±4 hours from blood culture had a sensitivity of 41.7%, specificity 89.9% and positive likelihood ratio 4.12 in diagnosis of EOS. When obtained 24–72 hours after blood culture, sensitivity of CRP increased (89.5%), but specificity (55.7%) and positive likelihood ratio (2.02) decreased. Comparing the periods with (n=4,977) and without (n=5,135) routine use of CRP, we observed lower rates of EOS evaluation (74.5% vs. 50.5%), antibiotic initiation (65.0% vs. 50.8%), and antibiotic prolongation in the absence of EOS (17.3% vs. 7.2%) in the later period. Rate and timing of EOS detection, transfer to a higher level of care, and in-hospital mortality were not different between periods.
Conclusions:
CRP diagnostic performance was not sufficient to guide decision-making in EOS. Discontinuation of routine CRP use was not associated with differences in patient outcomes despite lower rates of antibiotic administration.
Keywords: antibiotic stewardship, biomarker, clinical utility, newborn
C-reactive protein (CRP) is an acute phase reactant commonly used as a biomarker in evaluation for neonatal early-onset sepsis.(1–5) The clinical utility of a test can be measured using two approaches: diagnostic performance, and impact on patient outcomes.(6) Metrics for diagnostic performance include sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratios, and are calculated by comparing the results of the test with a “gold standard”. The gold standard used to diagnose EOS is pathogen isolation from blood or cerebrospinal fluid (CSF) culture, with or without additional clinical findings of infection.(7,8) Microbial cultures have limitations, and their use as the gold standard comparator for testing diagnostic performance of other biomarkers in neonatal sepsis has been debated.(9) The clinical utility of a test can also be measured by the extent to which its use guides decisions that improve patient outcomes, an approach that may circumvent the issue of an inadequate gold standard.(5,6) For EOS, relevant patient outcomes include early recognition of infection, prompt initiation of indicated antibiotic treatment, minimal antibiotic use among the uninfected, and reduction of mortality and morbidity. In this study, we assessed the clinical utility of CRP in the diagnosis of EOS using both the approaches – measuring diagnostic performance (Objective 1) and impact on patient outcomes with and without routine use (Objective 2), in a large cohort over a 12-year period.
Methods
This was a retrospective cohort study of all infants admitted within 3 days after birth to two neonatal intensive care units (NICUs) in the University of Pennsylvania Health System. The study centers share a common medical record system and aligned clinical practice. From 1/1/2009–12/31/2014, the centers used a categorical approach to neonatal EOS risk assessment based on previously published work, and utilized routine CRP measurement.(10) We determined the diagnostic performance of CRP in EOS for infants admitted during this time period (Objective 1). Between 2015–2017, both centers transitioned their approach of neonatal EOS risk assessment to the Neonatal Early-Onset Sepsis Calculator for term infants, and delivery criteria for preterm infants.(11,12) These new approaches did not require CRP values for decision making, and the new guideline did not incorporate its use.(13,14) By 2018, <10% of EOS evaluations included CRP measurement. To study the impact of CRP use on patient outcomes (Objective 2), comparisons were made between infants born 1/1/2012–12/31/2014 (Period 1: routine CRP use) and 1/1/2018–12/31/2020 (Period 2: minimal CRP use) (Figure 1). These time periods were selected to maximize capture of EOS cases for diagnostic performance analysis (Objective 1) and to minimize missing data elements in comparing patient outcomes (Objective 2; discharge disposition data was missing from hospital records during 2009–2011). Patient demographics, laboratory results, and medication data were obtained from the institutional data warehouse for 2009–2014 and from electronic medical records (Epic®, Epic Systems Corporation, Verona, WI) for 2018–2020. The study was approved by the Institutional Review Board of the University of Pennsylvania with a waiver of informed consent.
Figure 1. Study population.
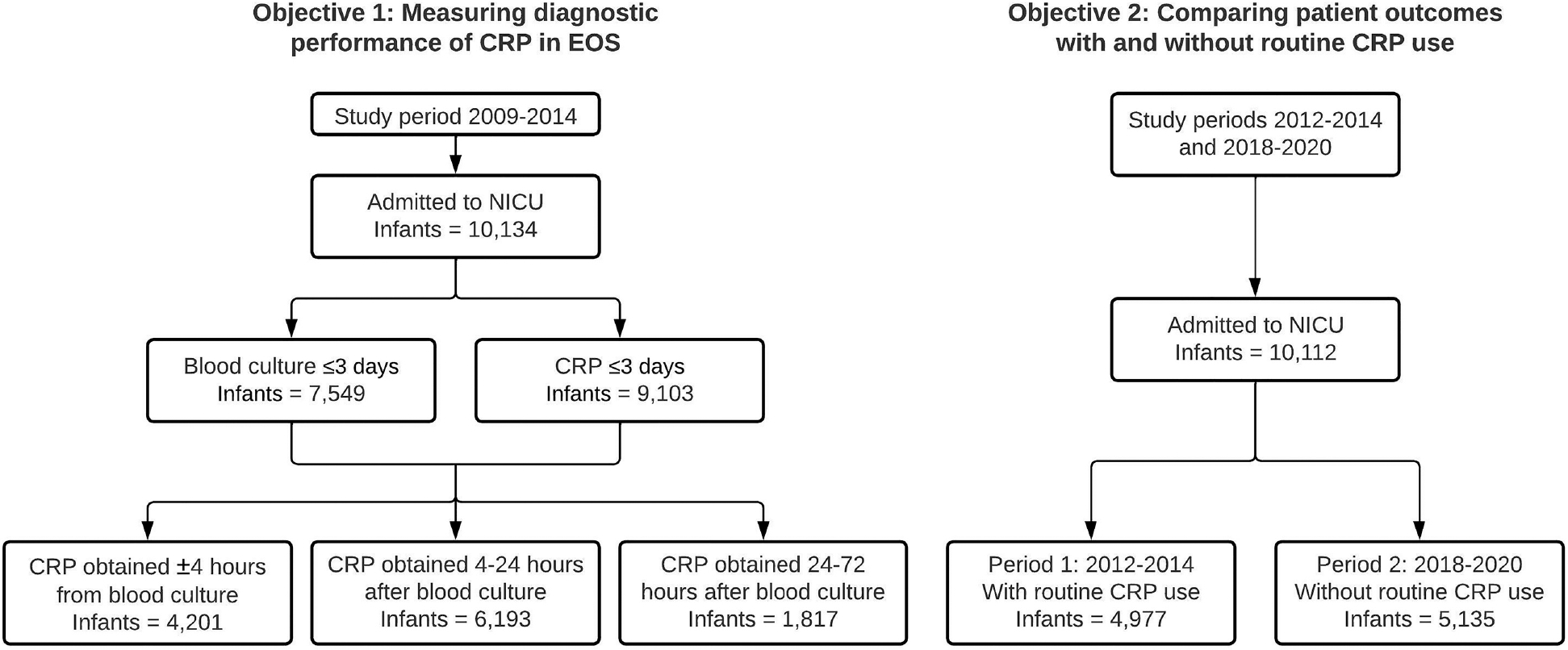
A. Derivation of cohort used in the analysis of diagnostic performance of CRP in EOS. The 3 categories of paired blood culture and CRP are not mutually exclusive – an infant could be present in more than one category if the infant had more than one CRP obtained within 72 hours of blood culture.
