Abstract
Purpose:
We examined the association between polypharmacy—an established risk factor for nonadherence in the elderly—and medication fill nonadherence in a large national sample of adolescent and young adult cancer survivors (AYAs) in the U.S.
Methods:
We pooled data (2008–2017) from the Medical Expenditure Panel Survey. We defined polypharmacy as ≥3 unique medications prescribed, based on self-report and pharmacy data, and medication fill nonadherence as self-reported delay or inability to obtain a necessary medication. We estimated prevalence of medication fill nonadherence among AYAs (age 18–39 years with a cancer history). We used logistic regression to estimate the association between 1) polypharmacy and medication fill nonadherence in AYAs, and 2) total number of medications prescribed and medication fill nonadherence, controlling for sex, number of chronic conditions, disability, and survey year.
Results:
AYAs (n=598) were predominantly female (76.2%) age 30–39 years (64.9%), and non-Hispanic White (72.1%). Nearly half were poor (19.0%) or near-poor/low income (21.6%). One in ten AYAs reported medication fill nonadherence (9.75%). Of these, more than 70% cited cost-related barriers as the reason. AYAs with polypharmacy had 2.49 times higher odds of medication fill nonadherence (95%CI 1.11–5.59), compared to those without polypharmacy. Odds of medication fill nonadherence increased by 16% with each additional medication prescribed (AOR 1.16, 95% CI 1.07–1.25).
Conclusions:
Polypharmacy may be an important risk factor for medication fill nonadherence in AYAs in the U.S.
Implications for Cancer Survivors:
Improving AYAs’ medication adherence requires eliminating cost-related barriers, particularly for those with polypharmacy.
Keywords: adolescent and young adult cancer, medication adherence, polypharmacy, cancer survivors, prescription drugs
Introduction
Adherence to medications to treat cancer and chronic conditions is critical to achieving optimal health outcomes for cancer survivors.1–5 The World Health Organization defines adherence as the “extent to which a person’s behavior—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed medical recommendations” (WHO, 2003, p. 3). Other definitions have emphasized the active and interactive nature of adherence (and conversely, nonadherence), in terms of the degree to which a patient assumes responsibility for and works to maintain health in collaboration with health care providers.6,7 The National Comprehensive Cancer Network (NCCN) and the National Cancer Institute (NCI) have identified nonadherence as a primary concern in adolescents and young adults diagnosed with cancer between the ages of 15 to 39 (hereafter, AYAs).6,8,9 Adherence to anticancer treatments is crucial to preventing poor response to therapy, recurrence/relapse, and progression.3,10–15 Many AYAs face long cancer maintenance phases with oral chemotherapy, immunotherapy, and/or targeted agents which can last months to years beyond intensive treatment.16–19 In addition, AYAs experience a high burden of early onset-chronic health conditions secondary to their cancer treatment; up to half of AYAs experience one or more comorbidities in addition to cancer.20–24 These conditions, such as cardiovascular disease, often require one or more medications.20,25 Yet nonadherence to medications ranges from 21% to 60% in AYAs during and after intensive treatment, compromising health, quality of life, and survival.1,4,5,26–28
Achieving treatment goals, including management of chronic conditions other than cancer, requires understanding drivers of nonadherence in this underserved population. Fifteen years ago, the NCI called for research identifying drivers of nonadherence in AYAs,6,9 yet to date, research specific to this population has predominantly been conducted in small samples29–38 and/or single-center studies.3,39 Polypharmacy—taking multiple medications—is a known risk factor for nonadherence in the elderly; for example, in cohort studies, polypharmacy resulted in 33–44% lower odds of medication adherence, and each additional medication reduced adherence by 16%.40–42 Recent studies demonstrate that AYAs have double the prevalence of polypharmacy compared to age- and sex-matched peers without cancer.20,25,43 Further, AYAs have a high financial burden of treatment, are frequently uninsured, and have reported cost as a barrier to accessing medications, suggesting that having more prescriptions to fill may impede adherence.27,44–47 However, to our knowledge, no studies have examined the association between polypharmacy and medication fill nonadherence in AYAs.
Research to determine whether polypharmacy and number of medications prescribed are risk factors for nonadherence can inform interventions and guide patient-provider discussions around AYAs’ prescription medications to facilitate adherence. Such knowledge is particularly needed given recent suggestions that prescription medications may be may be a primary strategy for reducing the burden of accelerated aging in AYAs.48 To address these gaps, we used the Medical Expenditure Panel Survey (MEPS) to examine the association between polypharmacy and medication fill nonadherence in a large, nationally representative sample of AYA cancer survivors in the United States. We focused on delay in obtaining or inability to obtain prescription medications (hereafter, “medication fill nonadherence”), one of the most commonly studied dimensions of nonadherence.49,50
Methods
Data source and population.
MEPS is a population-based survey of U.S. households, in which adults (age ≥ 18 years) are invited to participate in five interviews (hereafter, “Rounds”) over two years to collect detailed information on healthcare utilization, expenditures, and health status of each member of the household. Details of the survey design are reported elsewhere.51 Our cross-sectional study pooled data from 2008–2017 (Panels 13–21).52 To minimize missing data, we included only individuals who participated in both survey years (n=147,082).
AYAs.
