Graphical abstract
Keywords: Prescription, Oral anticoagulant, non-vitamin K oral anticoagulant, Warfarin, Atrial fibrillation, Stroke
Abstract
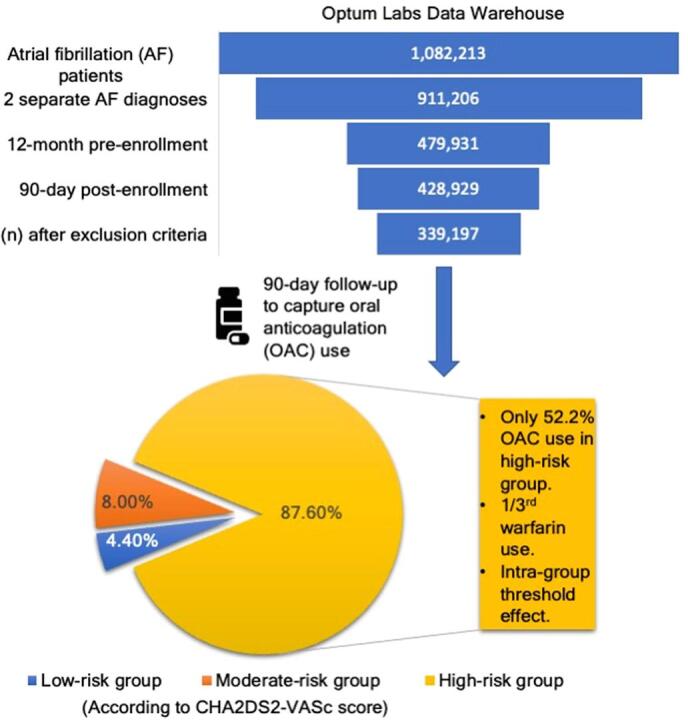
There is a need to reassess contemporary oral anticoagulation (OAC) trends and barriers against guideline directed therapy in the United States. Most previous studies were performed before major guideline changes recommended direct oral anticoagulant (DOAC) use over warfarin or have otherwise lacked patient level data. Data on overuse of OAC in low-risk group is also limited. To address these knowledge gaps, we performed a nationwide analysis to analyze current trends. This is a retrospective cohort study assessing non-valvular AF identified using a large United States de-identified administrative claims database, including commercial and Medicare Advantage enrollees. Prescription fills were assessed within a 90-day follow-up from the patient’s index AF encounter between January 1, 2016, and December 31, 2020. Among the 339,197 AF patients, 4.4%, 8.0%, and 87.6% were in the low-, moderate-, and high-risk groups (according to CHA2DS2-VASc score). An over (29.6%) and under (52.2%) utilization of OAC was reported in low- and high-risk AF patients. A considerably high frequency for warfarin use was also noted among high-risk group patients taking OAC (33.1%). The results suggest that anticoagulation use for stroke prevention in the United States is still comparable to the pre-DOAC era studies. About half of newly diagnosed high-risk non-valvular AF patients remain unprotected against stroke risk. Several predictors of OAC and DOAC use were also identified. Our findings may identify a population at risk of complications due to under- or over-treatment and highlight the need for future quality improvement efforts.
1. Introduction
In patients with atrial fibrillation, the net benefit of OAC therapy is almost unequivocal except for very low stroke risk patients. Even a high bleeding risk score (such as HAS-BLED score) should generally not preclude OAC therapy but rather be used to identify and treat bleeding risk factors.[3] However, despite a strong evidence of benefit, underuse of OAC therapy in real-world setting is common [4] and efforts to improve guideline-adherence need to be prioritized especially considering the rising incidence of cardioembolic strokes.[5] Since the approval of first Direct Oral Anticoagulant (DOAC) in 2011 the oral anticoagulation (OAC) trends have been changing steadily across countries as providers continue to gain confidence with the newer drugs and as evidence/guidelines for use became clearer.[1], [2] DOACs offer predictable pharmacokinetics and ease of use by eliminating frequent invasive monitoring requirements and superior outcomes in reducing stroke or systemic emboli when compared to warfarin. The first major guideline to reflect a preference for DOAC over warfarin was the 2016 ESC guidance statement (Class 1, Level A recommendation).[3], [6] Most prior studies assessing OAC rates were performed before this time, [7], [8], [9], [10] when there were several perceived reasons to choose DOAC over warfarin such as lack of provider confidence with newer drugs, less clear guidelines and evidence, lack of specific reversal drugs for DOAC. In United States, the few recent studies have otherwise lacked patient-level data to select for non-valvular atrial fibrillation (NVAF) patients or address factors influencing management decisions. Periodic assessment of OAC trends is critical to establish real-world feedback in various healthcare systems and to identify barriers to appropriate stroke prevention. Therefore, in the present study, we have assessed 1) Trends in OAC dispensing for newly diagnosed NVAF patients during a guideline based, DOAC preferred era (2016–2020) within the United States, especially focusing on low-risk and high-risk groups as stratified by CHA2DS2-VASc score. 2) Factors associated with OAC use, and 3) Factors associated with DOAC use over warfarin.
2. Methods
The study design was retrospective, population-based cohort analysis. For this we used de-identified administrative claims data from the Optum Labs Data Warehouse (OLDW), which includes medical and pharmacy claims, laboratory results, and enrollment records for commercial and Medicare Advantage (MA) enrollees. The database contains longitudinal health information for over 200 million enrollees and patients, representing a mixture of ages and geographical regions across the United States.[11] Since the data was de-identified, in compliance with the Health Insurance Portability and Accountability Act and customer requirements, Institutional Review Board approval or waiver of authorization was not required. This study conforms with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies.[12].
