Abstract
Background:
Oncostatin M (OSM) may promote type 2 inflammation in chronic rhinosinusitis with nasal polyps (CRSwNP) by inducing thymic stromal lymphopoietin (TSLP).
Objectives:
To study the impact of OSM on TSLP synthesis and release from nasal epithelial cells (NEC).
Methods:
OSM receptors (OSMR), IL-4 receptors (IL-4R), and TSLP were evaluated in mucosal tissue and primary NEC from CRSwNP patients by quantitative PCR (qPCR) and immunofluorescence. Air-liquid interface (ALI) cultured NEC were stimulated with cytokines including OSM, and qPCR, enzyme-linked immunosorbent assay (ELISA), western blot and flow cytometry were used to assess expression of OSMR, IL-4R and TSLP.
Results:
Increased levels of OSMRβ, IL-4Rα, and TSLP were observed in NP tissue and primary epithelial cells from NP of patients with CRSwNP, compared with control tissue or cells from control subjects. The level of expression of OSMRβ in tissue was correlated with levels of both IL-4Rα and TSLP. OSM stimulation of NEC increased expression of OSMRβ and IL-4Rα. Stimulation with IL-4 plus OSM augmented the production of TSLP; the response was suppressed by a STAT6 inhibitor. Stimulation of NEC with IL-4 plus OSM increased the expression of the proprotein convertase subtilisin/kexin3 (PCSK3), an enzyme that truncates and activates TSLP.
Conclusions:
OSM increases the expression of IL-4Rα and synergizes with IL-4 to induce the synthesis and release of TSLP in NEC. Since the combination of IL-4 and OSM also augmented expression of PCSK3, these results suggest that OSM can induce both synthesis and posttranslational processing/activation of TSLP, promoting type 2 inflammation.
Capsule summary:
OSM has dual effects in type 2 responses in CRS; it synergizes with IL-4 to induce the synthesis and release of TSLP in NEC and, in parallel, it stimulates NEC in combination with IL-4 to augment the expression of PCSK3, an enzyme that truncates and activates TSLP to promote type 2 inflammation.
Keywords: OSM, OSMRβ, IL-4Rα, TSLP, chronic rhinosinusitis, nasal polyps, nasal epithelial cells, type 2-dominant inflammation
Introduction
Chronic rhinosinusitis (CRS) is characterized by persistent inflammation of nasal and paranasal sinus mucosae and is one of the most common chronic disorder in adults in the United States.1 CRS is typically divided into 2 major subtypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), based on the presence or absence of nasal polyps (NP). CRSwNP is usually characterized by prominent type 2 inflammation with massive eosinophil infiltration, especially in the Caucasian population in western countries.1, 2 Type 2 cytokines promote the activation and/or recruitment of mast cells, B cells, and eosinophils to lesional nasal mucosae, which promotes type 2 inflammatory responses and further contributes to the hallmark features of CRSwNP.2–5
Oncostatin M (OSM), a pleiotropic cytokine belonging to the IL-6 family of cytokines, has been implicated to play a role in promotion of type 2 inflammation both in animal models and human disorders characterized by type 2 reactions and eosinophilic inflammation.6–10 Both OSM mRNA and protein have been reported to be significantly increased in NP tissue from patients with CRSwNP compared with uncinate tissue (UT) from control subjects.7 OSM has been demonstrated to promote airway mucosal epithelial barrier dysfunction,7 which may lead to more exposure of the tissue to environmental stimuli, including allergens, pathogens, and pollutants. However, the exact mechanism by which OSM is involved in type 2 inflammation in CRSwNP has not been fully elucidated. Interestingly, OSM has been observed to mainly be expressed locally by neutrophils in CRSwNP,11 which indicates that neutrophilic inflammation may have a role in the regulation of type 2 responses and eosinophilic inflammation in CRSwNP.
OSM has been shown to increase eotaxin-1 release in a dose-dependent manner in human airway smooth muscle cells and augments eotaxin-1 release induced by either IL-4 or IL-13 by increasing the expression of IL-4Rα expression.12 IL-4Rα has strong correlations with type 2 or eosinophilic inflammation. IL-4Rα, which is a component of receptors for both IL-4 and IL-13, has been reported to be expressed in airway epithelial cells including bronchial epithelial cells and nasal epithelial cells (NEC). 13,14 NEC not only act as a physical barrier, but also play a role in the regulation of immune responses by producing cytokines and chemokines and via interaction with immune cells.15 Whether OSM can induce IL-4Rα, and promote eosinophilic inflammation by inducing the important type 2 activating alarmins such as thymic stromal lymphopoietin (TSLP) in NEC is still unknown.
In particular, TSLP, an epithelial-derived cytokine, has been identified as an important switch for type 2 inflammation. TSLP-activated dendritic cells (DCs) induce naive allogeneic CD4+T cells to differentiate into Th2 cells.16 TSLP stimulation also causes myeloid DCs (mDCs) to produce large amounts of chemokines, such as eotaxin-2, which attracts eosinophils, as well as C-C motif chemokine ligand (CCL)17 and CCL22, which attract Th2 cells.17−19Additionally, TSLP induces type 2 cytokines generation by mast cells,20 type 2 innate lymphoid cells (ILC2s),21 and CD34+ hematopoietic progenitor cells,22 and can activate eosinophils23 and basophils as well.24
TSLP is highly elevated in epithelial cells of NP and the level of TSLP in CRSwNP is positively correlated with markers of eosinophilia and type 2 inflammation.25,26 It is now known that TSLP can be truncated in NP by proprotein convertases such as furin known as proprotein convertases subtilisin/kexin3 (PCSK3) and carboxypeptidase N, generating a functional dimerized form of TSLP (29–124 + 131–159), having a molecular weight of approximately 10–11 KDa in the denatured condition. The truncated form of TSLP has enhanced pro-type 2 activity on mast cells and ILC2 compared with the native long isoform of TSLP.27
We hypothesize that OSM may stimulate NEC to produce and release TSLP to promote type 2 inflammation in patients with CRSwNP. In this study, we show that OSM acts on OSMRβ in NEC to induce the expression of IL-4Rα, after which OSM synergizes with IL-4 in activating the synthesis, release, and posttranslational processing of TSLP.
METHODS
Patients and tissue sample collection
A total of 132 patients with CRS and control subjects were enrolled from the Allergy and Immunology and Otolaryngology clinics of the Northwestern Medical Faculty Foundation and the Northwestern Sinus Center at Northwestern Medicine. All CRS patients met the criteria of CRS diagnosis as defined by the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis.28 Control subjects were those who underwent septoplasty due to anatomic variations and had no other sinonasal diseases. NP tissue from patients with CRSwNP and uncinate tissues (UT) from CRS patients and control subjects were obtained during routine nasal and/or sinus surgery. Epithelial cells of UT from control subjects were collected by scraping and used for air-liquid interface (ALI) culture. Subjects’ characteristics are summarized in Table 1. The protocol for this study was approved by the Institutional Review Board of Northwestern University Feinberg School. All subjects signed an informed consent form.
Table 1.
Patients’ demographics
| Control subjects | Patients with CRSsNP | Patients with CRSwNP | |
|---|---|---|---|
|
| |||
| Total no. of subjects | n = 49 (20M) | n = 24 (15M) | n = 59 (36M) |
| Age(y), median (range) | 39 (20–87) | 42.5 (26–73) | 45 (24–71) |
| Y N U | Y N U | Y N U | |
| Atopy | 7 6 36 | 14 3 7 | 27 10 22 |
| Asthma | 0 49 0 | 6 18 0 | 23 33 3 |
| Methodology used | |||
| Tissue PCR | n = 15 (4M) | n = 13 (8M) | n = 35 (20M) |
| Age (y), median (range) | 52 (28–70) | 51 (26–73) | 50 (25–71) |
| Scraping NECs PCR | n = 12 (6M) | n = 11 (7M) | n = 24 (16M) |
| Age (y), median (range) | 33.5 (23–87) | 36 (26–72) | 42.5 (24–66) |
| Culture NECs | n = 22 (10M) | ||
| Age (y), median (range) | 36 (20–73) | ||
| Culture NHBE | n = 8(6M) | ||
| Age (y), median (range) | 30 (22–45) | ||
Y, yes; N, no; U, unknown; M, male.
