Summary
Despite extensive studies on kinematic features of impacting drops, the effect of mechanical stress on desiccated bacteria-laden droplets remains unexplored. In the present study, we unveiled the consequences of the impaction of bacteria-laden droplets on solid surfaces and their subsequent desiccation on the virulence of an enteropathogen Salmonella typhimurium (STM). The methodology elucidated the deformation, cell-cell interactions, adhesion energy, and roughness in bacteria induced by impact velocity and low moisture because of evaporation. Salmonella retrieved from the dried droplets were used to understand fomite-mediated pathogenesis. The impact velocity-induced mechanical stress deteriorated the in vitro viability of Salmonella. Of interest, an uninterrupted bacterial proliferation was observed in macrophages at higher mechanical stress. Wild-type Salmonella under mechanical stress induced the expression of phoP whereas infecting macrophages. The inability of STM ΔphoP to grow in nutrient-rich dried droplets signifies the role of phoP in sensing the mechanical stress and maintaining the virulence of Salmonella.
Subject areas: Applied physics, Microbiology, Biophysics
Graphical abstract
Highlights
-
•
Bacterial pathogens can persist in mechanically-stressed desiccated droplets
-
•
Bacterial cell walls deform flexibly under velocity-induced mechanical stresses
-
•
Impact velocity of bacteria laden-droplets could not hamper its virulence
-
•
Deleting phoP reduced bacterial survival in mechanically stressed dried droplets
Applied physics; Microbiology; Biophysics
Introduction
The bacterial droplets settling over inanimate surfaces are one of the leading causes of dissemination of microbial infection.1,2 The bioaerosols generated from contaminated water supply (like busted pipes), public restrooms, and wastewater treatment plants can be hotbeds to transmit airborne microbial infection.3 The bacteria-laden droplets from a contaminated water source or respiratory event of an infected person (cough, sneeze) usually impinge on various substrates (fomites) in the vicinity with specific velocities (kinetic energy). Such impact on substrates leads to severe deformations of the droplets because of significant mechanical stress inside the liquid phase. The bacteria inside the droplets experience these stress conditions for short durations (milliseconds). Furthermore, these droplets on substrates undergo evaporation under ambient airflow conditions, thereby creating desiccation stress (long timescales) on the bacteria, which leads to unique spatiotemporal assemblies and subsequent non-uniform bacterial deposit patterns. Even though harsh environmental stress conditions and nutrient limitations can decrease the microbial count, the transmission and virulence of bacterial pathogens recovered from fomites or dried droplets remain unaltered.4,5 However, how the mechanical (impact velocity) and desiccation stress in evaporating dried droplets shape the environmental persistence and long-term virulence of bacteria remains unclear. In the current study, we aimed to understand the stress response mechanism of bacteria present in bacteria-laden drops, which were subjected to varying stress conditions, and studied fomite-mediated bacterial pathogenesis. We present a unique combination of fluid mechanics studies combined with biological insights (including animal infection studies) to understand how stress translates to virulence.
Salmonella typhimurium (STM), one of the leading causes of self-limiting gastroenteritis and diarrhea in humans,6,7 has been used as a model system in the present study. However, the proposed experimental methodology is suitable for investigating any naturally dried bacterial droplets. There is no conclusive evidence that Salmonella infection occurs from the surfaces of fomites or mechanically stressed desiccated droplets. Salmonella is a common bacterial pathogen that severely impacts various food production industries.8 Recently, Salmonella was detected in the batch of chocolate produced by Barry Callebaut, the biggest chocolate factory in the world, having an average annual production of 2.2 million tons. Salmonella infection spreads to healthy individuals through contaminated food and water and causes conditions such as salmonellosis, which includes fever, diarrhea, and stomach cramps in the infected host.9,10 However, airway transmission of different Salmonella serovars, such as Enteritidis, Typhimurium, and Agona, has also been documented.11,12,13 Aerosolized Salmonella colonizes within the trachea, ceca, liver, and spleen of poultry broilers.14,15 Improper sterilization of medical equipment and aerosol-mediated transmission from contaminated feces lead to infections and associated outbreaks of Salmonella.16,17
Despite poor in vitro survival because of nutrient limitation, Salmonella Typhimurium retrieved from inanimate surfaces showed hyper-virulence in RAW264.7 cells.4 The ability of Salmonella to survive on inanimate surfaces in the presence of low moisture and fewer nutrients poses a risk of contamination of consumable food items. It has been reported that thousands of people die worldwide from Salmonella systemic infections, such as invasive non-typhoidal salmonellosis, Salmonella-induced meningitis, and neurological abnormalities.18,19,20,21,22 Moreover, Salmonella remains the second biggest cause of food-borne disease and the leading cause of hospitalization and death in the US.8,23 All these aspects add to the importance of understanding the effect of velocity-induced mechanical stress and evaporation-induced desiccation stress on Salmonella-laden droplets.
Each bacterium within the medium can experience contact force when acquainted with any surface. These contact forces can either be compressive or tensile, depending on whether the bacterial cell wall pushes itself onto or pulls away from the surface. Furthermore, they experience compressive forces when exposed to open atmosphere, deep-sea water environments, or inside the host cells. Moreover, inhalation, contact, and ingestion of bacteria-laden droplets and aerosols from polluted water can endanger human health. The bioaerosols or microdroplets from the infected persons can be altered by ambient airflow convection and transported much farther than 1.83m.24 A recent study indicates that the evaporation of droplets can result in aerosols that can drive the transmission of SARS-CoV-2.25 The aerosols expelled by the infected person during exhalation, coughing, and sneezing can deposit over inanimate surfaces exhibiting a wide range of droplet sizes and velocity scales. It can also act as a source of microbial contamination. Irrespective of the droplet size and the surface it impinges, the transmission ability of bacteria-laden droplets or fomites can lead to bacterial colonization inside the host.4 The impact dynamics profoundly depend on the physicochemical characteristics of impact substrate and droplet properties. The liquid droplet impact on surfaces is vital in various natural processes. The sudden burst of pipelines carrying sewage water and the velocity of droplets coming out from an infected person are some practical instances of drop impacts. These variations in impact velocities are characterized with the aid of several dimensionless numbers: The Reynolds number (Re = ρDV/μ), which is a dimensionless ratio of inertial to viscous force; Weber number (We = ρDV2/γ), which is the ratio of inertia to surface tension forces (γ), Ohnesorge number (Oh = μ/), and Capillary number (Ca = μV/γ) where μ is the viscosity, ρ is the density, D is the diameter, γ is the surface tension forces, and V is the impact velocity. The impact mechanism for water drops at exceptionally low velocities was initially reported by Worthington.26 The literature reports substantial work on droplet spreading, receding, splashing, and bouncing.27,28,29,30
In the current study, the physiological relevance of bacterial droplet impact was studied by following an experimental protocol with impact velocities ranging from 5 to 10 m/s. The conditions considered mimic the velocity range of the droplets expelled from the mouth and nose during sneezing or coughing. The fluid dynamic events demonstrated in this study led to alterations in bacterial colonization, morphology, in vitro viability, subsequent infection pathways in macrophages, and bacterial gene regulations.
Results
Global impact dynamics of bacteria-laden droplets signifies the dominance of inertial effects
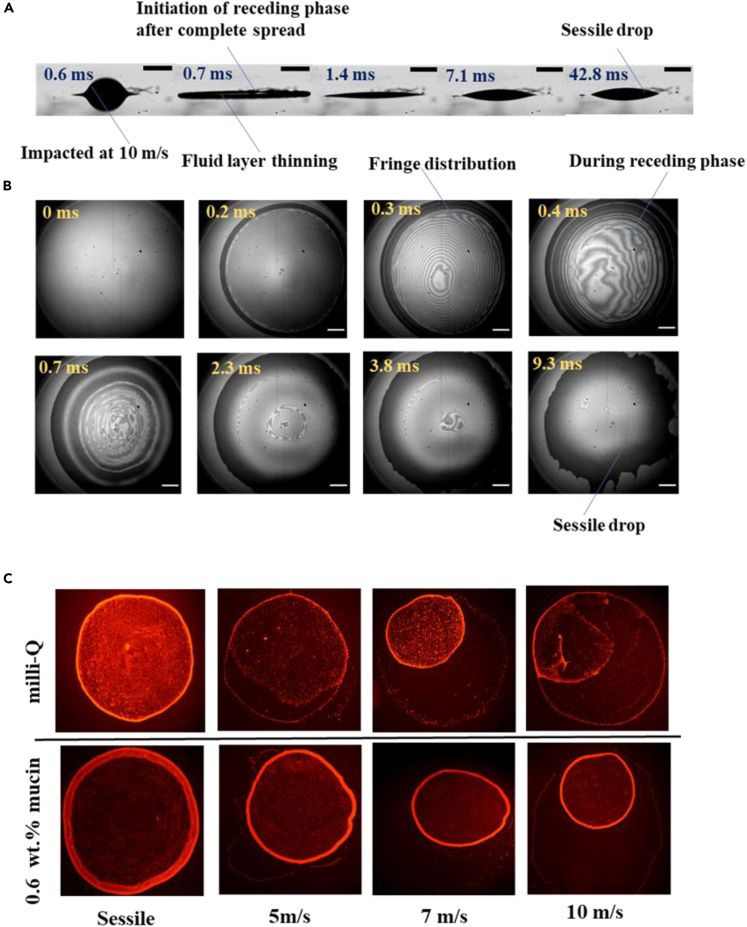
The droplet spread over glass substrate primarily depends on the inertial effects, based on the Weber number corresponding to impact velocities of 5, 7, and 10 m/s leading to shorter timescale mechanical stress. The side-view imaging of the droplet impact on the glass surface is presented at various time instants (Figure 1A). Droplet impact sequence from the point of contact on the surface to large scale spreading and subsequent retraction leading to the formation of a sessile drop (i.e., for a higher velocity of 10 m/s) is evident from the snapshots. Figure 1B represents the spreading dynamics of the droplet at a higher impact velocity and the variation of thin-film liquid layers captured using reflection interference microscopy (Figure 2A). The droplet spreading is entrenched with various parameters such as viscous dissipation, surface tension effects, surface roughness, and contact line dynamics. These factors get overshadowed when the inertia forces are dominant. In this context, the present study demonstrates the influence of inertia forces rather than viscous effects during the bacterial droplet impact and spreading. The bacterial droplet spreads after the first point of contact on the surface and reaches a maximum spreading diameter (dmax) until the initial kinetic energy is dissipated entirely. The spreading time also decreases with increased impact velocity because of the higher kinetic energy. Furthermore, when the drop attains dmax, the bacteria-laden drop retracts as the impact Weber number is significant, for which the maximum spreading diameter dmax is always greater than the equilibrium spreading diameter. Similarly, a higher receding velocity is observed for the higher impact velocity, as shown in Table 1. The interference patterns observed during the spread of the liquid layer signify the presence of a relatively thin liquid layer during the initial time instants for the maximum spread in the droplet. As time progress, retraction of the liquid layer is evident from the recorded images. Hence, the propagation of capillary waves of the receding liquid layer is seen as dark circular bands in the images from t = 0.3 to 0.7 ms (Figure 1B).