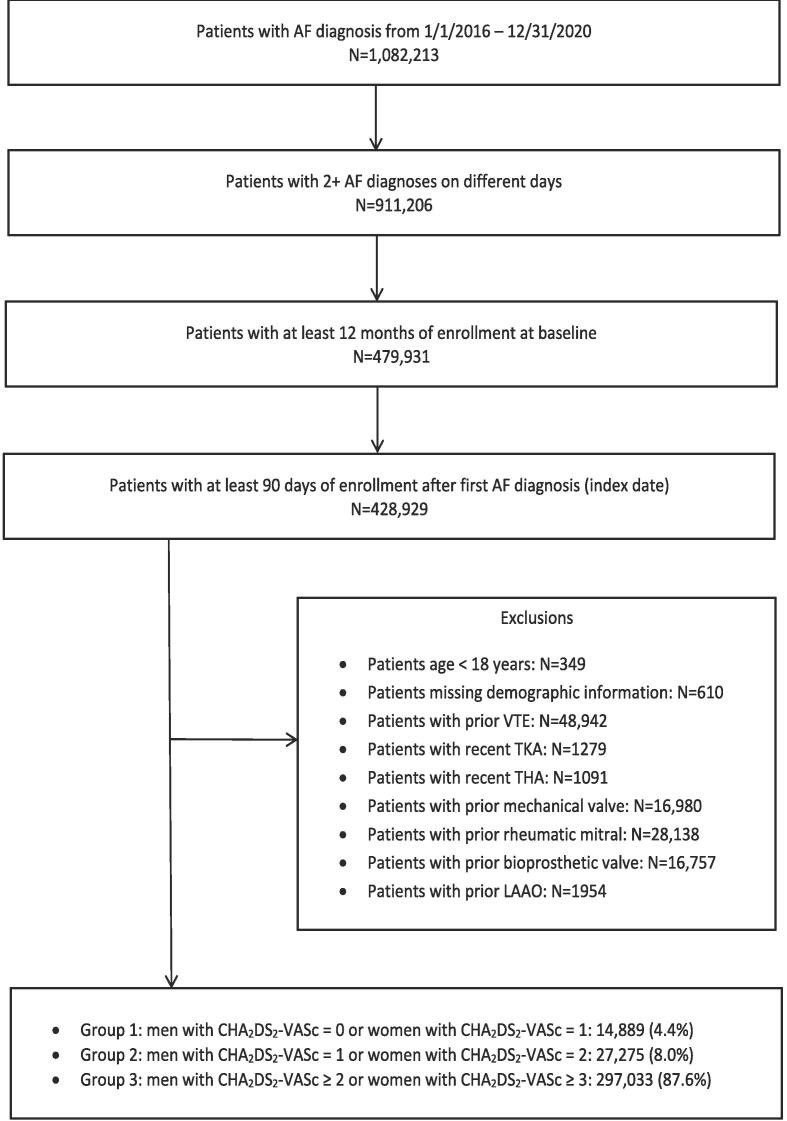
The study population included adult patients (≥18 years) with at least two diagnoses of AF between January 1, 2016, and December 31, 2020, on two different days. Outcome event was defined as OAC use within a 90-day follow-up window from the index AF encounter date. In addition, patients were required to have at least 12 months of continuous medical and pharmacy coverage (baseline period) before the first AF diagnosis (index date). Patients with independent valvular indications for warfarin, including mechanical or bioprosthetic valve placement and rheumatic mitral valve stenosis or left atrial appendage occlusion, were excluded. Patients with other independent OAC indications unrelated to AF, such as prior venous thromboembolism or recent (within two weeks) total knee or hip replacement, were also excluded (Fig. 1). These criteria were chosen to primarily include a NVAF population that is newly eligible to receive OAC therapy for AF diagnosis. The specific codes used for inclusion/exclusion criteria are provided in supplementary material (Table S1-S2). In a sensitivity analysis, patients with prior history of cardioversion, catheter ablation or surgical ablation/MAZE procedure were further excluded from the cohort with all other criteria kept identical to remove the transient OAC indication.
Fig. 1.
Flow diagram depicting stepwise exclusion criteria as applied to the initial cohort. Abbreviations: AF (atrial fibrillation), VTE, TKA (total knee arthroplasty), THA (total hip arthroplasty), LAAO.
The remaining cohort was divided mainly into two groups: (1) Low-risk group (CHA2DS2-VASc 0 in men or 1 in women), (2) High-risk group (CHA2DS2-VASc ≥ 2 in men or ≥ 3 in women). We also stratified antithrombotic use across a continuous CHA2DS2-VASc scores (0–9). These groups were further stratified according to treatment allocation (i) OAC vs no OAC and (ii) DOAC vs warfarin and assessed for association with various baseline patient characteristics.
Covariates included patient demographics (such as age, gender, and region) and type of health plan (commercial or Medicare Advantage). Comorbidities were ascertained using primary and secondary diagnostic codes from all medical claims during 12 months before the index date. Comorbidities assessed included CHA2DS2-VASc components, HAS-BLED components [hypertension, chronic kidney disease (CKD), liver disease, stroke, anemia/major bleed, age > 65, alcohol use, antiplatelet/NSAIDs use]. Labile international normalized ratio values were not available in the database, so it was excluded from the HAS-BLED score in our study. Specific codes used to calculate HAS-BLED score in this study are provided in supplementary material (Table S3-S4). Other comorbidities included falls, catheter/surgical ablation, hypertrophic cardiomyopathy, cardioversion, implanted device, and obesity. Relevant concurrent medication use, healthcare utilization rates, events (including ischemic stroke, systemic embolism, major or intracranial bleeding) within the previous 3-month and 12-month period were assessed as these can potentially influence OAC utilization.
Baseline patient characteristics were reported as proportion for categorical data and as mean with standard deviation (SD) for continuous variables. Means were compared using paired t-test and the proportions were compared using Pearson's chi-squared test. The analysis focused on the low and high-risk subgroups, where the guideline recommendations are relatively clear to allow interpretation. Lastly, the multivariable logistic regression model examined baseline characteristics associated with OAC use and DOAC use. Throughout present study, we have only assessed the drug dispensing rates (prescription fills) which may not reflect physician prescribing rates or actual use of the drug by patients which further depends upon compliance. Model covariates included those discussed previously. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary NC). X.Y. had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
3. Results
A total of 339,197 AF patients met the study criteria during the period of January 2016 to December 2020. (Fig. 1). Of the health plans examined, 88.6% of the claims were from the Medicare advantage plan, and the rest were from a commercial health plan. Distribution of cohort when risk stratified into low-, moderate-, and high-risk groups according to CHA2DS2-VASc score was 4.4%, 8.0%, and 87.6%, respectively. The mean age (SD) in these sub-groups was 51.2 (10.5), 59.9 (9.4), and 75.1 (8.8) years, respectively.
Proportion of patients that had OAC prescription fills within a 90-day follow up window of the index diagnosis date was 29.6% (low-risk group), 40.6% (moderate-risk group) and 52.2% (high-risk group). Of the patients on OAC, warfarin use was 10.7% (low-risk group) and 33.1% (high-risk group), while the rest utilized one of the DOAC drugs.
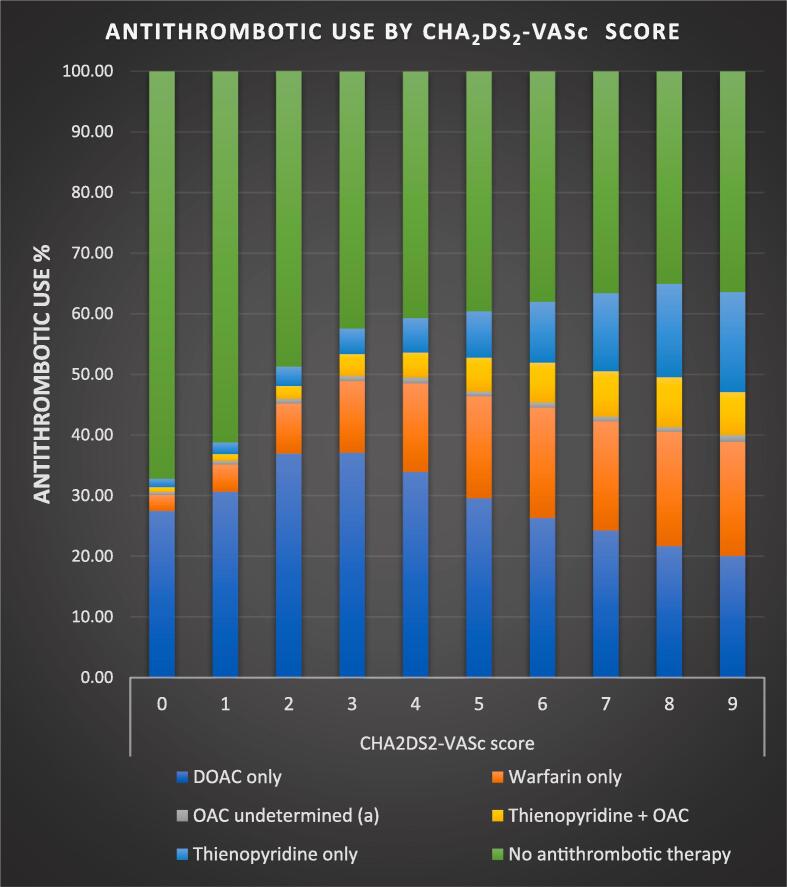
On a continuous scale (CHA2DS2-VASc score: 0–9), DOAC and warfarin use had a slightly decreasing and increasing trend respectively (Fig. 2). DOAC use was highest (37.1%) and lowest (20.1%) for CHA2DS2-VASc score 3 and 9 respectively. Warfarin use was highest with CHA2DS2-VASc scores > 4. Overall OAC use within the high-risk group showed a lack of variation across the CHA2DS2-VASc score. (Fig. 2).