We defined AYAs as individuals age 18–39 years in the year of the first survey (n=43,263) and who reported having ever been diagnosed with cancer or malignancy by a doctor or other healthcare professional (n=689). MEPS only collects cancer information for individuals age ≥ 18 years, and since 2012, age at diagnosis is not reported. Based on the subset of respondents for whom age at diagnosis is reported (i.e., surveys conducted before 2012), we estimate that more than 90% of our sample were diagnosed between the ages of 15 to 39 years, with the remainder diagnosed before age 15 years. Similar to other analyses,27,43,53 we excluded those who reported a history of non-melanoma skin cancer or skin cancer of an unknown type (n=88).
Sociodemographic characteristics.
Sociodemographics were measured in the year of the first survey and included age, sex, race and ethnicity, marital status, education, poverty status, health insurance, and usual source of care. MEPS includes poverty status categories based on family income as a percentage of the applicable poverty line, including poor (<100%), near poor (100% to less than 125%), low income (125% to less than 200%), middle income (200% to less than 400%), and high income (≥400%). For usual source of care, interviewers asked whether, for each household member, there is a particular doctor’s office, clinic, health center, or other place that the household member usually goes to if he/she is sick or needs advice about his/her health.
Clinical factors and health indicators.
We examined cancer types and health indicators including chronic conditions and disability. Cancer types included breast, cervix, melanoma, lymphoma, and uterine. Beginning in 2012, cancer types reported with a frequency of <20 per year among all MEPS participants or those that were considered clinically rare (defined by the National Institutes of Health’s list of rare diseases54) were categorized as “other” due to privacy concerns. Common cancers in AYAs that MEPS categorized as “other” included brain, leukemia, thyroid, and testicular cancers. Similarly, we categorized the following cancer types with frequency <20 in our sample as “other”: bladder, blood, colon, esophageal, kidney, larynx, liver, lung, mouth/tongue/lip, ovarian, pancreatic, prostate, rectal, muscle/soft tissue/fat, and stomach cancers. We derived chronic conditions from among priority conditions asked about in MEPS, a majority of which have demonstrated prevalence in AYAs.23,55,56 These included arthritis, asthma, diabetes, emphysema, heart disease (including angina, coronary heart disease, myocardial infarction, and other heart disease), hypercholesterolemia, hypertension, and stroke. We summed MEPS priority conditions for each individual to create a count of chronic conditions (0,1,2+). Disability (yes/no) was based on reporting any limitation in the following domains: instrumental activities of daily living; activities of daily living; physical function; work, school, or housework; or seeing and/or hearing.
Polypharmacy.
We used prescription drug information collected in Rounds 1–3 (approximately one calendar year) to estimate polypharmacy in AYAs. MEPS verifies self-reported information on prescription medications collected during interviews by contacting a subset of participants’ pharmacies. Non-prescription medications (e.g., over-the-counter, supplements) are not included. We used generic drug names reported for each individual to determine the number of unique prescribed medications per individual, so that drugs differing by dose or manufacturer were not double-counted. Consistent with our prior work on polypharmacy,43 we summed the number of unique prescribed medications across Rounds 1–3. Consistent with the literature suggesting that optimal cutpoints for polypharmacy vary by outcome,57 we examined the proportion of AYAs who were nonadherent by the number of medications prescribed and selected a threshold of ≥3 medications to define polypharmacy. This threshold has been used in the literature58 and increased the precision of estimates. We also examined the number of prescribed medications as a continuous variable in a separate model.
Medication fill nonadherence.
Adherence to medication is a complex behavior, consisting of deciding to take the medication in consultation with a provider, filling the medication, initiating and taking it as prescribed, and continuing to take it for the recommended course.59,60 Consistent with other studies of medication adherence/nonadherence,49,50,59,61 we focused on the behavior of filling the medication. In Round 4, MEPS asks participants if, in the past 12 months, they were 1) delayed in getting prescription medicines they or a doctor believed necessary; and 2) unable to get prescription medicines they or a doctor believed necessary. We measured medication fill nonadherence as a dichotomous variable; participants responding “yes” to either question in Round 4 were categorized as having medication fill nonadherence. Participants were also asked to identify, from among predetermined response options, the main reason for the delay and/or inability to get needed prescriptions. Response options included: could not afford care, insurance would not approve/cover/pay, doctor refused insurance plan, problems getting to doctor’s office, different language, couldn’t get time off work, didn’t know where to go to get care, was refused services, couldn’t get childcare, didn’t have time or took too long, or other.
Statistical analysis.
We described sociodemographic characteristics, clinical factors, and health indicators of AYAs. We estimated prevalence of medication fill nonadherence and reasons among AYAs.
We then used multivariable logistic regression to estimate the associations of polypharmacy (≥3 medications) and medication fill nonadherence (Model 1), as well as the continuous number of medications prescribed and medication fill nonadherence in AYAs (Model 2). We used a theory-based model62 consistent with Gellad et al.’s conceptual model of medication adherence in adults63 to guide the selection of potential confounders identified in the AYA literature.3,26,29,43,64,65,66 These included sex, chronic conditions, and disability. To control for secular trends, we also included year of first survey in the multivariable models.
Because of the complex survey design, we used survey weights, sampling strata, and sampling units in all analyses. We conducted analyses using SAS version 9.4 (SAS Institute, Cary, NC). Our study received Institutional Review Board exemption from the University of Texas Health Science Center at Houston (#HSC-SPH-20-0207).
Results
We excluded participants with missing data on the primary outcome (n=3) from the analysis. We identified 598 AYAs, the majority of whom were age ≥30 years, female, and non-Hispanic White (Table 1). Nearly half were either poor or near-poor/low income. Approximately one in four AYAs used Medicaid or other public insurance, and one in six were uninsured. More than one-fourth of AYAs did not have a usual source of care. The most common cancer type was “other,” followed by cervix and melanoma. Roughly half of AYAs reported having been diagnosed with a chronic condition other than cancer, and one-fourth reported a disability. More than half of AYAs had polypharmacy.
