Abstract
Background:
There is evidence supporting the association between Helicobacter pylori infection and colorectal cancer (CRC), but whether H. pylori eradication reduces the risk of CRC is still unknown.
Objectives:
To compare the incidence of CRC in subjects who had received H. pylori eradication therapy with general population.
Design:
A population-based retrospective cohort study.
Methods:
This study included all H. pylori-infected subjects who had received their first course of clarithromycin-containing triple therapy in 2003–2015 in Hong Kong. We compared the observed incidences of CRC in this H. pylori eradicated cohort with the expected incidences in the age- and sex-matched general population. The standardized incidence ratio (SIR) with 95% confidence interval (CI) was computed.
Results:
Among 96,572 H. pylori-eradicated subjects with a median follow-up of 9.7 years, 1417 (1.5%) developed CRC. Primary analysis showed no significant difference in the observed and expected incidences of CRC (SIR: 1.03, 95% CI: 0.97–1.09). However, when stratified according to the follow-up period, higher incidence of CRC was only observed in the first 5 years after eradication (SIR: 1.47, 95% CI: 1.39–1.55), but it was lower (SIR: 0.85, 95% CI: 0.74–0.99) than general population after 11 years. When stratified by tumor location, the observed incidence was higher for colon (SIR: 1.20, 95% CI: 1.12–1.29) but lower for rectal cancer (SIR: 0.90, 95% CI: 0.81–0.999) among H. pylori-eradicated subjects.
Conclusions:
H. pylori-infected subjects appeared to have a higher incidence of CRC initially, which declined progressively to a level lower than general population 10 years after H. pylori eradication, particularly for rectal cancer.
Keywords: clarithromycin-containing triple therapy, colorectal cancer, epidemiology, Helicobacter pylori, standardized incidence ratio
Introduction
Helicobacter pylori (H. pylori) infection is the major etiologic factor for chronic gastritis, peptic ulcer disease, and gastric cancer. Although the prevalence of H. pylori infection is declining in many regions, the disease burden, particularly in mid- and low-income countries with high background prevalence of infection, remains substantial. 1 While the stomach is the usual habitat of H. pylori and is considered as the primary target organ, there is emerging evidence suggesting the putative association between H. pylori infection and other extra-gastric diseases including colorectal cancer (CRC).2,3 The association between H. pylori infection and CRC as well as colorectal adenoma had been recently suggested. 4 Two meta-analyses showed that H. pylori infection was associated with a higher risk of colorectal adenoma and CRC, with the pooled odds ratio of 1.51 and 1.70, respectively.5,6 As yet, most of these data were based on case–control or cross-sectional studies, and H. pylori infection was mainly defined by positive serological tests. 6
Although the benefits of H. pylori eradication in prevention of gastric cancer development have been widely demonstrated, 7 it remains uncertain whether H. pylori eradication has any effects on the risk of subsequent CRC development to further strengthen the putative link between H. pylori infection and CRC. There is so far no study which addresses the role of H. pylori eradication on CRC development.
In this study, using a large population-based cohort of H. pylori-infected subjects who had received clarithromycin-containing triple therapy for H. pylori in Hong Kong, we aimed to compare the incidence of CRC in H. pylori-eradicated subjects with matched local general population to determine the potential effects of H. pylori eradication on subsequent risk of CRC.
Methods
Data source
We used the Clinical Data Analysis and Reporting System (CDARS), an electronic healthcare database of the Hong Kong Hospital Authority which is the only public healthcare provider serving the 7 million local population, to identify subjects who had previously received H. pylori eradication. The CDARS included patient’s demographics, diagnoses, out-patient attendance and hospitalization, drug prescriptions, and dispensing records from all public hospitals and clinics.8–10 All data from this database are anonymized and deidentified.
For incidences of CRC in the general population, we retrieved the number of new cases of CRC from the Hong Kong Cancer Registry. 11 The corresponding population demographics of that period were obtained from Hong Kong Annual Digest of Statistics with the mid-year population by 5-year age groups and sex over the study period. 12
Subjects
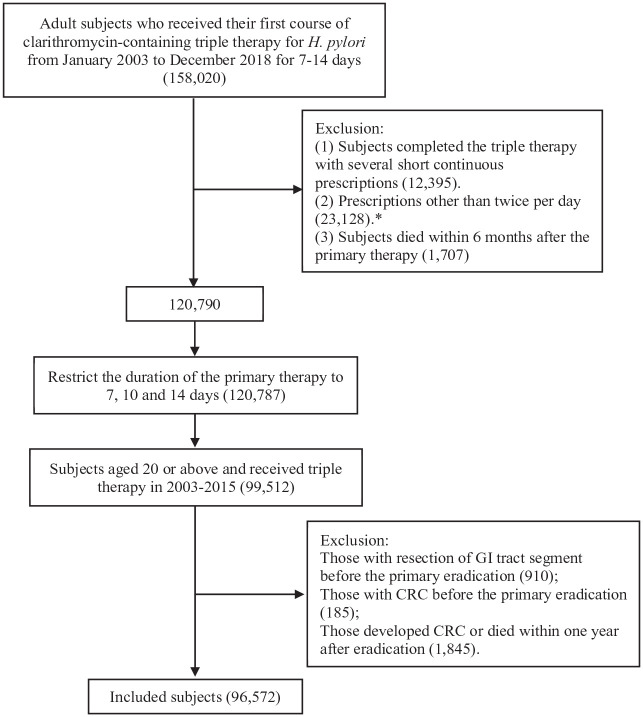
This is a retrospective population-based cohort study involving all subjects who had received their first course of clarithromycin-containing triple therapy for H. pylori eradication in Hong Kong between January 2003 and December 2015. Clarithromycin-containing triple therapy was identified by the co-prescription of proton pump inhibitor, clarithromycin and amoxicillin, or metronidazole with the same prescription start date and an overlapping prescription duration of 7, 10, or 14 days. 13 Subjects who received only a single course of clarithromycin-containing triple therapy were regarded as eradication success, while those who received retreatment for H. pylori were treated as retreatment group. 13 Retreatments after failure of the initial treatment included the repeated prescription of clarithromycin-containing triple therapy, subsequent prescription of the second-line or third-line therapy. We excluded subjects who had ever received resection of any gastrointestinal tract segment, those who had been diagnosed with CRC before the eradication, and those who developed CRC or died within 1 year after the eradication (Figure 1).