Quantitative RT-PCR
Real-time RT-PCR was performed using the TaqMan method as described previously.25 Primer and probe sets were synthesized by Applied Biosystems (Foster City, CA) (Table E1). Relative gene expression was calculated by using the 2-ΔΔCT method.. The mRNA expression levels were normalized to the expression of the house keeping genes, glucuronidase beta (GUSB) (in vivo experiments) or Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (in cell experiments). Details can be found in the Methods section in the Online Repository.
Immunofluorescence
Immunofluorescence was performed as previously described.11 The detailed protocol for immunofluorescence is provided in the Online Repository.
Cell culture and treatment
NEC from control UT were collected during surgery. NEC or normal human bronchial epithelial (NHBE) cells purchased from Lonza (Walkersville, MD) were cultured and expanded under ALI conditions as previously reported.29 The detailed protocol is provided in the online supplement section.
ELISA
The concentration of TSLP (R&D systems, Minneapolis, MN) in cell-free supernatants were measured by ELISA according to the manufacturer’s instructions. The minimal detection limit for TSLP is 31.2 pg/ml. Further detail is provided in the Methods section in the Online Repository.
TSLP cleavage assay and western blot analysis
TSLP is cleaved to form a fragment with increased activity.27 For the TSLP cleavage assay, 1.5 μg/ml recombinant TSLP (R&D systems) was incubated with 4 μg/ml recombinant PCSK3 (R&D systems) for 6 or 24 hours at 37 °C. Lysates of cultured NEC were evaluated with western blot analysis using the cleaved recombinant TSLP as a standard. Further details are provided in the Methods section in the Online Repository.
Flow cytometry
The viability of ALI cultured cells was evaluated by the Live/Dead™ Fixable Aqua Dead Cell Stain Kit (Thermo Fisher, Waltham, MA). Then cells were stained with APC-labeled IL-4Rα antibody (clone #25463) at a concentration 1:20 (R&D Systems) or isotype control APC-labeled IgG2A as previously reported.11 The detailed protocol is provided in the online supplement section.
Statistical analysis
All analyses were performed using software obtained from GraphPad Prism (La Jolla, CA). Almost all data were expressed as median with interquartile range. Differences between groups were analyzed using a nonparametric one-way ANOVA or Mann-Whitney test. Student’s t-test was used to compare in vitro experimental data. Correlations between the two groups were assessed by using the Spearman’s rank correlation. A p-value of less than 0.05 was accepted statistically significant.
RESULTS
The expression of OSMR and IL-4R; correlations between IL-4R, OSM, and OSMRβ in tissue
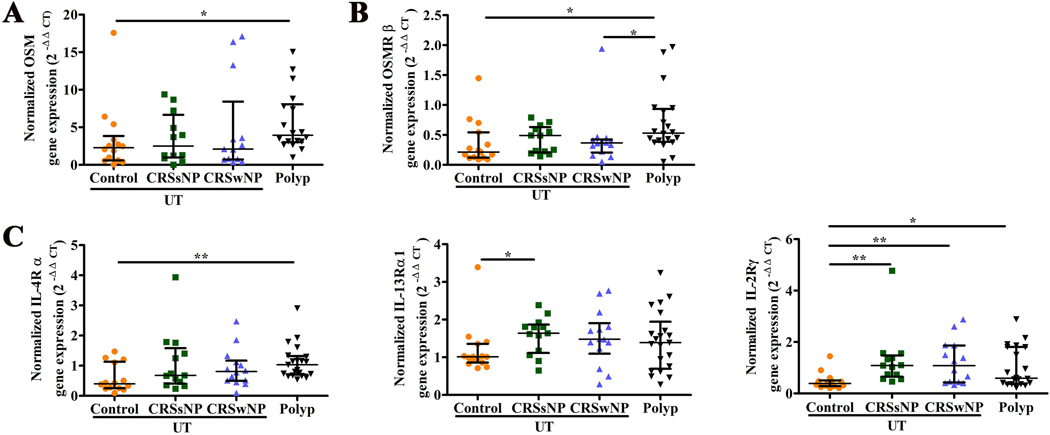
As in our earlier report,7 the level of OSM mRNA was significantly increased in NP tissue compared with the level in UT from control subjects (Figure 1A). OSM mainly signals through 2 receptors; the type 1 receptor is a heterodimer constituted by leukemia inhibitory factor receptor (LIFR) and gp130, and the type 2 receptor is a heterodimer constituted by OSM receptor β chain (OSMRβ) and gp130.30 As shown in Figure 1B, the level of OSMRβ mRNA was significantly increased in NP tissue compared with the level in UT from control subjects. While there was a trend toward an increase in the levels of gp130 mRNA between NP from patients with CRSwNP and UT from control subjects, UT from patients with CRSsNP had higher mRNA level of gp130 as compared with UT from control subjects (Figure E1). There was no difference in LIFR mRNA among the groups (Figure E1).
Figure 1.
The expression of OSM, OSMRβ and IL-4R in tissue. The mRNA expression of OSM in tissue (A); the mRNA expression of OSMRβ in tissue (B); the mRNA expression of IL-4Rα, IL-13Rα1, IL-2Rγ in tissue (C). *P < .05, **P < .01.
The IL-4R complex includes two distinct receptors; the type I IL-4R is composed of IL-4Rα and IL-2Rγ, whereas the type II IL-4R is composed of IL-4Rα and IL-13Rα1 chains, and also functions as the receptor for IL-13. Compared with UT from control subjects, the level of IL-4Rα mRNA was significantly increased in NP tissue from patients with CRSwNP, while the level of IL-13Rα1 mRNA was significantly increased in UT from patients with CRSsNP (Figure 1C). The level of mRNA for IL-2Rγ was significantly increased in NP tissue and UT from CRS patients compared to UT from control subjects (Figure 1C).
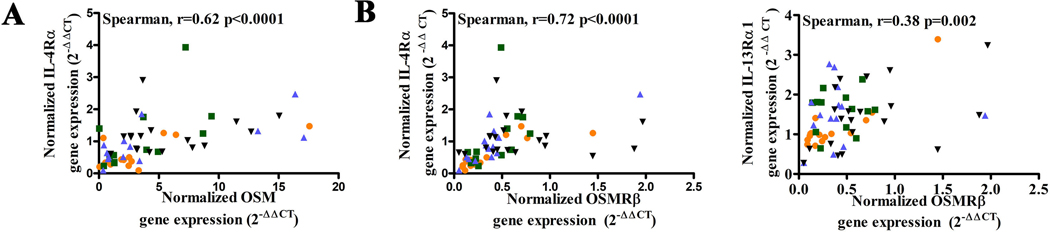
When correlation analysis was conducted, we found that the levels of mRNA of both OSM and OSMRβ were positively correlated with the levels of IL-4Rα mRNA in nasal mucosa (Figure 2A and B). In contrast, the levels of OSM mRNA did not correlate with the levels of mRNA of IL-13Rα1 or IL-2Rγ in nasal mucosa (Figure E2). The level of OSMRβ mRNA was moderately positively correlated with the mRNA level of IL-13Rα1 in nasal mucosa (Figure 2B), and had no correlation with the level of IL-2Rγ mRNA (Figure E2).
Figure 2.
The correlation between IL-4R and OSM, and OSMRβ in tissue. The correlation between IL-4Rα and OSM in tissue (A); the correlation between IL-4Rα, IL-13Rα1 and OSMRβ in tissue (B).
