Allergic bronchopulmonary aspergillosis (ABPA) occurs from a hypersensitivity reaction to the fungus Aspergillus and shares some pathogenesis with asthma (1). Oral corticosteroids (OCS) are usually the initial treatment of choice, but response is variable and side effects are common. For these reasons, there is a need to identify different treatment options for ABPA. Th2 inflammation is a shared pathway for many patients with severe asthma and ABPA (1). Small studies have shown promise using biologic therapies approved for asthma to treat ABPA, but large randomized trials are lacking. Dupilumab is a human monoclonal IgG4 antibody against the IL-4 receptor alpha subunit (IL-4rα) that blocks the activity of both IL-4 and IL-13 cytokine signaling pathways (2). The use of dupilumab in ABPA has been limited to case reports and series. Our group and others have reported improvement of symptoms, reduction of exacerbations, and a decrease in OCS use with dupilumab in patients with ABPA (3–6).
Like any other complex disease, genetics is one of the variables that could play a role in the clinical manifestation and response to treatment in patients with ABPA. Here, we present a unique case of genetically identical twin sisters with severe asthma and ABPA who had different clinical outcomes after only one agreed to be treated with biologics. Both twins met the Asano 2021 criteria for ABPA diagnosis (7). Their clinical characteristics are summarized in the Table E1. This table shows that before presentation, both twins had similar clinical courses, comorbidities, exposures, risk factors, and medications.
Online repository table E1:
Patients’ clinical characteristics, exposures, risk factors and medications.
| TWIN 1 | TWIN 2 | |
|---|---|---|
| SMOKING | Former smoker, quit in 1995, 5 pack-year smoking history. Never smoked or inhaled other products | Former smoker, quit in 1998. Never smoked or inhaled other products |
| COMORBIDITIES | Hypertension, hypercholesterolemia, type 2 diabetes mellitus, vitamin D deficiency, well-controlled GERD, mild allergic rhinitis, osteoarthritis | Hypertension, hypercholesterolemia, prediabetes, vitamin D deficiency, bilateral avascular necrosis of hips, colonic polyps, mild allergic rhinitis |