Table 1.
Characteristics of AYAs with a history of cancer, Medical Expenditures Panel Survey, 2008–2017 (n=598)
| n | weighted % | |
|---|---|---|
|
| ||
| Sociodemographics | ||
| Age in first survey year | ||
| 18–24 | 79 | 14.3 |
| 25–29 | 130 | 20.8 |
| 30–34 | 171 | 26.8 |
| 35–39 | 218 | 38.1 |
| Sex | ||
| Female | 479 | 76.2 |
| Male | 119 | 23.8 |
| Race/ethnicity | ||
| Hispanic | 165 | 15.5 |
| Non-Hispanic Black | 77 | 7.1 |
| Non-Hispanic White | 321 | 72.1 |
| Other Non-Hispanic | 35 | 5.3 |
| Marital status | ||
| Married | 246 | 45.8 |
| Not married | 352 | 54.2 |
| Education | ||
| Less than high school | 108 | 13.5 |
| High school degree | 170 | 24.7 |
| Some college | 165 | 26.9 |
| College degree or higher | 155 | 34.9 |
| Poverty status | ||
| Poor (<100% FPL) | 167 | 19.0 |
| Near poor and low income (100–199% FPL) | 153 | 21.6 |
| Middle income (200%-399% FPL) | 168 | 32.2 |
| High income (≥400% FPL) | 110 | 27.2 |
| Insurance | ||
| Private | 297 | 61.1 |
| Medicaid/other public | 188 | 23.7 |
| Uninsured (only) | 113 | 15.2 |
| Usual source of care | ||
| No | 177 | 27.1 |
| Yes | 416 | 71.8 |
| Unknown | 5 | 1.1 |
| Clinical factors | ||
| Cancer type | ||
| Breast | 42 | 6.6 |
| Cervix | 207 | 34.7 |
| Melanoma | 47 | 10.6 |
| Lymphoma | 28 | 4.4 |
| Uterinea | 45 | 5.3 |
| Otherb | 239 | 39.9 |
| Health indicators | ||
| Arthritisc | 111 | 18.0 |
| Asthma | 110 | 18.5 |
| Diabetes | 31 | 3.9 |
| Emphysema | 8 | 1.2 |
| Heart diseased | 67 | 13.2 |
| Hypercholesterolemia | 91 | 15.3 |
| Hypertension | 118 | 18.2 |
| Stroke | 23 | 2.8 |
| Number of chronic conditions | ||
| 0 | 293 | 49.0 |
| 1 | 161 | 27.1 |
| 2+ | 144 | 23.9 |
| Disabilitye | ||
| No | 411 | 72.6 |
| Yes | 180 | 26.5 |
| Unknown | 7 | 0.9 |
| Polypharmacyf | ||
| No | 296 | 47.7 |
| Yes | 302 | 52.3 |
NOTE: To account for the complex survey design, we used survey weights, sampling strata, and primary sampling units when calculating standard errors for weighted survey estimates
Uterine cancers reported in the year 2012 were grouped as “Other” because they appeared on National Institutes of Health’s list of rare diseases. Weighted percent based on n=530 for whom uterine cancer data are available.
“Other” cancers include bladder, blood, brain, colon, esophageal, kidney, larynx, leukemia, liver, lung, mouth/tongue/lip, ovarian, pancreatic, prostate, rectal, soft tissue, stomach, testicular, thyroid
Includes arthritis, gout, lupus, and fibromyalgia
Includes angina pectoris, myocardial infarction, coronary heart disease, or other heart disease
Includes any reported limitation in activities of daily living, instrumental activities of daily living, work, seeing and/or hearing
≥3 unique prescription medications
Approximately one in ten AYAs reported medication fill nonadherence (9.75%, 95% CI 6.76%–12.75%). Among those with medication fill nonadherence (n=48), reasons were similar for delaying and not obtaining medications (Figure 1). Specifically, more than a third of AYAs who delayed or did not obtain a needed medication reported that they could not afford it, and a similar proportion reported that insurance would not approve/cover/pay for it.
Figure 1.