The distribution and expression of OSMR and IL-4R in nasal polyp and NEC scrapings
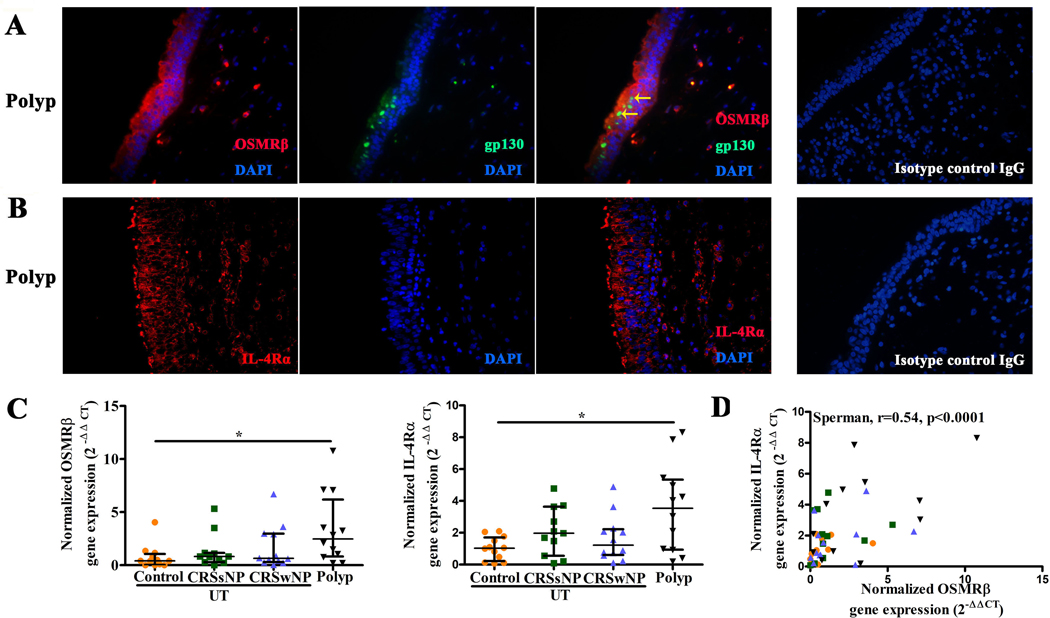
We next evaluated the distribution of OSMR and IL-4R in nasal polyp tissue. Immunofluorescent staining revealed that OSMRβ was mainly expressed by epithelial cells and sparse infiltrating cells in NP, while gp130 was found to be mainly expressed in epithelial cells (Figure 3A). Co-localization of OSMRβ and gp130 was observed in nasal epithelial cells (Figure 3A). No expression of LIFR protein, another IL-6 family member, was observed in nasal epithelial cells in NP tissue (data not shown). Immunofluorescent staining showed that IL-4Rα and IL-13Rα1 were expressed extensively by nasal epithelial cells in NP, and they were expressed by scattered infiltrating cells as well (Figure 3B and E3).
Figure 3.
The distribution of OSMRβ, IL-4Rα in nasal polyp, and the mRNA expression of OSMRβ and IL-4Rα, and the correlation between them in scraping NEC. The distribution of OSMRβ and gp130 in nasal polyp, the two yellow arrows represent the co-stains of OSMRβ and gp130 (A); the distribution of IL-4Rα in nasal polyp (B); the mRNA expression of OSMRβ, IL-4Rα in scraping NEC (C), the correlation between OSMRβ and IL-4Rα in scraping NEC from patients with CRS and control UT. NEC, nasal epithelial cells (D). *P < .05, ***P < .001, ****P < .0001.
We also investigated mRNA expression of OSMRβ, gp130, IL-4Rα and IL-13Rα1 in NEC from scrapings. As shown in Figure 3C, the level of mRNA for OSMRβ was significantly increased in epithelial cells from NP tissue compared with UT from control subjects. No significant difference was found in level of mRNA of gp130 or IL-13Rα1 in the epithelial cells from the different disease groups (Figure E4). Despite the elevation in NP, no significant difference was found in the level of IL-4Rα mRNA in the epithelial cells from UT from the CRS groups compared with UT from control subjects (Figure 3C). When correlation analysis was conducted, we found that the level of OSMRβ mRNA was positively correlated with the level of IL-4Rα mRNA in scraping NEC in four groups (Figure 3D).
Induction of IL-4R and OSMR in NEC by OSM stimulation
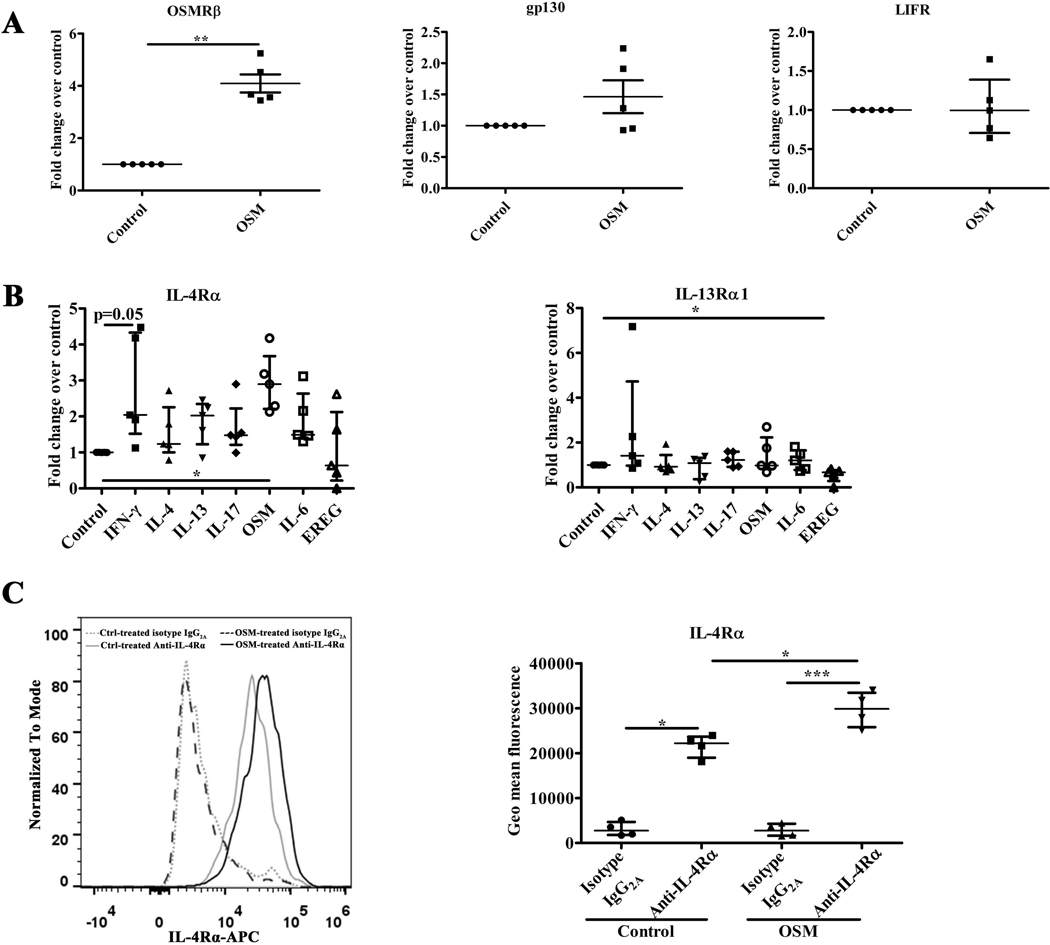
Based on the correlations of OSM, OSMRβ and IL-4R in the clinical samples, we suspected that OSM can induce expression of IL-4R. To test this possibility, we performed in vitro dose- and time-dependent stimulations with OSM of ALI cultured NEC using IL-4Rα expression as a readout. As shown in Figure E5, OSM induced mRNA for IL-4Rα; based on titration, the optimal effect of OSM on IL-4Rα mRNA production occurred at a concentration of 100 ng/ml and the optimal time duration was 12 hours. These findings support the hypothesis that the elevation of IL-4Rα observed in CRS may be due in part to the action of OSM. To further investigate the regulation of cytokine receptor expression in NEC, ALI cultured NEC were stimulated with OSM or the epidermal growth factor ligand epiregulin (EREG), another epithelial-mesenchymal transition (EMT)-inducing cytokine that is elevated in CRSwNP, at a concentration of 100 ng/ml.31 We also used a number of other cytokine stimuli in these experiments, including IFN-γ, IL-4, IL-13, IL-17, and IL-6, all at a concentration of 10 ng/ml for 12 hours. As shown in Figure 4A, OSM significantly upregulated the expression of OSMRβ mRNA in ALI cultured NEC, but did not induce either the signaling chain gp130 or the LIF receptor LIFR. In the IL-4R family, OSM significantly enhanced the expression of IL-4Rα mRNA, but not the expression of IL-13Rα1 mRNA in NEC (Figure 4B). Other cytokines known to activate various signaling pathways in epithelial cells, such as IL-4, IL-13, IL-17, IL-6 and EREG, had no obvious influence on the expression in NEC of mRNA for IL-4Rα, although IFN-γ trended to upregulate its expression (Figure 4B). None of the cytokines induced IL-13Rα1 in NEC, although EREG slightly reduced expression (Figure 4B). The influence of IFN-γ, IL-4, IL-13, IL-17, OSM, IL-6, and EREG on the expression of IL-13Rα2 in NEC was also investigated. Normal NEC constitutively expressed mRNA for IL-13Rα2. Both IL-4 and IL-13 trended to increase the expression of IL-13Rα2 mRNA in NEC, while IFN-γ, IL-17, OSM, IL-6, and EREG had no obvious impact on the expression of IL-13Rα2 mRNA in NEC (Figure E6).
