Abstract
Background:
Multiple monoclonal antibodies are currently approved for the treatment of asthma. However, there is limited evidence on their comparative effectiveness.
Objective:
To compare the effectiveness of omalizumab, mepolizumab, and dupilumab in individuals with moderate to severe asthma.
Methods:
We emulated a hypothetical randomized trial using electronic health records from a large US-based academic health care system. Participants aged ≥18 years with baseline immunoglobulin E levels between 30 and 700 IU/ml and peripheral eosinophil counts of ≥ 150 cells/microliter were eligible for study inclusion. The study period was March 2016 to August 2021. Outcomes included the incidence of asthma-related exacerbations and change in baseline forced expiratory volume in one second (FEV1) over 12 months of follow up.
Results:
Sixty-eight individuals on dupilumab, 68 on omalizumab, and 65 on mepolizumab met inclusion criteria. Over 12 months of follow-up, 31 exacerbations occurred over 68 person-years (0.46 exacerbations per person-year) in the dupilumab group, 63 over 68 person-years (0.93 per person-year) in the omalizumab group, and 86 over 65 person-years (1.32 per person-year) in the mepolizumab group (adjusted incidence rate ratios: dupilumab vs. mepolizumab 0.28, 95% confidence interval [CI] 0.09 – 0.84; dupilumab vs. omalizumab (0.36, CI 0.12–1.09); omalizumab vs. mepolizumab (0.78, CI 0.32–1.91). The difference in FEV1 change comparing patients receiving dupilumab to those receiving mepolizumab was 0.11 (CI −0.003 to 0.222) L, 0.082 (CI −0.040 to 0.204) L for dupilumab vs. omalizumab, and 0.026 (CI −0.083 to 0.140) L omalizumab vs. mepolizumab.
Conclusions:
Among patients with asthma and eosinophil counts of ≥150 cells/microliter and IgE levels of 30–700 kU/L, dupilumab was associated with greater improvements in exacerbation and FEV1 than omalizumab and mepolizumab.
Keywords: asthma, comparative effectiveness, monoclonal antibodies, target trial emulation, dupilumab, mepolizumab, omalizumab, eosinophilic, allergic
Capsule summary:
This emulation of a hypothetical trial shows that, in comparison to omalizumab and mepolizumab, dupilumab is associated with greater reductions in asthma exacerbations in patients with eosinophil counts of ≥150 cells/microliter and IgE levels of 30–700 kU/L.
INTRODUCTION
In December 2021, a sixth monoclonal antibody, or “biologic”, was approved for the treatment of severe asthma. However, head-to-head comparisons of the previously approved biologics are still lacking, thus limiting opportunities to optimize patient selection for these costly therapies.1 For individuals who meet prescribing criteria for only one of these therapies, the choice of therapy may be clear. However, for the many patients with moderate to severe asthma who have more than one phenotype concurrently, such as allergic and eosinophilic asthma, or those meeting prescribing criteria for multiple biologics (“multiply eligible”),2 the optimal choice of biologic is uncertain.
All currently approved biologic therapies have been shown to improve asthma-related outcomes in individuals with asthma uncontrolled on conventional therapy. These include omalizumab, an anti-immunoglobulin E which is approved for treatment of allergic asthma in individuals who have evidence of sensitivity to perennial allergens and immunoglobulin E (IgE) levels between 30 and 700 kU/L; dupilumab, an anti-interleukin 4 receptor alpha (anti-IL4R∝) that has been shown to be effective in both allergic and eosinophilic asthma; and mepolizumab, an anti-interleukin 5 (anti-IL5) that is effective in individuals with eosinophilic asthma defined as having peripheral blood eosinophils of ≥150 cells per microliter.3, 4
In the absence of head-to-head trials, observational data can be used to emulate a hypothetical randomized trial, a target trial, in order to generate evidence about comparative effectiveness of medications and to inform clinical decisions.5–7 In the absence of head-to-head trials, this generates important evidence to inform clinical decisions. We conducted a retrospective cohort study that emulated a target trial to compare the effectiveness of omalizumab, dupilumab, and mepolizumab in reducing asthma-related exacerbations and improving lung function in individuals with asthma.
METHODS
Data Source
We leveraged the integrated electronic health record from the Mass General Brigham (MGB) Research Patient Data Registry (RPDR). The RPDR is a centralized clinical data registry of patient-related data from hospitals within the MGB, the largest healthcare system in Massachusetts. This includes the Massachusetts General Hospital and the Brigham and Women’s Hospital which house the Mass General Brigham Asthma Center, specialized centers such as the Dana Farber Cancer Institute, and other affiliated hospitals such as the Faulkner Hospital. RPDR currently holds clinical information on about 6 million patients since 1980. The information within RPDR includes data from the electronic medical record systems, the billing systems and the clinical data repository, which includes laboratory data and radiology results.8 We conducted chart reviews to extract missing demographic and laboratory values, to verify the indication for biologic use and that these individuals received the biologics indicated, and to confirm comorbidities. This study was approved by the Mass General Brigham Institutional Review Board.
Study design and approach
Target trial emulation
Target trial emulation is a cohort study design that uses similar design parameters as a hypothetical randomized control trial, including eligibility criteria, treatment arms and protocol, start of follow-up and outcome assessment (Table E1).5–7 Given that non-experimental designs sometimes lead to biased estimates and spurious associations, explicit emulation of a target trial provides an opportunity to avoid common threats to validity from non-experimental studies and/or to identify how results from non-experimental studies may be biased in comparison to the hypothetical randomized trial.
