Abstract
Background
Although clinical features of Type 2 inflammation have been associated with poorer longitudinal outcomes in preschool children with recurrent wheezing, it remains difficult to predict which children are at highest risk for poor outcomes during a routine clinical encounter.
Objective
We tested the hypothesis that pre-specified cut points of blood eosinophil counts would predict exacerbation and treatment response outcomes in preschool children with recurrent wheezing and that prediction could be improved with the addition of a second biomarker.
Methods
Data from three clinical trials of 1,074 preschool children aged 12–71 months with recurrent wheezing were merged. The primary outcome was the occurrence of any exacerbation during follow-up. Secondary outcomes included the annualized rate of wheezing exacerbations and the occurrence of any exacerbation requiring hospitalization. Exploratory analyses focused on exacerbation outcomes, offline exhaled nitric oxide concentrations and caregiver reported asthma control scores after inhaled corticosteroid treatment initiation.
Results
Each blood eosinophil cut-point was associated with increased odds of exacerbation, higher exacerbation rate and greater hospitalization occurrence in preschool children with recurrent wheezing. However, outcome detection was improved with in children with more elevated blood eosinophil counts. Addition of a second biomarker of Type 2 inflammation improved outcome detection and was further associated with an improved response to initiation of daily inhaled corticosteroids in exploratory analyses. However, the specificity of blood eosinophils was poor.
Conclusion
Although validation studies are warranted, blood eosinophil cut points may be useful for clinical assessment and future studies of exacerbation and treatment response in preschool children with recurrent wheezing.
Keywords: Asthma in children, Asthma exacerbation, Blood eosinophils, Wheeze, Preschool child, Phenotype, Sensitization, Treatment response, Type 2 inflammation
Introduction
Preschool children with recurrent wheezing have frequent and troublesome respiratory symptoms, but unlike older children with asthma, can be very difficult to treat.1 Although it is clearly recognized that preschool children with recurrent wheezing are a heterogeneous group with differing underlying pathologies and disease trajectories,2–6 it remains difficult to predict which of these children are at highest risk for poor future clinical outcomes during a routine clinical encounter.7, 8 This is a critical practice gap since preschool children frequently have significant healthcare utilization needs for acute wheezing exacerbations.9, 10
Type 2 inflammation is driven by secretion of the T helper 2 cytokines interleukin (IL)-4, IL-5, and IL-13 and is associated with airway mucus production, bronchoconstriction, IgE synthesis and eosinophilia.11 In preschool children, several studies have shown that clinical features of Type 2 inflammation are associated with poorer longitudinal outcomes such as greater exacerbation likelihood and wheezing persistence.12 However, the concept of Type 2 inflammation has been poorly defined in young children for clinical application. Indeed, of the three externally validated tools for prediction of subsequent outcomes (i.e., persistent asthma) in preschool children, the direct indicators of Type 2 inflammation vary significantly, from eczema13–15 to ≥4% blood eosinophils.15 Other analyses have also focused on allergen sensitization and absolute blood eosinophil counts as predictors of outcomes, with little agreement among studies.16, 17
Although there is no current gold standard for measurement of Type 2 inflammation in clinical settings, blood eosinophils have emerged as a biomarker of choice, given that they are relatively simple and inexpensive to collect. In school-age children, elevated blood eosinophils (whether intermittent or persistent), signify an increased risk of future exacerbation18, 19 and are routinely used to guide biologic therapy for Type 2 inflammation.20, 21 We therefore sought to define the optimal cut point of blood eosinophils for prediction of clinical outcomes in preschool children with recurrent wheezing. Recognizing that wheezing exacerbations have a significant functional impact on affected families22 and are a primary driver of wheezing costs,23, 24 wheezing exacerbation was selected as the primary outcome of interest for our analyses. Leveraging a multicenter longitudinal cohort of preschool children, we tested the hypothesis that pre-specified cut points of blood eosinophil counts would predict exacerbation occurrence, and that prediction of exacerbation could be improved with the addition of a second biomarker. We also explored whether blood eosinophil cut points could be useful for discriminating inhaled corticosteroid treatment response.
Methods
Baseline and intervention period data were merged from three Phase 3 multi-center clinical trials involving preschool participants ages 12–71 months with recurrent wheezing housed in the National Heart, Lung and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center. Details of the included studies (i.e., Acute Intermittent Management Strategies (AIMS, NCT00319488),25 Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST, NCT00675584),26 and Azithromycin for Preventing the Development of Upper Respiratory Tract Illnesses into Lower Respiratory Tract Symptoms (APRIL, NCT01272635)27 were published previously and are listed in Table E1. Exclusion criteria for each of the studies included premature birth, other significant respiratory conditions, gastroesophageal reflux, recent antibiotic or systemic corticosteroid use within the previous 2–4 weeks, or a life-threatening wheezing episode. Written informed consent was obtained from all caregivers.
Participant characterization.
Each clinical center utilized the same manual of procedures for participant characterization. At the baseline visit of each trial, caregivers completed questionnaires to elicit data on demographics, family history, child allergy and respiratory symptoms, and treatment of symptoms including medications and healthcare utilization. Days with asthma symptoms were obtained during the run-in period from caregiver-completed diaries and were defined as full calendar days with any daytime or nighttime respiratory symptoms (cough, wheeze, difficulty breathing), any albuterol use, or any unscheduled healthcare visits for respiratory symptoms. Compliance with the diaries was used to estimate adherence and willingness to participate in the study; participants with unacceptable adherence (<75–80%) were ineligible for randomization.
Venipuncture was performed immediately after the run-in period prior to treatment randomization. Blood eosinophils were quantified from whole blood by means of an automated assay at each clinical site. Total serum IgE was quantified centrally (St. Louis Children’s Hospital, St. Louis, Mo; Advanced Diagnostic Laboratories, National Jewish Health, Denver, CO). In two studies (AIMS and MIST trials), skin testing to 8 common aeroallergens (house dust mite mixture [Dermatophagoides pteronyssinus and Dermatophagoides farinae], cockroach [American and German], dog [mixed breeds], cat [standardized], mold [mix no.1], grass [standardized Southern mix]. Tree [eastern 8 tree mix], and weed [national mix] and 3 foods [cow’s milk, chicken and whole egg, and peanut; Greer Laboratories, Lenoir, NC) was also performed prior to randomization using the Multi-test II (Lincoln Diagnostics, Decatur, IL) prick technique. Tests were considered positive if the prick resulted in a wheal with a mean diameter (mean of maximum and 90° midpoint diameters) that was at least 3 mm greater than that produced by the saline control.28, 29 In the APRIL study, specific IgE levels (ImmunoCAP; APRIL study) were performed for a nationally representative panel of 10 aeroallergens (cat dander [ImmunoCAP test code E1], dog dander [E5], mold mix [Mx1], German cockroach [i6], grass mix [gx2], tree mix [Tx4, Tx6], (9) weed mix [Wx1], giant ragweed [W3], Dermatophagoides pteronyssinus [D2], Dermatophagoides farinae [D2]) and 3 foods (milk [f2], egg, [f1], peanut [f13]) at a central laboratory (Advanced Diagnostic Laboratories, National Jewish Health, Denver, CO). Tests with levels >0.34 IU/mL were considered positive.
In the MIST study, caregivers also completed a modified version of the Asthma Control Questionnaire30 after randomization, which included equally-weighted questions about symptoms and short-acting bronchodilator use. Questions were summed and then averaged to obtain a total asthma control score. Exhaled nitric oxide concentrations were also quantified utilizing offline methods at baseline and after randomization as described previously.26
Standardization and supervision of asthma care.