Figure 4.
The expression of OSMR, IL-4R in ALI culture NEC from control UT stimulated by OSM or other cytokines. The mRNA expression of OSMRβ, gp130, LIFR in NEC stimulated by 100 ng/ml OSM for 12h, n = 5 (A); the mRNA expression of IL-4Rα, IL-13Rα1 in NEC stimulated by OSM or EREG at a concentration of 100 ng/ml, or by IFN-γ, IL-4, IL-13, IL-17, or IL-6 respectively at a concentration of 10 ng/ml for 12h, n = 5 (B); the protein expression of IL-4Rα detected by flow cytometry in NEC stimulated by OSM (100 ng/ml) for 24h, n = 4 (C); NEC, nasal epithelial cells; *P < .05, **P < .01, ***P < .001.
To assess the impact of epithelial cell differentiation on OSM responses, ALI cultured NEC were stimulated for various periods of time with OSM to determine the impact on IL-4Rα protein expression as assessed by flow cytometry. An incubation of 24 hours was selected as the optimal period for OSM induction of IL-4Rα (Figure E7). Data in the right panel of Figure 4C display the average of four independent experiments, and show that IL-4Rα protein was constitutively expressed by NEC, and OSM stimulation significantly increased the expression of IL-4Rα protein in differentiated NEC.
OSM alone and in combination with IL-4 induced TSLP expression in NEC; OSMRβ correlated with TSLP levels in vivo
During the course of these studies, we observed that OSM induced expression of the alarmin TSLP, which is known to profoundly promote type 2 responses. Previous studies by several groups, including ours, have shown that TSLP is highly elevated in CRS.25,26 As confirmation of this observation, Figure 5A shows that TSLP mRNA levels were significantly increased in NP tissue in comparison to control UT. The level of TSLP mRNA was also significantly increased in epithelial cells from NP tissue compared to UT from control subjects (Figure E8). We showed above (Figures 1B, 2B, 3C and D) that OSMRβ was elevated in NP tissue and in epithelial cells from NP tissue, and OSMRβ correlated with levels of IL-4Ra. We therefore tested whether elevations of TSLP and OSM mRNA in vivo might be correlated, and whether the level of mRNA for OSMRβ was correlated with the level of TSLP mRNA in nasal mucosa. We found a strong correlation between mRNA for TSLP and OSMRβ (r=0.45, P<.001) in the four tissue groups, supporting the hypothesis that OSM signaling may play a role in expression of TSLP in vivo (Figure 5B). Since OSM was able to upregulate the expression of IL-4Rα in epithelial cells (see above), we tested whether OSM could induce TSLP in vitro, alone or in combination with IL-4 or IL-13. As shown in Figure 5C, OSM alone significantly upregulated the expression of TSLP mRNA in NEC. Interestingly, the expression of TSLP mRNA induced by the combination of OSM plus IL-4 was significantly higher than that induced by only IL-4 or only OSM, suggesting additive effects. Although the same trend was evident with OSM plus IL-13, TSLP mRNA induced by OSM plus IL-13 was not significantly higher than that induced by OSM alone, although it was higher than IL-13 alone (Figure 5C). Induction of TSLP by OSM may be of importance in type 2 responses, prompting us to investigate the response further.
Figure 5.
The correlation between TSLP and OSM, OSMRβ, and IL-4Rα in tissue, and the expression of TSLP in ALI culture NEC from control UT stimulated by OSM or other cytokines. The mRNA expression of TSLP in tissue (A); the correlation between TSLP and OSM, OSMRβ, and IL-4Rα in tissue (B); the mRNA expression of TSLP in NEC stimulated by OSM or EREG at a concentration of 100 ng/ml, or by IFN-γ, IL-4, IL-13, IL-17, or IL-6 respectively at a concentration of 10 ng/ml, or IL-4 (10 ng/ml) plus OSM (100 ng/ml), or IL-13 (10 ng/ml) plus OSM (100 ng/ml) for 12h, n = 5 (C); NEC, nasal epithelial cells; *P < .05.