Figure 1.
Flow chart of patient selection.
*For metronidazole, prescriptions with frequency of three or four times per day were also included.
CRC incidences in matched general population
We computed the CRC incidences in the local general population using the number of new CRC cases from the Hong Kong Cancer Registry and the mid-year population by 5-year age group and sex from 2003 to 2019 (the latest available year). This cancer registry is a population-based registry committed to collecting data from all cancer cases and covering the entire local population in Hong Kong. Cancers from different sites were identified according to International Classification of Diseases (ICD)-9 and/or ICD-10 codes. The expected CRC cancer incidence of the age- and sex-matched local population was estimated based on the age- and sex-specific CRC incidence.
Outcome
The primary outcome was the observed incidence of CRC, including colon cancer and rectal cancer, which developed more than 1 year after eradication therapy in H. pylori-eradicated subjects versus the expected incidence in the matched general population. The date of diagnosis of CRC for H. pylori-eradicated subjects was the first date of inpatient or outpatient records for CRC workup or treatment, or death date when it was only identified from death certificate. The incident CRC was retrieved using ICD-9 (CRC: 153–154, colon cancer: 153; rectal cancer: 154) or ICD-10 (CRC: C18–21; colon cancer: C18; rectal cancer: C19–21).
Statistical analyses
Subjects were followed up from 1 year after H. pylori eradication until the date of diagnosis of CRC, death, or the end of follow-up on 31 December 2020, whichever came first. The observed incidence of CRC in H. pylori-eradicated subjects was compared with the expected incidence in the age- and sex-matched general population. The expected cumulative incidence and Kaplan–Meier survival curve for the matched general population were estimated using the methods from Finkelstein et al., 14 which was raised to compare the survival of a single sample to that of a defined reference population and had been used in previous studies.8,15 To estimate the expected CRC cases in the matched general population with specific age and sex, the probability of observing an incident CRC during the same follow-up period as that for the matched subjects in our cohort was calculated, based on local CRC incidence. Thus, the mean age- and sex-specific incidences in 2003–2019 in Hong Kong were used. In the sensitivity analysis, the mean incidences in 2003–2012 or 2010–2019 were used due to the potential changes in cancer incidences in the general population during the study period. The one-sample log-rank test and the standardized incidence ratio (SIR) with an exact 95% confidence interval (CI) were used to test the difference between the observed incidence of CRC in H. pylori-eradicated subjects and the expected incidence in the matched general population. Subcategory analyses by colon or rectal cancer were also performed. A sensitivity analysis using CRC from inpatient diagnosis or death record in H. pylori-eradicated subjects was performed.
To demonstrate the time trend of cancer incidence after H. pylori eradication, we compared the observed and the expected CRC incidences in different periods after eradication. The cumulative incidences and the corresponding SIRs in different periods (1–5, 6–10, and ⩾11 years) after eradication were computed. Furthermore, subgroup analyses were performed according to the age group (<40, 40–59, and ⩾60 years old), treatment outcome (eradication success and retreatment), and history of prior lower endoscopy before H. pylori eradication (yes/no). Tests with a two-sided p value <0.05 were considered statistically significant. All statistical analyses were performed using the R software, version 4.2.0 (Vienna, Austria).
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 16
Results
Patient characteristics
We included 96,572 H. pylori-infected subjects, aged 20 or above [median age: 54 years, interquartile range (IQR): 45–65; male: 46.8%], who had received clarithromycin-containing triple therapy for H. pylori in this study. Among them, 9781 (10.1%) subjects required retreatment for H. pylori and was treated as eradication failure. At baseline, there were 13,106 (13.6%) subjects who had received prior lower endoscopy.
CRC after H. pylori eradication versus matched general population
After a median follow-up of 9.7 (IQR: 6.4–13.2) years, 1417 H. pylori-eradicated subjects developed CRC with an incidence of 1.51 (95% CI: 1.43–1.59) per 1000 person-years. The expected incidence of CRC in the corresponding age- and sex-matched general population was 1.47 (95% CI: 1.39–1.55) per 1000 person-years, and there was no significant difference between the observed and expected CRC incidences with an SIR of 1.03 (95% CI: 0.97–1.09).
