Abstract
Recent research into the pathophysiology and treatment of atopic dermatitis (AD) showed notable progress. An increasing number of aspects of the immune system are being implicated in AD, including the epithelial barrier, TH2 cytokines, and mast cells. Major advances in therapeutics were made in biologic cytokine and receptor antagonists and among Janus kinase inhibitors. Herein, we focus on these areas and address new insights into AD epidemiology, biomarkers, endotypes, prevention, and comorbidities. Going forward, we expect future mechanistic insights and therapeutic advances to broaden physicians’ ability to diagnose and manage AD patients, and perhaps find a cure for this chronic condition.
Keywords: atopic dermatitis, TH2, epithelial barrier, janus kinase inhibitors
Introduction
Progress in our understanding of AD has advanced rapidly in the last year. This includes a deeper understanding of the epidemiology, treatment, genetics, and pathophysiology of AD. In particular, advances in understanding the mechanistic role of cytokine mediators, innate immunity, the epithelial barrier in AD pathogenesis will be emphasized. Furthermore, a wealth of clinical trial and other data on current and novel treatments for AD will be reviewed as well. Altogether, these advances will increase our ability to diagnose and treat patients with AD and provide hope for an eventual cure.
Epidemiology
The Global Burden of Disease Study estimated that the general prevalence of AD was 15-20% among children and up to 10% among adults (1). Although traditionally thought to have a lower prevalence in Africa compared to Europe, more recent studies have demonstrated AD occurring at increased frequency in children, as high as 31% in Ghana, which may possibly be connected to increased urbanization (2, 3). Population studies of AD in sub-Saharan Africa have also highlighted the increasing prevalence in this region, with over 15% of children affected (2). Given the limited resources in these regions, there is a need for additional cooperation with pharmaceutical companies to improve management in these areas (2). AD outcomes may also be influenced by structural racism, defined by Martinez et al. as “the totality of ways in which society fosters discrimination by creating and reinforcing inequitable systems through intentional policies and practices sanctioned by government and institutions.” For example, proximal pathways of structural racism include pollutant and other environmental hazard exposure, which may lead to “biologic embedding” through reinforcement of aberrant innate and adaptive immune responses (4, 5). What this construct emphasizes is the means by which generational exposure differences can reinforce aberrant immune responses, such as AD, through epigenetic and other mechanisms. This helps to emphasize how social and public policy decisions can have unintended adverse consequences on differential disease manifestations in populations long after such decisions are made. Biagini et al. evaluated phenotypic differences between White and Black children experiencing the atopic march. They found that Black children exhibit higher asthma risk despite having a more intact skin barrier as demonstrated by lower lesional and nonlesional transepidermal water loss and higher non-lesional FLG expression (6). In response to these issues, the American Academy of Allergy, Asthma & Immunology Committee on the Underserved provided a report highlighting the need for a multilevel approach across patients, healthcare providers, government, non-profits, and professional societies to better address disparities in atopy (7). Within AD, this report emphasized the lack of studies addressing disparities specifically within AD and suggests that effective intervential trials in AD need to improve reporting of and accounting for race and ethnicity in their results.
Environmental Pollutants
Recent work provided additional data on the role of pollutants in AD. In a cross-sectional study of 209,168 individuals from 2008–2013 from the Republic of Korea that examined the long-term average concentration of air pollutants before the diagnosis of AD, the authors identified significant associations between AD incidence and exposure to particulate matter (<2.5μm and <10μm in diameter), sulfur dioxide, nitrogen dioxide, and carbon monoxide. These associations held after adjusting for age, sex, income, comorbidities, and meteorologic variables (8). In another study from Tasmania, increasing nitrogen dioxide was associated with increased AD rates in males (9). These reports expand on the literature demonstrating the contributions of the anthropogenic environment to AD.
Clinical classification
The clinical classification of AD was recently thoroughly reviewed (10), providing detailed images of the clinical heterogeneity of eczematous lesions, as well as an overview of the clinical aspects of AD; this may be of value to clinicians seeking images of AD. We focus here on AD endotypes and biomarkers, as these were areas of notable progress.
Endotypes and Biomarkers:
Defining novel endotypes and biomarkers is expected to play a major role in AD diagnosis and treatment. However, multiple studies illustrated the profound immune complexity associated with such AD classifications. Two studies from Bakker et al. reported unbiased, principal components analyses of human AD serum cytokine profiles. The first study evaluated pediatric AD, identifying four clusters: TH2 cell/retinol-dominant, skin-homingdominant, TH1 cell/TH2 cell/TH17 cell/IL-1-dominant, and TH1 cell/IL-1/eosinophil-inferior. Disease severity was associated with a skin-homing dominant group (11). Only one of these clusters resembled a previously reported adult endotype. The second study identified four severe AD disease clusters: skin-homing chemokines/IL-1R1-dominant, TH1/TH2/TH17-dominant, TH2/TH22/PARC-dominant, and TH2/eosinophil-inferior (12). A third study evaluated humans across the lifespan using skin biopsies of lesional and non-lesional skin, demonstrating a shared TH2/TH22-signature that differs depending on the age of the patients, with greater TH17 skewing in infants and adults (13). A fourth study used a transcriptomic analysis of lesional and non-lesional skin biopsies to define another group of four clusters, similar to the aforementioned studies (14). While all these studies produce somewhat similar clustering results, we note that adult and pediatric AD as well as severe AD each demonstrate substantial immune heterogeneity when classified in this manner, and it is difficult to derive a specific classification system at this time.
While the complexity of these classifications renders the practical deployment of such unbiased approaches difficult, other studies evaluated hypothesis-driven or single-marker approaches to explain facets of AD. A study of 248 participants with varied AD severity showed that serum sphingosine-1-phosphate is elevated in AD and associated with severity (15). Another study evaluating children across the lifespan found that specific features, including SCORAD (SCORing Atopic Dermatitis) severity index at age three, trigger factors, and low vascular endothelial growth factor serum levels correlated with AD persistence (16). A third publication demonstrated that RNA-seq profiles from tape stripping of atopic skin in children correlated with clinical features and transepidermal water loss (TEWL). This suggests that non-invasive tape stripping might be a suitable alternative to biopsies for pediatric research in AD (17). Atopic dermatitis-specific lipid alterations have also been noted by tape stripping analysis, further supporting this sampling method (18). Lastly, one review evaluated the role of primary atopic disorders to describe how heritable monogenic allergic disorders can be detected using next-generation sequencing. The authors emphasized that in patients with red flags (e.g., short stature, infections, markedly elevated IgE), early onset of disease, or treatment refractoriness, sequencing can be considered for facilitated diagnosis (19).
Pathophysiology
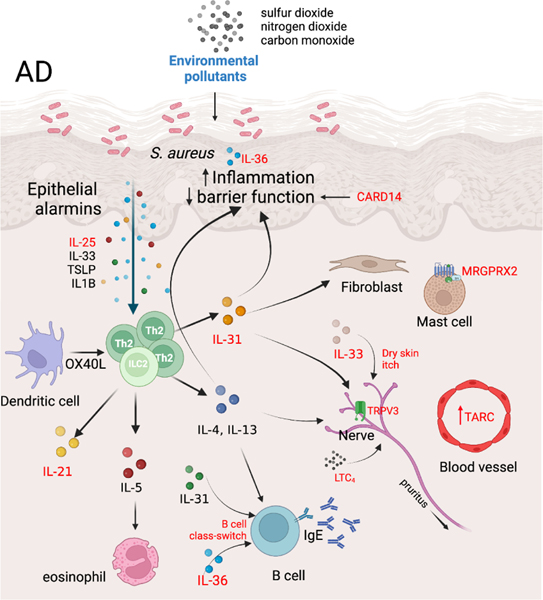
Recent advances provided multiple additions to our understanding of AD pathophysiology, categorized here by mediators, cell types, and the epithelial barrier (Figure 1).