Figure 1.
Global dynamics of bacterial droplet deposition
(A) Profile view Images captured at 10000 fps for Salmonella laden droplet impacted on glass surface with 10 m/s on the glass substrate. The scale bar is 400 μm.
(B) High-speed interference images captured at 10000 fps for Salmonella-laden droplet impacted on glass surface with 10 m/s on the glass substrate. The scale bar is 200 μm.
(C) Fluorescence intensity captured for dried droplet impacted at different Weber numbers.
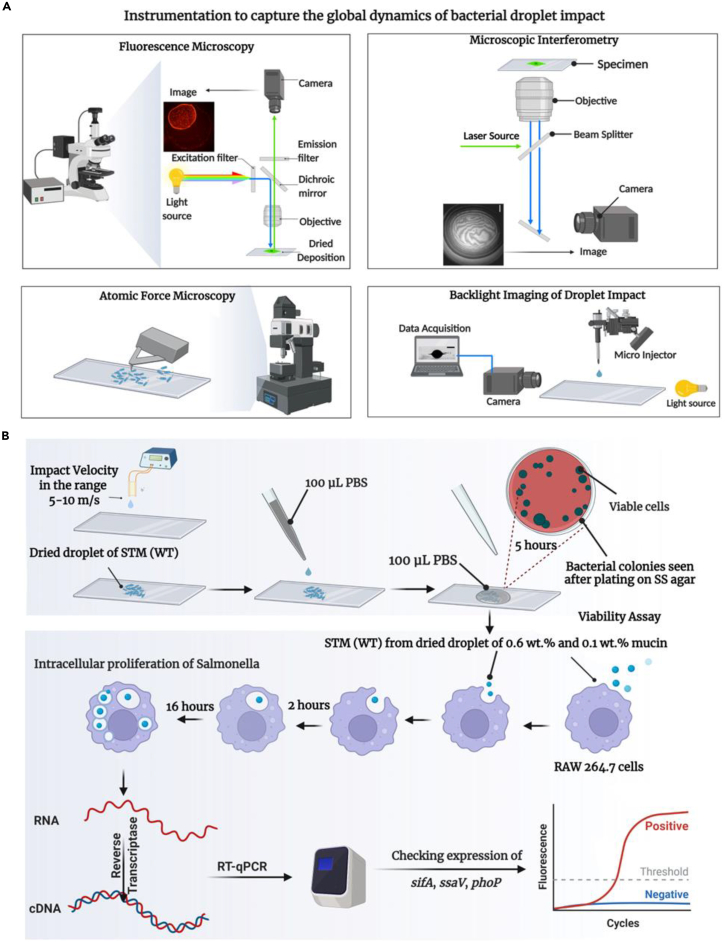
Figure 2.
Schematic layout of the experimental study
(A) The schematic representation to capture the global dynamics of droplet impact involving fluorescence microscopy, high-speed microscopic interferometry, atomic force microscopy, and backlight imaging of bacterial droplet impact.
(B) Experimental layout depicting the assessment of in vitro viability of bacteria, infection of macrophages by the bacteria retrieved from desiccated dried droplets, and quantification of the expression of virulent genes of bacteria during infection.
Table 1.
Operating conditions on which the experiments are carried out indicate the impact velocities with the maximum spread and diameter of the drop investigated
| Case number | Velocity (m/s) | Webernumber (-We) | Maximum spread factor (-β) | Average Receding velocity (m/s) |
|---|---|---|---|---|
| 1 | 5.0 ± 0.3 | 100 ± 10 | 3.09 | 0.0171 |
| 2 | 7.0 ± 0.3 | 250 ± 10 | 3.74 | 0.0079 |
| 3 | 10.0 ± 0.3 | 750 ± 20 | 4.75 | 0.0054 |
The increase in multiple waves can be seen in the receding process of the liquid layer, and the disintegration of such waves is seen once it attains the shape of a sessile drop. The fringe patterns during the spread process are because of the thin-film liquid layer at lower droplet contact angles. The distribution of the fringe pattern relies on the increase in contact angle from the receding point to the formation of a sessile drop (Figure 1B). The extent of spread of the droplet is mainly dependent on the impact Weber number, and the spreading diameter and variation of the impact velocities are presented in Table 1. For all impact velocities, similar variations of the fringe patterns were evident from the reflection interference microscope. The fringe pattern during the thin film formation of the impacted droplet has been processed by employing fast-frequency guided sequential demodulation (FFSD) method. Fast-frequency guided algorithms are most suitable for transient, complex data and extraction of two-dimensional phase field to intricate fringe patterns.31,32,33,34 During the initial period, the spread of the thin film is symmetric as can be observed from even distribution of thin film thickness from the center of the droplet. The edge of the droplet spread is considered as the minimum film thickness (the reference point for measurement) as the glass surface is seen from the images (Figure 1B). With increase in time, the uneven distribution of the variation of film-thickness is observed from the fringe patterns and possible film-thickness (Figures S1A and S1B). A similar variation of film thickness is observed at the initial time instants and a reduction in thickness at higher time instants varying between 9 and 14 μm.
The global dynamics of droplet spread remained almost similar for various inertial forces, where the viscous measurements of working fluid are nearly similar to conventional fluids, i.e., distilled water (Figures S2A and S2B). The side-view and interference microscopy imaging have been performed simultaneously with high-speed imaging systems as the impact, spreading, and retraction occur in rapid alternating regimes. The increase in impact velocity of the bacteria-laden droplet leads to a more extensive spread of the liquid layer, as presented in Table 1, because of the increase in inertial force. The impact energy of the droplet at different velocities and the increase in receding velocity assist in determining the unique bacterial deposition patterns and bacterial virulence.
The drying effects of bacteria-laden sessile drops after impact on the surface are inadequately addressed in the literature. The evaporation of impacted droplets leaves a different dried pattern on the glass substrate compared to sessile deposition. It is observed that the deposited pattern can range from a simple ring-like structure referred to as a coffee ring or multiple coffee rings with uniform deposition, depending on the shear force and flow characteristics. Correspondingly, the bacteria-laden fluids revealed various patterns on the glass substrate after the impacted drop evaporated (Figure 1C). A thick outer ring of particles was seen in the dried biological STM-mucin samples. For a sessile drop, the fluid flows radially outwards to replace the evaporated fluid at the base edge of the drop, maintaining a constant radius thus leading to the commonly seen coffee ring.35,36 The pinning of the contact line ensures outward capillary flow that helps in carrying the material toward the edge of the pinned surface, as represented in the literature.37,38,39,40 Moreover, capillary flow creates a thick outer ring at its maximum spread when the droplet impinges at a certain velocity for which the viscosity of the base fluid medium is accountable for transporting particles toward the contact line.41 During the spreading phase, the change in contact line or angle variation mainly depends on the liquid and the surface properties. Furthermore, when the droplet recedes after reaching maximum spread, it experiences a constant contact angle regime, and the bacterial deposition was found to be uniform inside the ring. The variation in deposition patterns primarily indicates differences in bacterial morphology with an increase in impact velocities which is systematically investigated in the following sections.
The dependence of the bacterial residue on the impact velocity can be addressed from the spread factor of the droplet with the time interval instants. The shape factor and non-dimensional time parameters were estimated using the correlations reported by Du et al.42 and Rioboo et al.43 as follows:
| (Equation 1) |
Where, in Equation 1, D0 is the initial diameter of the droplet (m), v is the impact velocity (m/s), and the t is the spatial time interval from the time of impact to constant contact line formation of the droplet (Figure S2B).