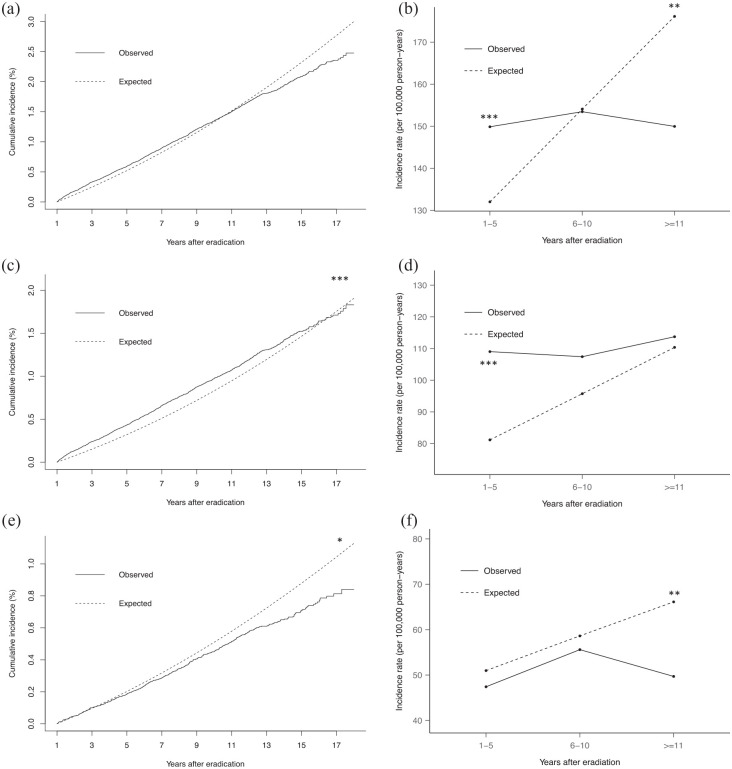
When considering CRC incidences according to different follow-up intervals after H. pylori eradication, higher incidence of CRC was observed in H. pylori-eradicated subjects than matched general population in the first 5 years (SIR: 1.14, 95% CI: 1.04–1.24). However, a lower incidence of CRC was observed after 11 years (SIR: 0.85, 95% CI: 0.74–0.99; Figure 2(a) and (b), Tables 1 and 2).
Figure 2.
Observed CRC incidence after H. pylori eradication compared with the expected incidence in the matched general population. (a) Cumulative incidence of CRC; (b) incidence rates of CRC according to follow-up duration; (c) cumulative incidence of colon cancer; (d) incidence rates of colon cancer according to follow-up duration; (e) cumulative incidence of rectal cancer; and (f) incidence rates of rectal cancer according to follow-up duration.*p < 0.05. **p < 0.02. ***p < 0.001. CRC, colorectal cancer.
Table 1.
Baseline characteristics of subjects.
| Characteristic | Value |
|---|---|
| N | 96,572 |
| Age, median (IQR) | 54.0 (45.0, 65.0) |
| Age group (%) | |
| ⩾60 years | 35,086 (36.3) |
| 40–59 years | 48,213 (49.9) |
| <40 years | 13,273 (13.7) |
| Male (%) | 45,200 (46.8) |
| Retreatment | 9,781 (10.1) |
| Prior lower endoscopy a | 13,106 (13.6) |
Including any colonoscopy and sigmoidoscopy before the primary eradication.
IQR, interquartile range.
Table 2.
CRC incidences after H. pylori eradication compared with the expected incidences in the matched general population.
| CRC | Colon cancer | Rectal cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Observation period | Person-years | Observed/expected number of cases | SIR (95% CI) | Person-years | Observed/expected number of cases | SIR (95% CI) | Person-years | Observed/expected number of cases | SIR (95% CI) |
| Overall | 937738.5 | 1417/1377.5 | 1.03 (0.97–1.09) | 939113.2 | 1026/854.6 | 1.20 (1.12–1.29) | 940742.9 | 476/528.0 | 0.90 (0.81–0.999) |
| Years after eradication | |||||||||
| 1–5 years | 461084.4 | 691/608.7 | 1.14 (1.04–1.24) | 461410.3 | 503/374.4 | 1.34 (1.22–1.48) | 461883.5 | 219/235.5 | 0.93 (0.80–1.08) |
| 6–10 years | 320616.2 | 492/494.0 | 1.00 (0.90–1.10) | 321182.3 | 345/307.4 | 1.12 (0.99–1.27) | 321898.6 | 179/188.7 | 0.95 (0.80–1.12) |
| ⩾11 years | 156037.9 | 234/274.8 | 0.85 (0.74–0.99) | 156520.6 | 178/172.7 | 1.03 (0.87–1.22) | 156960.8 | 78/103.8 | 0.75 (0.58–0.97) |
CI, confidence interval; CRC, colorectal cancer; SIR, standardized incidence ratio.
Separate analysis was performed for colon and rectal cancer. The observed incidence of colon cancer in H. pylori-eradicated subjects was significantly higher than the general population (SIR: 1.20, 95% CI: 1.12–1.29). However, analysis according to different follow-up durations showed significant difference in the first 5 years only (SIR: 1.34, 95% CI: 1.22–1.48), and the risk decreased with time to a level comparable to the general population afterwards (Figure 2(c) and (d), Table 2). For rectal cancer, H. pylori-eradicated subjects had an overall lower incidence rate than the general population (SIR: 0.90, 95% CI: 0.81–0.999). Notably, the incidence of rectal cancer in H. pylori-eradicated subjects was comparable to the matched general population in first 10 years but declined after 11 years of eradication (Figure 2(e) and (f), Table 2).