B. Derivation of cohort used in studying the impact of CRP use on clinical management and patient outcomes. Of note here, beginning in June 2016, well-appearing infants undergoing an EOS evaluation at one of the two study sites could have had blood cultures drawn and antibiotics started in the mother’s room. We included these infants along with NICU admissions to allow for comparison of all infants undergoing EOS evaluations between study periods.
Abbreviations – CRP, C-reactive protein; EOS, early-onset sepsis; NICU, neonatal intensive care unit.
Study definitions
EOS was defined as isolation of a pathogenic organism from blood and/or CSF culture obtained within 3 days after infant birth. Coagulase-negative staphylococci and other common skin commensal organisms from cultures obtained 0–3 days after birth were deemed as contaminants and included among negative cultures. Coagulase-negative staphylococci isolated from cultures obtained on day 4–7 after birth were considered pathogens if treated by the clinical team with antibiotics for ≥5 days. Prolonged antibiotics were defined as antibiotics administered for >2 days in the absence of pathogen isolation from blood or CSF culture.
CRP diagnostic performance
CRP testing was performed at the clinical laboratory at each site using latex particle immunoturbidimetry. We measured the diagnostic performance of CRP against the gold standard of culture-confirmed EOS. Blood culture practices for EOS at both sites included obtaining a minimum blood volume of 1 mL that was inoculated into a single pediatric aerobic blood culture bottle. An anaerobic blood culture bottle with a 1 mL inoculant was added in 2016 at one site.(15) Both CRP and blood culture results were reported in the electronic medical record system and interpreted by clinicians per standard care. We used a CRP cut-off value of ≥10 mg/L for the main analysis of diagnostic performance, but also analyzed diagnostic performance metrics at other thresholds that are commonly reported in the literature, ranging from 5 to 15 mg/L.(16) CRP tests that were resulted as ‘quantity not sufficient’ or cancelled by the laboratory were excluded from the analysis. Diagnostic performance of CRP was analyzed for tests ordered within ±4 hours of blood culture, and for tests ordered between 4–24 hours, and 24–72 hours after blood culture. If more than one CRP value was available during an analytic time frame, we chose the highest CRP value for analysis. Among infants without EOS, we compared birth characteristics, antibiotic initiation, type of antibiotics, antibiotic duration, frequency of CSF cultures in days 0–3, frequency of blood and CSF cultures in days 4–7, and hospital length of stay, between infants with CRP values above and below the cutoff value of 10 mg/L.
CRP and clinical outcomes
We defined clinically relevant patient outcomes as follows: proportions of all NICU infants from whom blood or CSF cultures were obtained on day 0–3 and 4–7 after birth; rates of positive blood or CSF cultures; time from birth to obtaining blood culture and antibiotic administration among culture-confirmed cases; rate of antibiotic initiation on day 0–3 and 4–7; duration of antibiotic use in the absence of positive cultures; hospital length of stay; transfer for higher level of NICU care in the first 7 days after birth; and all-cause in-hospital mortality overall, and in the first 7 days after birth.
Statistical analyses
We measured the diagnostic performance of CRP in EOS using sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios, and plotted receiver operating characteristic curves. Exact binomial confidence intervals for proportions were calculated by the method of Clopper & Pearson, and confidence intervals for ratios of proportions by the method of Koopman.(17,18) Associations between various characteristics and comparator groups were made using chi-squared test, Fisher’s exact text, and Mann-Whitney U test, as appropriate. Additionally, we used statistical process control p-charts to vizualize the changes in use of CRP and pre-specified outcomes over the study periods.(19) Proportions are presented monthly for all variables except deaths, which are presented quarterly due to the rarity of this outcome. The p-charts were generated using QI Macros for Excel developed by KnowWare International, Inc, Denver, CO. All other analyses were performed using Stata version 16 (StataCorp, College Station, TX).
Results
Diagnostic performance of CRP
From 2009–2014, 10,134 infants were admitted to the study NICUs (Figure 1); characteristics of the cohort are shown in Table I (onavailable at www.jpeds.com). The median gestational age was 37 weeks (IQR 34–39), median birth weight was 2,830 grams (IQR 2,050–3,415), 5,569 (55.0%) were male, and 5,019 (49.5%) were delivered by cesarean.
Table 1.
Characteristics and management of infants admitted to NICU from 2009–2014
| Characteristics | N = 10,134 |
|---|---|
| Sex (male) | 5,569 (55.0) |
| Gestational age, wk | 37 (34, 39) |
| Gestational age <37 wk | 4,551 (44.9) |
| Birth weight, g | 2830 (2050, 3415) |
| Birth weight | |
| <1500g | 1,239 (12.2) |
| 1500 to <2500 g | 2,746 (27.1) |
| ≥2500 g | 6,148 (60.7) |
| Cesarean delivery | 5,019 (49.5) |
| Length of stay by gestational age, d | 4 (3, 15) |
| ≥37 wk | 3 (2, 5) |
| <37 wk | 15 (6, 35) |
| Maternal race | |
| White/non-Hispanic | 3,066 (30.3) |
| Black/non-Hispanic | 5,475 (54.0) |
| Hispanic | 575 (5.7) |
| Other/Unknown | 1,018 (10.1) |
| Maternal age, y | 28 (23, 33) |
| Management on day 0–3 | |
| Blood culture obtained | 7,549 (74.5) |
| CSF culture obtained | 1,131 (11.2) |
| CRP obtained | 9,103 (89.8) |
| At least one CRP ≥10 mg/L | 2,085 (22.9) |
| Blood culture positive for a pathogen | 41 (0.4) |
| HOL positive blood culture was obtained | 1.4 (0.7, 2.8) |
| CSF culture positive for a pathogen | 1 (0.1) |
| Antibiotics initiated | 6,868 (67.8) |
| Antibiotics continued for >2 d | 1,698 (24.7) |
| Management on day 4–7 | |
| Blood culture obtained | 325 (3.2) |
| CSF culture obtained | 180 (1.8) |
| CRP obtained | 832 (8.2) |
| At least one CRP ≥10 mg/L | 172 (20.7) |
| Blood culture positive for pathogen | 35 (0.4) |
| HOL positive blood culture was obtained | 138.2 (107.4, 155.0) |
| CSF culture positive for pathogen | 1 (0.0) |
| Antibiotics initiated | 179 (1.8) |
| Antibiotics continued for >2 d | 78 (43.6) |
Data presented as n (%) or median (Q1, Q3). Missing data – Birth weight (1), Maternal age (163). Abbreviations – CRP, C-reactive protein; CSF, cerebrospinal fluid; HOL, hour of life; NICU, neonatal intensive care unit.
