SUMMARY
Background:
Epidemiologic monitoring of HIV among transgender women is extremely limited despite prioritization in the United States (US) Strategy to End the HIV/AIDS Epidemic. We aimed to estimate HIV incidence in a multi-site cohort of transgender women in eastern and southern US. Participant deaths were identified during follow-up; thus, we felt it was an ethical imperative to report mortality alongside HIV incidence.
Methods:
We established a multi-site cohort across two modes: (1) site-based, technology-enhanced mode in six cities; (2) exclusively digital mode spanning 72 cities. Trans feminine adults (≥18 years)who were not living with HIV were eligible and followed for ≥24 months. Participants completed surveys and oral fluid HIV testing with clinical confirmation. We ascertained deaths through community and clinical sources. We estimated HIV incidence and mortality using the number of HIV seroconversions and deaths, respectively, divided by person-years (py) accumulated from enrollment. Logistic regression models identified predictors of HIV seroconversion or death.
Findings:
Enrollment launched in March 2018 and January 2019 in the site-based and digital modes, respectively. We enrolled 1,312 participants with 83% retention as of May 2022. HIV incidence was 5·5/1,000py (15/2,730; 95CI: 2·7–8·3) and higher among Black participants and those living in the South. Mortality was 3·3/1,000py (9/2,730py; 95%CI:1·5–6·3) and higher among Latinx participants. Identical predictors of HIV seroconversion and death included: residence in the South, sexual partnerships with cisgender men, and use of stimulants. Participation in the digital cohort and seeking care for gender transition were inversely associated with both outcomes.
Interpretation:
As HIV research and interventions are increasingly delivered online, differences by mode highlight the need for continued community and location-based efforts to reach the most marginalized transgender women. Findings underscore community calls for interventions that address social and structural contexts that affect survival and other health concerns alongside HIV prevention.
Funding:
National Institutes of Health.
INTRODUCTION
Transgender women experience a disproportionately high burden of HIV and low access to healthcare. Multiple biological, behavioral, and interpersonal risks for HIV among transgender women are driven and reinforced by structural barriers that limit access to HIV prevention, testing, care, and other health services.1,2 Because of such vulnerabilities, recent studies among transgender women in the United States (US) have estimated HIV prevalence to range from 14–42%, with significant disparities across race and ethnicity.3,4 Gender-based discrimination and healthcare stigma are barriers to HIV testing, prevention, care, and treatment.5–7 As a result, transgender women are recognized as a priority population in the US Ending the HIV Epidemic (EHE) and National HIV/AIDS strategies.8,9
Multi-site cohorts focused on transgender women are needed to monitor epidemic trends, identify drivers of HIV acquisition, and assess if and how national health policies and HIV prevention efforts, including new pre-exposure prophylaxis (PrEP) modalities, impact HIV epidemic trends. To our knowledge, only one cohort in the US, the San Francisco site of the Trans National Study, has exclusively focused on transgender women to understand their unique risks for HIV acquisition.10 HIV cohort models are moving towards exclusively online (digital) methods to reduce infrastructure costs and facilitate inclusion of people otherwise excluded by geographic or other barriers to facilities;11,12 however, less is known of the tradeoffs of such transitions in cohort implementation.
Sustained cohorts also provide essential opportunities to monitor trends in other health outcomes that align with community and public health priorities, such as mortality, mental health, violence and safety, and resilience.13 Yet, few cohorts report mortality rates for populations not living with HIV, and none have reported mortality rates for transgender women in the US. Internationally, mortality rates among transgender women were reported from the Amsterdam cohort, which found that standardized mortality rates were 2·8-fold higher among transgender women for all causes of death and 6·1-fold higher for non-natural causes compared to national estimates for cisgender women.13
Our research team established a multi-site cohort study exclusively among transgender women in the eastern and southern US. This study aimed to assess HIV incidence and risks for infection that are unique to transgender women. We also evaluated how digital methods could be integrated to support participation. Throughout follow-up, we observed several premature deaths among participants. We report mortality rates alongside HIV incidence in this manuscript, given the urgency and importance of identifying how comprehensive HIV prevention programs can optimally support improvements in health and well-being beyond reducing HIV incidence.
METHODS
Study design and participants:
We informed and refined cohort design and methods using formative, qualitative research.14–16 We have previously described our methods.17,18 In brief, the cohort included two modes of participation: (1) site-based technology-enhanced mode in collaboration with research and clinical institutions in Atlanta, Baltimore, Boston, Miami, New York City, and Washington, DC, and (2) an exclusively digital (online) mode.17,18 This mode was geotargeted to 72 eastern and southern US cities that matched the original six cities based on population size and demographics.
Participation by mode differed in the following ways. The site-based mode used a quarterly assessment schedule, including HIV testing and questionnaires. Participants completed in-person assessments at baseline, 6, 12, and 24 months to foster rapport and complete annual laboratory-based STI testing. Participation in the digital mode was entirely remote. Due to resource constraints, we scheduled digital assessments semiannually; STI testing was unavailable.
Enrollment launched in March 2018 and January 2019 for the site-based and digital modes, respectively, and continued to August 2020. Thirty-six participants enrolled after March 13, 2020, when the COVID-19 pandemic was declared a national emergency.
We recruited participants via a mix of technology- and non-technology-infused methods.17,18 Individuals from the six site-based cities could enroll in either mode. Screening and consent were completed in-person for site-based participants and remotely for digital mode participants. Participants were required to complete electronic verification procedures to prevent fraudulent enrollment.17
Study eligibility included: aged ≥18 years; identified along the trans feminine spectrum based on a two-step measure of assigned sex at birth and current gender;19 spoke or understood English or Spanish; and resided within 50 miles of one of the study cities. We restricted cohort enrollment to individuals with a negative baseline HIV test. Individuals currently enrolled in a PrEP clinical trial were not eligible to participate.
Procedures:
Eligible candidates provided written consent to participate for 24 months. At the 24-month assessment, enrollees could consent to extend participation for up to three years. All study materials and activities were available in English and Spanish languages.
Retention efforts included a comprehensive locator form for current contact information, ongoing community outreach, networking, newsletters, and in-person and online events. These strategies permitted relationship-building, recontact, and event ascertainment. The study team regularly contacted participants using automated and on-demand text messages, emails, and phone calls to notify and remind them of study visits. We defined loss to follow-up as missing three or more consecutive assessments and not returning to the study by the time of data freeze. Staff initiated intensive outreach efforts when a participant missed two successive assessments.
We designed the questionnaire for electronic self-administration. We asked site-based participants with low literacy, identified by literacy screening, or limited capacity for self-administration to complete an interviewer-administered questionnaire.