Fig. 2.
Graphical representation of antithrombotic therapy utilization stratified by CHA2DS2-VASc score.(a) In a small proportion (n = 20) both DOAC (direct oral anticoagulant) and warfarin prescriptions were present likely due to a switch within the study window or a reporting error. (DOACs included apixaban, dabigatran, rivaroxaban and edoxaban; thienopyridines included anagrelide, cilostazol, clopidogrel, dipyridamole, prasugrel, ticagrelor, ticlopidine and vorapazar) Abbreviations: OAC (oral anticoagulant), DOAC (direct oral anticoagulant).
A comparison of baseline characteristics for OAC use vs no OAC, stratified into low-, and high-risk groups is shown in Table 1, Table 2. Most of the patient population was Caucasian (∼75%) with about 75% from South or Midwest regions of the United States. Only modest differences according to region were discernible among the two groups.
Table 1.
Patient Characteristics (low-risk group).
| Patient Characteristics | No OAC (N = 10,484) | OAC (N = 4405) | Total (N = 14,889) | p value |
|---|---|---|---|---|
| OAC use | ||||
| Warfarin | 0 (0.0%) | 473 (10.7%) | 473 (3.2%) | <0.0001 |
| NOAC | 0 (0.0%) | 4000 (90.8%) | 4000 (26.9%) | <0.0001 |
| Age (years) | <0.0001 | |||
| Mean (SD) | 50.1 (11.0) | 54.0 (8.9) | 51.2 (10.5) | |
| Median | 53 | 56 | 54 | |
| Q1, Q3 | 43.0, 59.0 | 50.0, 61.0 | 45.0, 60.0 | |
| Range | (18.0–64.0) | (18.0–64.0) | (18.0–64.0) | |
| Gender | <0.0001 | |||
| Female | 3396 (32.4%) | 1164 (26.4%) | 4560 (30.6%) | |
| Male | 7088 (67.6%) | 3241 (73.6%) | 10329 (69.4%) | |
| Region | 0.0029 | |||
| Midwest | 3338 (31.8%) | 1526 (34.6%) | 4864 (32.7%) | |
| Northeast | 1009 (9.6%) | 425 (9.6%) | 1434 (9.6%) | |
| South | 4029 (38.4%) | 1649 (37.4%) | 5678 (38.1%) | |
| Unknown | 81 (0.8%) | 21 (0.5%) | 102 (0.7%) | |
| West | 2027 (19.3%) | 784 (17.8%) | 2811 (18.9%) | |
| Health plan | 0.0112 | |||
| Commercial | 9904 (94.5%) | 4206 (95.5%) | 14110 (94.8%) | |
| Medicare Advantage | 580 (5.5%) | 199 (4.5%) | 779 (5.2%) | |
| HAS-BLED | <0.0001 | |||
| Mean (SD) | 0.4 (0.7) | 0.3 (0.6) | 0.4 (0.6) | |
| Median | 0 | 0 | 0 | |
| Q1, Q3 | 0.0, 1.0 | 0.0, 1.0 | 0.0, 1.0 | |
| Range | (0.0–4.0) | (0.0–4.0) | (0.0–4.0) | |
| HAS-BLED components | ||||
| Age > 65 years | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| CKD | 61 (0.6%) | 19 (0.4%) | 80 (0.5%) | 0.2515 |
| Stroke | 29 (0.3%) | 0 (0.0%) | 29 (0.2%) | 0.0005 |
| Ischemic stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| TIA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Anemia/Major bleed | 2204 (21.0%) | 732 (16.6%) | 2936 (19.7%) | <0.0001 |
| Anemia | 1361 (13.0%) | 457 (10.4%) | 1818 (12.2%) | <0.0001 |
| Major bleed | 1133 (10.8%) | 354 (8.0%) | 1487 (10.0%) | <0.0001 |
| Liver disease | 777 (7.4%) | 258 (5.9%) | 1035 (7.0%) | 0.0007 |
| Alcoholism | 447 (4.3%) | 133 (3.0%) | 580 (3.9%) | 0.0003 |
| Thienopyridine /NSAIDs | 784 (7.5%) | 294 (6.7%) | 1078 (7.2%) | 0.0841 |
| Thienopyridine | * | * | 16 (0.1%) | 0.1341 |
| NSAIDs | * | * | 1064 (7.1%) | 0.1288 |
| Other comorbidities | ||||
| Myocardial infarction | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Falls | 558 (5.3%) | 184 (4.2%) | 742 (5.0%) | 0.0034 |
| Hyperlipidemia | 3405 (32.5%) | 1373 (31.2%) | 4778 (32.1%) | 0.1184 |
| Hypertrophic cardiomyopathy | 44 (0.4%) | 33 (0.7%) | 77 (0.5%) | 0.0105 |
| Smoking | 1940 (18.5%) | 795 (18.0%) | 2735 (18.4%) | 0.5113 |
| Obesity | 1632 (15.6%) | 842 (19.1%) | 2474 (16.6%) | <0.0001 |
| COPD | 260 (2.5%) | 98 (2.2%) | 358 (2.4%) | 0.3535 |
| Obstructive sleep apnea | 1303 (12.4%) | 503 (11.4%) | 1806 (12.1%) | 0.0850 |
| Skilled nursing facility | 135 (1.3%) | 51 (1.2%) | 186 (1.2%) | 0.5148 |
| Extracranial bleeding | 1093 (10.4%) | 350 (7.9%) | 1443 (9.7%) | <0.0001 |
| ESRD requiring dialysis | * | * | * | 0.1123 |
| Gastrointestinal lesions | 277 (2.6%) | 89 (2.0%) | 366 (2.5%) | 0.0254 |
| End stage liver disease | 40 (0.4%) | 12 (0.3%) | 52 (0.3%) | 0.3030 |
| Interventions | ||||
| Catheter ablation | 215 (2.1%) | 76 (1.7%) | 291 (2.0%) | 0.1904 |
| Cardioversion | 404 (3.9%) | 216 (4.9%) | 620 (4.2%) | 0.0034 |
| Surgical ablation/MAZE | * | * | * | 0.8869 |
| Implanted device | 0.0467 | |||
| None | 10185 (97.1%) | 4315 (98.0%) | 14500 (97.4%) | |
| CRT defibrillator | * | * | * | |
| ICD | 85 (0.8%) | 25 (0.6%) | 110 (0.7%) | |
| CRT pacemaker | 65 (0.6%) | 17 (0.4%) | 82 (0.6%) | |
| Dual chamber pacemaker | 112 (1.1%) | 33 (0.7%) | 145 (1.0%) | |
| Single chamber pacemaker | * | * | * | |
| CABG | * | * | * | 0.4877 |
| PCI | * | * | * | 0.2057 |
| Healthcare Utilization | ||||
| Number of AF hospitalizations | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.8231 |
| Number of non-AF hospitalizations | 0.1 (0.3) | 0.0 (0.2) | 0.1 (0.3) | <0.0001 |
| All-cause hospital days | 7.3 (9.9) | 4.5 (3.7) | 6.6 (8.9) | 0.0011 |
| Events within past 3 months | ||||
| Ischemic stroke or systemic embolism | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Major bleed | * | * | * | 0.0407 |
| Intracranial bleed | * | * | * | 0.1472 |
| Number of events within past 12 months, mean (SD) | ||||
| Ischemic stroke or systemic embolism | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | – |
| Major bleed | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.7664 |
| Intracranial bleed | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1123 |
Baseline comparison of patient characteristics stratified according to oral anticoagulant use within the low-risk group. (*Cell size less than 11 are suppressed to protect patient confidentiality.).