A total of 7,549 (74.5%) infants had at least one blood culture obtained on day 0–3 after birth. Of them, 1,092 (14.5%) also had a CSF culture. Most blood cultures (7,083/7,549, 93.8%) were obtained within one day after birth. Among infants with a blood culture obtained on day 0–3, 41 (0.5%) were diagnosed with EOS. The most common organisms were group B Streptococcus (GBS, 39.0%) and Escherichia coli (39.0%). One infant with GBS bacteremia also had GBS isolated in the CSF. There were no infants with a positive CSF culture in the absence of a positive blood culture.
A total of 9,103 (89.8%) had one or more CRP obtained on day 0–3, and of them, 2,085 (22.9%) had at least one value ≥10 mg/L. Among 7,549 infants with a blood culture on day 0–3, 7,450 (98.6%) also had a CRP obtained in that timeframe. The diagnostic performance of CRP ≥10 mg/L in EOS is shown in Table II. With increasing duration between time when blood culture and CRP were obtained, sensitivity of CRP increased while specificity decreased. CRP obtained 4–24 hours after blood culture had the highest area under the curve on a receiver operating characteristic curve (Figure 2 available at www.jpeds.com). Alternate thresholds of CRP demonstrated similar patterns with the highest sensitivity at low cut-offs farther from time of blood culture and highest specificity for high cut-offs closer to the time of blood culture (Table III available at www.jpeds.com).
Table 2.
Diagnostic Performance of CRP in EOS
| CRP ±4 hours from blood culture N = 4,201 |
CRP 4–24 hours after blood culture N = 6,193 |
CRP 24–72 hours after blood culture N = 1,817 |
||||
|---|---|---|---|---|---|---|
| Blood culture positive | Blood culture negative | Blood culture positive | Blood culture negative | Blood culture positive | Blood culture negative | |
| CRP ≥10 mg/L | 10 | 422 | 24 | 1,482 | 17 | 796 |
| CRP <10 mg/L | 14 | 3,755 | 6 | 4,681 | 2 | 1,002 |
| Total | 24 | 4,177 | 30 | 6,163 | 19 | 1,798 |
| Diagnostic Performance Metrics | ||||||
| Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Sensitivity | 41.7% | 22.1% – 63.4% | 80.0% | 61.4% – 92.3% | 89.5% | 66.9% – 98.7% |
| Specificity | 89.9% | 88.9% – 90.8% | 76.0% | 74.9% – 77.0% | 55.7% | 53.4% – 58.0% |
| PPV | 2.3% | 1.1% – 4.2% | 1.6% | 1.0% – 2.4% | 2.1% | 1.2% – 3.3% |
| NPV | 99.6% | 99.4% – 99.8% | 99.9% | 99.7% – >99.9% | 99.8% | 99.3% – >99.9% |
| Positive LR | 4.12 | 2.55 – 6.68 | 3.33 | 2.77 – 4.00 | 2.02 | 1.72 – 2.38 |
| Negative LR | 0.65 | 0.46 – 0.91 | 0.26 | 0.13 – 0.54 | 0.19 | 0.05 – 0.70 |
EOS cases did not always have a paired CRP value for each time-period. Abbreviations – CI, confidence interval; CRP, C-reactive protein; EOS, early-onset sepsis; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Figure 2. ROC curves for CRP values in diagnosis of EOS.