The questionnaire spanned numerous domains of demographics; food security; immigration history; justice and carceral system involvement; gender-transition related care; sexual health history and access/uptake of HIV services including PEP and PrEP; primary care and social services utilization; barriers and facilitators to care; substance use; mental health symptoms; gender pride, affirmation; sexual relationships and behaviors; and social marginalization, stigma, discrimination, and violence victimization.17,18 Participants completed the questionnaire in an average of 30 minutes. Affirmative responses to questions indicating violence victimization, mental health needs, substance use, or HIV risk triggered referrals to local, gender-affirming services for the site-based mode and national resources for the digital mode.
We provided HIV testing at each assessment. In the site-based mode, participants performed the OraQuick HIV self-test (OraSure Technologies, Bethlehem, PA). This test was selected both to enable participants to test remotely and to empower participants to feel confident using HIV self-testing outside the study. HIV test results were available within 20 minutes. Site-based participants completed annual laboratory-based testing for syphilis, anorectal and vaginal gonorrhea, and chlamydia.
We asked digital mode participants to provide an oral fluid specimen, collected using the OraSure Oral Specimen Collection Device and tested at a central laboratory using enzyme immune assay with reflex to Western Blot for reactive preliminary results.20 We elected to use oral fluid specimen collection to ensure that study staff could receive the results and effectively provide interactive post-test counseling and support referrals for participants with reactive or indeterminate preliminary results. We based this decision on research that demonstrated low self-reported HIV self-test results in digital research.21
Participants who tested outside the study within a 30-day window before or after their assessment due date could submit HIV test result documentation instead of repeating the test. We referred all participants with a positive preliminary test result for confirmatory testing and care at a local and affirming facility of their preference. We asked these participants to provide confirmatory results to verify seroconversion.
We developed a web-based HIPAA-compliant digital platform hosted by the University’s central Information Technology department. The participant interface was available in English and Spanish as a hybrid application (app) for download for Android and iOS operating systems and through a web app for participants without smartphones or tablets. The app was tailored to each mode and provided secure access to the participant’s profile, study timeline, and assessment activities. The platform sent automated reminders and on-demand notifications (SMS and email) to participants in the participant’s preferred language. Staff had role- and mode-based access to the interface for tracking and data entry.
The Johns Hopkins Single Institutional Review Board (sIRB; IRB00142429) reviewed and approved this study. Johns Hopkins sIRB served as the IRB of record for all partner institutions in this multisite study. A community advisory board of 16 community members from study cities regularly reviewed and provided input to study methods, instruments, results, and dissemination.
Statistical analysis:
All incident HIV infections were laboratory-confirmed. We ascertained death through medical records, community reports, and/or obituaries. We attempted to confirm all deaths that were reported outside of medical records through a second source; all except one were confirmed by a second source. We estimated HIV incidence and mortality rates as the number of observed events divided by person-years (py) accumulated. Observations were censored at date of HIV seroconversion, loss to follow-up, or data freeze on May 25, 2022, whichever came first. We visualized Kaplan-Meier estimates of the cumulative incidence of HIV and death (separately). We found no substantive effect of the competing risk of death on HIV incidence estimates nor of HIV incidence on death. We stratified data by time before (enrollment to March 1, 2020) and during (March 2, 2020 – May 25, 2022) the pandemic to evaluate the potential impact of the COVID-19 pandemic on study outcomes.
We used logistic regression models to estimate relative risks (RR) of potential predictors of incident HIV or death from enrollment to censoring. Point estimates (i.e., odds ratios) from these models are interpreted as relative risks in longitudinal designs with rare outcomes. Logistic regression models were unadjusted due to the small number of events. We calculated RRs for individual, interpersonal, and structural variables that were conceptually and statistically associated with the outcome at p<0·10 in the appropriate test for differences. Independent variables were from the baseline assessment, with exceptions being measures of consistent phone connection, home internet access, and PrEP use during follow-up, which were time-fixed but measured after enrollment. We classified responses as having a home internet connection only if they reported such a connection at all assessments and classified responses as having “phone disconnected” if it was reported at any assessment. Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 4.1.2 (R Project for Statistical Computing) for visualizations.
Role of the funding source:
The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the report. The corresponding author had full access to all study data and responsibility for the decision to submit for publication. All authors reviewed and approved the manuscript for publication.
RESULTS
We enrolled 1,312 transgender women, with 734 (56%) in site-based and 578 (44%) in digital modes (Supplemental Figure 1, page 1). At the 24-month assessment, 59% of eligible participants consented to extending participation (at this analysis, 14 had not yet entered the 24-month assessment window). Overall retention at this analysis was 83% based on loss to follow-up. Throughout follow-up, 16 participants transferred sites and/or modes. As of May 2022, the cohort participants had contributed 2,730 accumulated person-years (py) to the analytic dataset.
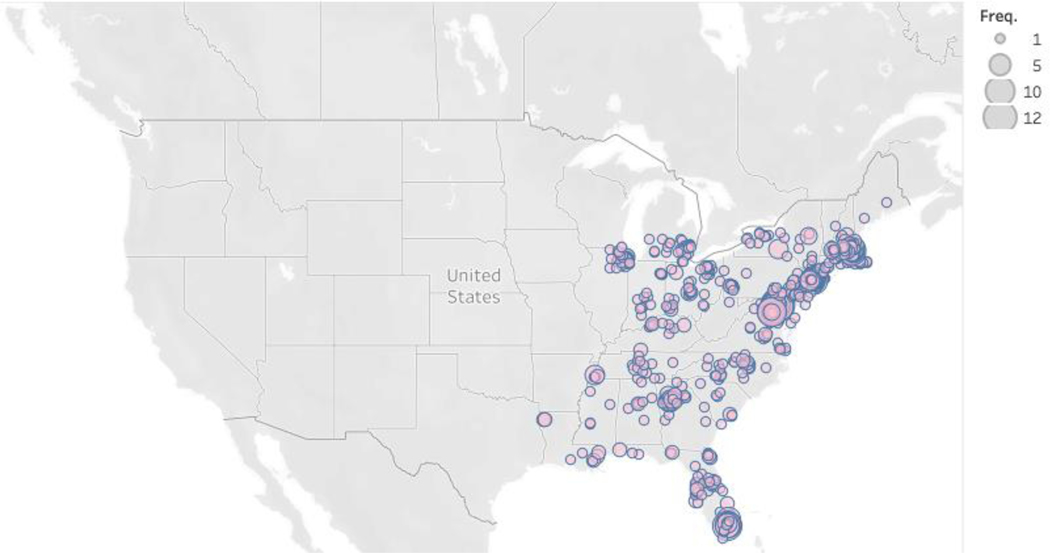
Table 1 displays participant characteristics. The study population was racially and ethnically diverse and well-distributed across the eastern and southern US (Figure 1). Six percent of participants (11% online) resided in a rural area. Differences in participant characteristics were observed across modes.
