Abstract
Background
Historical restrictions on take-home medications for opioid use disorder have generated considerable debate. The COVID-19 pandemic shifted the perceived risks and benefits of daily clinic attendance and led to widespread policy reform, creating an unprecedented opportunity to explore the impact of more flexible prescribing. We conducted a qualitative systematic review to synthesize the evidence on providers’ experiences with relaxing restrictions on take-home doses of medications prescribed for opioid use disorder during the COVID-19 pandemic.
Methods
The protocol for this systematic review was registered in PROSPERO (CRD42022360589; https://www.crd.york.ac.uk/prospero/). From Sept.–Nov. 2022, we searched Medline, Embase, CINAHL, PsycInfo, Web of Science, the Cochrane Register of Controlled Trials, and the grey literature from 2020 onward. Studies were eligible for inclusion if they used qualitative methods to investigate providers’ experiences with relaxed restrictions on take-home medications for opioid use disorder during the COVID-19 pandemic. We appraised study quality using the CASP qualitative checklist and used thematic synthesis and GRADE-CERQual to synthesize the results.
Results
We retrieved 13 articles representing 11 studies. Six were conducted in the United States and most focused on changes to methadone treatment. Providers’ experiences with increased flexibilities around take-homes were broadly positive, despite widespread initial concern over client safety and the potential for medication misuse. For a small number of providers, concerns about diversion were a specific manifestation of more general unease with loss of control over clients and the treatment process. Most providers appreciated increased flexibilities and described them as enabling more individualized, person-centered care.
Conclusion
Our findings support the continuation of flexibilities around take-homes and demonstrate that regulations and policies that reduce flexibility around take-homes conflict with person-centered approaches to care. Stronger guidance and support from professional regulatory agencies may help increase uptake of flexibilities around take-homes.
Keywords: Substance use, Opioid agonist treatment, Person-centered care
Introduction
Globally, an estimated 115,000 deaths were attributable to opioid overdose in 2017 (World Health Organization, 2021). Mortality rates are particularly high in North America, and increased dramatically during the COVID-19 pandemic. In Canada, for instance, there were 15,134 apparent opioid toxicity deaths in the first two years of the pandemic – a 91% increase compared with the 7,906 deaths in the two years prior to the pandemic (Special Advisory Committee on the Epidemic of Opioid Overdoses, 2022). This is consistent with provisional data from United States that shows 72,081 opioid-related drug overdose deaths in the first year of the pandemic (Mar. 2020–Feb. 2021) versus 51,999 in the year preceding the pandemic (Mar. 2019–Feb. 2020) (Ahmad et al., 2023). Unregulated street drugs have become increasingly toxic in recent years as the result of adulteration with fentanyl and other ultrapotent opioids (Tobias et al., 2022). In British Columbia, drug checking services have found that the illicit opioid supply is also highly contaminated with benzodiazepines and benzodiazepine analogues, leading to complex overdoses that may not respond to naloxone (British Columbia Centre on Substance Use, 2021).
Prescribing opioids of known concentration and purity to people who use drugs is one approach to reducing the harms associated with untreated opioid use disorder and dependence on an unregulated drug supply. Opioid agonist treatment using methadone or buprenorphine (OAT) is well-established and decreases rates of fatal overdose and infections associated with unsafe injection practices (Platt et al., 2018; Sordo et al., 2017). Individuals whose needs are not met by OAT may benefit from treatment with injectable diacetylmorphine or hydromorphone (Ferri et al., 2011; Oviedo-Joekes et al., 2016). Other alternatives, including fentanyl patches and hydromorphone tablets, have been provided to people who use substances as a public health measure (Bardwell et al., 2019; British Columbia Centre on Substance Use, 2023; Brothers et al., 2022; Olding et al., 2020; Young et al., 2022). This practice has been termed “safer supply” (Health Canada, 2021). While there is no universally accepted definition of safer supply – or the frequently used variant “safe supply” – the government of Canada defines it as “providing prescribed medications as a safer alternative to the toxic illegal drug supply to people who are at high risk of overdose” (Health Canada, 2021). As fentanyl and fentanyl analogues become more dominant in regional drug supplies, it will become increasingly necessary to expand the range of treatment options available to people who use opioids (Ciccarone, 2021, Morales et al., 2019).
Opioids prescribed for opioid use disorder are frequently required to be consumed under the direct observation of a health care professional, necessitating daily attendance at a clinic or pharmacy (Jin et al., 2020). This requirement is intended to reduce potential misuse, adverse events, and diversion, which has been defined as “the selling/trading, sharing or giving away of prescription medications to others” (Larance et al., 2011, p 239). Clients who meet certain criteria may be permitted to take a limited number of doses off-site and consume their medication without direct oversight. These doses are often referred to as “take-homes” (Department of Health and Social Care, 2021; Lam et al., 2020; Substance Abuse and Mental Health Services Administration [SAMHSA], 2015), although the term “home” does not cover the many possible locations where the medications may be used (e.g., safe consumption sites). Eligibility criteria for receiving take-homes vary substantially by region, but may include time-in-treatment requirements (e.g., three months), abstinence from alcohol and illicit drugs, physician assessments of client stability, and provider discretion (Jin et al., 2020; SAMHSA, 2015). Criteria may include an element of subjectivity, and are generally treated as ‘necessary but not sufficient’ conditions for receiving take-homes; Jin et al. (2020), in a global review of clinical practices in opioid agonist treatment, report that take-homes for clients meeting eligibility criteria are typically offered at the discretion of the provider.
Proponents of more flexibility around take-homes observe that supervised dosing requirements are burdensome for clients and widely perceived as degrading and stigmatizing (Anstice et al., 2009; Deering et al., 2011; Frank et al., 2021a; Madden et al., 2008), suggesting that clients have limited or no input into decisions around take-home privileges. Supervised dosing requirements have been posited as a contributor to low rates of treatment uptake and inequitable treatment coverage (Ritter & Di Natale, 2005; Russell et al., 2022). Moreover, evidence that supervised dosing mitigates the risks of medication misuse remains limited (Hov et al., 2016; Saulle et al., 2017). Consequently, restrictions on take-homes have generated considerable debate.
The COVID-19 pandemic shifted the perceived risks and benefits of take-home doses of medications for opioid use disorder. Physical distancing was quickly identified as critical in limiting the spread of the virus (World Health Organization, 2020), leading to scrutiny of supervised dosing requirements. High rates of structural vulnerability and comorbidities among people who use substances were expected to increase their susceptibility to contracting COVID-19 and experiencing poor health outcomes (Department of Health and Social Care, 2021; Farhoudian et al., 2020). Furthermore, national and regional lockdowns limited clients’ ability to travel and forced a general reduction in face-to-face contacts in substance use treatment (Radfar et al., 2021). The safety of health workers in substance use treatment settings was also a concern (Radfar et al., 2021). As a result, a number of countries saw amendments to regulations and guidelines that had previously limited access to take-home doses in OAT, including Australia (Lintzeris et al., 2020), Canada (Lam et al., 2020), England (Department of Health and Social Care, 2021), Italy (Vecchio et al., 2020), Spain (Departament de Salut, 2020), Switzerland (SSAM, 2020), and the United States (Substance Abuse and Mental Health Services Administration [SAMHSA], 2020).