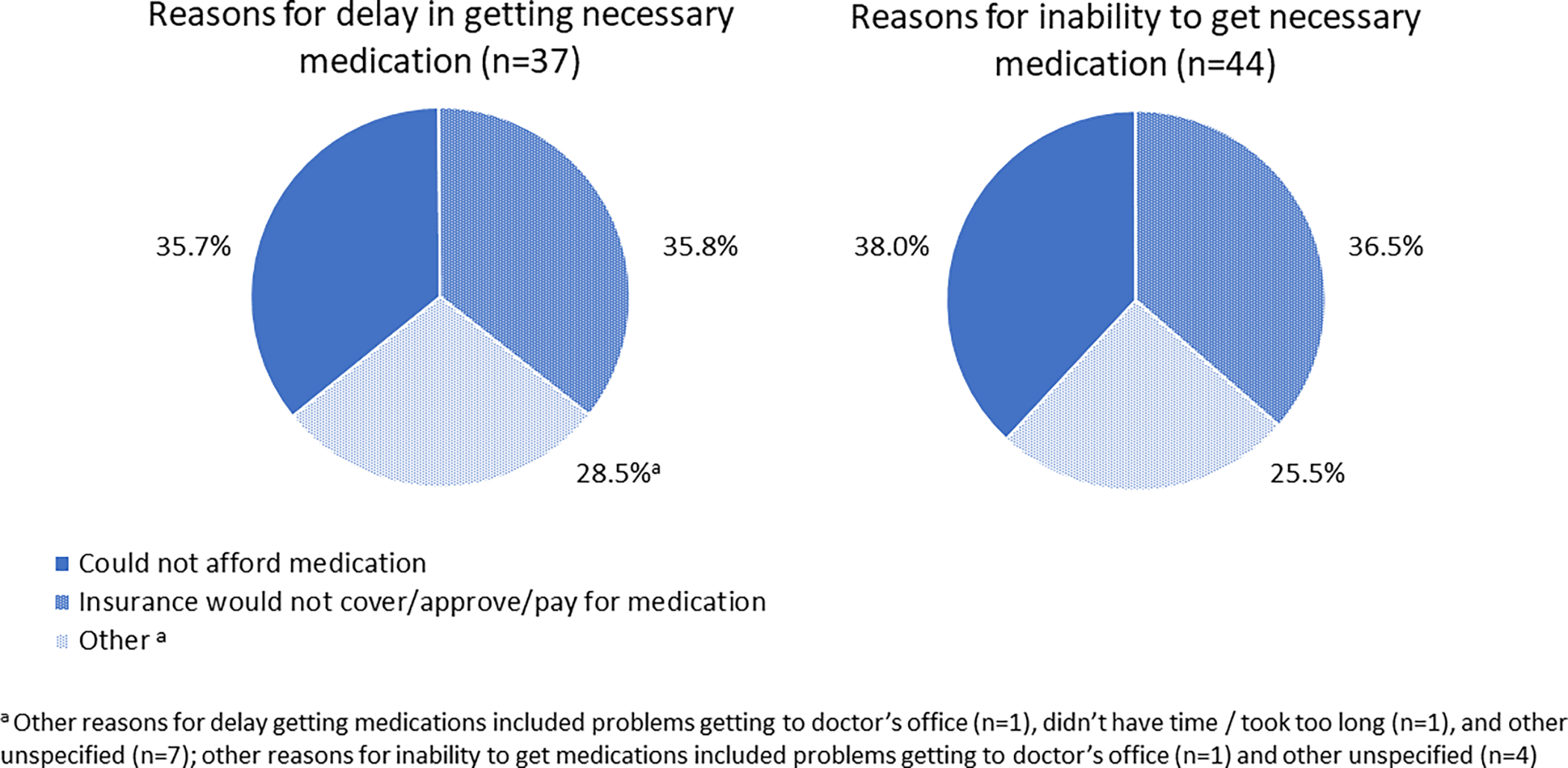
Reasons for delay in getting necessary medication (n=37) and inability to obtain necessary medication (n=44), among AYAs with a history of cancer (n=598)
As shown in Table 2, AYAs with polypharmacy had nearly two and a half times higher odds of medication fill nonadherence compared to those without polypharmacy (Model 1). When modeled as a continuous variable (Model 2), odds of medication fill nonadherence increased by 16% with each additional medication prescribed.
Table 2.
Association between polypharmacy and medication fill nonadherence (Model 1) and number of medications prescribed and medication fill nonadherence (Model 2) in AYAs with a history of cancer (n=598)
| Unadjusted | Model 1a | Model 2a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Characteristic | OR | 95% CI | P | AOR | 95% CI | P | AOR | 95% CI | P |
|
| |||||||||
| Female sex | 3.91 | 1.30–11.73 | 0.02 | 3.89 | 1.21–12.53 | 0.02 | 3.70 | 1.13–12.09 | 0.03 |
| Chronic conditions | 0.03 | 0.10 | 0.20 | ||||||
| 0 | REF | REF | REF | REF | REF | REF | |||
| 1+ | 2.57 | 1.09–6.07 | 2.07 | 0.88–4.86 | 1.82 | 0.73–4.49 | |||
| Disability | 0.08 | 0.95 | 0.46 | ||||||
| No/Unknown | REF | REF | REF | REF | REF | REF | |||
| Yes | 1.84 | 0.91–3.69 | 1.03 | 0.46–2.31 | 0.71 | 0.29–1.76 | |||
| Polypharmacy | <0.01 | 0.03 | |||||||
| No | REF | REF | REF | REF | |||||
| Yes | 3.35 | 1.51–7.40 | 2.49 | 1.11–5.59 | |||||
| Number of prescribed medications | 1.17 | 1.09–1.25 | <0.01 | 1.16 | 1.07–1.25 | <0.01 | |||
Adjusted for year of first survey, sex, number of chronic conditions, disability.
Discussion
In a large, nationally representative sample using one of the most comprehensive and robust sources of national data on healthcare utilization and expenditures,67–68 we found that AYA cancer survivors who were prescribed three or more medications were more than twice as likely to delay or not obtain needed medications, compared to those prescribed fewer medications. Furthermore, each additional medication conferred a 16% increase in odds of medication fill nonadherence. Thus managing the overall number of medications prescribed, with an eye toward cost, may facilitate adherence to critical medications in AYAs. While other studies have examined medication nonadherence due to cost,27,53 to our knowledge, our study is the first to identify polypharmacy as a driver of medication fill nonadherence in AYAs and to demonstrate negative consequences of being prescribed multiple medications. These findings fill a critical gap in the sparse literature on nonadherence in AYAs, identifying polypharmacy as a key risk factor that can be screened for in a clinical encounter.
