Abstract
FDA approval of dupilumab for moderate-to-severe AD shifted the paradigm from use of broad, systemic immunosuppressants to a safer, targeted treatment and led to the emergence of newer interleukin (IL)-4/IL-13 directed biologics and small molecule therapies, namely Janus kinase (JAK) inhibitors. Tralokinumab and emerging (not yet approved) lebrikizumab, which both target IL-13, are alternative biologics to dupilumab. Emerging anti-IL-31 receptor nemolizumab is likely to be used second-line to other biologics, primarily for pruritus. Three JAK inhibitors are currently in use for treating AD, two of which, abrocitinib and upadacitinib, are FDA-approved. This review provides an in-depth, practical discussion on use of these biologics and JAK inhibitors that are approved or have completed phase 3 clinical trials in pediatric patients and adults, comparing the groups of medications based on available efficacy and safety data.
Keywords: abrocitinib, atopic dermatitis, baricitinib, biologics, cytokine signaling, dupilumab, eczema, interleukin-4, interleukin-13, Janus kinase inhibitor, lebrikizumab, nemolizumab, tralokinumab, upadacitinib
Introduction
Atopic dermatitis (AD) is the most common inflammatory skin disorder, with approximately 80% of cases starting in childhood and 20% in adulthood. Overall, 10–20% of children and 3–7% of adults in developed countries have AD. Based on a US study, 58% have mild disease, 35% moderate and 7% severe, with adolescents and adults more often moderate-to-severe than younger children.1 AD is heterogenous in its age of onset, phenotypic differences based on ethnic background, risk of various cutaneous infections, including Herpes simplex virus (eczema herpeticum), trajectories in disease course, subsequent development of other atopic disorders (the atopic march with food allergy, allergic rhinitis and allergic asthma), and non-atopic comorbidities, particularly neuropsychiatric disorders (attention deficit-hyperactivity, anxiety, depression) and cardiovascular disease.2 Meaningful subgrouping of AD patients to explain or predict these associations has yet to be defined. Substantial effort has been undertaken to better understand the complex immunologic mechanisms of AD, its heterogeneity, and its dynamic course with unpredictable flares.
The wide spectrum of AD severity defies a one-size-fits-all therapy and most available guidelines advise a stepwise therapeutic approach. Management comprises 3 main pillars: (i) identifying and avoiding possible provocation factors, among them irritants and allergic triggers; (ii) addressing epidermal barrier dysfunction, including moisturization; and (iii) initiating efficacious anti-inflammatory therapy, which both treats flares and provides long-term control. Given evidence that an imbalance of the skin microbiome (dysbiosis) with high colonization by Staphylococcus aureus contributes to worsening of inflammatory reactivity and disease chronicity, new strategies have been developed to reverse the microbiome imbalance.
Anti-inflammatory therapies for skin disorders are applied topically or administered systemically. According to guidelines, topical therapies are classically approved and used for mild-to-moderate AD, while systemic therapies are typically recommended only for moderate-to-severe AD (with topicals as adjunctive agents).3, 4 This review focuses on biologics and systemic Janus kinase (JAK) inhibitors (JAKi, jakinibs) that are now FDA-approved or have completed phase 3 studies for pediatric and adult AD.
Biologics
In general, biologic therapies target one extracellular receptor subunit or cytokine, leading to more precise targeting than immunosuppressants and oral JAKi, leading to exceptional long-term safety and no required laboratory monitoring. Biologics are not metabolized by traditional cytochrome-based mechanisms or excreted renally, minimizing potential drug interactions. Limitations of biologics as a class include the need for frequent injections, conjunctivitis for interleukin (IL)-13 or IL-4/IL-13 blockade, the theoretical risk of anti-drug antibody formation with loss of efficacy over time, and difficulty in individualizing the dose. This review includes biologics that are approved or in late-stage development.
Dupilumab
In 2017, dupilumab became the first biologic therapy to receive approval from the U.S. Food & Drug Administration (FDA) for adults with moderate-to-severe AD and is the only approved biologic for pediatric patients (adolescents in 2019, children ≥ 6 years in 2020, and infants as young as 6 months old in June, 2022).5 Dupilumab is a human IgG4 monoclonal antibody that binds the widely expressed IL-4 receptor α (IL-4Rα). Because IL-4Rα is present in both type 1 and type 2 IL-4 complex receptors, dupilumab mediates dual blockade of IL-4 and IL-13 signaling.6–8 Current approvals or phase 3 studies for dupilumab include use for moderate-to-severe asthma characterized by an eosinophilic predominance, oral corticosteroid dependent asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, prurigo nodularis, hand dermatitis, chronic urticaria, and bullous pemphigoid.5, 9–14
Several phase 3 trials have demonstrated dupilumab’s safety, tolerability, and efficacy for moderate-to-severe AD failing topical therapies or for whom topical therapies were inadvisable. (Table 1).15–18 In SOLO 1 and SOLO 2, patients receiving 300mg dupilumab every two weeks achieved Investigator Global Assessment (IGA) of clear or almost clear (IGA 0/1) at 16 weeks, significantly more than placebo (36–38% versus 8–10%). Efficacy data were similar in patients receiving 300mg dupilumab every week (36–37%), supporting the recommendation of dosing every two weeks. Significant improvements were also observed in patient-reported symptoms, quality of life (QOL), use of rescue therapies, and symptoms of pre-existing anxiety or depression.15
Table 1.
Biologics for Atopic Dermatitis (Currently Approved or Completed Phase 3 Clinical Trials)
| Biologic | Study Type | Trial Identifier | Subject Number | Duration (weeks) | Dose (%) | Age (years) | Primary Endpoints Achieved | Secondary Endpoints Achieved | Notable Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Dupilumab | Phase 3 (monotherapy) | SOLO 1, NCT02277743 | 671 | 16 wks | 300mg Q1W, Q2W | ≥18 | IGA 0/1 | EASI 50/75/90, %EASI, BSA, SCORAD, PP-NRS 4, POEM, DLQI, HADS | Injection-site reactions, conjunctivitis |
| Phase 3 (monotherapy) | SOLO 2, NCT02277769 | 708 | 16 wks | 300mg Q1W, Q2W | ≥18 | IGA 0/1 | EASI 50/75/90, %EASI, BSA, SCORAD, PP-NRS 4, POEM, DLQI, HADS | Injection-site reactions, conjunctivitis | |
| Phase 3 (+/−TCS) | LIBERTY AD CHRONOS, NCT02260986 | 740 | 52 wks | 300mg Q1W, Q2W | ≥18 | IGA 0/1, EASI 75 | %EASI, BSA, SCORAD, PP-NRS 4, POEM, DLQI, HADS | Injection site reactions, conjunctivitis | |