| PHYSICAL ACTIVITY | 4–6 times per week, aerobics, weight training, 31–60 minutes | Walks 30 min slowly at least 5 times per week |
| AIRWAY CLEARANCE | Flutter valve | Unknown |
| INHALERS COMPLIANCE AND TECHNIQUE | Good | Good |
| GREW UP | Mississippi | Mississippi, Texas, Louisiana |
| LIVE IN | Atlanta, GA | Atlanta, GA |
| BMI | 33.5 | 32 |
| GERD OR DYSPHAGIA | GERD, well controlled with esomeprazole, no dysphagia | No |
| ENVIRONMENTAL EXPOSURES | None | None |
| WORK-RELATED EXPOSURES | None | None |
| EXTRAPULMONARY MANIFESTATIONS | Mild allergic rhinitis, now well controlled on dupilumab. She does not need intranasal corticosteroids anymore | Mild allergic rhinitis, controlled with intranasal corticosteroids |
| SIGNIFICANT TRAVEL-RELATED EXPOSURES | None | None |
| CURRENT MEDICATIONS | Budesonide-formoterol, albuterol, cholecalciferol, esomeprazole, hydrochlorothiazide-losartan, rosuvastatin, dupilumab | Fluticasone-vilanterol, albuterol, cholecalciferol, docusate, montelukast, triamcinolone nasal spray, hydrochlorothiazide-losartan |
| ASTHMA HISTORY BEFORE PRESENTATION TO OUR CLINIC | Asthma started during childhood. Well controlled from late teenage years to her early 40s. In her 40s she started with maintenance controller inhalers. In her 50s she started to have 3–4 exacerbations per year requiring corticosteroids. Never hospitalized or intubated for asthma. Intermittent use of daily prednisone 10 mg over the past several years | Had asthma during childhood but then went away at age 8. At 22 years of age her asthma symptoms presented again and started maintenance controller inhalers. She was well controlled until age 50 when she started to have 3–4 exacerbations per year requiring oral corticosteroids. Never hospitalized or intubated for asthma. Intermittent use of daily prednisone 5 to 10 mg for several years |
| ASTHMA MEDICATIONS USED BEFORE PRESENTATION TO OUR CLINIC (NO LONGER TAKING) | Montelukast, theophylline, fluticasone-salmeterol, mometasone-formoterol, aclidinium, cetirizine | Budesonide-formoterol, fluticasone-salmeterol |
| FAMILY HISTORY | Mother with asthma | Mother with asthma, daughter with asthma and eczema |
| WORK | Banker until 1999 and thereafter stay-at-home mother | Office manager and banker |