Study Population
Patients aged 18 years or older with a diagnosis of moderate to severe asthma as identified by the International Classification of Diseases, 9th (ICD-9) or 10th (ICD-10) revision who received biologics between 3/1/2016, when mepolizumab was added to the institutional formulary, and 8/31/2021, and who did not have a concomitant code for other chronic lung diseases including cystic fibrosis, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease were eligible for inclusion in the study. The index date was the date of initiation of the first biologic on or after 3/1/2016. However, we set the index date for dupilumab users as on or after 11/1/2018 when asthma was added to the institutional formulary as an indication for dupilumab following its approval for asthma in October 2018. We assumed individuals who initiated dupilumab prior to this date had initiated therapy primarily for atopic dermatitis and thus were excluded from the analyses.
To avoid wrongfully attributing asthma as the indication for therapy, we also excluded individuals with ICD-9 or 10 codes indicating other alternate indications for these biologics. Thus, we excluded individuals with ICD-9 or 10 codes for chronic spontaneous urticaria, Churg-Strauss disease, hyper-eosinophilic syndrome, chronic sinusitis, nasal polyposis, and atopic dermatitis or those in which dupilumab was prescribed by a dermatologist. Patients were followed from their index dates to 12 months later or 08/31/2021, whichever came first. (eFigure 1)
Reslizumab and benralizumab are rarely used in our center leading to extremely small sample sizes, and tezepelumab had not been added to the institutional formulary at the time of this writing. Thus, they are not included in this study.
Study outcomes
The primary outcomes of interest were the cumulative incidence and incidence rate of clinically significant exacerbations (composite of asthma exacerbation requiring steroids or hospitalization) over 12 months of follow up. We included change in pre-bronchodilator forced expiratory volume in the first second (FEV1) as a secondary outcome. Using previously validated methodology, we defined an exacerbation as an emergency room visit or a hospitalization event with a primary diagnostic code for asthma or an outpatient prescription for oral or intravenous corticosteroids.9–11 Prescriptions for corticosteroids within 7 days of a prior prescription were considered to belong to the same exacerbation event. Asthma-related emergency room visits or hospitalizations were encounters with a primary code for asthma, wheezing or bronchospasm which were spaced out at least 7 days apart (Table E2).
Statistical Analyses
Primary analyses were in the intention-to-treat population that included all patients who initiated biologic therapy and met study eligibility criteria regardless of subsequent switch to another biologic or discontinuation during the follow up period. Categorical variables are reported as counts and percentages; continuous variables are reported as means and standard deviations for normally distributed data and as medians and interquartile ranges for skewed data.
Covariate adjustment
We leveraged two approaches to account for non-random allocation between the groups and to emulate randomization at baseline. First, we focused on individuals for whom there was clinical equipoise: we restricted the study population to individuals with serum immunoglobulin (IgE) count between 30 and 700 IU/mL, the range of IgE used to determine eligibility for omalizumab, and eosinophil count of ≥150 cells per microliter, given mepolizumab and dupilumab’s approval for use in eosinophilic asthma.3, 12 Thereafter, we used overlap weighting for each pairwise comparison including potential confounders between biologic use and exacerbation rates as identified in a directed acyclic graph (DAG) constructed a priori. (Figure E2) Overlap weighting outperforms inverse probability treatment weighting, in terms of bias and variance, for continuous, binary, and time-to-event outcomes.13, 14 It limits the occurrence of extreme weights, and thus its benefit increases as the degree of covariate overlap between groups decrease, and is more robust to misspecification of the propensity score model.14 Relatedly, it has been shown to weight to an overlap population between groups being compared while balancing measured covariates.15 Variables used for weighting included age, sex, race/ethnicity, insurance, baseline asthma control using the annualized exacerbation rate pre-index date, pre-index eosinophil count, pre-index IgE level, baseline FEV1, use of ICS/LABA, body mass index (BMI), smoking status, presence of allergic rhinitis, Charlson comorbidity index, the season of biologic initiation, and the patient’s residence in the inner core. The inner core was as defined by the Metropolitan Area Planning Council in Massachusetts.16 We evaluated covariate balance by plotting the absolute standardized mean differences (ASMD).17 An ASMD of 0.10 or less was considered acceptable.18, 19
We used overlap-weighted negative binomial regression models to calculate incidence rate ratios for the exacerbations; we fit an overlap-weighted Cox proportional-hazards model and plotted cumulative incidence risk curves for time to first exacerbation. We evaluated changes from baseline to week 52 in prebronchodilator FEV1 comparing the biologics with the use of a mixed effects repeated-measures model. This model was adjusted for the abovementioned covariates along with the baseline FEV1, time, and interaction terms for time with treatment group and time with baseline FEV1. We also evaluated outcomes in patients within this cohort with eosinophil count ≥300 cells per microliter, a clinically significant threshold. All analyses were performed with R statistical software, version 3.6.0 (R Foundation for Statistical Computing).20
Sensitivity Analyses
We conducted several sensitivity analyses to evaluate the robustness of our results and to explore the possibility of spurious inferences due to residual confounding. First, our study period extended to periods following the onset of the Covid-19 pandemic, which may have influenced asthma admissions and/or ED utilization. Therefore, we tested the outcomes when limiting the sample to those who initiated biologic therapy on or before October 1, 2019, to allow a minimum of 6-month follow up through March 1, 2020. Secondly, we evaluated emergency room visits for non-asthma conditions in the one-year follow up period as a negative outcome control, expecting no difference between groups. Thirdly, we included a switch to another biologic as a failure event in the time-to-event analyses.21
RESULTS
Study Population
A total of 201 adult patients met inclusion criteria (Figure 1). This included 68 individuals who initiated dupilumab, 68 who initiated omalizumab, and 65 who initiated mepolizumab (Table 1). All measured covariates were well-balanced following overlap weighting with ASMD less than 0.10 (eFigure 3)
FIGURE 1:
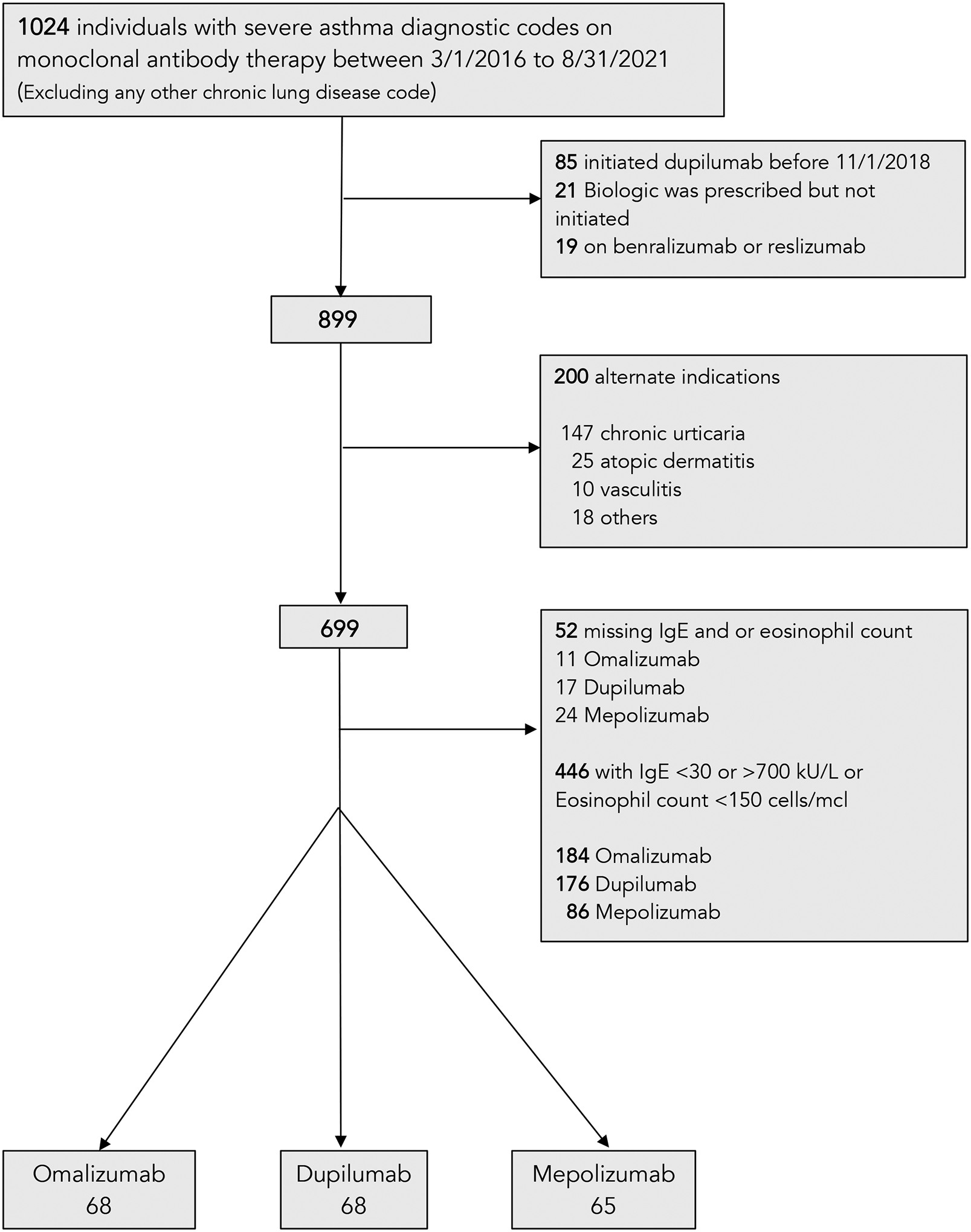
Flow chart showing the selection of the study population
Table 1:
Baseline characteristics of the study population
| Omalizumab | Mepolizumab | +Dupilumab | |
|---|---|---|---|
| Overall sample size, n | 68 | 65 | 68 |
| Age (mean, sd) | 47.7 (16.2) | 54.5 (13.6) | 51.7 (13.9) |
| Female sex, n (%) | 54 (79.4%) | 43 (66.2%) | 42 (61.8%) |
| Body mass index (mean, sd) | 30.1 (7.8) | 29.2 (7.5) | 28.3 (6.6) |
| Race: | |||
| White, n (%) | 52 (76.5%) | 49 (75.4%) | 54 (79.4%) |
| Black, n (%) | 3 (4.4%) | 9 (13.8%) | 4 (5.9%) |
| Asian, n (%) | 2 (2.9%) | 0 (0.0%) | 4 (5.9%) |
| Ethnicity: | |||
| Hispanic, n (%) | 2 (2.9%) | 2 (3.1%) | 1 (1.5%) |
