Abstract
Intestinal health is critically important for the digestion and absorption of nutrients and thus is a key factor in determining performance. Intestinal health issues are very common in high performing poultry lines due to the high feed intake, which puts pressure on the physiology of the digestive system. Excess nutrients which are not digested and absorbed in the small intestine may trigger dysbiosis, i.e. a shift in the microbiota composition in the intestinal tract. Dysbiosis as well as other stressors elicit an inflammatory response and loss of integrity of the tight junctions between the epithelial cells, leading to gut leakage. In this paper, key factors determining intestinal health and the most important nutritional tools which are available to support intestinal health are reviewed.
Keywords: Poultry, Intestinal health, Inflammation, Gut leakage, Microbiota, Feed additive
1. Introduction
The complete ban on the use of antimicrobial growth promoters (AGP) in animal feed in the E.U. came into force on January 1, 2006 (Regulation 1831/2003/EC). It signaled the start of similar initiatives in many different countries all over the world. Following this ban, gut health issues, collectively called dysbacteriosis or dysbiosis, became apparent, especially in broilers. This was most obvious in those countries where, together with the AGP ban, the use of ionophore coccidiostats was also banned.
In hindsight, it is remarkable that there is still no consensus about the mode of action of AGP, even after decades of use (Danzeisen et al., 2011). Different and sometimes contradictory hypotheses have been put forward, most of which, however, were not underpinned by experimental evidence. Nevertheless, there is agreement that AGP somehow seem to dampen intestinal inflammation and reduce bile salt deconjugation (Lin, 2014), thus masking gut health issues (Smith, 2019).
The ban on AGP has triggered a renewed scientific interest in the intestinal health of animals. Whilst in the past, the focus of gut health research was almost exclusively on the veterinary aspects of pathogenic organisms invading the intestine and/or intestinal tissues (helminths, protozoa, bacteria and viruses), causing severe damage to the host mucosa and resulting in clinical symptoms of disease, the current focus is on the fundamental aspects of the numerous complex and subtle interactions between the host mucosa, the intestinal content and all organisms residing in the intestinal tract.
By investigating the mechanisms of gut health, people have come to realize that, from now on, progress in poultry nutrition can only be made by taking into account the effects on gut health that may come from any change in feed formulation and/or processing.
In less than 20 years, this new field of research has grown to become a scientific area of its own: the science of the intestinal ecosystem. It has been boosted by new and advanced research tools becoming available, especially the so-called omics technologies (Dehau et al., 2022; Goossens et al., 2022).
Intestinal health is an extremely complex topic with numerous different aspects to it. It is not possible and therefore also not the aim of this review to cover all these aspects, but rather to highlight some of the most important areas that are directly linked to, and can be influenced by nutrition. This review thus focuses on the current state of knowledge regarding the possible impact of feed formulation on the intestinal ecosystem. First, some basic principles of poultry intestinal physiology and immunology, which play a role in the maintenance of gut health, are highlighted. Then the question of why and how gut health is under pressure in the modern broiler will be addressed, with particular emphasis on the three main and interrelated drivers of poor intestinal health, namely dysbiosis, mucosal barrier leakage and inflammation.
2. The physiology of gut health
The modern broiler is characterized by highly efficient feed conversion and fast growth. Such performance requires massive feed intake, which puts enormous pressure on the physiology of the gastrointestinal (GI) tract (Svihus, 2014). Through evolution, the anatomy and physiology of the GI tract of birds were not designed to cope with a continuous flow of copious amounts of feed, but rather to extract a maximum amount of nutrients from limited amounts of poor quality feed. Indeed, in nature there is a continuous selection of those individuals that survive episodes of starvation. The survivors are those who are able to efficiently extract the highest amount of nutrients from often poorly digestible feedstuffs. Millions of years of selection pressure have led to a consolidation of the different steps of the digestive process, characterized by initial moisturization and acidification of the feed, together with mechanical size reduction of the feed particles. Then a series of enzymes take care of a stepwise breakdown of the nutrients into small molecules that become suitable ligands for selective receptor-mediated uptake by the small intestinal epithelial cells. Finally, the undigestible fraction is partly fermented by a host-adapted microbial population located in the hindgut (with the notable exception of ruminants, which also have an important fermentative microbiome in their forestomachs).
When focusing on the different segments of the GI tract it is remarkable that the first segment, i.e. the oral cavity, in birds is merely designed to pass the feed particles from the beak into the pharynx and further down the oesophagus, as opposed to in mammals, where the more developed tongue must push the feed between the teeth for mechanical particle reduction. After swallowing, a considerable fraction of the feed is temporarily stored in the crop in birds, where it is moisturized and acidified and slightly fermented by lactobacilli (Classen et al., 2016). Passage into the proventriculus and gizzard compartment ensures further acidification and initial enzymatic breakdown of the feed proteins in the proventriculus and particle size reduction in the gizzard (Svihus, 2011). Gizzard development and function is enhanced by the presence of insoluble fiber in the feed (Sacranie et al., 2012). The function of this entire proximal segment of the GI tract, collectively named the foregut, can be manipulated by different feeding strategies (Rodrigues and Choct, 2018).
Once past the proventriculus and gizzard complex, the feed reaches the duodenum, where it is admixed with bile and pancreatic enzymes, further preparing the nutrients for absorption. Close to the epithelial surface, enzymes secreted by the epithelial cells, including the aminopeptidases, ensure the final preparation of the nutrients for absorption. Aminopeptidase Ey was recently shown to be a potential marker of intestinal health which could be detected in intestinal content and faeces of broilers (De Meyer et al., 2019).
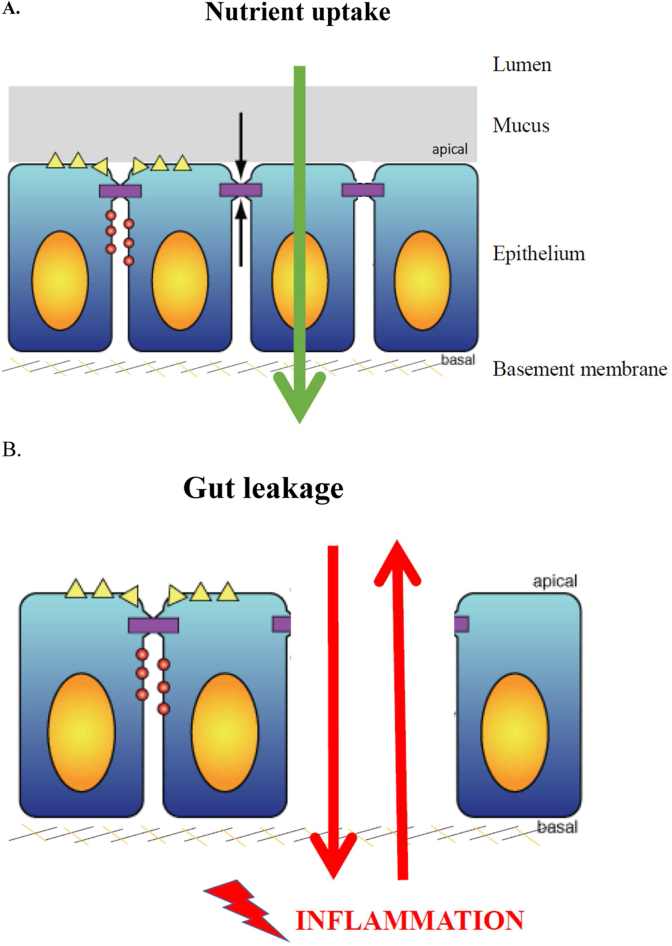
The epithelial cells in the small intestine form a continuous layer, and the space between the cells is sealed by tight junctions (Fig. 1). These tight junctions are a critical element of the gut barrier, maintained at a high energy cost by the epithelial cells (von Bucholz et al., 2022). Changes in the expression level and functioning of tight junctions cause gut leakage, characterized by body fluids leaking into the intestinal lumen, which may ultimately result in wet faeces (Binienda et al., 2020).