At enrollment, all caregivers received a written action plan that detailed instructions for administration of albuterol sulfate (90 mcg/actuation) when a pre-specified threshold of symptoms was met. Albuterol sulfate and a valved holding chamber were provided by each study and were dispensed at the first visit, then as needed at each subsequent study visit. Additionally, caregivers received a home supply of oral prednisolone in the event of an asthma exacerbation that was also dispensed at the first study visit. Children whose symptoms did not resolve or who required albuterol treatments for more than 24 hours were first instructed by action plan to call the study medical staff, who were available by phone 24 hours a day, and then to initiate a 4-day burst of prednisolone (2 mg/kg/day for 2 days followed by 1 mg/kg/day for 2 days). The action plan was reviewed and reinforced with caregivers at each study visit. Physician discretion for prednisolone administration outside of the action plan parameters was also permitted provided that a specific reason for the initiation was documented. Two courses of systemic corticosteroids had to be separated by at least one week to count as two exacerbations.
Outcomes.
The primary outcome was the occurrence of an exacerbation during the follow-up period. The definition of exacerbation was consistent with that proposed by a National Institutes of Health Working Group31 and was defined as respiratory symptoms resulting in treatment with systemic corticosteroids (prednisolone). Secondary outcomes included the annualized rate of wheezing exacerbations during the study intervention period and the occurrence of any exacerbation requiring hospitalization. Exploratory analyses focused on inhaled corticosteroid treatment responses and were conducted in MIST study participants treated with daily inhaled corticosteroid versus daily placebo. Exploratory outcomes were any exacerbation occurrence, annualized exacerbation rate, change in offline exhaled nitric oxide concentrations after daily inhaled corticosteroid treatment initiation, and caregiver reported asthma control scores after treatment initiation.
Outcome analyses.
Analyses were performed with IBM SPSS software, version 28. Group differences were analyzed with Chi-Square tests and analysis of variance. Variable associations were assessed with Pearson and Spearman correlation. Multivariate logistic regression was performed to obtain odds ratios for outcomes of interest. Predictors included blood eosinophils (≥150, ≥200, ≥250, ≥300, ≥350 cells/microliter) and sensitization patterns. Generalized linear models were used for the outcome of annualized exacerbation rate. Models were adjusted for daily inhaled corticosteroid treatment, intermittent inhaled corticosteroid treatment sex, age group (<3 years versus ≥3 years), and tobacco smoke exposure. Receiver operating curves were generated to assess sensitivity and specificity of selected cut points. For exploratory analyses, offline exhaled nitric oxide concentrations after randomization (averaged for each participant) were subtracted from the averaged concentrations before randomization. Caregiver reported asthma control scores were obtained by averaging the score for each response. Asthma control scores at each available time point were then averaged for each participant. Analyses utilized a 0.05 significance level without adjustment for multiple comparisons.
Results
The sample for analysis consisted of 1,074 preschool children with recurrent wheezing who had complete blood eosinophil, sensitization, and outcome data for analysis. Features of the sample are shown Table 1. Blood eosinophil counts (mean ± standard deviation) were 341.1 ± 286.9, with a median of 240.1 and range of 50.4 to 1836 cells/microliter (Figure E1A). Blood eosinophil counts were strongly correlated with blood eosinophil percentages (Figure E1B). For each of the prespecified blood eosinophil count cut points, blood eosinophil percentages were as follows (median [25th-75th percentile]): ≥150 eosinophils, 4% [3–7%]; ≥200 eosinophils, 5% [3.1–7.3%]; ≥250 eosinophils, 5.7% [4–8%]; ≥300 eosinophils, 6% [4.5–8.3%]; ≥350 eosinophils/microliter, 7% [5–9%]. Blood eosinophil counts were also slightly higher in males versus females (360.6 ± 305.6 [median=258.0] vs. 307.1 ± 248.3 [median=225.0], p=0.003) and children ≥3 years versus children <3 years (368.0 ± 306.2 [median=266.7] vs. 309.3 ± 259.3 [median=224.0], p<0.001). Blood eosinophil counts did not differ by Hispanic ethnicity, race, or receipt of inhaled corticosteroids or leukotriene receptor antagonists at any time during the previous year (Figure E2). Blood eosinophil counts were weakly associated with the average days per week with asthma symptoms in the study run-in periods immediately prior to blood collection (Figure 1A) and the number of oral corticosteroid bursts in the previous year (no burst vs. any burst: 325.6 ± 296.6 [median=223.0] vs. 349.9 ± 281.2 [median=267.5], p=0.335) (Figure 1B). Blood eosinophil counts were also higher in children with wheezing-related hospitalizations in the previous year (no hospitalization [n=929], 332.4 ± 281.0 [median=234.0] vs. one hospitalization [n=126], 388.4 ± 281.2 [median=293.0] vs. two hospitalizations [n=19], 450.9 ± 321.9 [median=365.7], p=0.012). As expected, blood eosinophil counts were also higher in children with a history of eczema (378.6 ± 309.7 [median=274.2] vs. 306.8 ± 260.1 [median=224.4], p<0.001), children with aeroallergen sensitization (456.0 ± 328.8 [median=370.0] vs. 246.7 ± 204.0 [median=190.6], p<0.001), and children with food sensitization (459.3 ± 339.7 [median=369.6] vs. 275.0 ± 227.7 [median=205.0], p<0.001). Associations between blood eosinophil counts and total serum IgE, the percentage of positive aeroallergens (of 8), and the percentage of total positive allergens (of 11 total aeroallergens and foods) were also significant and are shown in Figure E3A–C.
Table 1.
Features of the participants. Data represent the mean ± standard deviation or the number of participants (%).
| Feature | Participants (N=1,074) |
|---|---|
| Age (months) | 32.7 ± 14.7 |
| Age of symptom onset (months) | 12.9 ± 10.6 |
| Male | 681 (63.4) |
| Self-reported race | |
| White | 733 (68.2) |
| Black | 235 (21.9) |
| Asian | 22 (2.0) |
| More than one race | 60 (5.6) |
| Reported as “other” | 24 (2.2) |
| Hispanic ethnicity | 283 (26.4) |
| Family medical history | |
| Mother with asthma | 352 (32.8) |
| Mother with allergies | 280 (26.1) |
| Father with asthma | 397 (37.0) |
| Father with allergies | 308 (28.7) |
| Household exposures | |
| Tobacco smoke | 293 (27.3) |
| Cat | 169 (15.7) |
| Dog | 325 (30.3) |
| Self-reported triggers | |
| Inhaled allergens | 566 (52.7) |
| Exercise | 524 (48.8) |
| Respiratory infections | 1,027 (95.6) |
| Chemicals/irritants | 219 (20.4) |
| Weather changes | 765 (71.2) |
| Cold air | 549 (51.1) |
| Emotions | 328 (30.5) |
| Tobacco | 265 (24.7) |
| Food | 51 (4.7) |
| Ever had eczema | 513 (47.8) |
| Medication use in past year (at any time) | |
| Inhaled corticosteroid | 459 (42.7) |
| Leukotriene receptor antagonist | 113 (10.5) |
| Exacerbation history (past year) | |
| Any oral corticosteroid burst | 684 (63.7) |
| Number of oral corticosteroid bursts | 1.3 ± 1.3 |
| Any unscheduled visit | 1,037 (96.6) |
| Number of unscheduled visits | 3.5 ± 3.3 |
| Any Emergency Department visit | 801 (74.6) |
| Number of Emergency Department visits | 2.1 ± 2.0 |
| Any hospitalization | 126 (11.7) |
Figure 1.