In the United States, for example, the Substance Abuse and Mental Health Administration (SAMHSA) issued a federal exemption that dramatically increased providers’ ability to dispense take-home doses of methadone (SAMHSA, 2020). Pre-pandemic guidelines in the United States restricted take-homes to clients meeting specific criteria (e.g., absence of illicit drug and alcohol use; regular clinic attendance) and carried time-in-treatment requirements that limited clients in their first six months of treatment to 1–2 take-home doses per week (SAMHSA, 2015). Under the COVID-19 exemption, clients meeting SAMHSA's eight-point definition of “stable” (60 days in treatment with negative toxicology tests, among other criteria) could receive up to 28 days of take-homes (SAMHSA, 2020). Clients considered “less stable” (30 days in treatment with negative toxicology tests, among other criteria) could receive up to 14 days of take-homes. Providers were urged to use their clinical judgement to decide whether a client could “safely handle” this amount of medication (SAMHSA, 2020). Though the maximum length of buprenorphine prescriptions remained unchanged, the American Society of Addiction Medicine urged providers to extend prescription durations for individual clients if it was possibly to do so safely (ASAM COVID-19 Task Force, 2020). In Canada, provincial guidance on OAT was updated to support greater use of take-home doses of methadone and buprenorphine and there was increased federal and provincial support for safer supply initiatives (British Columbia Centre on Substance Use, 2020a,b; Hajdu, 2020; Lam et al., 2020). As in the United States, providers were also expected to exercise their clinical judgment in deciding whether take-homes were appropriate for individual clients (British Columbia Centre on Substance Use, 2020b; Lam et al., 2020).
These changes created an unprecedented opportunity to explore the impact of more flexible prescribing of opioid agonist treatment and safe supply medications. To this end, the objective of this systematic review was to synthesize the evidence on providers’ experiences with relaxing restrictions on take-home medications for opioid use disorder during the COVID-19 pandemic. Though there is a substantial body of research examining the impact of COVID-19-related changes to substance use treatment (e.g., Bouck et al., 2022; Garg et al., 2022; Krawczyk et al., 2022a; Lintzeris et al., 2022; May et al., 2022), this is, to our knowledge, the first systematic review of international scope to focus on providers’ experiences. Knowledge of these experiences is necessary to understand the effects of increased flexibility in prescribing take-home medications for opioid use disorder during COVID-19, to explain differences in the uptake of regulatory changes, and to inform post-pandemic policies and guidelines. Changes to guidance on take-homes have now been rolled back in a number of jurisdictions (e.g., Department of Health and Social Care, 2021; Lam et al., 2020), despite calls for sustained reform (e.g., Frank et al., 2021b; Joseph et al., 2021; Krawczyk et al., 2020). High-quality evidence on provider experiences is urgently needed to inform these decisions. We recognize that client experiences are also of critical importance and are synthesizing these data in a separate review, registered in PROSPERO, which is currently underway (CRD42022352310; https://www.crd.york.ac.uk/prospero/).
Methods
Design
We conducted a qualitative systematic review using thematic synthesis (Thomas & Harden, 2008) to address the research question “What were providers’ experiences with the relaxation of restrictions on take-home medications prescribed for opioid use disorder during the COVID-19 pandemic?” We conducted the searches from September–November 2022 and limited our results to material published after January 1, 2020. The protocol for this systematic review was registered in PROSPERO (CRD42022360589; https://www.crd.york.ac.uk/prospero/). The protocol was designed by AA in collaboration with EOJ. This review is reported in accordance with the PRISMA statement for reporting systematic reviews (Page et al., 2021).
Search strategy
We used a comprehensive, pre-planned search strategy to seek all available evidence addressing our research question. We used the PICo (Population, phenomenon of Interest, Context) framework to structure our research question and search strategy. We defined our population as health care professionals prescribing or dispensing opioids for opioid use disorder. Our phenomenon of interest was provider experiences (e.g., views on policy changes; perceived advantages and disadvantages of changes). Our context was defined as the relaxation of restrictions on take-home medications for opioid use disorder during the COVID-19 pandemic. The search was limited to material published after 1 January 2020 because the review focuses on actions taken in response to the Covid-19 pandemic, which was not formally declared by the World Health Organization until March 2020. No published search filters were used, and no language restrictions were applied at the search stage.
To identify peer-reviewed literature, we conducted searches of the following electronic databases and registers from September 16–19, 2022: Medline (Ovid), Embase (Ovid), CINAHL Complete (EBSCOhost), PsycInfo (EBSCOhost), Web of Science Core Collection (Web of Science), and the Cochrane Central Register of Controlled Trials (Ovid).
We conducted grey literature searches from October 24–November 7, 2022 to mitigate publication bias. We searched PAIS, PsycExtra, and the websites of 18 government, research, and policy organizations working in substance use and addiction. We also searched selected websites from CADTH's ‘Grey Matters’ tool, which is a checklist designed to assist researchers and information specialists in searching for health-related grey literature (CADTH, 2019). We searched Google (www.google.com) and Google Scholar (www.googlescholar.com) in incognito mode from Canada. One reviewer (AA) browsed the first 100 results from Google and the first 200 results from Google Scholar. We conducted citation chaining from 1–2 December 2022. For backward citation chaining, we manually screened the reference lists of all included articles. For forward citation chaining, we searched the title of each included article in Google Scholar and used the ‘Cited by’ function to identify studies citing that article.
The search strategy was developed by a member of the research team with expertise in systematic searching (AA) and was peer-reviewed by a professional research librarian at the University of British Columbia. The search strategy used in Ovid Medline is shown as an example in Supplementary Material. All other search strategies and searches “as run” are available from the OSF data repository (https://osf.io/mgdpe/).
Screening
Search results were imported into Covidence, a web-based software platform for supporting systematic reviews (Veritas Health Innovation, 2021). Results were automatically deduplicated in Covidence prior to screening. In the first stage of screening, we reviewed titles and abstracts against eligibility criteria (Table 1 ) and excluded articles that were clearly not relevant. In the second stage, we screened the full text of all remaining articles against our eligibility criteria. Those that met our eligibility criteria were included in the review. At both stages, each article was screened by two reviewers working independently (AA, RF). To reconcile disagreements, the two reviewers discussed the article until consensus was reached or referred the decision to a third member of the research team (JL, SB).
Table 1.