Fig. 1.
The intestinal epithelial cell is the orchestrator of gut health. (A) Nutrients, prepared for absorption by the digestive enzymes, are taken up through the transcellular pathway (green arrow) by a receptor-mediated (yellow triangles) process, while the intercellular spaces are sealed by tight junctions (black arrows). (B) Gut leakage is due to opening-up of the intercellular pathway (red arrows) following tight junction loss or even complete epithelial cell loss. The ensuing uncontrolled inside-out and outside-in fluxes allow contact of potentially harmful intestinal content with the Toll-like and other receptors that trigger inflammation.
Absorption of nutrients by epithelial cells in the small intestine is a transcellular, receptor-mediated process, characterized by different receptors for different nutrients being expressed on the luminal side of the plasma membrane of the villus epithelial cells. At the same time, exclusion of passive diffusion of potentially harmful small molecules from the intestinal lumen through the intestinal barrier is taken care of by a family of receptors collectively named ABC transporters, of which P-glycoprotein is the most important member. Expression of P-glycoprotein has been demonstrated in the GI tract of the chicken, with the highest expression in the small intestine (Barnes, 2001; Haritova et al., 2010). The expression and function can be downregulated and inhibited by certain drugs in chickens (Barnes, 2001) and by naringin from grapefruit in humans, leading to loss of integrity of the mucosal barrier. This might explain the disadvantage of grapefruit peel as opposed to orange peel as a feed ingredient in broiler diets (Vlaicu et al., 2020).
Glutamine is used as the primary fuel source for the highly metabolically active cells of the small intestinal villus epithelium in different animal species (Labow and Souba, 2000). Under challenge conditions, the available glutamine (endogenous and from feed) is insufficient, leading to energy deficit in the intestinal epithelial cells, as has been shown many years ago in a rat model (Fox et al., 1988). As a consequence, there is not enough energy to maintain the tight junctions between the epithelial cells, leading to gut leakage. In chickens, glutamine supplementation has been shown to protect intestinal mucosal morphology and function under conditions of intestinal stress, such as necrotic enteritis (Xue et al., 2018). Injection of glutamine in ovo on day 17 of incubation stimulates small intestinal maturation (Reicher et al., 2022).
As mentioned above, high performing commercial chicken breeds are characterized by extremely high feed intake. This puts a lot of stress on the physiology of the entire GI tract, but particularly so on the absorptive epithelial cells of the small intestine. In order to cope with stress and damage, this population of cells needs to be renewed continuously. New enterocytes are formed by rapid cell division in the crypts, with the newly formed cells then moving up the villus while maturing and expressing the receptors and enzymes needed for the job. At the end of the maturation process and triggered by different stressors, the epithelial cells located at the tips of the villi undergo apoptosis and are released into the intestinal lumen. Increased damage will lead to higher cell loss on the villi and result in shortening of villi and elongation of crypts in an attempt to compensate for the cell loss by increasing multiplication of cells in the crypts, hence the value of measuring villus to crypt ratio as a criterion of intestinal health. As an example, we showed that replacing inorganic zinc sulphate in the diet of broilers with a zinc amino acid complex increased villus length and villus to crypt ratio, suggesting that even inorganic zinc may place increased (oxidative) stress on epithelial cells (De Grande et al., 2020).
The ileum in birds is the small intestinal segment between Meckel's diverticulum and the caeca. Here the villi are smaller. Content from the ileum, colon and cloaca is intermittently transported into the caeca by peristaltic and antiperistaltic movements of the ileum and colon. Caecal development and motility is influenced by the fiber content of the feed (Svihus et al., 2013). The caeca constitutes the true fermentation vessel of the GI tract in birds, with around 1011 cfu/mL of bacteria in the lumen. Intestinal health in this segment of the GI tract is therefore strongly influenced by microbe-host interactions. At the end of the fermentation process, caecal content is directly shed into the environment as caecal droppings. The caeca are also the niche where non-host specific serotypes of Salmonella reside, hence the strategy of sampling caecal droppings as the most sensitive way of detecting non-host specific Salmonella carriage in birds (Heyndrickx et al., 2002). The damage to intestinal health caused by non-host specific Salmonella serotypes such as Salmonella Enteritidis is, however, usually very limited (Desmidt et al., 1998).
3. The immunology of gut health
The intestinal immune system has two different and seemingly conflicting tasks: building a defensive immune response against pathogenic invaders, as well as establishing tolerance towards symbiotic microbes and foreign antigens contained in feed. The latter is called ‘oral tolerance’.
Oral tolerance is defined as a state of active suppression of immune responses to ingested soluble antigens mediated by the gut-associated lymphoid tissue (Tordesillas and Berin, 2018). Contact of the immune system with these antigens, which are present in the intestinal lumen, is ensured i. a. by antigen presenting cells which sample across the intestinal mucosal barrier (Allen et al., 2016). These antigens are subsequently presented to the immune system in the mesenteric lymph nodes in mammals and in the spleen in birds. Oral tolerance is initiated in these organs i. a. by interleukin 10 and a specific lymphocyte subset, namely the regulatory T-lymphocytes in mammals (Nowak-Wegrzyn and Chatchatee, 2017). Recent studies on T-cell lineages have confirmed Th17 cells and regulatory T-cells play a role in maintaining oral tolerance and gut homeostasis in chickens (fore review see: Kim et al., 2019a), thereby underscoring the highly conserved nature of these mechanisms across distant animal species. In mammals, these regulatory T-cells are induced by the presence of butyrate-producing Ruminococcaceae and Lachnospiraceae (formerly Clostridium clusters IV and XIVa) in the intestinal lumen (Atarashi et al., 2010). These same two families of strictly anaerobic, butyrate-producing, gram positive bacteria also constitute the majority of the microbiota residing in the caeca of chickens (Rychlik, 2020). It is therefore assumed that they are also involved in the induction of oral tolerance in the chicken. In addition, a population of tissue-resident T-lymphocytes, residing in between the intestinal epithelial cells, the so-called intraepithelial lymphocytes, is considered to play a prominent role in gut health and homeostasis in humans and other mammals (Olivares-Villagomez and Van Kaer, 2018). We recently showed that this population of intraepithelial lymphocytes can be stimulated in the small intestinal mucosa of chickens by adding a peptidoglycan-degrading enzyme to the feed (Wang et al., 2021). The enzymatic degradation of bacterial peptidoglycan has been shown to generate nucleotide-binding oligomerization domain (NOD)-activating muramyl dipeptides. In humans it is well established that recognition of the intestinal microbiota by NOD2 is necessary for the homeostasis of the intraepithelial lymphocyte population (Jiang et al., 2013).
Innate immune defense lines together form the so-called ‘gut barrier’. Mucus is the first line of defense, which largely precludes adhesion and permeation of microorganisms and toxins through the gut barrier, thereby preventing the development of inflammation (Johansson and Hansson, 2016). In the lower intestinal tract (in the chicken, the caeca), however, more copious amounts of mucus are secreted and also serve to feed the microbiota (Marcobal et al., 2013; Schroeder, 2019).