The results were consistent when using either the mean cancer incidences in 2003–2012 or 2010–2019 to estimate the expected cancer incidences (Supplemental Tables 1 and 2). When using CRC from inpatient diagnosis or death record in H. pylori-eradicated subjects, the result was also consistent (Supplemental Table 3).
Subgroup analyses by subjects’ characteristics
Further subgroup analyses were performed according to patient’s age, H. pylori eradication success and prior colonoscopy at baseline. The results were consistent among subjects aged 60 or above, in which higher incidences of all CRC, and colon cancer, were observed in the first 5 years after eradication among H. pylori-eradicated subjects (SIR for CRC: 1.19, 95% CI: 1.08–1.31; SIR for colon cancer: 1.39, 95% CI: 1.24–1.56). For rectal cancer, the incidence was lower in H. pylori-eradicated subjects, aged 60 or above, after 11 years of the eradication (SIR: 0.64, 95% CI: 0.43–0.97). However, the incidences of CRC were comparable to the age-matched general population among H. pylori-eradicated subjects <40 years and 40–59 years throughout the follow-up period (Table 3).
Table 3.
Subgroup analyses of CRC incidences after H. pylori eradication compared with the expected incidence in the matched general population.
| Subgroups | CRC | Colon cancer | Rectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years | Observed/expected number of cases | SIR (95% CI) | Person-years | Observed/expected number of cases | SIR (95% CI) | Person-years | Observed/expected number of cases | SIR (95% CI) | |
| Age ⩾60 | 294452.7 | 934/874.0 | 1.09 (0.99–1.15) | 295195.5 | 701/565.4 | 1.24 (1.14–1.35) | 296329.4 | 290/312.5 | 0.93 (0.81–1.06) |
| 1–5 years | 159423.9 | 516/434.7 | 1.19 (1.08–1.31) | 159628.0 | 386/277.4 | 1.39 (1.24–1.56) | 160011.0 | 153/158.4 | 0.97 (0.81–1.16) |
| 6–10 years | 96417.6 | 302/303.3 | 1.00 (0.88–1.13) | 96735.0 | 217/197.5 | 1.10 (0.94–1.28) | 97234.0 | 107/107.5 | 1.00 (0.80–1.24) |
| ⩾11 years | 38611.2 | 116/135.9 | 0.85 (0.69–1.05) | 38832.5 | 98/90.5 | 1.08 (0.86–1.36) | 39084.4 | 30/46.6 | 0.64 (0.43–0.97) |
| Age 40–59 | 497280.6 | 457/479.5 | 0.95 (0.85–1.06) | 497876.9 | 309/276.4 | 1.12 (0.98–1.27) | 498349.6 | 175/204.2 | 0.86 (0.72–1.02) |
| 1–5 years | 235894.7 | 165/167.8 | 0.98 (0.83–1.17) | 236006.0 | 111/93.8 | 1.18 (0.96–1.46) | 236097.4 | 61/74.2 | 0.82 (0.62–1.09) |
| 6–10 years | 173226.1 | 179/182.0 | 0.98 (0.83–1.16) | 173462.4 | 121/105.3 | 1.15 (0.94–1.41) | 173668.6 | 68/77.1 | 0.97 (0.81–1.16) |
| ⩾11 years | 88159.8 | 113/129.7 | 0.87 (0.71–1.08) | 88408.5 | 77/77.3 | 1.00 (0.77–1.28) | 88583.7 | 46/52.9 | 0.87 (0.63–1.21) |
| Age <40 | 146005.2 | 26/24.1 | 1.08 (0.70–1.67) | 146040.8 | 16/12.7 | 1.26 (0.72–2.18) | 146064.0 | 11/11.3 | 0.97 (0.50–1.89) |
| 1–5 years | 65765.8 | 10/6.2 | 1.62 (0.81–3.25) | 65776.3 | 6/3.2 | 1.87 (0.77–4.53) | 65775.2 | 5/3.0 | 1.69 (0.64–4.43) |
| 6–10 years | 50972.5 | 11/8.7 | 1.26 (0.65–2.45) | 50984.8 | 7/4.6 | 1.52 (0.67–3.46) | 50996.1 | 4/4.1 | 0.97 (0.33–2.84) |
| ⩾11 years | 29267.0 | 5/9.2 | 0.55 (0.21–1.43) | 29279.7 | 3/4.9 | 0.61 (0.18–2.06) | 29292.7 | 2/4.3 | 0.47 (0.11–2.01) |
| Retreatment | 95437.9 | 152 / 127.2 | 1.20 (1.00–1.43) | 95611.0 | 110 / 78.6 | 1.40 (1.13–1.73) | 95736.5 | 54 / 49.1 | 1.10 (0.81–1.49) |
| 1–5 years | 47312.9 | 70/55.8 | 1.25 (0.96–1.64) | 47351.5 | 45/34.1 | 1.32 (0.95–1.84) | 47375.7 | 28/21.8 | 1.29 (0.84–1.96) |
| 6–10 years | 32578.8 | 53/46.1 | 1.15 (0.85–1.56) | 32656.7 | 41/28.6 | 1.43 (1.01–2.03) | 32710.3 | 17/17.7 | 0.96 (0.56–1.64) |