Table 1.
Characteristics of LITE cohort participants at enrollment and by mode of participation
| Characteristic at enrollment | Site-based mode n = 734 | Digital mode n = 578 | Overall cohort N = 1,312 | p-value2 |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | n (%) | ||
|
| ||||
| Median age, years (IQR) | 29 (24, 37) | 27 (22, 34) | 28 (23, 35) | <0·0001 |
|
| ||||
| Region | <0·0001 | |||
|
| ||||
| North | 382 (52%) | 273 (47%) | 655 (50%) | |
|
| ||||
| Mid-Atlantic | 194 (26%) | 107 (19%) | 301 (23%) | |
|
| ||||
| South | 158 (22%) | 198 (34%) | 356 (27%) | |
|
| ||||
| Race and ethnic identity | <0·0001 | |||
|
| ||||
| Non-Hispanic White | 266 (36%) | 431 (75%) | 697 (53%) | |
|
| ||||
| Non-Hispanic Black | 147 (20%) | 26 (4%) | 173 (13%) | |
|
| ||||
| Hispanic White | 67 (9%) | 19 (3%) | 86 (7%) | |
|
| ||||
| Hispanic Black | 23 (3%) | 0 (0%) | 23 (2%) | |
|
| ||||
| Non-Hispanic and more than one or other race | 110 (15%) | 73 (13%) | 183 (14%) | |
|
| ||||
| Hispanic and more than one or other race | 111 (15%) | 22 (4%) | 133 (10%) | |
|
| ||||
| Unknown | 10 (1%) | 7 (1%) | 17 (1%) | |
|
| ||||
| Spanish language (ref: English) | 57 (8%) | 2 (0%) | 59 (4%) | <0·0001 |
|
| ||||
| Born in the US (ref: no) | 596 (82%) | 553 (96%) | 1,149 (88%) | <0·0001 |
|
| ||||
| Sexual orientation | <0·0001 | |||
|
| ||||
| Straight/heterosexual | 243 (34%) | 56 (10%) | 299 (23%) | |
|
| ||||
| Lesbian | 82 (11%) | 127 (22%) | 209 (16%) | |
|
| ||||
| Gay | 48 (7%) | 11 (2%) | 59 (5%) | |
|
| ||||
| Bisexual | 117 (16%) | 134 (23%) | 251 (20%) | |
|
| ||||
| Queer, pansexual, other | 224 (31%) | 244 (43%) | 468 (36%) | |
|
| ||||
| Education | <0·0001 | |||
|
| ||||
| High school diploma/GED or less | 259 (36%) | 103 (18%) | 362 (28%) | |
|
| ||||
| Some college or higher | 468 (64%) | 470 (82%) | 938 (72%) | |
|
| ||||
| High literacy (ref: low; site-based mode only) | 634 (89%) | |||
|
| ||||
| Current employment status | <0·0001 | |||
|
| ||||
| Unemployed | 330 (46%) | 193 (35%) | 523 (41%) | |
|
| ||||
| Employed full-time | 223 (31%) | 246 (44%) | 469 (37%) | |
|
| ||||
| Employed part-time | 164 (23%) | 117 (21%) | 281 (22%) | |
|
| ||||
| Income | <0·0001 | |||
|
| ||||
| Above the federal poverty level | 332 (45%) | 366 (63%) | 698 (53%) | |
|
| ||||
| Below the federal poverty level | 283 (39%) | 132 (23%) | 415 (32%) | |
|
| ||||
| Unknown | 119 (16%) | 79 (14%) | 198 (15%) | |
|
| ||||
| Health insurance | <0·0001 | |||
|
| ||||
| Uninsured | 71 (10%) | 54 (10%) | 125 (10%) | |
|
| ||||
| Public insurance | 353 (52%) | 147 (27%) | 500 (41%) | |
|
| ||||
| Private insurance | 258 (38%) | 348 (63%) | 606 (49%) | |
|
| ||||
| Method of recruitment (multiple options) | ||||
|
| ||||
| Friend | 235 (32%) | 121 (21%) | 356 (28%) | <0·0001 |
|
| ||||
| Healthcare organization | 304 (42%) | 38 (7%) | 342 (26%) | <0·0001 |
|
| ||||
| 52 (7%) | 54 (9%) | 106 (8%) | 0·1318 | |
|
| ||||
| 27 (14%) | 245 (45%) | 272 (37%) | <0·0001 | |
|
| ||||
| Google ad | 7 (4%) | 104 (19%) | 111 (15%) | <0·0001 |
|
| ||||
| Dating app | 30 (4%) | 12 (2%) | 42 (3%) | 0·0417 |
|
| ||||
| Other | 51 (7%) | 56 (10%) | 107 (8%) | 0·0702 |
Notes: Ref: Reference group not displayed for binary variables; Pearson’s Chi-squared test used to detect differences by mode of participation
Figure 1:
Geographic distribution of cohort participants by Zip Code of residence reported at enrollment
We confirmed 15 participants with seroconversions. Table 2 displays incidence and mortality rates overall and by subgroup. Supplemental Appendix Table 1 (page 2) displays subgroup rates stratified by cohort mode. HIV incidence was 5·5 (95%CI 2·7–8·3) per 1,000py overall. HIV incidence was higher in the site-based mode (IR: 8·7/1,000py) than in the digital mode (IR: 1·6/1,000py), though confidence intervals overlapped. Incidence rates differed by race and PrEP indication, based on criteria adapted for transgender women,22 and were qualitatively different by age, region, and time relative to the onset of COVID-19. Of the 15 participants who seroconverted during follow-up, seven were PrEP-naïve at the time of seroconversion, seven were former PrEP users, and one declined to answer questions on PrEP use.
Table 2.