Eligibility criteria used in screening.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
ALL of the following:
|
ANY of the following:
|
Refer to Supplementary Material (p. 2) for details.
Data extraction and quality assessment
Data extraction and quality assessment were performed in Covidence by two reviewers working independently (AA, RF). Data reconciliation was completed through discussion and consensus or by a third member of the research team (JL, SB). For data extraction, we created a standardized form to extract information on study characteristics and findings (e.g., study region; study aim/objectives; study design; sample characteristics; conclusions). To pilot the form, three members of the research team (AA, RF, TM) extracted data from test articles. This process resulted in the deletion of a redundant field, the refinement of two similar fields, and minor re-ordering to improve usability. The final version of the form can be accessed in the OSF data repository (https://osf.io/mgdpe/).
We used the CASP Qualitative Checklist to appraise study quality (Critical Appraisal Skills Programme, 2018). This checklist is commonly used in qualitative evidence syntheses (Noyes et al., 2018a). It consists of 10 questions addressing various aspects of study quality, including validity, study design, recruitment strategy, methods of data collection, rigor of data analysis, consideration of the relationship between researchers and participants, and clarity of findings.
Synthesis
We chose to synthesize qualitative data using thematic synthesis (Thomas & Harden, 2008). Thematic synthesis is a three-step process consisting of (1) line-by-line coding of study findings, (2) grouping related codes into broader descriptive themes, and (3) moving from descriptive themes to “analytical themes” that “generate new interpretive constructs, explanations or hypotheses” about the phenomenon under investigation (Thomas & Harden, 2008). This approach is considered well-aligned with the principles of systematic review. It is compatible with qualitative studies undertaken from diverse epistemological positions and can accommodate qualitative data of varying degrees of conceptual and contextual richness (Noyes et al., 2022).
All coding was completed in NVivo 1.7 (QSR International Pty Ltd., 2020). Two members of the research team (AA, SB) conducted line-by-line coding of all findings addressing our study question, including first-order findings (participant quotes) and second-order findings (researchers’ interpretations). Three studies were coded by both coders to create an initial set of free codes, which were subsequently grouped under broader descriptive themes. All studies were then coded or re-coded by one coder. After all articles had been coded, each article was reviewed by the second coder to check for consistency and completeness. The coders met frequently throughout this process to refine free codes and descriptive themes. In the final, interpretive stage of the thematic analysis, the coders explored connections between and within the descriptive themes to develop analytical themes that together “made sense” of providers’ experiences. This process was facilitated by extensive discussion and collaborative mind-mapping. The analytical themes were further refined following consultations with other members of the research team (EOJ, SM), including a practicing addiction medicine physician with extensive experience prescribing medications for opioid use disorder.
Certainty of findings
We used the GRADE-CERQual (Confidence in Evidence from Reviews of Qualitative research) or ‘CERQual’ approach to assess the strength of the evidence supporting each analytical theme. GRADE-CERQual was developed by the Grading of Recommendations Assessment, Development, and Evaluation Working Group “to support the use of findings from qualitative evidence syntheses in decision-making” (Lewin et al., 2018a). Our approach was informed by a series of guidance papers from GRADE (Colvin et al., 2018; Glenton et al., 2018; Lewin et al., 2018a,b; Munthe-Kaas et al., 2018; Noyes et al., 2018b) and is briefly summarized below.
To make CERQual assessments, one member of the research team (AA) examined the group of individual studies contributing to each review finding (i.e., each analytical theme). For each finding, the body of evidence was first evaluated in terms of the four components of CERQual: methodological limitations, coherence, adequacy of data, and relevance to the research question. The impact of each component on our confidence in individual review findings was assessed as No or very minor concerns, Minor concerns, Moderate concerns, or Serious concerns. No or very minor concerns are those that are unlikely to reduce confidence in the review finding, whereas serious concerns are considered “very likely” to reduce confidence in the review finding (Lewin et al., 2018b, p. 15).
Next, the four CERQual components were used to make overall assessments of level of confidence in each review finding (high/moderate/low/very low). Confidence levels in GRADE-CERQual indicate “the extent to which a review finding is reasonable representation of the phenomenon of interest” (Lewin et al., 2018a, p. 3), and are intended to assist policymakers in deciding how to incorporate qualitative evidence into decision-making. All assessments were reviewed and discussed with a second member of the review team (SB) prior to finalization.
Results
Study characteristics
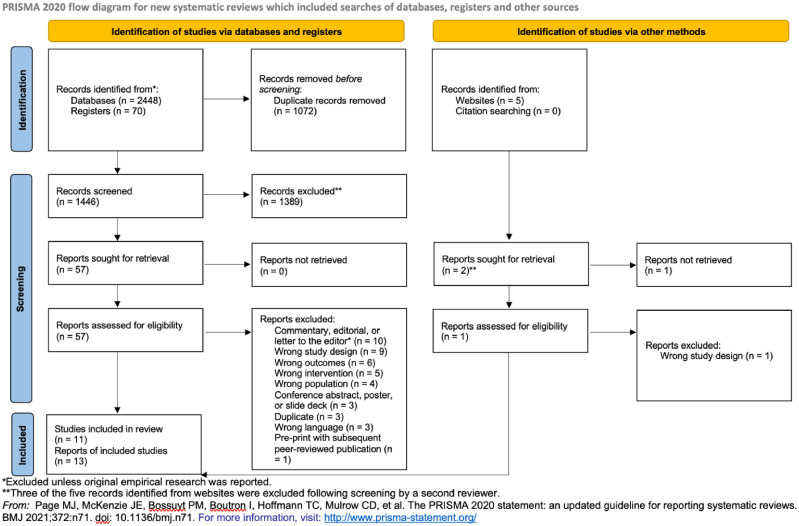
Our search retrieved 2518 records from databases and registers and five records from other sources. See Fig. 1 for a PRISMA 2020 flow diagram (Page et al., 2021). After title/abstract and full text screening (Fig. 1), we identified 13 articles (representing 11 studies) that met our eligibility criteria. Two studies were associated with multiple reports (Glegg et al., 2022/McCrae et al., 2022; Suen et al., 2022/Wyatt et al., 2022). Study characteristics are shown in Table 2 .
Fig. 1.
PRISMA 2020 flow diagram.
Table 2.