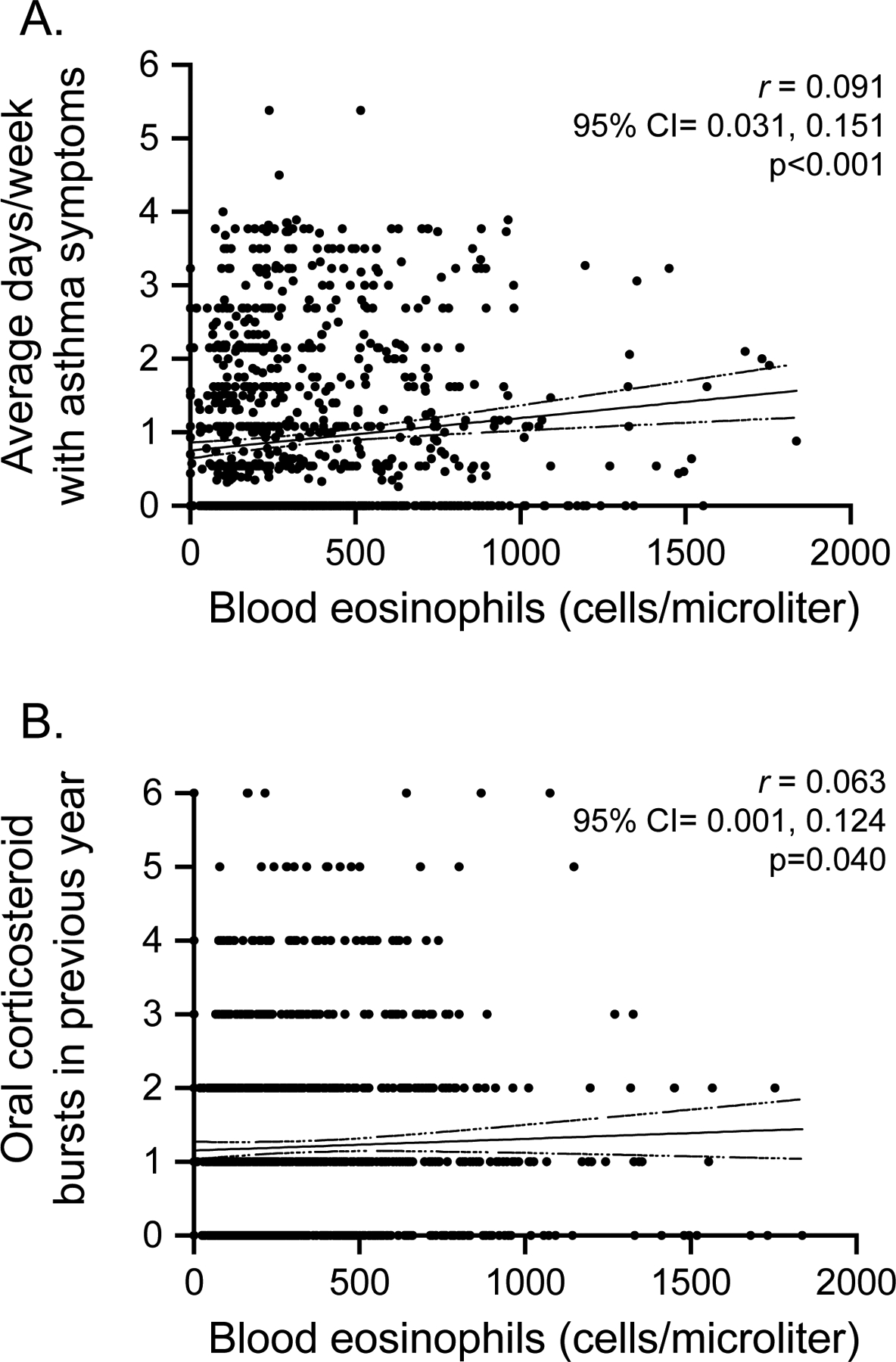
Associations between blood eosinophil counts and (A) the average number of days per week with asthma symptoms during the study run-in periods, and (B) the number of oral corticosteroid bursts in the previous year. CI=confidence interval (shown as dashed line).
Outcome occurrence.
The primary outcome of interest, occurrence of an asthma exacerbation with receipt of systemic corticosteroids before study completion, occurred in 370 (34.5%) children, with an overall annualized exacerbation rate of 0.51 ± 0.87 exacerbations per child (range=0–6 exacerbations). In children with an exacerbation, the average annualized exacerbation rate was 1.45 ± 0.87 exacerbations per child (median=1). Hospitalizations occurred in 43 (11.6%) children with an exacerbation.
Prediction of exacerbation outcomes.
Crude and adjusted associations between exacerbation occurrence and blood eosinophil count cut points and sensitization variables, as single predictor variables, are shown in Table 2. Higher blood eosinophil cut points were associated with higher odds of exacerbation, with some loss of sensitivity. Further analyses therefore focused on whether detection of exacerbation outcomes could be improved with a second variable. For these analyses, blood eosinophil and sensitization predictor variables were modeled as interaction terms. The results are provided in Table 3. The addition of a second sensitization-related prediction variable resulted in fewer children with the exacerbation outcome of interest, but improved exacerbation detection by up to ~29% for aeroallergen sensitization variables and up to ~32% for food sensitization variables (Table 3). Results were similar after further adjustment for an exacerbation treated with oral corticosteroids in the previous year (Table E2). Receiver operator characteristic curves of blood eosinophil cut points plus sensitization variables for the detection of exacerbation occurrence demonstrated improved sensitivity with the additional predictor variables, but specificity was quite low (Table E3). This low specificity resulted in low area under the receiver operator curves, as shown in Figure E4, suggesting that several exacerbations were not adequately detected.
Table 2.
Associations between single blood eosinophil and sensitization predictor variables and exacerbation occurrence (primary outcome) in the sample of 1,074 participants.
| Predictor | N with exacerbation | Crude odds ratio (95% CI) | Adjusted odds ratio1 (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Blood eosinophils | |||||
| ≥ 150 cells/microliter (N=793) | 286 | 1.32 (0.99, 1.78) | 1.35 (1.01, 1.83) | 0.772 | 0.282 |
| ≥ 200 cells/microliter (N=652) | 250 | 1.57 (1.20, 2.04) | 1.55 (1.18, 2.03) | 0.673 | 0.433 |
| ≥ 250 cells/microliter (N=521) | 203 | 1.48 (1.15, 1.91) | 1.44 (1.11, 1.87) | 0.550 | 0.548 |
| ≥ 300 cells/microliter (N=441) | 179 | 1.58 (1.22, 2.04) | 1.54 (1.18, 2.00) | 0.484 | 0.629 |
| ≥ 350 cells/microliter (N=378) | 156 | 1.58 (1.22, 2.05) | 1.51 (1.16, 1.97) | 0.421 | 0.685 |
| Sensitization | |||||
| Any food or aeroallergen sensitization (N=601) | 233 | 1.55 (1.20, 2.01) | 1.54 (1.18, 2.01) | ||
| Any aeroallergen sensitization (N=484) | 199 | 1.71 (1.32, 2.21) | 1.72 (1.31, 2.24) | ||
| Any food sensitization (N=385) | 154 | 1.46 (1.13, 1.89) | 1.49 (1.14, 1.94) | ||
| Multiple aeroallergen sensitization (N=303) | 134 | 1.80 (1.37, 2.36) | 1.92 (1.44, 2.56) | ||
| Multiple food sensitization (N=201) | 87 | 1.60 (1.17, 2.18) | 1.64 (1.19, 2.25) | ||
| Food and aeroallergen sensitization (N=268) | 120 | 1.80 (1.36, 2.39) | 1.84 (1.37, 2.46) |
CI = confidence interval;
Adjusted for daily inhaled corticosteroid treatment, intermittent inhaled corticosteroid treatment, sex, age group (<3 years versus ≥3 years), and tobacco smoke exposure
Table 3.