Crude incidence rates and mortality rates among N=1,312 participants with 2,730 person-years of follow-up
| Group | Incident HIV | Death |
|---|---|---|
| Rate per 1,000py (95%CI) | Rate per 1,000py (95%CI) | |
| All | 5·5 (2·7–8·3) | 3·3 (1·5–6·3) |
| Cohort mode | ||
| Digital | 1·6 (0·2–5·8) | 0·8 (0·0–4·5) |
| Site-based | 8·7 (4·0–13·5) | 5·4 (2·3–10·6) |
| Race & ethnicity 1 | ||
| White | 1·6 (0·3–4·7) | 3·2 (1·2–7·0) |
| Black | 19·3 (8·4–30·2) | 4·8 (1·0–14·1) |
| Hispanic/Latinx ethnicity | 9·9 (3·2–23·1) | 9·9 (3·2–23·1) |
| Geographic region | ||
| North | 3·0 (0·8–7·7) | 0·0 (0·0–2·8) |
| Mid-Atlantic | 4·8 (1·0–14·1) | 4·8 (1·0–14·1) |
| South | 10·3 (4·5–20·3) | 7·7 (2·8–16·8) |
| Age category | ||
| 18–24 | 8·5 (3·4–17·5) | 3·6 (0·8–10·6) |
| >=25 | 4·2 (1·8–8·3) | 3·2 (1·2–6·9) |
| PrEP indicated at baseline 2 | ||
| No | 0·7 (0·0–3·8) | |
| Yes | 11·2 (5·3–17·0) |
Notes:
Categories are not mutually exclusive, as participants may report multiple race or ethnic identities;
PrEP indication based on an adapted version of CDC criteria for transgender women22
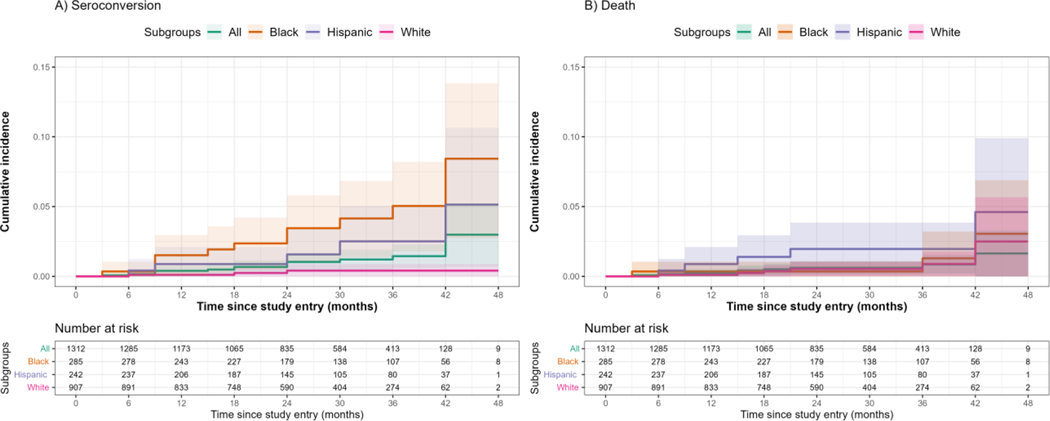
Nine participants died during the study. These deaths were attributed to homicide (n=1), suicide (n=1), overdose (n=2), cardiac arrest of unknown etiology (n=1), other health condition (n=1) and unknown causes (n=3). Mortality rates were 3·3 (95%CI 1·5–6·3) per 1000py. Rates were qualitatively different by cohort mode, race, and ethnicity. Figure 2 displays the cumulative incidence densities by race and ethnicity.
Figure 2.
Cumulative incidence of a) HIV seroconversion and b) death among cohort participants, overall and by race and ethnicity
Note: 95% confidence intervals represented by shaded areas
Table 3 displays the relative risk estimates of characteristics and experiences associated with seroconversion and death (Supplemental Appendix Table 2, page 4, displays the associated p-values). Baseline variables associated with both outcomes, included recent sexual relationships with cisgender men, laboratory-confirmed STI infection, and region of residence. When we reclassified geographic location to US Census regions (i.e., reclassifying mid-Atlantic to North or South by state), residence in the South had a higher risk of seroconversion (RR: 3·4, 95%CI 1·1, 12·2) and perfectly predicted deaths. Cocaine or methamphetamine use were associated with increased risk of HIV seroconversion, while any stimulant use was associated with increased risk of death. Participants in the digital mode and those seeking gender-transition-related care had a lower risk of HIV seroconversion and death.
Table 3.
Predictors of HIV incidence and mortality among transgender women in eastern and southern U·S·(N=1,312), March 2018-May 2022
| HIV incidence | Mortality | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic at baseline | Overall N = 1,312 | No seroconversion N = 1,297 | Seroconversion N = 15 | RR1 (95%CI) | Alive N = 1,303 | Deceased N = 9 | RR1 (95%CI) |
|
| |||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | |||
|
| |||||||
| DEMOGRAPHICS | |||||||
| Digital mode participant (ref: site-based) | 578 (44%) | 576 (44%) | 2 (13%) | 0·2 (0·0–0·7) | 577 (44%) | 1 (11%) | 0·2 (0·0–0·9) |
| Race & ethnicity 1 | |||||||
| White | 907 (70%) | 904 (70%) | 3 (20%) | 0·1 (0·0, 0·3) | 901 (70%) | 6 (67%) | |
| Black | 285 (22%) | 273 (21%) | 12 (80%) | 14·8 (4·7–65·4) | 282 (22%) | 3 (33%) | |
| Hispanic / Latinx | 242 (19%) | 237 (18%) | 5 (36%) | 237 (18%) | 5 (62%) | 7·4 (1·8, 36·4) | |
| Region 5 | |||||||
| North | 655 (50%) | 651 (50%) | 4 (27%) | Ref | 655 (50%) | 0 (0%) | RR not calculated due to zero events in cell |
| Mid-Atlantic | 301 (23%) | 298 (23%) | 3 (20%) | 1·6 (0·3–7·5) | 298 (23%) | 3 (33%) | |
| South | 356 (27%) | 348 (27%) | 8 (53%) | 3·7 (1·2–14·1) | 350 (27%) | 6 (67%) | |
| SOCIOECONOMIC | |||||||
| Currently employed full or part-time (ref: unemployed) | 750 (59%) | 743 (59%) | 7 (50%) | 749 (59%) | 1 (11%) | 0·1 (0·0, 0·5) | |
| Phone disconnected during follow-up (ref: no; n=983) | 137 (14%) | 133 (14%) | 4 (50%) | 6·3 (1·5–27·1) | 137 (14%) | 0 (0%) | |
| Internet connection at home (ref: no; n=983) | 924 (94%) | 919 (94%) | 5 (62%) | 0·1 (0·0–0·5) | 923 (94%) | 1 (50%) | |
| RISK ENVIRONMENTS | |||||||
| Homeless last 6 months (ref: no) | 137 (11%) | 135 (11%) | 2 (13%) | 134 (10%) | 3 (33%) | 4·3 (0·9–16·4) | |
| Arrested last 12 months (ref: no) | 63 (5%) | 62 (5%) | 1 (7%) | 60 (5%) | 3 (33%) | 10·1 (2·1–39·3) | |
| Lifetime sex work (ref: no) | 492 (38%) | 486 (38%) | 6 (43%) | 486 (38%) | 6 (67%) | 3·3 (0·9–15·7) | |
| VIOLENCE VICTIMIZATION | |||||||
| Psychological violence (ref: no) | 1,101 (85%) | 1,095 (86%) | 6 (46%) | 0·1 (0·1–0·4) | 1,095 (85%) | 6 (75%) | |
| Sexual violence (ref: no) | 557 (43%) | 555 (44%) | 2 (15%) | 0·2 (0·0–0·9) | 553 (43%) | 4 (44%) | |
| GENDER AFFIRMATION & HEALTH HISTORY | |||||||
| Sought gender-transition-related care (ref: no, past 12 mo.) | 1,082 (83%) | 1,072 (84%) | 10 (67%) | 0·4 (0·1, 1·3) | 1,078 (84%) | 4 (50%) | 0·2 (0·1–0·8) |
| Has a personal doctor/health care provider (ref: no) | 919 (71%) | 912 (71%) | 7 (47%) | 0·4 (0·1–1·0) | 912 (71%) | 7 (78%) | |
| Health insurance 3 | |||||||
| Uninsured or public insurance | 625 (51%) | 617 (51%) | 8 (67%) | 616 (50%) | 9 (100%) | RR not calculated | |
| Private insurance | 606 (49%) | 602 (49%) | 4 (33%) | 606 (50%) | 0 (0%) | due to zero events in cell |
|
| Stigma-related barriers to care | 733 (56%) | 725 (56%) | 8 (53%) | 731 (56%) | 2 (22%) | 0·2 (0·0–0·9) | |