Characteristics of included studies.
| No. | Study | Region | Aim | Sample | No. of Providers in Sample | Opioid Medication(s) | Data Collection and Qualitative Approach |
|---|---|---|---|---|---|---|---|
| 1 | (a) Glegg et al., 2022/(b) McCrae et al., 2022 | Canada | "to: 1) describe the operational and clinical characteristics of iOAT, TiOAT and safer supply sites across Canada before and early on in the COVID-19 pandemic; and 2) explain the reasons for the emerging changes in safer supply implementation during the pandemic's first wave." (p. 2) | Healthcare providers from 103 iOAT, TiOAT, safer supply, and risk mitigation sites across Canada. | 50 | Any form of OAT, iOAT, tiOAT, or safer supply | Data collection: Interviews; survey with open-ended questions Data collection period: Mar.-May 2020 Approach: Qualitative analysis using “interpretive epistemological lens" (p. 3) |
| 2 | Goldsamt et al., 2021 | United States | "to understand how OTPs adapted to structural, behavioral and regulatory changes, implemented due to the COVID-19 pandemic, including the reactions of staff to these changes. This paper focuses on OTP clinic directors' perspectives of the regulatory changes instituted during the early part of the pandemic in the U.S." (p. 2) | Directors of methadone treatment programs in the US, including eight programs in the Northeast, eight in the South, six in the Midwest and three in the West. | 25 | Methadone | Data collection: Interviews Data collection period: Jun.-Aug. 2020 Approach: Qualitative description1 |
| 3 | Hatch-Maillette et al. 2021 | United States | "The pandemic incited rapid changes in standard OTP methadone take-home privileges . . . we comment on the immediate impact of these changes on OTP providers and patients" (p. 1) | Medical providers from an OTP in the American Pacific Northwest. | NR | Methadone | Data collection: Not reported Data collection period: Mar.-May 2020 Approach: Qualitative description1 |
| 4 | Hunter et al., 2021 | United States | "To better understand how OTPs have responded to the pandemic, we conducted a qualitative study to describe how clinicians working in OTPs responded to the changes in regulations, particularly the use of telemedicine, as well as challenges to treatment delivery and implications for the quality and safety of care." (p. 2) | Clinicians licensed to dispense methadone at OTPs in 13 US states. | 20 | Methadone | Data collection: Interviews Data collection period: May-Jun. 2020 Approach: “Rapid thematic content analysis” (p. 2) |
| 5 | Madden et al., 2021 | United States | "to explore how providers working in community-based substance use treatment perceive MMT regulations, specifically take-home policies, and deregulation [. . .] provide context to better understand how providers are responding to calls extending COVID-19 MMT policies and to identify considerations for meaningful adoption of longer-term policy" (p. 2). | Medical professionals working in Texas or New Mexico, including physicians, licensed counselors, program administrators, and peer support workers. | 59* (8 contributing data on COVID-19 MMT policies) | Methadone, buprenorphine | Data collection: Interviews Data collection period: Feb. 2017-Aug. 2020 Approach: Grounded theory |
| 6 | May et al., 2022 | United Kingdom | To "[investigate] the longer-term impacts of the pandemic on the health and wellbeing (including drug-related harms and risk behaviours) and everyday lives of PWID, as well as their experiences of treatment changes from the perspectives of both PWID and service providers" (p. 2). | Service providers and people who use drugs recruited through drug and homelessness charities providing treatment services and healthcare in England and Scotland. | 17 | Methadone, buprenorphine | Data collection: Interviews Data collection period: May-Sept. 2021 Approach: Qualitative description1 |
| 7 | Meteliuk et al., 2020 | Ukraine | "We describe the consequences of this interim guidance [including larger take-home allowances] for managing patients with opioid use disorder (OUD) who are treated with opioid agonist therapies (OAT) in the Ukrainian context." (p. 1). | Chief Narcologists from each administrative region of Ukraine, as well as OAT clients. | NR | NR | Data collection: Weekly calls with Chief Narcologists Data collection period: Jan.-Jun. 2020 Approach: Qualitative description1 |
| 8 | (a) Suen et al., 2022; (b) Wyatt et al., 2022 | United States | "to describe the MOUD treatment experiences of patients and providers at an OTP in San Francisco, California, to inform future research and policy as the US shifts toward recovery from COVID-19." (p. 1148) | MOUD providers and clients at one OTP in San Francisco, California. | 10 | Methadone, buprenorphine | Data collection: Interviews Data collection period: Aug.-Sep. 2020 (for provider participants) Approach: Modified grounded theory |
| 9 | Treitler et al., 2022 | United States | "Study aims were to: 1) Describe MOUD practice changes induced by the pandemic; 2) Understand provider experiences with those practice changes; and 3) Elicit provider perspectives on which (if any) changes should be sustained long-term." (p. 2) | MOUD practitioners drawn from a pool of 12 OTP and 58 OBAT [office-based addiction treatment] providers, including prescribers and behavioral health clinicians. | 20 | Methadone, buprenorphine | Data collection: Interviews Data collection period: Sep.-Nov. 2020 Approach: Pragmatic qualitative inquiry framework; rapid analysis with deductive and inductive coding |
| 10 | Vicknasingam et al., 2021 | Malaysia | "to collect information and qualitative and quantitative data on the potential impact of the MCO [Movement Control Orders] on PWUDs in Malaysia to evaluate how service providers and recipients of these services were adapting and coping during this period in Malaysia." (p. 2) | MMT personnel and clients at MMT programs, HIV clinics, and NGO services in the Malaysian states of Penang, Kelantan, Selangor, and Melaka | 5* (3 contributing data on COVID-19 MMT policies) | Methadone | Data collection: Interviews Data collection period: Mar.-Aug. 2020 Approach: Qualitative description1 |
| 11 | Walters et al., 2022 | United States | "This article examines how the first wave of the COVID-19 pandemic in the US, and the COVID-19 mitigation strategies which were implemented, affected the lives of people who use drugs in relation to MOUD." (p. 1145) | MOUD service providers and people who use drugs in the Northeast region of the U.S. | 15 | Methadone, buprenorphine | Data collection: Interviews Data collection period: Jun. 2020-[not reported] Approach: Qualitative description;1 insider research |
Abbreviations: iOAT, injectable opioid agonist treatment; MMT, methadone maintenance treatment; MOUD, medications for opioid use disorder; NR, not reported; OTP, opioid treatment program; PWID, people who inject drugs; PWUD, people who use drugs; tiOAT, tablet-based injectable opioid agonist treatment
No specific methodology, but qualitative data collection and analysis with hybrid [inductive and deductive] thematic analysis (Hong et al., 2018).
The majority of studies (7) were conducted in the United States. Other study locations included Canada (1), Malaysia (1), Ukraine (1), and the United Kingdom (1). In most studies (7), content on COVID-19 related changes to take-homes focused on methadone. One study reported on providers’ experiences with take-home opioids for unwitnessed consumption in the broader context of risk mitigation prescribing in British Columbia (McCrae et al., 2022/Glegg et al., 2022). While all studies included findings that addressed our research question, there was considerable variation in the depth and richness of usable data.
The overall quality of the included articles was variable. See Supplementary Table 1 for the results of our assessments using the CASP Qualitative Checklist. All studies provided clear statements of research aims and findings and made appropriate use of qualitative methodology. However, we identified limitations related to recruitment strategies and rigor of data analysis in approximately half of the studies. In addition, only one study included adequate consideration of the relationship between researchers and participants. The results of the quality appraisal are reflected in our GRADE-CERQual assessments of methodological limitations, which are incorporated into in the overall level of confidence assigned to each finding.