Associations between combined blood eosinophil and sensitization predictor variables and exacerbation occurrence (primary outcome) in the sample of 1,074 participants.
| Model | N | N with exacerbation | Unadjusted OR (95% CI) | Adjusted OR1 (95% CI) | Change in adjusted OR |
|---|---|---|---|---|---|
| Blood eosinophils ≥150 cells/microliter, plus: | |||||
| Any food or aeroallergen sensitization | 492 | 195 | 1.53 (1.19, 1.97) | 1.59 (1.22, 2.07) | 15.1% |
| Any aeroallergen sensitization | 413 | 169 | 1.59 (1.23, 2.05) | 1.63 (1.24, 2.13) | 17.2% |
| Any food sensitization | 326 | 137 | 1.60 (1.22, 2.10) | 1.67 (1.26, 2.20) | 19.2% |
| Multiple aeroallergen sensitization | 273 | 120 | 1.73 (1.30, 2.29) | 1.89 (1.41, 2.55) | 28.6% |
| Multiple food sensitization | 175 | 80 | 1.78 (1.28, 2.47) | 1.82 (1.30, 2.54) | 25.8% |
| Food and aeroallergen sensitization | 247 | 111 | 1.79 (1.34, 2.39) | 1.82 (1.35, 2.46) | 25.8% |
| Blood eosinophils ≥200 cells/microliter, plus: | |||||
| Any food or aeroallergen sensitization | 438 | 177 | 1.56 (1.21, 2.01) | 1.61 (1.23, 2.10) | 3.7% |
| Any aeroallergen sensitization | 375 | 156 | 1.61 (1.24, 2.10) | 1.65 (1.26, 2.18) | 6.1% |
| Any food sensitization | 295 | 127 | 1.67 (1.27, 2.20) | 1.75 (1.31, 2.32) | 11.4% |
| Multiple aeroallergen sensitization | 254 | 112 | 1.72 (1.29, 2.29) | 1.88 (1.39, 2.55) | 17.6% |
| Multiple food sensitization | 166 | 76 | 1.77 (1.27, 2.48) | 1.84 (1.31, 2.59) | 15.8% |
| Food and aeroallergen sensitization | 232 | 106 | 1.84 (1.37, 2.48) | 1.91 (1.40, 2.59) | 18.8% |
| Blood eosinophils ≥250 cells/microliter, plus: | |||||
| Any food or aeroallergen sensitization | 368 | 151 | 1.55 (1.20, 2.02) | 1.61 (1.22, 2.13) | 10.6% |
| Any aeroallergen sensitization | 323 | 136 | 1.61 (1.23, 2.11) | 1.68 (1.26, 2.23) | 14.3% |
| Any food sensitization | 253 | 115 | 1.86 (1.39, 2.48) | 1.95 (1.45, 2.63) | 26.2% |
| Multiple aeroallergen sensitization | 223 | 98 | 1.68 (1.24, 2.26) | 1.86 (1.35, 2.56) | 22.6% |
| Multiple food sensitization | 147 | 70 | 1.91 (1.35, 2.72) | 2.01 (1.40, 2.88) | 28.4% |
| Food and aeroallergen sensitization | 208 | 100 | 2.05 (1.51, 2.79) | 2.16 (1.57, 2.97) | 33.3% |
| Blood eosinophils ≥300 cells/microliter, plus: | |||||
| Any food or aeroallergen sensitization | 324 | 135 | 1.56 (1.19, 2.05) | 1.62 (1.22, 2.15) | 4.9% |
| Any aeroallergen sensitization | 296 | 126 | 1.62 (1.23, 2.13) | 1.70 (1.27, 2.27) | 9.4% |
| Any food sensitization | 222 | 104 | 1.94 (1.44, 2.62) | 2.02 (1.48, 2.76) | 23.8% |
| Multiple aeroallergen sensitization | 206 | 92 | 1.71 (1.26, 2.33) | 1.89 (1.36, 2.61) | 18.5% |
| Multiple food sensitization | 133 | 67 | 2.14 (1.48, 3.09) | 2.25 (1.55, 3.27) | 31.6% |
| Food and aeroallergen sensitization | 194 | 95 | 2.11 (1.54, 2.89) | 2.22 (1.60, 3.08) | 30.6% |
| Blood eosinophils ≥350 cells/microliter, plus: | |||||
| Any food or aeroallergen sensitization | 283 | 124 | 1.73 (1.31, 2.28) | 1.77 (1.32, 2.36) | 14.7% |
| Any aeroallergen sensitization | 257 | 115 | 1.79 (1.34, 2.38) | 1.85 (1.37, 2.49) | 18.4% |
| Any food sensitization | 202 | 97 | 2.03 (1.49, 2.77) | 2.06 (1.50, 2.84) | 26.7% |
| Multiple aeroallergen sensitization | 182 | 85 | 1.87 (1.35, 2.58) | 2.03 (1.44, 2.84) | 25.6% |
| Multiple food sensitization | 119 | 60 | 2.12 (1.45, 3.12) | 2.17 (1.47, 3.21) | 30.4% |
| Food and aeroallergen sensitization | 176 | 88 | 2.18 (1.58, 3.03) | 2.25 (1.60, 3.15) | 32.9% |
CI = confidence interval, OR = odds ratio
Adjusted for daily inhaled corticosteroid treatment, intermittent inhaled corticosteroid treatment, sex, age group (<3 years versus ≥3 years), and tobacco smoke exposure
Annualized exacerbation rates and hospitalization occurrence are shown in Figure 2. Each of the blood eosinophil cut points was associated with a significantly higher annualized exacerbation rate when used as a single predictor variable. However, exacerbation detection was improved with the addition of a second aeroallergen sensitization variable (Figure 3A). Similarly, hospitalization occurrence for the exacerbation was also greater in children with the each of the pre-specified blood eosinophil cut points plus additional aeroallergen sensitization variables (Figure 3B). Annualized exacerbation rates and hospitalization occurrence, with addition of food sensitization variables, are shown in Figure E5 and followed similar trends.
Figure 2.