| Residence in inner city, n% | 11 (16.2%) | 8 (12.3%) | 13 (19.1%) |
| *Private insurance | 48 (70.6%) | 47 (72.3%) | 55 (80.9%) |
| Concomitant medications | |||
| ICS/LABA, n (%) | 35 (51.5%) | 39 (60.0%) | 42 (61.8%) |
| LAMA, n (%) | 17 (25.0%) | 24 (36.9%) | 14 (20.6%) |
| OCS, n (%) | 4 (5.9%) | 4 (6.2%) | 2 (2.9%) |
| Baseline eosinophil count (cells/mcL), median (IQR) | 305 (190 – 472) | 630 (400 – 1010) | 410 (278 – 642) |
| Baseline Immunoglobulin E (IU/ml), median (IQR) | 144 (80 – 276) | 120 (65 – 295) | 166 (74 – 285) |
| Pre-index annualized exacerbation rate (mean, sd) | 0.8 (1.6) | 1.1 (1.4) | 0.8 (1.2) |
| Pre-bronchodilator FEV1, liters (median, IQR) | 2.1 (1.7–2.7) | 2.2 (1.7–2.8) | 2.0 (1.5–2.6) |
| Pre-bronchodilator FEV1 percent predicted, % (median, IQR) | 83 (69 – 92) | 72 (62– 83) | 81 (69 – 93) |
| Charlson Comorbidity Index, mean (sd) | 1.2 (0.8) | 1.3 (0.7) | 1.1 (1.1) |
| Smoking status | |||
| Current, n (%) | 2 (2.9%) | 3 (4.6%) | 5 (7.4%) |
| Former, n (%) | 13 (19.1%) | 9 (13.8%) | 15 (22.1%) |
| Never, n (%) | 44 (64.7%) | 41 (63.1%) | 37 (54.4%) |
| Unknown, n (%) | 9 (13.2%) | 12 (18.5%) | 11 (16.2%) |
| Allergic rhinitis, n (%) | #63 (92.6%) | 48 (73.8%) | 55 (80.9%) |
| Season of initiation in Winter | |||
| Winter, n (%) | 9 (13.2%) | 13 (20.0%) | 26 (38.2%) |
Abbreviations: IQR, interquartile range; ICS/LABA, inhaled corticosteroids/long-acting beta-agonists; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroids.
66 (97%) of the 68% patients on dupilumab were using the 300 mg every 2-week dose.
No patient within this cohort was uninsured
The five patients on omalizumab “without allergic rhinitis” with missing perennial allergic rhinitis as shown in Table 1 all had IgE within the accepted level for omalizumab dosing and noted diagnosis of perennial rhinitis based on history in chart. However, we did not see the objective evidence of testing to perennial allergens.
Comparison of incidence rates of exacerbations
The median duration of follow up was 1.6 years for dupilumab (interquartile range, IQR: 1.2–2.0), 3.1 years for omalizumab (IQR: 1.9–4.2), and 3.0 years for mepolizumab (IQR: 1.9–4.1). Over 12 months of follow up, 31 exacerbations occurred over 68 person-years (0.46 exacerbations per person-year) in the dupilumab group, 63 over 68 person-years (0.93 exacerbations per person-year) in the omalizumab group, and 86 over 65 person-years (1.32 exacerbations per person-year)in the mepolizumab group (adjusted incidence rate ratios [IRR] for dupilumab vs. mepolizumab 0.28, 95% confidence interval [CI] 0.09 to 0.84; dupilumab vs. omalizumab (IRR 0.36, 95% CI 0.12 to 1.08); omalizumab vs. mepolizumab (IRR 0.78, 95% CI 0.32 to 1.91). (Table 2). In patients with eosinophils ≥300 cells per microliter, the IRR comparing dupilumab to mepolizumab was 0.26, 95% CI 0.08–0.82 and dupilumab vs. omalizumab, 0.33, 95% CI 0.09–1.24. (Table E3).
Table 2:
Incidence rate ratios (IRR) of exacerbations
| EXACERBATION RATE RATIOS IRR (95% CI) |
MEPOLIZUMAB | OMALIZUMAB | DUPILUMAB |
|---|---|---|---|
| MEPOLIZUMAB (reference) | 1.00 | 0.78 (0.32 – 1.91) | 0.28 (0.09 – 0.84) |
| OMALIZUMAB (reference) | 1.00 | 0.36 (0.12 – 1.08) | |
| DUPILUMAB (reference) | 1.00 |
Comparison of cumulative incidence of exacerbations
Over 12 months of follow up, asthma-related exacerbations occurred in 17 (25.0%) patients in the dupilumab group, 28 (43.1%) in the mepolizumab group, and 27 (39.7%) in the omalizumab group (adjusted hazard ratios, HR, 95% CI: dupilumab vs. mepolizumab, 0.35, 95% CI 0.18–0.71; dupilumab vs. omalizumab, 0.42, 95% CI 0.20–0.87; omalizumab vs. mepolizumab, 0.84, 95% CI 0.47–1.50) (Figure 2). In patients with eosinophils ≥300 cells per microliter, the HR comparing dupilumab to mepolizumab was 0.26, 95% CI 0.10–0.67 and dupilumab vs. omalizumab, 0.24, 95% CI 0.09–0.63. (Figure E4).