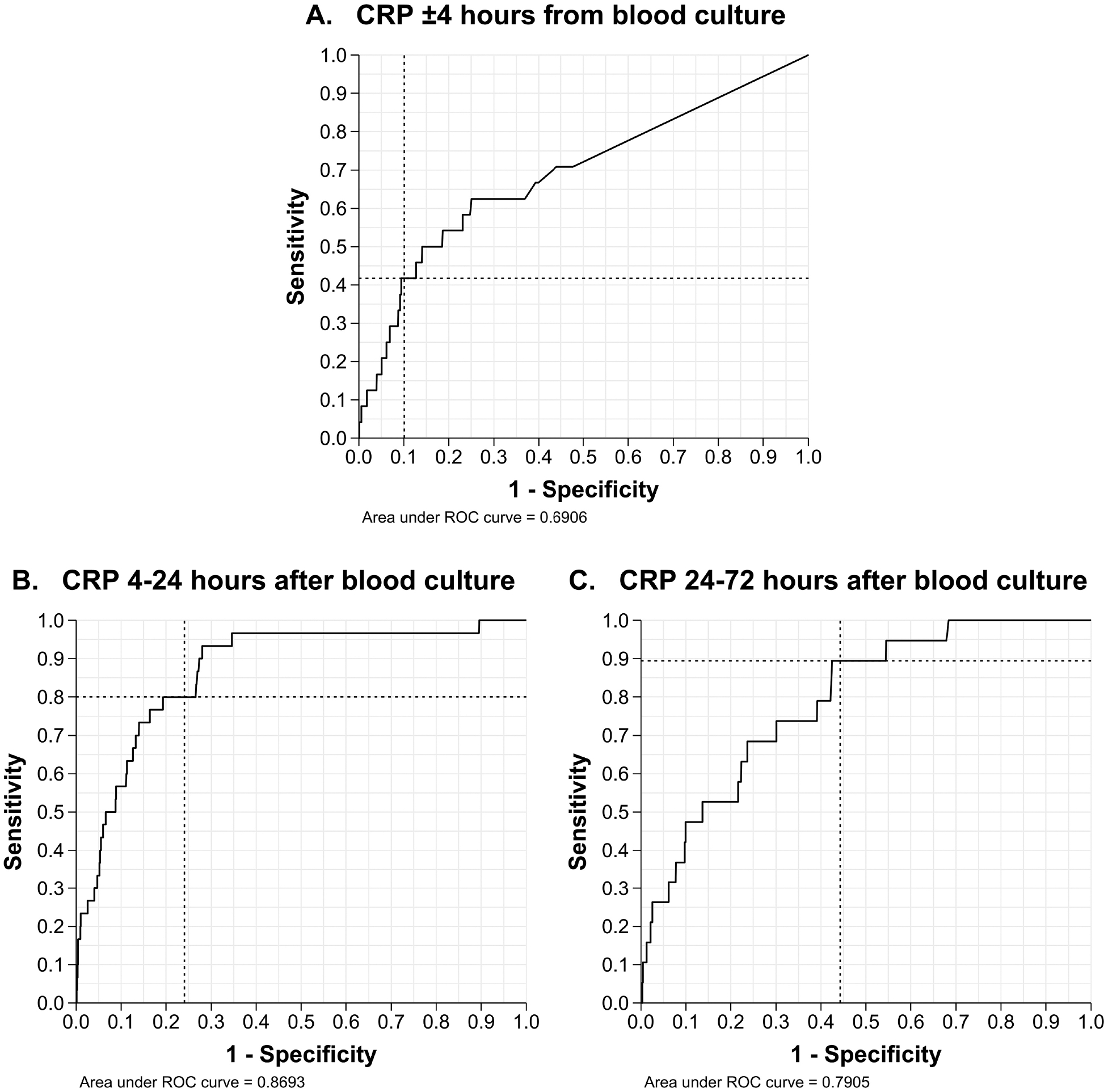
A. ROC curve of CRP values obtained ±4 hours from blood culture.
B. ROC curve of CRP values obtained 4–24 hours after blood culture.
C. ROC curve of CRP values obtained 24–72 after blood culture.
The dotted intersecting lines in all 3 ROC curves mark the sensitivity and 1-specificity for a CRP value of 10 mg/L. When plotting ROC curves, CRP values below the lower detection limit reported as <0.20 mg/L, were converted to 0.10 mg/L (half of the lower detection limit) and included in analysis.
Abbreviations – CRP, C-reactive protein; EOS, early-onset sepsis; ROC, receiver operating characteristic.
Table 3.
Diagnostic accuracy of CRP in EOS for varying cut-off values and varying duration between time CRP and blood culture
| CRP cut-off value | True positive | False positive | False negative | True negative | Sensitivity | Specificity | Accuracy1 | Positive LR | Negative LR |
|---|---|---|---|---|---|---|---|---|---|
| CRP ±4 h from blood culture | |||||||||
| ≥5 mg/L | 12 | 623 | 12 | 3,554 | 50.0% | 85.1% | 84.9% | 3.35 | 0.59 |
| ≥7 mg/L | 10 | 526 | 14 | 3,651 | 41.7% | 87.4% | 87.1% | 3.31 | 0.67 |
| ≥10 mg/L | 10 | 422 | 14 | 3,755 | 41.7% | 89.9% | 89.6% | 4.12 | 0.65 |
| ≥15 mg/L | 7 | 326 | 17 | 3,851 | 29.2% | 92.2% | 91.8% | 3.74 | 0.77 |
| CRP 4–24 h after blood culture | |||||||||
| ≥5 mg/L | 29 | 2,209 | 1 | 3,954 | 96.7% | 55.9% | 36.1% | 2.70 | 0.05 |
| ≥7 mg/L | 28 | 1,835 | 2 | 4,328 | 93.3% | 70.2% | 70.3% | 3.13 | 0.09 |
| ≥10 mg/L | 24 | 1,482 | 6 | 4,681 | 80.0% | 76.0% | 76.0% | 3.33 | 0.26 |
| ≥15 mg/L | 23 | 1,131 | 7 | 5,032 | 76.7% | 81.7% | 81.6% | 4.18 | 0.29 |
| CRP 24–72 h after blood culture | |||||||||
| ≥5 mg/L | 18 | 1,084 | 1 | 714 | 94.7% | 39.7% | 40.3% | 1.57 | 0.13 |
| ≥7 mg/L | 17 | 966 | 2 | 832 | 89.5% | 46.3% | 46.7% | 1.67 | 0.23 |
| ≥10 mg/L | 17 | 796 | 2 | 1,002 | 89.5% | 55.7% | 56.1% | 2.02 | 0.19 |
| ≥15 mg/L | 14 | 593 | 5 | 1,205 | 73.7% | 67.0% | 67.1% | 2.23 | 0.39 |
Accuracy calculated as .
Abbreviations – CRP, C-reactive protein; EOS, early-onset sepsis; FN, false negative; FP, false positive; LR, likelihood ratio; TN, true negative; TP, true positive.
Clinical management of infants without EOS
Of the 7,508 infants without EOS, 7,410 (98.7%) had a CRP obtained on day 0–3, and of these, 1,934 (26.1%) had at least one CRP ≥10 mg/L. Infants with CRP ≥10 mg/L were more frequently males born via vaginal delivery at higher gestational ages and with higher birth weights (Table IV). They were also more likely to be started on empiric antibiotics, receive prolonged antibiotics despite negative blood or CSF cultures, receive antibiotics other than penicillin, ampicillin, and gentamicin, have a CSF culture obtained on day 0–3 or 4–7, and have a longer hospital length of stay.
Table 4.
Influence of CRP measurements on management of infants without EOS
| CRP high1 N = 1,934 | CRP low2 N = 5,476 | P value | |
|---|---|---|---|
| Characteristics | |||
| Sex (male) | 1,184 (61.2) | 2,981 (54.4) | <0.001 |
| Cesarean delivery | 890 (46.0) | 2,741 (50.1) | 0.002 |
| Gestational age, wk | 39 (36, 40) | 36 (33, 39) | <0.001 |
| Birth weight, g | 3250 (2550, 3640) | 2680 (1931, 3330) | <0.001 |
| Length of stay by gestational age, d | |||
| <37 wk | 38 (13, 69) | 17 (8, 37) | <0.001 |
| ≥37 wk | 4 (3, 7) | 3 (2, 4) | <0.001 |
| Management and outcomes in day 0–3 | |||
| CSF culture obtained | 636 (32.9) | 424 (7.7) | <0.001 |
| CSF culture positive for a pathogen | 0 | 0 | N/A |
| Antibiotics initiated | 1,884 (97.4) | 4,777 (87.2) | <0.001 |
| Antibiotics continued for >2 d | 821 (43.6) | 788 (16.5) | <0.001 |
| Antibiotics other than penicillin, ampicillin and gentamicin given | 125 (6.6) | 159 (3.3) | <0.001 |
| Vancomycin | 83 (66.4) | 130 (81.8) | 0.003 |
| Cephalosporins | 95 (76.0) | 101 (63.5) | 0.02 |
| Metronidazole | 8 (6.4) | 8 (5.0) | 0.62 |
| Management and outcomes in day 4–7 | |||
| Blood culture obtained | 62 (3.2) | 202 (3.7) | 0.33 |
| CSF culture obtained | 71 (3.7) | 83 (1.5) | <0.001 |
| Blood culture positive for a pathogen | 4 (0.2) | 24 (0.4) | 0.20 |
| CSF culture positive for a pathogen | 1 (0.1) | 0 | 0.26 |
Data presented as n (%) or median (Q1, Q3). All infants in the table had a blood culture obtained in days 0–3 which was not positive for a pathogen.