| PETS | None | None |
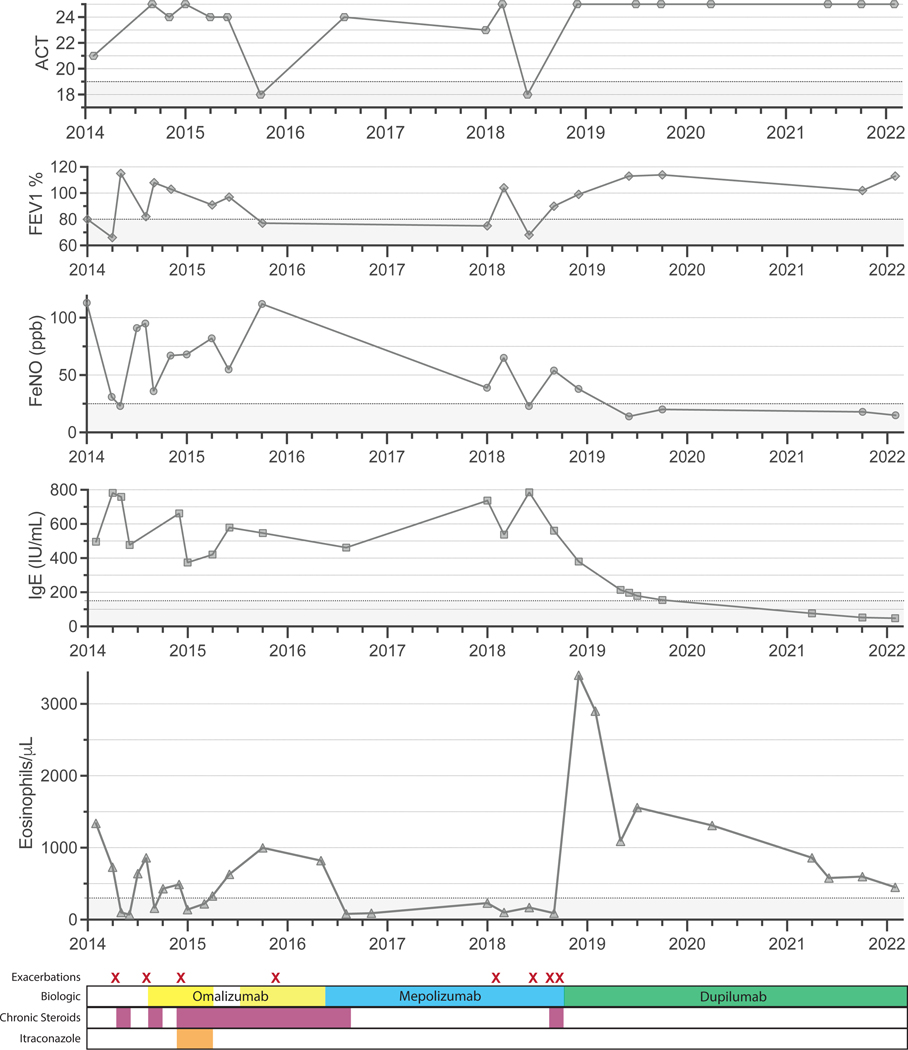
Twin 1 is a 61-year-old female with long-standing asthma presented to our clinic in 2014. She was found to have airflow limitation with a forced expiratory volume in the first second (FEV1) of 65% of predicted, a total IgE of 496 IU/mL with a blood eosinophil count of 1340 cells/μL (Figure 1), central bronchiectasis on computed tomography (CT) of the chest (Figure E1), along with a positive Aspergillus-specific IgE and skin test. She was diagnosed with ABPA and started on daily prednisone, itraconazole, and omalizumab. Despite treatment adherence, her symptoms persisted, and she continued to have exacerbations, airflow limitation, airway inflammation, and blood Th2 biomarker elevation (Figure 1). She was then switched to mepolizumab with good response and was able to discontinue daily prednisone. However, after two years on mepolizumab, she had several exacerbations and was restarted on daily prednisone. She was then transitioned to dupilumab followed by a rapid and substantial clinical improvement. Shortly after dupilumab was started she experienced asymptomatic hypereosinophilia that self-improved over time. Almost 4 years after initiating dupilumab, she has remained free of exacerbations and has not needed regular prednisone (Figure 1). She also has minimal symptoms, normal lung function, evidence of low airway inflammation, and total serum IgE below 150 IU/mL (Figure 1) and undetectable Aspergillus-specific IgE.
Figure 1. Clinical course of twin 1.
A) Clinical parameters from top to bottom: Asthma Control Test (ACT), forced expiratory volume in the first second (FEV1) percent of predicted, fractional exhaled nitric oxide (FeNO), parts per billion (ppb), total immunoglobulin E (IgE) IU/mL, eosinophils/μL. B) Biologic therapy (top), daily prednisone (middle), and antifungal treatment (bottom). Exacerbations are noted with an “X”.
Online repository figure E1. Chest CT findings of both patients.
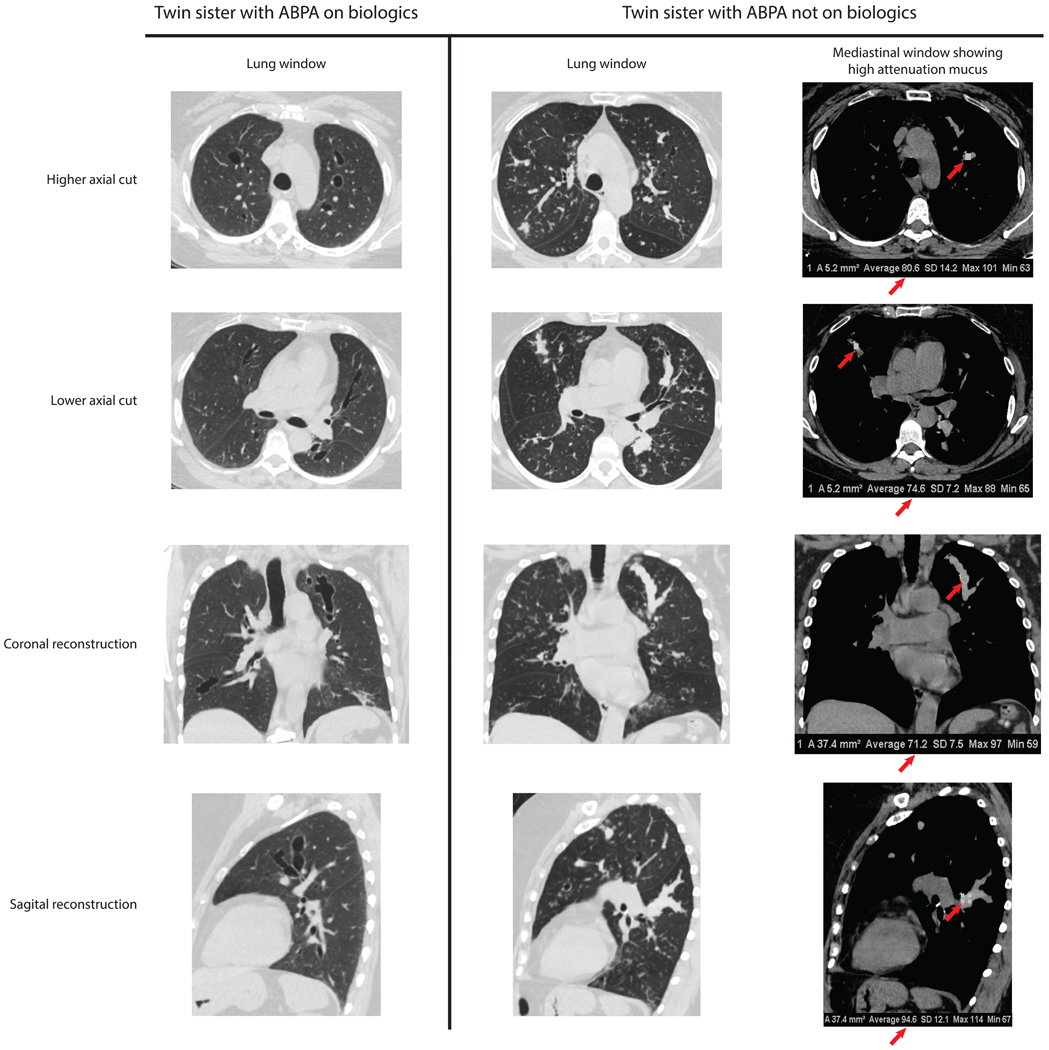