Figure 1. Novel insights and additions to AD pathogenesis.
This figure highlights novel insights and additions to AD pathogenesis (shown in red), and environmental contribution (blue), and their relevance to key AD associated features including TH2 immune responses, epidermal inflammation and barrier function, mast cell activation, and pruritus. LTC4, leukotriene C4; MRGPRX2, Mas-related G protein-coupled receptor-X2; TARC, thymus and activation-regulated chemokine; TRPV3, transient receptor potential vanilloid subfamily V member 1; TSLP, thymic stromal lymphopoietin.
Mediator data
While the roles of IL-4 and IL-13 are well characterized in AD, novel insights were reported on their role in AD pathogenesis. One study that included both human samples and mechanistic modelling demonstrated that IL-4 and IL-13 modulate abnormalities in sex steroid hormone synthesis via 3β-hydroxysteroid dehydrogenase 1, and may partly underlie the disrupted AD skin barrier (20). Another study used AD patient peripheral eosinophils to demonstrate how IL-4 upregulates, via the JAK/STAT pathway, expression and function of the histamine receptor 4, which is highly expressed on such eosinophils (21). These studies notably connect type 2 cytokines to structural and pathologic aspects of AD and help explain how immune signaling promotes disease outcomes.
Multiple studies evaluated the role of other cytokines in AD. One manuscript used CyTOF to study T cell polarization in the blood of AD patients versus controls, finding a correlation of IL-21 expression in IL-13+ T cells with AD severity, thus implying a role for IL-21 in AD (22). IL-25 (IL-17E), an IL-17 family member, has received attention as an epithelial barrier damage “alarmin.” The role of IL-25 in epithelial immunology was outlined, emphasizing its role as a key driver of type 2 innate lymphoid cell (ILC2) and TH2 production of cytokines, including IL-4 and IL-13 (23).
IL-31, a cytokine in the gp130/IL-6 family, is a pruritogen previously implicated in AD itch; one group performed a drug screen in multiple cell lines for IL-31 transcriptional inhibitors and demonstrated a role for 4-(2-(4-isopropylbenzylidene)hydrazineyl)benzoic acid in antagonizing IL-31 production and function in itch in AD (24). Another manuscript evaluated TRP vanilloid channel 3 (TRPV3), an epidermal-keratinocyte localized cation channel warm temperature detector. TRPV3 was noted to be upregulated in lesional AD skin and to mediate IL-31-induced itching in a serpin E1-dependent manner (25).
Several studies increased our understanding of the role of the extended IL-1 family of cytokines including IL-33, IL-36, and IL-37 in AD pathogenesis. Trier et al. demonstrated that IL-33 receptor expression on sensory neurons is not required for AD pruritus but is required for dry skin itch using human plasma samples and a mouse model (26). Kindi et al. used human keratinocytes in culture and human skin explants to show that the S. aureus virulence factor second immunoglobulin-binding protein is essential for inducing IL-33 and downstream type 2 cytokine responses (27). Keratinocyte-derived IL-36 appears to provide a key mechanism for IgE development and B cell class switching in AD (28). IL-37 and its receptor (IL-18R) expression is reduced in AD, suggesting that IL-37 may play a regulatory role (29).
Leukotriene C4 was shown to be a potent itch inducer acting through its physiological receptor CysLT2R in sensory neurons in a model of dermatitis (30). Lastly, one study showed that peripheral blood CCL17 (thymus- and activation-regulated chemokine) elevation was associated with presence of AD, though sensitivity and specificity were not adequate to provide diagnostic conclusions with high reliability (31).
Cellular data
Mast cells and basophils
Mast cells and basophils play a major role in atopy, including AD. Antagonism between TH1 and TH2 immune processes mediated by mast cells was recently described by Levya-Castillo et al. The study demonstrated how a TH2 cytokine, IL-13, produced by cutaneous mast cells in response to tape stripping, directly inhibits dendritic cell expression of IL-12, in turn decreasing IFN-γ release from CD4+ T cells (32, 33). Another study provided evidence that a microRNA, miR103a-3p, contained in mast cell-derived extracellular vesicles, may play a role in AD by promoting innate lymphoid cell (ILC) 2 upregulation of IL-5, especially in the presence of IL-33 (34). Basophil biology received further attention, with one study using a mouse AD model to show that basophils supply a substantial amount of IL-4 in the skin during AD to induce barrier dysfunction (35).
The role of ILCs is increasingly recognized in AD. Evidence for ILC lineage changes (i.e., between ILC1, ILC2, and ILC3/17) or “infidelity” has increased (36). Alkon et al. used single cell RNA sequencing to demonstrate that in AD ILCs commonly co-express both type 2 (GATA3, IL13) and type 3/17 (RORC, IL22, IL26) genes within the same cell, particularly in AD lesional skin (37). This lineage plasticity perhaps relates to the protean immune profiles of AD noted in “Endotypes” above and cautions against reductionist approaches to simpler classifications.
Innate immunity
As our understanding of innate immunity’s role as arbiter of immune responses continues to grow, multiple articles described insights into the role of innate immune responses and AD. CXCR4+ natural killer T cells (NKT cells), seen as a bridge between innate and adaptive immunity, were shown to be enriched in AD skin, facilitating a tissue-resident status contributing to lesional pathogenesis (38). Another study used human skin transcriptomics to suggest that natural killer cells (vs. NKT cells), are enriched and dysfunctional in both non-lesional and lesional AD skin (39). Mucosal associated invariant T (MAIT) cells, another T cell at the innate/adaptive interface, were implicated in AD pathogenesis, and ablation of non-polymorphic MHC class I related-1 molecule (MR1, a critical receptor for antigen presentation to MAIT cells) prevented AD development in the MC903 mouse model of AD (40). Notably, phototherapy appeared to model this effect of the MR1 receptor, likely through photodegradation of folate into inhibitory MR1 ligands (40). These studies strongly link innate/adaptive overlap T and NK cell types to key roles in AD.
Another study addressed the role of Langerhans cells (LCs) and follicular helper T cells (TFH) in AD pathogenesis using two AD mouse models, one driven by thymic stromal lymphopoietin (TSLP) overproduction and the other by ovalbumin (OVA) sensitization. This led to the observation that LCs drive TFH responses when stimulated directly by TSLP, but that LCs inhibit TFH responses during OVA sensitization (41).
A recent article profiled transcriptomes of developing human fetal skin, healthy adult skin, and adult skin with AD and psoriasis (42). This study noted in situ reemergence of prenatal vascular endothelial cell and macrophage cellular programs in AD and psoriasis lesional skin (42). Another study used peripheral blood from AD patients and controls to identify a circular RNA, hsa_circ_0004287, which inhibited macrophage activation in AD and psoriasis in a subsequent mouse model. The authors posited this as a therapeutic target for AD (43).
More data became available to demonstrate the role of JAK signaling in AD. One study demonstrated that inflammatory dendritic epidermal cell differentiation and function is impaired by JAK inhibition, further rationalizing the utility of JAK inhibitors in AD (44).
Finally, a study suggested a proinflammatory role for p62 (sequestrome 1), a multifunctional adaptor protein target of rapamycin (45). With the prior NK and T cell studies, these works emphasize the role of purely innate cells, such as dendritic cells and monocyte-derived cells, in AD pathogenesis.