| Phase 3 (+TCS) | LIBERTY AD CAFÉ, NCT02755649 | 325 | 16 wks | 300mg Q1W, Q2W | ≥18 | EASI 75 | IGA 0/1, EASI 50/90, %EASI, BSA, SCORAD, PP-NRS 4, POEM, DLQI, HADS | Injection site reactions, conjunctivitis | |
| Phase 3 (monotherapy) | SOLOCONTINUE, NCT02395133 | 422 | 36 wks | 300mg Q1W, Q2W, Q4W, Q8W | ≥18 | %EASI, EASI 75 | IGA 0/1, %EASI, EASI 50, PP-NRS 3 | Injection site reactions, conjunctivitis | |
| Phase 3 (monotherapy) | NCT03912259 | 165 | 16 wks | 300mg | ≥18 | IGA 0/1 | IGA 0/1, EASI 75, PP-NRS 4, BSA, DLQI, POEM | Conjunctivitis, nasopharyngitis, upper respiratory infection | |
| Phase 3 (monotherapy) | NCT03054428 | 251 | 16 wks | 300mg Q2W, Q4W 200mg Q2W | 12–17 | IGA 0/1 | EASI 50/75/90, %PP-NRS, PP-NRS 3, PP-NRS-4, SCORAD, CDLQI, POEM, HADS | Injection site pain, conjunctivitis | |
| Phase 3 (+TCS) | NCT03345914 | 367 | 16 wks | 300mg Q4W, 200mg Q2W, 100mg Q2W | 6 – 11 | IGA 0/1 | EASI 50/75/90, %EASI, %PP-NRS, PROMISanxiety/depression, SCORAD, CDLQI, POEM, DFI | Injection site pain, conjunctivitis | |
| Phase 3 (+TCS) | NCT03346434 | 162 | 16 wks | 200mg Q4W | 6 mo – 5 yrs | IGA 0/1 | EASI 50/75/90, %EASI, %PP-NRS, PP-NRS 4, SCORAD, CDLQI, POEM, | Injection site pain, conjunctivitis | |
| Tralokinumab | Phase 3 (monotherapy) | ECZTRA 1, NCT03131648 | 802 | 52 wks (16 wks initial + 36 wks maintenance) | 300mg Q2W, Q4W | ≥18 | IGA 0/1, EASI 75 | EASI 50/90, SCORAD 50/75, PP-NRS 4, DLQI | Conjunctivitis, nasopharyngitis, upper respiratory infection |
| Phase 3 (monotherapy) | ECZTRA 2, NCT03160885 | 794 | 52 weeks (16 wks initial + 36 wks maintenance) | 300mg Q2W, Q4W | ≥18 | IGA 0/1, EASI 75 | EASI 50/90, SCORAD 50/75, PP-NRS 4, DLQI, IGA (maintenance), EASI 75 (maintenance) | Conjunctivitis, upper respiratory infection | |
| Phase 3 (+TCS) | ECZTRA 3, NCT03363854 | 380 | 32 weeks (16 wks initial + 16 wks continuation) | 300mg Q2W, Q4W | ≥18 | IGA 0/1, EASI 75 | EASI 50/90, SCORAD 50/75, PP-NRS 4, DLQI, TCS use | Conjunctivitis, upper respiratory infection, oral herpes, headache | |
| Phase 3 (+TCS) | ECZTRA 7, NCT03761537 | 277 | 26 weeks | 300mg Q2W | ≥18 | EASI 75 | IGA 0/1, SCORAD, DLQI | Injection site pain, conjunctivitis, headache | |
| Phase 3 (monotherapy) | NCT03526861 | 289 | 52 wks (16 wks initial + 36 wks maintenance) | 150mg Q2W, Q4W, 300 mg Q2W, Q4W | 12–17 | IGA 0/1, EASI 75 | EASI 50/90, %PP-NRS,PP-NRS 4,SCORAD, CDLQI,POEM | Injection site pain, conjunctivitis, headache, upper respiratory infection | |
| Lebrikizumab | Phase 3 (monotherapy) | ADvocate 1, NCT04146363 | 424 | 52 weeks (16 wks induction + 36 wks maintenance) | 250mg Q2W, Q4W | ≥12 | IGA 0/1, EASI 75 | %EASI, EASI 90, SCORAD, BSA, %PP-NRS, PP-NRS 4, sleep loss, DLQI, CDLQI, POEM | Conjunctivitis |
| Phase 3 (monotherapy) | ADvocate 2, NCT04178967 | 445 | 52 weeks (16 wks induction + 36 wks maintenance) | 250mg Q2W, Q4W | ≥12 | IGA 0/1, EASI 75 | %EASI, EASI 90, SCORAD, BSA, %PP-NRS, PP-NRS 4, sleep loss, DLQI, POEM | Conjunctivitis | |
| Phase 3 (+TCS) | ADhere, NCT04250337 | 228 | 16 weeks | 250mg Q2W | 12–17 | IGA 0/1, EASI 75 | %EASI, EASI 90, SCORAD, BSA, %PP-NRS, PP-NRS 4, sleep loss, DLQI, POEM | Conjunctivitis, headache | |
| Nemolizumab | Phase 3 (+TCS/TCI) | JapicCTI-173740 | 215 | 68 weeks (16 wks + 52 wks OLE) | 60mg Q4W | ≥13 | %VAS pruritus | Conclusions limited: no adjustment for multiple comparisons | Injection site reactions, cytokine abnormalities, increased creatinine kinase |
The 52-week LIBERTY AD CHRONOS trial examined dupilumab plus topical corticosteroid (TCS) or topical calcineurin inhibitor (TCI) dual-therapy for adults. At week 16, 39% of patients on 300mg dupilumab every two weeks achieved IGA 0/1 vs 12% receiving placebo, while 69% of patients on dupilumab every two weeks achieved 75% improvement in the Eczema Area and Severity Index score (EASI-75) compared with 23% on placebo. This effect was durable at 52 weeks.18 A second dual-therapy trial with TCS in patients resistant or intolerant to cyclosporine A reported similar efficacy data.19
In pediatrics, three pivotal, 16-week, randomized, controlled trials20–22 demonstrated efficacy and significant reduction in signs of inflammation, itch, and likelihood of skin infections,23 each leading to their respective age-group approval (Table 1). In adolescents, 24% reached IGA 0/1 with dupilumab monotherapy vs 2% on placebo.20 For ages 6 – 11 years, dupilumab plus concomitant TCS use led to IGA 0/1 for 30% using 300mg every 4 weeks (for patients < 30kg) vs 13% on placebo and IGA 0/1 for 39% using 200mg every 2 weeks (for patients ≥ 30kg) vs 10% on placebo.21 In infants and children ages 6 months to < 6 years, 28% on dupilumab plus very low potency TCS reached IGA 0/1 vs 4% on placebo with monthly administration.22
Subcutaneous dosing options of 200mg and 300mg are available as pre-filled syringe and, for adolescents and adults, pre-filled pen. For adults, the recommended dose is 300mg every two weeks after a 600mg loading dose. For pediatrics, dosing is age- and weight-based: 300 mg every 2 weeks for ≥60 kg, 200 mg every 2 weeks for 30 to <60 kg, 300 mg every 4 weeks for 15 to <30 kg, and 200 mg every 4 weeks for 5 to <15 kg. An initial loading dose (double the weight-appropriate dose) should be administered for all patients ≥ 6 years.
Needle phobia and injection site pain can cause parents and patients to fear its initiation. Several tactics and tips can be helpful for clinicians and patients. First, for patients requiring loading doses, the initial injection can be given by a healthcare provider and the second by the parent/caregiver, allowing teaching in a supervised clinic setting and greater parental comfort with administering the injection. If too difficult to administer at home, providing access to monthly in-office administration can ensure treatment administration. To reduce pain, dupilumab should be given at room temperature (removed from the refrigerator for a minimum of 45 minutes, ideally a few hours, with stability at room temperature before administration up to 14 days). Pre-treatment topical anesthetic can be applied to the injection site until children become less frightened about the injection. During administration, parents can hold their child in a bear hug to soothe and limit movement. Use of distraction techniques before and during the injection, such as audio or visual media, toys or non-pharmacological vibratory or tactile devices (eg, Buzzy® or ShotBlocker®) can be helpful. The pen formulation has the familiarity of an epinephrine pen with no visible needle, and is both faster and easier to administer, including self-administer; however, some report more pain with its use, even when gently squeezing the injection site skin to prevent intramuscular delivery. The pen formulation is currently being tested in 6-to-11-year-olds.