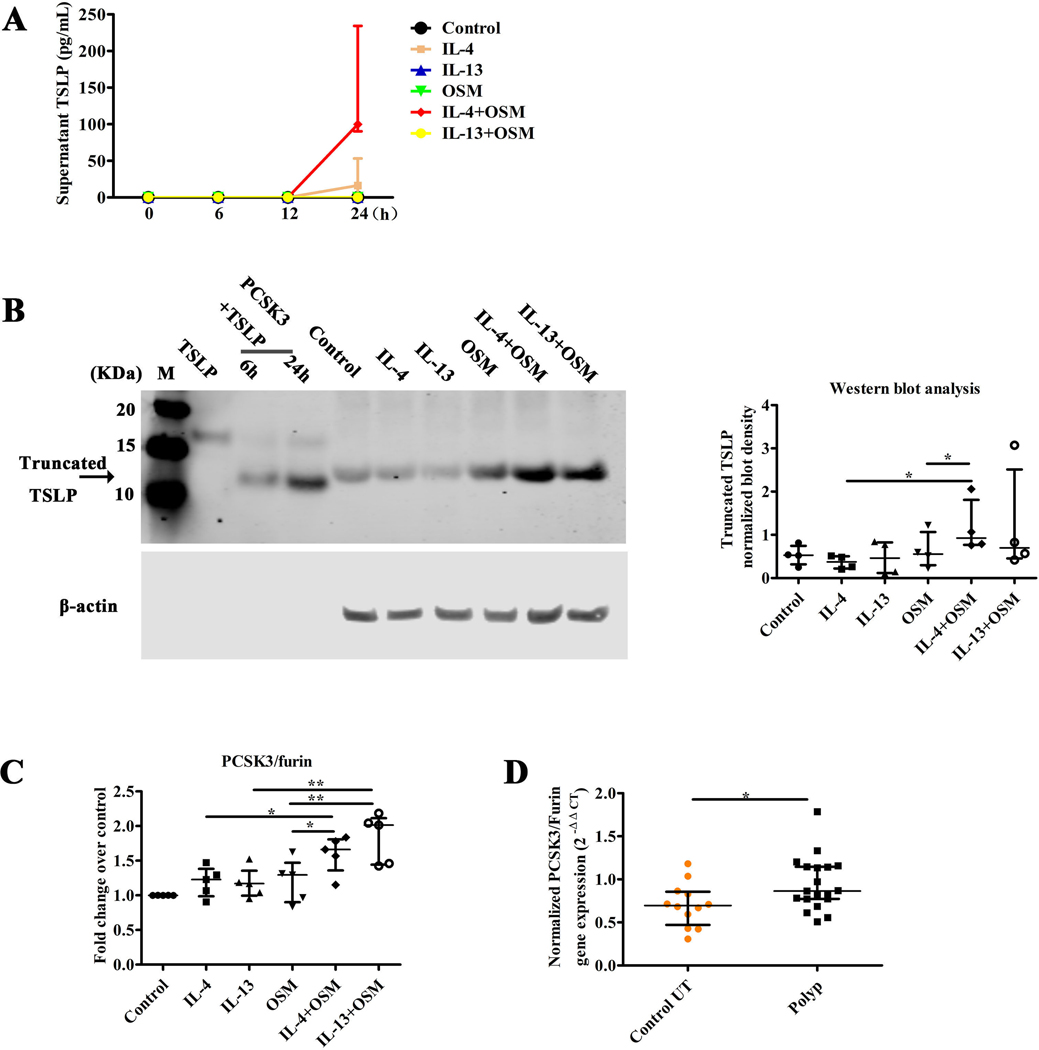
Previous studies by Kato et al. in our group have shown that TSLP is released after stimulation with STAT6 activating cytokines and after stimulation of TLR3 by dsRNA or respiratory virus; these two classes of stimuli synergistically induce TLSP.32 Other studies by Kato have shown that TSLP can be truncated and activated by PCSK3.25, 27 In order to confirm the induction of TSLP protein by OSM, and to test whether additive, or possibly synergistic, induction of TSLP mRNA by OSM plus IL-4 occurs at the protein level, we assessed the levels of TSLP in the supernatants of stimulated NEC from control UT by ELISA. We also assessed levels of the truncated form of TSLP in stimulated NEC by western blot. We first determined the kinetics of TSLP release in ALI cultured NEC exposed to various stimuli. The highest levels of TSLP release were observed at 24 hours after stimulation, the longest period tested (Figure 6A). These data confirm the qPCR findings at the protein level, and also indicate that OSM combined with IL-4 synergistically induced TSLP production and release at the protein level. Interestingly, the ELISA assay failed to detect TSLP in supernatants from cells stimulated with IL-13 or IL-13 plus OSM, even though TSLP was detected after IL-13 stimulation by western blot (see below). Figure 6B shows the appearance of native TSLP on a western blot (far left) and truncated TSLP after incubation for 6h or 24h with PCSK3 (see legend); also shown is TSLP formed by epithelial cells after no stimulation (control) or simulation with the cytokines under investigation, alone and in combination. As can be seen in Figure 6B, a truncated form of TSLP was detectable in both untreated and stimulated NEC and made up the bulk of the detected TSLP. However, the intensity of the truncated TSLP band measured in 4 experiments using densitometry was dramatically increased in the NEC stimulated by IL-4 plus OSM when compared with stimulation by IL-4 or OSM only (right side of Figure 6B). Compared with stimulation by IL-13 or OSM alone, IL-13 plus OSM also increased the expression of truncated TSLP, though the effect was variable and did not reach statistical significance (Figure 6B). In a study by Nagarkar DR et al, it was determined that the cleavage product is only very weakly recognized in the ELISA assay.25
Figure 6.
The expression of truncated TSLP in ALI cultured NEC from control UT stimulated by OSM, IL-4, IL-13, IL-4 plus OSM, or IL-13 plus OSM. The release of TSLP in supernatant of NEC stimulated by IL-4 (10 ng/ml), IL-13 (10 ng/ml), OSM (100 ng/ml), IL-4 (10 ng/ml) plus OSM (100 ng/ml), or IL-13 (10 ng/ml) plus OSM (100 ng/ml) in time-dependent manner for 0, 6, 12, 24h, was analyzed by ELISA, n = 4 (A); the expression of native TSLP and TSLP after incubation with PCSK3/Furin for 6h or 24h. The expression of truncated TSLP in lysate of NEC stimulated by IL-4 (10 ng/ml), IL-13(10 ng/ml), OSM (100 ng/ml), IL-4 (10 ng/ml) plus OSM (100 ng/ml), or IL-13 (10 ng/ml) plus OSM (100 ng/ml) for 24h, n =4 (B); the mRNA expression of PCSK3 in the NEC stimulated by IL-4 (10 ng/ml), IL-13 (10 ng/ml), OSM (100 ng/ml), IL-4 (10 ng/ml) plus OSM (100 ng/ml), or IL-13 (10 ng/ml) plus OSM (100 ng/ml) for 12h, n =5 (C); and the mRNA expression of PCSK3 in control UT and NP tissue (D); NEC, nasal epithelial cells; UT; N.D, no detection; *P < .05, **P < .01.
In order to elucidate the mechanism by which the truncated form of TSLP could be produced in NEC, we investigated the expression of PCSK3, the furin protease previously implicated in TSLP post translational processing in NEC.27 As shown in Figure 6C, NEC constitutively expressed PCSK3 mRNA. Stimulation with IL-4 plus OSM increased the level of PCSK3 mRNA significantly, compared with stimulation with IL-4 or OSM alone (Figure 6C). A similar pattern was observed with OSM plus IL-13, which trended toward even higher levels of the protease. Nasal polyp tissue manifested significantly higher expression of PCSK3 mRNA compared with UT from control subjects (Figure 6D).
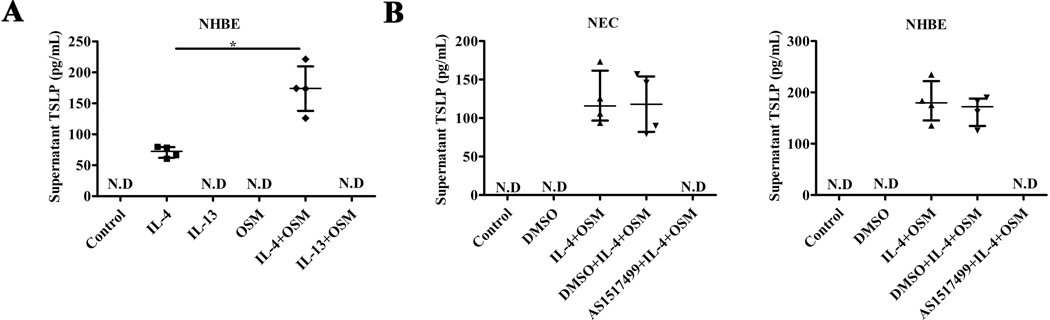
To assess the generalizability of the effect observed in NEC, we used ELISA to test whether IL-4 plus OSM can also induce the release of TSLP protein in NHBE. As shown in Figure 7A, IL-4 significantly increased TSLP release from NHBE, and IL-4 plus OSM further increased the release of TSLP compared with stimulation by IL-4 or OSM only. As with the earlier time course experiment (Figure 6A), IL-13 alone and OSM alone or in combination with IL-13 failed to release TSLP detectable by the ELISA.
Figure 7.