Abbreviations: Oral anticoagulant (OAC); Direct oral anticoagulant (DOAC); coronary artery disease (CAD); peripheral artery disease (PAD); chronic kidney disease (CKD); transient ischemic attack; non-steroidal anti-inflammatory drug (NSAID); chronic obstructive pulmonary disease (COPD); end-stage renal disease (ESRD); implantable cardioverter-defibrillator (ICD); coronary artery bypass graft (CABG); percutaneous coronary intervention (PCI).
Table 2.
Patient Characteristics (high-risk group).
| Patient Characteristics |
No OAC (N = 141,966) | OAC (N = 155,067) | Total (N = 297,033) | p value |
|---|---|---|---|---|
| OAC use | ||||
| Warfarin | 0 (0.0%) | 51309 (33.1%) | 51309 (17.3%) | <0.0001 |
| NOAC | 0 (0.0%) | 106619 (68.8%) | 106619 (35.9%) | <0.0001 |
| Age (years) | <0.0001 | |||
| Mean (SD) | 75.1 (9.2) | 75.2 (8.4) | 75.1 (8.8) | |
| Median | 76 | 76 | 76 | |
| Q1, Q3 | 70.0, 84.0 | 70.0, 82.0 | 70.0, 83.0 | |
| Range | (18.0–88.0) | (19.0–88.0) | (18.0–88.0) | |
| Gender | <0.0001 | |||
| Female | 67456 (47.5%) | 71900 (46.4%) | 139356 (46.9%) | |
| Male | 74510 (52.5%) | 83167 (53.6%) | 157677 (53.1%) | |
| Region | <0.0001 | |||
| Midwest | 39164 (27.6%) | 48865 (31.5%) | 88029 (29.6%) | |
| Northeast | 22623 (15.9%) | 25699 (16.6%) | 48322 (16.3%) | |
| South | 67265 (47.4%) | 67132 (43.3%) | 134397 (45.2%) | |
| Unknown | 113 (0.1%) | 57 (0.0%) | 170 (0.1%) | |
| West | 12801 (9.0%) | 13314 (8.6%) | 26115 (8.8%) | |
| Health plan | 0.9244 | |||
| Commercial | 16112 (11.3%) | 17616 (11.4%) | 33728 (11.4%) | |
| Medicare Advantage | 125854 (88.7%) | 137451 (88.6%) | 263305 (88.6%) | |
| CHA2DS2-VASc | <0.0001 | |||
| Mean (SD) | 4.7 (1.7) | 4.6 (1.6) | 4.7 (1.7) | |
| Median | 5 | 4 | 4 | |
| Q1, Q3 | 3.0, 6.0 | 3.0, 6.0 | 3.0, 6.0 | |
| Range | (2.0–9.0) | (2.0–9.0) | (2.0–9.0) | |
| CHA2DS2-VASc components | ||||
| Heart failure | 48527 (34.2%) | 52162 (33.6%) | 100689 (33.9%) | 0.0018 |
| Systolic | 18381 (12.9%) | 20895 (13.5%) | 39276 (13.2%) | <0.0001 |
| Diastolic | 20834 (14.7%) | 22886 (14.8%) | 43720 (14.7%) | 0.5214 |
| Hypertension | 133413 (94.0%) | 146510 (94.5%) | 279923 (94.2%) | <0.0001 |
| Diabetes mellitus | 60388 (42.5%) | 66523 (42.9%) | 126911 (42.7%) | 0.0460 |
| Thromboembolism | 30743 (21.7%) | 32548 (21.0%) | 63291 (21.3%) | <0.0001 |
| CAD or PAD | 87189 (61.4%) | 88846 (57.3%) | 176035 (59.3%) | <0.0001 |
| CAD | 80241 (56.5%) | 82123 (53.0%) | 162364 (54.7%) | <0.0001 |
| PAD | 26123 (18.4%) | 24068 (15.5%) | 50191 (16.9%) | <0.0001 |
| HAS-BLED | <0.0001 | |||
| Mean (SD) | 3.2 (1.2) | 3.1 (1.1) | 3.1 (1.2) | |
| Median | 3 | 3 | 3 | |
| Q1, Q3 | 2.0, 4.0 | 2.0, 4.0 | 2.0, 4.0 | |
| Range | (0.0–8.0) | (0.0–8.0) | (0.0–8.0) | |
| HAS-BLED components | ||||
| Age > 65 years | 80874 (57.0%) | 88680 (57.2%) | 169554 (57.1%) | – |
| CKD | 31797 (22.4%) | 30878 (19.9%) | 62675 (21.1%) | <0.0001 |
| Stroke | 22570 (15.9%) | 23719 (15.3%) | 46289 (15.6%) | <0.0001 |
| Ischemic stroke | 21488 (15.1%) | 23134 (14.9%) | 44622 (15.0%) | 0.0978 |
| TIA | 16047 (11.3%) | 17291 (11.2%) | 33338 (11.2%) | 0.1877 |
| Anemia/Major bleed | 85694 (60.4%) | 84990 (54.8%) | 170684 (57.5%) | <0.0001 |
| Anemia | 74152 (52.2%) | 72003 (46.4%) | 146155 (49.2%) | <0.0001 |
| Major bleed | 40604 (28.6%) | 37543 (24.2%) | 78147 (26.3%) | <0.0001 |
| Liver disease | 23447 (16.5%) | 21781 (14.0%) | 45228 (15.2%) | <0.0001 |
| Alcoholism | 8547 (6.0%) | 7034 (4.5%) | 15581 (5.2%) | <0.0001 |
| Thienopyridine/NSAIDs | 29703 (20.9%) | 25016 (16.1%) | 54719 (18.4%) | <0.0001 |
| Thienopyridine | 16818 (11.8%) | 11839 (7.6%) | 28657 (9.6%) | <0.0001 |
| NSAIDs | 14776 (10.4%) | 14478 (9.3%) | 29254 (9.8%) | <0.0001 |
| Other comorbidities | ||||
| Myocardial infarction | 27743 (19.5%) | 26099 (16.8%) | 53842 (18.1%) | <0.0001 |
| Falls | 36265 (25.5%) | 30623 (19.7%) | 66888 (22.5%) | <0.0001 |
| Hyperlipidemia | 122022 (86.0%) | 136726 (88.2%) | 258748 (87.1%) | <0.0001 |
| Hypertrophic cardiomyopathy | 1265 (0.9%) | 1846 (1.2%) | 3111 (1.0%) | <0.0001 |
| Smoking | 54472 (38.4%) | 54776 (35.3%) | 109248 (36.8%) | <0.0001 |
| Obesity | 44904 (31.6%) | 56340 (36.3%) | 101244 (34.1%) | <0.0001 |
| COPD | 23179 (16.3%) | 21896 (14.1%) | 45075 (15.2%) | <0.0001 |
| Obstructive sleep apnea | 27754 (19.5%) | 35534 (22.9%) | 63288 (21.3%) | <0.0001 |