Side effects have been reported, particularly injection site reactions and conjunctivitis in all age groups.15, 18, 19 A systematic review analyzing more than 3300 patients on dupilumab reported 26.1% incidence of conjunctivitis overall.24 In pediatric trials, up to 11% of adolescents were affected by conjunctivitis,20 21% of those 6–11 years of age,21 and 5% 6 months - 5 years.22 Given that increased occurrence of ocular surface disease from dupilumab has been restricted to AD cohorts in studies, the mechanism likely involves dupilumab-related reduction of goblet cells in an already compromised ocular barrier.25 Of note, conjunctivitis is also seen in patients treated with tralokinumab26 or lebrikizumab,27 further linking goblet cell depletion to IL-13 inhibition. Most cases of conjunctivitis are mild and can be treated using artificial tears or antihistamine drops without disruption of dupilumab administration. More severe reactions warrant ophthalmology referral and anti-inflammatory medication. Increased AD severity and prior history of ocular inflammation are risk factors for development of medication-associated conjunctivitis28, 29 The prevalence of adverse reactions is higher than seen in clinical trials.30 In a review of real-world data, conjunctivitis was seen in up to 62% of patients treated with dupilumab.31 Other side effects reported post-approval include persistent or new onset facial erythema32–36 (reported in up to 20% of patients), psoriasiform dermatitis37, and inflammatory arthritis.38–40 Proposed mechanisms for facial erythema include unmasking of existing allergic contact dermatitis or psoriasis, topical steroid withdrawal41, dupilumab-induced Malassezia hypersensitivity, or Demodex proliferation resulting in rosacea. Concomitant treatment of these adverse events often allows dupilumab continuation. Although alopecia areata has developed during dupilumab therapy, dupilumab improved alopecia areata in others. In both clinical trials and post-marketing data,42 dupilumab temporarily induces eosinophilia, especially at 4–8 weeks after initiation, but has not been clinically relevant or requiring intervention, making laboratory monitoring unnecessary. In our experience, dupilumab has high durability in real-life use, consistent with the minimal formation of neutralizing anti-drug antibodies.
Dupilumab administration to infants and children with AD and its approval for use in other diseases, including asthma and eosinophilic esophagitis, not only allows earlier initiation of systemic treatment as needed, but also has the potential to reduce the risk of or concurrently treat other atopic diseases. In younger children with AD, treatment coincides with childhood vaccine administrations. Consensus guidelines suggest that vaccines should be administered 4 weeks prior to dupilumab initiation, if possible.43 Especially for children ≤6 years of age, who require routine administration of rotavirus, measles, mumps, and rubella (MMR), and varicella live vaccines, the risks of live vaccines are unknown and their administration during dupilumab use should be considered on a case-by-case basis. Dupilumab can be continued during administration of inactivated vaccines, including seasonal influenza injections and COVID 19 immunization. While adult studies have helped to reassure that dupilumab does not interfere with development of appropriate antibody titers, these studies were not performed in children; specific antibody levels can be measured to ensure serologic protection after vaccination if important.
Long-term data is available for adolescents from a phase 3 open-label extension; 43% on 300mg of dupilumab every 2 or 4 weeks reached IGA 0/1 by 52 weeks. Of these, 29% maintained IGA 0/1 for 12 consecutive weeks (weeks 40 – 52) and discontinued dupilumab. After a mean of 18 weeks, 43% remained clear/almost clear using TCS alone.44 Long-term safety was maintained. Additional post-approval studies showed IL-4/IL-13 blockade to repair the skin barrier, reduce skin infections and S. aureus colonization, and improve comorbidities.45–47
Tralokinumab
Tralokinumab was approved in the US and Europe for adults with moderate-to-severe AD in 2021. It is a human IgG4 monoclonal antibody that neutralizes cytokine IL-13 and interrupts activation of the IL-13Rα1 and, to a lesser extent, IL-13Rα2 receptor subunits.48, 49 Growing evidence suggests that IL-13, found in greater amounts in AD skin than IL-4, is the central cytokine involved in AD pathogenesis, causing disruption of the skin barrier, keratinocyte-mediated amplification of the inflammatory response, and activation of the neuronal itch response.50–53 The recommended dose for adults with moderate-to-severe AD is 300mg SC every two weeks after the 600mg loading dose (Table 1).
ECZTRA 1 and ECZTRA 2 studied tralokinumab monotherapy in adults over 52 weeks. At 16 weeks, tralokinumab 300mg every two weeks was superior to placebo in achieving IGA 0/1 (15.8% vs. 7.1% and 22.2% vs. 10.9%, respectively) and EASI-75 (25.0% vs. 12.7% and 33.2% vs. 11.4%, respectively). In the second phase, patients with good response were re-randomized to tralokinumab every two weeks, tralokinumab every four weeks, or placebo. At 52 weeks, durable responses occurred in 51%−60% of patients on tralokinumab every two weeks and 39%−51% of patients treated every four weeks. Of patients re-randomized to placebo, 21%−47% met IGA or EASI endpoints at 52 weeks, suggesting persistent treatment effect.54 A network meta-analysis confirmed the efficacy of tralokinumab, but found it slightly lower than dupilumab at 16 weeks.55 During the first 16 weeks of the ECZTRA 6 study, significantly more adolescents with moderate-to-severe AD receiving tralokinumab 150mg or 300mg achieved IGA 0/1 and EASI-75 without rescue than placebo-treated adolescents. Conjunctivitis occurred in 3–4% on tralokinumab vs. 2% on placebo.56
The 32-week, phase 3 ECZTRA 3 trial evaluated tralokinumab plus TCS versus placebo plus TCS in adults. At week 16, tralokinumab responders were re-randomized to receive tralokinumab every two weeks or every four weeks thereafter. Both regimens achieved IGA 0/1 or EASI-75 after 16 weeks compared to placebo (39% vs 26% for every two weeks and 56% vs 37% every four weeks, respectively). Similar trends were observed with concomitant TCS use in the ECZTRA 7 trial of adults who previously used cyclosporine.57 Because biologics are frequently prescribed with topical agents, pragmatic dual-therapy trials may better reflect expected outcomes in clinical practice.54, 58, 59
Overall, tralokinumab is well-tolerated, with conjunctivitis occurring in up to 13.1% in phase 3 trials.54, 57, 58 A review estimated an overall prevalence of 7.5%, possibly lower than reported for dupilumab.60 Patients on dual therapy with TCS had slightly higher rates of headache57, 58, injection site reaction, and upper respiratory tract infection (URI)58 compared to placebo. Tralokinumab is not currently under development for other type 2 diseases.
Lebrikizumab
Lebrikizumab also targets IL-13, disrupting formation of the IL-13Rα1/IL-4Rα complex and downstream signal transduction.61 In two recent phase 3 trials, lebrikizumab 250mg every two weeks demonstrated efficacy at 16 weeks, achieving IGA 0/1 (33–43%) and EASI-75 (51%−59%) compared to placebo (11–13% and 16–18%, respectively) (Table 1). Improvements in itch, sleep loss, and quality of life were also reported.62, 63 A 16-week dual-therapy trial with TCS reported significant improvements in IGA and EASI-75.64 Lebrikizumab’s safety profile is similar to tralokinumab, with up to 7.5% incidence of conjunctivitis in phase 3 trials.
Nemolizumab
Nemolizumab is an investigational human monoclonal antibody that antagonizes the receptor for IL-31, a cytokine associated with pruritus.65, 66 IL-31 receptors are expressed on cutaneous sensory neurons and, when activated, promote itch signals.67 A phase 3 trial of Japanese patients on 60mg nemolizumab every four weeks plus TCS/TCI showed 43% reduction in pruritus per visual analogue scale (VAS) compared to 21% for patients on placebo at 16 weeks (Table 1). Percent improvement in EASI scores from baseline was 46% on nemolizumab group versus 33% on placebo. Injection site reactions (8%) and elevated creatine phosphokinase (CPK) levels (3%) occurred.68 One study noted increased dose-dependent occurrence of predominantly mild asthma events.69 Studies of 30mg nemolizumab with TCS are ongoing.