| ⩾11 years | 15546.2 | 29/25.3 | 1.15 (0.76–1.74) | 15602.8 | 24/15.8 | 1.52 (0.96–2.39) | 15650.4 | 9/9.6 | 0.94 (0.45–1.95) |
| Eradication success | 842300.6 | 1265/1250.4 | 1.01 (0.95–1.08) | 843502.2 | 916/776.0 | 1.18 (1.10–1.27) | 845006.4 | 422/478.9 | 0.88 (0.79–0.98) |
| 1–5 years | 413771.5 | 621/552.9 | 1.12 (1.03–1.23) | 414058.7 | 458/340.3 | 1.35 (1.21–1.49) | 414507.8 | 191/213.7 | 0.89 (0.76–1.05) |
| 6–10 years | 288037.4 | 439/447.9 | 0.98 (0.88–1.09) | 288525.6 | 304/278.8 | 1.09 (0.96–1.24) | 289188.3 | 162/171.0 | 0.95 (0.79–1.13) |
| ⩾11 years | 140491.7 | 205/249.6 | 0.82 (0.70–0.96) | 140917.9 | 154/156.9 | 0.98 (0.82–1.18) | 141310.4 | 69/94.2 | 0.73 (0.56–0.96) |
| Prior lower endoscopy a | 115713.0 | 130/200.0 | 0.65 (0.53–0.79) | 115840.4 | 90/124.7 | 0.72 (0.57–0.91) | 115977.8 | 45/75.8 | 0.59 (0.43–0.83) |
| 1–5 years | 62404.1 | 75/98.7 | 0.76 (0.59–0.98) | 62443.9 | 50/61.0 | 0.82 (0.60–1.12) | 62498.9 | 27/37.9 | 0.71 (0.46–1.09) |
| 6–10 years | 38659.7 | 40/71.1 | 0.56 (0.40–0.80) | 38710.8 | 29/44.6 | 0.65 (0.43–0.98) | 38774.3 | 13/26.7 | 0.49 (0.26–0.90) |
| ⩾11 years | 14649.1 | 15/30.2 | 0.50 (0.28–0.88) | 14685.7 | 11/19.2 | 0.57 (0.30–1.11) | 14704.6 | 5/11.2 | 0.45 (0.17–1.18) |
| No prior lower endoscopy | 822025.6 | 1287/1177.5 | 1.09 (1.03–1.16) | 823272.8 | 936/729.9 | 1.28 (1.19–1.38) | 824765.1 | 431/452.2 | 0.95 (0.86–1.06) |
| 1–5 years | 398680.3 | 616/510.0 | 1.21 (1.10–1.32) | 398966.3 | 453/313.4 | 1.45 (1.30–1.61) | 399384.6 | 192/197.6 | 0.97 (0.83 1.14) |
| 6–10 years | 281956.5 | 452/422.9 | 1.07 (0.96–1.19) | 282471.5 | 316/262.9 | 1.20 (1.06–1.36) | 283124.3 | 166/162.0 | 1.02 (0.86–1.22) |
| ⩾11 years | 141388.8 | 219/244.6 | 0.90 (0.77–1.04) | 141835.0 | 167/153.6 | 1.09 (0.91–1.29) | 142256.2 | 73/92.6 | 0.79 (0.61–1.02) |
Including any colonoscopy, sigmoidoscopy, or polypectomy before the primary eradication.
CI, confidence interval; CRC, colorectal cancer; SIR, standardized incidence ratio.
Consistent results were observed in subgroups of subjects with H. pylori eradication success (Table 3). There was no decreasing trend of the incidence of CRC among subjects with initial eradication failure who received retreatment for H. pylori.
In subgroup of subjects who had prior lower endoscopy at baseline, the incidences of CRC, including colon cancer and rectal cancer, were lower than matched general population (Table 3). Furthermore, the incidence of CRC in H. pylori-eradicated subjects who had prior lower endoscopy showed a progressive decreasing trend during the extended follow-up period when compared to the matched general population (SIR in 1–5 years: 0.76, 95% CI: 0.59–0.98; SIR in 6–10 years: 0.56, 95% CI: 0.40–0.80; SIR after 11 years: 0.50, 95% CI: 0.28–0.88).
Discussion
In this population-based study with a median follow-up of 9.7 years, we found that H. pylori-eradicated subjects had an overall higher incidence of CRC, colon cancer in particular, when compared with matched general population, which was mainly observed in the first 5 years after treatment for H. pylori. This finding was in line with previous studies that H. pylori-infected subjects was associated with a higher risk of CRC.4,6 However, with lengthening of the follow-up duration after treatment for H. pylori, the CRC incidence declined to a level comparable with, and even lower than, the general population in the long term. Furthermore, apart from testifying the cancer prevention effects of colonoscopy, the risk of CRC further declined with time after H. pylori eradication suggesting the potential additional benefits of H. pylori eradication.