| MENTAL AND BEHAVIORAL HEALTH | |||||||
| Cocaine use (ref: no) | 136 (11%) | 132 (10%) | 4 (29%) | 3·5 (0·9–10·6) | 134 (10%) | 2 (22%) | |
| Meth, speed, amphetamines (ref: no) |
73 (6%) | 70 (5%) | 3 (21%) | 4·7 (1·1–15·5) | 72 (6%) | 1 (11%) | |
| Any stimulant use (ref: none) | 211 (16%) | 207 (16%) | 4 (29%) | 207 (16%) | 4 (44%) | 4·2 (1·0–15·9) | |
| Lifetime suicidal ideation (ref: no) | 971 (77%) | 966 (77%) | 5 (36%) | 0·2 (0·1–0·5) | 966 (77%) | 5 (62%) | |
| SEXUAL HEALTH AND HIV PREVENTION | |||||||
| Laboratory-confirmed STI (site-based only) | 80 (11%) | 75 (11%) | 5 (38%) | 5·3 (1·6–16·3) | 76 (11%) | 4 (50%) | 8·4 (2·0–36·2) |
| Self-reported STI (ref: no) | 264 (20%) | 258 (20%) | 6 (43%) | 3·0 (1·0–8·6) | 261 (20%) | 3 (33%) | |
| Indicated for PrEP based on criteria for transgender women (ref: no) | 619 (47%) | 605 (47%) | 14 (93%) | 16·0 (3·2–291·0) | 614 (47%) | 5 (56%) | |
| PARTNER CHARACTERISTICS | |||||||
| Gender(s) of casual and regular partners (past 3 mo.)3,4 | |||||||
| Cis woman or trans person only | 259 (30%) | 259 (30%) | 0 (0%) | RR not calculated due to zero events in cell | 259 (30%) | 0 (0%) | RR not calculated due to zero events in cell |
| Cis man only | 364 (42%) | 356 (42%) | 8 (73%) | 359 (42%) | 5 (100%) | ||
| Multiple genders | 244 (28%) | 241 (28%) | 3 (27%) | 244 (28%) | 0 (0%) | ||
| Partner PrEP use (ref: no) | 193 (15%) | 188 (15%) | 5 (36%) | 3·1 (1·0–9·2) | 191 (15%) | 2 (22%) | |
Notes: Characteristics displayed and relative risks (RRs) calculated for variables significant at p<0·05 using the appropriate Pearson’s Chi-squared test, Fisher’s exact test, or Wilcoxon rank sum test for either or both outcomes (Supplemental table 2 displays all tested variables and p-values); bolded relative risks are those with 95%CIs that do not cross 1·0;
Original select-all categories for race are used in this table to avoid small cells; responses to race categories may overlap for participants with multiracial identities;
Relative risks not calculated due to zero events in some categories;
Gender of regular and casual partners missing for 4 deceased participants for the following reasons: 2 reported no sex in last 12 months, 1 reported sex work clients only, and 1 reported sex in previous 12 months, but no sex partners in past 3 months.
Characteristics and experiences associated with HIV incidence included: race, phone disconnected, self-reported history of STI, number of recent sexual partners, PrEP indication, and partner PrEP use. A home internet connection, primary care provider, experience of psychological, physical, or sexual violence, and lifetime suicidal ideation were inversely associated with HIV seroconversion.
Characteristics and experiences associated with premature death during follow-up included: Hispanic/Latinx ethnicity and recent arrest. All deaths occurred among participants who were uninsured or publicly insured. Full or part-time employment and stigma-related barriers to healthcare were inversely associated with death.
DISCUSSION
We describe HIV incidence and mortality among a diverse, multi-site cohort study exclusively focused on transgender women in the US. HIV incidence was high at 5·5/1,000py. Unexpectedly, mortality rates were similar at 3·3/1,000py overall. HIV incidence and mortality rates were highest among Black- and Latinx-identified participants and those residing in the South. Predictors of HIV incidence and death underscored individual and contextual factors that impact health outcomes for transgender women. Notably, sexual partnerships with cisgender men and the use of stimulants were shared predictors of HIV seroconversion and death, while participation in the digital mode and seeking gender-transition-related care were inversely associated with these outcomes.
High mortality rates and causes of death reflect the burden of violence victimization, poor mental health, and substance misuse among transgender women, which stem from persistent stigma, discrimination, incarceration, and barriers to healthcare.23,24 Despite a wealth of longitudinal data collected in HIV prevention research, mortality rates are rarely reported in the scientific literature for comparison, though deaths are reported in clinical trials. During the non-inferiority trial of long-acting injectable PrEP among transgender women and cisgender men, 11 participant deaths were reported—comparable to the number of seroconversions (n=13).25 Eight of those deaths were attributed to overdose or trauma.25 While the primary goal of HIV observational research and trials is to evaluate HIV outcomes, these findings collectively raise ethical questions of why death—particularly violent and preventable deaths—are not given greater attention in HIV research. It also raises questions about how benefits of research participation can be enhanced to address related vulnerabilities.
Predictors of incident HIV infection point to clear intervention targets, particularly multi-level HIV prevention interventions. Structural interventions may indirectly aid in sustained access to various health services, including HIV prevention and mental and behavioral health services, and ultimately improve long-term survival. Examples of structural interventions include policy changes to reduce disproportionate arrest and incarceration, reduce employment discrimination, increase insurance access, support stable housing and safety, develop culturally competent care, and permit insurance coverage for gender-affirming care.