JAK inhibitors (JAKi or jakinibs)
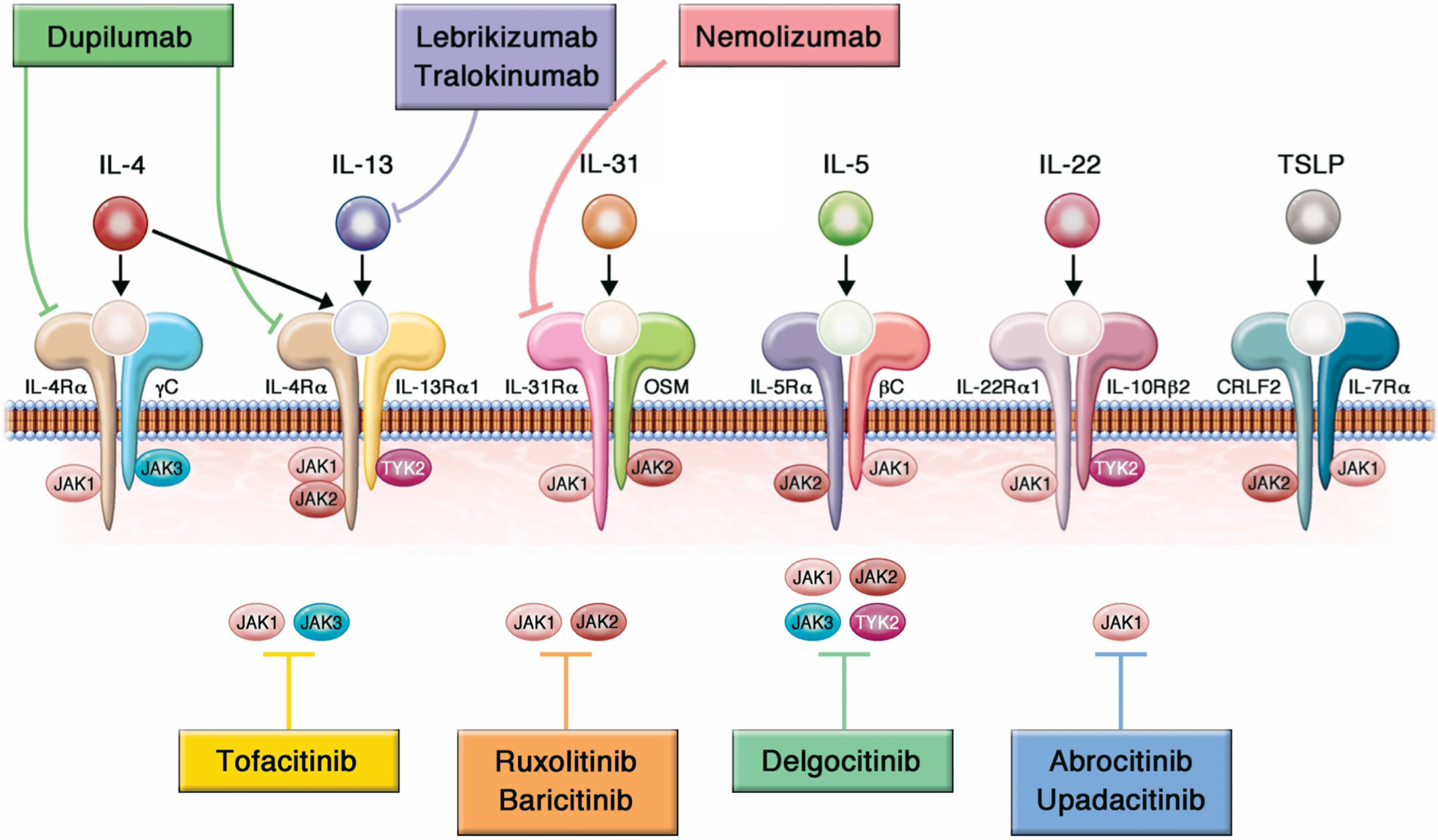
JAK-STAT signaling (particularly JAK1) is downstream of activation of type 2 cytokines (IL-4, IL-13, IL-31) (Figure 1).70, 71 JAKi in murine models impaired IL-4– and IL-13–dependent TH2 cell differentiation, improved skin barrier function, and decreased pruritus. Clinical trials have similarly shown reduction in inflammation and itch.72–74 First-generation JAKi (such as tofacitinib and baricitinib) interfere with more than 50 cytokines and thus affect numerous tissues. JAKi to treat AD now include second-generation inhibitors, which potentially have a narrower range of inhibition (JAK1 selectivity) and thus may have a better safety profile and higher efficacy than first-generation JAKi. Three systemic JAKi are approved for treatment of AD: baricitinib (European Medicines Agency (EMA)-approved for AD but currently only FDA-approved for alopecia areata) and second-generation JAKi abrocitinib and upadacitinib (both JAK1-selective; both FDA- and EMA-approved). Upadacitinib is also FDA-approved for moderate-to-severe rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, non-radiographic ankylosing spondyloarthritis, and ulcerative colitis, and baricitinib for rheumatoid arthritis.
Figure 1.
A. JAK-STAT pathway, ultimately leading to regulation of gene transcription in the nucleus. B. Cytokine binding and activation of a variety of receptors leads to signaling through the JAK-STAT pathway to cause inflammation. Modified figure reprinted with permission from Chovatiya and Paller. J Allergy Clin Immunol 2021;148:927–940.
Overall oral JAKi can be used in children 12 years and older (upadacitinib) and adults 18 years and older (abrocitinib, upadacitinib and barictinib) who have moderate-to-severe atopic dermatitis that has been refractory to systemic and topical therapy or who are candidates for systemic therapy (Table 2 for pivotal trials). Caution should be used in those greater than 65 years of age, with increased risk for cardiovascular disease, smokers or long-term former smokers, with increased risk for thrombosis, and with increased risk for cancer unless there is no suitable alternative. JAKi should be used with caution in combination with other immunosuppressants, strong CYP3A4 inhibitors (such as clarithromycin and ketoconazole, particularly relevant for upadacitinib), and CYP2C19 metabolizers or strong inhibitors (particularly relevant for abrocitinib). Examples of strong CYP2C19 inhibitors are azole antifungals (eg fluconazole), selective serotonin receptor inhibitors (eg fluoxetine and fluvoxamine), tricyclic antidepressants (eg amitriptyline), proton-pump inhibitors (eg lansoprazole) and gemfibrozil. Abrocitinib is contraindicated with concomitant use of antiplatelet therapy (except for low-dose aspirin < 81mg daily) during the first three months of treatment due to risk of thrombocytopenia. Although not tested in clinical trials, relative indications for considering JAKi preferentially may be for short-term use, including seasonal AD flares, and if concomitant alopecia areata or vitiligo. JAKi provide more rapid improvement in both signs and symptoms of AD than biologics, given their broader suppression of immune activation, including of the itch-related IL-31 pathway, making them a good choice for rapid control, especially in biologic-resistant patients. JAK inhibitor dosing and frequency of these oral drugs can be varied and tailored to an individual patient’s needs more easily than biologics. They also have a shorter half-life with more rapid clearance than biologics and no associated risk of anti-drug antibodies. In contrast to available biologics, which require no laboratory monitoring, JAKi require monitoring for tuberculosis and abnormalities of blood counts and lipids at baseline and during the treatment course. In addition, all JAKi for AD have an increased risk of herpes infections, particularly herpes zoster and serious infections. The boxed warning on all JAKi was based on the ORAL Surveillance study (NCT02092467), which evaluated the safety of tofacitinib in patients who were 50 years of age or older and had at least one additional cardiovascular risk factor.75 While these events have rarely occurred with more selective JAKi for AD, they remain theoretical risks and limit their use primarily to otherwise healthy patients without significant risk factors.
Table 2.