The release of TSLP in supernatant of ALI cultured NHBE cells stimulated by OSM, IL-4, IL-13, IL-4 plus OSM, or IL-13 plus OSM, and the synergistic effect of IL-4 plus OSM on TSLP was suppressed in NEC from control UT and NHBE cells by an inhibitor of STAT6. The release of TSLP in supernatant of NHBE cells stimulated by IL-4 (10 ng/ml), IL-13(10 ng/ml), OSM (100 ng/ml), IL-4 (10 ng/ml) plus OSM (100 ng/ml), or IL-13 (10 ng/ml) plus OSM (100 ng/ml) for 24h, n = 4 (A). NEC or NHBE cells stimulated by IL-4 (10 ng/ml) plus OSM (100 ng/ml) for 24h, or pretreated with DMSO or STAT6 inhibitor (AS1517499, 50 nM) for 2 hours, then IL-4 (10 ng/ml) plus OSM (100 ng/ml) for further 24h. TSLP release in culture supernatant was analyzed by ELISA, n =4(B). N.D, no detection. *P < .05.
The synergistic effect of IL-4 plus OSM on TSLP was suppressed by an inhibitor of STAT6
NEC or NHBE cells were pre-treated with the STAT6 inhibitor AS1517499 or equivalent DMSO buffer alone for 2 hours, and then stimulated with IL-4 and OSM together for 24 hours. As shown in Figure 7B, the level of TSLP measured by ELISA in the supernatants of NEC or NHBE stimulated by IL-4 plus OSM was dramatically increased compared with that in the supernatants of control-treated cells. However, the release of TSLP from the NEC or NHBE cells stimulated by IL-4 plus OSM was completely blocked by the STAT6 inhibitor AS1517499 but not the DMSO buffer control (Figure 7B). The inhibitor also reduced expression of truncated TSLP when the stimulus was the combination of OSM and IL-13 (data not shown). As a negative control, NHBE cells were pre-treated with STAT6 inhibitor AS1517499 or equivalent DMSO buffer control for 2 hours, stimulated with dsRNA for 24 hours, and then IL-8 was measured in the supernatant by ELISA. The release of IL-8 was not blocked by the STAT6 inhibitor AS1517499, suggesting that the inhibitory effect is not non-specific (data not shown).33
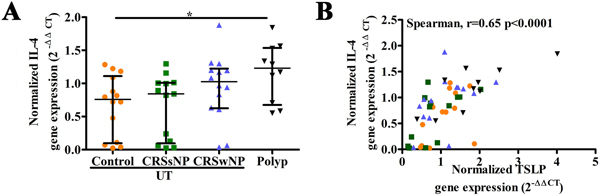
The expression of IL-4 mRNA in tissue correlates with TSLP
As in our former report,25 the level of IL-4 mRNA was significantly increased in NP tissue compared with UT from control subjects (Figure 8A). Moreover, we found that the level of TSLP mRNA was strongly positively correlated with the level of IL-4 mRNA in nasal mucosae (r= 0.65, p< .0001; Figure 8B). These data support a hypothesis that there is a positive feedback between TSLP and IL-4 in nasal polyps that promotes type 2 inflammation.
Figure 8.
The expression of IL-4 in tissue, and the correlation between IL-4 and TSLP in tissue. The mRNA expression of IL-4 in tissue (A); the correlation between IL-4 and TSLP in tissue (B).
DISCUSSION
OSM has been implicated in type 2 and eosinophilic inflammation of the airways in diseases such as asthma and CRSwNP; however, the underlying mechanisms and receptor utilized are not fully elucidated.6–10 Our interest has been in CRSwNP, where we suspect that OSM plays a role in EMT and epithelial dysfunction. In the present study, we demonstrated that among OSM receptors, OSMRβ/gp130 (type 2 OSMR) is elevated in disease, while LIFR/gp130 (type 1 OSMR) is not, suggesting that the former may mediate OSM responses and confirming our earlier studies.7 OSM stimulation in vitro increased OSMRβ expression in NEC, indicating that OSM may augment its own function in an autologous manner by induction of its own receptor in NEC. Increased levels of OSM and OSMRβ were mainly observed in NP tissue from patients with CRSwNP. Higher levels of OSMRβ mRNA were also found in epithelial cell scrapings taken from NP compared to epithelial cells scraped from UT from CRS patients and control subjects, and immunofluorescent study showed OSMRβ was mainly expressed by epithelial cells, indicating that OSM may exert its effects on the relevant nasal epithelial cells in CRSwNP through OSMRβ. Though the distribution of OSMRβ and gp130 were somewhat distinct within epithelial cells, according to our former study, gp130 is expressed in nasal polyps.34 Therefore, gp130 may combine with OSMRβ in the epithelial cell membrane.
IL-4Rα, which can dimerize with IL-13Rα1 to form the type 2 receptor activated by both IL-4 and IL-13, plays an important role in the pathogenesis of disorders characterized by type 2 inflammation. The anti-IL-4Rα antibody dupilumab has been approved for treatment of moderate to severe atopic dermatitis and uncontrolled asthma.35 Dupilumab has also received approval from the Food and Drug Administration (FDA) for the treatment of inadequately controlled CRSwNP in the United States.36 This strongly suggests that one or both IL-4/IL-13 receptors that utilize IL-4Rα are centrally involved in CRS.
We report here that OSM stimulation increased expression of IL-4Rα mRNA and protein in NEC, as previously reported in human airway smooth muscle cells,12 suggesting the possibility that OSM may enhance the action of IL-4 and IL-13 on NEC by increasing the expression of IL-4Rα. OSM has been reported to increase the release of eotaxin in mouse fibroblasts and human airway smooth muscle cells, but we did not observe this effect in NEC (data not shown).12, 37 In clinical samples from CRSwNP patients, we found higher IL-4Rα expression in NP tissue in comparison with UT from control subjects, and its expression was mainly in epithelial cells. In addition, levels of mRNA for both OSM and OSMRβ in tissue were positively correlated with the level of IL-4Rα mRNA, indicating a potential relationship between higher IL-4Rα expression in NP tissue and OSM signaling. These findings also support the possibility that IL-4Rα is induced in vivo by the action of OSM in CRSwNP. In total, these findings suggest that OSM may be part and parcel of a type 2 response in CRSwNP. Further supporting this hypothesis, we found that OSM up-regulates the expression of TSLP mRNA in NEC, even though it did not increase TSLP release or intracellular expression of TSLP protein in NEC. This finding indicates that OSM may only be a partial stimulus and may only regulate the transcription of TSLP but not the translation of TSLP in NEC. Interestingly however, we found that OSM was additive with or synergized with IL-4 to increase both the expression of TSLP mRNA (additive) and synthesis of TSLP protein (synergistic) in NEC. In contrast, we found that IL-13 gave variable results compared to IL-4 in combination with OSM; in some cases, it increased the signal (expression of TSLP mRNA, TSLP protein fragments) and in others it did not (ELISA assay for protein). Since the cleavage product was very weakly detected by the commercial ELISA kit, as reported by Nagarkar et al., we speculate that the ELISA data reflect the residual, non-cleaved, portion of TSLP in the samples and that for some reason, there is little or no residual native TSLP remaining when IL-13 is used along with OSM.
The enhancement of TSLP synthesis and release in NEC by the combination of stimulation with IL-4 and OSM is clearly shown in Figures 5C, 6A and 6B. Since OSM increased the expression of IL-4Rα, this effect may in part have occurred secondary to the induction of the IL-4Rα by OSM in NEC. Since IL-4 and IL-13 utilize distinct receptors (both type I and II for IL-4 and only type II for IL-13), we considered the possibility that the differential effects of IL-4 and IL-13 may reflect different receptor usage in epithelial cells. Our studies of receptor expression revealed that IL-2Rγ mRNA was expressed in tissue and elevated in disease groups. Future studies would be warranted to determine whether the effects of IL-4 to promote TSLP synthesis and release were mediated via the type II receptor (IL-4Rα/IL-13Rα1) or the type I receptor (IL-4Rα/IL-2Rγ) in NEC.