Epithelial barrier
The skin epithelial barrier serves as the visible canvas for AD, and interest in the role of barrier function and its related phenomena figured prominently in recent work. This was especially in relation to research gaps in AD genetic susceptibility, epigenetic responses to microbiota, and how barrier restoration and microbiota manipulations affect AD (46). One study addressed the role of genome wide association study (GWAS)-derived single-nucleotide polymorphisms (SNPs) on skin barrier pathology using targeted chromosome conformation capture. This study showed that many SNPs identified by GWAS may unexpectedly affect distal genes, as only 35% of target genes were the nearest gene to known GWAS variants (47). Using a GWAS-based approach with followon mechanistic work, DeVore et al. defined Caspase Recruitment Domain Family Member 14 (CARD14) as a regulator of FLG expression in the skin of children with AD and further showed that CARD14 regulates FLG homeostasis in a manner dependent upon rs11652075 (48), a variant in the CARD14 gene that intriguingly is also associated with psoriasis (49).
In a study evaluating the systemic proteomic differences between mild and moderate/severe AD, He et al. noted that mild AD showed high levels of TH2/TH22 cell activation localized to the skin and lacked the systemic inflammation of moderate/severe AD (50). The same group used RNA-sequencing to show that tape stripping is capable of differentiating among AD, psoriasis, and normal skin (51). Another study suggested that tape stripping demonstrates the ability to detect TH2 skin response in AD and correlates with severity of AD better than skin biopsy (52). Skin-based impedance spectroscopy may offer a future in vivo technique for assessing epithelial barrier component integrity, including for claudin 1 and 4 dysfunction (53). This may provide a noninvasive measure of therapeutic effect in AD (54). Another study used RNA sequencing to evaluate existing biopsy specimens from trials of cyclosporine and dupilumab to demonstrate a “core” AD RNA signature involving itch and keratinocyte dysfunction, along with a “dynamic” signature dominated by type 2 cytokines responsive to both therapies (55).
Atopic itch, which is generated in part at the epithelial barrier, has long been known as both a symptom and enhancer of AD. Self-DNA liberated by barrier disruption, presumably due to physical disruption from scratching, was shown to interfere with antimicrobial peptides (56). In another study, overexpression of PAR2, which acts as a sensor for proteolytic enzymes, enhanced house dust mite-induced itch in a mouse model (57). Coupled with the IL-31 and IL-33 data above(26), our understanding of the pathogenic role of pruritus in AD continues to grow.
Microbiome & Metabolism
Related to barrier defects, microbiota alteration has been observed in multiple AD studies (46). The most prominent example is S. aureus, which attaches to the stratum corneum by binding to the N-terminal region of corneodesmosin (58), and also produces biofilms that are significantly associated with AD severity (59). S. aureus can also interact with the host immune system and affected tissue by inducing pro-inflammatory responses from TH2 cells (60), or working with TH2 and its cytokines to induce allergic inflammation and phenotypes (61).
Besides S. aureus, S. epidermidis also acts similarly to affect AD, and recent work highlighted that its cysteine protease EcpA can degrade desmoglein-1 and LL-37 and disrupt the skin barrier. Expression of these molecules is associated with disease severity (62). Therefore, the distributions and expression of different skin microbial species can serve as signatures to stratify the AD patients with different phenotypes, degree of severity, and treatment response, due to microbial networking with the host immune system (63).
Microbiota in other tissues can also play role in AD. A study investigating the microbiome of airway and gut showed that infancy in urban area increases risk of asthma, AD, and allergic sensitization at a later age, suggesting a predisposed microbial composition (64). Another study of school-age children found a less consistent association between microbial diversity in stool with atopic diseases; association of α-diversity with risk of eczema was non-significant (65). Bacteriotherapy efforts have attempted to use S. hominis A9 as topical therapy for AD. Though disease severity was not improved, S. aureus composition was significantly decreased (66). However, the immune system and microbiome interactions are complicated, particularly given microbiome variability across skin sites (67). These studies highlight the importance of gathering samples from diverse backgrounds and the necessity of joint efforts spanning microbiology and systems biology to study the causal relationships and disease mechanisms of the microbiome in AD.
Genetics
AD is a complex condition, and previous GWAS have highlighted multiple disease susceptibility regions (68). Recent studies have focused on using existing resources to advance our understanding of the AD genetic architecture as well as unravel disease-causing variants. One study used biobank resources to successfully identify 30 genome-wide significant regions, including 5 novel loci. This work highlights the feasibility of deploying different emerging biobank datasets in future genetic AD studies (69). Differences in the linkage disequilibrium between ethnic groups can enhance resolution in fine-mapping causal variants. A trans-ethnic meta-analysis using Caucasian and Japanese cohorts was able to identify putative causal variants: a missense variant (R243W) with a deleterious effect in NLRP10 and a variant altering expression of CCDC80 (70). Information from chromatin interaction has also been used to infer the mechanism for AD-associated signals, highlighting the need to integrate multi-omic information in understanding their biological effects (71). AD genetic studies also facilitate efforts for other sub-phenotypes or related traits, including the identification and understanding of genetic signals associated with eczema herpeticum (72) and total IgE level (73). One important translational implication for AD GWAS is to use polygenic risk scores to model the disease risk. One study showed that genetic scores derived from GWAS are predictive of AD with up to an odds ratio (OR) of 3.86 for severe disease (74). Another study demonstrated the importance in using a cohort with similar demographics as the target population in model training, achieving an area under the receiver operating characteristic curve (AUROC) of 0.88 when predicting moderate-to-severe AD (75).
Prevention
Work in AD prevention has continued apace. There is a longstanding interest in whether prophylactic skin barrier enhancement via emollient application prevents AD. A large systematic review and meta-analysis of prophylactic emollients within 6 weeks of birth reported a benefit only in those at high risk for AD when emollients were used continuously (76). A Cochrane review concluded that “emollients during the first year of life in healthy infants are probably not effective for preventing eczema”(77). This conclusion was supported by the PreventADALL study, a cluster-randomized trial of 2,400 infants, which evaluated whether either early food introduction or prophylactic emollients could prevent food allergy or AD among the general population. While early feeding reduced the risk of food allergy, early feeding did not impact AD development, and emollient prophylaxis showed no significant effect on food allergy or AD (78). However, one study of maternal diet during pregnancy did find that offspring of mothers with greater yogurt and/or vegetable intake displayed reduced odds of AD, although causation still has yet to be established (79). Thus, while emollient use may not provide definitive AD prevention, other avenues, including immune metabolic regulation remain open for investigation.
Novel treatments
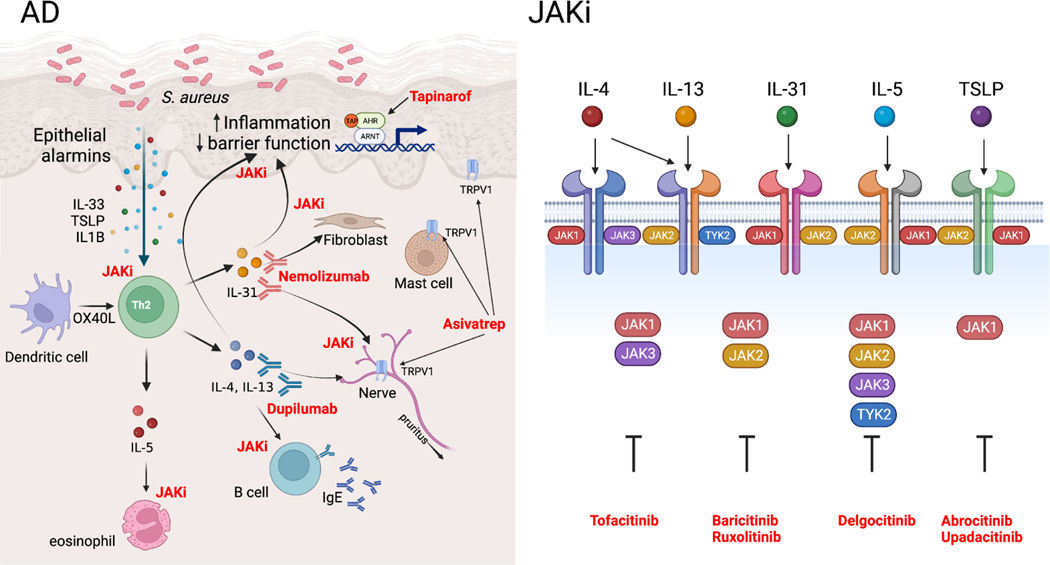
Progress on novel treatments also continued in the past year (Figure 2, Table 1). Tapinarof, a topical therapeutic aryl hydrocarbon receptor modulating agent, was evaluated in a phase 2b, double-blind, randomized, vehicle-controlled study of adolescents and adults with AD. Participants received tapinarof cream or vehicle for 12 weeks, which resulted in improvements in Eczema Area and Severity Index (EASI) and Patient-Oriented Eczema Measure (POEM) (80), corroborating results from a 2019 study (81).