Approximately one in ten AYAs in our study reported that they delayed or were unable to obtain medications they perceived to be necessary. Our study focused on medication fill nonadherence, and nonadherence is likely higher when other dimensions of use (e.g., deciding not to take the medication, skipping doses, taking fewer doses than prescribed) are considered. The association between polypharmacy and nonadherence may persist or be even stronger in AYAs when these dimensions are assessed because individuals with polypharmacy may have more opportunities to miss doses and more complex medications regimens.60 Importantly, nonadherence may also vary depending on the conditions being treated and the types of medications studied.60,64 For example, in a retrospective cohort study of breast cancer survivors’ adherence to adjuvant endocrine therapy (AET),58 survivors with polypharmacy who were frequently taking antihypertensives or lipid-lowering drugs were more likely to adhere to AET, while those frequently taking anxiolytics/antipsychotics, antidepressants, insulin, or opioids were less likely to adhere to AET. Notably, AYAs experience a high burden of mental health conditions, which have been linked to nonadherence.29,69 Future research should examine the association of polypharmacy and nonadherence in terms of medication use (versus fill) and specific medications of interest in AYAs, such as oral anticancer medications and cardiovascular agents. In addition, variation by factors such as mental health conditions should be explored.
More than 70% of AYAs who delayed or did not obtain a medication in our study attributed this to cost-related barriers—most commonly an inability to afford the medication and, secondarily, problems with insurance coverage. These findings reinforce those of two other national studies demonstrating cost-related medication nonadherence in up to one-fourth of AYAs.27,53 Notably, those studies used a measure of adherence that included skipping medication doses to save money, taking less medicine to save money, and delaying filling a prescription to save money. Affordability is influenced by financial resources, and about 40% of AYAs in our sample were poor, near poor, or low income, and one in six were uninsured. This is consistent with other studies showing high poverty and lack of insurance in AYAs.70–77 A large literature documents the financial burden of cancer treatment in AYAs, whose education, early career development, earnings, and eligibility for employer-based insurance may be disrupted by cancer at the same time that they incur considerable out of pocket medical costs,44,45,47,55,78–82 including rising prescription costs.83 Although the Patient Protection and Affordable Care Act introduced key provisions to reduce uninsurance in AYAs,44,84 many remain underinsured or have high out-of-pocket expenses.44,47
Our study’s findings underscore recent NCCN Guidelines for AYAs85 and a growing literature recommending clinical assessment of medication adherence and reasons for nonadherence and provision of resources to address financial barriers.26,27,47,86 Evidence supports the routine clinical use of valid patient-reported measures of adherence,87 such as the brief Morisky Adherence Questionnaire88,89 or the interview-based Medication Adherence Measure.90 While important to determining polypharmacy, medication reviews in which patients are asked if they take a medication (yes/no) do not adequately capture adherence. Consideration should also be given to the clinical workflow and visibility of polypharmacy and adherence assessments to the medical provider, as nurses or medical assistants often assess medication use at the beginning of an appointment when the provider is not present. In addition, technology may be leveraged to assess polypharmacy and medication fill nonadherence. For example, regionally integrated electronic health record platforms may facilitate accurate medication review, given that AYAs’ care is often fragmented,91–93 and integration with pharmacy systems could enable providers to be notified when a medication has not been filled. When cost is identified as a barrier, AYAs should be connected to resources such as medication assistance programs and financial counseling;47,85 NCCN offers tools for locating these resources including a searchable app (available at https://www.nccn.org/business-policy/business/virtual-reimbursement-resource-room-and-appresources). Finally, reform at the payor-level is urgently needed to prevent financial toxicity of cancer treatment and ensure affordability of medications for AYAs in the United States, who may live with the physical, psychosocial, and financial costs of cancer for many years.
Our findings using a national sample of AYAs in the U.S. cannot be generalized to AYAs in other countries, where health care systems differ. Further, our findings should be considered in light of limitations. Notably, MEPS does not collect information on cancer stage, treatment status, or time since diagnosis, and further research is needed to identify differences in polypharmacy and its association with medication fill nonadherence by key clinical factors, such as whether patients are on or off active cancer treatment. In addition, our measure of medication fill nonadherence was based on self-report and may underestimate prevalence of medication fill nonadherence in AYAs. Finally, while our models controlled for chronic conditions and disability, it is important to note that these factors frequently co-occur with polypharmacy. Qualitative studies should explore how AYAs, including those with chronic conditions and disabilities, experience polypharmacy and nonadherence.
Conclusions
Polypharmacy may be an important risk factor for medication fill nonadherence in AYAs. Financial support and cost-reducing measures are needed for AYAs taking multiple medications to increase adherence, improve health outcomes, and enhance quality of life for this population experiencing late effects of cancer and its treatment. Future studies should develop and test interventions to improve adherence to medications among AYAs, with specific attention to affordability and other medications co-prescribed.
Funding:
A.C.B. was supported by the American Cancer Society (ACS; RSG-21-0460) and the Cancer Control Research Training Program, UTHealth School of Public Health (National Cancer Institute/National Institutes of Health Grant T32 CA057712). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, NIH, or ACS.
Footnotes
Financial interests: A.C.B. reports consulting for Substack, Inc. C.C.M. reports consulting for Freenome. L.A.S., B.A.B., M.A., M.R., and C.M. have no disclosures.
Ethics approval: The study received an exemption from the Institutional Review Board at the University of Texas Health Science Center (#HSC-SPH-20-0207), as all data used in the study are publicly available.
Consent to participate/publish: Not applicable.
Data availability:
The data underlying this article were derived from sources in the public domain: IPUMS Health Surveys, Medical Expenditure Panel Survey, Version 1.1 https://doi.org/10.18128/D071.V1.1, and Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, https://meps.ahrq.gov/mepsweb/.