| Skilled nursing facility | 42456 (29.9%) | 33149 (21.4%) | 75605 (25.5%) | <0.0001 |
| Extracranial bleeding | 37334 (26.3%) | 35418 (22.8%) | 72752 (24.5%) | <0.0001 |
| ESRD requiring dialysis | 2256 (1.6%) | 1493 (1.0%) | 3749 (1.3%) | <0.0001 |
| Gastrointestinal lesions | 9868 (7.0%) | 8716 (5.6%) | 18584 (6.3%) | <0.0001 |
| End stage liver disease | 2371 (1.7%) | 1357 (0.9%) | 3728 (1.3%) | <0.0001 |
| Interventions | ||||
| Catheter ablation | 1985 (1.4%) | 2885 (1.9%) | 4870 (1.6%) | <0.0001 |
| Cardioversion | 5610 (4.0%) | 12718 (8.2%) | 18328 (6.2%) | <0.0001 |
| Surgical ablation/MAZE | 74 (0.1%) | 110 (0.1%) | 184 (0.1%) | 0.0396 |
| Implanted device | <0.0001 | |||
| None | 122975 (86.6%) | 131864 (85.0%) | 254839 (85.8%) | |
| CRT defibrillator | 459 (0.3%) | 806 (0.5%) | 1265 (0.4%) | |
| ICD | 6880 (4.8%) | 8242 (5.3%) | 15122 (5.1%) | |
| CRT pacemaker | 1770 (1.2%) | 1959 (1.3%) | 3729 (1.3%) | |
| Dual chamber pacemaker | 7585 (5.3%) | 9274 (6.0%) | 16859 (5.7%) | |
| Single chamber pacemaker | 2297 (1.6%) | 2922 (1.9%) | 5219 (1.8%) | |
| CABG | 16110 (11.3%) | 16241 (10.5%) | 32351 (10.9%) | <0.0001 |
| PCI | 19233 (13.5%) | 19174 (12.4%) | 38407 (12.9%) | <0.0001 |
| Concurrent medication | ||||
| Amiodarone | 10616 (7.5%) | 13242 (8.5%) | 23858 (8.0%) | <0.0001 |
| Other AADs | 11833 (8.3%) | 16582 (10.7%) | 28415 (9.6%) | <0.0001 |
| Calcium channel blockers as rate control drugs | 21622 (15.2%) | 28342 (18.3%) | 49964 (16.8%) | <0.0001 |
| Aspirin | 2923 (2.1%) | 2719 (1.8%) | 5642 (1.9%) | <0.0001 |
| Digitalis | 11011 (7.8%) | 17521 (11.3%) | 28532 (9.6%) | <0.0001 |
| Baseline (years), mean (SD) | 4.2 (3.1) | 4.1 (3.1) | 4.2 (3.1) | <0.0001 |
| Follow-up (years), mean (SD) | 2.3 (1.5) | 2.6 (1.6) | 2.5 (1.5) | <0.0001 |
| Health utilization within past 12 months, mean (SD) | ||||
| Number of AF emergency room visits | 0.0 (0.1) | 0.0 (0.2) | 0.0 (0.2) | <0.0001 |
| Number of non-AF emergency room visits | 0.6 (1.4) | 0.5 (1.0) | 0.5 (1.2) | <0.0001 |
| Number of AF hospitalizations | 0.0 (0.1) | 0.0 (0.2) | 0.0 (0.1) | <0.0001 |
| Number of non-AF hospitalizations | 0.4 (0.9) | 0.3 (0.7) | 0.4 (0.8) | <0.0001 |
| All-cause hospital days | 9.9 (12.8) | 8.3 (9.9) | 9.2 (11.6) | <0.0001 |
| Patients with Events within past 3 months | ||||
| Ischemic stroke or systemic embolism | 1868 (1.3%) | 1850 (1.2%) | 3718 (1.3%) | 0.0026 |
| Major bleed | 1750 (1.2%) | 656 (0.4%) | 2406 (0.8%) | <0.0001 |
| Intracranial bleed | 535 (0.4%) | 125 (0.1%) | 660 (0.2%) | <0.0001 |
| Number of events within past 12 months, mean (SD) | ||||
| Ischemic stroke or systemic embolism | 0.0 (0.2) | 0.0 (0.2) | 0.0 (0.2) | <0.0001 |
| Major bleed | 0.0 (0.2) | 0.0 (0.1) | 0.0 (0.2) | <0.0001 |
| Intracranial bleed | 0.0 (0.1) | 0.0 (0.0) | 0.0 (0.1) | <0.0001 |
Baseline comparison of patient characteristics stratified according to oral anticoagulant use within the high-risk group.
Abbreviations: Oral anticoagulant (OAC); Direct oral anticoagulant (DOAC); coronary artery disease (CAD); peripheral artery disease (PAD); chronic kidney disease (CKD); transient ischemic attack; non-steroidal anti-inflammatory drug (NSAID); chronic obstructive pulmonary disease (COPD); end-stage renal disease (ESRD); cardiac resynchronization therapy (CRT); implantable cardioverter-defibrillator (ICD); coronary artery bypass graft (CABG); percutaneous coronary intervention (PCI); antiarrhythmic drug (AAD).
The mean CHA2DS2-VASc and HAS-BLED scores were marginally higher for patients without an OAC prescription fill. Among the individual CHA2DS2-VASc score components in the high-risk group, patients with coronary and peripheral vascular disease were less likely to use OAC (61.4% vs 57.3%; P <.001). Among the HAS-BLED score components, history of CKD, stroke, anemia, major bleeding history, alcoholism, liver disease, thienopyridine and NSAID use were all negatively associated with OAC use (P <.001).
Most other comorbidities were also negatively associated with OAC use in the high-risk group including myocardial infarction, falls, gastrointestinal lesions, extracranial bleeding, smoking, chronic obstructive pulmonary disease, end-stage renal and liver disease (P <.001). Whereas obesity, hyperlipidemia and obstructive sleep apnea were positively associated with OAC use (P <.001).