Figure 2. Novel therapeutic advances in AD.
Left panel, Intersection between therapeutic development and atopic dermatitis (AD) pathogenesis with therapeutic agents shown in red next to their biologic mechanisms of action. Right panel, Janus kinase (JAK) family members dimerize to mediate different cytokine responses. JAK inhibitors (JAKi) inhibit multiple aspects of AD pathogenesis through targeting of select JAK family members (right panel modified from Chovatiya et. al. (93)).
Table 1:
Novel immunomodulators for AD
| Name | Route of delivery | Mechanism of action | Specific target |
|---|---|---|---|
| Dupilumab | Injection | Cytokine antagonist | IL-4Rα |
| Nemolizumab | Injection | Cytokine antagonist | IL-31 |
| Secukinumab | Injection | Cytokine antagonist | IL-17A |
| Asivatrep | Topical | Cation channel antagonist | TRPV1 |
| Tapinarof | Topical | Aryl hydrocarbon receptor modulator | |
| Abrocitinib | Oral | JAKi | JAK1 |
| Baricitinib | Oral | JAKi | JAK1, JAK2 |
| Delgocitinib | Topical | JAKi | JAK1, JAK2, JAK3, TYK2 |
| Ruxolitinib | Topical | JAKi | JAK1, JAK2 |
| Tofacitinib | Oral | JAKi | JAK1, JAK3 |
| Udapacitinib | Oral | JAKi | JAK1 |
A phase 3 trial of asivatrep, a transient receptor potential vanilloid subfamily V member 1 (TRPV1) antagonist, reported global improvements in AD symptomatology at 8 weeks among participants on twice-daily 1.0% application of drug compared to placebo (82). TRPV1 is a nonselective cation channel expressed in keratinocytes, mast cells, and cutaneous sensory nerves.
Nemolizumab, a monoclonal antibody targeting the IL-31 receptor, also underwent clinical trials. In a post hoc analysis of a phase 2b trial of moderate-to-severe AD among participants with EASI ≥ 16 at baseline, nemolizumab therapy resulted in improvements in inflammation, pruritus, and sleep (83). A 24-week, double-blind, multicenter nemolizumab dose-finding study in which participants were randomized to placebo, 10, 30, or 90 mg subcutaneous monthly injections showed similar improvements, with a maximal dose effect at 30 mg (84). A 16-week, double-blind, phase 3 trial reported that nemolizumab treatment in AD induced a greater reduction in pruritus than placebo plus topical agents (85). Lastly, a meta-analysis of 14 cohorts among 6 randomized/controlled studies of nemolizumab in AD showed “a promising effect based on the difference in the average change in pruritus visual analog score and EASI versus placebo”(86).
A prospective, uncontrolled, multicenter cohort examining dupilumab, an IL-4 receptor inhibitor, showed a 70% decrease in EASI at 16 weeks and a 76.6% decrease at 52 weeks (87). A meta-analysis identified dupilumab as having the highest quality trial evidence at 1 year in adults for all treatments in all AD populations (88). Indeed, when dupilumab is ineffective, there is limited data on the optimal next step of therapy (89). One study reported a real-world 6-month observation of dupilumab, where dupilumab showed elevated rates of conjunctivitis over mycophenolate, methotrexate, or cyclosporine, but was not associated with increased infections (90). An intriguing study of longitudinal immune responses to dupilumab suggested that not just TH2 but also TH17 responses correlate with EASI, suggesting that non-TH2 immune responses (such as TH17) play a role in AD pathophysiology and dupilumab responses (91).
Secukinumab is a monoclonal antibody that binds IL-17A. In a phase 2, randomized, double-blinded trial, secukinumab did not improve AD among 41 subjects (92). While the role of TH17 signaling in AD is of interest, deployment of TH17 antagonism in AD has not been successful.
Janus kinase (JAK) inhibitors (JAKi) hold promise for AD treatment (93). Upadacitinib is an oral JAK1-selective JAKi with a rapid onset of action (94). The AD Up study reported favorable results at both 16 weeks (95) and 52 weeks (96) for this medication in combination with topical corticosteroids. Similar results were reported from the Measure Up 1 and 2 studies (97). Abrocitinib is another oral JAK1-selective JAKi. One phase 3, double-blinded trial compared abrocitinib to placebo or dupilumab. Abrocitinib was overall comparable to dupilumab but more effectively reduced itch (98). In another study, abrocitinib responders were assigned to blinded continuation vs. discontinuation. Those with flares were effectively rescued with the drug, suggesting that therapy holidays are feasible (99). The third JAKi currently in clinical trials for AD, baricitinib, is an oral JAK1/2 inhibitor. In a phase 3 trial of adults with moderate-to-severe AD who responded inadequately or were intolerant to topical therapy, baricitinib improved EASI vs. placebo at 16 weeks of therapy (100). Delgocitinib is a topical pan-JAKi (inhibiting all four members of the JAK family: JAK1, JAK2, JAK3 and TYK2) clinically approved for AD in Japan (101). One delgocitinib study of pediatric patients reported substantial improvements in EASI versus vehicle at 4 weeks that were maintained with continued application at one year during a study extension (102). Ruxolitinib cream is a topical JAK1/JAK2 JAKi. Two phase 3 trials reported 8-week efficacy for ruxolitinib in Investigator’s Global Assessment (IGA) and itch reduction vs. vehicle (103).