FIGURE 2:
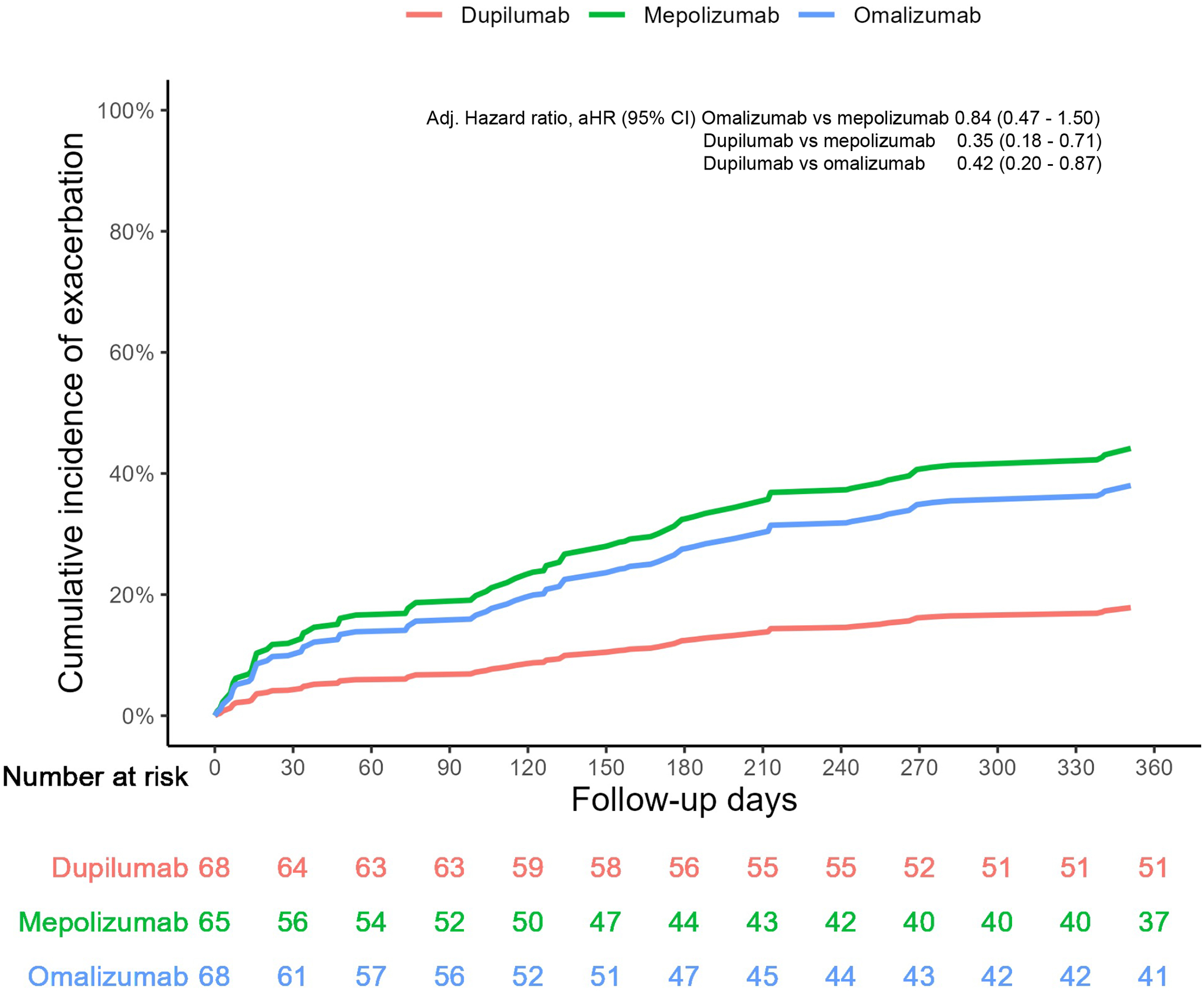
Cumulative incidence of exacerbations over 12 months of follow up
Counting switch to another biologic as a failure event
Eleven (18.6%) omalizumab, 1 (1.4%) dupilumab, and 16 (25.0%) mepolizumab patients switched therapy during the period of follow up. In analyses including switch to other biologics as a failure event, HR and 95% CI comparing dupilumab to mepolizumab was 0.44, 95% CI 0.28–0.71; dupilumab vs. omalizumab, 0.72, 95% CI 0.43–1.21; omalizumab vs. mepolizumab, 0.62, 95% CI 0.38–1.00. (Figure 3).
FIGURE 3:
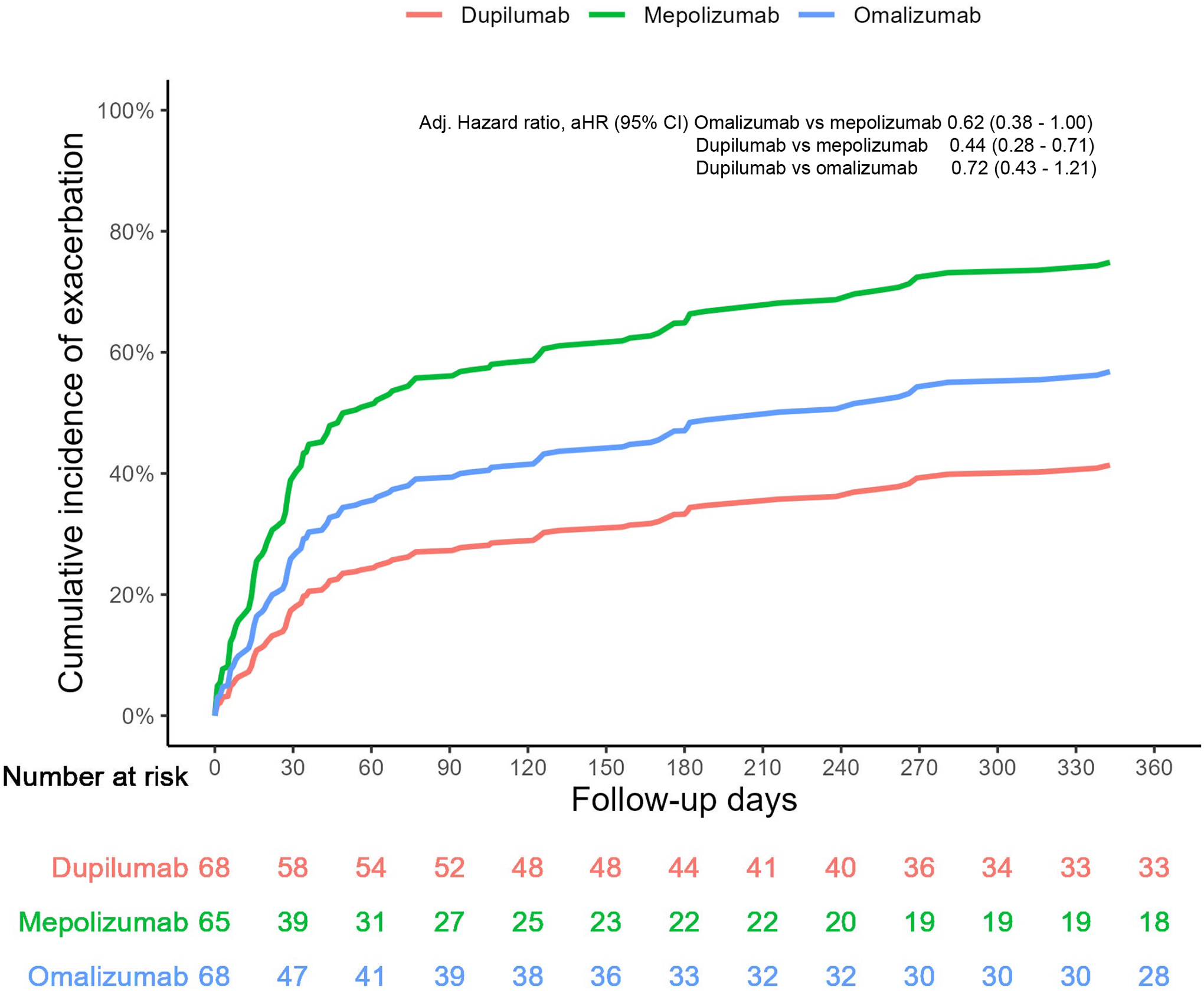
Cumulative incidence of exacerbations over 12 months of follow up including switch as a failure event
Change from baseline in pre-bronchodilator FEV1
At 12 months of follow up, the change from baseline in the prebronchodilator FEV1 was greater in patients receiving dupilumab than in those receiving mepolizumab (mean difference, 0.110L; 95% CI, −0.003 to 0.222, p =0.056) or omalizumab (0.082L; −0.040 to 0.204, p=0.118). However, these changes were not statistically significant. (Table 3). Results in subgroup with eosinophils ≥300 cells per microliter was consistent. (Table E4)
Table 3:
Change from Baseline to 1 year in Pre-bronchodilator FEV1
| MEAN DIFFERENCE IN LITERS (95% CI) |
MEPOLIZUMAB | OMALIZUMAB | DUPILUMAB |
|---|---|---|---|
| MEPOLIZUMAB (reference) | 0 | 0.028 (−0.083 to 0.140) | 0.110 (−0.003 to 0.222) |
| OMALIZUMAB (reference) | 0 | 0.082 (−0.040 to 0.204) | |
| DUPILUMAB (reference) | 0 |
Sensitivity Analyses and negative outcome