Varying stress conditions immensely altered the bacterial morphology
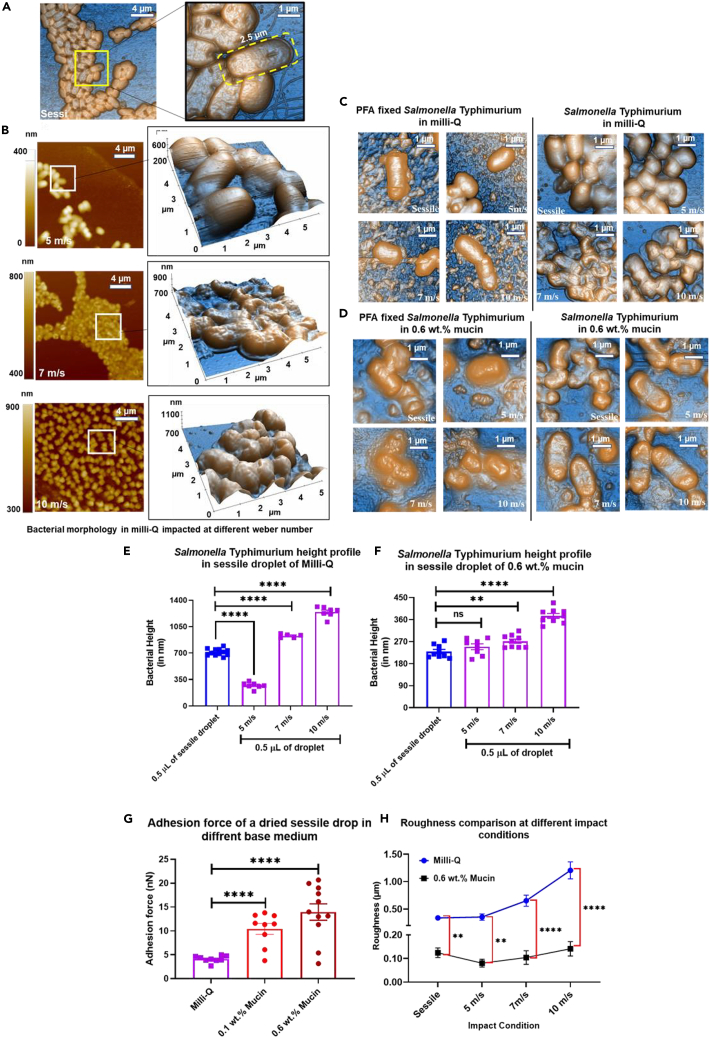
From the discussions in the earlier section, inertial force is found to have a significant role in altering bacterial morphology. The topographic images of the bacterial cell wall at three impact Weber numbers (100, 250 and, 750) corresponding to 5, 7 and, 10 m/s are acquired using Atomic Force Microscopy (AFM) in nutrient-neutral (milli-Q) and nutrient-rich medium (0.6 wt % mucins). The coupling of mechanical forces with microscopic characterization is essential for comprehending physiological changes (Figures 2A and B). The qualitative analysis of the droplet impact with rapidly changing conditions significantly affects the surface morphology of the bacteria. Like other Gram-negative pathogens, Salmonella has a rigid cell wall made of peptidoglycan, a repeating unit of N-acetyl muramic acid and N-acetyl glucosamine connected by pentapeptide linkage.44,45 In response to environmental stimuli, Salmonella modifies its peptidoglycan layer.46 Also, when subjected to a certain velocity, the shape regulation of the Salmonella cell wall is profoundly affected by the external forces that affect the rate of cell wall synthesis.47 Apart from the impact stress, the bacterial cell wall is subjected to evaporative stress, which does not allow the bacteria to snap back to its initial cell wall shape (Figure 3A), as vividly observed for We = 250 (5 m/s) and 750 (7 m/s) for milli-Q and nutrient-rich medium48(Figures 3B–3D).
Figure 3.
Atomic force microscopy identifies higher stress induction and greater surface damage at a higher impact velocity
(A) AFM images for Salmonella in milli-Q in a sessile drop
(B) AFM images of the bacteria-laden droplet with milli-Q as the base medium at three different impact velocities in a 5 μm square area.
(C and D) (C) AFM images of the bacteria-laden droplet with milli-Q and (D) 0.6 wt % Mucin as the base medium at three different impact velocities in a 5 μm square area for PFA fixed cells.
(E) AFM images of the bacterial-laden droplet with milli-Q and (F) 0.6 wt % mucin as a base medium compared with PFA fixed bacteria at three different impact velocities along with their height profiles in 5 μm square area.
(G) Adhesion energy in nutrient-neutral (milli-Q) and nutrient-rich (0.6 wt % Mucin) base fluid medium experienced on a dried STM (WT) sessile drop.
(H) Roughness measurements presented as RMS roughness at different impact conditions for milli-Q and 0.6 wt % mucins. (P) ∗<0.05, (P) ∗∗<0.005, (P) ∗∗∗<0.0005, (P) ∗∗∗∗<0.0001, ns= non-significant, (Student’s t test-unpaired).
The maximum bacterial height of Salmonella when subjected to desiccation stress and the mechanical stress increased from 712.6 (sessile) to 1244.3 nm (10 m/s) in milli-Q medium (Figure 3E). A similar trend is seen when milli-Q is replaced with a nutrient-rich, 0.6 wt % mucin solution (Figure 4F), indicating flexible behavior of the cell walls (Figures 3C and 3D). AFM data also supports the view that the cell walls of Salmonella Typhimurium deform flexibly in the milli-Q and mucin medium (Figure 3C) because of nutrient deficiency and evaporative stress. Moreover, the flow shear stress is induced in the cell walls of the bacteria at higher impact velocities (Figures S3A and S3B). The height profile of a randomly selected bacterial cell (Figures S4A and S4B) indicates the presence of peaks and valleys over the cell wall. The deformation in the cell wall can be quantified using the roughness parameter.
Figure 4.
Assessment of in vitro viability of Salmonella retrieved from desiccated droplets of different base solutions impacted on a solid surface with or without mechanical stress induced by impact velocity
The viability of Salmonella Typhimurium (in log10 scale) recovered from dried sessile droplets of different base solutions impacted on solid glass surface with or without velocities (5, 7, and 10 m/s). As base solutions.
(A–F) milli-Q, (B) glucose 5 wt %, (C) 10% glycerol, (D) LB broth, (E and F) mucin 0.1 and 0.6 wt %, were used (N≥3). Data are represented as mean ± SEM. (P) ∗< 0.05, (P) ∗∗< 0.005, (P) ∗∗∗< 0.0005, (P) ∗∗∗∗< 0.0001, ns= non-significant, (Student’s t test-unpaired).
The root means square roughness (Rq) is calculated for Salmonella Typhimurium in milli-Q, and 0.6 wt % mucins are calculated based on the following Equation 2 for sessile and impact conditions (5, 7, and 10 m/s).
| (Equation 2) |
where Rq is the root-mean-square roughness at a height ‘z’ for the pixel ‘k’ in the image. The Rq indicates the distance between peaks and valleys or the average height variations from the mean line. Physically, Rq demonstrates the extent of surface damage which is more significant in milli-Q than in 0.6 wt % mucins for Weber number greater than 250 (5 m/s) (Figure 3G). The stress experienced on the deposition surface, calculated from the force exerted by AFM probe, shows higher surface stress (Figures S3A and S3B) for a velocity greater than 7 m/s with increased surface roughness. The nano-intender used for AFM measures the adhesion energy for nutrient-rich and nutrient-neutral samples by quantifying the forces between the tip and the sample.
The force-displacement spectroscopy for all the samples indicates that the force experienced during approach and retraction of the probe over the dried bacterial sample is significantly different, depicting greater adhesion between the tip and the cell walls, especially for velocity greater than 7 m/s (Figure 3H). Furthermore, higher adhesion energy indicates enhanced interaction of cells with the adjacent cells and the substrate. The enhanced cell-cell interaction leads to local stiffening of the dried deposit resulting in variations in the adhesion energy and resisting the deformation caused because of the receding velocity (dβ/dt) at different impact conditions. This enhanced interaction between the cells and substrate cushions the bacteria, keeping it viable even in adverse conditions.49 The increased surface roughness has a pertinent role in microbial adhesion.50,51 The undulations on the surface of bacterial deposits caused because of surface roughness enhanced their adhesion on glass substrate because of increased surface area and fissures at higher impact velocities. Moreover, cell-cell interaction or cushioning of bacteria happens when the adhesion energy is large, especially in the case of nutrient-rich 0.6 wt % mucins compared to a nutrient-deficient milli-Q medium (Figure 3G).
The cushioning effect on the cell membranes is quantitatively assessed using roughness kurtosis. The roughness kurtosis (Rku), acquired from AFM, provides the sharpness of spikes on the surface, which physically denotes that a grooved or pitted surface could shelter the bacteria by providing a cushioning effect. The numerical value of Rku greater than three indicates a presence of a spiky surface, and Rku less than three denotes a bumpy surface52 given by Equation 3.
| (Equation 3) |
where Rq is the root-mean-square roughness at a height ‘z’ for the pixel ‘k’ in the image. The average roughness kurtosis is ≈5.1 in the case of bacteria-laden 0.6 wt % mucins, ≈4.2 in 0.1 wt % mucins, and ≈1.1 in milli-Q sessile drop. The spiky and bumpy surfaces are evident from the AFM images (Figures 3A, 3C, and 3D). This indicates that mucin (0.1 and 0.6 wt %) provides a better cushioning effect to the bacteria trapped between the spiky surfaces than milli-Q and influences the in vitro viability of the bacteria. However, the viability of the bacteria is also subjected to nutrient availability in the medium, which requires further investigation, as discussed in the subsequent sections.