Includes infants who had at least one CRP value ≥10 mg/L in days 0–3.
Includes infants with all CRP values <10 mg/L in days 0–3.
Abbreviations – CRP, C-reactive protein; CSF, cerebrospinal fluid; EOS, early-onset sepsis.
Comparing outcomes in time periods with and without routine use of CRP
We compared 4,977 infants admitted to the study sites from 2012–2014 (Period 1: routine CRP use) with 5,135 infants admitted from 2018–2020 (Period 2: minimal CRP use) (Table V). Change in CRP use and pre-specified outcomes over time are shown in Figure 3 (available at www.jpeds.com). Proportion of infants with CRP on day 0–3 decreased significantly from 89.2% in Period 1 to 7.7% in Period 2. The proportion of infants with a blood or CSF culture obtained on day 0–3 and 4–7 also decreased in Period 2. A smaller proportion of admitted infants were administered antibiotics on day 0–3 and 4–7 in Period 2 compared with Period 1. Among infants with a blood culture on day 0–3, a higher proportion of infants were started on antibiotics in Period 2 as compared with Period 1 (98.3% vs 86.1%; P <0.001). In Period 2, among infants for whom antibiotics were initiated on day 0–3, a smaller proportion of infants were administered prolonged antibiotics in the setting of negative cultures.
Table 5.
Characteristics, management, and infant outcomes during period with and without routine CRP use in EOS
| Period 1 N = 4,977 | Period 2 N = 5,135 | P value | |
|---|---|---|---|
| Demographics and delivery characteristics | |||
| Maternal age, y | 29 (23, 33) | 31 (26, 34) | <0.001 |
| Race/ethnicity | <0.001 | ||
| White/Non-Hispanic | 1,555 (31.2) | 1,786 (34.8) | |
| Black/Non-Hispanic | 2,552 (51.3) | 2,388 (46.5) | |
| Hispanic | 297 (6.0) | 400 (7.8) | |
| Asian/Other/Unknown | 573 (11.5) | 561 (10.9) | |
| Sex (male) | 2,712 (54.5) | 2,840 (55.3) | 0.40 |
| Caesarean delivery | 2,519 (50.6) | 2,340 (45.6) | <0.001 |
| Gestational age, wk | 37 (34, 39) | 37 (34, 39) | 0.06 |
| Birth weight, g | 2820 (2060, 3420) | 2810 (2090, 3400) | 0.99 |
| Management in days 0–3 | |||
| Blood culture obtained | 3,709 (74.5) | 2,592 (50.5) | <0.001 |
| CSF culture obtained | 431 (8.7) | 63 (1.2) | <0.001 |
| CRP obtained | 4,441 (89.2) | 393 (7.7) | <0.001 |
| At least one CRP ≥10 mg/L | 984 (22.2) | 190 (48.4) | <0.001 |
| Infants with >2 CRP obtained | 593 (13.4) | 16 (4.1) | <0.001 |
| Blood culture positive for a pathogen | 23 (0.5) | 15 (0.3) | 0.16 |
| HOL blood culture obtained, h | 1.0 (0.5, 2.3) | 1.2 (1.0, 5.1) | 0.11 |
| HOL antibiotics started, h | 2.5 (1.6, 5.0) | 2.2 (1.4, 4.5) | 0.76 |
| CSF culture positive for a pathogen | 1 (0.0) | 0 | 0.49 |
| Antibiotics initiated in 0–3 days1 | 3,233 (65.0) | 2,607 (50.8) | <0.001 |
| Antibiotic continued for >2 d when blood/CSF cultures did not grow a pathogen2 | 548/3,170 (17.3) | 183/2,532 (7.2) | <0.001 |
| Management in days 4–7 | |||
| Blood culture obtained | 146 (2.9) | 109 (2.1) | 0.009 |
| CSF culture obtained | 77 (1.6) | 17 (0.3) | <0.001 |
| CRP obtained | 389 (7.8) | 123 (2.4) | <0.001 |
| At least one CRP ≥10 mg/L | 79 (20.3) | 46 (37.4) | <0.001 |
| Infants with >2 CRP obtained | 31 (8.0) | 3 (2.4) | 0.04 |
| Blood culture positive for a pathogen | 17 (0.3) | 9 (0.2) | 0.10 |
| HOL blood culture obtained, h | 138.2 (113.8, 155.0) | 117.0 (105.4, 150.4) | 0.40 |
| HOL antibiotics started3, h | 130.0 (114.3, 162.4) | 134.4 (90.5, 158.1) | 0.37 |
| CSF culture positive for a pathogen | 0 | 2 (0.0) | 0.50 |
| New antibiotic course started in 4–7 days4 | 88 (1.8) | 54 (1.1) | 0.002 |
| Antibiotic continued for >2 d when blood/CSF cultures did not grow a pathogen2 | 18/70 (25.7) | 7/46 (15.2) | 0.18 |
| Disposition | |||
| Length of stay by gestational age, d | 4 (3, 15) | 4 (3, 15) | 0.84 |
| <37 wk | 15 (6, 36) | 15 (6, 32) | 0.37 |
| ≥37 wk | 3 (2, 4) | 3 (2, 4) | 0.64 |
| Transferred for higher level of care in ≤7 days after birth | 158 (3.2) | 165 (3.2) | 0.91 |
| Deceased in ≤7 days after birth | 24 (0.5) | 33 (0.6) | 0.28 |
| Deceased before discharge | 38 (0.8) | 44 (0.9) | 0.60 |
Data presented as n (%) or median (Q1, Q3). Missing data – Maternal age (98); Sex (2); Gestational age (2).
More infants may have received antibiotics than infants with blood culture because some infants were born and transferred from outside centers.
The denominator includes all infants with a negative blood/CSF culture who received antibiotics in the respective time frame.
Seven infants (4 in Period 1 and 3 in Period 2) with a positive blood culture in days 4–7 had antibiotics started in days 0–3 after birth; these 7 infants were excluded from comparison of HOL antibiotics started.
New antibiotic course defined as new antibiotics started after a gap of >2 days from the stop date of previous antibiotic course.