Fifty-one of the 68 (75.0%) individuals on dupilumab, 62 of the 65 (95.4%) on mepolizumab, and 61 of the 68 (89.7%) on omalizumab had initiated therapy on or before October 1, 2019. The HR for exacerbations comparing dupilumab to mepolizumab was 0.25 (95% CI 0.11 to 0.59); dupilumab vs. omalizumab, 0.22 (95% CI 0.09 to 0.54); omalizumab vs. mepolizumab, 1.12 (95% CI 0.63 to 2.01) (eFigure 5). The HRs of emergency room visits for non-asthma conditions in the 1-year period after baseline were not significantly different between the groups. (eFigure 6).
DISCUSSION
Despite the essential role that biologics play in the treatment of moderate to severe asthma, little is known regarding the real-world comparative effectiveness of these products. Given the costs of these products,22 and that individuals with severe asthma bear a disproportionate burden of asthma-related morbidity and mortality, the opportunity costs for choosing a less effective biologic in an individual with severe asthma are significant and delay could be fatal.23, 24 In this retrospective cohort study using electronic health records data from a large healthcare system, we found that dupilumab was associated with a lower hazard of asthma-related exacerbations compared to omalizumab or mepolizumab in individuals with IgE levels between 30 and 700 kU/L, the range of IgE for which omalizumab is approved, and eosinophil counts of ≥150 cells per mcl. In addition, we found that patients with dupilumab had greater improvements in FEV1 compared to patients in the mepolizumab and omalizumab groups. However, the differences in FEV1 were not statistically significant. The results were similar in the subgroup of patients with eosinophil of ≥ 300 cells per mcl. Our conclusions remained unchanged in multiple sensitivity analyses.