Findings
We identified three analytical themes (Fig. 2 ) representing providers’ experiences with the relaxation of restrictions on take-home medications for opioid use disorder during the COVID-19 pandemic:
-
•
Theme #1: Initial caution yielding to support.Providers were initially cautious of changes to established practices around take-homes. After observing few or no negative consequences, many came to support increased flexibilities.
-
•
Theme #2: Striving to balance risks.Providers developed new processes to balance risks assumed by clients, risks assumed by society, and risks assumed by providers, including greater use of team-based decision-making. However, a small number of providers remained uneasy with the loss of control over client medication and the reduction in client monitoring.
-
•
Theme #3: Shifting towards person-centered care.Providers found that structural reforms removed impediments to person-centered care. Increased flexibility around take-homes facilitated individualized care, client autonomy, and provider-client relationships.
Fig. 2.
Graphical display of findings.
The first theme describes a common, overarching trajectory in providers’ reactions to increased flexibility around take-homes. The second and third themes focus on the mechanisms underlying this attitudinal shift. Theme #2 explores changes to clinical processes and highlights challenges in balancing risks and sharing control, while Theme #3 describes the perceived impact of increased flexibility around take-homes on client care. Based on our GRADE-CERQual assessments, we had moderate confidence in the evidence supporting each theme. Moderate confidence reflects the belief that “it is likely that the review finding is a reasonable representation of the phenomenon of interest” (Lewin et al., 2018a, Table 3 ). Our assessments are summarized in Table 3. Additional details are shown in Supplementary Table 2.
Table 3.
CERQual evidence profile.
| Review Finding | CERQual Assessment of Confidence in the Evidence | Explanation of CERQual Assessment | Studies Contributing to Review Finding |
|---|---|---|---|
| Theme #1: Initial caution yielding to support. Providers were initially cautious of changes to established practices around take-homes. After observing few or no negative consequences, many came to support increased flexibilities. | Moderate confidence | Moderate concerns regarding relevance; minor concerns regarding methodological limitations and adequacy. | 1, 2, 4–6, 8–10 |
| Theme #2: Striving to balance risks. Providers developed new processes to balance risks assumed by clients, risks assumed by society, and risks assumed by providers, including greater use of team-based decision-making. However, a small number of providers remained uneasy with the loss of control over client medication and the reduction in client monitoring. | Moderate confidence | Moderate concerns regarding relevance; minor concerns regarding methodological limitations and coherence. | 1–10 |
| Theme #3: Shifting towards person-centered care. Providers found that structural reforms removed impediments to person-centered care. Increased flexibility around take-homes facilitated individualized care, client autonomy, and provider-client relationships. | Moderate confidence | Moderate concerns regarding adequacy and relevance; minor concerns regarding coherence. | 1–6, 8, 9, 11 |
Theme #1: Initial caution yielding to support. Providers were initially cautious of changes to established practices around take-homes. After observing few or no negative consequences, many came to support increased flexibilities.
Initial reactions of providers
Providers reacted to the relaxation of restrictions on take-home medication for opioid use disorder with wariness, hesitation, and occasional incredulity (2, 4, 5, 6, 8, 9). Changes to longstanding practices around eligibility for take-homes were approached with caution. Although the need to adapt processes to mitigate the risk of COVID-19 was not widely questioned, providers expressed concerns over modifying their approach to take-homes (2, 4, 5, 6, 8, 9):
One interviewee reported that they use “more take-homes than we were comfortable with” (Northeast [provider], 220 patients) based on guidelines issued by their state, and another described their discomfort with take-home medications as, “Powerful medications in the wrong hand that can kill...very anxiety causing.” (Northeast [provider], 16,000 patients).” ([2], p. 3).
“We did an exercise giving a full month out. Which I think was totally crazy. You don't give someone brand new in treatment 28 bottles of methadone.” (Provider in [5], p. 4)
In a study of opioid treatment programs in the US, Suen et al. (2022) suggest a “culture of conservative dosing” as one factor contributing to providers’ cautious reactions to changes ([8a], p. 1149).
“Our corporate compliance person was just like ‘We've got to give them [take-homes], we've got to give them.’ I'm like ‘I don't feel comfortable.’ So, as the weeks went by, we just kind of went back to our old process” (Provider in [9], p. 5)
Few or no negative consequences observed
Despite providers’ concerns, they observed few or no negative consequences to the expansion of take-homes (2, 8, 9, 10) after developing new processes for determining client eligibility:
“I haven't seen any negativity or repercussions for the ones [clients] that we've selected [to receive take-homes]. There are people that have been wanting take-homes even before COVID that are now pushing it even more and we still are not able to give it to them because of their using choices.” (Provider in [8a], p. 1151)
While a small number of providers noted cases of suspected misuse (2, 6), others were reassured to see limited or no evidence of increased diversion (2, 9) overdose (8, 9), or treatment discontinuation (10):
Whilst reports of serious harm were limited, some instances of misuse were described [. . .] However, service providers reported how these occurrences were largely kept under control by reverting clients to previous prescribing regimes if misuse or safety concerns were identified: “We gradually worked out who needed let's say closer supervision for their own safety, right?” ([6], p. 6)
“We didn't see a whole bunch of people just die. I mean, that certainly was our fear, like, ‘Oh, my God, we're going to give all these people take homes. Within a month, they're all going to be dead.’ That didn't happen.” (Provider in [9], p. 4)
Growing support for relaxed regulations
Providers became more comfortable with the changes when their initial fears failed to materialize. At the same time, many observed that the changes had enabled them to offer better, more person-centered care (see Theme #3). Ultimately, a majority of providers grew to support increased flexibilities around take-homes and wanted them to be extended or made permanent (2, 6, 8, 9, 10):
Interviewed MMT personnel indicated that they would prefer to continue with the relaxed rules for the methadone take-home dosing to continue [sic] even after the COVID-19 restrictions are ultimately lifted. ([10], p. 3)
In light of experience with the COVID-19-era flexibilities, pre-pandemic federal regulatory restrictions were generally seen as overly rigid, burdensome, and unconducive to individualized, person-centered treatment processes. ([9], p. 7)
Providers from safer supply programs in British Columbia presented the reduced risk of death from the toxic drug supply as a compelling reason to continue with flexibilities:
“As long as the black market is contaminated, we don't see why we should stop [. . .] we all discussed together that, yeah, there's no plan on withdrawing the practice when COVID is finished.” (Provider in [1a], p. 7)
Theme #2: Striving to balance risks. Providers developed new processes to balance risks assumed by clients, risks assumed by society, and risks assumed by providers, including greater use of team-based decision-making. However, a small number of providers remained uneasy with the loss of control over client medication and the reduction in client monitoring.