The epithelial cells immediately below the mucus layer together with the intraepithelial lymphocyte population form the second line of defense (Van Camelbeke and Vermeire, 2017).
Candidatus Savagella (formerly: segmented filamentous bacteria) is yet another group of organisms that has been shown to be a potent inducer of the intestinal immune system, at least in the mouse model. Candidatus Savagella is segmented and filamentous in shape and attaches to the epithelium of the ileum. These organisms are also present in the chicken ileum (Redweik et al., 2020).
Innate immune mechanisms are particularly well developed in the small intestine. They are meant to protect not only against potentially harmful and invading pathogens, but also against any bacteria coming in close contact with the epithelial cells or the underlying lamina propria. Alongside rapid feed passage and the presence of bile salts and digestive enzymes, innate immunity contributes to the remarkably low density of microbiota in the duodenum and jejunum. The innate immune mechanisms include a protective mucus layer, secretory IgA, host defense peptides such as β-defensins and cathelicidins (Yacoub et al., 2015; Veldhuizen et al., 2013) and lysozyme, all of which are designed to defend against invading microorganisms. The sensors that alert the host about imminent invasion, the Toll-like receptors, are even more elaborate in chickens than in humans (Swiderska et al., 2018). It is clear that, under conditions of intestinal stress or overwhelming presence of invaders, these innate immune defense mechanisms are insufficient. Under these conditions it may be beneficial to support these endogenous mechanisms using exogenous supplements. Such beneficial effects have been documented in experimental necrotic enteritis in broilers (Liu et al., 2010).
The rich and complex microbiota residing in the lumen of the caeca and colonizing the caecal mucus layer play a key role in the overall development of the intestinal adaptive immunity, which is characterized by tolerance towards feed- and luminal microbial antigens and a protective response against pathogenic microorganisms (Kogut et al., 2020).
4. Intestinal health and the microbiome
It is generally accepted now that the microbiota plays a crucial role in intestinal health. Diversity and density of the microbiome vary greatly in different regions of the GI tract. In the crop and in the ileum, members of the Lactobacillaceae family dominate, usually representing more than 90% of the microbial population. These populations have not been investigated in great detail, as opposed to the caecal microbiome, which has been the subject of numerous studies (Rychlik, 2020).
4.1. Establishment of the caecal microbiome
Initial colonization of the caeca appears to be dominated by members of the Enterobacteriaceae family (Videnska et al., 2014). By the end of the first week after hatching, a considerable fraction of Enterobacteriaceae is replaced by butyrate-producing members of the families Lachnospiraceae and Ruminococcaceae, which become dominant, often representing more than 60% of the microbial population in the caecal lumen (Videnska et al., 2014). Contact with the mother hen appears to be important for colonization of the caeca by the phyla Actinobacteria and Bacteriodota. The latter can be completely absent when there is no contact with the hen (Kubasova et al., 2019). Once fully established, the caecal microbiota of poultry is composed of a rich and dense population of bacteria belonging to many different genera and species, but largely belonging to only four different phyla, namely Firmicutes, Bacteriodota, Proteobacteria and Actinobacteria. Over the years, several epidemiological studies have tried to correlate the presence and abundance of certain genera and families with performance, especially in broilers (Stanley et al., 2016). Although no causal relationships can be inferred from these studies, they may represent a useful reference for further attempts to try and influence the microbiome for improved gut health or perhaps to identify new candidate probiotic bacteria.
4.2. Microbial metabolites as gut health modulating signals
When taking into consideration that intestinal microbiota in vertebrates have been shaped by millions of years of coevolution, it is not surprising that the microbe–microbe interactions and microbe-host interactions in the intestinal ecosystem are extremely complex and only a small fraction of it has been discovered and investigated so far. Nevertheless, these interactions have a determining role not only in gut health but also in the health of animals and humans in general. As mentioned above, the intestinal commensal microbes are immune modulators. They are, however, much more than that. The interaction with the host can be direct, as in the case of bacteria attaching to the epithelium or being taken up by antigen presenting cells. The interaction can also be indirect, through ‘interkingdom signals’. These signals are mostly metabolites produced by the bacteria, and sensed by the host. Many of these signals are sensed through epithelial G-protein coupled receptors (GPR). In humans and laboratory animals, an increasing number of these GPR and their ligands have been identified. Even if not all of these GPR as known in humans do have a counterpart in the chicken, it is clear that the GPR family and their ligands of bacterial origin have an important part to play in gut health, including that of poultry. In this review, only a selection of the bacterial metabolites and their respective GPR that are most relevant to gut health are discussed.
GPR41, GPR43 and GPR109a are receptors for the short chain fatty acids acetate, propionate and butyrate (Brown et al., 2003). Butyrate is one of the most important end metabolites of the lower intestinal microbial metabolic network, which is highly conserved across animal species. It is considered an ‘interkingdom signaling molecule’, a signal produced by the microbial kingdom and sensed by the animal kingdom. It exerts a myriad of effects not only on intestinal health but also on general health (Guilloteau et al., 2010). Butyrate is also, together with propionate, the preferred energy source for the epithelial cells of the lower GI tract. We showed that stimulating butyrate production in the caeca of broilers by adding xylanases to a wheat based diet was associated with an increased density of L-cells in the ileum (Yacoubi et al., 2018). These enteroendocrine L-cells, which are present in the epithelium throughout the lower intestinal tract, release glucagon-like peptide 2 into the blood when stimulated by short chain fatty acids, especially butyrate, as was shown in studies on mammals (Tappenden et al., 2003). Glucagon-like peptide-2 is a known hormonal stimulant of the growth and differentiation of small intestinal epithelial cells. In this way, production of short chain fatty acids in the lower intestinal tract is supporting gut health in the upper intestinal tract.
Indole is another well known interkingdom signaling molecule. It is synthesized by a large number of different bacterial species from tryptophan (Lee et al., 2015). Adding indole derivatives to the feed of chickens was shown to protect against the damaging effects of coccidiosis (Kim et al., 2019b). These and other (immunomodulatory) effects of indole and closely related molecules in the host are mediated through the aryl hydrocarbon receptor (Gao et al., 2018; Kim et al., 2019b, Kim et al., 2019a). These effects were confirmed in a mouse model, where indole was shown to reinforce the tight junctions between the intestinal epithelial cells and dampen inflammation (Bansal et al., 2010).
Lactate is an important intermediate metabolite in the intestinal microbial network. We showed that lactobacilli, which produce lactate on a substrate of xylooligosaccharide prebiotics, cross feed to members of the Lachnospiraceae family which can consume the lactate to produce butyrate in broilers (De Maesschalck et al., 2015), thereby generating an intestinal health promoting effect. In a mouse model, the microbial lactate also exerts a direct beneficial effect on the intestinal mucosa, mediated by the receptor GPR81 (Ranganatan et al., 2018). Lactate is, however, a double edged sword. It is produced by many different bacterial species, but the ability to utilize lactate as a nutrient source seems to be restricted to a limited number of the anaerobic lower intestinal bacterial species, thus holding the risk of lactate accumulation (Sheridan et al., 2022). Indeed, in case of excessive production, lactate has been shown to promote hydrogen sulfide formation in the colon in humans, and to promote reactive oxygen species production in the intestine of Drosophila (Latsenko et al., 2018; Marquet et al., 2009). The harmful (pro-inflammatory) effects of excessive microbial lactate formation have hitherto remained largely unexplored in poultry.