JAK Inhibitors for Atopic Dermatitis (Currently Approved or Completed Phase 3 Clinical Trials)
| Biologic | Study Type | Trial Identifier | Subject Number | Duration (weeks) | Dose (%) | Age (years) | Primary Endpoints Achieved | Key Secondary Endpoints Achieved | Notable Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Abrocitinib(JAK1) | Phase 3 (monotherapy) | JADE MONO-1, NCT 03349060 | 387 | 12 wks | 200mg, 100mg | 12+ | IGA 0/1, EASI-75 | PP-NRS4, %PSAAD | Nausea, vomiting, elevated CPK, headache, herpes simplex, folliculitis, nasopharyngitis transient thrombocytopenia |
| Phase 3 (monotherapy) | JADE MONO-2, NCT 03575871 | 391 | 12 wks | 200mg, 100mg | 12+ | IGA 0/1, EASI-75 | PP-NRS4, %PSAAD | ||
| Phase 3 (monotherapy) | JADE-REGIMEN, NCT 03627767 | 1,233 | 52 wks | 200mg, 100mg | 12+ | Flare, loss of EASI-50, IGA | IGA 0/1 | ||
| Phase 3 (+TCS) | JADE-TEEN,NCT 03796676 | 285 | 12 wks | 200mg, 100mg | 12–17 | IGA 0/1, EASI-75 | PP_NRS4, %PSAAD | ||
| Phase 3 (+TCS) | JADE EXTEND, NCT 03422822 | 3154 | Variable | 200mg, 100mg | 12 | Safety multiple | IGA, EASI-50,75,90, iNRS, Pt-GA, BSA, DLQI, POEM | ||
| Phase 3 (+TCS vs DUPI 300 q2wks) | JADECOMPARE, NCT 03720470 | 838 | 16 wks | 200mg, 100mg | >18 | IGA 0/1 wk 12, EASI-75 wk 12 | PP-NRS4 vs placebo, DUPI, IGA 0/1 vs placebo, DUPI, EASI-75 vs placebo, DUPI* | ||
| Baricitinib | Phase 3 (monotherapy) | BREEZE-AD1,NCT03334396 | 624 | 16 wks | 4mg, 2mg, 1mg | >18 | vIGA-AD 0/1 | vIGA-AD 0/1, %EASI, EASI-75, 90, SCORAD-75, i-NRS, %skin pain-NRS, Item 2-ADSS | Headache, herpes simplex, increased CPK |
| Phase 3 (monotherapy) | BREEZE-AD2,NCT03334422 | 615 | 16 wks | 4mg, 2mg, 1mg | >18 | vIGA-AD 0/1 | vIGA-AD 0/1, %EASI, EASI-75, 90, SCORAD-75, i-NRS, %skin pain-NRS, Item 2-ADSS | Headache, increased CPK | |
| Phase 3 (+TCS) | BREEZE-AD3, NCT03334435 | 1645 | 52 wks | 4mg, 2mg | >18 | VIGA-AD 0/1 (wks 16, 32,52) | IGA- 0/1/2, IGA 0/1 (non-responders), EASI-75, i-NRS, Skin-pain NRS4, Item2-ADSS1.5 | No change from Breeze, 1, 2 | |
| Phase 3 (+TCS) | BREEZE-AD4, NCT03428100 | 463 | 16 wks | 4mg, 2mg, 1mg | >18 | EASI-75 | %EASI, EASI-90, vIGA 0/1, SCORAD-75, iNRS, %skinpain-NRS, Item2-ADSS | Headache, influenza, nasopharyngitis | |
| Phase 3 (monotherapy) | BREEZE-AD5, NCT03435081 | 440 | 16 wks | 2mg, 1mg | >18 | EASI-75 | EASI-90, vIGA, i-NRS, % skin-pain-NRS, % item2-ADSS, SCORAD 75 | Herpes simplex, diarrhea, nasopharyngitis, upper respiratory infection, nausea | |
| Phase 3 (monotherapy) | BREEZE-AD6, NCT03559270 | 374 | 16 wks | 4mg, 2mg | >18 | EASI-75 | IGA- 0/1, BSA<3%, Itch-NRS4 | Headache, herpes simplex, upper respiratory infection | |
| Phase 3 (+TCS) | BREEZE-AD7, NCT03733301 | 329 | 16 wks | 4mg, 2mg | >18 | vIGA-AD 0/1 | EASI-75,90, SCORAD-75, i-NRS, %skin pain-NRS, %item 2-ADSS | ||
| Upadacitinib | Phase 3 (monotherapy) | Measure UP 1, NCT03569293 | 847 | 16 wks | 30mg, 15mg | 12–75 | EASI-75, vIGA 0/1 | EASI 90/100, worst itch-NRS4, POEM4, DLQI4, DLQI 0/1 | Acne, headache, nasopharyngitis, herpes zoster, increased CPK, upper respiratory infection |
| Phase 3 (monotherapy) | Measure Up 2, NCT03607422 | 836 | 16 wks | 30mg, 15mg | 12–75 | EASI-75, vIGA 0/1 | EASI 90/100, worst itch-NRS4, POEM4, DLQI4, DLQI 0/1 | Acne, headache, nasopharyngitis, herpes zoster, increased CPK, upper respiratory infection | |
| Phase 3 (+TCS) | AD Up, NCT03568318 | 300 | 16 wks | 30mg, 15mg | 12–75 | EASI-75, vIGA 0/1 | EASI90/100, worst itch-NRS4 | Acne, headache, nasopharyngitis, herpes zoster, increased CPK, upper respiratory infection, eczema herpeticum | |
| Phase 3 (monotherapy vs.DUPI 300 q2 wks) | Heads Up, NCT 03738397 | 692 | 16 wks | 30mg | 18–75 | EASI-75 | EASI 90/100, worst itch-NRS4, % worst itch-NRS 30 mg dose superior to DUPI | Rates of acne, serious infection, eczema herpeticum, herpes zoster, and laboratory-related adverse events were higher for upadacitinib Rates of conjunctivitis and injection-site reactions were higher for DUPI |
200mg (not 100mg) of ABRO was superior to DUPI for itch scores only (not IGA or EASI-75).
Baricitinib
The JAK1/2 selective inhibitor baricitinib was the first JAKi approved (in Europe) for systemic treatment of AD (Table 2). BREEZE-AD1, AD2, AD4, and AD7 showed that >50% of patients administered baricitinib 4mg daily, as monotherapy or in combination with TCS, achieved primary endpoints IGA 0/1 and EASI-75 at week 16 compared to placebo.76 Notably, itch relief occurred by week 1 of treatment at the 4mg dosage.77, 78 Nasopharyngitis, URIs, CPK elevations, and headache were the most frequently reported adverse events. In Breeze-AD3, a 52-week double-blind extension study of 2 randomized clinical trials, baricitinib 4mg and 2mg demonstrated sustained long-term efficacy in adults.79
Abrocitinib
Abrocitinib is FDA-approved for adults unresponsive to other systemic therapy at a starting dose of 100mg, with the option to advance to 200mg if response in inadequate. JAK1-selective inhibitor abrocitinib in phase 3 JADE trials (Mono-1 and 2) demonstrated IGA 0/1 responses in affected adults and adolescents at 200mg, 100mg and placebo of Mono-1 44%/Mono-2 38%, 24%/28%, and 8%/9%, respectively (Table 2).80, 81 Like baricitinib, abrocitinib was effective in improving sleeping patterns and overall quality of life in adolescents and adults.82 The most frequent dose-related, drug-related side effect of abrocitinib is nausea. Others, typically mild, are headaches, dizziness, nasopharyngitis, URI symptoms, vomiting, CPK increase (without rhabdomyolysis), folliculitis and acne, and herpes zoster infection. Severe treatment-related events included eczema herpeticum, herpangina and pneumonia, acute pancreatitis, and chronic inflammatory bowel disease; occasional patients receiving 200mg abrocitinib had thrombocytopenia. Transient and usually mild elevations in serum aminotransferase were not linked to clinically apparent acute liver injury.83 In a 12-week head-to-head comparison of abrocitinib to dupilumab 300mg every 2 weeks in adults, both the 100mg and 200mg doses of abrocitinib (FDA-approved as starting dose) was similar in efficacy but the 200mg dose was superior in Worst Itch NRS ≥4 points at 2 weeks.73 JADE-TEEN, a phase 3 trial for 12–17 year olds, showed that abrocitinib plus TCS greatly improved signs and symptoms of AD compared to TCS alone; >70% of treated teens showed improved sleep and Patient-Oriented Eczema Measure (POEM) score.82, 84 Of note, the plasma concentration of abrocitinib is greatly increased by co-administration with inhibitors of cytochrome P450 (CYP2C19/fluvoxamine; CYP2C9 and CYP3A/systemic azole antifungals and macrolide family antibiotics) and is reduced by CYP inducers, such as rifampin.85
Upadacitinib
Upadacitinib in the phase 3 Measure Up 1 and 2 clinical trials demonstrated efficacy and safety through 16 weeks, which was maintained through week 52 in adults and adolescents (Table 2).72, 86 IGA 0/1 scores and Worst Itch NRS score improvement of ≥4 were achieved at 16 weeks in 40–60% (vs 5–12% for placebo), with dose-dependent efficacy (15mg vs 30mg). Meaningful improvements also occurred at week 16 for quality of life, anxiety and depression, and sleep.86 The most frequently reported treatment-emergent adverse event with upadacitinib is acne, but other changes (except lack of nausea) resemble those of abrocitinib: cough, headache, urinary tract infection, URI, nasopharyngitis, and transient elevation in CPK levels. Infections included herpes zoster, eczema herpeticum, herpangina, and one case each of pneumonia and tuberculosis. Non-melanoma skin cancer occurred within 3 months of initiation in a few subjects.86 A 260-week follow-up study is ongoing.86 In head-to-head comparison of upadacitinib (30mg daily) to dupilumab (300mg every 2 weeks), upadacitinib performed superiorly at 16 weeks for skin clearance (EASI-75) and itch relief (Worst Itch NRS ≥4).87 Rates of serious infection including eczema herpeticum and herpes zoster were higher for patients who received upadacitinib, whereas the rate of conjunctivitis was higher for patients who received dupilumab. The 15mg dose of upadacitinib is FDA-approved for initiation in adults and adolescents ≥40kg with 30mg dose escalation allowed for failure to improve adequately. Of note, strong cytochrome P450 (CYP3A) inhibition from antifungals and macrolides (as for abrocitinib), can greatly increase levels of upadacitinib, whereas broad inducers of CYP (such as rifampin) can decrease levels, although without clinical adverse events.88