Weighing risks assumed by clients, society, and providers
Providers were forced to make immediate decisions around take-home privileges in a complex risk environment arising from the dual health crises of the opioid epidemic and the COVID-19 pandemic. Though the impact of the former was well documented, the risks of the latter were largely unknown. Providers weighed the perceived risks of take-homes against client susceptibility to COVID-19:
“… It's been an interesting balance to strike regarding making sure that our patients are getting not too much methadone that could potentially be dangerous or diverted, but also ensuring we're reducing everyone's exposure, especially those who are higher risk.” (Provider in [4], p. 4)
This balance was complicated by considerations around personal liability if increased take-homes resulted in medication misuse, client nonadherence, polysubstance use, or overdose (1, 2, 4, 5, 8, 9). In the context of social and physical distancing, some providers were also concerned that limiting client services (e.g., group meetings, daily clinic visits) would further isolate an already-marginalized population (2, 5, 6, 7).
Developing new processes through team-based decision making
To ensure a consistent and supportable approach, providers developed new processes for balancing risks to individual clients, society as a whole, and the providers themselves. The tensions generated by providers’ responsibility to uphold the ethical principle of non-maleficence were addressed by evaluating the “unique risk profiles” of individual clients ([3], p. 2). Factors influencing decisions on client eligibility for take-homes included client history, patterns of substance use, housing status, engagement in treatment, vulnerability to COVID-19, and client preference (3, 8, 9):
Providers articulated a desire to be equitable and provide take-home opportunities. “If I had a client and she was like “Oh, I want take-homes,” and I'm like, “Okay, well, you have a pretty extensive history of continued [opioid] use so why don't we give you a random UA and then we'll take it from there.” So those are more of the cases, is when they request it themselves.” [Provider D]” ([8], p. 1150)
Interdisciplinary consultation featured more prominently in clinical decision-making (1, 3, 6, 8). In the absence of explicit top-down guidance, assessments of client suitability for take-homes drew on the perspectives of multiple providers (e.g., physicians, counselors, recovery workers). This holistic, team-based approach reduced the burden on individual providers and led to more informed treatment decisions (1, 6, 8).
“Because it's a novel program, we're coming up with never-before-seen circumstances or marginal cases that need discussion, so it really helps a lot [to be connected]. And having a variety of different perspectives [. . .]” (Provider in [1a], p. 6)
Unease over reduced monitoring
Though increased flexibility around take-homes motivated providers to come up with creative solutions for continued care provision, others experienced discomfort with toeing an invisible threshold of acceptable risk. Some providers indicated that structure was a critical component of treatment (5, 6, 9) and suggested that less frequent clinic attendance and reduced accountability could destabilize clients. At times, providers made changes to treatment plans that may not have manifested as quickly under different conditions (1, 2, 9):
“[For] some of my clients, I moved a little faster than I wanted to out of daily dosing because I didn't want them, at the height of COVID, to have to be going into pharmacies on a daily basis.” (Provider in [1b], p. 7)
The rapidity of the changes made in response to COVID-19 left some providers challenged by their inability to assess the clinical impact of expanding take-homes (2, 4, 6, 9):
“The chances of abuse of the medication itself is so much higher … we just find it to be a huge liability on our part” (Provider in [4], p. 3)
Unease over loss of control
The relaxation of restrictions on take-homes upset the balance of power between providers and clients, as some providers viewed medication as “the best control that we have” (provider in [9], p. 4). Some providers were concerned that offering take-home doses was “giving [clients] what they want” (provider in [2], p. 3):
“As a contingency management tool, we've lost the ability to grant or remove take-home dosages from patients, either as an incentive for doing better or as something they would lose if they did worse. So, we've definitely lost a lot of tools.” (Provider in [9], p. 2)
The conceptualization of medication as an instrument of control was strongly endorsed by providers who were opposed to continuing with increased flexibilities around take-homes after the pandemic was over (4, 9).
Inconsistent implementation of flexibilities
As a result of these reservations, some clinics only partially exploited the new flexibilities around take-homes (4, 5, 8, 9). In one study, 28% of OTP clinicians reported that their place of work had not made any changes to take-home dosing (4). Other providers heavily restricted access to take-homes or reverted to pre-pandemic practices after the first phase of the pandemic had passed (2, 3, 6, 9):
“And when people say, ‘Why are you putting me [back] in daily supervise? That's a punishment.’ I'd say, ‘No, it's not a punishment, this is for your own good, this is, I hate to say this, this is to keep you safe.’” (Provider in [6], p. 6)
Theme #3: Shifting towards person-centered care. Providers found that structural reforms removed impediments to person-centered care. Increased flexibility around take-homes facilitated individualized care, client autonomy, and provider-client relationships.
Enhanced person-centered care
For providers, increased flexibility around take-homes changed treatment in ways that facilitated person-centered care. Person-centered care, also referred to as patient-centered care, seeks to enhance client engagement by encouraging mutual participation in the creation of treatment (Marchand et al., 2019). The core principles of person-centered care include “the integration of a holistic or bio-psycho-social approach; an individualized focus on clients’ unique needs, goals and preferences; shared power and responsibility between the client and health care provider as with collaborative care or shared decision-making; and a therapeutic alliance” (Marchand et al., 2019, p. 2). Health care providers circled around these principles in describing the impact of increased flexibilities on care provision.
Provider-client relationships
Increased flexibility around take-homes was seen as conducive to better provider-client relationships (1, 5, 8), with one provider describing a sense of relief at being able to offer clients more trust:
“I feel like it's proven in a lot of case[s] that [patients] could handle the methadone and it's been nice to give them more trust in managing their methadone and not having to come in every day. I think it's beneficial for the relationship.” (Provider in [8a], p. 1150)
Providing individualized care
With increased clinical discretion, providers had more freedom to consider individual client circumstances and to make decisions around take-home doses on a case-by-case basis. This individualized approach was seen as reducing treatment burden for clients, particularly those who had difficulty with transportation or had previously struggled to balance treatment with work or other obligations (1, 2, 4, 5, 8).
“[. . .] Someone can come in and grab their meds for the day in the morning and then go to the work for the rest of the day. They don't have to leave work 3 times a day. So, there's the dignity in choice in giving people back their schedule and their life, and also just trusting that those who are accessing it know what's best for them.” (Provider in [1a], p. 6)
Client autonomy
With less frequent clinic visits, providers saw clients able to devote more time and energy to other important areas of their lives (8, 11). In this way, increased flexibilities around take-homes were consistent with a biopsychosocial approach to health.