Our findings extend those from two recent indirect treatment comparisons showing dupilumab may be more effective than omalizumab and mepolizumab in decreasing asthma-related exacerbations and in improving lung function.25, 26 However, in those studies which used aggregate-level data from published randomized placebo-controlled trials, the differences between these therapies did not meet clinically important thresholds.26–29 In this study, using individual-level data from a healthcare system, we found reductions by half or greater in the risk of exacerbations comparing dupilumab to mepolizumab and omalizumab. For FEV1, the mean difference in FEV1 improvement comparing dupilumab to mepolizumab in these patients with eosinophilic/allergic asthma, was over 100 milliliters, although the 95% confidence interval crossed the null value of 0. There are however currently no validated clinically important differences for exacerbation rates and FEV1 reduction in asthma and these differences need to be evaluated in other clinical cohorts.30 Additionally, differences between the populations recruited into those randomized placebo-controlled trials and our clinic population may account for these differences.31 For instance, the mean exacerbation rate in the year prior to biologic initiation in this study was one compared to two in the dupilumab trials and greater than three in the seminal mepolizumab trials.26 Furthermore, we limited our study cohort by IgE level and eosinophil count. However, in the seminal randomized trials of dupilumab, baseline eosinophil count was higher than in the mepolizumab trials, and two-thirds of patients in the dupilumab trials were reported as having allergic rhinitis. These may potentially influence response to these therapies accounting for some of the differences between those indirect comparisons and this study.29 While indirect comparisons can be useful when evidence from head-to-head trials are unavailable, the results may be limited by differences in the study populations of the therapies being compared and unavailability of individual patient data.32 Moreover, results of indirect comparisons may still differ from results of studies of clinic populations given that many of these trials had strict inclusion and exclusion criteria. For instance, obese patients were less likely to have been recruited into these asthma trials of biologics in individuals with asthma.31 Nonetheless, taken altogether, the evidence to date suggests that dupilumab may be more clinically effective than mepolizumab and omalizumab in improving exacerbations and lung function.
The better effectiveness of dupilumab may be related to its mechanism of action. Dupilumab is a broad-spectrum “type 2” biologic. It blocks both IL4 and IL13 signaling thereby decreasing B cell class switch to IgE.33 In addition, it prevents differentiation of naïve T-helper cells to T-helper 2 cells thus decreasing canonical Th2 cytokines, like IL5, and IL-5 induced eosinophil recruitment, the mechanism deployed by the anti-IL5, mepolizumab.3, 34 By blocking IL13, dupilumab may also impact the airway hyperreactivity, goblet cell hyperplasia, and smooth muscle dysfunction associated with asthma and may account for dupilumab’s remarkable effect in improving prebronchodilator FEV1.35
The strengths of this study include the use of data from an integrated health system which provides the opportunity to capture clinical variables and laboratory data, including eosinophil count, which are important when comparing these biologics, and to capture outcomes including exacerbations and lung function. While the patients prescribed each biologic may be different, this data source provided the opportunity to balance important covariates across biologic groups. Using an innovative non-experimental design, we addressed a question of interest with important clinical relevance to the management of patients with moderate to severe asthma for which there is little to no evidence to date. Furthermore, we use real-world data which may be more reflective of how these biologics perform in a usual care setting rather than in a monitored trial setting.
Our study also has limitations. First, we have a relatively small sample size and power in detecting differences may have been limited especially in comparisons of omalizumab and mepolizumab. Furthermore, as with any observational study, the subgroups of patients using these medications differed at baseline and there is a risk of residual confounding. Additionally, there may be temporal trends in asthma outcomes, care, or assessment over calendar time. However, we tried to mitigate these concerns by emulating a hypothetical trial and adjusting for measured confounders. We were able to achieve covariate balance using propensity score weighting of overlap weights. Our results were generally robust to multiple sensitivity analyses and demonstrate biological plausibility given our current understanding of type 2 inflammation. Furthermore, analyses limited to the pre-COVID-19 pandemic era also provided similar conclusions. Secondly, while real world data may be more representative than trial populations, our study population is small and drawn from a single healthcare system in the Northeastern region of the U.S. Only ~10–15% of individuals identified as belonging to underrepresented minority groups and fewer than 30% were publicly insured. Furthermore, we excluded children, those with concomitant comorbidities, and restricted by baseline eosinophil count and IgE level. Thus, results may be limited in generalizability to pediatric populations with asthma, those with comorbidities, and/or those with IgE level and eosinophil count outside the ranges used in this study. Thus, additional work is needed to generate evidence in more diverse and representative populations. Thirdly, patients may have been prescribed biologics but did not use it and adherence to background asthma therapy while on biologics may be associated with improved outcomes on these biologics. However, we included patients with two or more prescriptions for the index biologic and included baseline use of maintenance ICS/LABA in the statistical models. Finally, we have not considered safety events although the optimal choice of biologic includes a delicate balance between the safety and effectiveness. While dupilumab was most effective, there is emerging evidence on additional safety events associated with dupilumab with a recent labeling change to include arthralgias and avoidance of live vaccines.36 Thus, more research on dupilumab’s long term safety is needed.
In summary, this study using data from a single integrated health care system suggests that dupilumab has a lowest overall risk of asthma-related exacerbations compared to omalizumab and mepolizumab in individuals with eosinophils ≥150 cells per mcl and with IgE between 30 and 700 kU/L. This data adds to indirect comparisons of clinical trials to suggest that dupilumab may be a better choice for multiply eligible patients. Additional research including multiply eligible individuals or individuals who meet criteria for both eosinophilic and allergic asthma is needed to generate evidence about whether there is a hierarchy of phenotypes in these individuals. That is, if the allergic phenotype should be targeted before the eosinophilic phenotype in these individuals or vice versa. Such a hierarchy would have important implications for the stepwise approach to initiation of monoclonal antibody in asthma treatment.
Supplementary Material
Key messages:
In this clinical cohort of patients with eosinophil counts of ≥150 cells/microliter and IgE levels of 30–700 kU/L:
Dupilumab was associated with greater reductions in exacerbations than mepolizumab and omalizumab.
Dupilumab was associated with greater than 100 mL improvement in FEV1 compared to mepolizumab, but this did not meet statistical significance.
Funding
Dr. Akenroye is supported by the NIH/NIMHD K99/R00 MOSAIC (K99MD015767) and the BWH Minority Faculty Career Development Award, Dr. Foer by the NIH/NHLBI (K23HL161332), Dr. Li by the NIH/NIAID (K23AI163371), Dr. Jackson by the NIH/NHLBI (K01HL145320), and Dr. Segal by NIH/NIA (K24AG049036).