Succinate is an intermediate metabolite in microbial propionate synthesis (Reichardt et al., 2014). GPR91 is the receptor for succinate in mice (Diehl et al., 2016). In newly hatched broiler chicks we showed beneficial effects on intestinal health from feeding amorphous cellulose, characterized by the expansion of the succinate-producing genus Alistipes in the caeca (De Maesschalck et al., 2019). Succinate produced by the microbiota and taken up by the intestinal epithelium is, however, not used as energy source by the epithelial cells, but rather acts as a substrate for intestinal gluconeogenesis in mice (De Vadder et al., 2016).
For a range of other microbial metabolites, including methane, hydrogen sulfide, carbon dioxide, formate, hydrogen and nitric oxide, effects on intestinal health have been described in laboratory animals, but their role in poultry gut health needs further investigation (Boros et al., 2012; Farugia and Szurszewski, 2014; Oliphant and Allen-Vercoe, 2019).
Commensal gut bacteria also produce considerable amounts of metabolites which, in the host, are important neurotransmitters, such as serotonin and γ-aminobutyric acid (GABA). GABA production has been reported for a number of intestinal bacteria, including lactic acid bacteria, Escherichia coli and members of the phylum Bacteroidetes (Dhakal et al., 2012; Medvecky et al., 2018). GABA receptors are present in the enteric nervous system of mouse colon (Seifi et al., 2014), suggesting possible regulatory effects of microbiota-derived neurotransmitters on numerous gastrointestinal functions and thus also on intestinal health. The role of microbiota-derived neurotransmitters in poultry intestinal health is still largely unexplored.
4.3. Dysbiosis, a shift in the microbiota towards production of proinflammatory signals
The question ‘which are the good bugs and which are the bad guys?’ has intrigued many scientists and field practitioners alike. The answer is not so simple and straightforward. Results of numerous experimental, epidemiological and comparative studies, however, have revealed some trends for which there is consensus. For example, untoward expansion of the phylum Proteobacteria seems to be harmful in different animal species and in humans (Shin et al., 2015). Many proteobacteria are known producers of hydrogen sulfide which, in high concentrations, is toxic for epithelial cells and a trigger of inflammation (Lim et al., 2022). Conversely, depletion of butyrate-producing taxa, especially those belonging to the families of Lachnospiraceae and Ruminococcaceae, leads to alterations in epithelial cell metabolism and loss of the anti-inflammatory protection of high butyrate production (Rivera-Chavez et al., 2016). As it happens, both the expansion of Proteobacteria and loss of butyrate producers are typical effects seen in dysbiosis.
5. Intestinal inflammation and gut leakage
It is now well established that even in perfectly healthy animals (and humans) there is always a certain level of ‘physiologic inflammation’ in the GI tract (Rabinowitz and Mayer, 2012; Kogut et al., 2018). This allows for immediate response in case of an imminent threat, which is important for the intestinal mucosa, as it is continuously exposed to numerous potentially harmful substances and (micro)organisms that are ingested. For the same reason, the entire mucosa of the GI tract is equipped with sensors that can rapidly alert and activate a powerful defensive inflammatory response, through the nuclear factor kappa B (NFκB) cascade. These sensors include the Toll-like receptors, mentioned above (Velova et al., 2018), and a range of other receptors.
5.1. Proinflammatory signals
Many different triggers can lead to rapid upregulation of intestinal inflammation.
One such trigger is the presence of pathogenic microorganisms, which may damage the gut barrier and/or invade through the mucosa and become life-threatening for the host. Mucosal damage will expose the Toll-like receptors, which are present in the mucosal lamina propria, to their ligands, which are present in the intestinal lumen (lipopolysaccharides, peptidoglycan, flagellin, etc).
The inflammatory trigger can, however, be much more subtle. It has even been established that any (environmental) stressor which induces dysfunction of the gut barrier will trigger inflammation (Lambert, 2009). Most of these stressors will cause oxidative stress to the intestinal epithelial cells (Durand et al., 2022). One such stressor which is of particular importance in poultry production is heat stress (Ahmad et al., 2022). Nutritional stressors can also trigger intestinal inflammation, such as excess protein in the feed. When dietary protein is not efficiently digested and absorbed it becomes a substrate for the microbiota and triggers dysbiosis, a shift in the intestinal microbiome characterized by the expansion of proinflammatory microbial populations. The exact mechanisms by which these microbes exert their proinflammatory effects in chickens are still under investigation (Gilbert et al., 2018). Metabolome studies in human inflammatory bowel disease patients, however, point towards a microbial metabolite-mediated dysregulation of the intestinal barrier, leading to leaky gut (Iyer and Corr, 2021).
5.2. Anti-inflammatory signals
In the healthy animal, the intestinal immune system is able to control the inflammatory environment of the gut, despite continuous exposure to high levels of bacteria, through negative feedback mechanisms on existing inflammation.
Powerful negative feedback mechanisms allow for precise regulation of intestinal inflammation through a range of regulatory pathways which are modulated by commensal bacteria. Amongst these regulatory pathways, the NOD receptor pathway (Philpott et al., 2014) and the peroxisome proliferator-activated receptor γ (PPARγ) pathway (Nepelska et al., 2017) are best documented for their negative feedback on intestinal inflammation. In the case of NOD, continuous activation will dampen down inflammation, while temporary activation will enhance inflammation (Watanabe et al., 2014). When taking into consideration that high performing birds tend to have a continuous elevated level of intestinal inflammation, it may be useful to support these negative feedback mechanisms. In line with this strategy, we recently showed that a microbial muramidase added to broiler feed was able to break down peptidoglycan from the intestinal microbiome into muramyl dipeptide, a NOD ligand, thereby dampening down inflammation in the duodenum of broilers (Wang et al., 2021).
PPARγ is an intracellular butyrate receptor. Interaction of butyrate with this receptor has been shown to inhibit expansion of Enterobacteriaceae (Byndloss et al., 2017). Numerous papers report the use of butyrate supplements in feed or other feed supplements stimulating endogenous butyrate production by caecal microbiota in order to protect gut health and thereby improving performance in poultry (Onrust et al., 2015).
Activation of GPR43 by microbial short chain fatty acids is yet another regulatory mechanism dampening down intestinal inflammation in the mouse model (Yang et al., 2018). The anti-inflammatory effects of butyrate (and propionate) are also well documented in poultry, in spite of a gap in knowledge regarding the avian receptors involved (Zou et al., 2019).
5.3. The intestinal inflammatory phenotype
Triggering of the inflammation cascade by proinflammatory signals leads to a shift in the entire intestinal ecosystem in which a fundamental change in the metabolism of the epithelial cells seems to play a pivotal role. Whereas the metabolism of the epithelial cells of the lower intestinal tract (colon and caecum in mammals, caeca in chickens) in healthy organisms is characterized by high oxygen consumption, these epithelial cells switch to low oxygen consumption/high lactate production in the case of inflammation (Litvak et al., 2018). This leads to increased oxygen in the lumen, which is reported as being a driver of dysbiosis (Rivera-Chavez et al., 2017). Since dysbiosis is a known trigger of inflammation, and inflammation in turn triggers dysbiosis, a vicious cycle evolves. The inflammatory state is associated with leakage of the tight junctions, which allows passage of pro-inflammatory compounds from the intestinal lumen across the gut barrier into the portal vein, finally reaching the liver. When this phenomenon is severe, the hepatocytes change their profile of protein secretion, commonly known as the acute phase response (O'Reilly and Eckersall, 2014). The newly secreted proteins pass into the general circulation and, due to the gut leakage, may end up in the intestinal content. We showed that one such acute phase protein, ovotransferrin, can be detected in intestinal content and even in faeces in the case of severe gut barrier damage in chickens (Goossens et al., 2018).