The impact velocity reduced the in vitro viability of Salmonella Typhimurium in nutrient-rich desiccated droplets
Earlier, we reported that irrespective of the presence of nutrients, the in vitro viability of wild-type Salmonella inside the sessile droplets is severely compromised post-evaporation. Moreover, the bacteria recovered from dried nutrient-rich sessile droplets hyper-proliferates inside the RAW264.7 macrophages.4 However, the effect of mechanical stress because of the impact velocities of the droplets on the in vitro viability of the bacteria has not been investigated before. In this study, we have used neutral (milli-Q), low-nutrient (glucose 5 wt %, and 10% glycerol), and nutrient-rich (LB broth, mucin 0.1 wt %, and 0.6 wt %) base solutions for impacting the bacterial droplet on the glass surface with specific velocities (5, 7, and 10 m/s) as mentioned earlier. 0.5 μL of the planktonic culture of wild-type Salmonella in different base solutions gave rise to 105 to 106 CFU of viable bacteria (Figures 4A–4F; red bar). In the dried sessile droplet of 0.5 μL volume, the viability of STM (WT) reduced drastically to 102 from 104 CFU (Figures 4A–4F; blue bar). The loss of moisture because of evaporation restricted the availability of nutrients to the bacteria in the dried droplet and decreased the bacterial burden compared to the liquid-phase culture. In milli-Q (Figures 4A) and 5% glucose solutions (Figure 4B), the impact of droplets carrying wild-type Salmonella on the glass surface did not create any significant difference in its viability (∼102 CFU) compared to the sessile droplet (∼102 CFU). Salmonella can survive in glycerol by upregulating the genes associated with lipopolysaccharide biosynthesis and gluconate metabolism.53 When the bacteria were allowed to impact the glass slide with glycerol (10%) as the base medium (Figure 4C), we observed a significant decrease in the in vitro viability of Salmonella (∼102 CFU) compared to the sessile droplet (∼104 CFU) under all three velocities (Figure 4C). The reduction in the bacterial viability under velocity is attributed to mechanical stress induced by impact velocity (Figures S2A and S2B). Similar observations were obtained when bacteria-laden nutrient-rich-base solutions such as LB broth (Figure 4D), 0.1, and 0.6 wt % mucins (Figures 4E and 3F) were subjected to impact velocity. The viability of STM (WT) in the sessile droplet (∼103 CFU) of LB broth was significantly reduced to ∼102 CFU when exposed to mechanical stress (Figure 4D). A more prominent velocity-induced reduction of in vitro viability was observed in 0.1 wt % mucin solution (Figure 4E). Mucins are heavily glycosylated O-linked glycoproteins synthesized and secreted by the epithelial cells.54 The availability of nutrients in mucin improved the survival of the bacteria in the sessile droplet (∼104 CFU) even when subjected to an impact velocity of 5 m/s (∼104 CFU) velocity (Figure 4E). With an increase in the impact velocity from 5 to 7 m/s, the viability of wild-type Salmonella decreased from ∼104 CFU to ∼102 CFU and remained constant at 10 m/s (∼102 CFU) (Figure 4E). Furthermore, when we used 0.6 wt % of mucin, we observed that the higher nutrient content overshadowed the effect of impact velocity on the in vitro survival of Salmonella (Figure 4F). Unlike 0.1 wt % mucin concentration, no significant change in the in vitro viability of the bacteria was observed at 5 and 7 m/s impact velocities (Figure 6F). However, the viability of STM (WT) reduced from ∼104 CFU to ∼103 CFU with an increase in the impact velocity from 7 to 10 m/s (Figure 6F). Collectively the present data suggest that in addition to desiccation and nutrient limitation stress, the mechanical stress induced by the impact velocity also reduces the bacterial burden in dried droplets post-evaporation.
Figure 6.
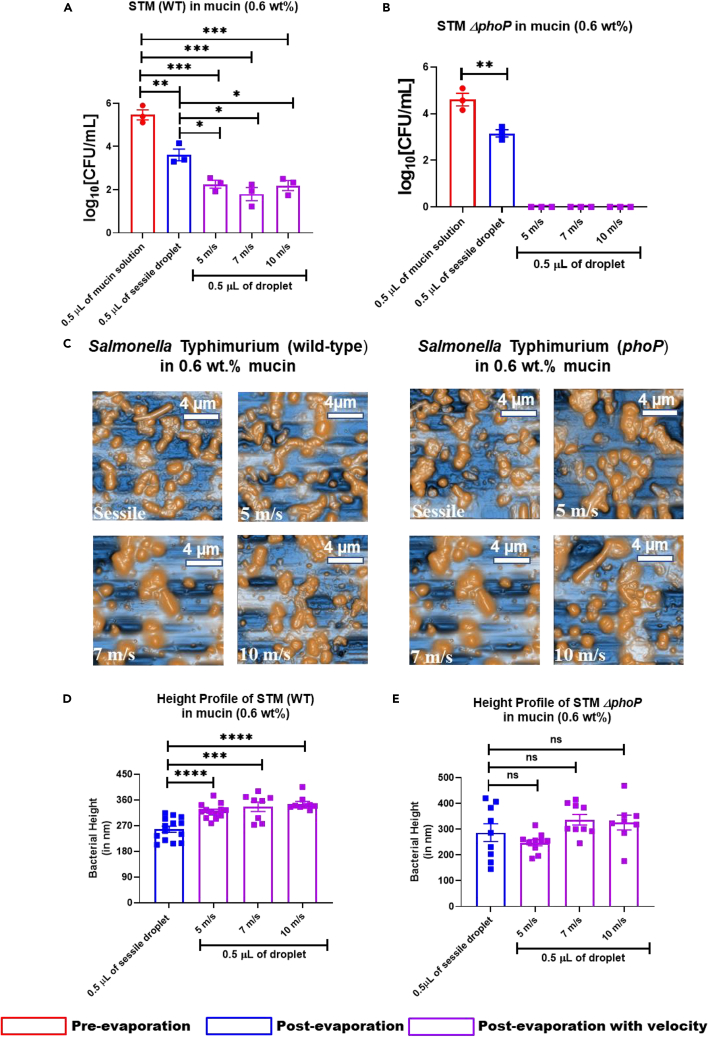
Deleting phoP reduced the in vitro viability of Salmonella Typhimurium in desiccated droplet of mucin (0.6 wt %) impacted on glass surface impacted with velocity 5, 7, and 10 m/s
(A–E) The in vitro viability of (A) STM (WT) and (B)ΔphoP (log10 scale) recovered from dried sessile droplets of mucin (0.6 wt %) impacted solid glass surface with or without velocities (5, 7, and 10 m/s) (N≥3). (C) AFM images of bacteria (STM WT and ΔphoP) laden droplets in mucin (0.6 wt %) on the glass surface. Height profile of (D) STM (WT) and (E)ΔphoP from the desiccated droplets of mucin (0.6 wt %) on the glass surface. Data are represented as mean ± SEM. (P) ∗< 0.05, (P) ∗∗< 0.005, (P) ∗∗∗< 0.0005, (P) ∗∗∗∗< 0.0001, ns = non-significant, (Student’s t test-unpaired).