Abbreviations – CRP, C-reactive protein; CSF, cerebrospinal fluid; EOS, early-onset sepsis; HOL, hour of life.
Figure 3. Statistical process control charts of infection evaluation, antibiotic use and outcomes during study Period 1 and 2.
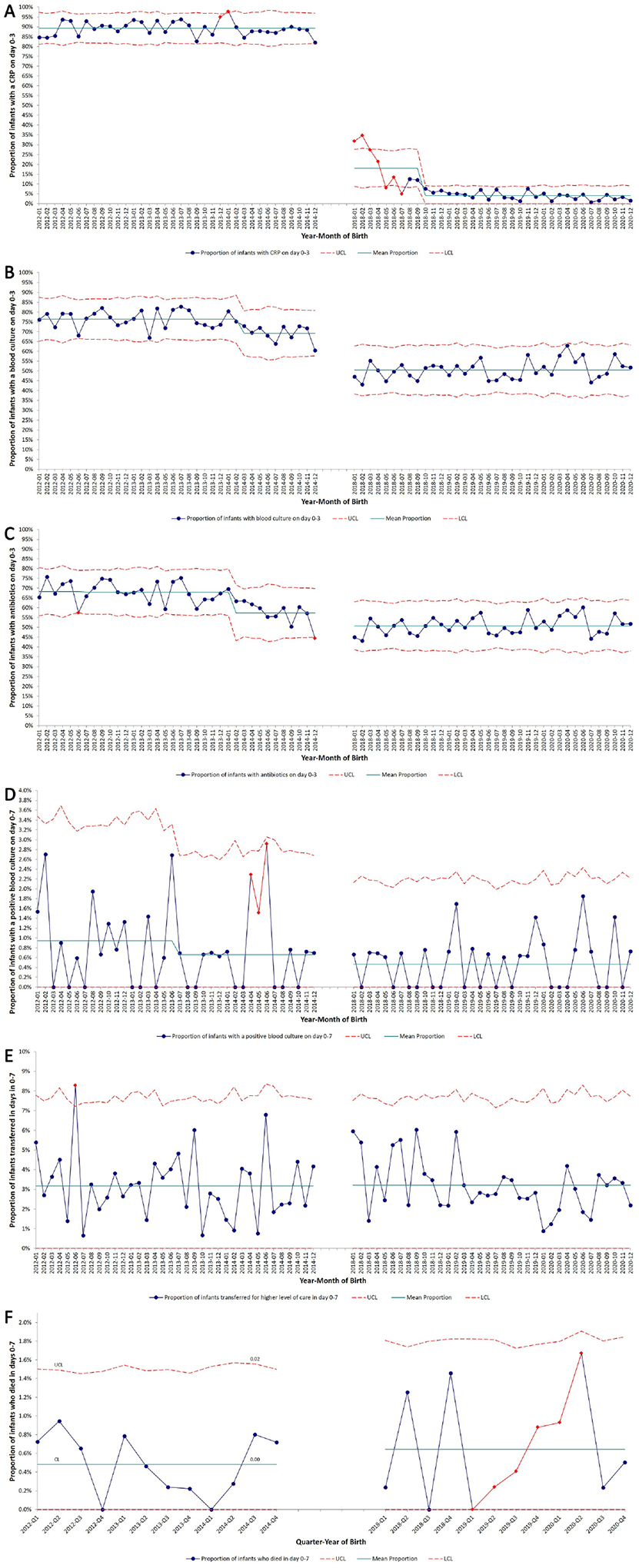
The blue dots (●) and red diamonds (♦) represent the proportion of all NICU infants with the outcome of interest in a particular month. The results of the stability analysis are marked by red diamonds for “unstable” data points which do not follow the expected pattern. These points could be flagged for one or more of the following reasons – 1) Point above UCL or below LCL, 2) Two or more consecutive points above or below 2 standard deviations from mean, 3) Six or more consecutive points trending up or down, and 4) 15 or more consecutive points within 1 standard deviation of mean. The red dashed lines (---) represent upper and lower control limits. Special cause variation within a study period was identified when 8 consecutive data points were above or below the mean line, and the mean line subsequently adjusted.
A. SPC chart of monthly proportion of infants with at least one CRP obtained on day 0–3 over the study period.
B. SPC chart of monthly proportion of infants with blood culture obtained on day 0–3 over the study period.
C. SPC chart of monthly proportion of infants started on empiric antibiotics on day 0–3 over the study period.
D. SPC chart of monthly proportion of infants with at least one positive blood culture growing a pathogen on day 0–7 over the study period.
E. SPC chart of monthly proportion of infants transferred for higher level of care in days 0–7 over the study period.
F. SPC chart of quarterly proportion of infants who died in days 0–7 over the study period.
Abbreviations – CRP, C-reactive protein; CSF cerebrospinal fluid; EOS, early-onset sepsis; LCL, lower control limit; NICU, neonatal intensive care unit; SPC, statistical process control; UCL, upper control limit.
The incidence of EOS and of blood or CSF culture confirmed infection on day 4–7 was not different between the two periods. Among culture-confirmed infection cases, the time from birth to when blood culture was collected, and empiric antibiotics initiated, was not different between the two periods. There was no difference in the hospital length of stay between the two periods overall, or among term and preterm infants considered separately. The proportions of infants who died or were transferred for higher level of care in the first week after birth, and the proportion of infants who died any time during hospitalization was also not different between the two periods.
Discussion
In this study, we measured the clinical utility of CRP in EOS risk assessment in two ways: diagnostic performance in the detection of culture-confirmed EOS and differences in patient outcomes with and without routine use of CRP. CRP had low sensitivity when obtained within ±4 hours of a blood culture, and a modest positive likelihood ratio; with increasing time between blood culture and CRP measurement, sensitivity increased but positive likelihood ratio and specificity decreased. Overall, our results are aligned with other studies where sensitivity of CRP early in EOS evaluation varies from 30% to 74%, and increases with time.(16,20–22) These results support existing data that CRP should not be used to decide initiation of empiric antibiotics.(23) Given that sensitivity improves over the same period of time culture results are reported, we suggest that CRP has little role in early identification of infants with EOS. Nonetheless, it is possible that CRP could function to modify clinical decisions and actions in a manner that would improve patient outcomes. This hypothesis arises from the concept that recognition of the presence or absence of an inflammatory state could have clinical value, and potentially compensate for an undefined “failure rate” of blood culture to detect true infection. In comparing 3-year periods in our centers marked by ~90% use of CRP with EOS evaluation and <10% use, we found no significant clinical harm as measured by change in time to detection of infection, time to antibiotic initiation among EOS cases, infection rates, transfer for higher level of care or in-hospital mortality in the first week after birth. Overall, we found that CRP did not improve care delivery in EOS management, but instead was associated with higher rates of diagnostic testing (blood cultures and lumbar punctures) and higher rates of antibiotic administration.