“The ability to use some real judgment, some human judgment—we've given some people take-home medications that has allowed them, I think, the same flexibility in their life. [...] Like they're doing other things with their time now. They're seeing family or they've chosen to enter some kind of like quarantine pod, and they're doing social things because we were able to sort of fudge it and say like, “We know you don't meet [pre-COVID-19] criteria but that's not the current criteria.” [...] I would say we've seen some positive changes.” (Provider in [8a], p. 1150)
Providers observed that clients who were given more take-home doses demonstrated an increased sense of responsibility (1, 2, 3, 8), with some appearing more motivated to meet treatment goals (2, 9):
“This was the most surprising thing . . . getting the take-home medications that they have not earned, actually motivated them to change that they are now meeting the criteria . . . So that for them it's no longer a pandemic bottle, it is another bottle that I have earned” (Provider in [9], p. 5)
In other cases, clients who felt that take-homes were not helping them exercised their autonomy by voluntarily relinquishing take-home privileges:
While most patients relished greater take-home allowances, some who were judged suitable clinically and given appropriate take-home doses came back into the clinic and returned the medication, demonstrating insight into their own ability to manage these changes. ([2], p. 5)
These occurrences suggest that flexibilities around take-homes allowed providers and clients to adapt treatment to meet individual needs and preferences.
Discussion
This qualitative systematic review synthesized providers’ experiences with relaxing restrictions on take-home doses of opioids prescribed for opioid use disorder during the COVID-19 pandemic. Despite initial concerns, most providers were ultimately supportive of the increased flexibilities. Although providers grappled with considerable uncertainty in developing new processes for making decisions around take-homes, these changes were generally experienced as enabling more individualized, person-centered care. The results of this review are consistent with experiences reported by providers at opioid treatment programs in New York, who found that pandemic-related flexibilities improved person-centred care and advocated for their continuation (Joseph et al., 2021).
This finding is noteworthy because person-centred approaches to health are widely viewed as the standard of care, as evidenced by endorsement from the WHO and numerous professional medical associations (e.g., Australian Medical Association, 2021; Canadian Medical Association, 2008; Institute of Medicine, 2001). Commentators have observed that person-centered care “may be of particular importance when addressing the needs of people who are socially marginalized and lack strong advocates” (Kolind & Hesse, 2017, p. 465) and have identified a need for greater use of person-centred approaches in treatment for substance use disorders (Brothers & Bonn, 2019; Deering et al., 2011; Kolind & Hesse, 2017; Strike & Guta, 2017).
Clients in opioid agonist treatment report interactions with the health care system that leave them feeling demeaned, mistrusted, and judged (Anstice et al., 2009). Deering et al. (2011), in a New Zealand study, found that people who use opioids saw opioid agonist treatment as marked by “paternalistic and inflexible services” (p. 640) rather than consideration of individual treatment needs. Treatment norms in opioid agonist treatment may be partly attributable to negative attitudes towards people who use illicit substances (Lloyd, 2013; Merrill et al., 2002) and the pervasive view of substance use as a moral failure or criminal problem instead of a health issue (Livingston et al., 2012). Moreover, institutional approaches to substance use treatment are enmeshed in colonial and racialized histories (Matsuzaka & Knapp, 2020; Goldenberg et al., 2022) that have lasting effects on the way certain ethnic groups engage with healthcare (Urbanoski, 2017). Person-centered care provides a framework for providers to examine how intersecting client identities can facilitate or complicate client needs and program access.
Interventions that increase the accessibility of safer alternatives to the illicit drug supply, such as those implemented during COVID-19, are urgently needed. Use of opioid agonist treatment is associated with a dramatic reduction in the risk of overdose-related mortality (Sordo et al., 2017), yet a global systematic review found that, on average, just 19% of people who inject opioids were enrolled in opioid agonist treatmentwhere such programs were available (Larney et al., 2017). Retention rates in opioid agonist treatment are low: at 4–6 months, an estimated 57% of buprenorphine clients and 66% of methadone clients remain in treatment (Klimas et al., 2021). Although literature on person-centred care in the context of opioid agonist treatment is limited (Marchand et al., 2019), research suggests that person-centered care is associated with increased use of treatment services for substance use disorder (Park et al., 2020). Notably, increased use of take-homes and other changes to opioid agonist treatment during the COVID-19 pandemic have been associated with improved retention in several recent studies (Cunningham et al., 2022; Farid et al., 2022; Gomes et al., 2022).
When given the flexibility to support individualized decision-making around take-homes, providers in the present review made decisions on take-home eligibility by weighing client needs and preferences against three types of risk: risks to self (i.e., prescriber liability), risks to clients, and risks to society. Of these, risks to society – namely, diversion – appeared to be the primary factor in providers’ initial caution around take-homes. It is generally acknowledged that some selling or sharing of opioid agonist medications will occur regardless of dispensing conditions (Fountain et al., 2000). However, the extent of the resulting harm is unclear and can be expected to vary with time and place (Bell, 2010). Though diversion can have undesirable consequences (Reimer et al., 2016), a growing body of research offers a more nuanced picture of the phenomenon by exploring the characteristics of the market for opioid agonist medication, reasons for selling or sharing doses, and the potential for diverted medication to reduce harm.
Research suggests that the market for opioid agonist medications consists largely of people who already use illicit substances and is driven in part by insufficient access to treatment (Johnson & Richert, 2015; Roche et al., 2008; Schuman-Olivier et al., 2010). Clients share or sell their medication for a variety of reasons, including compassion for people in pain or withdrawal, coercion, and the need to obtain money or resources to meet subsistence needs (Harris & Rhodes, 2013; National Safer Supply Community of Practice, 2022). To the extent that illicit use of opioid agonist medication displaces the use of more harmful drugs, diversion may reduce harm for people who use substances (Harris & Rhodes, 2013). This point is particularly relevant in North America, where the risks posed by substances of known purity and concentration must be weighed against the unprecedented toxicity of the current supply of street drugs contributing to the opioid overdose crisis (Tyndall, 2018).
Our study found that, for a small number of providers, concerns about diversion were a specific manifestation of more general unease with loss of control over clients and the treatment process. While increased flexibilities around take-homes created an opportunity for clients and providers to share power and responsibility in treatment, the perspective of some providers in our review was that treatment demanded a degree of client monitoring (Goldsamt et al., 2021; Treitler et al., 2022) and that the loss of daily clinic visits deprived them of opportunities to “keep an eye on” clients (Treitler et al., 2022, p. 3) through in-person assessment and urine toxicology testing. In these instances, the general shift towards person-centered care was perceived as threatening rather than liberating. While some clients may also prefer daily contact with providers (Notley et al., 2014), increased flexibility in care provision does not remove this option for clients who desire it. Engaging clients in treatment may improve clinical outcomes (Marshall et al., 2022) and respects client autonomy.