Given the potential risks of systemic JAKi, topicals are an appealing alternative to oral administration. Although outside of the scope of this review, it should be noted that two topical JAKi, delgocitinib (pan-JAK) and ruxolitinib (JAK1/2 inhibitor), are currently available for mild-to-moderate AD.89, 90 89, 91 Delgocitinib is only approved and available in Japan, whereas ruxolitinib is U.S. FDA-approved. Packaging limits delgocitinib to 5g per application and ruxolitinib 1.5% cream to 20% body surface area because of concerns regarding systemic absorption.92–96
In summary, several biologics are now FDA-approved or emerging that offer therapy that targets specific cytokines or receptors recognized to be central to the pathogenesis of AD. These biologics are all injected subcutaneously with a frequency of once every two weeks or less. JAKi target a broader array of immune signaling and thus carry greater safety risks, despite their high potential efficacy (Table 3). As JAKi are small molecular inhibitors, they are able to be administered orally, allowing for greater dosing flexibility. As with biologics for psoriasis during the past 20 years, it is possible that biologics in the future will achieve greater efficacy and/or a requirement for less frequent administration than currently available IL4R- and IL-13-targeting biologics. Second-generation JAKi are more selective than first-generation JAKi, potentially leading to greater efficacy and safety. Regardless, patients may not respond to currently available biologics or small molecule therapy as single agents. Combining a biologic, such as dupilumab, with a broader systemic agent, such as methotrexate or a JAKi, may control AD in patients who do not improve on a single agent alone or during flares. The safety, feasibility, and cost effectiveness of combination therapy needs to be better studied in a carefully selected patient population. Finally, given the cost and potential risks of both biologics and JAKi, future discovery of biomarkers that predict response to specific biologics and JAKi could optimize patient-specific treatment choice.
Table 3.
Benefits and Risks of Biologics vs JAK Inhibitors
| Medication | Benefits | Risks | Convenience/Desirability | Comments |
|---|---|---|---|---|
| Biologics | More precise targeting than immunosuppressants and oral JAK inhibitors No lab monitoring required |
Associated pain, risk of injection site reaction | Requires refrigeration and special shipping | All AD biologics administered subcutaneously |
| Dupilumab | High efficacy for inflammation and itch No increased risk of systemic infection Fewer skin infections Excellent safety to date Requires no lab monitoring Available for 6 months and older with AD |
Conjunctivitis Red face syndrome reported in real-life experience, but not trials Rare arthritis Rare psoriasiform dermatitis |
Given every 2–4 wks Pain with injection |
Longest duration of experience in AD Only new systemic medication shown to be efficacious for other atopic disorders and thus can have potential benefits on other atopic conditions |
| Tralokinumab | Moderate efficacy for inflammation and itch No increased risk of systemic infection or herpes Fewer skin infections High safety to date Requires no lab monitoring |
Excellent safety- Conjunctivitis is adverse event but may be less than with dupilumab | Given every 2–4 wks Usually 2 syringes per dose Pain with injection |
Although no head-to-head trials, phase 3 trial results suggest lower efficacy short-term than dupilumab or lebrikizumab Efficacy improves with time Only available for adults |
| Lebrikizumab | High efficacy for inflammation and itch No increased risk of systemic infection or herpes Fewer skin infections High safety to date Requires no lab monitoring |
Conjunctivitis is adverse event | Given every 2–4 wks Pain with injection |
Not yet FDA-approved |
| Nemolizumab | High efficacy for itch in some studies | High creatine kinase levels Possible worsening or new onset asthma |
Given every 4 wks | Not yet FDA-approved Lower efficacy for inflammation than other biologics Role in treating AD is unclear, given availability of other biologics with greater anti-inflammatory efficacy Only published phase 3 study from Japan and 60mg dosing (vs 30mg in ongoing studies) |
| JAK Inhibitors | Small molecule inhibitors with rapid clearance | Require lab monitoring All with boxed warning based on tofacitinib data (malignancy, thrombosis, cardiovascular issues, serious infections, death) |
Oral Easy to stop and start (eg, for short-term/seasonal use) Multiple doses available to tailor to patient needs |
Broader range of effects, which means potential value for other concomitant non-atopic disorders (eg alopecia areata and vitiligo), but also higher risk for side effects |
| Baricitinib | Currently FDA-approved for adults with alopecia areata (4mg) More modest efficacy than other JAK inhibitors, but also fewer tolerability issues |
Boxed warning Not as targeted as JAK1 selective medications but less adverse events than other JAKi in trials Headache, nasopharyngitis, increased infections, including herpetic |
Daily administration Does not require refrigeration |
Not FDA-approved for AD (approved in Europe) |
| Abrocitinib | High efficacy suggests use for severe AD Flexibility in dosing – start at 100mg daily and can increase to 200mg daily |
Boxed warning Nausea/vomiting, headache, nasopharyngitis, infections, including herpes simplex and zoster, acne Abnormal platelet counts, increased transaminases, high creatine kinase, hyperlipidemia |
Daily administration Easy to stop and start Does not require refrigeration |
12-week head-to-head trial in adults suggests that only 200mg dose is more efficacious than dupilumab 300mg every 2 wks for itch (and not for IGA 0/1/EASI-75) |
| Upadacitinib | High efficacy suggests use for severe AD Flexibility in dosing – start at 15mg daily and can increase to 30mg daily |
Boxed warning Acne, headache, nasopharyngitis, infections, including herpes simplex and zoster Abnormal blood counts, increased transaminases, high creatine kinase, hyperlipidemia |
Daily administration Easy to stop and start Does not require refrigeration |
16-week head-to-head trial in adults suggests that 30mg dose is more efficacious than dupilumab 300mg every 2 wks |
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest:
Dr. Sneha Butala and Rishi Seshadri have no conflicts of interest to report.
Dr. Leslie Castelo-Soccio has previously received a consultant fee in March 2021 for alopecia areata and funding in support of an autoimmune fellowship from in September 2020, both from Pfizer. She serves on the medical advisory board for the National Alopecia Areata Foundation.
Dr. Eric L. Simpson reports grants and/or personal fees from AbbVie, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, FortéBio, Galderma, Incyte, Kyowa Kirin, LEO Pharma, MedImmune, Menlo Therapeutics, Merck, Novartis, Ortho Dermatologics, Pfizer, Pierre Fabre Dermo Cosmetique, Regeneron, Sanofi, Tioga, and Valeant.
Dr. John J. O’Shea and the National Institutes of Health hold patents related to therapeutic targeting of JAKs, for which Dr. O’Shea also receives royalties.
Dr. Thomas Bieber was speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, DIECE Therapeutics, Domain Therapeutics, EQRx, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, LÓréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences”.
Dr. Amy S. Paller has been an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, and UCB; a consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer-Ingelheim, Bristol Myers Squibb, Castle Creek, Eli Lilly, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi/Genzyme, Seanergy, TWI Biotechnology, and UCB, on the data safety monitoring board for AbbVie, Abeona, Catawba, Galderma, and InMed.