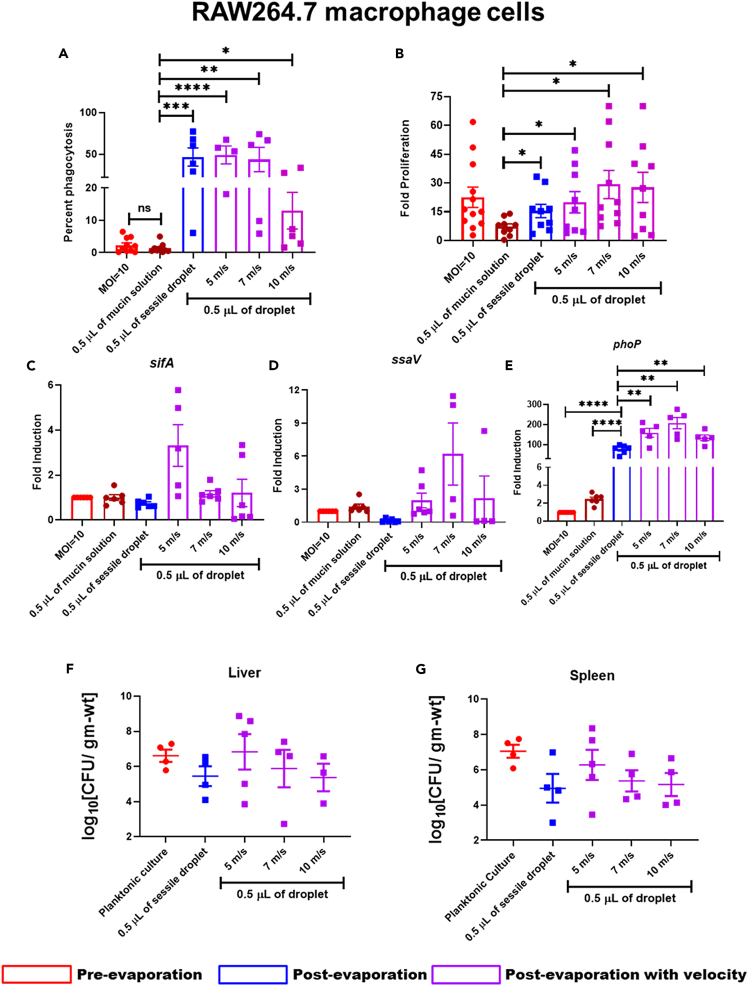
Wild-type Salmonella recovered from the desiccated droplet of mucin subjected to higher impact velocity showed hyperproliferation in RAW264.7 cells
We further investigated the effect of velocity-induced mechanical stress in the intracellular virulence of Salmonella retrieved from the dried droplet of mucin. After crossing the intestinal mucosal barrier, Salmonella interacts with phagocytic immune cells such as macrophages, dendritic cells, neutrophils, etc.10 Professional antigen-presenting cells like macrophages phagocytose invading pathogens thereby limiting their spread throughout the host body.55,56 Our study revealed that Salmonella recovered from dried sessile and velocity-impacted mucin (0.1 and 0.6 wt %) droplets are prone to phagocytosis by RAW264.7 cells compared to the planktonic culture (Figures 5A and S5A), suggesting a better clearance of the bacteria and subsequent restriction of the infection by macrophages. Surprisingly, these phagocytosed bacteria hyper-proliferated inside the macrophages (Figure 5B), indicating that the bacteria droplet, even when subjected to high mechanical stress because of impact velocity, can efficiently maintain their virulence while infecting the host cells. Wild-type Salmonella recovered from the velocity-impacted post-evaporated dried droplets of high mucin concentration (0.6 wt %) exhibited a similar phenomenon while infecting the RAW264.7 cells (Figure S5B). Inside the host cells, Salmonella stays inside a membrane-bound acidic compartment called Salmonella-containing vacuole (SCV).57 The acidic pH of SCV is sensed by the PhoQ/PhoP two-component system (TCS) of Salmonella, which uses Salmonella pathogenicity island-2 (SPI-2) encoded virulent factors such as sifA, ssaV, etc., for its successful replication inside the host cell.58,59,60 We hypothesized that the velocity-induced mechanical stress upregulated the expression of PhoQ/PhoP TCS and SPI-2 genes in Salmonella, which helped in the successful proliferation of the bacteria inside the macrophages. With an increase in the impact velocity (5–10 m/s) of Salmonella retrieved from 0.1 (Figures 4C–4E) and 0.6 wt % (Figures S5C–S5E) mucin droplets, showed an increased transcript-level expression of sifA (Figure 5C), ssaV (Figure 5D), and phoP (Figure 5E), which explains the reason behind their enhanced proliferation inside the macrophages. The uninterrupted proliferation and greater expression of sifA, ssaV, and phoP in intracellularly growing Salmonella further suggested intact in vivo pathogenesis. To validate this hypothesis, the animal infection study was carried out on C57BL/6 mice with Salmonella recovered from the bacteria-laden dried droplets (with or without impingement) and compared the bacterial burden of liver and spleen with the mice infected with a planktonic culture of Salmonella (Figures 5F and 5G). The comparable bacterial load of Salmonella retrieved from the bacteria-laden dried droplets (impacted the solid surface with or without velocity) in the liver and spleen of infected C57BL/6 mice suggested that the in vivo pathogenesis of Salmonella is not compromised due to mechanical and desiccation stress (Figures 5F and 5G).
Figure 5.
The virulence of Salmonella Typhimurium retrieved from desiccated mucin droplet (0.1 wt %) impacted on a solid surface at different velocities
(A–G) Salmonella Typhimurium recovered from desiccated mucin (0.1 wt %) droplet impacted solid glass surface with or without specific velocities (5, 7, and 10 m/s) were used to infect RAW264.7 cells to determine the (A)percent phagocytosis and (B) intracellular proliferation (n = 3, N = 2). Determining the transcript-level expression of (C)sifA,(D)ssaV, and (E)phoP from intracellular Salmonella by RT-qPCR (n = 3, N = 2). Salmonella retrieved from the bacteria-laden dried droplet (mucin 0.1 wt %) impacted solid glass surface with or without velocities were used to infect C57BL/6 mice. On sixth day post-infection, the mice were sacrificed, and the bacterial burden in the (F) Liver and (G) Spleen was enumerated. Data are represented as mean ± SEM. (P) ∗< 0.05, (P) ∗∗< 0.005, (P) ∗∗∗< 0.0005, (P) ∗∗∗∗< 0.0001, ns= non-significant, (Student’s t test-unpaired).
Survival of wild-type Salmonella in the velocity-impacted desiccated mucin droplets depends upon phoP
As wild-type Salmonella recovered from the dried droplet of mucins (for sessile and impact velocities 5, 7, and 10 m/s) indicated a consistent upregulation in the expression of phoP while infecting macrophages, we hypothesized that the survival of Salmonella Typhimurium in the nutrient restricted environment of dried velocity-impacted droplets depends on phoP. To test this hypothesis, the in vitro viability of STM (WT) (Figure 6A) and ΔphoP (Figure 6B) was measured in sessile droplets of high concentration mucin (0.6 wt %) to the impact Weber numbers of 100, 250, and 750. As discussed earlier, the viability of STM (WT) (∼106 CFU–∼104 CFU) and ΔphoP (∼105 CFU–∼103 CFU) was compromised in the sessile droplet of mucin after evaporation (Figures 6A and 5B). The bacteria-laden mucin droplets subjected to 5, 7, and 10 m/s velocities further reduced the viability of STM (WT) to ∼102 CFU because of the generation of mechanical stress (Figure 6A). On the contrary, STM ΔphoP struggled to survive in the desiccated mucin droplets for impact Weber numbers of 100–750 (Figure 6B). The inability of STM ΔphoP to survive in the stressed environment of velocity-impacted desiccated droplets showed a novel role of phoP in assisting bacterial pathogens in tolerating mechanical stress. The deformation of the bacterial cell walls is an intrinsic aspect of impact velocity that affects the bacterial cell-cell interactions and surface topography. As demonstrated earlier, bacterial cell deformation depends on critical parameters such as impact velocity, evaporative stress, and adhesion energy. In the case of an impact velocity of 5 m/s, the stretched time scales delayed the deformation of the bacterial cell phase because of lower receding velocity (Table 1). Likewise, the increase in height profiles with an increase in the impact velocity results in enhanced squeezing of bacterial cell walls. Of interest, the height profile of STM (WT) (Figure 6D) was strikingly different from the STM ΔphoP (Figure 6E). The STM ΔphoP exhibited an unresponsive height variation for the impact velocity, 5 m/s, and the sessile drop (Figure 6E). Similarly, a negligible increase was noted for STM ΔphoP even when the velocity is ramped up to 7 and 10 m/s. This indicates that the change in height profiles of Salmonella in desiccated droplets depends profoundly on phoP when subjected to velocity-induced mechanical stress.
Discussion
Earlier, multiple studies reported a reduction in the viability of bacterial pathogens when exposed to antibacterial surfaces.61,62,63 However, the present study demonstrated that investigating bacteria-laden drop impact on surfaces is highly relevant from the disease transmission perspective. This novel approach to droplet impact study opens a new dimension that brought out the effect of impact velocity-induced mechanical stress and evaporation-induced desiccation stress on the survival of bacteria in the nutrient-rich and nutrient-deficient medium. The spread of Salmonella from contaminated fomites or via aerosol is emerging as an important route of infections.14,64 Our study has discovered a few of the essential virulent genes of Salmonella that are required for their survival in desiccated droplets by bridging visible and microscopic perspectives. Even though the bacterial cell wall deformation is extensive at the higher impact velocity, the parameters such as receding velocity and adhesion energy also play an essential role in controlling the deformation. An increase in the bacterial cell height profile was noted for a higher impact Weber number because of high receding velocity occurring in smaller time scales. Compared to milli-Q, the enhanced adhesion energy in a nutrient-rich medium (0.1 and 0.6 wt % mucins) provides cushioning effect to the bacterial cell walls to resist the mechanical stress induced by the impact velocities. The better survival of wild-type Salmonella in the dried droplet of mucin (0.1 and 0.6 wt %) than in milli-Q, glucose, and glycerol indicated that the bacterial viability subjected to desiccation and mechanical stress also depends on nutrient availability. However, compared to the sessile droplets, the reduction in the bacterial viability on induction of velocity suggested a prominent role of mechanical stress in limiting the environmental persistence of pathogenic bacteria. Salmonella Typhimurium retrieved from the velocity-induced dried droplet showed intact intracellular proliferation within macrophages and C57BL/6 mice. Multiple studies showed that Salmonella requires PhoQ/PhoP two-component system to survive within the host cells.65,66,67 Compared to the macrophages infected with the planktonic culture of Salmonella (grown in LB and mucin solutions), the enhanced and consistent expression of phoP from the bacteria recovered from velocity-induced or uninduced desiccated droplets delineated the mystery behind their intact intra-host virulence. The inability of phoP-deficient Salmonella to grow in evaporated nutrient-rich base solution (mucin 0.6 wt %) with high impact velocity suggested the paramount importance of phoP in assisting Salmonella to sense mechanical stress. The bacteria-laden droplet impacting the surface with specific velocities significantly reduced the viability of bacteria during desiccation with the assistance of additional mechanical stress. Earlier our group reported that Salmonella surviving in the dried fomites could efficiently proliferate in the host macrophages.4 In the current study, we showed that even when subjected to high mechanical stress, the ability of the pathogen recovered from the dried fomites to cause successful infection is not hampered. To the best of our knowledge, our study proposed a novel and unique role of phoP, a two-component system gene of Salmonella Typhimurium, to sense the mechanical stress during the formation of dried fomites. Available reports suggest that many other pathogenic bacteria, such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae, can persist on inanimate solid surfaces in a nutrient-deprived environment for a very long time.68,69,70 These bacteria are often associated with hospital-acquired infections in humans. Our findings open a promising avenue for understanding the complex connection of naturally dried Salmonella droplets subjected to differential mechanical and evaporative stress with the association of their virulent genes in fomite-mediated environmental persistence and intra-host pathogenesis, which could be extended to investigate other bacterial pathogens as well.