Comorbidities
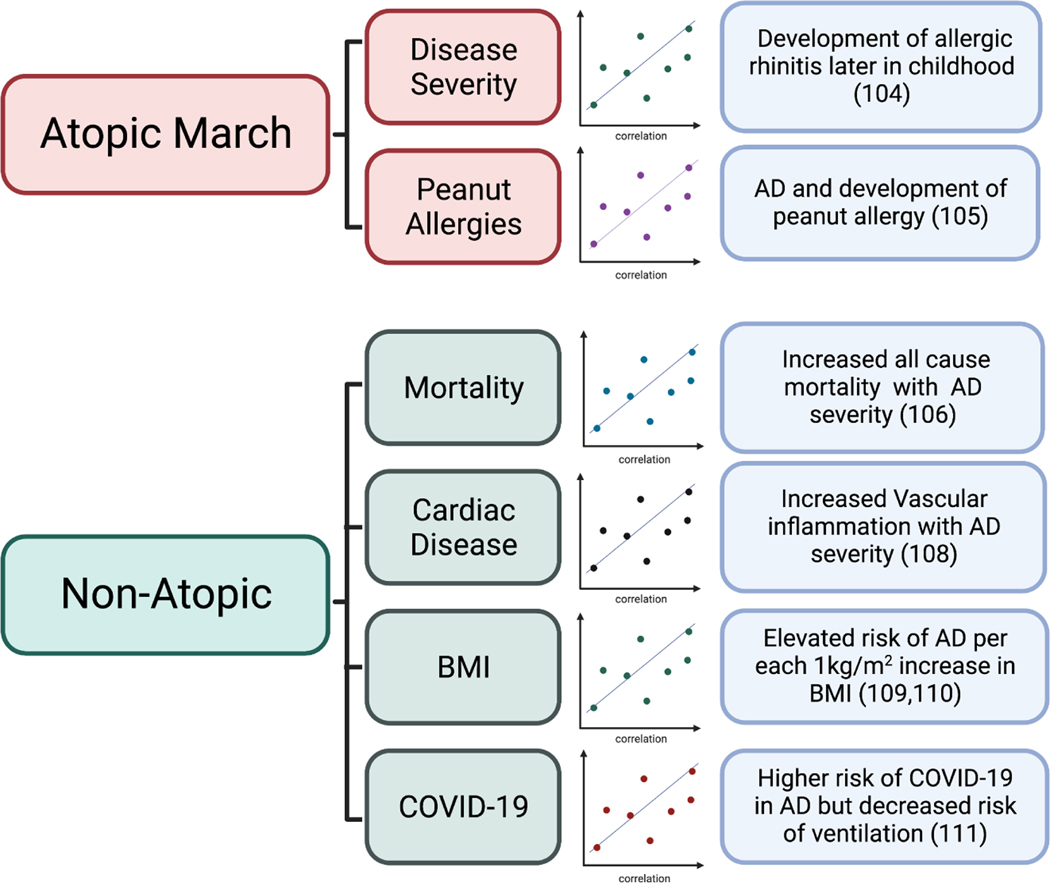
AD is the first step of the atopic march development of food allergies, allergic rhinitis, and asthma. Increasing severity of early-onset AD, but not late-onset, is associated with aeroallergen sensitization and allergic rhinitis later in childhood (104). In a study of 321 infants at risk for peanut allergy, AD was strongly associated with peanut allergy development, whereas other factors such as family history were not a major risk factor without concomitant AD (105). A visual summary of these and other AD comorbidities can be found in Figure 3.
Figure 3. Novel Insights into AD Comorbidities.
Highlighted comorbidities associated with “Atopic March” or “Non-Atopic” aspects of AD pathogenesis. This includes association of AD disease severity with development of allergic rhinitis and association between AD and development of peanut allergy. Non-Atopic associated comorbidities include association between AD and all-cause mortality, AD associated vascular inflammation, influence of body mass index (BMI) with AD risk, and COVID-19.
Despite the substantial population prevalence of AD, previously unrecognized comorbidities continue to be identified. In a 3-million-person study from the United Kingdom from 1998–2016, AD was linked with significantly elevated all-cause mortality (hazard ratio 1.04), which was higher for infectious, digestive, and genitourinary causes of death. Furthermore, individuals with severe AD had a hazard ratio of 1.62 vs. those without AD (106). In another study of adults with AD using insurance claims data, the authors noted increased odds of anxiety, autoimmune diseases, infections, malignancies, atherosclerosis, and metabolic syndrome in AD patients (107). While such studies cannot establish causation, these observations may help explain why increased mortality is associated with AD (106). Indeed, cardiac disease has been linked to AD, as vascular inflammation in AD is associated with enhanced TH2 responses and clinical severity, which may explain the cardiovascular comorbidities observed in AD populations (108).
Elevated body mass index (BMI) is also associated with AD. Using 30,608 cases and 389,849 controls in the UK Biobank Resource, one group demonstrated a small but significant OR of 1.02 elevated risk of AD per each 1kg/m2 increase in BMI (109). Children with elevated BMI in early childhood were noted in another study to have a higher rate of subsequent AD later in childhood (110).
In a year defined by COVID-19, one meta-analysis analyzed AD and COVID-19, finding that skin conditions such as AD were associated with a greater risk of COVID-19 (OR 1.55) but a decreased risk of mechanical ventilation (OR 0.22) (111). Together, the studies above together suggest a complex, possibly bidirectional association between mortality-associated conditions and AD that requires more evaluation.
Conclusions
In summary, progress in AD research remains strong, and with increasing data on epidemiology, pathophysiology, and therapy, we are poised to further combat this challenging condition. Ongoing areas of investigation include improvements in prevention, approaches to deconvolute the immune and genetic complexity of AD, and methods to mitigate comorbid conditions. In the future, the areas of greatest need include those areas listed in Table 2. Future work will continue to address these gaps in the field even as new areas of need become better understood.
Table 2:
Areas of need for future work
| Area | Specific improvement | Population in need |
|---|---|---|
| Epidemiology | Inclusion of disadvantaged groups in clinical trials and other clinical studies. | Disadvantaged groups |
| Endotypes | Simple definitions for endotyping accessible to all institutions. | All patients |
| Prevention | Low-impact interventions to prevent AD in all risk groups, especially from infancy. | Primarily children |
| Comorbidity | Improved understanding of the mechanisms of comorbidity development to facilitate mitigation. | All patients |
Funding and conflict of interest:
CS receives support from the Gerber Foundation and from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI162661. JEG is supported by the Taubman Medical Research Institute, and NIH grants P30AR075043. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AD
atopic dermatitis
- TH
T helper cell
- IL
interleukin
- TEWL
transepidermal water loss
- Ig
immunoglobulin
- SCORAD
SCORing Atopic Dermatitis
- ILC
innate lymphoid cell
- NK T cell
natural killer T cell
- MR1
MHC class I related-1 molecule
- TRPV3
TRP vanilloid channel 3
- MAIT
mucosal associated invariant T
- GWAS
genome wide association study
- TSLP
thymic stromal lymphopoietin
- OVA
ovalbumin
- AUROC
area under the receiver operating characteristic curve
- EASI
Eczema Area and Severity Index
- POEM
Patient-Oriented Eczema Measure
- TRPV1
transient receptor potential vanilloid subfamily V member 1
- JAKi
Janus kinase (JAK) inhibitors
- BMI
body mass index
- IGA
investigator’s global assessment
Footnotes
The rest of the authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–9. [DOI] [PubMed] [Google Scholar]
- 2.Skevaki C, Ngocho JS, Amour C, Schmid-Grendelmeier P, Mmbaga BT, Renz H. Epidemiology and management of asthma and atopic dermatitis in Sub-Saharan Africa. J Allergy Clin Immunol. 2021;148(6):1378–86. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum BE, Klein R, Hagan PG, Seadey MY, Quarcoo NL, Hoffmann R, et al. Dermatology in Ghana: a retrospective review of skin disease at the Korle Bu Teaching Hospital Dermatology Clinic. Pan Afr Med J. 2017;26:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez A, de la Rosa R, Mujahid M, Thakur N. Structural racism and its pathways to asthma and atopic dermatitis. J Allergy Clin Immunol. 2021;148(5):1112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croce EA, Levy ML, Adamson AS, Matsui EC. Reframing racial and ethnic disparities in atopic dermatitis in Black and Latinx populations. J Allergy Clin Immunol. 2021;148(5):1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biagini JM, Kroner JW, Baatyrbek Kyzy A, Gonzales A, He H, Stevens M, et al. Longitudinal atopic dermatitis endotypes: An atopic march paradigm that includes Black children. J Allergy Clin Immunol. 2022;149(5):1702–10.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis CM, Apter AJ, Casillas A, Foggs MB, Louisias M, Morris EC, et al. Health disparities in allergic and immunologic conditions in racial and ethnic underserved populations: A Work Group Report of the AAAAI Committee on the Underserved. J Allergy Clin Immunol. 2021;147(5):1579–93. [DOI] [PubMed] [Google Scholar]
- 8.Park SK, Kim JS, Seo HM. Exposure to air pollution and incidence of atopic dermatitis in the general population: A national population-based retrospective cohort study. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 9.Lopez DJ, Lodge CJ, Bui DS, Waidyatillake NT, Su JC, Perret JL, et al. Association between ambient air pollution and development and persistence of atopic and non-atopic eczema in a cohort of adults. Allergy. 2021;76(8):2524–34. [DOI] [PubMed] [Google Scholar]