The positive association between H. pylori infection and CRC has been reported in previous studies. 4 To date, majority of previous studies were case–control or cross-sectional in design, which failed to demonstrate the potential causality between H. pylori infection and CRC. 4 Although H. pylori infection and its related complications may increase patients’ visits to physicians and the chance of receiving CRC screening, we found that the increase in risk was mainly observed for colon cancer but not rectal cancer, which could not be explained by simple health seeking and screening effects. A retrospective population-based cohort study showed that H. pylori infection was associated with a significantly higher risk of CRC with an adjusted HR of 1.87. 17 A recent large nested case–control study based on 10 prospective cohorts found that seropositivity to 4 out of 13 H. pylori proteins was associated with a 10–11% increased risk of CRC. 18 This was however disputed by others.19,20 In our study involving all H. pylori-eradicated subjects, we found that H. pylori-eradicated subjects had a higher incidence of CRC, particularly in the first 5 years after treatment. This finding supports that H. pylori-infected subjects have an increased risk of CRC. However, after treatment for H. pylori, the incidence declined to a level comparable to general population, indicating that H. pylori eradication may have long-term protective effect on CRC development. In a secondary analysis of a randomized controlled trial on H. pylori treatment and gastric cancer, H. pylori treatment was also associated with a lower long-term risk of CRC specific death after controlling baseline gastric histology (hazard ratio: 0.25, 95% CI: 0.07–0.89), and a protective trend was also observed in the multivariate model. 21
The association between H. pylori and the site of colorectal neoplasia had also been evaluated previously. Hong et al. 22 reported that positive association between H. pylori infection and colonic adenoma or advanced adenoma was confined to proximal adenoma. In contrast, Zhang et al. 23 found that H. pylori infection may be associated with the increased risk of CRC in the left colon. Further studies are needed to confirm and evaluate the potential different effects of H. pylori infection on colon and rectal cancer.
Despite the reported association between H. pylori infection and CRC, the underlying mechanism remains unclear.4,24H. pylori could have direct and/or indirect effects on colorectal carcinogenesis. 4 Although colon is not the usual habitat of H. pylori, the bacterium could traverse the colon and rectum and it was also detected in colorectal lesions.25,26 Studies have reported that components of H. pylori or specific strains may promote DNA synthesis and cell proliferation in small intestinal epithelial cell line.27,28H. pylori may also promote colorectal carcinogenesis in an indirect manner. First, colorectal carcinogenesis might be caused by dysbiosis of the gut microbiota induced by H. pylori infection.29,30H. pylori infection may result in changes in gut microbiota by altering gastric acidity and host–microbe interactions. 29 Studies have shown that treatments for H. pylori with antibiotics significantly reduced the alpha-diversity of the gut microbiota transiently. 29 After H. pylori eradication, short-chain fatty acids-producing bacteria were enriched,31,32 which has been shown to have anticancer effect on CRC.33,34 Guo et al. 31 reported that the Bifidobacterium-related taxa was enriched after successful eradication, which is a well-known probiotic and has potential CRC prevention effect. 35 Several studies have also reported increased abundance of putative CRC-associated gut bacteria in H. pylori-positive patients.32,36 Whether H. pylori eradication therapy could reduce presumed CRC-associated bacteria in the long term remains unknown. Second, H. pylori infection-related gastric and systemic inflammation may promote colorectal carcinogenesis. H. pylori infection was reported to modulate the production and activity of cyclooxygenase 2 and consequently prostaglandin E2, 37 that have been associated with CRC risk.38,39H. pylori infection induces different signal transduction processes with the production of pro-inflammatory cytokines like tumor necrosis factor alpha, interferon gamma, interleukin (IL)-1, IL-6, IL-8, leading to the development and progression of gastric inflammation and carcinogenesis, 40 which may also promote colorectal carcinogenesis. 41 Third, H. pylori infection and related gastritis increase the secretion of gastrin, which can act as a promoter of gastric and colorectal carcinogenesis.42,43 Several studies reported that elevated serum gastrin level was associated with an increased risk of colorectal adenoma and CRC.44,45 However, CRC tumor cells themselves have been shown to secrete gastrin in an autocrine manner.46,47
Our study has several strengths. First, this is a large population-based cohort study involving subjects that had received eradication therapy for H. pylori infection with long-term follow-up period, allowing us to evaluate the temporal trend of CRC risk after treatment for H. pylori. Second, this study is based on a comprehensive healthcare database in Hong Kong, where the majority of local residents received medical attention due to the easy availability of high-quality medical care at heavily subsided cost. Therefore, almost all diagnosed cancers are recorded in this system.
This study has limitations. First, H. pylori-infected subjects who had never received treatment should be ideally included. Nevertheless, due to the high background incidence of gastric cancer and peptic ulcer disease, H. pylori is generally eradicated once detected in local practice. As H. pylori test result is not recorded in the electronic system, we were unable to identify any untreated H. pylori-infected cohort in this database. However, we have included subjects who required retreatment or failure of H. pylori eradication as an internal control. The accuracy of the retreatment-inferred eradication failure had been validated previously with a sensitivity of 91.3% and a specificity of 98.7%. 13 Second, we only included H. pylori-eradicated subjects who had received clarithromycin-containing triple therapy as the primary therapy. It was the most commonly prescribed first-line eradication therapy in Hong Kong, due to a relatively low prevalence of clarithromycin resistance, approximately 10%. 48 Third, the association between H. pylori and CRC might also be altered by genetic heterogeneity of H. pylori strains, 18 which was not evaluated in our study. Serum-based tests for H. pylori infection, test for specific H. pylori antigen in particular, were not generally conducted in the local clinical practice in a population with predominant cagA-positive strains. 49
Conclusion
H. pylori-eradicated subjects had a higher incidence of CRC, colon cancer in particular, compared with the matched general population, which was mainly observed in the first 5 years after treatment for H. pylori. Importantly, the CRC incidence progressively declined to a level comparable to the general population after treatment for H. pylori, and the rectal cancer incidence was found to be lower than general population after more than 10 years of eradication.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231170943 for Long-term effect of Helicobacter pylori eradication on colorectal cancer incidences by Chuan-Guo Guo, Feifei Zhang, Fang Jiang, Lingling Wang, Yijun Chen, Wenxue Zhang, Anni Zhou, Shutian Zhang and Wai K. Leung in Therapeutic Advances in Gastroenterology
Acknowledgments
We would like to thank the Hospital Authority of Hong Kong for the use of clinical data from the Clinical Data Analysis and Reporting System and all staff members for their work on the maintenance of the system.