Abbreviations:
- AD
atopic dermatitis
- CPK
creatine phosphokinase
- CYP
cytochrome P
- EASI
Eczema Area and Severity Index score
- FDA
Food & Drug Administration
- IL
interleukin
- IGA
Investigator Global Assessment
- JAK
Janus kinase
- JAKi, jakinibs
JAK inhibitors
- MMR
measles, mumps, and rubella
- POEM
Patient-Oriented Eczema Measure
- TCS
topical corticosteroid
- TCI
topical calcineurin inhibitor
- URI
upper respiratory tract infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–28 e2. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51. [DOI] [PubMed] [Google Scholar]
- 3.Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regeneron. DUPIXENT® (dupilumab) injection, for subcutaneous use. Tarrytown, NY. 2022. [Google Scholar]
- 6.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullens DM, Kasran A, Peng X, Lorré K, Ceuppens JL. Effects of anti-IL-4 receptor monoclonal antibody on in vitro T cell cytokine levels: IL-4 production by T cells from non-atopic donors. Clin Exp Immunol. 1998;113(3):320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junttila IS. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol. 2018;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–50. [DOI] [PubMed] [Google Scholar]
- 10.NCT04180488. Dupilumab for the Treatment of Chronic Spontaneous Urticaria in Patients Who Remain Symptomatic Despite the Use of H1 Antihistamine and Who Are naïve to, Intolerant of, or Incomplete Responders to Omalizumab (LIBERTY-CSU CUPID). 2024.
- 11.NCT04681729. Dupilumab for the Treatment of Chronic Inducible Cold Urticaria in Patients Who Remain Symptomatic Despite the Use of H1-antihistamine (LIBERTY-CINDU CUrIADS). 2022.
- 12.NCT04183335. Study of Dupilumab for the Treatment of Patients With Prurigo Nodularis, Inadequately Controlled on Topical Prescription Therapies or When Those Therapies Are Not Advisable (LIBERTY-PN PRIME). 2021.
- 13.NCT04202679. Study of Dupilumab for the Treatment of Patients With Prurigo Nodularis, Inadequately Controlled on Topical Prescription Therapies or When Those Therapies Are Not Advisable (PRIME2). 2021.
- 14.NCT03633617. Study to Determine the Efficacy and Safety of Dupilumab in Adult and Adolescent Patients With Eosinophilic Esophagitis (EoE). 2021.
- 15.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375(24):2335–48. [DOI] [PubMed] [Google Scholar]
- 16.Thaçi D ELS , Deleuran M, Kataoka Y, Chen Z, Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019;94(2):266–75. [DOI] [PubMed] [Google Scholar]
- 17.Cork MJ, Eckert L, Simpson EL, Armstrong A, Barbarot S, Puig L, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat. 2020;31(6):606–14. [DOI] [PubMed] [Google Scholar]
- 18.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303. [DOI] [PubMed] [Google Scholar]
- 19.De Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical t. British Journal of Dermatology. 2018;178(5):1083–101. [DOI] [PubMed] [Google Scholar]
- 20.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2020;156(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paller AS, Siegfried EC, Thaci D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–93. [DOI] [PubMed] [Google Scholar]
- 22.Paller AS, Simpson EL, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2022;400(10356):908–19. [DOI] [PubMed] [Google Scholar]
- 23.Paller AS, Beck LA, Blauvelt A, Siegfried EC, Cork MJ, Wollenberg A, et al. Infections in children and adolescents treated with dupilumab in pediatric clinical trials for atopic dermatitis-A pooled analysis of trial data. Pediatr Dermatol. 2022;39(2):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139–47. [DOI] [PubMed] [Google Scholar]
- 25.Bakker DS, Ter Linde JJM, Amini MM, Ariens LFM, van Luijk CM, de Bruin-Weller MS, et al. Conjunctival inflammation in dupilumab-treated atopic dermatitis comprises a multicellular infiltrate with elevated T1/T17 cytokines: A case series study. Allergy. 2021;76(12):3814–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wollenberg A, Beck LA, de Bruin Weller M, Simpson EL, Imafuku S, Boguniewicz M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 27.Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020;156(4):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narla S, Silverberg JI, Simpson EL. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol. 2022;86(3):628–36. [DOI] [PubMed] [Google Scholar]
- 30.Neagu N, Dianzani C, Avallone G, Dell’Aquila C, Morariu SH, Zalaudek I, et al. Dupilumab ocular side effects in patients with atopic dermatitis: a systematic review. J Eur Acad Dermatol Venereol. 2022;36(6):820–35. [DOI] [PubMed] [Google Scholar]
- 31.Kamata M, Tada Y. A Literature Review of Real-World Effectiveness and Safety of Dupilumab for Atopic Dermatitis. JID Innov. 2021;1(3):100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wijs LEM, Nguyen NT, Kunkeler ACM, Nijsten T, Damman J, Hijnen DJ. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: a case series. Br J Dermatol. 2020;183(4):745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldman RA, DeWane ME, Sloan B, Grant-Kels JM. Characterizing dupilumab facial redness: A multi-institution retrospective medical record review. J Am Acad Dermatol. 2020;82(1):230–2. [DOI] [PubMed] [Google Scholar]
- 34.McKenzie PL, Rangu S, Treat JR, Castelo-Soccio L. Experience using dupilumab for pediatric atopic dermatitis at a tertiary care c enter: Inadequate response and adverse events. Pediatr Dermatol. 2021;38(5):1178–84. [DOI] [PubMed] [Google Scholar]
- 35.Jo CE, Finstad A, Georgakopoulos JR, Piguet V, Yeung J, Drucker AM. Facial and neck erythema associated with dupilumab treatment: A systematic review. J Am Acad Dermatol. 2021;84(5):1339–47. [DOI] [PubMed] [Google Scholar]
- 36.Muzumdar S, Skudalski L, Sharp K, Waldman RA. Dupilumab Facial Redness/Dupilumab Facial Dermatitis: A Guide for Clinicians. American Journal of Clinical Dermatology. 2022;23(1):61–7. [DOI] [PubMed] [Google Scholar]
- 37.Napolitano M, Scalvenzi M, Fabbrocini G, Cinelli E, Patruno C. Occurrence of psoriasiform eruption during dupilumab therapy for adult atopic dermatitis: A case series. Dermatol Ther. 2019;32(6):e13142. [DOI] [PubMed] [Google Scholar]
- 38.Willsmore ZN, Woolf RT, Hughes C, Menon B, Kirkham B, Smith CH, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181(5):1068–70. [DOI] [PubMed] [Google Scholar]
- 39.Jay R, Rodger J, Zirwas M. Review of dupilumab-associated inflammatory arthritis: An approach to clinical analysis and management. JAAD Case Rep. 2022;21:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy MJ, Cohen JM, Vesely MD, Damsky W. Paradoxical eruptions to targeted therapies in dermatology: A systematic review and analysis. J Am Acad Dermatol. 2022;86(5):1080–91. [DOI] [PubMed] [Google Scholar]
- 41.Ahn J, Lee DH, Na CH, Shim DH, Choi YS, Jung HJ, et al. Facial erythema in patients with atopic dermatitis treated with Dupilumab - a descriptive study of morphology and Aetiology. J Eur Acad Dermatol Venereol. 2022. [DOI] [PubMed] [Google Scholar]
- 42.Kreeshan FC, Al-Janabi A, Warren RB, Hunter HJA. Real-World Experience and Laboratory Monitoring of Dupilumab in Patients with Moderate to Severe Atopic Dermatitis in a Tertiary Centre. Dermatol Ther (Heidelb). 2021;11(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Cabriales SA, Kirchhof MG, Constantinescu CM, Murguia-Favela L, Ramien ML. Recommendations for Vaccination in Children with Atopic Dermatitis Treated with Dupilumab: A Consensus Meeting, 2020. Am J Clin Dermatol. 2021;22(4):443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blauvelt A, Guttman-Yassky E, Paller AS, Simpson EL, Cork MJ, Weisman J, et al. Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23(3):365–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, et al. IL-4Rα Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis. J Invest Dermatol. 2020;140(1):191–202.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boguniewicz M, Beck LA, Sher L, Guttman-Yassky E, Thaçi D, Blauvelt A, et al. Dupilumab Improves Asthma and Sinonasal Outcomes in Adults with Moderate to Severe Atopic Dermatitis. J Allergy Clin Immunol Pract. 2021;9(3):1212–23.e6. [DOI] [PubMed] [Google Scholar]