References
- 1.Drzayich Antol D, Waldman Casebeer A, Khoury R, et al. The relationship between comorbidity medication adherence and health related quality of life among patients with cancer. Journal of patient-reported outcomes. 2018;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adherence to long-term therapies : evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 3.Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive Evaluation of Adherence to Therapy, Its Associations, and Its Implications in Patients With Chronic Myeloid Leukemia Receiving Imatinib. Clinical lymphoma, myeloma & leukemia. 2016;16(6):366–371.e363. [DOI] [PubMed] [Google Scholar]
- 4.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. British journal of cancer. 2013;108(7):1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. British journal of cancer. 2008;99(11):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(32):4800–4809. [DOI] [PubMed] [Google Scholar]
- 7.Kyngas H, Duffy ME, Kroll T. Conceptual analysis of compliance. Journal of clinical nursing. 2000;9(1):5–12. [DOI] [PubMed] [Google Scholar]
- 8.Coccia PF, Pappo AS, Beaupin L, et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(1):66–97. [DOI] [PubMed] [Google Scholar]
- 9.Albritton K, Caligiuri M, Anderson B, Nichols C, Ulman D. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Bethesda, MD: National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance. 2006. [Google Scholar]
- 10.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(14):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117(14):3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. [DOI] [PubMed] [Google Scholar]
- 13.Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(14):2423–2429. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia S, Landier W, Hageman L, et al. Systemic Exposure to Thiopurines and Risk of Relapse in Children With Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. JAMA oncology. 2015;1(3):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(17):2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasvolsky O, Shimony S, Yeshurun M, et al. Maintenance therapy after allogeneic hematopoietic transplant for acute myeloid leukemia: a systematic review and meta-analysis. Acta Oncologica. 2021;60(10):1335–1341. [DOI] [PubMed] [Google Scholar]
- 17.Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3–internal tandem duplication mutation (SORMAIN). Journal of Clinical Oncology. 2020;38(26):2993–3002. [DOI] [PubMed] [Google Scholar]
- 18.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. The Lancet. 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LHRH-agonists in Early Breast Cancer Overview group. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. The Lancet. 2007;369(9574):1711–1723. [DOI] [PubMed] [Google Scholar]
- 20.Betts AC, Murphy CC, Shay LA, Balasubramanian BA, Markham C, Allicock M. Polypharmacy and prescription medication use in a population-based sample of adolescent and young adult cancer survivors. Journal of cancer survivorship : research and practice. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaul S, Veeranki SP, Rodriguez AM, Kuo YF. Cigarette smoking, comorbidity, and general health among survivors of adolescent and young adult cancer. Cancer. 2016;122(18):2895–2905. [DOI] [PubMed] [Google Scholar]
- 22.Chao C, Bhatia S, Xu L, et al. Chronic comorbidities among survivors of adolescent and young adult cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(27):3161–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu XC, Prasad PK, Landry I, et al. Impact of the AYA HOPE comorbidity index on assessing health care service needs and health status among adolescents and young adults with cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(12):1844–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao C, Xu L, Bhatia S, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors Study. Journal of Clinical Oncology. 2016;34(14):1626–1633. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CD, Nichols HB, Lund JL. Polypharmacy and medication use by cancer history in a nationally representative group of adults in the USA, 2003–2014. Journal of cancer survivorship : research and practice. 2021:Online ahead of print. doi: 10.1007/s11764-11021-01059-x. [DOI] [PubMed] [Google Scholar]
- 26.McGrady ME, Pai ALH. A Systematic Review of Rates, Outcomes, and Predictors of Medication Non-Adherence Among Adolescents and Young Adults with Cancer. Journal of adolescent and young adult oncology. 2019;8(5):485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul S, Avila JC, Mehta HB, Rodriguez AM, Kuo YF, Kirchhoff AC. Cost-related medication nonadherence among adolescent and young adult cancer survivors. Cancer. 2017;123(14):2726–2734. [DOI] [PubMed] [Google Scholar]
- 28.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast cancer research and treatment. 2011;126(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennard BD, Stewart SM, Olvera R, Bawdon RE, Lewis CP, Winick NJ. Nonadherence in adolescent oncology patients: preliminary data on psychological risk factors and relationships to outcome. Journal of Clinical Psychology in Medical Settings. 2004;11(1):31–39. [Google Scholar]