Footnotes
ORCID iD: Chuan-Guo Guo  https://orcid.org/0000-0002-0657-473X
https://orcid.org/0000-0002-0657-473X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chuan-Guo Guo, Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China; Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Feifei Zhang, National Institute of Health Data Science at Peking University, Beijing, China.
Fang Jiang, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Lingling Wang, Department of Gastroenterology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China.
Yijun Chen, Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Wenxue Zhang, Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Anni Zhou, Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Shutian Zhang, Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Wai K. Leung, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong 999077, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (Reference Number: UW 21-431). As deidentified data from the healthcare system and public data were used, patient consent was not required by the Institutional Review Board.
Consent for publication: Not applicable.
Author contribution(s): Chuan-Guo Guo: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Software; Validation; Visualization; Writing – original draft.
Feifei Zhang: Formal analysis; Investigation; Methodology; Software; Visualization; Writing – review & editing.
Fang Jiang: Data curation; Investigation; Validation; Writing – review & editing.
Lingling Wang: Investigation; Writing – review & editing.
Yijun Chen: Investigation; Writing – review & editing.
Wenxue Zhang: Investigation; Writing – review & editing.
Anni Zhou: Investigation; Writing – review & editing.
Shutian Zhang: Investigation; Resources; Supervision; Writing – review & editing.
Wai K. Leung: Conceptualization; Funding acquisition; Investigation; Project administration; Resources; Supervision; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by National Natural Science Foundation of China (82204120).
The authors declare that there is no conflict of interest.
Availability of data and materials: The clinical data that support the findings of this study were from the Clinical Data Analysis and Reporting System of the Hong Kong Hospital Authority, which were used under license for this study. The cancer statistics are openly available from the Hong Kong Cancer Registry at https://www3.ha.org.hk/cancereg/.
References
- 1.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017; 153: 420–429. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi F, Covino M, Roubaud Baudron C.Review: Helicobacter pylori and extragastric diseases. Helicobacter 2019; 24: e12636. [DOI] [PubMed] [Google Scholar]
- 3.Venerito M, Vasapolli R, Rokkas T, et al. Helicobacter pylori, gastric cancer and other gastrointestinal malignancies. Helicobacter 2017; 22: e12413. [DOI] [PubMed] [Google Scholar]
- 4.Butt J, Epplein M.Helicobacter pylori and colorectal cancer-A bacterium going abroad? PLoS Pathog 2019; 15: e1007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko HJ, Lin YC, Chen CC, et al. Helicobacter pylori infection and increased diabetes prevalence were the risks of colorectal adenoma for adults: a systematic review and meta-analysis (PRISMA-compliant article). Medicine (Baltimore) 2021; 100: e28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Y, Jing Z, Bie M, et al. Association between Helicobacter pylori infection and the risk of colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2020; 99: e21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford AC, Yuan Y, Moayyedi P.Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020; 69: 2113–2121. [DOI] [PubMed] [Google Scholar]
- 8.Leung WK, Wong IOL, Cheung KS, et al. Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology 2018; 155: 67–75. [DOI] [PubMed] [Google Scholar]
- 9.Guo CG, Cheung KS, Zhang F, et al. Incidences, temporal trends and risks of hospitalisation for gastrointestinal bleeding in new or chronic low-dose aspirin users after treatment for Helicobacter pylori: a territory-wide cohort study. Gut 2020; 69: 445–452. [DOI] [PubMed] [Google Scholar]
- 10.Guo CG, Cheung KS, Zhang F, et al. Delay in retreatment of Helicobacter pylori infection increases risk of upper gastrointestinal bleeding. Clin Gastroenterol Hepatol 2021; 19: 314–322.e312. [DOI] [PubMed] [Google Scholar]
- 11.Hong Kong Hospital Authority. Hong Kong cancer registry (HKCaR), https://www3.ha.org.hk/cancereg/ (2022, accessed 17 May 2022).
- 12.Census and Statistics Department Hong Kong Special Administrative Region. Hong Kong annual digest of statistics, https://www.censtatd.gov.hk/en/ (2022, accessed 17 May 2022).