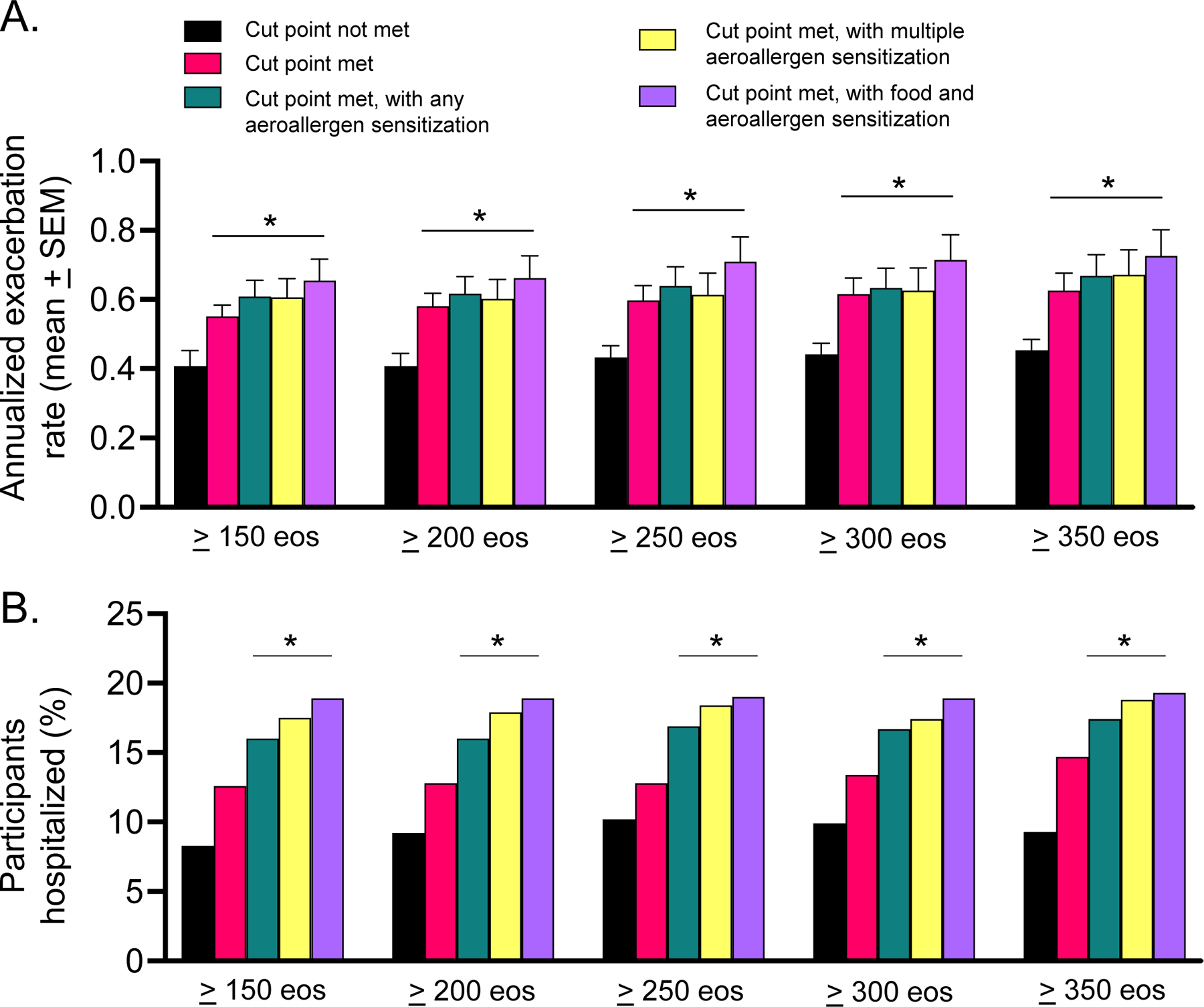
(A) Annualized exacerbation rate (mean ± standard error of the mean) and (B) percentage of children hospitalized for an exacerbation, by blood eosinophil (eos) grouping. *p < 0.05 vs. cut point not met.
Figure 3.
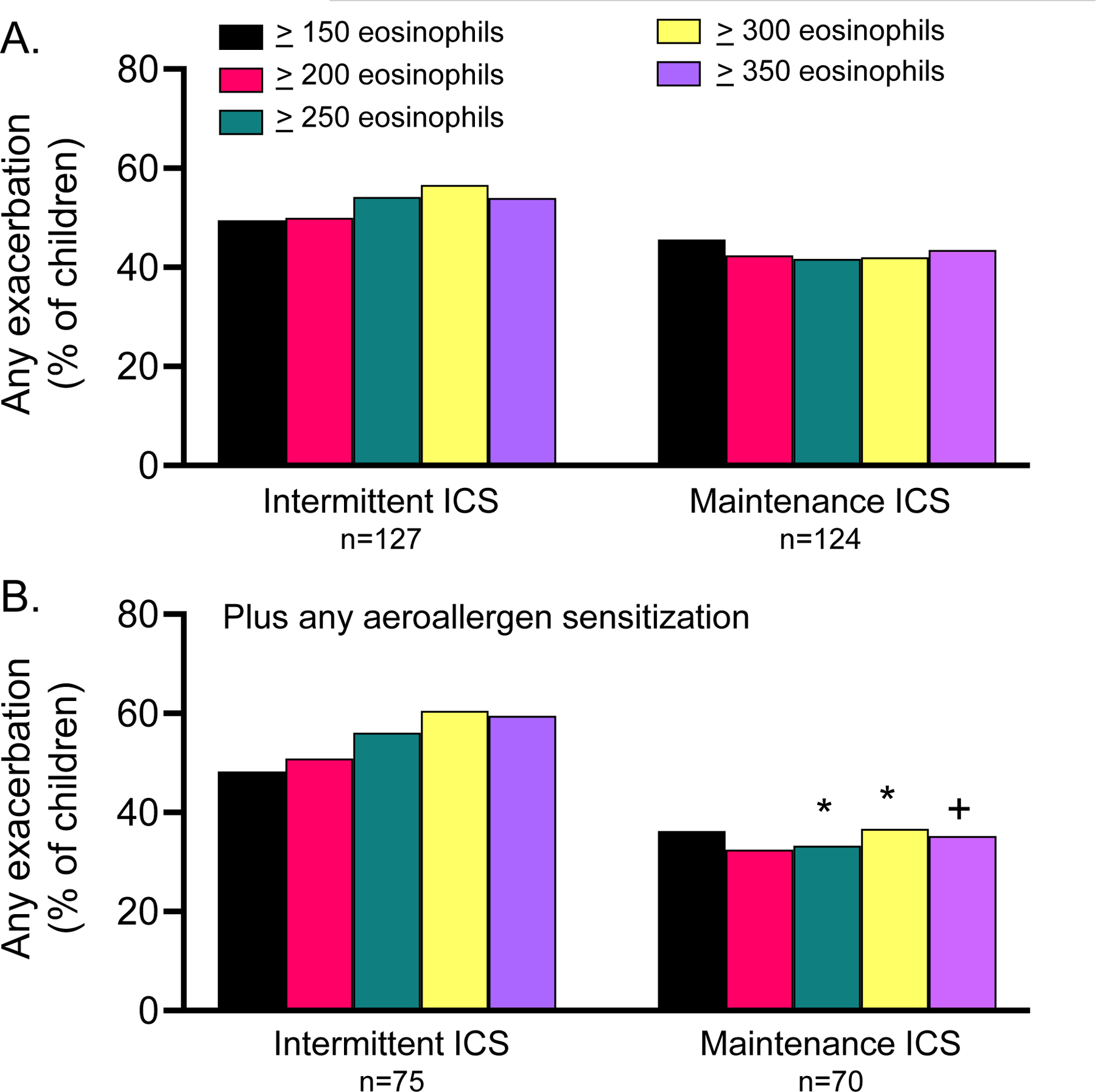
(A) Occurrence of any exacerbation in children treated with intermittent versus maintenance inhaled corticosteroid (ICS) in the MIST study, by blood eosinophil cut point and by (B) blood eosinophil cut point in children with any aeroallergen sensitization. *p<0.05 vs. intermittent ICS, +p=0.06 vs. intermittent ICS
Exploratory outcomes.