A) Twin 1 on biologic therapy. Central bronchiectasis are seen without mucus impaction and without parenchymal abnormalities. B) Twin 2 not on biologic therapy. Lung windows (left) reveal central cylindrical bronchiectasis, mucus impaction, and tree-in-bud nodularity. Mediastinal windows (right) show high attenuation mucus with Hounsfield units > 70 (red arrows).
Twin 2 presented to our clinic in early 2021 with long-standing eosinophilic asthma and frequent exacerbations. Due to having required repetitive courses of OCS for many years, she had developed avascular necrosis of bilateral hips requiring bilateral hip replacements. In our clinic, she was found to have airflow limitation with an FEV1 of 58% of predicted, a peripheral blood eosinophil count of 2,350 cells/μL (off OCS), a total IgE of 842 IU/mL, and an Aspergillus-specific IgE of 18.6 IU/mL. CT of the chest showed central bronchiectasis and high attenuation mucus impaction (Figure E1). Like her twin sister, she was diagnosed with ABPA, and was started on daily OCS. However, she declined to start a biologic or antifungal therapy. One year later, she continues to require daily OCS and unfortunately has developed prediabetes.
Illustrating the role of genetics in ABPA, we compare the unique cases of twin sisters with ABPA. One twin was treated with three different biologic therapies over the course of 8 years, while the other declined biologic treatment. The debate on how much genetics and environment impact ABPA is ongoing. Interestingly, both sisters were born and raised in the Southeastern United States with similar but non-identical environmental exposures. Some fungi, especially Aspergillus, are known to be ubiquitous in this part of the world, making exposure to them essentially unavoidable. Thus, for ABPA, genetics may seem to play an important role in disease susceptibility.
In this report of two patients with genetic homogeneity, the long-term effectiveness of dupilumab to treat ABPA in Twin 1 is encouraging. Although control was not achieved with omalizumab or mepolizumab, Twin 1 is now exacerbation free with minimal symptoms for almost 4 years on dupilumab. She has evidence of bronchiectasis due to permanent scarring from previous ABPA exacerbations, but she has no signs of current airway inflammation or mucus impaction on imaging (Figure E1), and her markers of Th2 inflammation have greatly improved.