- 10.Ständer S Atopic Dermatitis. N Engl J Med. 2021;384(12):1136–43. [DOI] [PubMed] [Google Scholar]
- 11.Bakker DS, de Graaf M, Nierkens S, Delemarre EM, Knol E, van Wijk F, et al. Unraveling heterogeneity in pediatric atopic dermatitis: Identification of serum biomarker based patient clusters. J Allergy Clin Immunol. 2022;149(1):125–34. [DOI] [PubMed] [Google Scholar]
- 12.Bakker DS, Nierkens S, Knol EF, Giovannone B, Delemarre EM, van der Schaft J, et al. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J Allergy Clin Immunol. 2021;147(1):189–98. [DOI] [PubMed] [Google Scholar]
- 13.Renert-Yuval Y, Del Duca E, Pavel AB, Fang M, Lefferdink R, Wu J, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol. 2021;148(1):148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefèvre-Utile A, Saichi M, Oláh P, Delord M, Homey B, Soumelis V. Transcriptome-based identification of novel endotypes in adult atopic dermatitis. Allergy. 2022;77(5):1486–98. [DOI] [PubMed] [Google Scholar]
- 15.Sakai T, Herrmann N, Maintz L, Nümm TJ, Welchowski T, Claus RA, et al. Serum sphingosine-1-phosphate is elevated in atopic dermatitis and associated with severity. Allergy. 2021;76(8):2592–5. [DOI] [PubMed] [Google Scholar]
- 16.Lauffer F, Baghin V, Standl M, Stark SP, Jargosch M, Wehrle J, et al. Predicting persistence of atopic dermatitis in children using clinical attributes and serum proteins. Allergy. 2021;76(4):1158–72. [DOI] [PubMed] [Google Scholar]
- 17.Pavel AB, Renert-Yuval Y, Wu J, Del Duca E, Diaz A, Lefferdink R, et al. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy. 2021;76(1):314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merleev AA, Le ST, Alexanian C, Toussi A, Xie Y, Marusina AI, et al. Biogeographic and disease-specific alterations in epidermal lipid composition and single-cell analysis of acral keratinocytes. JCI Insight. 2022;7(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaseghi-Shanjani M, Smith KL, Sara RJ, Modi BP, Branch A, Sharma M, et al. Inborn errors of immunity manifesting as atopic disorders. J Allergy Clin Immunol. 2021;148(5):1130–9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Chinnappan M, Prestwood CA, Edwards M, Artami M, Thompson BM, et al. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc Natl Acad Sci U S A. 2021;118(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaper-Gerhardt K, Köther B, Wolff L, Kabatas A, Gehring M, Nikolouli E, et al. The H(4) R is highly expressed on eosinophils from AD patients and IL-4 upregulates expression and function via the JAK/STAT pathway. Allergy. 2021;76(4):1261–4. [DOI] [PubMed] [Google Scholar]
- 22.Czarnowicki T, Kim HJ, Villani AP, Glickman J, Duca ED, Han J, et al. High-dimensional analysis defines multicytokine T-cell subsets and supports a role for IL-21 in atopic dermatitis. Allergy. 2021;76(10):3080–93. [DOI] [PubMed] [Google Scholar]
- 23.Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40–52. [DOI] [PubMed] [Google Scholar]
- 24.Kamikaseda Y, Uruno T, Kunimura K, Harada A, Saiki K, Oisaki K, et al. Targeted inhibition of EPAS1-driven IL-31 production by a small-molecule compound. J Allergy Clin Immunol. 2021;148(2):633–8. [DOI] [PubMed] [Google Scholar]
- 25.Larkin C, Chen W, Szabó IL, Shan C, Dajnoki Z, Szegedi A, et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J Allergy Clin Immunol. 2021;147(3):1110–4.e5. [DOI] [PubMed] [Google Scholar]
- 26.Trier AM, Mack MR, Fredman A, Tamari M, Ver Heul AM, Zhao Y, et al. IL-33 signaling in sensory neurons promotes dry skin itch. J Allergy Clin Immunol. 2022;149(4):1473–80.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Kindi A, Williams H, Matsuda K, Alkahtani AM, Saville C, Bennett H, et al. Staphylococcus aureus second immunoglobulin-binding protein drives atopic dermatitis via IL-33. J Allergy Clin Immunol. 2021;147(4):1354–68.e3. [DOI] [PubMed] [Google Scholar]
- 28.Patrick GJ, Liu H, Alphonse MP, Dikeman DA Youn C, Otterson JC, et al. Epicutaneous Staphylococcus aureus induces IL-36 to enhance IgE production and ensuing allergic disease. J Clin Invest. 2021;131(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou T, Tsang MS, Chu IM, Kan LL, Hon KL, Leung TF, et al. Skewed inflammation is associated with aberrant interleukin-37 signaling pathway in atopic dermatitis. Allergy. 2021;76(7):2102–14. [DOI] [PubMed] [Google Scholar]
- 30.Voisin T, Perner C, Messou MA, Shiers S, Ualiyeva S, Kanaoka Y, et al. The CysLT(2)R receptor mediates leukotriene C(4)-driven acute and chronic itch. Proc Natl Acad Sci U S A. 2021;118(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Himadri, George R, Mathew L, Shanmugam V, Mani T, Jeyaseelan L. The role of thymus and activation-regulated chemokine as a marker of severity of atopic dermatitis. J Am Acad Dermatol. 2021;84(2):545–7. [DOI] [PubMed] [Google Scholar]
- 32.Leyva-Castillo JM, Das M, Artru E, Yoon J, Galand C, Geha RS. Mast cell-derived IL-13 downregulates IL-12 production by skin dendritic cells to inhibit the T(H)1 cell response to cutaneous antigen exposure. J Allergy Clin Immunol. 2021;147(6):2305–15.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theoharides TC, Conti P. Mast cells to dendritic cells: Let IL-13 shut your IL-12 down. J Allergy Clin Immunol. 2021;147(6):2073–4. [DOI] [PubMed] [Google Scholar]
- 34.Toyoshima S, Sakamoto-Sasaki T, Kurosawa Y, Hayama K, Matsuda A, Watanabe Y, et al. miR103a-3p in extracellular vesicles from FcεRI-aggregated human mast cells enhances IL-5 production by group 2 innate lymphoid cells. J Allergy Clin Immunol. 2021;147(5):1878–91. [DOI] [PubMed] [Google Scholar]
- 35.Pellefigues C, Naidoo K, Mehta P, Schmidt AJ, Jagot F, Roussel E, et al. Basophils promote barrier dysfunction and resolution in the atopic skin. J Allergy Clin Immunol. 2021;148(3):799–812.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s. J Allergy Clin Immunol. 2021;147(5):1531–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkon N, Bauer WM, Krausgruber T, Goh I, Griss J, Nguyen V, et al. Single-cell analysis reveals innate lymphoid cell lineage infidelity in atopic dermatitis. J Allergy Clin Immunol. 2022;149(2):624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, Kim JH, Kim SH, Kim HR, Zhang K, Pan Y, et al. Skin-resident natural killer T cells participate in cutaneous allergic inflammation in atopic dermatitis. J Allergy Clin Immunol. 2021;147(5):1764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möbus L, Rodriguez E, Harder I, Schwarz A, Wehkamp U, Stölzl D, et al. Elevated NK-cell transcriptional signature and dysbalance of resting and activated NK cells in atopic dermatitis. J Allergy Clin Immunol. 2021;147(5):1959–65.e2. [DOI] [PubMed] [Google Scholar]
- 40.Naidoo K, Woods K, Pellefigues C, Cait A, O’Sullivan D, Gell K, et al. MR1-dependent immune surveillance of the skin contributes to pathogenesis and is a photobiological target of UV light therapy in a mouse model of atopic dermatitis. Allergy. 2021;76(10):3155–70. [DOI] [PubMed] [Google Scholar]