6. Steering the microbiome for improved gut health
In recent years numerous attempts have been made to modulate the intestinal microbiome through nutritional or management interventions with the aim to improve intestinal health in poultry (Kogut, 2019). Enzymes, prebiotics, probiotics, synbiotics and postbiotics all have been used in many different studies with the aim to support eubiosis and avoid dysbiosis in poultry. The effects of feed enzymes on the intestinal microbiome in chickens have been reviewed recently (Ducatelle et al., 2022). The effects of prebiotics on the intestinal health in poultry and pigs have also been reviewed recently (Azad et al., 2020; Ducatelle et al., 2015). Probiotics may have effects not only on the intestinal health of poultry (Ducatelle et al., 2015; Yacoob et al., 2022; Yousaf et al., 2022) but also general health effects, including improvement in bone metabolism (Chen et al., 2022). All of the above mentioned feed additives have documented effects on the gut microbiota, but it is not always absolutely clear whether the changes in intestinal microbiota are responsible for the observed beneficial effects on intestinal health. Further mechanistic studies are definitely warranted. Nevertheless, some fundamental underlying microbiota-mediated mechanisms are already fairly well documented, including those mediated by non-starch polysaccharides (NSP-fiber, mostly from plant cell walls) and protein utilization by the microbiota.
The microbiota residing in different sections of the GI tract are strongly influenced by the flow of undigested feed components. In high performing broilers, feed protein utilization in the small intestine is incomplete. On average 84 g/kg crude protein ends up in the faeces when broilers are fed ad libitum (Kim et al., 2022a). Heat stress further reduces protein retention in broilers (Habashy et al., 2017), hence the beneficial effects that can be obtained from protease supplementation in the diet (McCafferty et al., 2022). The interactions between the intestinal microbiota and protein have been reviewed by Apajalahti and Vienola (2016). Excess protein can be utilized by lactobacilli (Engels et al., 2022). Within physiological limits, this may foster expansion of butyrate-producing and lactate-consuming members of the Lachnospiraceae family. In very young broilers, which lack a fully established caecal microbiome, a similar microbial cross-feeding-based (lactobacilli–Lachnospiraceae), intestinal health supporting effect can be obtained also by supplying xylooligosaccharides in the feed (De Maesschalck et al., 2015). As mentioned above, however, excessive and untoward expansion of lactobacilli may lead to accumulation of lactate, a drop in pH and inhibition of Lachnospiraceae (Brownlie et al., 2022). Accumulation of lactate may also support Salmonella growth (Gillis et al., 2018). Residual protein that is fermented by putrefactive bacteria in the caeca results in the formation of compounds which, in high amounts, are toxic and pro-inflammatory, such as phenols, cresol, ammonia, amines and indoles (Apajalahti and Vienola, 2016). Adjusting the feed formula for optimal digestion of protein thus may help prevent dysbiosis and support intestinal health.
NSP in feed is undigestible. Depending on the ingredient composition (e.g. wheat-based vs corn-based), different amounts and types of NSP are delivered to different segments of the GI tract (Kim et al., 2022b). These NSP constitute the main substrate for the intestinal microbiota in the ileum and caeca. The insoluble fraction of NSP has especially been shown to exert beneficial effects on intestinal health through enhancing microbiota activity in the hind gut (Kheravii et al., 2018). These beneficial effects of fiber depend to some extent on the physical form and in particular on the size of the particles, and interactions between particle size and fiber have been reported (Kheravii et al., 2017). Using wheat bran that was reduced to different particle sizes, we showed that particles of around 180 μm are preferentially colonized by lactate- and butyrate-producing caecal microbial populations, excluding colonization by members of the Enterobacteriaceae family (Vermeulen et al., 2018). This enhances butyrate production in the caeca, making the birds less susceptible to Salmonella (Vermeulen et al., 2017). The beneficial effects of (moderate amounts of) fiber on intestinal health and performance also depend on the fiber source (Mateos et al., 2012). This points towards differential effects of NSP fractions with different chemical characteristics. The arabinoxylan fraction, which is the dominant fiber fraction in wheat, may be either indirectly (following initial degradation by members of the Bacteroidetes phylum) or directly used by members of the Ruminococcaceae family to generate butyrate. In feed, xylanase supplements can support expansion of Faecalibacterium, one of the most important genera in this family (Ravn et al., 2017). The cellulose fraction of NSP has documented beneficial effects on intestinal health and performance through succinate production by Alistipes spp., as mentioned above. Further work on the roles of different fiber fractions in intestinal health is ongoing and may provide additional tools for steering the microbiome.
7. Beyond the gut
Damage to the gut barrier allows leakage of potentially harmful compounds and microorganisms from the intestinal lumen into the portal circulation and from there directly to the liver. As mentioned above, this causes a change in the metabolism of the hepatocytes known as the acute phase response. Since the hepatocytes synthesize most of the plasma proteins, this will also lead to a change in the composition of plasma in the general circulation, thus potentially impacting on all organ systems in the body. The vascular route is, however, not the only route by which intestinal health has an impact on the different organ systems. The interaction of the digestive system with other organ systems is of particular interest in human medicine. Considerable research efforts have been made to unravel the mechanisms behind the gut–brain axis. More recently, investigations on these effects beyond the gut have been initiated in poultry as well, since it has been realized that this may explain why even minor deficiencies in intestinal health may have deleterious effects on general health and performance of birds (Bindari and Gerber, 2022).
Within the limits of this review it is not possible, however, to discuss all of the numerous effects of gut health issues on the different organ systems. Just as an example, effects on meat quality have been shown in heat stress-induced gut barrier damage (Cao et al., 2021). In humans, it has been well known for many years that chronic intestinal inflammation is often accompanied by reduced serum levels of retinol and retinol-binding protein (Janczewska et al., 1991). In an experimental model of gut leakage in broilers, using host proteome analyses of intestinal content samples, we recently found increased concentrations of retinol-binding protein 4 in the lumen of the ileum (De Meyer et al., 2019). Leakage of retinol-binding protein from the blood into the intestine may result in loss of anti-oxidant vitamin A and thus exacerbate the pro-oxidative effects of heat stress, which ultimately may also reflect on meat quality (Savaris et al., 2021). The extraintestinal effects of poor intestinal health, beyond those due to the negative effects of feed digestion and absorption, may be mediated by bacterial translocation (Baxter et al., 2019) and by bacterial metabolites. The latter have been reviewed for butyrate by Guilloteau et al. (2010).
8. Conclusions and perspectives
Many of the key mechanisms regulating gut health are highly conserved through evolution, as indicated by the similarities between birds and mammals, even if the genes involved in these processes are often only distantly related. In the chicken, as in other animal species, a basal level of ‘physiologic’ inflammation is rapidly upregulated by a wide range of nutritional and environmental stressors. Such stressors range from obvious gut pathogenic microorganisms to minor imbalances in nutrient composition of feed. Cross-talk between the gut microbiota and the host through metabolite sensing plays an essential role in the control of intestinal inflammation. In poultry gut health research, the present focus appears to be mainly on nutritional steering of the intestinal (essentially caecal) microbiome towards expansion of beneficial genera and species with the objective of avoiding dysbiosis and excessive gut inflammation/gut leakage in high performing commercial breeds. The next step is nutritional steering of the microbial metabolome towards production of beneficial ‘interkingdom signals’. Already, a range of different nutritional tools are available to steer the microbiome towards enhanced endogenous microbial butyrate production in the caeca of poultry (Onrust et al., 2015). Further efforts in this direction are to be expected.