We also explored whether the blood eosinophil cut points of could discriminate inhaled corticosteroid treatment response in the MIST study. Two hundred fifty-one children were included in this analysis (intermittent inhaled corticosteroid, n=127; daily maintenance inhaled corticosteroid, n=124). Given the small sample size, any aeroallergen sensitization, which had higher sensitivity than any food or aeroallergen sensitization in the previous analyses, was selected as the second predictor variable.
Exacerbations occurred in 112 children (n=44.6%). There were no differences in exacerbation occurrence by treatment group using the blood eosinophil cut point alone (Figure 3A), but in children with any aeroallergen sensitization, exacerbation occurrence was decreased with maintenance inhaled corticosteroid in children with blood eosinophil counts ≥250 cells/microliter or greater (Figure 3B). In the subset of children who had offline exhaled nitric oxide concentrations measured, nitric oxide concentrations were reduced in children with each of the blood eosinophil cut points after initiation of daily maintenance inhaled corticosteroid therapy (Figure 4A) and were further reduced in children with aeroallergen sensitization (Figure 4B). Reductions in offline exhaled nitric oxide correlated with caregiver reported asthma control scores in children treated with daily maintenance but not intermittent inhaled corticosteroid (Table E4).
Figure 4.
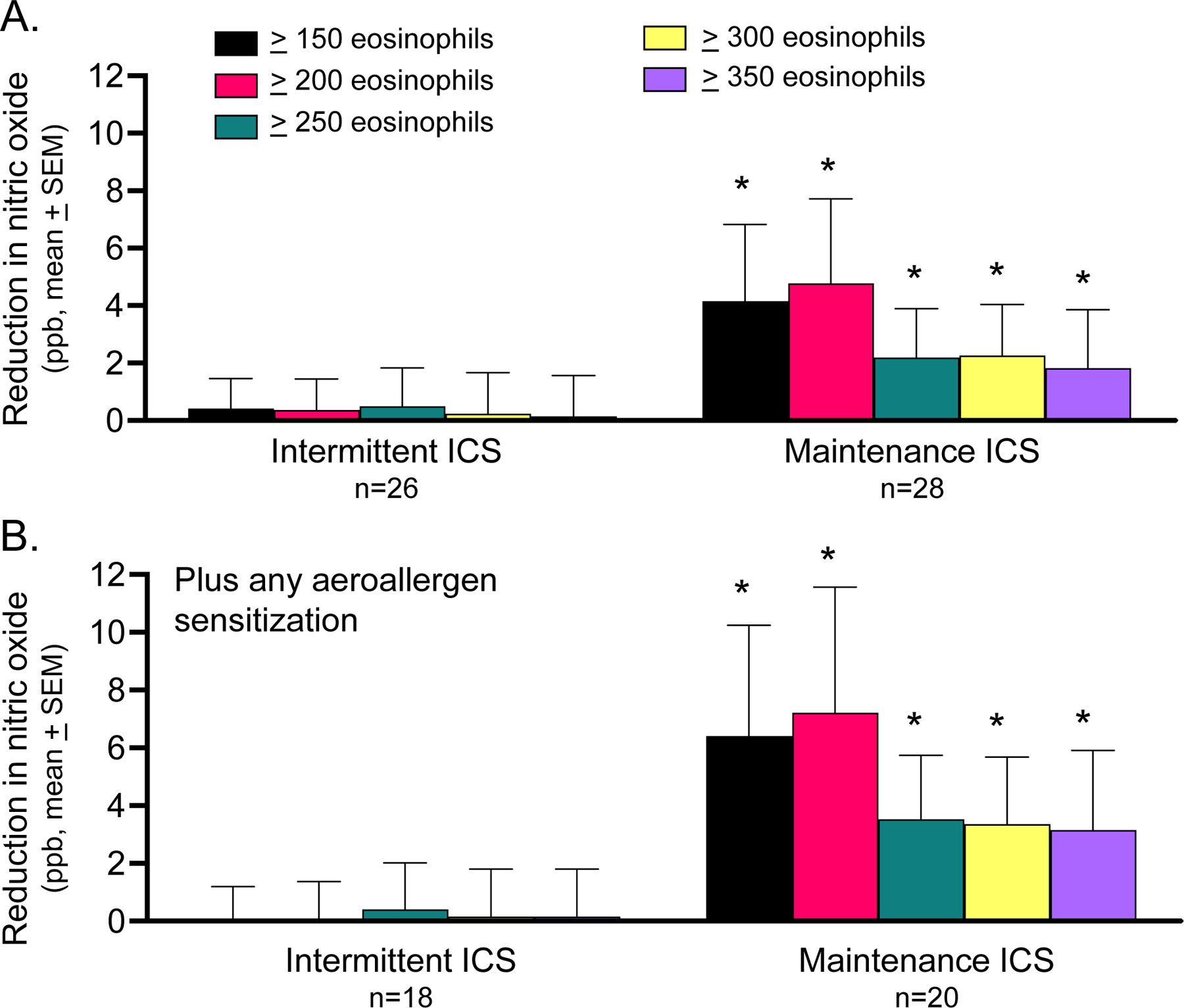
(A) Reduction in offline exhaled nitric oxide concentration in children treated with intermittent versus maintenance inhaled corticosteroid (ICS) in the MIST study, by blood eosinophil cut point and by (B) blood eosinophil cut point in children with any aeroallergen sensitization. *p<0.05 vs. intermittent ICS
Discussion
In this multicenter cohort of preschool children with recurrent wheezing, elevated blood eosinophils were associated with higher odds of exacerbation over 12–18 months of follow-up. Addition of second biomarkers of Type 2 inflammation (i.e., sensitization), improved exacerbation outcome detection and discriminated responses to the initiation of daily maintenance inhaled corticosteroid treatment in exploratory analyses. These results suggest that pre-specified blood eosinophil cut points may be useful for clinical assessment and future studies of exacerbation and treatment response in preschool children with recurrent wheezing. However, the specificity of blood eosinophils as a biomarker is quite poor given that exacerbations also occur in preschool children with non-Type 2 inflammation.2
Nonetheless, our findings do have plausibility. Other phenotypic analyses of independent populations of preschool children have similarly shown more frequent wheezing, greater airflow obstruction and greater respiratory morbidity including more frequent hospitalizations in children with allergic sensitization and elevated blood eosinophil counts.3, 5, 32 Although airway samples would have been ideal for a more comprehensive assessment of Type 2 inflammation in the present study, other studies have shown discordance between systemic and airway Type 2 inflammation in this population. Indeed, bronchoalveolar lavage eosinophils do not necessarily correlate well with peripheral eosinophils in preschool children33 and tend to be low (i.e., <1% of the total cell count) in this age group.34–37 Similar studies of airway tissue have likewise shown a sparsity of airway eosinophils in preschool children,38 with no significant differences between nonatopic and atopic children when analyzed as groups.39, 40 However, those individual preschool children with airway eosinophilia tend to have more refractory asthma features including a greater likelihood of treatment with high-dose inhaled corticosteroids.41 These children may also have an altered predisposition to airway rhinovirus infection,33, 41 which may further exaggerate Type 2 immune pathways.42 Thus, there is likely a developmental progression of airway eosinophilia in children, since preschool children with recurrent wheezing do have increased airway smooth muscle area which is associated with development of atopic asthma by school age.43
Admittedly, there is no gold standard cut point for blood eosinophils in children. Moreover, the optimal cut point may differ as a function of age.19 Whereas the Individualized Therapy for Asthma in Toddlers study utilized a cut point of ≥300 blood eosinophils for prediction of exacerbation and differential treatment response, that study was limited to children with persistent asthma necessitating daily treatment,17 as opposed to children with recurrent wheezing who were evaluated in the present study. Moreover, the sample sizes for prediction of differential treatment response in that study were quite small.17 A separate large study of preschool children with recurrent wheezing found that a blood eosinophil cut point of 322 cells/microliter corresponded to the third quartile threshold of the population studied.32 Another report of blood eosinophil counts stratified by age group likewise found that a blood eosinophil cut point of >300 cells corresponded to just below the 75th percentile in children with asthma age 13–48 months.44 Other cut points of blood eosinophils evaluated in the present study may provide greater sensitivity and may be more useful in population-based studies or in studies of biologics therapies. For example, in one study of older children, a blood eosinophil cut point of ≥150 cells/microliter was associated with increased odds of subsequent exacerbation and a shorter time to first acute visit for asthma in children 5 to 11 years.19 A separate study also showed that children age 6 to 11 years with ≥150 blood eosinophils had a significant reduction in severe asthma exacerbations, greater improvement in lung function, and greater improvement in asthma control after treatment with dupilumab.20
Strengths of the present study include the large sample of well characterized children, the multicenter design, and standardization of data collection procedures and wheezing management. Nonetheless, this study does have limitations. First, it is recognized that blood eosinophilia and Type 2 high phenotypes are unlikely static phenomena.18 Unfortunately, blood samples were not collected at subsequent visits which prohibits assessment of biomarker and phenotype stability. However, a recent analysis of Type 2 inflammation in preschool children based on blood eosinophils and atopy found that 73% of preschool children assigned to the Type 2 “high” phenotype retained that phenotype designation after one year of follow-up.45 These children were also more likely to develop asthma at the age of 6 years.45 A separate study in adults also showed that multiple measurement of blood eosinophils only marginally increased sensitivity, with a single measurement of ≥150 blood eosinophils predicting subsequent measurements of ≥150 blood eosinophils in 85% of the population.46