EOS evaluation is typically performed soon after birth and is based on presence of perinatal risk factors, clinical status, and results of laboratory tests.(24) Laboratory tests are expected to provide information that is additive to risk-based history and clinical status in order to improve decision making.(6) For example, microbiological cultures provide information about the causative pathogen and guide antibiotic choice. Host response inflammatory markers such as CRP can be viewed as analogues to the physical exam, where deviations from a physiological ‘normal’ is an indication for disease, much like an abnormal exam finding. Given that newborns can manifest abnormalities due to physiologic transition as well as due to infection, there are several decision-making points where CRP could be useful. The first scenario where CRP may influence EOS management is the identification of infants for whom empiric antibiotics should be initiated. The low sensitivity of CRP precludes withholding antibiotics based on normal results.(21,23,25–28) The opposite, however, is not true. During 2009–2014, infants in this study with an abnormal CRP obtained within one day after birth were uniformly started on antibiotics (1,357/1,437, 94.4%). Although we cannot isolate how many infants were started on antibiotics solely due to an abnormal CRP, it is possible that the CRP result influenced the decision to initiate empiric antibiotic therapy. The second scenario in which CRP may influence EOS management is the use of serial values to drive antibiotic initiation, given the rising sensitivity of CRP with increasing time from blood culture collection.(28) During 2009–2014, of the 6,868 study infants with antibiotics initiated on day 0–3 after birth, 211 infants (3.1%) had antibiotics initiated only after a new elevation in the second or third CRP value. This low fraction highlights that this information may not be useful in real-world practice. Most EOS cases become symptomatic within the first few hours to days after birth.(29) Therefore, as time from birth increases, the need for a test to determine well-being decreases.
The final scenario in which CRP may affect EOS management is the determination of duration of empiric antibiotic therapy.(28,30) This utility relies on the rising sensitivity of CRP with time from evaluation in addition to clinical context at the time of blood culture result. For example, a normal CRP may support early antibiotic discontinuation when a possible contaminant (such as Coagulase-negative staphylococci) is reported.(31) More commonly, concern for ‘culture-negative’ infection makes it likely that a negative culture result is considered unreliable.(28) This may be due to perceived poor sensitivity of blood cultures, lack of confidence in the technique of culture collection or other factors concerning for infection, such as severity of illness. A high CRP in such a context is often interpreted as an indicator of ‘missed’ infection. This use is demonstrated in our study (Table IV) where infants with a negative blood culture and an abnormal CRP were more likely to receive prolonged antibiotics. However, the poor specificity of CRP at 24–72 hours suggests that by using this approach, a substantial proportion of infants without infection will be mislabeled.(2,5) It is also noteworthy that approximately half of the infants with an abnormal CRP did not receive prolonged antibiotics, suggesting that clinicians used information other than CRP for decision-making.
In each scenario where CRP may have altered decision making, the most important question is whether that change improved care. For instance, would the absence of a CRP result alter the frequency of prolonged antibiotic administration among infants with negative blood/CSF cultures, and in turn change outcomes? In the absence of CRP, clinicians could still decide to prolong antibiotics due to other factors; or the proportion with prolonged antibiotics may increase due to uncertainty created by not having a CRP result; or the antibiotics could be stopped. For the latter, if an infant is truly infected and partially treated, the infant may subsequently decompensate and require repeat evaluation. When we compared outcomes between time periods where CRP was, and was not, routinely used, we found that neither repeat evaluations nor adverse outcomes increased in the first week and clinicians did not continue to administer prolonged antibiotics, suggesting no apparent benefits of prolonged antibiotic courses administered during the routine CRP period.
Multiple studies report increase in procedures and antibiotic use in sepsis evaluations with CRP use.(3,4,32,33) We also found increased use of procedures and antibiotic use during the period of routine CRP use. In our study and others, discontinuation of routine CRP use was associated with reduced antibiotic use.(34) However, at our study centers, the decision to stop obtaining CRP routinely was made in conjunction with changes in how we conducted EOS evaluations.(13–15) Therefore, we cannot attribute the reduction in antibiotic use solely to discontinuation of CRP use, and instead attribute it to the change in EOS evaluations as a whole. Within this context, we found sustained compliance with eliminating routine CRP, and had no major adverse impacts. In the absence of demonstrable benefit, and with the possibility of triggering antibiotic overuse and unnecessary evaluations, we are unable to justify routine CRP use in EOS evaluations.
Our study has limitations. We chose to use a convenience sample to enhance the contrast in CRP use between the pre-post period, as observational studies may incur unmeasured confounding. There are other types of test utility that we did not measure.(6,35) For instance, even if clinicians made the same decisions, but did it more efficiently in the presence of a test, the test may prove to be useful. We did not assess clinician perspective on CRP use. Similarly, the utility is context dependent. We studied the use of CRP testing routinely applied to all evaluations. It is possible that in some patients where ambiguity is greater, new and useful knowledge can be obtained from a correctly timed CRP. Finally, we studied discontinuation of routine CRP in the setting of larger changes in EOS evaluation approach and our experience may not be generalizable to other settings where this was not done.
In this pre-post cohort analysis, initial CRP testing for EOS evaluations was not sufficiently sensitive to support decisions to withhold empiric antibiotic treatment. Although later CRP measurements were more sensitive, they were too nonspecific to support decisions to continue treatment. Discontinuation of routine CRP use during EOS evaluation was not associated with changes in rate or promptness of EOS detection or management. Furthermore, discontinuation of routine CRP use was not associated with more serious adverse outcomes, despite an associated reduction in rates of antibiotic use. Using the aforementioned methods of test utility determination, we did not identify a clear advantage of using CRP in EOS evaluation.
Supplementary Material
Acknowledgments
S.M. receives funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development from the National Institutes of Health (K23HD088753). DDF receives funding from the Agency for Healthcare Research and Quality (K08HS027468). The authors declare no conflicts of interest.
Abbreviations:
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- EOS
early-onset sepsis
- GBS
group B Streptococcus
- NICU
neonatal intensive care unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this study were presented at the American Academy of Pediatrics National Conference & Exhibition in Orlando, FL in November 2017, and at the Pediatric Academic Societies annual meeting in Baltimore, MD in April 2019.