Author contributions
Richard Ducatelle: Writing – original draft. Evy Goossens: Writing – review and editing. Venessa Eeckhaut: Writing – review and editing. Filip Van Immerseel: Writing – review and editing. All of the authors have read and approved the final version of this manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
Evy Goossens received a grant (PRIDIV2021001601) from Hankija Oy, Hyvinkää, Finland. Venessa Eeckhaut received a grant (D/01709/02) from Christian Hansen, Horsholm, Denmark.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Ahmad R., Yu Y.H., Hsiao F.S.H., Su C.H., Liu H.C., Tobin I., et al. Influence of heat stress on poultry growth performance, intestinal inflammation, and immune function and potential mitigation by probiotics. Animals. 2022;12:2297. doi: 10.3390/ani12172297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen F., Tong A.A., Huang A.Y. Unique transcompartmental bridge: antigen-presenting cells sampling across endothelial and mucosal barriers. Front Immunol. 2016;7:231. doi: 10.3389/fimmu.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalathi J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim Feed Sci Technol. 2016;221:323–330. [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Moose Y., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (Wash D C) 2010;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.A.K., Goa J., Ma J., Li T., Tan B., Huang X., et al. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim Nutr. 2020;6:379–388. doi: 10.1016/j.aninu.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D.M. Expression of P-glycoprotein in the chicken. Comp Biochem Physiol A. 2001;130:301–310. doi: 10.1016/s1095-6433(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Baxter M.F.A., Dridi S., Koltes D., Latorre J.D., Bottje W.G., Greene E.S., et al. Evaluation of intestinal permeability and liver bacterial translocation in two modern broilers and their jungle fowl ancestors. Front Genet. 2019;10:480. doi: 10.3389/fgene.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindari Y.R., Gerber P.F. Factors affecting chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poultry Sci. 2022;101 doi: 10.1016/j.psj.2021.101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binienda A., Twardowska A., Makaro A., Salaga M. Dietary carbohydrates and lipids in the pathogenesis of leaky gut syndrome: an overview. Int J Mol Sci. 2020;21:8368. doi: 10.3390/ijms21218368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros M., Ghyczy M., Erces D., Varga G., Tokes T., Kupal K., et al. The anti-inflammatory effects of methane. Crit Care Med. 2012;40:1269–1278. doi: 10.1097/CCM.0b013e31823dae05. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–21119. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Brownlie E.J.E., Chaharlangi D., Wong E.O., Kim D., Navarre W.W. Acids produced by lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut Microb. 2022;14 doi: 10.1080/19490976.2022.2046452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byndloss M.X., Olson E.E., Rivera-Chavez F., Tiffany C.R., Cevallos S.A., Lokken K.L., et al. Microbiota-activated PPAR-γ-signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Chowdhury V.S., Cline M.A., Gilbert E.R. The microbiota-gut-brain axis during heat stress in chickens: a review. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.752265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Xu T.T., Zhang C.D., Tong X.S., Shaucat A., He I.F., et al. Effects of probiotics and gut microbiota on bone metabolism in chickens: a review. Metabolites. 2022;12:1000. doi: 10.3390/metabo12101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen H.L., Apajalahti J., Svihus B., Choct M. The role of the crop in poultry production. World Poult Sci J. 2016;72:456–472. [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grande A., Leleu S., Delezie E., Rapp C., De Smet S., Goossens E., et al. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poultry Sci. 2020;99:441–453. doi: 10.3382/ps/pez525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehau T., Ducatelle R., Van Immerseel F., Goossens E. Omics technologies in poultry health and productivity – part 1: current use in poultry research. Avian Pathol. 2022;51:407–417. doi: 10.1080/03079457.2022.2086447. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., et al. The effects of xylo-oligosaccharides on performance and microbiota in broiler chickens. Appl Environ Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Daube G., et al. Amorphous cellulose feed supplement alters broiler caecal microbiome. Poultry Sci. 2019;98:3811–3817. doi: 10.3382/ps/pez090. [DOI] [PubMed] [Google Scholar]
- De Meyer F., Eeckhaut V., Ducatelle R., Dhaenens M., Daled S., De Deurwaerder A., et al. Host intestinal biomarker identification in a gut leakage model in broilers. Vet Res. 2019;50:46. doi: 10.1186/s13567-019-0663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmidt M., Ducatelle R., Haesebrouck F. Immunohistochemical observations in the ceca of chickens infected with Salmonella Enteritidis phage type four. Poultry Sci. 1998;77:73–74. doi: 10.1093/ps/77.1.73. [DOI] [PubMed] [Google Scholar]
- De Vadder F., Kovathceva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mitchieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metabol. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Dhakal R., Bajpai V.K., Baek K.-H. Production of GABA (γ – aminobutyric acid) by microorganisms: a review. Braz J Microbiol. 2012;43 doi: 10.1590/S1517-83822012000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J., Gries B., Pfeil U., Goldenberg A., Mermer P., Kummer W., Paddenberg R. Expression and localization of GPR91 and GPR99 in murine organs. Cell Tissue Res. 2016;364:245–262. doi: 10.1007/s00441-015-2318-1. [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Eeckhaut V., Haesebrouck F., Van Immerseel F. A review of prebiotics and probiotics for the control of dysbiosis: present status and future perspectives. Animals. 2015;9:43–48. doi: 10.1017/S1751731114002584. [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Van Immerseel F., Eeckhaut V., Goossens E. Enzymes in farm animal nutrition. 3rd ed. CAB Int. 2022. Enzymes and the microbiome in the post-antibiotic era. [Chapter 15] pp. 254–265. [Google Scholar]
- Durand D., Colin A., Merlot E., Baeza E., Guilloteau L.A., Le Foc’h N., Toma A., Fontané-Dicharry S., Gondret F. Implication of redox balance in animal health and performance at critical periods, insights from different animal species. Animal. 2022;16 doi: 10.1016/j.animal.2022.100543. [DOI] [PubMed] [Google Scholar]
- Engels W., Siu J., van Schalkwijk S., Wesselink W., Jacobs S., Bachmann H. Metabolic conversions by lactic acid bacteria during plant protein fermentations. Foods. 2022;11:1005. doi: 10.3390/foods11071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G., Szurszewski J.H. Carbon dioxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterol. 2014;147:303–313. doi: 10.1053/j.gastro.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.D., Kripke S.A., De Paula J., Berman J.M., Settle R.G., Rombeau J.L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. J Parenter Enteral Nutr. 1988;12:325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Gao J., Xu K., Liu H., Bai M., Peng C., Li T., Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M.S., Ijssenagger N., Kies A.K., van Mil S.W.C. Protein fermentation in the gut: implications for intestinal dysfunction in humans, pigs, and poultry. Am J Physiol Gastrointest Liver Physiol. 2018;315:G159–G170. doi: 10.1152/ajpgi.00319.2017. [DOI] [PubMed] [Google Scholar]
- Gillis C.C., Hughes A.R., Spiga L., Winter M.G., Zhu W.H., de Carvalho T.F., et al. Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe. 2018;23:54–64. doi: 10.1016/j.chom.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens E., Debyser G., Callens C., Degussem M., Dedeurwaerder A., Devreese B., et al. Elevated faecal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens. Vet Res. 2018;49:51. doi: 10.1186/s13567-018-0548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens E., Dehau T., Ducatelle R., Van Immerseel F. Omics technologies in poultry health and productivity – part 2: future applications in the poultry industry. Avian Pathol. 2022;51:418–423. doi: 10.1080/03079457.2022.2085545. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Fuller A.L., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int J Biometeorol. 2017;61:2111–2118. doi: 10.1007/s00484-017-1414-1. [DOI] [PubMed] [Google Scholar]