Limitations of the study
The mechanical characterization has brought out a global picture of impact dynamics of bacteria-laden droplets. However, sophisticated experimental techniques are needed to investigate the local effects of bacterial-laden droplets specially to study the variations of interferometric patterns. Furthermore, the study is limited to impact dynamics over a glass substrate and hence need to be investigated for surfaces with different properties. In addition, the regulation of bacterial gene network under velocity-induced mechanical stress and desiccation stress could have been studied in great details. In the current study, the bacteria recovered from the desiccated droplets (with or without impact velocity) were used for macrophage infection. The expression of bacterial genes associated with virulence was quantified from infected macrophages. Similar kind of gene expression study should be done after the recovery of the bacteria from the dried droplets before using them for infection. In the current study, the significant reduction in the in vitro viability of bacteria because of mechanical and desiccation stress was a major obstacle in direct isolation of RNA from the dried droplets.
Ethical statement
The Institutional Animal Ethics Committee (IAEC) at Indian Institute of Science, Bangalore, India (Registration No: 48/1999/CPCSEA) approved all the animal experiment. The guidelines of CPCSEA were followed while conducting the animal experiments. The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) was formed under Chapter 4, Section 15(1) of the Prevention of Cruelty to Animals Act 1960. Ethical clearance number used to conduct the study is CAF/Ethics/670/2019.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Salmonella enterica serovar Typhimurium ATCC strain14028S | Gifted by Prof. M. Hensel | Wild-type (WT) |
| S. Typhimurium ΔphoP | Laboratory stock | ChlR |
| Chemicals, peptides, and recombinant proteins | ||
| Luria-Bertani broth | HIMEDIA | M575-500G |
| D-Glucose | Qualigens | Q15405 |
| Mucin | Sigma-Aldrich | M1778-100G |
| Salmonella-Shigella agar | HIMEDIA | M108-500G |
Resource availability
Lead contact
Any information and request related to reagents and resources should be directed to Prof. Saptarshi Basu (sbasu@iisc.ac.in).
Materials availability
This study didn’t generate any new or unique resource or reagent.
Method details
Figure 2A depicts the pictorial representation of the instrumentation for measuring impact velocity, spreading, receding diameters, and the impact droplet deposition patterns captured using side-view imaging, fluorescence microscopy, and reflection interference microscopy. Further, the in vitro bacterial viability after evaporation (environmental persistence), the infectivity of the bacteria recovered from the impacted dried droplets (in cell line and mouse infection model), and the effect of mechanical stress on the expression of bacterial genes during infection (by RNA isolation, cDNA synthesis and RT-qPCR method) were studied, as depicted in Figure 2B.
Preparation of bacterial samples
Bacterial solutions were prepared by modifying a protocol as demonstrated earlier.4 Briefly, overnight grown (10–12 hours old) stationary-phase cultures of STM (WT) and ΔphoP (corresponding to 108 CFU/mL) were centrifuged at 5000 rpm for 10 minutes. The bacterial pellets were washed twice with double autoclaved milli-Q water and finally resuspended in 5 mL of milli-Q (neutral), 5% glucose (low-nutrient), 10% glycerol (low-nutrient), LB broth (nutrient-rich), 0.1 and 0.6 wt.% mucin (nutrient-rich) solutions to prepare the base medium.
Side-view visualization
The LED light source (3W) is aligned in the direction of the Phantom Miro 110 camera to capture the drop impact phenomena for varying impact velocities. The images have been recorded at 10000 frames per second to measure droplet impact velocities and in-situ visualization of the droplet. The droplet impact velocities considered in this study (5, 7, and 10 m/s) were verified using this technique. The uncertainty of impact velocities is in the range of ±0.5 m/s.
Interference imaging
The plain glass slides (Bluestar©) kept in propan-2-ol, sonicated for 10 minutes, followed by rinsing with Kimwipes (Kimberly Clark International), were used to impact the bacterial-laden droplet at different velocities. The bacteria-laden droplet spreading dynamics were captured using the reflection interference microscope with interferometric optics, including a Navitar zoom lens, dichroic mirror, and beam splitter, and a microscope objective (5×,10×) aligned for capturing the impact of a droplet on the glass surface for varying impact velocities from the bottom view. A laser light source of wavelength 640 nm (Cavitar, Cavilux smart UHS, 400W power) is in-line alignment with a beam splitter. An objective lens is placed at its focal length between the glass slide and the beam splitter for the fine focus of the light beam. The reflected beam from the beam splitter passes through the bottom of the glass surface, and then the reflected light travels through the beam splitter to develop an interference fringe pattern, which is captured using high-speed imaging systems. The spreading dynamics of the drop impact have been recorded at 10000 frames per second (Photron, SA5 camera) simultaneously with the side-view imaging.
Atomic Force Microscopy
The dried precipitate patterns over the plain glass slides are viewed under a bio-Atomic Force Microscopy (AFM) (Park System, South Korea) integrated with an optical microscope with an X-Y flat scanner to observe the bacterial physiology. The scanner is used in both contact and non-contact modes to acquire microscopic, scanned images, adhesion energy, and variation of force-distance spectroscopy data for different samples. The XEI, XEP software is integrated with the instrument used to operate, analyze, and store data to estimate various mechanical parameters. The technique of AFM measurement is based on laser beam deflection, where the laser beam is reflected from the rear side of the cantilever onto a position-sensitive detector. The position and force given to the sample are regulated using the instrumental software. ACTA cantilever with high stiffness and the resonance frequency is used to get the topographical images of the sample. The contact mode AFM is carried out using CONTSCR (256px, scan rate 0.5 Hz) with less than an 8 nm scanning radius. The stiffness of the cantilever is 0.2 N/m with a resonance frequency of 25 kHz for acquiring the force indentation curve. Cantilever sensitivity and the spring constant are calibrated before each experiment runs.
Atomic force microscopy analysis
The initial AFM data acquired from the bacterial sample is analyzed using XEI software provided by Park System. The force-indentation curves were processed for baseline correction to nullify the cantilever-bending to accurately determine the tip-sample contact point and indentation force. The roughness was calculated by averaging the RMS roughness of three independent 5 μm2 areas.
Assessment of the in vitro viability of the bacteria
The in vitro viability of the bacteria was measured by following a protocol as demonstrated earlier.4 0.5 μL of the bacterial samples, prepared in different base mediums, were dispensed on a sterile glass slide with the help of a micropipette. The bacteria droplet is impacted at three different velocities on the glass substrate using a PICO Pμlse micro dispensing system (Nordson, USA) by applying suitable fluid pressure to achieve the desired velocity (5,7, and 10 m/s). The experiments are conducted at room temperature 25°C and relative humidity of 45%. Five hours after the desiccation, 100 μL sterile PBS was added to the top of the slide to resuspend the dried bacterial droplets of different solutions. After resuspension, this 100 μL PBS was directly plated on Salmonella-Shigella (SS) agar. 100 μL from the overnight-grown culture and freshly prepared bacterial suspensions were plated to calculate the bacterial count in 0.5 μL of planktonic culture. After incubating the plates for sixteen hours at 37°C temperature, the viable bacterial load was enumerated.
Infection of RAW264.7 macrophages
The infection of the RAW264.7 macrophages was done following a protocol as demonstrated earlier.4 Wild-type Salmonella Typhimurium was retrieved from the desiccated dried droplet of mucin as described earlier and used to infect RAW264.7 macrophage cells. The infected cells were incubated in a 5% CO2 incubator for 30 minutes at 37°C temperature, followed by washing with sterile PBS to clear the unattached bacteria. The cells were further incubated with 100 μg/mL of gentamycin for an hour and 25 μg/mL of gentamycin till the end of the experiment. The cells were lysed with 0.1% Triton-X100 at 3 hours and 18 hours. The lysates were serially diluted with PBS and plated on SS agar. To calculate the bacterial fold proliferation, the CFU was obtained from 18hr. was divided with the CFU from 3 hr. The CFU obtained from 3 hr. was further divided with the pre-inoculum CFU to calculate the percent phagocytosis of macrophages.
Isolation of RNA from infected macrophages
The isolation of RNA from infected macrophages was performed using a protocol as demonstrated earlier.71,72 The infected macrophage cells were treated with 1 mL of TRIzol solutions for lysis and kept at −80°C overnight. The lysates were further subjected to chloroform extraction, and to the isolated aqueous layer, an equal volume of isopropanol was added. The RNA was precipitated by centrifuging the mixture of an aqueous layer and isopropanol at 14000 rpm for 20 minutes. The RNA pellet was washed with 70% ethanol and resuspended in DEPC water. The RNA was treated with RNase-free DNase and converted into cDNA with the PrimeScriptTM cDNA synthesis kit. The cDNA was subjected to RT-qPCR using SYBR green RT-qPCR kit to estimate the transcript-level expression of sifA, ssaV, and phoP.
Determining the bacterial burden from the organs of C57BL/6 mice
The bacterial load from the organs of infected animals was determined by following a protocol demonstrated earlier.22,71,73 Wild-type Salmonella Typhimurium was retrieved from the desiccated dried droplet of mucin (0.1 wt.%) as described earlier4 and used to infect 4–6 weeksold C57BL/6 mice. 106 CFU of overnight grown stationary phase planktonic culture of Salmonella was used as control. On the 6th day post-infection, the animals were sacrificed, and the liver and spleen were collected. The infected organs were homogenized, and the lysates were serially diluted for plating on Salmonella-Shigella agar. The CFU obtained from each organ was normalized with the weight of the tissues used from homogenization.