Concurrent medications use including amiodarone (8.5% vs 7.5%), other antiarrhythmic drugs (10.7% vs 8.3%), calcium channel blockers (18.3% vs 15.2%) and digitalis (11.3% vs 7.8%) were all associated with OAC use in the high-risk subgroup (P <.001). Furthermore, patients on OAC were also more likely to have utilized other cardiac-related interventions such as catheter and surgical ablations, cardioversion, permanent pacemaker/defibrillator devices, prior percutaneous coronary intervention or coronary artery bypass graft (P <.001).
The number of all-cause hospital days and non-AF-related emergency room visits or hospitalizations in the last 12 months were slightly higher in the non-OAC group in high-risk patients (P <.001). Also, within the previous 3 months of the index date, patients in the non-OAC group had a slightly higher number of patients with ischemic stroke/systemic embolism, major bleeding, and intracranial bleeding episodes.
Results from the multivariable logistic regression model for low and high-risk groups are detailed in Table 3, Table 4. In terms of insurance coverage, Medicare advantage plan was a negative predictor of OAC and DOAC compared to commercial plan in both low and high-risk groups.
Table 3.
Predictors of OAC and DOAC use (low-risk group).
| Variables |
OAC use (vs no OAC) |
DOAC use (vs warfarin) |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age group | ||||
| 18–44 years | ref | ref | ref | ref |
| 45–54 years | 1.76 (1.58, 1.97) | <0.0001 | 0.84 (0.58, 1.22) | 0.3678 |
| 55 + years | 2.74 (2.47, 3.02) | <0.0001 | 0.72 (0.51, 1.01) | 0.0575 |
| Gender | ||||
| Female | ref | ref | ref | ref |
| Male | 1.35 (1.24, 1.46) | <0.0001 | 0.99 (0.78, 1.26) | 0.9539 |
| Health plan | ||||
| Commercial | ref | ref | ref | ref |
| Medicare Advantage | 0.73 (0.62, 0.87) | 0.0003 | 0.22 (0.16, 0.31) | <0.0001 |
| HAS-BLED components | ||||
| Anemia/Major bleed | 0.78 (0.70, 0.85) | <0.0001 | 0.70 (0.53, 0.91) | 0.0076 |
| Liver disease | 0.84 (0.72, 0.97) | 0.0191 | 1.47 (0.90, 2.39) | 0.1246 |
| Alcoholism | 0.79 (0.64, 0.97) | 0.0221 | 1.73 (0.84, 3.57) | 0.1343 |
| Antiplatelets/NSAIDs | 0.91 (0.79, 1.05) | 0.1867 | 1.78 (1.07, 2.95) | 0.0273 |
| Other comorbidities | ||||
| Falls | 0.86 (0.72, 1.02) | 0.0908 | 2.56 (1.27, 5.16) | 0.0087 |
| Hypertrophic cardiomyopathy | 2.52 (1.56, 4.07) | 0.0002 | 1.03 (0.35, 3.02) | 0.9553 |
| Cardioversion | 1.45 (1.20, 1.73) | 0.0001 | 1.10 (0.66, 1.84) | 0.7141 |
| Implanted device | 0.71 (0.55, 0.91) | 0.0069 | 0.24 (0.14, 0.39) | <0.0001 |
| Concurrent medication | ||||
| Amiodarone/other AADs | 0.70 (0.62, 0.80) | <0.0001 | 0.78 (0.54, 1.11) | 0.1708 |
Multivariable model assessing the association of clinically relevant variables to oral anticoagulant use within the low-risk group.
Abbreviations: non-steroidal anti-inflammatory drug (NSAID); antiarrhythmic drug (AAD).
Table 4.
Predictors of OAC use (high-risk group).
| Variables |
OAC use (vs no OAC) |
DOAC use (vs warfarin) |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age group | ||||
| 18–64 years | ref | ref | ref | ref |
| 65–74 years | 1.41 (1.37, 1.46) | <0.0001 | 1.06 (1.00, 1.11) | 0.0436 |
| 75 + years | 1.51 (1.46, 1.56) | <0.0001 | 0.85 (0.81, 0.90) | <0.0001 |
| Gender | ||||
| Female | ref | ref | ref | ref |
| Male | 1.03 (1.01, 1.04) | 0.0006 | 0.81 (0.79, 0.83) | <0.0001 |
| Health plan | ||||
| Commercial | ref | ref | ref | ref |
| Medicare Advantage | 0.91 (0.89, 0.94) | <0.0001 | 0.47 (0.44, 0.49) | <0.0001 |
| CHA2DS2-VASc components | ||||
| Heart failure | 1.02 (1.00, 1.04) | 0.0184 | 0.62 (0.60, 0.63) | <0.0001 |
| Diabetes mellitus | 1.07 (1.05, 1.08) | <0.0001 | 0.92 (0.90, 0.94) | <0.0001 |
| Thromboembolism | 1.07 (1.04, 1.10) | <0.0001 | 0.88 (0.84, 0.92) | <0.0001 |
| CAD or PAD | 0.87 (0.85, 0.88) | <0.0001 | 0.84 (0.82, 0.86) | <0.0001 |
| HAS-BLED components | ||||
| CKD | 0.89 (0.87, 0.91) | <0.0001 | 0.96 (0.93, 0.98) | 0.0015 |
| Stroke | 1.03 (1.00, 1.07) | 0.0557 | 0.90 (0.85, 0.94) | <0.0001 |
| Anemia/Major bleed | 0.83 (0.82, 0.85) | <0.0001 | 0.86 (0.84, 0.88) | <0.0001 |
| Liver disease | 0.88 (0.86, 0.90) | <0.0001 | 1.21 (1.17, 1.25) | <0.0001 |
| Alcoholism | 0.82 (0.79, 0.84) | <0.0001 | 1.21 (1.14, 1.28) | <0.0001 |
| Antiplatelets/NSAIDs | 0.75 (0.74, 0.77) | <0.0001 | 1.86 (1.80, 1.93) | <0.0001 |
| Other comorbidities | ||||
| Falls | 0.74 (0.73, 0.75) | <0.0001 | 1.02 (0.99, 1.05) | 0.2745 |
| Obesity | 1.32 (1.30, 1.34) | <0.0001 | 1.26 (1.23, 1.29) | <0.0001 |
| Intervention | ||||
| Catheter ablation/surgical ablation/MAZE | 0.89 (0.84, 0.95) | 0.0005 | 1.03 (0.95, 1.12) | 0.5133 |
| Cardioversion | 2.14 (2.07, 2.22) | <0.0001 | 0.92 (0.88, 0.96) | 0.0001 |
| Implanted device | 1.18 (1.15, 1.20) | <0.0001 | 0.72 (0.69, 0.74) | <0.0001 |
| Concurrent medication | ||||
| Amiodarone/other AADs | 1.04 (1.02, 1.06) | 0.0006 | 0.67 (0.64, 0.69) | <0.0001 |
Multivariable model assessing the association of clinically relevant variables to oral anticoagulant use within the high-risk group.
Abbreviations: coronary artery disease (CAD); peripheral artery disease (PAD); chronic kidney disease (CKD); antiarrhythmic drug (AAD).