- Haritova A.M., Schrickx J., Fink-Gremmels J. Expression of drug efflux transporters in poultry tissues. Res Vet Sci. 2010;89:104–107. doi: 10.1016/j.rvsc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Heyndrickx M., Vandekerchove D., Herman L., Rollier I., Gryspeerdt K., De Zutter L. Routes for Salmonella contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect. 2002;129:253–265. doi: 10.1017/s0950268802007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer N., Corr S.C. Gut microbial metabolite-mediated regulation of the intestinal barrier in: the pathogenesis of inflammatory bowel disease. Nutrients. 2021;13:4259. doi: 10.3390/nu13124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewska I., Bartnik W., Butruk E., Tomecki R., Kazik E., Ostrowski J. Metabolism of vitamin A in inflammatory bowel disease. Hépato-Gastro. 1991;38:391–395. [PubMed] [Google Scholar]
- Jiang W., Wang X., Zeng B., Liu L., Tardivel A., Wei H., Han J., Robson MacDonald H., Tschopp J., Tian Z., Zhou R. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210:2465–2476. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Morgan N.K., Swick R.A., Choct M., Wu S.-B. Roles of dietary fibre and ingredient particle size in broiler nutrition. World Poult Sci. 2018;74:301–316. [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poultry Sci. 2017;96:3272–3281. doi: 10.3382/ps/pex123. [DOI] [PubMed] [Google Scholar]
- Kim E., Morgan N.K., Moss A.F., Li L., Ader P., Choct M. Characterisation of undigested components throughout the gastrointestinal tract of broiler chickens fed either a wheat- or maize-based diet. Anim Nutr. 2022;8:153–159. doi: 10.1016/j.aninu.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Morgan N.K., Moss A.F., Li L., Ader P., Choct M. The flow of non-starch polysaccharides along the gastrointestinal tract of broiler chickens fed either a wheat- or maize-based diet. Anim Nutr. 2022;9:138–142. doi: 10.1016/j.aninu.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Chaudhari A.A., Lillehoj H.S. Involvement of T-cell immunity in avian coccidiosis. Front Immunol. 2019;10:2732. doi: 10.3389/fimmu.2019.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Lillehoi H.S., Min W. Indole treatment alleviates intestinal tissue damage induced by chicken coccidiosis through activation of the aryl hydrocarbon receptor. Front Immunol. 2019;10:560. doi: 10.3389/fimmu.2019.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim Feed Sci Technol. 2019;250:32–40. [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poultry Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Lee A., Santin E. Microbiome and pathogen interaction with the immune system. Poultry Sci. 2020;99:1906–1913. doi: 10.1016/j.psj.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., et al. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow B.I., Souba W.W. Glutamine. World J Surg. 2000;24:1503–1513. doi: 10.1007/s002680010269. [DOI] [PubMed] [Google Scholar]
- Lambert G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Latenko I., Boquete J.-P., Lemaitre B. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens Drosphila lifespan. Immunology. 2018;49:922–942. doi: 10.1016/j.immuni.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Wood T.K., Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23:707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Lim D.R.X., Chen Y.H., Ng L.F., Gruber J., Gan Y.H. Glutathione catabolism by Enterobacteriaceae species to hydrogen sulphide adversely affects the viability of host systems in the presence of 5’fluoro-deoxyuridine. Mol Microbiol. 2022;117:1089–1103. doi: 10.1111/mmi.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Antibiotic growth promoters enhance animal production by targeting intestinal bile acid hydrolase and its producers. Front Microbiol. 2014;5:33. doi: 10.3389/fmicb.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M.X., Bäumler A.J. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362 doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo Y., Wang Z., Yuan J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010;39:17–24. doi: 10.1080/03079450903447404. [DOI] [PubMed] [Google Scholar]
- Marcobal A., Southwick A.M., Earle K.A., Sonnenburg J.L. A refined palate: bacterial consumption of host glycans in the gut. Glycobiology. 2013;23:1038–1046. doi: 10.1093/glycob/cwt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet P., Duncan S.H., Chassard C., Bernalier-Donadille A., Flint H.J. Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol Lett. 2009;299:128–134. doi: 10.1111/j.1574-6968.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Jimenez-Moreno E., Serrano M.P., Lazaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical charcteristics. J Appl Poultry Res. 2012;21:156–174. [Google Scholar]
- McCafferty K.W., Toghyani M., Morgan N.K., Cowieson A.J., Choct M., Moss A.F. Effect of protease supplementation and diet type on jejunal and ileal digestibility and total tract metabolisability of nitrogen, starch, and energy in broilers. Br Poultry Sci. 2022;63:386–394. doi: 10.1080/00071668.2021.1975260. [DOI] [PubMed] [Google Scholar]
- Medvecky M., Cejkova D., Polansky O., Karasova D., Kubasova T., Cizek A., Rychlik I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 2018;19:561. doi: 10.1186/s12864-018-4959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepelska M., Wouters T.D., Jacouton E., Béguet-Crepsel F., Lapaque N., Doré J., et al. Commensal gut bacteria modulate phosphorylation-dependent PPAR-g transcriptional activity in human intestinal epithelial cells. Sci Rep. 2017;7 doi: 10.1038/srep43199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak-Wegrzyn A., Chatchatee P. Oral tolerance is a state of active non responsiveness to ingested soluble antigens mediated by gut-associated intestinal lymphoid tissue. Ann Nutr Metab. 2017;70(suppl 2):7–24. doi: 10.1159/000457915. [DOI] [PubMed] [Google Scholar]
- Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagomez D., Van Kaer L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. 2018;39:264–275. doi: 10.1016/j.it.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrust L., Ducatelle R., Van Driessche K., De Maesschalck C., Vermeulen K., Haesebrouck F., Eeckhaut V., et al. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front Vet Sci. 2015;2:75. doi: 10.3389/fvets.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly E.L., Eckersall P.D. Acute phase proteins: a review of their function, behavior and measurement in chickens. World Poultrymeat J. 2014;70:27–43. [Google Scholar]
- Philpott D.J., Sorbara M.T., Robertson S.J., Croitoru K., Girardin S.E. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- Rabinowitz K., Mayer L. Working out mechanisms of controlled/physiologic inflammation in the GI tract. Immunol Res. 2012;54:14–24. doi: 10.1007/s12026-012-8315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P., Shanmugam A., Swafford D., Suryawanchi A., Bhattacharjee P., Husseim M.S., et al. GPR81, a cell surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J Immunol. 2018;200:1781–1789. doi: 10.4049/jimmunol.1700604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn J.L., Thogersen J.C., Eklöf J., Pettersson D., Ducatelle R., Van Immerseel F., et al. GH11 xylanase increases prebiotic oligosaccharides from wheat bran favouring butyrate-producing bacteria in vitro. Anim Feed Sci Technol. 2017;226:113–123. [Google Scholar]
- Redweik G.A., Kogut M.A., Arsenault R.J., Mellata M. Oral treatment with ileal spores triggers immunometabolic shifts in chicken gut. Front Vet Sci. 2020;7:629. doi: 10.3389/fvets.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation N° 1831/2003/EC of the European union; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1831.