- 30.Hullmann SE, Brumley LD, Schwartz LA. Medical and psychosocial associates of nonadherence in adolescents with cancer. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2015;32(2):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaroff MH, Festa RS, Adesman AR, Walco GA. Therapeutic adherence to oral medication regimens by adolescents with cancer. II. Clinical and psychologic correlates. The Journal of pediatrics. 1992;120(5):812–817. [DOI] [PubMed] [Google Scholar]
- 32.Linder LA, Wu YP, Macpherson CF, et al. Oral medication adherence among adolescents and young adults with cancer before and following use of a smartphone-based medication reminder app. Journal of adolescent and young adult oncology. 2019;8(2):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau RC, Matsui D, Greenberg M, Koren G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Medical and Pediatric Oncology: The Official Journal of SIOP—International Society of Pediatric Oncology (Societé Internationale d’Oncologie Pédiatrique 1998;30(2):85–90. [DOI] [PubMed] [Google Scholar]
- 34.Pizzo PA, Robichaud KJ, Edwards BK, Schumaker C, Kramer BS, Johnson A. Oral antibiotic prophylaxis in patients with cancer: a double-blind randomized placebo-controlled trial. The Journal of pediatrics. 1983;102(1):125–133. [DOI] [PubMed] [Google Scholar]
- 35.Pai ALH, Drotar D, Kodish E. Correspondence Between Objective and Subjective Reports of Adherence Among Adolescents With Acute Lymphoblastic Leukemia. Children’s Health Care. 2008;37(3):225–235. [Google Scholar]
- 36.Jaime-Pérez JC, Jiménez-Castillo RA, Pinzón-Uresti MA, et al. Real-world outcomes of treatment for acute lymphoblastic leukemia during adolescence in a financially restricted environment: results at a single center in Latin America. Pediatric blood & cancer. 2017;64(7):e26396. [DOI] [PubMed] [Google Scholar]
- 37.Wu YP, Stenehjem DD, Linder LA, et al. Adherence to oral medications during maintenance therapy among children and adolescents with acute lymphoblastic leukemia: a medication refill analysis. Journal of Pediatric Oncology Nursing. 2018;35(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tebbi CK, Zevon MA, Richards ME, Cummings KM. Attributions of responsibility in adolescent cancer patients and their parents. Journal of Cancer Education. 1989;4(2):135–142. [DOI] [PubMed] [Google Scholar]
- 39.Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. American journal of hematology. 2011;86(6):471–474. [DOI] [PubMed] [Google Scholar]
- 40.Chapman RH, Petrilla AA, Benner JS, Schwartz JS, Tang SS. Predictors of adherence to concomitant antihypertensive and lipid-lowering medications in older adults: a retrospective, cohort study. Drugs & aging. 2008;25(10):885–892. [DOI] [PubMed] [Google Scholar]
- 41.van Bruggen R, Gorter K, Stolk RP, Zuithoff P, Klungel OH, Rutten GE. Refill adherence and polypharmacy among patients with type 2 diabetes in general practice. Pharmacoepidemiology and drug safety. 2009;18(11):983–991. [DOI] [PubMed] [Google Scholar]
- 42.Gray SL, Mahoney JE, Blough DK. Medication adherence in elderly patients receiving home health services following hospital discharge. The Annals of pharmacotherapy. 2001;35(5):539–545. [DOI] [PubMed] [Google Scholar]
- 43.Murphy CC, Fullington HM, Alvarez CA, et al. Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer. 2018;124(13):2850–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons SK, Kumar AJ. Adolescent and young adult cancer care: Financial hardship and continued uncertainty. Pediatric blood & cancer. 2019;66(4):e27587. [DOI] [PubMed] [Google Scholar]
- 45.Smith AW, Keegan T, Hamilton A, et al. Understanding care and outcomes in adolescents and young adults with cancer: A review of the AYA HOPE study. Pediatric blood & cancer. 2019;66(1):e27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corrigan KL, Fu S, Chen YS, et al. Financial toxicity impact on younger versus older adults with cancer in the setting of care delivery. Cancer. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salsman JM, Bingen K, Barr RD, Freyer DR. Understanding, measuring, and addressing the financial impact of cancer on adolescents and young adults. Pediatric blood & cancer. 2019;66(7):e27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armenian SH, Gibson CJ, Rockne RC, Ness KK. Premature aging in young cancer survivors. Journal of the National Cancer Institute. 2019;111(3):226–232. [DOI] [PubMed] [Google Scholar]
- 49.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. The American journal of geriatric pharmacotherapy. 2011;9(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Medical care. 2013;51(8 Suppl 3):S11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen JW, Monheit AC, Beauregard KM, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry : a journal of medical care organization, provision and financing. 1996;33(4):373–389. [PubMed] [Google Scholar]
- 52.IPUMS Health Surveys: Medical Expenditure Panel Survey, Version 1.1. IPUMS; 2019. https://meps.ipums.org. Accessed Accessed March 12, 2020. [Google Scholar]
- 53.Lee M, Salloum RG. Racial and ethnic disparities in cost-related medication non-adherence among cancer survivors. Journal of cancer survivorship : research and practice. 2016;10(3):534–544. [DOI] [PubMed] [Google Scholar]
- 54.National Institutes of Health. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases. Accessed February 21, 2021.