Second, it is also possible that the observed associations between blood eosinophils and exacerbation and treatment response outcomes in the present study are due to unmeasured sources of confounding, despite adjustment of our models. Indeed, each of the included studies utilized different treatment approaches. These timescale over which these studies were undertaken is also potentially significant since the standard of care has changed over the past 20 years. Home visits were not performed in the included studies, so the conditions of the home that contributed to sensitization and blood eosinophilia are not adequately addressed. Likewise, environmental factors such as air pollution, which have been associated with airway eosinophil infiltration in young children with wheezing,47 were also not addressed. Airway physiology measures and genetic analyses were also not performed.
Third, although the present study involved a multicenter design, the results may not necessarily be generalizable to the broader population of preschool children with recurrent wheezing. Most participants were recruited at specialty clinics within academic medical centers and may therefore not be entirely representative of the preschool wheezing population encountered in primary care. Indeed, the patients included in the analysis had adequate access to medical care (evidenced by continued participation in the parent studies) and were also adherent to the prescribed therapies. In contrast, a recent study found that inhaled corticosteroid treatment determined by blood eosinophils did not impact urgent healthcare visits at 4 months,48 but adherence to inhaled corticosteroids in that study was also quite poor and consistent with other real-world populations of children with asthma.49
In summary, pre-specified blood eosinophil cut points were associated with higher odds of exacerbation, higher exacerbation rate, and greater hospitalization occurrence over 12–18 months of follow-up in preschool children with recurrent wheezing. Outcome detection was greater in children with higher cut points. Addition of sensitization variables to the model improved sensitivity of outcome detection and also discriminated responses to initiation of daily maintenance inhaled corticosteroids in exploratory analyses. While validation is warranted, these results suggest that pre-specified blood eosinophil cut points may be useful in clinical settings and future studies for prediction of subsequent exacerbations and treatment responses in preschool children with recurrent wheezing.
Supplementary Material
Highlights Box.
What is already known about this topic?
In prepubertal children with asthma, elevated blood eosinophils predict future exacerbations and are used to guide biologic therapy. The optimal blood eosinophil cut points for prediction of exacerbation and treatment response in preschool children are unknown.
What does this article add to our knowledge?
Cut points of ≥150 blood eosinophils/microliter predicted exacerbation outcomes in preschool children, but exacerbation detection was greater with higher cut points. Addition of sensitization variables improved detection of exacerbations and responses to daily inhaled corticosteroids in exploratory analyses.
How does this study impact current management guidelines?
Blood eosinophil cut points combined with sensitization variables may be useful for clinical assessment and future studies of exacerbation and treatment response in preschool children with recurrent wheezing.
This study was supported in part by:
R01 NR017939, K24 NR018866, and UL 1TR002378
Abbreviations
- AIMS
Acute Intermittent Management Strategies
- APRIL
Azithromycin for Preventing the Development of Upper Respiratory Tract Illnesses into Lower Respiratory Tract Symptoms
- IL
Interleukin
- MIST
Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
Anne M. Fitzpatrick, Jocelyn R. Grunwell, Kirsten A. Cottrill, Abby D. Mutic and David T. Mauger have no disclosures pertaining to the submitted work.
Study registrations:
AIMS ClinicalTrials.gov number, NCT00319488
APRIL ClinicalTrials.gov number, NCT01272635
MIST ClinicalTrials.gov number, NCT0067558
References
- 1.Bacharier LB, Guilbert TW, Jartti T, Saglani S. Which Wheezing Preschoolers Should be Treated for Asthma? J Allergy Clin Immunol Pract 2021; 9:2611–8. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Bacharier LB, Guilbert TW, Jackson DJ, Szefler SJ, Beigelman A, et al. Phenotypes of Recurrent Wheezing in Preschool Children: Identification by Latent Class Analysis and Utility in Prediction of Future Exacerbation. J Allergy Clin Immunol Pract 2019; 7:915–24 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai R, Miliku K, Gaddipati S, Choi J, Ambalavanan A, Tran MM, et al. Wheeze trajectories: Determinants and outcomes in the CHILD Cohort Study. J Allergy Clin Immunol 2021. [DOI] [PubMed] [Google Scholar]
- 4.Oksel C, Granell R, Haider S, Fontanella S, Simpson A, Turner S, et al. Distinguishing Wheezing Phenotypes from Infancy to Adolescence. A Pooled Analysis of Five Birth Cohorts. Ann Am Thorac Soc 2019; 16:868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O’Connor GT, et al. Longitudinal Phenotypes of Respiratory Health in a High-Risk Urban Birth Cohort. Am J Respir Crit Care Med 2019; 199:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo C, Blais L, Brownell M, Quail JM, Sadatsafavi M, Forget A, et al. Association Between Asthma Control Trajectories in Preschoolers and Long-Term Asthma Control. J Allergy Clin Immunol Pract 2022. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Rodriguez JA, Cifuentes L, Martinez FD. Predicting Asthma Using Clinical Indexes. Front Pediatr 2019; 7:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colicino S, Munblit D, Minelli C, Custovic A, Cullinan P. Validation of childhood asthma predictive tools: A systematic review. Clin Exp Allergy 2019; 49:410–8. [DOI] [PubMed] [Google Scholar]
- 9.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3 2012:1–58. [PubMed] [Google Scholar]
- 10.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in Children - United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018; 67:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coverstone AM, Seibold MA, Peters MC. Diagnosis and Management of T2-High Asthma. J Allergy Clin Immunol Pract 2020; 8:442–50. [DOI] [PubMed] [Google Scholar]
- 12.Feleszko W, Jartti T, Bacharier LB. Current strategies for phenotyping and managing asthma in preschool children. Curr Opin Allergy Clin Immunol 2022; 22:107–14. [DOI] [PubMed] [Google Scholar]