Quantification and statistical analysis
Each experiment has been independently repeated several times, as indicated in the figure legends (n = technical replicates and N = biological replicates). GraphPad Prism 8.4.3 software was used to generate the graphs and perform the statistical tests. All statistics were performed in the manuscript by unpaired students t-test, as mentioned in the figure legends. The p-values below 0.05 were considered significant in all the studies.
Acknowledgments
We acknowledge MNCF (a characterization center in CENSE at IISc) for allowing access to atomic force microscopy equipment. We also acknowledge the esteemed reviewers for their valuable suggestion. We thank the central animal facility of Indian Institute of Science for proving us BALB/c mice to conduct our experiments.
Author contributions
Conceptualization: S.B. and D.C.; Methodology: S.B., D.C., V.H., A.R.C., and S.R.S.; Investigation: V.H., A.R.C., and S.R.S.; Visualization: V.H., A.R.C., and S.R.S.; Funding acquisition: D.C. and S.B.; Project administration: D.C. and S.B.; Supervision: D.C. and S.B.; Writing— original draft: V.H., A.R.C., and S.R.S.; Writing: V.H., A.R.C., and S.R.S.; Editing and revision: S.B., D.C., V.H., A.R.C., and S.R.S.
Declaration of interests
The authors declare no competing interests.
Published: April 8, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106580.
Contributor Information
Dipshikha Chakravortty, Email: dipa@iisc.ac.in.
Saptarshi Basu, Email: sbasu@iisc.ac.in.
Supplemental information
Data and code availability
-
•
This study didn’t generate datasets/codes.
-
•
All relevant data are within the paper and its Supporting Information files.
-
•
Further information related to the data is available upon request to the lead contact, Prof. Saptarshi Basu (sbasu@iisc.ac.in).
References
- 1.Niazi S., Hassanvand M.S., Mahvi A.H., Nabizadeh R., Alimohammadi M., Nabavi S., Faridi S., Dehghani A., Hoseini M., Moradi-Joo M., et al. Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East. Environ. Sci. Pollut. Res. Int. 2015;22:16014–16021. doi: 10.1007/s11356-015-4793-z. [DOI] [PubMed] [Google Scholar]
- 2.Joung Y.S., Ge Z., Buie C.R. Bioaerosol generation by raindrops on soil. Nat. Commun. 2017;8:14668–14710. doi: 10.1038/ncomms14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou M., Liu S., Gu C., Hu H., Tang Z., Zhang Y., Xu C., Li F. The bioaerosols emitted from toilet and wastewater treatment plant: a literature review. Environ. Sci. Pollut. Res. Int. 2021;28:2509–2521. doi: 10.1007/s11356-020-11297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majee S., Chowdhury A.R., Pinto R., Chattopadhyay A., Agharkar A.N., Chakravortty D., Basu S. Spatiotemporal evaporating droplet dynamics on fomites enhances long term bacterial pathogenesis. Commun. Biol. 2021;4:1173. doi: 10.1038/s42003-021-02711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green E.R., Fakhoury J.N., Monteith A.J., Pi H., Giedroc D.P., Skaar E.P. Bacterial hydrophilins promote pathogen desiccation tolerance. Cell Host Microbe. 2022;30:975–987.e7. doi: 10.1016/j.chom.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajra D., Nair A.V., Roy Chowdhury A., Mukherjee S., Chatterjee R., Chakravortty D. Salmonella Typhimurium U32 peptidase, YdcP, promotes bacterial survival by conferring protection against in vitro and in vivo oxidative stress. Microb. Pathog. 2022;173:105862. doi: 10.1016/j.micpath.2022.105862. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury A.R., Mukherjee D., Singh A.K., Chakravortty D. Loss of outer membrane protein A (OmpA) impairs the survival of Salmonella Typhimurium by inducing membrane damage in the presence of ceftazidime and meropenem. J. Antimicrob. Chemother. 2022;77:3376–3389. doi: 10.1093/jac/dkac327. [DOI] [PubMed] [Google Scholar]
- 8.Harrison O.L., Rensing S., Jones C.K., Trinetta V. Salmonella enterica 4,[5], 12: i:-an emerging threat for the swine feed and pork production industry. J. Food Protect. 2022;85:660–663. doi: 10.4315/JFP-21-400. [DOI] [PubMed] [Google Scholar]
- 9.Salzman N.H., Ghosh D., Huttner K.M., Paterson Y., Bevins C.L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 10.Garai P., Gnanadhas D.P., Chakravortty D. Salmonella enterica serovars Typhimurium and Typhi as model organisms: revealing paradigm of host-pathogen interactions. Virulence. 2012;3:377–388. doi: 10.4161/viru.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira C.J.B., Carvalho L.F.O.S., Garcia T.B. Experimental airborne transmission of Salmonella Agona and Salmonella Typhimurium in weaned pigs. Epidemiol. Infect. 2006;134:199–209. doi: 10.1017/S0950268805004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallapura G., Morgan M.J., Pumford N.R., Bielke L.R., Wolfenden A.D., Faulkner O.B., Latorre J.D., Menconi A., Hernandez-Velasco X., Kuttappan V.A., et al. Evaluation of the respiratory route as a viable portal of entry for Salmonella in poultry via intratracheal challenge of Salmonella Enteritidis and Salmonella Typhimurium. Poultry Sci. 2014;93:340–346. doi: 10.3382/ps.2013-03602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface. 2009;6:S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal A., Riggs M.R., Urrutia A., Osborne R.C., Jackson A.P., Bailey M.A., Macklin K.S., Price S.B., Buhr R.J., Bourassa D.V. Investigation of the potential of aerosolized Salmonella Enteritidis on colonization and persistence in broilers from day 3 to 21. Poultry Sci. 2021;100:101504. doi: 10.1016/j.psj.2021.101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbaugh E., Trampel D., Wesley I., Hoff S., Griffith R., Hurd H.S. Rapid aerosol transmission of Salmonella among turkeys in a simulated holding-shed environment. Poultry Sci. 2006;85:1693–1699. doi: 10.1093/ps/85.10.1693. [DOI] [PubMed] [Google Scholar]
- 16.Mori M., Hamamoto A., Takahashi A., Nakano M., Wakikawa N., Tachibana S., Ikehara T., Nakaya Y., Akutagawa M., Kinouchi Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Biol. Eng. Comput. 2007;45:1237–1241. doi: 10.1007/s11517-007-0263-1. [DOI] [PubMed] [Google Scholar]
- 17.Cevallos-Cevallos J.M., Gu G., Danyluk M.D., Dufault N.S., van Bruggen A.H.C. Salmonella can reach tomato fruits on plants exposed to aerosols formed by rain. Int. J. Food Microbiol. 2012;158:140–146. doi: 10.1016/j.ijfoodmicro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Sarichai P., Buddhasiri S., Walters G.E., Khantawa B., Kaewsakhorn T., Chantarasakha K., Tepaamorndech S., Thiennimitr P. Pathogenicity of clinical Salmonella enterica serovar Typhimurium isolates from Thailand in a mouse colitis model. Microbiol. Immunol. 2020;64:679–693. doi: 10.1111/1348-0421.12837. [DOI] [PubMed] [Google Scholar]
- 19.GBD 2017 Non-Typhoidal Salmonella Invasive Disease Collaborators The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019;19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang D.B., DuPont H.L. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect. Dis. 2005;5:341–348. doi: 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- 21.Robicsek A., Jacoby G.A., Hooper D.C. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 2006;6:629–640. doi: 10.1016/S1473-3099(06)70599-0. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri D., Roy Chowdhury A., Biswas B., Chakravortty D. Salmonella typhimurium infection leads to colonization of the mouse brain and is not completely cured with antibiotics. Front. Microbiol. 2018;9:1632. doi: 10.3389/fmicb.2018.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S., Subbiah J., Dvorak B. Environmental and occupational impacts from US beef slaughtering are of same magnitude of beef foodborne illnesses on human health. Environ. Int. 2019;129:507–516. doi: 10.1016/j.envint.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Finlay W.H. Academic Press; 2001. The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction. [Google Scholar]
- 25.Stadnytskyi V., Anfinrud P., Bax A. Breathing, speaking, coughing or sneezing: what drives transmission of SARS-CoV-2? J. Intern. Med. 2021;290:1010–1027. doi: 10.1111/joim.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worthington H.R. Worthington; 1876. The Worthington Steam Pumping Engine: History of its Invention and Development. [Google Scholar]
- 27.Lee J.S., Park S.J., Lee J.H., Weon B.M., Fezzaa K., Je J.H. Origin and dynamics of vortex rings in drop splashing. Nat. Commun. 2015;6:8187–8188. doi: 10.1038/ncomms9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quetzeri-Santiago M.A., Castrejón-Pita A.A., Castrejón-Pita J.R. The effect of surface roughness on the contact line and splashing dynamics of impacting droplets. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-51490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q., Lo J.H.Y., Li Y., Liu Y., Zhao J., Xu L. The role of drop shape in impact and splash. Nat. Commun. 2021;12:3068. doi: 10.1038/s41467-021-23138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin Z., Hobbs P.V. Splashing of water drops on solid and wetted surfaces: hydrodynamics and charge separation. Phil. Trans. Roy. Soc. Lond. Math. Phys. Sci. 1971;269:555–585. [Google Scholar]