IL-4 signaling via IL-4Rα/IL-13Rα1 activates the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, specifically employing STAT6.38,39 It has been reported that the IL-4Ra–STAT6 axis plays an important role in development of the asthma phenotype in animal models of allergic airway disease, as neutralization of STAT6 inhibits eosinophilia, remodeling, and hyperresponsiveness (AHR) of airway.40,41 In the current study, we demonstrate that the combination of IL-4 plus OSM induces significant TSLP release, suggesting that the presence of OSM, in an inflammatory environment that is already skewed toward type 2, can potentiate type 2 inflammation further by inducing this important type 2 activating alarmin. In our studies, the effect of the combination was blocked by a STAT6 inhibitor, which supports the role of IL-4 receptors in driving TSLP production. We also found that IL-4 plus OSM induced the release of TSLP from NHBE as well as NEC, suggesting that this response may be relevant in both the upper and lower airways.
We were at first surprised that the ELISA experiments failed to detect production of TSLP when the cells were stimulated with IL-13 plus OSM but did detect it when IL-4 plus OSM was used as the stimulus. If accepted at face value, this result would seem to suggest that IL-4 is signaling in a different manner than IL-13 in the epithelial cells. Current dogma holds that structural cells, such as epithelial cells, fibroblasts, endothelial cells etc., primarily express the type 2 receptor, that responds to both cytokines, while many lymphoid and myeloid cells express the type 1 receptor or both receptors. The finding that only IL-4 plus OSM induces TSLP detectable by ELISA would suggest that perhaps the type 1 receptor is present and influencing the response that we have observed. This conclusion doesn’t fit with our observation that IL-13 plus OSM induced TSLP mRNA just as well as IL-4 plus OSM, that IL-13 plus OSM induced TSLP detected by western blot just as well and that IL-13 plus OSM induced PCSK3 just as well (or better) than IL-4 plus OSM. We also found that OSM plus IL-13-induced truncated TSLP was blocked by the STAT6 inhibitor, suggesting that IL-13 is signaling through STAT6 in this system. Together, these findings suggest that a more likely explanation of the discrepancy mentioned above is that the ultimate form of TSLP produced when IL-13 plus OSM is the stimulus may be processed slightly differently than that made after stimulation with IL-4 plus OSM, and that using IL-13 plus OSM the product is nearly completely converted to the fragment. Since the fragment is very weakly detected by the antibody used in the ELISA experiments, but is detected by the antibody used in the western blot studies,25 this would lead to the conclusion that IL-13 is equal in strength as a stimulus to IL-4, alone or in combination with OSM, and that for an unknown reason, TSLP is more completely processed when IL-13 is the STAT6 activating stimulus. Further studies evaluating the samples for TSLP biological activity and using mass spectrometry-based sequencing to identify the molecular forms of native and processed TSLP will be necessary to resolve this important question.
The reason why the truncated form of TSLP is detected in the lysate of both untreated and stimulated NEC may be that NEC constitutively express PCSK3, which can truncate the long-isoform of TSLP into a truncated form with enhanced pro-type 2 capacity.27 Both IL-4 plus OSM and IL-13 plus OSM increased the expression of PCSK3 in NEC, leading to the appearance of truncated TSLP. In addition, in this study our western blot assay failed to detect much of the long (full length) isoform of TSLP (15KDa) in the lysate of either untreated or stimulated NEC, as reported by Nagarkar et al and Poposki et al.25, 27 The conditions of the cultures in the present study appear to have promoted processing of TSLP. A truncated TSLP band approximately 10–11 KDa was detected in both treated and untreated NEC. We hypothesize that IL-13 may be a slightly better inducer of PCSK3 than IL-4, leading to more complete processing. We did not perform protein sequencing or mass spectrometry to determine the sequence of the truncated TSLP in this study and to quantitate the conversion rate, so it’s identity has yet to be established.
Refractory CRSwNP is often characterized by severe eosinophilic inflammation mixed with neutrophils.42,43,44 And it has been also reported that neutrophils in CRSwNP make it more resistant to steroid therapy.45 Neutrophilia in patients with asthma associates with more severe diseases as well, perhaps because neutrophils are not particularly responsive to corticosteroid therapy.46 However, little is known about the exact pathophysiological role of neutrophils in eosinophilic mucosal diseases like CRSwNP and asthma. Here we demonstrate that OSM, which we have previously shown is secreted by neutrophils, may exacerbate type 2 responses and eosinophilic inflammation in CRSwNP, in part by collaborating with IL-4 to induce TSLP, and inducing PCSK3 to cause metabolism of the TSLP generated to an active fragment.
As a member of the IL-6 family of cytokines, OSM can be generated by innate immune response to infection or environmental stimuli. Whether the OSM pathway is activated once nasal polyps have formed or just as part of a secondary innate immune response to colonizing bacteria or other stimuli in the nasal cavity needs to be elucidated. TSLP is regarded as a switch for type 2 inflammation, and its expression can be affected by many factors. Although dupilumab, an anti-IL-4Rα antibody, is effective in treating diseases characteristic of type 2 inflammation and has been approved for inadequately controlled CRSwNP by the FDA, whether it suppresses the expression of TSLP is not clear. An antibody against TSLP was reported to reduce asthma exacerbations and decrease type 2 inflammatory biomarkers in patients with asthma with or without NP47, so TSLP is considered to be a promising target for the treatment of CRSwNP.
When the 75th percentile of normal controls was used as the cut-off value, we found that NP from CRSwNP patients with high OSMRβ mRNA levels indeed had more revision surgeries than those with lower OSMRβ levels (Table E2). Since we had very limited data on allergy, asthma and CRS exacerbations and biological treatments in this cohort of patients, we are interested in exploring the relationship between OSMRβ and presence of allergy and asthma and CRS exacerbations and benefit of biological treatments in future studies.
In summary, our results have shown that OSM, predominantly secreted by neutrophils in CRSwNP, can synergize with IL-4, and perhaps IL-13, to induce the synthesis and release of TSLP in nasal and bronchial epithelial cells. IL-4 plus OSM augments the expression of PCSK3 in NEC, which can truncate the TSLP, producing a more effective type 2 inflammation-promoting cytokine. TSLP has been implicated as an inducer of type 2 responses and eosinophilic inflammation in both CRSwNP and asthma.25,27,48 The present study suggests that some of these actions may result from enhancement of responses to IL-4 and IL-13 by OSM-induction of IL-4Rα in epithelial cells. Concurrently, OSM can augment its own function by inducing the expression of its receptor OSMRβ in epithelium and can act in concert and/or synergistically with IL-4 and IL-13. This study may have uncovered a highly interactive feed forward system CRS and asthma.
Supplementary Material
Key messages:
The type 2 OSM receptor (OSMRβ/gp130) is expressed in epithelial cells and is increased in nasal polyps.
OSM induces the expression of IL-4Rα in ALI cultured NEC.
IL-4 synergizes with OSM to induce the synthesis and release of TSLP in NEC, a response blocked by a STAT6 inhibitor.
Stimulation of NEC with both IL-4 and OSM augmented the expression of PCSK3, an enzyme that truncates and activates TSLP; effects expected to promote type 2 inflammation.
Sources of support:
This research was supported in part by NIH grants (KL2 TR001424, K23 AI141694, R01 AI104733, 24 U19 AI106683, and P01 145818), by grants from the Parker B. Francis Fellowship Foundation 25, a HOPE APFED/AAAAI Pilot Grant Award, by the Ernest S. Bazley Foundation and by the National Natural Science Foundation of China (81800888, 82171113).