- 41.Marschall P, Wei R, Segaud J, Yao W, Hener P, German BF, et al. Dual function of Langerhans cells in skin TSLP-promoted T(FH) differentiation in mouse atopic dermatitis. J Allergy Clin Immunol. 2021;147(5):1778–94. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371(6527). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Fu J, Han X, Zhang C, Xia L, Zhu R, et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N(6)-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J Allergy Clin Immunol. 2022;149(6):2021–33. [DOI] [PubMed] [Google Scholar]
- 44.Klaeschen AS, Nümm TJ, Herrmann N, Leib N, Maintz L, Sakai T, et al. JAK1/2 inhibition impairs the development and function of inflammatory dendritic epidermal cells in atopic dermatitis. J Allergy Clin Immunol. 2021;147(6):2202–12.e8. [DOI] [PubMed] [Google Scholar]
- 45.Sukseree S, Bakiri L, Palomo-Irigoyen M, Uluçkan Ö, Petzelbauer P, Wagner EF. Sequestosome 1/p62 enhances chronic skin inflammation. J Allergy Clin Immunol. 2021;147(6):2386–93.e4. [DOI] [PubMed] [Google Scholar]
- 46.Darlenski R, Kozyrskyj AL, Fluhr JW, Caraballo L. Association between barrier impairment and skin microbiota in atopic dermatitis from a global perspective: Unmet needs and open questions. J Allergy Clin Immunol. 2021;148(6):1387–93. [DOI] [PubMed] [Google Scholar]
- 47.Sahlén P, Spalinskas R, Asad S, Mahapatra KD, Höjer P, Anil A, et al. Chromatin interactions in differentiating keratinocytes reveal novel atopic dermatitis- and psoriasis-associated genes. J Allergy Clin Immunol. 2021;147(5):1742–52. [DOI] [PubMed] [Google Scholar]
- 48.DeVore SB, Stevens ML, He H, Biagini JM, Kroner JW, Martin LJ, et al. Novel role for caspase recruitment domain family member 14 and its genetic variant rs11652075 in skin filaggrin homeostasis. J Allergy Clin Immunol. 2022;149(2):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012;90(5):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He H, Del Duca E, Diaz A, Kim HJ, Gay-Mimbrera J, Zhang N, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol. 2021;147(4):1369–80. [DOI] [PubMed] [Google Scholar]
- 51.He H, Bissonnette R, Wu J, Diaz A, Saint-Cyr Proulx E, Maari C, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol. 2021;147(1):199–212. [DOI] [PubMed] [Google Scholar]
- 52.Andersson AM, Sølberg J, Koch A, Skov L, Jakasa I, Kezic S, et al. Assessment of biomarkers in pediatric atopic dermatitis by tape strips and skin biopsies. Allergy. 2022;77(5):1499–509. [DOI] [PubMed] [Google Scholar]
- 53.Mannweiler R, Bergmann S, Vidal YSS, Brandner JM, Günzel D. Direct assessment of individual skin barrier components by electrical impedance spectroscopy. Allergy. 2021;76(10):3094–106. [DOI] [PubMed] [Google Scholar]
- 54.Rinaldi AO, Korsfeldt A, Ward S, Burla D, Dreher A, Gautschi M, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy. 2021;76(10):3066–79. [DOI] [PubMed] [Google Scholar]
- 55.Möbus L, Rodriguez E, Harder I, Stölzl D, Boraczynski N, Gerdes S, et al. Atopic dermatitis displays stable and dynamic skin transcriptome signatures. J Allergy Clin Immunol. 2021;147(1):213–23. [DOI] [PubMed] [Google Scholar]
- 56.Kopfnagel V, Dreyer S, Zeitvogel J, Pieper DH, Buch A, Sodeik B, et al. Free human DNA attenuates the activity of antimicrobial peptides in atopic dermatitis. Allergy. 2021;76(10):3145–54. [DOI] [PubMed] [Google Scholar]
- 57.Braz JM, Dembo T, Charruyer A, Ghadially R, Fassett MS, Basbaum AI. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc Natl Acad Sci U S A. 2021;118(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Towell AM, Feuillie C, Vitry P, Da Costa TM, Mathelié-Guinlet M, Kezic S, et al. Staphylococcus aureus binds to the N-terminal region of corneodesmosin to adhere to the stratum corneum in atopic dermatitis. Proc Natl Acad Sci U S A. 2021;118(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez T, Stevens ML, Baatyrbek Kyzy A, Alarcon R, He H, Kroner JW, et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy. 2021;76(1):302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farag AK, Roesner LM, Wieschowski S, Heratizadeh A, Eiz-Vesper B, Kwok WW, et al. Specific T cells targeting Staphylococcus aureus fibronectin-binding protein 1 induce a type 2/type 1 inflammatory response in sensitized atopic dermatitis patients. Allergy. 2022;77(4):1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murai-Yamamura M, Garcet S, Yamamura K, Gonzalez J, Miura S, Li X, et al. TH 2 cytokines and Staphylococcus aureus cooperatively induce atopic dermatitis-like transcriptomes. Allergy. 2021;76(11):3534–7. [DOI] [PubMed] [Google Scholar]
- 62.Cau L, Williams MR, Butcher AM, Nakatsuji T, Kavanaugh JS, Cheng JY, et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J Allergy Clin Immunol. 2021;147(3):955–66 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tay ASL, Li C, Nandi T, Chng KR, Andiappan AK, Mettu VS, et al. Atopic dermatitis microbiomes stratify into ecologic dermotypes enabling microbial virulence and disease severity. J Allergy Clin Immunol. 2021;147(4):1329–40. [DOI] [PubMed] [Google Scholar]
- 64.Lehtimäki J, Thorsen J, Rasmussen MA, Hjelmsø M, Shah S, Mortensen MS, et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J Allergy Clin Immunol. 2021;148(1):234–43. [DOI] [PubMed] [Google Scholar]
- 65.Hu C, van Meel ER, Medina-Gomez C, Kraaij R, Barroso M, Kiefte-de Jong J, et al. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J Allergy Clin Immunol. 2021;148(2):612–20. [DOI] [PubMed] [Google Scholar]
- 66.Nakatsuji T, Hata TR, Tong Y, Cheng JY, Shafiq F, Butcher AM, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27(4):700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottman N, Barrientos-Somarribas M, Fyhrquist N, Alexander H, Wisgrill L, Olah P, et al. Microbial and transcriptional differences elucidate atopic dermatitis heterogeneity across skin sites. Allergy. 2021;76(4):1173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown SJ. What Have We Learned from GWAS for Atopic Dermatitis? J Invest Dermatol. 2021;141(1):19–22. [DOI] [PubMed] [Google Scholar]
- 69.Sliz E, Huilaja L, Pasanen A, Laisk T, Reimann E, Magi R, et al. Uniting biobank resources reveals novel genetic pathways modulating susceptibility for atopic dermatitis. J Allergy Clin Immunol. 2022;149(3):1105–12 e9. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka N, Koido M, Suzuki A, Otomo N, Suetsugu H, Kochi Y, et al. Eight novel susceptibility loci and putative causal variants in atopic dermatitis. J Allergy Clin Immunol. 2021;148(5):1293–306. [DOI] [PubMed] [Google Scholar]
- 71.Sahlen P, Spalinskas R, Asad S, Mahapatra KD, Hojer P, Anil A, et al. Chromatin interactions in differentiating keratinocytes reveal novel atopic dermatitis- and psoriasis-associated genes. J Allergy Clin Immunol. 2021;147(5):1742–52. [DOI] [PubMed] [Google Scholar]