In the low-risk group, older men were more likely to be on OAC therapy. Among comorbidities when adjusted for other variables, anemia/major bleeding was a negative predictor for OAC and DOAC therapy. Among comorbidities when adjusted for other variables, anemia/major bleeding was a negative predictor for OAC and DOAC therapy. Liver disease and alcoholism were negative predictors of OAC but were not associated with DOAC. Falls were a negative predictor of OAC use and DOAC were preferred in those with a history of fall. HCM and cardioversion were predictors of OAC use but not for a choice of DOAC over warfarin. Implanted device was negatively associated with OAC and DOAC use. Lastly, antiarrhythmic drug use was negatively associated with OAC use.
In the high-risk group, older men with NVAF were more likely to be on OAC but less likely to be on DOAC. Heart failure, Diabetes Mellitus were positive predictors of OAC but negative predictors of DOAC. CAD/PAD was negatively associated with OAC and DOAC use. HAS-BLED components were mostly negative predictors to OAC use. Further, warfarin was preferred with history of CKD, stroke or anemia/major bleeding. DOAC was preferred with history of liver disease, alcoholism, antiplatelet/NSAIDs use. Falls were a negative predictor to OAC use while obesity was associated with both OAC and DOAC use. Lastly, Cardioversion, Implanted device, and antiarrhythmic drug were positively associated with OAC use and negatively with DOAC use.
In the sensitivity analysis, 4,153 (29.5%) patients had OAC use in the low-risk group (N = 14,096) while 141,196 (51.1%) patients had OAC use noted in the high-risk group (N = 276,450). Predictors of OAC use for the sensitivity analysis are detailed in Table S5 and S6. Briefly, results for the low-risk group were similar to the main analysis and in the high-risk group, heart failure and amiodarone/other antiarrhythmic drug use were no longer significant predictors of OAC use while falls were noted as significant predictor of DOAC use.
4. Discussion
In this study, using the nationwide data of OLDW, we provide several key insights into the problem of under and over-prescription of OAC. Considerable changes have been made in the guidelines of AF management in the last decade and through this work we highlight the need to strengthen evidence-based care in NVAF patients at the point of care.
Within the low-risk group, a significant proportion (about 30%) of patients had OAC prescription fills. Majority of this group composed of relatively young (mean age 54 years) males (74%). OAC use primarily comprised of NOAC (91%) in this group. In this low-risk NVAF subgroup, independent indications for OAC use such as venous thromboembolism and recent total knee or hip replacement were excluded. Other possible indications for OAC use had low prevalence during previous 12 months such as cardioversion (5%) and catheter ablation (1.7%). Also, in the sensitivity analysis, the OAC utilization rates remained similar to the main analysis. Further, there was minimal AF and non-AF related hospital days and healthcare utilization in this subgroup. Overall, these findings suggest an overutilization of OAC in low-risk CHA2DS2-VASc group. As per guidelines, OAC is not recommended in this group due to the unjustifiable incremental increase in major bleeding risk compared to the annual absolute risk reduction for ischemic stroke risk.[3] Among independent predictors of OAC use in low-risk group, antiarrhythmic drugs and implanted device were negatively associated with OAC use and may suggest a better guideline adherence in patients under specialized care.
Among the high-risk group, only about half the patients (52%) had OAC prescription fills. Further, 33% of OAC use in this group was warfarin instead of DOAC. These results reflect a high proportion of patients that are still left unprotected from stroke risk or are otherwise not on guideline directed OAC therapy. Here we did not consider the adherence and persistence rates [13] for OAC therapy which are further likely to reduce the number of AF patients left unprotected against stroke. Although not directly comparable, these numbers seem comparable to the pre-DOAC era studies in the United States that were predominantly comprised of patients using VKA.[7], [9], [14] In comparison, some other high-income countries during the post-DOAC era have reported considerably higher OAC rates in the high-risk group approaching 84.3% in a large prospective Belgian cohort.[15] Among the independent predictors older men, patients with heart failure, diabetes mellitus, CAD/PAD, CKD, stroke/thromboembolism, anemia/major bleeding were more likely to receive warfarin instead of DOAC. This may suggest a relative avoidance of DOAC by clinicians in patients with a higher tendency for bleeding and is consistent with findings from a previous study.[16] DOAC use was more likely in patients with liver disease and alcoholism likely reflecting the metabolism of these drugs.
A high proportion of warfarin use suggests that the grade 1 A recommendation [3], [17] of DOAC use over warfarin is either underrecognized or inadequately realized. The major clinical settings in which VKA is the agent of choice include: 1) moderate-severe mitral stenosis or bioprosthetic/mechanical heart valve (both excluded from analysis). 2) Severe CKD patients, when physicians usually opt for either VKA or apixaban (note: this group included only 1% of end-stage renal disease patients). 3) When additional patient costs are not justified (e.g., out of pocket payments or lower bracket incomes). By exclusion, underutilization may relate to the direct higher costs associated with DOAC relative to warfarin. Inadequacies of Medicare advantage plan such as high-out of pocket maximum; hassles of prior authorization and referral requirements could have discouraged patients from starting long term OAC treatment.[18] Indeed, Medicare Advantage plan in this study was a negative predictor of OAC and DOAC use compared to commercial plan. The negative association of Medicare plan for DOAC use relative to private insurance plan has previously been reported.[19].
The OAC utilization within the high-risk group was relatively unaffected by the CHA2DS2-VASc score (threshold effect) and is consistent with previous research [7]. This may suggest an influence of other unknown factors on management decision within this group (e.g., risks not well-captured in our dataset, patients/physician preference or cost). Among the CHA2DS2-VASc score components, particularly in a high-risk group, all components increased the odds of OAC use except CAD/PAD which might be due to the clinical challenge of balancing benefit versus harm in these patients posed by concurrent dual or triple oral antithrombotic therapy. Most of the HAS-BLED score components were negatively associated with OAC use. Additionally, there was a higher prevalence of most comorbidities in the non-OAC group. Patients in the non-OAC group were more likely to have utilized non-AF-related hospital facilities, such as skilled nursing facilities, higher non-AF emergency room visits, hospitalization, and all-cause hospital days. Thus, it is reasonable to speculate that patients not on OAC represented a sicker population with a higher prevalence of most non-AF related comorbidities, potentially adding to the bleeding risk. From a patient’s perspective, these findings may suggest a weariness and an ambivalent nature towards accepting further long-term primary preventive treatments without providing immediate symptomatic benefits or a cure to their ongoing problems. This is similar to some prior studies that have linked polypharmacy and worsening of health outcomes with low adherence to OAC therapy.[13] Thus, we emphasize a need to engage patients in a shared decision-making process and help direct them towards more beneficial treatment options or revisit the decision later.[20] Lastly, patients on OAC were more likely to have utilized other cardiac-related interventions such as catheter/surgical ablations, cardioversion, permanent pacemaker/defibrillator devices, prior percutaneous coronary intervention, or coronary artery bypass graft. These findings suggest that patients under specialty care may have higher rates of OAC utilization.
To our knowledge, this is the only study to have studied exclusively NVAF patients in the US during this time period. Further, limited prior data is available for overutilization of OAC in the low-risk group. Some recent studies have otherwise lacked patient level data to exclude independent indications for OAC therapy or study predictors and barriers to OAC therapy.[21], [22], [23].