- 47.Eichenfield LF, Bieber T, Beck LA, Simpson EL, Thaçi D, de Bruin-Weller M, et al. Infections in Dupilumab Clinical Trials in Atopic Dermatitis: A Comprehensive Pooled Analysis. Am J Clin Dermatol. 2019;20(3):443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popovic B, Breed J, Rees DG, Gardener MJ, Vinall LM, Kemp B, et al. Structural Characterisation Reveals Mechanism of IL-13-Neutralising Monoclonal Antibody Tralokinumab as Inhibition of Binding to IL-13Rα1 and IL-13Rα2. J Mol Biol. 2017;429(2):208–19. [DOI] [PubMed] [Google Scholar]
- 49.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–41. [DOI] [PubMed] [Google Scholar]
- 50.Tazawa T, Sugiura H, Sugiura Y, Uehara M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295(11):459–64. [DOI] [PubMed] [Google Scholar]
- 51.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122(7):2590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell. 2017;171(1):217–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic Dermatitis Is an IL-13-Dominant Disease with Greater Molecular Heterogeneity Compared to Psoriasis. J Invest Dermatol. 2019;139(7):1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drucker AM, Morra DE, Prieto-Merino D, Ellis AG, Yiu ZZN, Rochwerg B, et al. Systemic Immunomodulatory Treatments for Atopic Dermatitis: Update of a Living Systematic Review and Network Meta-analysis. JAMA Dermatol. 2022;158(5):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paller A, Blauvelt A, Soong W, et al. , editor Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. Presented at Fall Clinical meeting; Oct. 21–24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutermuth J, Pink AE, Worm M, Soldbro L, Bjerregård Øland C, Weidinger S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br J Dermatol. 2022;186(3):440–52. [DOI] [PubMed] [Google Scholar]
- 58.Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bieber T Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54–62. [DOI] [PubMed] [Google Scholar]
- 60.Wollenberg A, Beck LA, de Bruin Weller M, Simpson EL, Imafuku S, Boguniewicz M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186(3):453–65. [DOI] [PubMed] [Google Scholar]
- 61.Ultsch M, Bevers J, Nakamura G, Vandlen R, Kelley RF, Wu LC, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol. 2013;425(8):1330–9. [DOI] [PubMed] [Google Scholar]
- 62.Eli L, Company, Dermira I. Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis (ADvocate1). 2021.
- 63.Eli L, Company, Dermira I. Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis. 2021.
- 64.Eli L, Company, Dermira I. Safety and Efficacy of Lebrikizumab (LY3650150) in Combination With Topical Corticosteroid in Moderate-to-Severe Atopic Dermatitis. 2021.
- 65.Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500–8.e24. [DOI] [PubMed] [Google Scholar]
- 66.Datsi A, Steinhoff M, Ahmad F, Alam M, Buddenkotte J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy. 2021;76(10):2982–97. [DOI] [PubMed] [Google Scholar]
- 67.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabashima K, Matsumura T, Komazaki H, Kawashima M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N Engl J Med. 2020;383(2):141–50. [DOI] [PubMed] [Google Scholar]
- 69.Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–82. [DOI] [PubMed] [Google Scholar]
- 70.Furue M Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4JAKSTAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J Clin Med. 2020;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klaeschen AS, Numm TJ, Herrmann N, Leib N, Maintz L, Sakai T, et al. JAK1/2 inhibition impairs the development and function of inflammatory dendritic epidermal cells in atopic dermatitis. J Allergy Clin Immunol. 2021;147(6):2202–12 e8. [DOI] [PubMed] [Google Scholar]
- 72.Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–68. [DOI] [PubMed] [Google Scholar]
- 73.Bieber T, Simpson EL, Silverberg JI, Thaci D, Paul C, Pink AE, et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N Engl J Med. 2021;384(12):1101–12. [DOI] [PubMed] [Google Scholar]
- 74.Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–55. [DOI] [PubMed] [Google Scholar]
- 75.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316–26. [DOI] [PubMed] [Google Scholar]
- 76.Radi G, Simonetti O, Rizzetto G, Diotallevi F, Molinelli E, Offidani A. Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare (Basel). 2021;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simpson EL, Forman S, Silverberg JI, Zirwas M, Maverakis E, Han G, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62–70. [DOI] [PubMed] [Google Scholar]
- 78.Rosmarin D, Casillas M, Chen S, Dawson Z, Pierce E, Zhang H, et al. Onset of Symptom Relief Reported in Daily Diaries of Patients With Atopic Dermatitis Treated With Baricitinib in a United States Clinical Trial (BREEZE-AD5). J Cutan Med Surg. 2022;26(3):262–6. [DOI] [PubMed] [Google Scholar]
- 79.Silverberg JI, Simpson EL, Wollenberg A, Bissonnette R, Kabashima K, DeLozier AM, et al. Long-term Efficacy of Baricitinib in Adults With Moderate to Severe Atopic Dermatitis Who Were Treatment Responders or Partial Responders: An Extension Study of 2 Randomized Clinical Trials. JAMA Dermatol. 2021;157(6):691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–66. [DOI] [PubMed] [Google Scholar]
- 81.Gooderham MJ, Chu CY, Rojo R, Valdez H, Biswas P, Cameron MC, et al. Economic impact of abrocitinib monotherapy and combination therapy in patients with moderate-to-severe atopic dermatitis: Results from JADE MONO-2 and JADE COMPARE. JAAD Int. 2021;4:46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cork MJ, McMichael A, Teng J, Valdez H, Rojo R, Chan G, et al. Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: an analysis of patient-reported outcomes. J Eur Acad Dermatol Venereol. 2022;36(3):422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang EQ, Le V, O’Gorman M, Tripathy S, Dowty ME, Wang L, et al. Effects of Hepatic Impairment on the Pharmacokinetics of Abrocitinib and Its Metabolites. J Clin Pharmacol. 2021;61(10):1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA Dermatol. 2021;157(10):1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Dowty ME, Wouters A, Tatulych S, Connell CA, Le VH, et al. Assessment of the Effects of Inhibition or Induction of CYP2C19 and CYP2C9 Enzymes, or Inhibition of OAT3, on the Pharmacokinetics of Abrocitinib and Its Metabolites in Healthy Individuals. Eur J Drug Metab Pharmacokinet. 2022;47(3):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC, Katoh N, et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol. 2022;158(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021;157(9):1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohamed MF, Jungerwirth S, Asatryan A, Jiang P, Othman AA. Assessment of effect of CYP3A inhibition, CYP induction, OATP1B inhibition, and high-fat meal on pharmacokinetics of the JAK1 inhibitor upadacitinib. Br J Clin Pharmacol. 2017;83(10):2242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854–62. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Oda M, Kabashima K, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(6):1575–83. [DOI] [PubMed] [Google Scholar]
- 91.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823–31. [DOI] [PubMed] [Google Scholar]
- 92.Bissonnette R, Call RS, Raoof T, Zhu Z, Yeleswaram S, Gong X, et al. A Maximum-Use Trial of Ruxolitinib Cream in Adolescents and Adults with Atopic Dermatitis. Am J Clin Dermatol. 2022;23(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong X, Chen X, Kuligowski ME, Liu X, Liu X, Cimino E, et al. Pharmacokinetics of Ruxolitinib in Patients with Atopic Dermatitis Treated With Ruxolitinib Cream: Data from Phase II and III Studies. Am J Clin Dermatol. 2021;22(4):555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–72. [DOI] [PubMed] [Google Scholar]
- 95.Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145(2):572–82. [DOI] [PubMed] [Google Scholar]
- 96.Kim BS, Sun K, Papp K, Venturanza M, Nasir A, Kuligowski ME. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82(6):1305–13. [DOI] [PubMed] [Google Scholar]