- 72.Bin L, Malley C, Taylor P, Preethi Boorgula M, Chavan S, Daya M, et al. Whole genome sequencing identifies novel genetic mutations in patients with eczema herpeticum. Allergy. 2021;76(8):2510–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daya M, Cox C, Acevedo N, Boorgula MP, Campbell M, Chavan S, et al. Multiethnic genome-wide and HLA association study of total serum IgE level. J Allergy Clin Immunol. 2021;148(6):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arehart CH, Daya M, Campbell M, Boorgula MP, Rafaels N, Chavan S, et al. Polygenic prediction of atopic dermatitis improves with atopic training and filaggrin factors. J Allergy Clin Immunol. 2022;149(1):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simard M, Madore AM, Girard S, Waserman S, Duan Q, Subbarao P, et al. Polygenic risk score for atopic dermatitis in the Canadian population. J Allergy Clin Immunol. 2021;147(1):406–9. [DOI] [PubMed] [Google Scholar]
- 76.Zhong Y, Samuel M, van Bever H, Tham EH. Emollients in infancy to prevent atopic dermatitis: A systematic review and meta-analysis. Allergy. 2022;77(6):1685–99. [DOI] [PubMed] [Google Scholar]
- 77.Kelleher MM, Cro S, Cornelius V, Lodrup Carlsen KC, Skjerven HO, Rehbinder EM, et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database Syst Rev. 2021;2:CD013534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skjerven HO, Lie A, Vettukattil R, Rehbinder EM, LeBlanc M, Asarnoj A, et al. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet. 2022;399(10344):2398–411. [DOI] [PubMed] [Google Scholar]
- 79.Venter C, Palumbo MP, Glueck DH, Sauder KA, O’Mahony L, Fleischer DM, et al. The maternal diet index in pregnancy is associated with offspring allergic diseases: the Healthy Start study. Allergy. 2022;77(1):162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paller AS, Stein Gold L, Soung J, Tallman AM, Rubenstein DS, Gooderham M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84(3):632–8. [DOI] [PubMed] [Google Scholar]
- 81.Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80(1):89–98 e3. [DOI] [PubMed] [Google Scholar]
- 82.Park CW, Kim BJ, Lee YW, Won C, Park CO, Chung BY, et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J Allergy Clin Immunol. 2022;149(4):1340–7.e4. [DOI] [PubMed] [Google Scholar]
- 83.Silverberg JI, Pinter A, Alavi A, Lynde C, Bouaziz JD, Wollenberg A, et al. Nemolizumab is associated with a rapid improvement in atopic dermatitis signs and symptoms: subpopulation (EASI >/= 16) analysis of randomized phase 2B study. J Eur Acad Dermatol Venereol. 2021;35(7):1562–8. [DOI] [PubMed] [Google Scholar]
- 84.Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–82. [DOI] [PubMed] [Google Scholar]
- 85.Kabashima K, Matsumura T, Komazaki H, Kawashima M, Nemolizumab JPSG. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N Engl J Med. 2020;383(2):141–50. [DOI] [PubMed] [Google Scholar]
- 86.Liang J, Hu F, Dan M, Sang Y, Abulikemu K, Wang Q, et al. Safety and Efficacy of Nemolizumab for Atopic Dermatitis With Pruritus: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Front Immunol. 2022;13:825312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ariëns LFM, van der Schaft J, Spekhorst LS, Bakker DS, Romeijn GLE, Kouwenhoven TA, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-Week results from the Dutch BioDay registry. J Am Acad Dermatol. 2021;84(4):1000–9. [DOI] [PubMed] [Google Scholar]
- 88.Siegels D, Heratizadeh A, Abraham S, Binnmyr J, Brockow K, Irvine AD, et al. Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy. 2021;76(4):1053–76. [DOI] [PubMed] [Google Scholar]
- 89.Narla S, Silverberg JI, Simpson EL. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol. 2022;86(3):628–36. [DOI] [PubMed] [Google Scholar]
- 90.Schneeweiss MC, Kim SC, Wyss R, Schneeweiss S, Merola JF. Dupilumab and the risk of conjunctivitis and serious infection in patients with atopic dermatitis: A propensity score-matched cohort study. J Am Acad Dermatol. 2021;84(2):300–11. [DOI] [PubMed] [Google Scholar]
- 91.Trichot C, Faucheux L, Karpf L, Grandclaudon M, Pattarini L, Bagot M, et al. T(H) cell diversity and response to dupilumab in patients with atopic dermatitis. J Allergy Clin Immunol. 2021;147(2):756–9. [DOI] [PubMed] [Google Scholar]
- 92.Ungar B, Pavel AB, Li R, Kimmel G, Nia J, Hashim P, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J Allergy Clin Immunol. 2021;147(1):394–7. [DOI] [PubMed] [Google Scholar]
- 93.Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021;157(9):1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–81. [DOI] [PubMed] [Google Scholar]
- 96.Silverberg JI, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Costanzo A, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977–87.e14. [DOI] [PubMed] [Google Scholar]
- 97.Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–68. [DOI] [PubMed] [Google Scholar]
- 98.Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N Engl J Med. 2021;384(12):1101–12. [DOI] [PubMed] [Google Scholar]
- 99.Blauvelt A, Silverberg JI, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104–12. [DOI] [PubMed] [Google Scholar]
- 100.Simpson EL, Forman S, Silverberg JI, Zirwas M, Maverakis E, Han G, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62–70. [DOI] [PubMed] [Google Scholar]
- 101.Dhillon S Delgocitinib: First Approval. Drugs. 2020;80(6):609–15. [DOI] [PubMed] [Google Scholar]
- 102.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854–62. [DOI] [PubMed] [Google Scholar]
- 103.Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–72. [DOI] [PubMed] [Google Scholar]
- 104.Schoos AM, Chawes BL, Bønnelykke K, Stokholm J, Rasmussen MA, Bisgaard H. Increasing severity of early-onset atopic dermatitis, but not late-onset, associates with development of aeroallergen sensitization and allergic rhinitis in childhood. Allergy. 2022;77(4):1254–62. [DOI] [PubMed] [Google Scholar]
- 105.Keet C, Pistiner M, Plesa M, Szelag D, Shreffler W, Wood R, et al. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. J Allergy Clin Immunol. 2021;147(3):984–91.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silverwood RJ, Mansfield KE, Mulick A, Wong AYS, Schmidt SAJ, Roberts A, et al. Atopic eczema in adulthood and mortality: UK population-based cohort study, 1998–2016. J Allergy Clin Immunol. 2021;147(5):1753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roh YS, Huang AH, Sutaria N, Choi U, Wongvibulsin S, Choi J, et al. Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol. 2022;86(4):835–45. [DOI] [PubMed] [Google Scholar]
- 108.Villani AP, Pavel AB, Wu J, Fernandes M, Maari C, Saint-Cyr Proulx E, et al. Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy. 2021;76(10):3107–21. [DOI] [PubMed] [Google Scholar]
- 109.Budu-Aggrey A, Watkins SH, Brumpton B, Løset M, Tyrrell J, Modalsli EH, et al. Assessment of a causal relationship between body mass index and atopic dermatitis. J Allergy Clin Immunol. 2021;147(1):400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manjunath J, Silverberg JI. Association of obesity in early childhood with atopic dermatitis in late childhood and adolescence. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 111.Patrick MT, Zhang H, Wasikowski R, Prens EP, Weidinger, Gudjonsson JE, et al. Associations between COVID-19 and skin conditions identified through epidemiology and genomic studies. J Allergy Clin Immunol. 2021;147(3):857–69.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]