- 55.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smitherman AB, Anderson C, Lund JL, Bensen JT, Rosenstein DL, Nichols HB. Frailty and Comorbidities Among Survivors of Adolescent and Young Adult Cancer: A Cross-Sectional Examination of a Hospital-Based Survivorship Cohort. Journal of adolescent and young adult oncology. 2018;7(3):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner JP, Jamsen KM, Shakib S, Singhal N, Prowse R, Bell JS. Polypharmacy cut-points in older people with cancer: How many medications are too many? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2016;24(4):1831–1840. [DOI] [PubMed] [Google Scholar]
- 58.Calip GS, Xing S, Jun DH, Lee WJ, Hoskins KF, Ko NY. Polypharmacy and Adherence to Adjuvant Endocrine Therapy for Breast Cancer. Journal of oncology practice. 2017;13(5):e451–e462. [DOI] [PubMed] [Google Scholar]
- 59.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. British journal of clinical pharmacology. 2012;73(5):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcum ZA, Gellad WF. Medication adherence to multidrug regimens. Clinics in geriatric medicine. 2012;28(2):287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattler EL, Lee JS, Perri M 3rd. Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs & aging. 2013;30(6):383–399. [DOI] [PubMed] [Google Scholar]
- 62.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gellad WF, Grenard J, McGlynn EA. A review of barriers to medication adherence: a framework for driving policy options. RAND; Santa Monica, CA; 2009. [Google Scholar]
- 64.Zeber JE, Manias E, Williams AF, et al. A systematic literature review of psychosocial and behavioral factors associated with initial medication adherence: a report of the ISPOR medication adherence & persistence special interest group. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16(5):891–900. [DOI] [PubMed] [Google Scholar]
- 65.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast cancer research and treatment. 2011;125(1):191–200. [DOI] [PubMed] [Google Scholar]
- 66.Kondryn HJ, Edmondson CL, Hill J, Eden TO. Treatment non-adherence in teenage and young adult patients with cancer. The Lancet Oncology. 2011;12(1):100–108. [DOI] [PubMed] [Google Scholar]
- 67.Hill SC, Zuvekas SH, Zodet MW. Implications of the accuracy of MEPS prescription drug data for health services research. Inquiry : a journal of medical care organization, provision and financing. 2011;48(3):242–259. [DOI] [PubMed] [Google Scholar]
- 68.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Medical care. 2009;47(7 Suppl 1):S44–50. [DOI] [PubMed] [Google Scholar]
- 69.De R, Sutradhar R, Kurdyak P, et al. Incidence and Predictors of Mental Health Outcomes Among Survivors of Adolescent and Young Adult Cancer: A Population-Based Study Using the IMPACT Cohort. Journal of Clinical Oncology. 2021;39(9):1010–1019. [DOI] [PubMed] [Google Scholar]
- 70.Murphy CC, Lupo PJ, Roth ME, Winick NJ, Pruitt SL. Disparities in cancer survival among adolescents and young adults: a population-based study of 88,000 patients. Journal of the National Cancer Institute. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fintel AE, Jamy O, Martin MG. Influence of insurance and marital status on outcomes of adolescents and young adults with acute lymphoblastic leukemia. Clinical lymphoma, myeloma & leukemia. 2015;15(6):364–367. [DOI] [PubMed] [Google Scholar]
- 73.Robbins AS, Lerro CC, Barr RD. Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer. 2014;120(8):1212–1219. [DOI] [PubMed] [Google Scholar]
- 74.Rosenberg AR, Kroon L, Chen L, Li CI, Jones B. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer. 2015;121(8):1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keegan TH, Tao L, DeRouen MC, et al. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? Journal of cancer survivorship : research and practice. 2014;8(2):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeRouen MC, Parsons HM, Kent EE, Pollock BH, Keegan THM. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer causes & control : CCC. 2017;28(8):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parsons HM, Schmidt S, Harlan LC, et al. Young and uninsured: Insurance patterns of recently diagnosed adolescent and young adult cancer survivors in the AYA HOPE study. Cancer. 2014;120(15):2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keegan TH, Lichtensztajn DY, Kato I, et al. Unmet adolescent and young adult cancer survivors information and service needs: a population-based cancer registry study. Journal of cancer survivorship : research and practice. 2012;6(3):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guy GP Jr., Yabroff KR, Ekwueme DU, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff. 2014;33(6):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118(20):5155–5162. [DOI] [PubMed] [Google Scholar]
- 81.Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA pediatrics. 2016;170(5):495–501. [DOI] [PubMed] [Google Scholar]
- 82.Smith AW, Parsons HM, Kent EE, et al. Unmet support service needs and health-related quality of life among adolescents and young adults with cancer. Frontiers in oncology. 2013;3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kesselheim AS, Avorn J, Sarpatwari A. The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform. Jama. 2016;316(8):858–871. [DOI] [PubMed] [Google Scholar]
- 84.Jemal A, Lin CC, Davidoff AJ, Han X. Changes in insurance coverage and stage at diagnosis among nonelderly patients with cancer after the Affordable Care Act. Journal of Clinical Oncology. 2017;35(35):3906–3915. [DOI] [PubMed] [Google Scholar]
- 85.National Comprehensive Cancer Network. Adolsecent and Young Adult Oncology (Version 2.2022). nccn.org/professionals/physician_gls/pdf/aya.pdf. Accessed April 15, 2022.
- 86.Pai ALH, McGrady ME. Assessing Medication Adherence as a Standard of Care in Pediatric Oncology. Pediatric blood & cancer. 2015;62(S5):S818–S828. [DOI] [PubMed] [Google Scholar]
- 87.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Translational behavioral medicine. 2015;5(4):470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. The journal of clinical hypertension. 2008;10(5):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986:67–74. [DOI] [PubMed] [Google Scholar]
- 90.Zelikovsky N, Schast AP. Eliciting accurate reports of adherence in a clinical interview: development of the Medical Adherence Measure. Pediatric Nursing. 2008;34(2):141–146. [PubMed] [Google Scholar]
- 91.Smits-Seemann RR, Kaul S, Zamora ER, Wu YP, Kirchhoff AC. Barriers to follow-up care among survivors of adolescent and young adult cancer. Journal of cancer survivorship : research and practice. 2017;11(1):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118(23):5964–5972. [DOI] [PubMed] [Google Scholar]
- 93.Roser K, Baenziger J, Mader L, Christen S, Dehler S, Michel G. Attendance to Follow-Up Care in Survivors of Adolescent and Young Adult Cancer: Application of the Theory of Planned Behavior. Journal of adolescent and young adult oncology. 2018;7(5):584–591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were derived from sources in the public domain: IPUMS Health Surveys, Medical Expenditure Panel Survey, Version 1.1 https://doi.org/10.18128/D071.V1.1, and Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, https://meps.ahrq.gov/mepsweb/.