Individual-level interventions may include substance use and sexual health services. Substance use interventions may reduce the harmful effects of use and HIV acquisition risk associated with methamphetamines and cocaine.12 Sexual health predictors of HIV acquisition, including STI infection and number of sexual partners, can be met with prevention strategies to increase availability of combined STI and HIV testing with linkage to PrEP services.
The non-use of PrEP observed among participants who seroconverted, specifically never initiating PrEP or discontinuing use, suggests that a thoughtful approach to PrEP delivery for transgender women is needed. Extant literature shows that knowledge of and willingness to use PrEP is high among transgender women, but there is a gap in use.26 To explore this, a group-based multi-trajectory model identified five longitudinal behavioral- and interpersonal-risk patterns mapped to daily oral PrEP use over 18 months of follow-up.27 Two patterns demonstrated consistent (15%) or dynamic risk for HIV (47%) but no reported PrEP use, while the other patterns showed PrEP use consistent with risk. Youth (ages 18–24 years) and uninsured participants were more likely to have dynamic risk and to report never using PrEP. Those experiencing homelessness were more likely to have dynamic risk patterns and PrEP use.27 These results suggest that many transgender women use PrEP during periods of risk; however, basic livelihood and survival may take priority over PrEP use and persistence, even if considered an effective prevention option. More research is needed to understand how to support PrEP use among transgender women who can benefit from PrEP, particularly youth and people experiencing structural vulnerabilities. Research on whether recently approved long-acting injectable PrEP resolves these barriers is also needed.
Participants who reported sexual partner PrEP use at baseline were more likely to experience HIV seroconversion. This finding may be due to low adherence, discontinued use by the partner, or acquisition from a different partner not using PrEP. It could also reflect prior reports of discrepant sexual agreements among some transgender women and their cisgender male sexual partners, which were associated with intra-and extra-dyadic sexual risk.28 One pilot study of a couples-focused HIV prevention program for transgender women and their partners showed acceptability and improved prevention practices within sexual partnerships.29 Because partnerships with cisgender men were associated with an increased likelihood of incident HIV and death, such partner-level interventions may be leveraged to support HIV prevention, supportive relationships, and safety.
Our findings reflect US HIV epidemic trends, though limited incidence estimates are available to compare study findings. The incidence rate among the full sample in our study is comparatively lower than the facility-based cohort in San Francisco (N=415; IR: 1·3/100py; 95%CI: 0·7–2·7). However, rates are similar when comparing estimates from our site-based mode to the San Francisco cohort.10 This highlights the qualitative differences observed in HIV incidence and mortality estimates between site-based and digital modes, though with overlapping confidence intervals. Inconsistent access to communication technology was also associated with incident infection in our study. These findings support the digital divide concept (i.e., participants with technology access and comfort using it may have more education, social capital, and fewer economic vulnerabilities) and the need to address this divide in HIV research.
Hybrid cohort models that combine site-based and digital modes may facilitate sampling that is representative and generalizable to the target population, which is critical to monitoring epidemiologic trends. Each mode accrued and retained study populations with different profiles. When combined, however, the study population reflected the race and ethnic distributions of the 2020 Census.30 National survey efforts increasingly use mixed-mode approaches (e.g., National Survey on Drug Use and Health) to minimize response bias and measurement error.31 These efforts have promise for alternative methods in HIV research designs. Therefore, strategies beyond online delivery could increase inclusion of people with limited or inconsistent technology access and who may experience health vulnerabilities. Failure to recognize and address disparities in technology access within HIV research and prevention services could maintain or exacerbate health inequities.
Our findings should be interpreted in light of study limitations. First, this cohort was exclusive to the eastern and southern US, so it may not be generalizable to transgender women in the broader US and territories. The observed differences across cohort mode reflect differences in characteristics of individuals reached through such strategies, i.e., digital mode participants may have lower individual, inter-personal and structural vulnerabilities that relate to HIV acquisition risk, but site-based participants may have better access to HIV prevention and gender affirming services. The mixed-mode approach, coupled with the inclusion of all trans feminine people regardless of reported behavioral risk, likely maximizes the generalizability of our results to transgender women in the region. We interpret HIV incidence, mortality rates, and corresponding regression models with caution due to the small number of events. Incidence and mortality could be underestimated, particularly for digital mode participants, given the limited community and clinical connections, which are critical to reconnecting with participants or ascertaining events. National datasets used to verify a participant’s death, such as the National Death Index, are of limited utility for transgender participants, given the commonality of name changes to align with one’s gender. Limitations exist for digital research as participants are less likely to share multiple identifiers (e.g., social security number) in digital survey platforms, given security concerns. Finally, increased stigma in health facilities was inversely associated with premature death. As we report unadjusted associations, this unexpected association may be confounded by increased engagement in health facilities. Likewise, we were surprised to observe inverse associations between violence victimization and suicidal ideation with incident infection. Epidemiologically, this may reflect survival bias; sociologically, it may reflect participants’ resilience.
Conclusion:
HIV incidence among a cohort study of transgender women in eastern and southern US underscore the prioritization of transgender women in national HIV strategies.8,9 Mortality rates and shared predictors of HIV incidence and premature death emphasize community calls for attention to structural factors and other threats to health and well-being alongside HIV for transgender women, particularly transgender women of color. National strategies8 such as colocated service delivery, expansion of prescribing authority to pharmacists and other providers, and telehealth may reduce barriers to effective daily oral and long-acting PrEP and other HIV preventions. Partner-level and multi-level interventions delivered across digital and in-person modalities to support safety, housing, employment, and substance use treatment alongside HIV prevention will likely be strategies that change the trajectories of the HIV epidemic and premature death among transgender women.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Transgender women are a priority population in the United States’ Ending the HIV/AIDS Strategy. We searched scientific databases, PubMed and Google Scholar, using Medical Subject Headings and keyword terms for publications reporting HIV incidence and mortality in transgender women in the United States in the last ten years (January 2012 – July 2022).
Becasen and colleagues’ most recent systematic review identified 13 articles reporting laboratory-confirmed HIV prevalence among transgender women in the US, published between 2006 and 2017. They estimated laboratory-confirmed HIV prevalence at 14·1% (95%CI = 8·7, 22·2; I2= 96%). Prevalence differed by race and ethnicity, though with high heterogeneity across studies. The authors estimated that 55% of included studies were representative of the general transgender population, while the rest demonstrated evidence that study samples over-represented people with greater vulnerability to HIV. No cohort studies were described in that review.
Only one cohort has reported HIV incidence exclusively for transgender women in the US. McFarland and colleagues published a brief report describing HIV incidence among transgender women recruited via long-chain peer referral in the San Francisco site of the Trans National Study. Among 415 participants, HIV incidence was 1·3/100 person-years (95%CI: 0·7–2·7), with elevated rates among transgender women of color, aged 18–24, with a history of incarceration, and without health insurance.