- 13.Caudri D, Wijga A, CM AS, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol 2009; 124:903–10 e1–7. [DOI] [PubMed] [Google Scholar]
- 14.Pescatore AM, Dogaru CM, Duembgen L, Silverman M, Gaillard EA, Spycher BD, et al. A simple asthma prediction tool for preschool children with wheeze or cough. J Allergy Clin Immunol 2014; 133:111–8 e1–13. [DOI] [PubMed] [Google Scholar]
- 15.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000; 162:1403–6. [DOI] [PubMed] [Google Scholar]
- 16.Chang TS, Lemanske RF Jr., Guilbert TW, Gern JE, Coen, Evans MD, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract 2013; 1:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016; 138:1608–18 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Custovic A, Siddiqui S, Saglani S. Considering biomarkers in asthma disease severity. J Allergy Clin Immunol 2022; 149:480–7. [DOI] [PubMed] [Google Scholar]
- 19.Shah SP, Grunwell J, Shih J, Stephenson S, Fitzpatrick AM. Exploring the Utility of Noninvasive Type 2 Inflammatory Markers for Prediction of Severe Asthma Exacerbations in Children and Adolescents. J Allergy Clin Immunol Pract 2019; 7:2624–33 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacharier LB, Maspero JF, Katelaris CH, Fiocchi AG, Gagnon R, de Mir I, et al. Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N Engl J Med 2021; 385:2230–40. [DOI] [PubMed] [Google Scholar]
- 21.Bush A, Fitzpatrick AM, Saglani S, Anderson WC 3rd, Szefler SJ. Difficult-to-Treat Asthma Management in School-Age Children. J Allergy Clin Immunol Pract 2022; 10:359–75. [DOI] [PubMed] [Google Scholar]
- 22.Jensen ME, Mendelson MJ, Desplats E, Zhang X, Platt R, Ducharme FM. Caregiver’s functional status during a young child’s asthma exacerbation: A validated instrument. J Allergy Clin Immunol 2016; 137:782–8 e6. [DOI] [PubMed] [Google Scholar]
- 23.Bui AL, Dieleman JL, Hamavid H, Birger M, Chapin A, Duber HC, et al. Spending on Children’s Personal Health Care in the United States, 1996–2013. JAMA Pediatr 2017; 171:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry R, Braileanu G, Palmer T, Stevens P. The Economic Burden of Pediatric Asthma in the United States: Literature Review of Current Evidence. Pharmacoeconomics 2019; 37:155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF Jr., et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol 2008; 122:1127–35 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF Jr., et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med 2011; 365:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015; 314:2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol 2004; 114:1282–7. [DOI] [PubMed] [Google Scholar]
- 29.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF Jr., Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials 2004; 25:286–310. [DOI] [PubMed] [Google Scholar]
- 30.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005; 99:553–8. [DOI] [PubMed] [Google Scholar]
- 31.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Just J, Saf S, Guiddir T, Cottel N, Amat F, Lambert N, et al. Determinants of blood eosinophilia in moderate and severe asthmatic patients during childhood: Evidence from the severe asthma molecular phenotype (SAMP) cohort. Pediatr Allergy Immunol 2021; 32:1217–25. [DOI] [PubMed] [Google Scholar]
- 33.Robinson PFM, Fontanella S, Ananth S, Martin Alonso A, Cook J, Kaya-de Vries D, et al. Recurrent Severe Preschool Wheeze: From Prespecified Diagnostic Labels to Underlying Endotypes. Am J Respir Crit Care Med 2021; 204:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezmigna D, Brown M, Daines C, Morgan W. Bronchoalveolar lavage profiles in uncontrolled wheezy children compared by asthma predictive index. Pediatr Pulmonol 2022; 57:293–9. [DOI] [PubMed] [Google Scholar]
- 35.Gut G, Armoni Domany K, Sadot E, Soferman R, Fireman E, Sivan Y. Eosinophil cell count in bronchoalveolar lavage fluid in early childhood wheezing: is it predictive of future asthma? J Asthma 2020; 57:366–72. [DOI] [PubMed] [Google Scholar]
- 36.Guiddir T, Saint-Pierre P, Purenne-Denis E, Lambert N, Laoudi Y, Couderc R, et al. Neutrophilic Steroid-Refractory Recurrent Wheeze and Eosinophilic Steroid-Refractory Asthma in Children. J Allergy Clin Immunol Pract 2017; 5:1351–61 e2. [DOI] [PubMed] [Google Scholar]
- 37.Snijders D, Agostini S, Bertuola F, Panizzolo C, Baraldo S, Turato G, et al. Markers of eosinophilic and neutrophilic inflammation in bronchoalveolar lavage of asthmatic and atopic children. Allergy 2010; 65:978–85. [DOI] [PubMed] [Google Scholar]
- 38.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med 2005; 171:722–7. [DOI] [PubMed] [Google Scholar]
- 39.Turato G, Barbato A, Baraldo S, Zanin ME, Bazzan E, Lokar-Oliani K, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am J Respir Crit Care Med 2008; 178:476–82. [DOI] [PubMed] [Google Scholar]
- 40.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007; 176:858–64. [DOI] [PubMed] [Google Scholar]
- 41.Teague WG, Lawrence MG, Williams S, Garrod AS, Froh D, Early SV, et al. Novel Treatment-Refractory Preschool Wheeze Phenotypes Identified by Cluster Analysis of Lung Lavage Constituents. J Allergy Clin Immunol Pract 2021; 9:2792–801 e4. [DOI] [PubMed] [Google Scholar]
- 42.Jackson DJ, Gern JE. Rhinovirus Infections and Their Roles in Asthma: Etiology and Exacerbations. J Allergy Clin Immunol Pract 2022; 10:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Reilly R, Ullmann N, Irving S, Bossley CJ, Sonnappa S, Zhu J, et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age. J Allergy Clin Immunol 2013; 131:1024–32, 32 e1–16. [DOI] [PubMed] [Google Scholar]
- 44.Levina D, Leontjeva M, Abbasova N, Petrova Y, Bitieva R, Erdes SI, et al. Changes in blood eosinophil levels in early childhood and asthma development: A case-control study. Pediatr Allergy Immunol 2022; 33:e13734. [DOI] [PubMed] [Google Scholar]
- 45.Maison N, Omony J, Illi S, Thiele D, Skevaki C, Dittrich AM, et al. T-high asthma phenotypes across life span. Eur Respir J 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014; 11:531–6. [DOI] [PubMed] [Google Scholar]
- 47.Bonato M, Gallo E, Bazzan E, Marson G, Zagolin L, Cosio MG, et al. Air Pollution Relates to Airway Pathology in Children with Wheezing. Ann Am Thorac Soc 2021; 18:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saglani S, Bingham Y, Balfour-Lynn I, Goldring S, Gupta A, Banya W, et al. Blood eosinophils in managing preschool wheeze: Lessons learnt from a proof-of-concept trial. Pediatr Allergy Immunol 2022; 33:e13697. [DOI] [PubMed] [Google Scholar]
- 49.Drouin O, Smyrnova A, Betinjane N, Ducharme FM. Adherence to inhaled corticosteroids prescribed once vs twice daily in children with asthma. Ann Allergy Asthma Immunol 2022; 128:423–31 e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