- 13.Guo CG, Jiang F, Cheung KS, et al. Timing of prior exposure to antibiotics and failure of Helicobacter pylori eradication: a population-based study. J Antimicrob Chemother 2022; 77: 517–523. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein DM, Muzikansky A, Schoenfeld DA.Comparing survival of a sample to that of a standard population. J Natl Cancer Inst 2003; 95: 1434–1439. [DOI] [PubMed] [Google Scholar]
- 15.Spector LG, Menk JS, Knight JH, et al. Trends in long-term mortality after congenital heart surgery. J Am Coll Cardiol 2018; 71: 2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 17.Liu IL, Tsai CH, Hsu CH, et al. Helicobacter pylori infection and the risk of colorectal cancer: a nationwide population-based cohort study. QJM 2019; 112: 787–792. [DOI] [PubMed] [Google Scholar]
- 18.Butt J, Varga MG, Blot WJ, et al. Serologic response to Helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology 2019; 156: 175–186.e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XZ, Schottker B, Castro FA, et al. Association of Helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: a ten-year follow-up of the ESTHER cohort study. Oncotarget 2016; 7: 17182–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blase JL, Campbell PT, Gapstur SM, et al. Prediagnostic Helicobacter pylori antibodies and colorectal cancer risk in an elderly, caucasian population. Helicobacter 2016; 21: 488–492. [DOI] [PubMed] [Google Scholar]
- 21.Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019; 366: l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SN, Lee SM, Kim JH, et al. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci 2012; 57: 2184–2194. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Hoffmeister M, Weck MN, et al. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol 2012; 175: 441–450. [DOI] [PubMed] [Google Scholar]
- 24.Papastergiou V, Karatapanis S, Georgopoulos SD.Helicobacter pylori and colorectal neoplasia: is there a causal link? World J Gastroenterol 2016; 22: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M, Helliwell P, Pritchard C, et al. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol 2007; 5: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soylu A, Ozkara S, Alis H, et al. Immunohistochemical testing for Helicobacter pylori existence in neoplasms of the colon. BMC Gastroenterol 2008; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bark J, Enroth H, Engstrand L, et al. Cancer-associated strains of Helicobacter pylori stimulate DNA synthesis in IEC-6 cells. Eur J Gastroenterol Hepatol 1998; 10: 837–841. [DOI] [PubMed] [Google Scholar]
- 28.Brannstrom J, Zachrisson K, Hulten K, et al. Helicobacter pylori stimulates DNA synthesis in a small intestinal cell line in vitro. Digestion 1998; 59: 33–39. [DOI] [PubMed] [Google Scholar]
- 29.Chen CC, Liou JM, Lee YC, et al. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021; 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020; 158: 322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Zhang Y, Gerhard M, et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020; 69: 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dash NR, Khoder G, Nada AM, et al. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS One 2019; 14: e0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu NN, Jiao N, Tan JC, et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat Microbiol 2022; 7: 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou H, Chen D, Zhang K, et al. Gut microbiota-derived short-chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett 2022; 526: 225–235. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo-Cantabrana C, Delgado S, Ruiz L, et al. Bifidobacteria and their health-promoting effects. Microbiol Spectr 2017; 5: 1–19. [DOI] [PubMed] [Google Scholar]
- 36.Iino C, Shimoyama T, Chinda D, et al. Influence of Helicobacter pylori infection and atrophic gastritis on the gut microbiota in a Japanese population. Digestion 2020; 101: 422–432. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Wen S, Wang X, et al. Helicobacter pylori modulates cyclooxygenase-2 and 15-hydroxy prostaglandin dehydrogenase in gastric cancer. Oncol Lett 2017; 14: 5519–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenport JR, Cai Q, Ness RM, et al. Evaluation of pro-inflammatory markers plasma C-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Mol Carcinog 2016; 55: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konturek PC, Kania J, Burnat G, et al. Prostaglandins as mediators of COX-2 derived carcinogenesis in gastrointestinal tract. J Physiol Pharmacol 2005; 56: 57–73. [PubMed] [Google Scholar]
- 40.Wessler S, Krisch LM, Elmer DP, et al. From inflammation to gastric cancer–the importance of Hedgehog/GLI signaling in Helicobacter pylori-induced chronic inflammatory and neoplastic diseases. Cell Commun Signal 2017; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthusami S, Ramachandran IK, Babu KN, et al. Role of inflammation in the development of colorectal cancer. Endocr Metab Immune Disord Drug Targets 2021; 21: 77–90. [DOI] [PubMed] [Google Scholar]
- 42.Renga M, Brandi G, Paganelli GM, et al. Rectal cell proliferation and colon cancer risk in patients with hypergastrinaemia. Gut 1997; 41: 330–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartwich A, Konturek SJ, Pierzchalski P, et al. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis 2001; 16: 202–210. [DOI] [PubMed] [Google Scholar]
- 44.Thorburn CM, Friedman GD, Dickinson CJ, et al. Gastrin and colorectal cancer: a prospective study. Gastroenterology 1998; 115: 275–280. [DOI] [PubMed] [Google Scholar]
- 45.Selgrad M, Bornschein J, Kandulski A, et al. Helicobacter pylori but not gastrin is associated with the development of colonic neoplasms. Int J Cancer 2014; 135: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 46.Charnley RM, Thomas WM, Stanley J, et al. Serum gastrin concentrations in colorectal cancer patients. Ann R Coll Surg Engl 1992; 74: 138–140. [PMC free article] [PubMed] [Google Scholar]
- 47.Bombski G, Gasiorowska A, Orszulak-Michalak D, et al. Elevated plasma gastrin, CEA, and CA 19-9 levels decrease after colorectal cancer resection. Int J Colorectal Dis 2003; 18: 148–152. [DOI] [PubMed] [Google Scholar]
- 48.Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 707–715. [DOI] [PubMed] [Google Scholar]
- 49.Wong BC, Lam SK, Ching CK, et al. Seroprevalence of cytotoxin-associated gene A positive Helicobacter pylori strains in Changle, an area with very high prevalence of gastric cancer in South China. Aliment Pharmacol Ther 1999; 13: 1295–1302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231170943 for Long-term effect of Helicobacter pylori eradication on colorectal cancer incidences by Chuan-Guo Guo, Feifei Zhang, Fang Jiang, Lingling Wang, Yijun Chen, Wenxue Zhang, Anni Zhou, Shutian Zhang and Wai K. Leung in Therapeutic Advances in Gastroenterology