In contrast, Twin 2 who declined biologic therapy continues to be OCS dependent despite suffering from corticosteroid related adverse effects. Her most recent CT of the chest shows cylindrical bronchiectasis with multiple areas of high-attenuation mucus impaction and tree-in-bud micronodules consistent with active airway inflammation (Figure E1).
With six different biologics FDA approved for treating severe asthma (2), one important question is whether one is superior for the management of ABPA. Both omalizumab and IL-5 targeted therapies may benefit symptoms in ABPA, but only IL-5 signaling blockade has been shown to clear mucus plugs (8). Recently, there have been two reports of favorable clinical response to dupilumab after failure of anti-IL-5 therapy (4, 6), suggesting interruption of upstream T2 mediators such as IgE may be critical to prevent exacerbations. Dupilumab may specifically benefit patients with ABPA by the following mechanisms: 1) inhibition of IL-13 that upregulates the Muc5ac gene implicated in the generation of airway mucus (9) and/or 2) reduction of IgE production by inhibition of local B cell differentiation into IgE antibody secreting cells. Dupilumab likely interrupts the initial pathogenic mechanism of IgE-mediated immune cell activation.
This study shows how genetics and environmental exposures play a role in ABPA, and the benefits of dupilumab in patients with uncontrolled ABPA.
Clinical Implications.
We describe the cases of identical twins with ABPA, but only one of them received biologic therapies. We used their genetic homogeneity as a basis for assessing the effectiveness of dupilumab in treating ABPA.
Funding Information
This work was supported by the following National Institutes of Health awards: U01AI141993 (FEL) and P01AI125180 (FEL) and T32HL116271 (PAL, MR, and NS)
FEL is the founder of Micro-Bplex, Inc. FEL serves on the scientific advisory board of Be Biopharma, is a recipient of grants from the BMGF and Genentech, Inc. FEL has also served as a consultant for Astra Zeneca.
Footnotes
Conflicts of interest
PAL, MR, NS, FCF, AS, and JP have no conflicts of interest to disclose.
Statement of informed consent obtained.
We obtained written informed consent from both patients in our study for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43(8):850–73. [DOI] [PubMed] [Google Scholar]
- 2.Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. 2022;386(2):157–71. [DOI] [PubMed] [Google Scholar]
- 3.Eldaabossi SAM, Awad A, Anshasi N. Mepolizumab and dupilumab as a replacement to systemic glucocorticoids for the treatment of Chronic Eosinophilic Pneumonia and Allergic Bronchopulmonary Aspergillosis - Case series, Almoosa specialist hospital. Respir Med Case Rep. 2021;34:101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikura S, Saraya T, Yoshida Y, Oda M, Ishida M, Honda K, et al. Successful Treatment of Mepolizumab- and Prednisolone-resistant Allergic Bronchopulmonary Aspergillosis with Dupilumab. Intern Med. 2021;60(17):2839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Veer T, Dallinga MA, van der Valk JPM, Kappen JH, In ‘t Veen J, van der Eerden MM, et al. Reduced exacerbation frequency and prednisone dose in patients with ABPA and asthma treated with dupilumab. Clin Transl Allergy. 2021;11(10):e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramonell RP, Lee FE, Swenson C, Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: A case series. J Allergy Clin Immunol Pract. 2020;8(2):742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano K, Hebisawa A, Ishiguro T, Takayanagi N, Nakamura Y, Suzuki J, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147(4):1261–8 e5. [DOI] [PubMed] [Google Scholar]
- 8.Asano K, Ueki S, Tamari M, Imoto Y, Fujieda S, Taniguchi M. Adult-onset eosinophilic airway diseases. Allergy. 2020;75(12):3087–99. [DOI] [PubMed] [Google Scholar]
- 9.Suzaki I, Kawano S, Komiya K, Tanabe T, Akaba T, Asano K, et al. Inhibition of IL-13-induced periostin in airway epithelium attenuates cellular protein expression of MUC5AC. Respirology. 2017;22(1):93–100. [DOI] [PubMed] [Google Scholar]