- Reichardt N., Duncan S.H., Young P., Belenguer A., Mc William Leith C., Scott K.P., et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicher N., Melkman-Zihavi T., Dayan J., Wong E.A., Uni Z. Nutritional stimulation by in ovo feeding modulates cellular proliferation and differentiation in the small intestinal epithelium of chicks. Anim Nutr. 2022;8:99–101. doi: 10.1016/j.aninu.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez F., Lopez C.A., Bäumler A. Oxygen as a driver of dysbiosis. Free Radic Biol Med. 2017;105:93–101. doi: 10.1016/j.freeradbiomed.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Rivera-Chavez F., Zhang L.F., Faber F., Lopez C.A., Byndloss M.X., Olsan E.E., et al. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe. 2016;19:443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues I., Choct M. The foregut and its manipulation via feeding practices in the chicken. Poultry Sci. 2018;97:3188–3206. doi: 10.3382/ps/pey191. [DOI] [PubMed] [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals. 2020;10:103. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacranie A., Svihus B., Denstadli V., Moen B., Iji P.A., Choct M. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility and performance of broiler chickens. Poultry Sci. 2012;91:693–700. doi: 10.3382/ps.2011-01790. [DOI] [PubMed] [Google Scholar]
- Savaris V.D.L., Broch J., de Souza C., Rolhof Junior N., Sanchez de Avila A., Polese C., et al. Effects of vitamin A on carcass and meat quality in broilers. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.O. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep. 2019;7:3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi M., Brown J.F., Mills J., Bhandari P., Belelli D., Lambert J.J., et al. Molecular and function diversity of GABA-A receptors in the enteric nervous system of the mouse colon. J Neurosci. 2014;34:10361–10378. doi: 10.1523/JNEUROSCI.0441-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P.O., Louis P., Tsompanidou E., Shaw S., Harmsen H.J., Duncan S.H., et al. Distribution, organization and expression of genes concerned with anaerobic lactate utilization in human intestinal bacteria. Microb Genom. 2022;8 doi: 10.1099/mgen.0.000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae L.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Smith J.A. Broiler production without antibiotics: United States field perspectives. Anim Feed Sci Technol. 2019;250:93–98. [Google Scholar]
- Stanley D., Hughes R.J., Geier M.S., Moore R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol. 2016;7:187. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J Appl Poultry Res. 2014;23:306–314. [Google Scholar]
- Svihus B. The gizzard: function, influence of diet structure and effects on nutrient availability. World Poult Sci J. 2011;67:207–223. [Google Scholar]
- Svihus B., Choct M., Classen H.L. Function and nutritional roles of the avian caeca: a review. World Poult Sci J. 2013;69:249–263. [Google Scholar]
- Swiderska Z., Smidova A., Buchtova L., Bryjova A., Fabianova A., Munclinger P., et al. Avian Toll-like receptor allelic diversity far exceeds human polymorphism: an insight from domestic chicken breeds. Sci Rep. 2018;8 doi: 10.1038/s41598-018-36226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden K.A., Albin D.M., Bartholome A.L., Mangian H.F. Glucagon-like peptide-2 and short chain fatty acids: a new twist to an old story. J Nutr. 2003;133:3717–3720. doi: 10.1093/jn/133.11.3717. [DOI] [PubMed] [Google Scholar]
- Tordesillas L., Berin M.C. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. 2018;55:107–117. doi: 10.1007/s12016-018-8680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Exp Rev Gastroenterol Hepathol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E.J.A., Brouwer E.C., Schneider V.A.F., Fluit A.C. Chicken cathelicidins display antimicrobial activity against multiresistant bacteria without inducing strong resistance. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velova H., Gutovska-Ding M.W., Burt D.W., Vinkler M. Toll-like receptor evolution in birds: gene duplication, pseudogenization, and diversifying selection. Mol Biol Evol. 2018;35:2170–2184. doi: 10.1093/molbev/msy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen K., Verspreet J., Courtin C.M., Haesebrouck F., Baeyen S., Haegeman A., et al. Reduced-particle-size wheat bran is efficiently colonized by a lactic acid producing community and reduces levels of Enterobacteriaceae in the cecal microbiota of broilers. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.01343-18. 013433-e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen K., Verspreet J., Courtin C.M., Haesebrouck F., Ducatelle R., Van Immerseel F. Reduced particle size wheat bran is butyrogenic and lowers Salmonella colonization, when added to poultry feed. Vet Microbiol. 2017;198:64–71. doi: 10.1016/j.vetmic.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldinova M., Gerzova L., Ceikova D., et al. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaicu P.A., Untea A.E., Panaite T.D., Turcu R.P. Effect of dietary orange and grapefruit peel on growth performance, health status, meat quality and intestinal microflora of broiler chickens. Ital J Anim Sci. 2020;19:1394–1405. [Google Scholar]
- Von Bucholz J.S., Ruhnau D., Hess C., Aschenbach J.R., Hess M., Awad W.A. Paracellular intestinal permeability of chickens induced by DON and/or C. jejuni is associated with alterations in tight junction mRNA expression. Microb Pathog. 2022;168 doi: 10.1016/j.micpath.2022.105509. [DOI] [PubMed] [Google Scholar]
- Wang Y., Goossens E., Eeckhaut V., Perez Calvo E., Lopez-Ulibarri R., Eising I., et al. Dietary muramidase degrades bacterial peptidoglycan to NOD-activating muramyl dipeptides and reduces duodenal inflammation in broiler chickens. Br J Nutr. 2021;126:641–651. doi: 10.1017/S0007114520004493. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Asano N., Meng G., Yamashita K., Arai Y., Sakurai T., et al. NOD2 downregulates colonic inflammation by IRF4-mediated inhibition of K63-linked polyubiquitination of RICK and TRAF6. Mucosal Immunol. 2014;7:1312. doi: 10.1038/mi.2014.19. 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.D., Barekatian R., Wu S.B., Choct M., Swick R.A. Dietary L-glutamine supplementation improves growth performance, gut orphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poultry Sci. 2018;97:1334–1341. doi: 10.3382/ps/pex444. [DOI] [PubMed] [Google Scholar]
- Yacoob M.U., Wang G., Wang M. An updated review on probiotics as an alternative to antibiotics in poultry – a review. Anim Biosci. 2022;35:1109–1120. doi: 10.5713/ab.21.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub H.A., Elazzazy A.M., Abuzinadah O.A.H., Al-Heijn A.M., Mahmoud M.M., Harakeh S.M. Antimicrobial activities of chicken β-defensins (4 and 10) peptides against pathogenic bacteria and fungi. Front Cell Infect Microbiol. 2015;5:36. doi: 10.3389/fcimb.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi N., Saulnier L., Bonnin E., Devillard E., Eeckhaut V., Rhayat L., et al. Short chain arabinoxylans prepared from enzymatically treated wheat grain exert prebiotic effects during the broiler starter period. Poultry Sci. 2018;97:412–424. doi: 10.3382/ps/pex297. [DOI] [PubMed] [Google Scholar]
- Yang G., Chen S., Deng B., Tan C., Deng J., Zhu G., et al. Implication of G protein-coupled receptor 43 in intestinal inflammation: a mini-review. Front Immunol. 2018;9:1434. doi: 10.3389/fimmu.2018.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf S., Nouman H.M., Ahmed I., Husain S., Waseem M., Nadeem S., et al. A review of probiotic applications in poultry: improving immunity and having beneficial effects on production and health. Adv Microbiol. 2022;61:115–123. [Google Scholar]
- Zou X., Ji J., Qu H., Wang J., Shu D.M., Wang Y., et al. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poultry Sci. 2019;98:4449–4456. doi: 10.3382/ps/pez279. [DOI] [PubMed] [Google Scholar]