Potential limitations of this study are as follows. Administrative claims might be subject to misclassification and claims data may be driven by reimbursement concerns. However, the results would still represent the true utilization patterns among patients even if prescription rates by physicians may have been higher than prescription fills. The guidelines recommend OAC prescription based on CHA2DS2-VASc score, the actual treatment decisions are individualized for each patient and include patient’s preferences, values, and insurance coverage. We have attempted to incorporate some of these possibilities while assessing the inferences of our results. Provider specialty and hospital setting in which AF was diagnosed is not assessed in this study and may be further predictors of OAC use. Some of the independent transient indications for OAC use such as ablation and cardioversion were included in the main analysis but removed in a separate sensitivity analysis. Finally, the study population included patients with commercial health insurance, and Medicare Advantage, and may not generalize to those with no coverage.
5. Conclusions
Despite the added benefits DOAC use over VKA, the guideline based OAC use in AF patients seems comparable to pre-DOAC era studies. Both under and over treatment patterns were notable findings with a significant proportion of high-risk patients still treated with warfarin. Although the nuances of clinical practice that drive individual patient and clinician decisions are not possible to resolve from observational data sources alone, our findings highlight the need for future systematic processes to optimize OAC utilization especially considering the rising incidence of embolic strokes.
Sources of Funding
This work was supported by the Department of Cardiovascular Medicine at Mayo Clinic in Rochester, MN. The authors also acknowledge support by NIH T32 HL007111.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Anthony H. Kashou reports financial support was provided by Mayo Clinic Minnesota.].
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101212.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Vora P., Morgan Stewart H., Russell B., Asiimwe A., Brobert G. Time Trends and Treatment Pathways in Prescribing Individual Oral Anticoagulants in Patients with Nonvalvular Atrial Fibrillation: An Observational Study of More than Three Million Patients from Europe and the United States. Int. J. Clin. Pract. 2022;2022:6707985. doi: 10.1155/2022/6707985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiyovich A., Shalev V., Chodick G., Tirosh M., Katz A., Klar M.M., et al. Shifting from vitamin K antagonists to non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: predictors, patterns and temporal trends. BMC Cardiovasc. Disord. 2021;21(1):493. doi: 10.1186/s12872-021-02295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016;50(5):e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg B.A., Kim S., Thomas L., Fonarow G.C., Hylek E., Ansell J., et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129(20):2005–2012. doi: 10.1161/CIRCULATIONAHA.114.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamel H., Healey J.S. Cardioembolic Stroke. Circ. Res. 2017;120(3):514–526. doi: 10.1161/CIRCRESAHA.116.308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer A., Flierl U., Berliner D., Bauersachs J. Anticoagulants for Stroke Prevention in Atrial Fibrillation in Elderly Patients. Cardiovasc. Drugs Ther. 2020;34(4):555–568. doi: 10.1007/s10557-020-06981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu J.C., Maddox T.M., Kennedy K.F., Katz D.F., Marzec L.N., Lubitz S.A., et al. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights From the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1(1):55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 8.Wilke T., Groth A., Mueller S., Pfannkuche M., Verheyen F., Linder R., et al. Oral anticoagulation use by patients with atrial fibrillation in Germany. Adherence to guidelines, causes of anticoagulation under-use and its clinical outcomes, based on claims-data of 183,448 patients. Thromb. Haemost. 2012;107(6):1053–1065. doi: 10.1160/TH11-11-0768. [DOI] [PubMed] [Google Scholar]
- 9.Lubitz S.A., Khurshid S., Weng L.C., Doros G., Keach J.W., Gao Q., et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am. Heart J. 2018;200:24–31. doi: 10.1016/j.ahj.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarese G., Sartipy U., Friberg L., Dahlstrom U., Lund L.H. Reasons for and consequences of oral anticoagulant underuse in atrial fibrillation with heart failure. Heart. 2018;104(13):1093–1100. doi: 10.1136/heartjnl-2017-312720. [DOI] [PubMed] [Google Scholar]
- 11.Wallace P.J., Shah N.D., Dennen T., Bleicher P.A., Bleicher P.D., Crown W.H. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Farinha J.M., Jones I.D., Lip G.Y.H. Optimizing adherence and persistence to non-vitamin K antagonist oral anticoagulant therapy in atrial fibrillation. Eur. Heart J. Suppl. 2022;24(Suppl A):A42–A55. doi: 10.1093/eurheartj/suab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowan S.B., Bailey D.N., Bublitz C.E., Anderson R.J. Trends in anticoagulation for atrial fibrillation in the U.S.: an analysis of the national ambulatory medical care survey database. J. Am. Coll. Cardiol. 2007;49(14):1561–1565. doi: 10.1016/j.jacc.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Cools F., Wollaert B., Vervoort G., Verstraete S., Voet J., Hermans K., et al. Treatment patterns in anticoagulant therapy in patients with newly diagnosed atrial fibrillation in Belgium: results from the GARFIELD-AF registry. Acta Cardiol. 2019;74(4):309–318. doi: 10.1080/00015385.2018.1494089. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg B.A., Shrader P., Thomas L., Ansell J., Fonarow G.C., Gersh B.J., et al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II) Am. Heart J. 2017;189:40–47. doi: 10.1016/j.ahj.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 17.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66–e93. doi: 10.1016/j.hrthm.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Neuman P., Jacobson G.A. Medicare Advantage Checkup. New England J Med. 2018;379(22):2163–2172. doi: 10.1056/NEJMhpr1804089. [DOI] [PubMed] [Google Scholar]
- 19.Yong C.M., Liu Y., Apruzzese P., Doros G., Cannon C.P., Maddox T.M., et al. Association of insurance type with receipt of oral anticoagulation in insured patients with atrial fibrillation: A report from the American College of Cardiology NCDR PINNACLE registry. Am. Heart J. 2018;195:50–59. doi: 10.1016/j.ahj.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Mehawej J., Saczynski J., Abu H.O., Gagnier M., Bamgbade B.A., Lessard D., et al. Factors Associated With Patient Engagement in Shared Decision-Making for Stroke Prevention Among Older Adults with Atrial Fibrillation. Can Geriatr J. 2021;24(3):174–183. doi: 10.5770/cgj.24.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheelock K.M., Ross J.S., Murugiah K., Lin Z., Krumholz H.M., Khera R. Clinician Trends in Prescribing Direct Oral Anticoagulants for US Medicare Beneficiaries. JAMA Netw. Open. 2021;4(12):e2137288. doi: 10.1001/jamanetworkopen.2021.37288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troy A., Anderson T.S. National Trends in Use of and Spending on Oral Anticoagulants Among US Medicare Beneficiaries From 2011 to 2019. JAMA Health Forum. 2021;2(7):e211693. doi: 10.1001/jamahealthforum.2021.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duvalyan A., Pandey A., Vaduganathan M., Essien U.R., Halm E.A., Fonarow G.C., et al. Trends in Anticoagulation Prescription Spending Among Medicare Part D and Medicaid Beneficiaries Between 2014 and 2019. J. Am. Heart Assoc. 2021;10(24):e022644. doi: 10.1161/JAHA.121.022644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.