Transgender women have participated in many domestic and international facility-based and online HIV cohort studies and trials. Aggregation with other populations has resulted in enrollment numbers often insufficient for transgender women-specific estimations of HIV incidence, risks, and mortality.
Community advocacy emphasizes attention to the burden of violence, trauma, and premature death of transgender women. Prospective research cohorts provide an opportunity to measure health trends and mortality. However, few cohorts report mortality rates for populations not living with HIV, and none have reported mortality rates for transgender women in the US. An Amsterdam cohort, however, provides some insight. Among 2,927 transgender women enrolled in their gender clinic between 1972–2018 and contributing 40,232 person-years, cumulative mortality was 10·8%—significantly higher than among cisgender women (standardized mortality ratio [SMR]: 2·8, 95%CI: 2·5–3·1)—with no change in rates across decades. Cause-specific rates were higher among transgender women than cisgender women for cardiovascular disease (SMR: 2·6, 95%CI: 1·9–3·4), cancer (SMR: 1·6, 95%CI: 1·3–2·0), infection (SMR: 8·7, 95%CI: 4·7–14·1), and non-natural causes (SMR: 6·1, 95%CI: 4·2–8·4).
Added value of this study:
We report epidemiologic data from a multi-site cohort study exclusively for transgender women in the eastern and southern US. Using a mixed-mode approach, we enrolled 1,312 participants between March 2018 to August 2020 in a technology-enhanced site-based or fully digital mode. We followed the participants for at least 24 months, accruing 2,730 person-years.
Overall HIV incidence was 5·5/1,000py (15/2,730; 95CI: 2·7–8·3) and higher among Black participants and those living in the South. Overall mortality was 3·3/1,000py (95%CI:1·5-6·3) and higher among Latinx participants. Shared predictors of HIV seroconversion and death included: residence in the South, sexual partnerships with cisgender men, and use of stimulants. Participation in the online cohort and seeking gender transition-related care were inversely associated with both outcomes. To our knowledge, this study provides the first multi-site estimates of HIV incidence and mortality among transgender women in the United States – a population that is prioritized in the national strategy but for whom little prospective, population-specific data are available. Our study findings also provide an opportunity to evaluate the tradeoffs of transitioning from site-based to fully digital cohort modalities.
Implications of available evidence:
HIV incidence and mortality are high in transgender women in the US, particularly among transgender women of color. Differences across cohort modes suggest that mixed-mode approaches may support the recruitment and retention of more representative samples. They also highlight the need for continued community and location-based efforts as HIV research and interventions are increasingly delivered online. Collectively, the evidence underscores community calls for multi-level, combination approaches specific to transgender women. Such approaches should support social and structural determinants of health and other health concerns, including survival, alongside HIV prevention. A singular focus on HIV prevention is a missed opportunity to address other threats to the lives of people prioritized in HIV services and programming.
Acknowledgments:
The authors would like to express their gratitude to the transgender women who participated in this study. This study would not be possible without their experiences and participation. We appreciate the continued involvement and contributions of the Community Advisory Board that supports and guides this study. We also want to thank all the research staff who spent their time and effort to actualize this study and connect with study participants in meaningful ways; Shannon Seopaul, Chloe Jordan, Maryam Roosta, Genesis Valera, Blanca Esquivel, Ali Harris, Amiyah Guerra, Valeria Botero, Jessica Orr, Nicho Herrera, Melissa Estrada, Alexandra Beem, Jessica Castaneda, Jeffrey Herman, and Barrett Crawford. Research reported in this publication was jointly supported by the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, and the National Institute of Child Health and Human Development of the National Institutes of Health under Award Number UG3/UH3AI133669 (Wirtz/Reisner). Research reported in this publication was also supported by HIV/AIDS, Hepatitis, STD, and TB Administration (HAHSTA), Washington DC Department of Health. The LITE study also appreciates support from the Centers for AIDS Research at partner institutions, including JHU (P30AI094189), Emory University (P30AI050409), Harvard University (P30AI060354), DC CFAR (AI117970), and the University of Miami (P30AI073961). A predoctoral fellowship from the National Institute of Mental Health (F31MH124582) supports EEC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or HAHSTA.
Footnotes
Data sharing:
De-identified individual data and data dictionary will be made available upon reasonable request after approval of a proposal and signing of a data use agreement. There is a formal process for external users to request access to LITE data, which involves review and approval by Principal Investigators from each study site as well as the Community Advisory Board; further details and forms can be obtained by emailing Dr. Andrea Wirtz (awirtz1@jhu.edu) and Dr. Sari Reisner (sreisner@bwh.harvard.edu).
Declarations of Interest:
KN Althoff is a consultant for the All of Us Research Program.
Tonia Poteat is a consultant for ViiV Healthcare and received an honorarium for a lecture provided to Merck & Co staff.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrea L Wirtz, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Center for Public Health and Human Rights Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
Elizabeth Humes, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
Keri N Althoff, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
Tonia C Poteat, Center for Health Equity Research, University of North Carolina School of Medicine, Chapel Hill, NC, United States.
Asa Radix, Callen-Lorde Community Health Center, New York, NY, United States.
Kenneth H Mayer, Beth Israel Deaconess Medical Center, Harvard Medical School, Harvard University, Boston, MA, United States; The Fenway Institute, Fenway Health, Boston, MA, United States.
Jason S Schneider, Emory University School of Medicine, Atlanta, GA, United States.
J Sonya Haw, Emory University School of Medicine, Atlanta, GA, United States.
Andrew J Wawrzyniak, Department of Psychiatry and Behavioral Sciences, Miller School of Medicine, University of Miami, Miami, FL, United States.
Christopher M Cannon, Whitman-Walker Institute, Washington, DC, United States.
Meg Stevenson, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Center for Public Health and Human Rights Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
Erin E Cooney, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Center for Public Health and Human Rights Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
Dee Adams, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States.
James Case, School of Nursing, Johns Hopkins University, Baltimore, MD, United States.
Chris Beyrer, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Center for Public Health and Human Rights Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Duke Global Health Institute, Duke University, Durham, NC, United States.
Oliver Laeyendecker, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, United States; Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, United States.
Allan E Rodriguez, Division of Infectious Diseases, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, United States.
Sari L Reisner, The Fenway Institute, Fenway Health, Boston, MA, United States.
American Cohort to Study HIV Acquisition Among Transgender Women (LITE) Study Group, Division of Endocrinology, Diabetes, and Hypertension, Brigham Women’s Hospital, Boston, MA, United States; Department of Medicine, Harvard Medical School, Harvard University, Boston, MA, United States; Department of Epidemiology, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, United States.