Disclosures
Dr. Alexander is past Chair and a current member of FDA’s Peripheral and Central Nervous System Advisory Committee; is a co-founding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a past member of OptumRx’s National P&T Committee. Dr. Segal has received unrelated grant funding from Bristol Myers Squibb and has conducted unrelated consultancy for Sanofi and Provention Bio. All other authors have no conflicts of interest to disclose.
Abbreviations used
- ACQ
Asthma Control Questionnaire
- ASMD
Absolute standardized mean differences
- CI
Confidence interval
- COVID-19
Coronavirus disease-19
- FEV1
Forced Expiratory Volume in 1 second
- ICD
International Classification of Diseases
- IgE
Immunoglobulin E
- IL
Interleukin
- MD
Mean difference
- IRR
Incidence rate ratio
- HR
Hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Drazen JM, Harrington D. New Biologics for Asthma. N Engl J Med. 2018;378(26):2533–4. [DOI] [PubMed] [Google Scholar]
- 2.Akenroye A, McCormack M, Keet C. Severe Asthma in the US Population and Eligibility for Monoclonal Antibody Therapy. J Allergy Clin Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. 2022;386(2):157–71. [DOI] [PubMed] [Google Scholar]
- 4.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. The Journal of allergy and clinical immunology. 2015;135(2):299–310; quiz 1. [DOI] [PubMed] [Google Scholar]
- 5.Hernan MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183(8):758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernán MA. Methods of Public Health Research - Strengthening Causal Inference from Observational Data. N Engl J Med. 2021;385(15):1345–8. [DOI] [PubMed] [Google Scholar]
- 8.Liao KP, Cai T, Savova GK, Murphy SN, Karlson EW, Ananthakrishnan AN, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. Bmj. 2015;350:h1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am J Respir Crit Care Med. 2021;203(7):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane SJ, Petersen H, Seltzer JM, Blanchette CM, Navaratnam P, Allen-Ramey F, et al. Moderate symptom-based exacerbations as predictors of severe claims-based exacerbations in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2013;50(6):642–8. [DOI] [PubMed] [Google Scholar]
- 11.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3 Suppl):S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xolair prescribing information 2021. [updated 07/2021. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf.
- 13.Cheng C, Li F, Thomas LE. Addressing Extreme Propensity Scores in Estimating Counterfactual Survival Functions via the Overlap Weights. Am J Epidemiol. 2022. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Matsouaka RA, Thomas L. Propensity score weighting under limited overlap and model misspecification. Stat Methods Med Res. 2020;29(12):3721–56. [DOI] [PubMed] [Google Scholar]
- 15.Thomas LE, Li F, Pencina MJ. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. Jama. 2020;323(23):2417–8. [DOI] [PubMed] [Google Scholar]
- 16.Metropolitan Area Planning Council (MAPC): Massachusetts community types. 2021.
- 17.Zhang Z, Kim HJ, Lonjon G, Zhu Y, written on behalf of AMEB-DCTCG. Balance diagnostics after propensity score matching. Ann Transl Med 72019. p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–S90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RC. R: A language and environment for statistical computing.: R Foundation for Statistical Computing, Vienna, Austria.; 2019. [Available from: URL http://www.R-project.org/. [Google Scholar]
- 21.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. [DOI] [PubMed] [Google Scholar]
- 22.Mauger D, Apter AJ. Indirect treatment comparisons and biologics. The Journal of allergy and clinical immunology. 2019;143(1):84–6. [DOI] [PubMed] [Google Scholar]
- 23.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15(3):348–56. [DOI] [PubMed] [Google Scholar]
- 24.Global Asthma Network: The Global Asthma Report 2018.
- 25.Bateman ED, Khan AH, Xu Y, Guyot P, Chao J, Kamat S, et al. Pairwise indirect treatment comparison of dupilumab versus other biologics in patients with uncontrolled persistent asthma. Respir Med. 2022;191:105991. [DOI] [PubMed] [Google Scholar]
- 26.Akenroye A, Lassiter G, Jackson JW, Keet C, Segal J, Alexander GC, et al. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. The Journal of allergy and clinical immunology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agache I, Rocha C, Beltran J, Song Y, Posso M, Solà I, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1043–57. [DOI] [PubMed] [Google Scholar]
- 28.Agache I, Beltran J, Akdis C, Akdis M, Canelo-Aybar C, Canonica GW, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–42. [DOI] [PubMed] [Google Scholar]
- 29.Nopsopon T, Lassiter G, Chen ML, Alexander GC, Keet C, Hong H, et al. Comparative Efficacy of Tezepelumab to Mepolizumab, Benralizumab, and Dupilumab in Eosinophilic Asthma: A Bayesian Network Meta-analysis. The Journal of allergy and clinical immunology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonini M, Di Paolo M, Bagnasco D, Baiardini I, Braido F, Caminati M, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev. 2020;29(156). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akenroye A, Keet C. Underrepresentation of blacks, smokers, and obese patients in studies of monoclonal antibodies for asthma. The journal of allergy and clinical immunology In practice. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 33.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11(12):958–72. [DOI] [PubMed] [Google Scholar]
- 34.Israel E, Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med. 2017;377(10):965–76. [DOI] [PubMed] [Google Scholar]
- 35.Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol. 2013;31(1):3–10. [PubMed] [Google Scholar]
- 36.Dupixent [Dupilumab] FDA Labels, Warnings, Reactions: FDA Report; 2022. [Available from: https://fda.report/SrLC/1709.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