- 31.Kai L., Kemao Q. Fast frequency-guided sequential demodulation of a single fringe pattern. Opt. Lett. 2010;35:3718–3720. doi: 10.1364/OL.35.003718. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Kemao Q. Frequency guided methods for demodulation of a single fringe pattern. Opt. Express. 2009;17:15118–15127. doi: 10.1364/oe.17.015118. [DOI] [PubMed] [Google Scholar]
- 33.Chattopadhyay A., Sampathirao S.R., Hegde O., Basu S. Malleable patterns from the evaporation of a colloidal liquid bridge: coffee ring to the scallop shell. Langmuir. 2022;38:5590–5602. doi: 10.1021/acs.langmuir.2c00196. [DOI] [PubMed] [Google Scholar]
- 34.Fedorov A.V., Melville W.K., Rozenberg A. An experimental and numerical study of parasitic capillary waves. Phys. Fluids. 1998;10:1315–1323. [Google Scholar]
- 35.Yunker P.J. University of Pennsylvania; 2012. Coffee-rings and Glasses: Colloids Out of Equilibrium. [Google Scholar]
- 36.Choi Y., Han J., Kim C. Pattern formation in drying of particle-laden sessile drops of polymer solutions on solid substrates. Kor. J. Chem. Eng. 2011;28:2130–2136. [Google Scholar]
- 37.Deegan R.D., Bakajin O., Dupont T.F., Huber G., Nagel S.R., Witten T.A. Contact line deposits in an evaporating drop. Phys. Rev. 2000;62:756–765. doi: 10.1103/physreve.62.756. [DOI] [PubMed] [Google Scholar]
- 38.Rasheed A., Sharma S., Kabi P., Saha A., Chaudhuri S., Basu S. Precipitation dynamics of surrogate respiratory sessile droplets leading to possible fomites. J. Colloid Interface Sci. 2021;600:1–13. doi: 10.1016/j.jcis.2021.04.128. [DOI] [PubMed] [Google Scholar]
- 39.Bansal L., Chakraborty S., Basu S. Confinement-induced alterations in the evaporation dynamics of sessile droplets. Soft Matter. 2017;13:969–977. doi: 10.1039/c6sm02429g. [DOI] [PubMed] [Google Scholar]
- 40.Bhardwaj R., Fang X., Somasundaran P., Attinger D. Self-assembly of colloidal particles from evaporating droplets: role of DLVO interactions and proposition of a phase diagram. Langmuir. 2010;26:7833–7842. doi: 10.1021/la9047227. [DOI] [PubMed] [Google Scholar]
- 41.Iqbal R., Majhy B., Shen A.Q., Sen A.K. Evaporation and morphological patterns of bi-dispersed colloidal droplets on hydrophilic and hydrophobic surfaces. Soft Matter. 2018;14:9901–9909. doi: 10.1039/c8sm01915k. [DOI] [PubMed] [Google Scholar]
- 42.Du J., Zhang Y., Min Q. Numerical investigations of the spreading and retraction dynamics of viscous droplets impact on solid surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2021;609:125649. [Google Scholar]
- 43.Rioboo R., Marengo M., Tropea C. Time evolution of liquid drop impact onto solid, dry surfaces. Exp. Fluid. 2002;33:112–124. [Google Scholar]
- 44.Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vollmer W., Blanot D., de Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 46.Hernández S.B., Castanheira S., Pucciarelli M.G., Cestero J.J., Rico-Pérez G., Paradela A., Ayala J.A., Velázquez S., San-Félix A., Cava F., García-Del Portillo F. Peptidoglycan editing in non-proliferating intracellular Salmonella as source of interference with immune signaling. PLoS Pathog. 2022;18:e1010241. doi: 10.1371/journal.ppat.1010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheffers D.-J., Pinho M.G. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford R.J., Webb H.K., Truong V.K., Hasan J., Ivanova E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012;179:142–149. doi: 10.1016/j.cis.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Sousa C., Teixeira P., Oliveira R. Influence of surface properties on the adhesion of Staphylococcus epidermidis to acrylic and silicone. Int. J. Biomater. 2009;2009:718017. doi: 10.1155/2009/718017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teughels W., Van Assche N., Sliepen I., Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoda I., Koseki H., Tomita M., Shida T., Horiuchi H., Sakoda H., Osaki M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014;14:234–237. doi: 10.1186/s12866-014-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan C.-W., Carson L., Smith G.C., Morelli A., Lee S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl. Surf. Sci. 2017;404:67–81. [Google Scholar]
- 53.Finn S., Rogers L., Händler K., McClure P., Amézquita A., Hinton J.C.D., Fanning S. Exposure of Salmonella enterica serovar typhimurium to three humectants used in the food industry induces different osmoadaptation systems. Appl. Environ. Microbiol. 2015;81:6800–6811. doi: 10.1128/AEM.01379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niv Y., Ho S.B., Rokkas T. Mucin secretion in cystic fibrosis: a systematic review. Dig. Dis. 2021;39:375–381. doi: 10.1159/000512268. [DOI] [PubMed] [Google Scholar]
- 55.Hirayama D., Iida T., Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017;19:92. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acharya D., Li X.R.L., Heineman R.E.S., Harrison R.E. Complement receptor-mediated phagocytosis induces proinflammatory cytokine production in murine macrophages. Front. Immunol. 2019;10:3049. doi: 10.3389/fimmu.2019.03049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 2008;11:38–45. doi: 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalebroux Z.D., Miller S.I. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr. Opin. Microbiol. 2014;17:106–113. doi: 10.1016/j.mib.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Figueira R., Holden D.W. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology (Read.) 2012;158:1147–1161. doi: 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- 60.Hindle Z., Chatfield S.N., Phillimore J., Bentley M., Johnson J., Cosgrove C.A., Ghaem-Maghami M., Sexton A., Khan M., Brennan F.R., et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 2002;70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen C., Zverina L., Ehtiati K., Thormann E., Mordhorst H., Pamp S.J., Madsen N.J., Daugaard A.E. Antimicrobial PDMS surfaces prepared through fast and oxygen-tolerant SI-SARA-ATRP, using Na(2)SO(3) as a reducing agent. ACS Omega. 2021;6:14551–14558. doi: 10.1021/acsomega.1c01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stepulane A., Rajasekharan A.K., Andersson M. Multifunctional surface modification of PDMS for antibacterial contact killing and drug-delivery of polar, nonpolar, and amphiphilic drugs. ACS Appl. Bio Mater. 2022;5:5289–5301. doi: 10.1021/acsabm.2c00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tripathy A., Kumar A., Chowdhury A.R., Karmakar K., Purighalla S., Sambandamurthy V., Chakravortty D., Sen P. A nanowire-based flexible antibacterial surface reduces the viability of drug-resistant nosocomial pathogens. ACS Appl. Nano Mater. 2018;1:2678–2688. doi: 10.1021/acsanm.8b00397. [DOI] [Google Scholar]
- 64.Wathes C.M., Zaidan W.A., Pearson G.R., Hinton M., Todd N. Aerosol infection of calves and mice with Salmonella typhimurium. Vet. Rec. 1988;123:590–594. [PubMed] [Google Scholar]
- 65.Groisman E.A. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu X., Latifi T., Bougdour A., Gottesman S., Groisman E.A. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc. Natl. Acad. Sci. USA. 2006;103:13503–13508. doi: 10.1073/pnas.0606026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia Véscovi E., Soncini F.C., Groisman E.A. The role of the PhoP/PhoQ regulon in Salmonella virulence. Res. Microbiol. 1994;145:473–480. doi: 10.1016/0923-2508(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 68.Hrenovic J., Durn G., Goic-Barisic I., Kovacic A. Occurrence of an environmental Acinetobacter baumannii strain similar to a clinical isolate in paleosol from Croatia. Appl. Environ. Microbiol. 2014;80:2860–2866. doi: 10.1128/AEM.00312-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Abreu P.M., Farias P.G., Paiva G.S., Almeida A.M., Morais P.V. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol. 2014;14:118. doi: 10.1186/1471-2180-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pantel A., Richaud-Morel B., Cazaban M., Bouziges N., Sotto A., Lavigne J.P. Environmental persistence of OXA-48-producing Klebsiella pneumoniae in a French intensive care unit. Am. J. Infect. Control. 2016;44:366–368. doi: 10.1016/j.ajic.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Roy Chowdhury A., Sah S., Varshney U., Chakravortty D. Salmonella Typhimurium outer membrane protein A (OmpA) renders protection from nitrosative stress of macrophages by maintaining the stability of bacterial outer membrane. PLoS Pathog. 2022;18:e1010708. doi: 10.1371/journal.ppat.1010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chowdhury A.R., Hajra D., Chakravortty D. The extracellular loops of Salmonella Typhimurium outer membrane protein A (OmpA) maintain the stability of Salmonella containing vacuole (SCV) in murine macrophages and protect the bacteria from autophagy-dependent lysosomal degradation. bioRxiv. 2021 doi: 10.1101/2021.11.07.467609. Preprint at. [DOI] [Google Scholar]
- 73.Chandra K., Roy Chowdhury A., Chatterjee R., Chakravortty D. GH18 family glycoside hydrolase Chitinase A of Salmonella enhances virulence by facilitating invasion and modulating host immune responses. PLoS Pathog. 2022;18:e1010407. doi: 10.1371/journal.ppat.1010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This study didn’t generate datasets/codes.
-
•
All relevant data are within the paper and its Supporting Information files.
-
•
Further information related to the data is available upon request to the lead contact, Prof. Saptarshi Basu (sbasu@iisc.ac.in).