REFERENCES
- 1.James S, Herman JL, Ranklin S, Keisling M, Mottet LA, Anafi M. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality,, 2016. [Google Scholar]
- 2.Wilson EC, Hernandez CJ, Arayasirikul S, et al. In Their Own Words: How Trans Women Acquired HIV Infection. AIDS and Behavior 2022; 26(6): 2091–8. [DOI] [PubMed] [Google Scholar]
- 3.Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the Prevalence of HIV and Sexual Behaviors Among the US Transgender Population: A Systematic Review and Meta-Analysis, 2006–2017. American Journal of Public Health 2019; 109(1): e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. Atlanta, 2021. [Google Scholar]
- 5.Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and Facilitators to Engagement and Retention in Care among Transgender Women Living with Human Immunodeficiency Virus. Ann Behav Med 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirtz A, Poteat T, Malik M, Glass N. Gender-Based Violence Against Transgender People in the United States: A Call for Research and Programming. Trauma Violence Abuse 2020; 21(2): 227–41. [DOI] [PubMed] [Google Scholar]
- 8.The White House. National HIV/AIDS Strategy for the United States 2022–2025. Washington, DC: White House Office of National AIDS Policy,, 2021. [Google Scholar]
- 9.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA 2019; 321(9): 844. [DOI] [PubMed] [Google Scholar]
- 10.McFarland W, Wesson P, Turner C, et al. High HIV Incidence Among Young and Racial/Ethnic Minority Transgender Women in San Francisco: Results of a Longitudinal Cohort Study. JAIDS Journal of Acquired Immune Deficiency Syndromes 2020; 84(1): e7–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rendina HJ, Talan AJ, Tavella NF, et al. Leveraging Technology to Blend Large-Scale Epidemiologic Surveillance with Social and Behavioral Science Methods: Successes, Challenges, and Lessons Learned Implementing the UNITE Longitudinal Cohort Study of HIV risk factors among Sexual Minority Men in the U.S. Am J Epidemiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grov C, Westmoreland D, Morrison C, Carrico AW, Nash D. The Crisis We Are Not Talking About: One-in-Three Annual HIV Seroconversions Among Sexual and Gender Minorities Were Persistent Methamphetamine Users. J Acquir Immune Defic Syndr 2020; 85(3): 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Blok CJM, Wiepjes CM, van Velzen DM, et al. Mortality trends over five decades in adult transgender people receiving hormone treatment: a report from the Amsterdam cohort of gender dysphoria. The Lancet Diabetes & Endocrinology 2021; 9(10): 663–70. [DOI] [PubMed] [Google Scholar]
- 14.Akinola M, Wirtz AL, Chaudhry A, Cooney E, Reisner SL. Perceived acceptability and feasibility of HIV self-testing and app-based data collection for HIV prevention research with transgender women in the United States. AIDS Care 2021: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisner SL, Chaudhry A, Cooney E, Garrison-Desany H, Juarez-Chavez E, Wirtz AL. ‘It all dials back to safety’: A qualitative study of social and economic vulnerabilities among transgender women participating in HIV research in the USA. BMJ Open 2020; 10(1): e029852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirtz AL, Cooney EE, Chaudhry A, Reisner SL, American Cohort To Study HIVAATW. Computer-Mediated Communication to Facilitate Synchronous Online Focus Group Discussions: Feasibility Study for Qualitative HIV Research Among Transgender Women Across the United States. J Med Internet Res 2019; 21(3): e12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirtz A, Cooney E, Stevenson M, et al. Digital Epidemiologic Research on Multilevel Risks for HIV Acquisition and Other Health Outcomes Among Transgender Women in Eastern and Southern United States: Protocol for an Online Cohort. JMIR research protocols 2021; (29152). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirtz AL, Poteat T, Radix A, et al. American Cohort to Study HIV Acquisition Among Transgender Women in High-Risk Areas (The LITE Study): Protocol for a Multisite Prospective Cohort Study in the Eastern and Southern United States. JMIR research protocols 2019; 8(10): e14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tate CC, Ledbetter JN, Youssef CP. A two-question method for assessing gender categories in the social and medical sciences. Journal of sex research 2013; 50(8): 767–76. [DOI] [PubMed] [Google Scholar]
- 20.Holmström P, Syrjänen S, Laine P, Valle SL, Suni J. HIV antibodies in whole saliva detected by ELISA and western blot assays. J Med Virol 1990; 30(4): 245–8. [DOI] [PubMed] [Google Scholar]
- 21.De Boni RB, Veloso VG, Fernandes NM, et al. An Internet-Based HIV Self-Testing Program to Increase HIV Testing Uptake Among Men Who Have Sex With Men in Brazil: Descriptive Cross-Sectional Analysis. J Med Internet Res 2019; 21(8): e14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone J, Reisner S, Cooney E, Wirtz A, American Cohort to Study HIV Acquisition among Transgender Women STudy Group. Perceived HIV acquisition risk and low uptake of pre-exposure prophylaxis (PrEP) among Transgender women with PrEP indication in the United States: Results from the LITE Study. JAIDS 2021; 88: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poteat T, Humes E, Althoff K, et al. Characterizing arrest and incarceration in a prospective cohort of transgender women. Journal of Correctional Health Care 2022; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter S, Settle E, Wylie K, et al. Synergies in health and human rights: a call to action to improve transgender health. The Lancet 2016; 388(10042): 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. New England Journal of Medicine 2021; 385(7): 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poteat T, Wirtz A, Malik M, et al. A Gap Between Willingness and Uptake: Findings From Mixed Methods Research on HIV Prevention Among Black and Latina Transgender Women. J Acquir Immune Defic Syndr 2019; 82(2): 131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooney EE, Reisner SL, Saleem HT, et al. Prevention-effective adherence trajectories among transgender women indicated for PrEP in the United States: a prospective cohort study. Annals of epidemiology 2022; 70: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamarel KE, Reisner SL, Darbes LA, et al. Dyadic dynamics of HIV risk among transgender women and their primary male sexual partners: the role of sexual agreement types and motivations. AIDS Care 2016; 28(1): 104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Operario D, Gamarel KE, Iwamoto M, et al. Couples-Focused Prevention Program to Reduce HIV Risk Among Transgender Women and Their Primary Male Partners: Feasibility and Promise of the Couples HIV Intervention Program. AIDS Behav 2017; 21(8): 2452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones N, Marks R, Ramirez R, Ríos-Vargas M. 2020 Census Illuminates Racial and Ethnic Composition of the Country: US Census Bureau,, 2021. [Google Scholar]
- 31.Edith DD. Mixed-Mode: Past, Present, and Future. Survey Research Methods 2018; 12(2). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.