References
- (1).Hofer N The Role of C-Reactive Protein in the Diagnosis of Neonatal Sepsis. In: Wilhelm Müller, editor. Neonatal Bacterial Infection Rijeka: IntechOpen; 2013. p. Ch. 4. [Google Scholar]
- (2).Hofer N, Müller W, Resch B. Non-infectious conditions and gestational age influence C-reactive protein values in newborns during the first 3 days of life. Clin Chem Lab Med 2011. Feb;49(2):297–302. [DOI] [PubMed] [Google Scholar]
- (3).Kiser C, Nawab U, McKenna K, Aghai ZH. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics 2014. Jun;133(6):992–998. [DOI] [PubMed] [Google Scholar]
- (4).Mukherjee A, Davidson L, Anguvaa L, Duffy DA, Kennea N. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed 2015. May;100(3):248. [DOI] [PubMed] [Google Scholar]
- (5).Tiozzo C, Mukhopadhyay S. Noninfectious influencers of early-onset sepsis biomarkers. Pediatr Res 2022. Jan;91(2):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bossuyt PM, Reitsma JB, Linnet K, Moons KG. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem 2012. Dec;58(12):1636–1643. [DOI] [PubMed] [Google Scholar]
- (7).Flannery DD, Edwards EM, Puopolo KM, Horbar JD. Early-Onset Sepsis Among Very Preterm Infants. Pediatrics 2021. Oct;148(4):e2021052456. doi: 10.1542/peds.2021-052456. Epub 2021 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr 2020. Jul 1;174(7):e200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med 2014. Jul;15(6):523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gerdes JS, Polin R. Early diagnosis and treatment of neonatal sepsis. Indian J Pediatr 1998;65(1):63–78. [DOI] [PubMed] [Google Scholar]
- (11).Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018. Dec;142(6):e20182896. doi: 10.1542/peds.2018-2896. [DOI] [PubMed] [Google Scholar]
- (12).Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018. Dec;142(6):e20182894. doi: 10.1542/peds.2018-2894. [DOI] [PubMed] [Google Scholar]
- (13).Dhudasia MB, Mukhopadhyay S, Puopolo KM. Implementation of the Sepsis Risk Calculator at an Academic Birth Hospital. Hosp Pediatr 2018. May;8(5):243–250. [DOI] [PubMed] [Google Scholar]
- (14).Garber SJ, Dhudasia MB, Flannery DD, Passarella MR, Puopolo KM, Mukhopadhyay S. Delivery-based criteria for empiric antibiotic administration among preterm infants. J Perinatol 2021. Feb;41(2):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Woodford EC, Dhudasia MB, Puopolo KM, Skerritt LA, Bhavsar M, DeLuca J, et al. Neonatal blood culture inoculant volume: feasibility and challenges. Pediatr Res 2021. Nov;90(5):1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hedegaard SS, Wisborg K, Hvas AM. Diagnostic utility of biomarkers for neonatal sepsis--a systematic review. Infect Dis (Lond) 2015. Mar;47(3):117–124. [DOI] [PubMed] [Google Scholar]
- (17).Rosner BA. Fundamentals of Biostatistics. : Thomson-Brooks/Cole; 2006. [Google Scholar]
- (18).Koopman PAR. Confidence Intervals for the Ratio of Two Binomial Proportions. Biometrics 1984;40(2):513–517. [Google Scholar]
- (19).Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care 2010. Oct;22(5):402–407. [DOI] [PubMed] [Google Scholar]
- (20).Arnon S, Litmanovitz I, Regev RH, Bauer S, Shainkin-Kestenbaum R, Dolfin T. Serum amyloid A: an early and accurate marker of neonatal early-onset sepsis. J Perinatol 2007. May;27(5):297–302. [DOI] [PubMed] [Google Scholar]
- (21).Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, Assumma M, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem 2003. Jan;49(1):60–68. [DOI] [PubMed] [Google Scholar]
- (22).Hansen AB, Verder H, Staun-Olsen P. Soluble intercellular adhesion molecule and C-reactive protein as early markers of infection in newborns. J Perinat Med 2000;28(2):97–103. [DOI] [PubMed] [Google Scholar]
- (23).Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998. Oct;102(4):E41. [DOI] [PubMed] [Google Scholar]
- (24).Mukhopadhyay S, Puopolo KM. Neonatal Early-Onset Sepsis: Epidemiology and Risk Assessment. Neoreviews 2015;16(4):e221–e230. [Google Scholar]
- (25).Eschborn S, Weitkamp JH. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol 2019. Jul;39(7):893–903. [DOI] [PubMed] [Google Scholar]
- (26).Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology 2012;102(1):25–36. [DOI] [PubMed] [Google Scholar]
- (27).Kawamura M, Nishida H. The usefulness of serial C-reactive protein measurement in managing neonatal infection. Acta Paediatr 1995. Jan;84(1):10–13. [DOI] [PubMed] [Google Scholar]
- (28).Philip AG, Mills PC. Use of C-reactive protein in minimizing antibiotic exposure: experience with infants initially admitted to a well-baby nursery. Pediatrics 2000. Jul;106(1):E4. [DOI] [PubMed] [Google Scholar]
- (29).Achten NB, Plötz FB, Klingenberg C, Stocker M, Bokelaar R, Bijlsma M, et al. Stratification of Culture-Proven Early-Onset Sepsis Cases by the Neonatal Early-Onset Sepsis Calculator: An Individual Patient Data Meta-Analysis. J Pediatr 2021. Jul;234:77–84.e8. [DOI] [PubMed] [Google Scholar]
- (30).Neonatal infection: antibiotics for prevention and treatment | Guidance | NICE. Available at: https://www.nice.org.uk/guidance/ng195. Accessed Oct 27, 2022.
- (31).Hemels MA, van den Hoogen A, Verboon-Maciolek MA, Fleer A, Krediet TG. Shortening the antibiotic course for the treatment of neonatal coagulase-negative staphylococcal sepsis: fine with three days? Neonatology 2012;101(2):101–105. [DOI] [PubMed] [Google Scholar]
- (32).Macallister K, Smith-Collins A, Gillet H, Hamilton L, Davis J. Serial C-Reactive Protein Measurements in Newborn Infants without Evidence of Early-Onset Infection. Neonatology 2019;116(1):85–91. [DOI] [PubMed] [Google Scholar]
- (33).Sturgeon JP, Zanetti B, Lindo D. C-Reactive Protein (CRP) levels in neonatal meningitis in England: an analysis of national variations in CRP cut-offs for lumbar puncture. BMC Pediatr 2018. Dec 3;18(1):380–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Singh N, Gray JE. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J Perinatol 2021. Oct;41(10):2488–2494. [DOI] [PubMed] [Google Scholar]
- (35).Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991;11(2):88–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


