Abstract
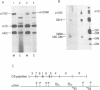
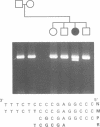
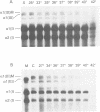
Previous studies have shown that Ehlers-Danlos syndrome type IV (EDS IV) is caused by mutations of type III collagen (COL3A1). Here we have characterised the most amino-terminal glycine substitution so far described in a patient with EDS IV. A combination of peptide mapping and chemical cleavage analysis of cDNA localised the mutation in cyanogen bromide peptide CB5. Sequence analysis showed a G to A mutation, converting glycine 661 to arginine, which was a new dominant mutation. Analysis of type III collagen secreted by cultured fibroblasts showed an overmodified mutant protein with normal thermal stability. However, the intracellularly retained form melted 2 degrees C lower than normal. This indicated that molecules resulting from the same mutation can differ in their thermal stabilities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Romanic A. M., Prockop D. J. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990 Nov;86(5):1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Substitution of aspartate for glycine 1018 in the type III procollagen (COL3A1) gene causes type IV Ehlers-Danlos syndrome: the mutated allele is present in most blood leukocytes of the asymptomatic and mosaic mother. Am J Hum Genet. 1992 Sep;51(3):497–507. [PMC free article] [PubMed] [Google Scholar]
- Narcisi P., Wu Y., Tromp G., Earley J. J., Richards A. J., Pope F. M., Kuivaniemi H. Single base mutation that substitutes glutamic acid for glycine 1021 in the COL3A1 gene and causes Ehlers-Danlos syndrome type IV. Am J Med Genet. 1993 May 15;46(3):278–283. doi: 10.1002/ajmg.1320460308. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., De Paepe A., Narcisi P., Dalgleish R., De Keyser F., Matton M., Pope F. M. Linkage of a polymorphic marker for the type III collagen gene (COL3A1) to atypical autosomal dominant Ehlers-Danlos syndrome type IV in a large Belgian pedigree. Hum Genet. 1988 Mar;78(3):276–281. doi: 10.1007/BF00291676. [DOI] [PubMed] [Google Scholar]
- Nuytinck L., Narcisi P., Nicholls A., Renard J. P., Pope F. M., De Paepe A. Detection and characterisation of an overmodified type III collagen by analysis of non-cutaneous connective tissues in a patient with Ehlers-Danlos syndrome IV. J Med Genet. 1992 Jun;29(6):375–380. doi: 10.1136/jmg.29.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Martin G. R., Lichtenstein J. R., Penttinen R., Gerson B., Rowe D. W., McKusick V. A. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Narcisi P., Ward P. N., Nicholls A. C., De Paepe A., Pope F. M. A 27-bp deletion from one allele of the type III collagen gene (COL3A1) in a large family with Ehlers-Danlos syndrome type IV. Hum Genet. 1992 Jan;88(3):325–330. doi: 10.1007/BF00197268. [DOI] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Ward P. N., De Paepe A., Narcisi P., Pope F. M. Characterisation of a glycine to valine substitution at amino acid position 910 of the triple helical region of type III collagen in a patient with Ehlers-Danlos syndrome type IV. J Med Genet. 1991 Jul;28(7):458–463. doi: 10.1136/jmg.28.7.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Ward P. N., Narcisi P., Nicholls A. C., Lloyd J. C., Pope F. M. A single base mutation in the gene for type III collagen (COL3A1) converts glycine 847 to glutamic acid in a family with Ehlers-Danlos syndrome type IV. An unaffected family member is mosaic for the mutation. Hum Genet. 1992 Jun;89(4):414–418. doi: 10.1007/BF00194313. [DOI] [PubMed] [Google Scholar]
- Stolle C. A., Pyeritz R. E., Myers J. C., Prockop D. J. Synthesis of an altered type III procollagen in a patient with type IV Ehlers-Danlos syndrome. A structural change in the alpha 1(III) chain which makes the protein more susceptible to proteinases. J Biol Chem. 1985 Feb 10;260(3):1937–1944. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Nov 15;264(32):19313–19317. [PubMed] [Google Scholar]