WS served on an advisory board for GlaxoSmithKline. BT reports personal fees from Sanofi Regeneron/Genzyme, and OptiNose. AP reports personal fees from Sanofi Regeneron and personal fees and grants from AstraZeneca. LG reports personal fees from Astellas Pharmaceuticals. RK reports personal fees from Sanofi, Novartis, Lyra Pharmaceutical, and Neurent. AK reports a consultant fee from Astellas Pharma and a gift for his research from Lyra Therapeutics. RS reports personal fees from Intersect ENT, Merck, GlaxoSmithKline, Sanofi, AstraZeneca/Medimmune, Genentech, Actobio Therapeutics, Lyra Therapeutics, Astellas Pharma Inc., Allakos Inc. and Otsuka Inc. RS also receives royalties from Siglec-8 and Siglec-8 ligand related patents licensed by Johns Hopkins to Allakos.
Abbreviations used:
- ALI
air-liquid interface
- CRS
chronic rhinosinusitis
- CRSsNP
chronic rhinosinusitis without nasal polyps
- CRSwNP
chronic rhinosinusitis with nasal polyps
- NP
nasal polyps
- UT
uncinate tissue
- OSM
oncostatin M
- TSLP
thymic stromal lymphopoietin
- ECP
eosinophil cationic protein
- EMT
epithelial-mesenchymal transition
- OSMRβ
OSM receptor β chain
- IL-4Rα
IL-4 receptor alpha
- LIFR
leukemia inhibitory factor receptor
- IL-13Rα1
IL-13 receptor alpha 1
- IL-13Rα2
IL-13 receptor alpha 2
- PCSK3
proprotein convertases subtilisin/kexin3
- CCL
C-C motif chemokine ligand
- RT
room temperature
Footnotes
Conflicts of interest:
PPC, BFW, JN, JP, AIK, LS, RC, JH, DC, KW and ZL report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016; 6 Suppl 1:S22–209. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012; 3:1–298. [PubMed] [Google Scholar]
- 3.Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol 122 (1), 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2013; 131:1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato A Immunopathology of chronic rhinosinusitis. Allergol Int 2015; 64 (2), 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritz DK, Kerr C, Fattouh R, Llop-Guevara A, Khan WI, Jordana M, et al. A mouse model of airway disease: oncostatin M-induced pulmonary eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness are STAT6 dependent, and interstitial pulmonary fibrosis is STAT6 independent. J Immunol 2011; 186:1107–18. [DOI] [PubMed] [Google Scholar]
- 7.Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol 2015; 136:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp Lung Res 2009; 35:781–94. [DOI] [PubMed] [Google Scholar]
- 9.Kang HJ, Kang JS, Lee SH, Hwang SJ, Chae SW, Woo JS, et al. Upregulation of oncostatin m in allergic rhinitis. Laryngoscope 2005; 115:2213–16. [DOI] [PubMed] [Google Scholar]
- 10.Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol 2007; 178:4615–22. [DOI] [PubMed] [Google Scholar]
- 11.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol 2017; 139 (6), 1966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, et al. Oncostatin M Causes eotaxin-1 Release From Airway Smooth Muscle: Synergy With IL-4 and IL-13. J Allergy Clin Immunol 2005; 115 (3), 514–20. [DOI] [PubMed] [Google Scholar]
- 13.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999; 162(10):6233–7. [PubMed] [Google Scholar]
- 14.van der Velden VH, Naber BA, Wierenga-Wolf AF, Debets R, Savelkoul HF, Overbeek SE, et al. Interleukin 4 receptors on human bronchial epithelial cells. An in vivo and in vitro analysis of expression and function. Cytokine. 1998;10(10):803–13. [DOI] [PubMed] [Google Scholar]
- 15.Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol 2015; 136:1442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YJ. Thymic Stromal Lymphopoietin: Master Switch for Allergic Inflammation. J Exp Med 2006; 203 (2), 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673–680. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Liu YJ, Arima K. Cellular and Molecular Mechanisms of TSLP Function in Human Allergic disorders--TSLP Programs the “Th2 Code” in Dendritic Cells. Allergol Int 2012; 61 (1), 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007; 204(2):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara N, Poposki JA, Klingler1 AI, Tan BK, KE Hulse1, Stevens WW, et al. Role of RANK-L as a potential inducer of ILC2-mediated type 2 inflammation in chronic rhinosinusitis with nasal polyps. Mucosal Immunol 2020; 13 (1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol 2009; 123(2):472–8. [DOI] [PubMed] [Google Scholar]
- 23.Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFα increase Thymic Stromal Lymphopoietin Receptor (TSLPR) expression on eosinophils and enhance TSLP stimulated degranulation. Clin Mol Allergy 2012; 10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nature medicine 2013; 19(8):1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Huls KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2013; 132:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao B, Cao PP, Zeng M, Zhen Z, Wang H, Zhang YN, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy 2015; 70:1169–80. [DOI] [PubMed] [Google Scholar]
- 27.Poposki JA, Klingler AI, Stevens WW, Peters AT, Hulse KE, Grammer LC, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol 2017; 139 (5), 1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016; 6 Suppl 1:S22–209. [DOI] [PubMed] [Google Scholar]
- 29.Seshadri S, Lu x, Purkey MR, Homma T, Choi AW, Carter R, et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2015; 136 (6), 1548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermanns HM. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015; 26 (5), 545–58. [DOI] [PubMed] [Google Scholar]
- 31.Homma T, Kato A, Sakashita M, Takabayashi T, Norton JE, Suh LA, et al. Potential Involvement of the Epidermal Growth Factor Receptor Ligand Epiregulin and Matrix Metalloproteinase-1 in Pathogenesis of Chronic Rhinosinusitis. Am J Respir Cell Mol Biol 2017; 57(3):334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179(2):1080–7.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009; 41(5):516–24. [DOI] [PubMed] [Google Scholar]
- 34.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125(2):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsunaga K, Katoh N, Fujieda S, Lzuhara K, Oishi K. Dupilumab: basic aspects and applications to allergic diseases. Allergol Int 2020; 69:187–196. [DOI] [PubMed] [Google Scholar]
- 36.Laidlaw TM, Buchheit KM. Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol 2020; 124(4):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langdon C, Kerr C, Tong L, Richards CD. Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 mice. J Immunol 2003; 170:548–55. [DOI] [PubMed] [Google Scholar]
- 38.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002; 8:885–9. [DOI] [PubMed] [Google Scholar]
- 39.Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, et al. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol 1999; 163(12):6876–83. [PubMed] [Google Scholar]
- 40.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 1998(6); 187: 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomkinson A, Kanehiro A, Rabinovitch N, Joetham A, Cieslewicz G, Gelfand EW The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. Am J Respir Crit Care Med 1999(4); 160: 1283–91. [DOI] [PubMed] [Google Scholar]
- 42.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016; 137:1449–56. [DOI] [PubMed] [Google Scholar]
- 43.Ryu G, Kim DK, Dhong HJ, Eun KM, Lee KE, Kong IG, et al. Immunological characteristics in refractory chronic rhinosinusitis with nasal polyps undergoing revision surgeries. Allergy Asthma Immunol Res 2019; 11:664–76.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poposki JA, Klingler AI, Stevens WW, Suh LA, Tan BK, Peters AT, et al. Elevation of activated neutrophils in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2021; doi: 10.1016/j.jaci.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 2012; 129:1522–28. [DOI] [PubMed] [Google Scholar]
- 46.Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther 1989; 250(2):598–605. [PubMed] [Google Scholar]
- 47.Emson C, Corren J, Sałapa K, Hellqvist Å, Parnes JR, Colice G. Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma with and without Nasal Polyposis: A Post Hoc Analysis of the Phase 2b PATHWAY Study. J Asthma Allergy 2021;14:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377(10):936–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.