Providers who expressed persistent, ongoing discomfort with increased flexibilities around take-home were a minority. Most providers became more comfortable prescribing take-homes after observing little or no increase in diversion, overdose, or other adverse events. This perception is broadly supported by a rapidly growing body of literature on client outcomes (e.g., Amram et al., 2021; Bouck et al., 2022; Corace et al., 2022; Ezie et al., 2022; Garg et al., 2022; Lintzeris et al., 2022). Though the impact of supervised dosing on diversion and client health outcomes has been examined in previous systematic reviews, findings have been inconclusive (Hov et al., 2016; Saulle et al., 2017). In finding that a majority of providers wanted flexibilities to be extended or made permanent, the present review is consistent with surveys finding that 77-79% of methadone and buprenorphine providers wished to retain flexibilities around take-homes beyond the COVID-19 pandemic (Corace et al., 2022; Krawczyk et al., 2022b).
Interestingly, despite observing few negative consequences, feeling enabled to offer more person-centred care, and expressing a desire to retain pandemic-related flexibilities, some providers continued to exercise these flexibilities conservatively (Hunter et al., 2021; Madden et al, 2021; Suen et al., 2022; Treitler et al., 2022). This, too, is consistent with data from recent surveys of treatment providers (Krawczyk et al., 2022b; Levander et al., 2022). Given that providers appeared generally reassured of the safety of changes for clients and the community, concerns over professional and legal liability may have deterred them from taking full advantage of the new flexibilities (Madden et al., 2021).
Strengths and limitations
A strength of this review is the rigor of its methodology, its relevance to current policy issues, and its contribution to a substantial body of research supporting more person-centred approaches in substance use treatment. Our literature search was comprehensive and peer-reviewed. Screening and data extraction were completed in duplicate by two reviewers working independently. We used GRADE-CERQual to make explicit and transparent assessments of our level of confidence in our findings, increasing their potential value to decision-makers.
The review has several limitations. Although we defined our population of interest as health care professionals prescribing or dispensing opioids for opioid use disorder, it was not feasible to limit the review to studies focusing exclusively on this subgroup of health care providers. In six of the included studies, findings represented an amalgamation of views from an assortment of provider types working in settings where opioids were prescribed for opioid use disorder (1, 5, 6, 8, 9, 11). Where possible, we excluded direct quotes from providers who did not prescribe or dispense opioids. However, the conclusions of this review are drawn in part from the experiences of other provider groups, including behavioral health clinicians, treatment coordinators, social workers, and peer workers. We took this limitation into account in the ‘Relevance’ component of our GRADE-CERQual assessments.
We did not conduct subgroup analysis because of limited variation in the regions and treatment types represented in included studies. In addition, we were unable to consider studies that were published in languages other than English, French, Spanish, Portuguese, or Italian. Most of the studies that we retrieved were conducted in the United States and focused on methadone. Our limited ability to explore sources of variation in experience and incorporate studies from other geographical regions may reduce the generalizability of our findings.
Our search strategy deviated from our protocol in that we did not re-run database searches. Nor did we contact subject matter experts to solicit unpublished manuscripts. However, given the recency of the original database searches (Sept. 2022) and citation chaining (Dec. 2022) and the inclusion of preprints in several of the databases that we searched (e.g., Ovid MEDLINE ALL; Embase), we do not believe that this omission would significantly increase retrieval or alter review findings.
Our research team is dedicated to understanding the treatment of substance use disorders and exploring the role of person-centred care in the treatment of addictions. The authors responsible for this review have diverse racial and gender identities and represent various institutions and clinical professions. The authors involved in the coding and initial analysis of the presented data include Caucasian, female and gender-fluid people with academic backgrounds and previous experience working alongside people who use substances. We sought to limit bias in study selection and data extraction by using systematic review methods. We acknowledge that we bring multiple perspectives to this work and that our complex, intersecting identities and experiences influence our engagement with research and our interpretation of the results of this review.
Conclusions
Our findings support the continuation of flexibilities around take-homes and suggest that regulations and policies that reduce flexibility around take-homes stand in active opposition to person-centered care. Treatment practices dictated by fear of diversion come at the cost of humane, accessible care for people with opioid use disorder (Doernberg et al., 2019; Frank et al., 2021a). Person-centered care promotes client autonomy and enhances treatment engagement, therapeutic relationships between clients and providers, and treatment outcomes. However, the success of person-centered care is contingent on providers and clients sharing power, responsibility, and the attendant risks.
Stronger guidance and support from professional regulatory agencies may help increase uptake of flexibilities around take-homes. This may include addressing liability concerns, offering peer support (prescriber-to-prescriber), and securing strong support from governing institutions. Future research should explore providers’ suggestions on how to develop and disseminate useful, context-specific tools to promote person-centered clinical decision-making.
Data availability statement
Templates of data collection forms, data extracted from included studies, data used in analyses, and other materials used in this review are publicly available and can be obtained from the authors upon reasonable request.
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics approval
The authors declare that the work reported herein did not require ethics approval because it did not involve animal or human participation.
Funding sources
This research received funding from the following sources
This review is part of the PORTIA study and is supported by the Canadian Institutes of Health Research Grant [number CIHR 159685] and the Canada Research Chairs Program (MTS; EOJ). The funder had no role in study design, the collection, analysis, and interpretation of data, writing the report, or the decision to submit the article for publication.
Acknowledgements
This review is part of the PORTIA study and is supported by the Canadian Institutes of Health.
Research Grant [number CIHR 159685] and the Canada Research Chairs Program (MTS; EOJ). We thank Jacob Lee for assistance in screening and data reconciliation, Tianna Magel for assistance in piloting the data extraction form, and Ursula Ellis for peer-review of the search strategy. The authors respectfully acknowledge that this manuscript was created on the unceded, traditional, and contemporary territories of the Coast Salish Peoples, including the territories of xʷməθkʷəýəm (Musqueam), Skwxwú7mesh (Squamish), səliĺilw̓ətaʔɬ (Tsleil-Waututh), and Snuneymuxw Nations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2023.104058.
Appendix. Supplementary materials
References
- Ahmad F.B., Cisewski J.A., Rossen L.M., Sutton P. National Center for Health Statistics; 2023. Provisional drug overdose death counts. [Google Scholar]; Retrieved from https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed February 16, 2023.
- Amram O., Amiri S., Panwala V., Lutz R., Joudrey P.J., Socias E. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. The American Journal of Drug and Alcohol Abuse. 2021;47(6):722–729. doi: 10.1080/00952990.2021.1979991. [DOI] [PubMed] [Google Scholar]
- Anstice S., Strike C.J., Brands B. Supervised methadone consumption: Client issues and stigma. Substance Use & Misuse. 2009;44(6):794–808. doi: 10.1080/10826080802483936. [DOI] [PubMed] [Google Scholar]
- ASAM COVID-19 Task Force. (2020). Access to buprenorphine in office-based settings. Retrieved from https://www.asam.org/quality-care/clinical-recommendations/covid/access-to-bureniphrine-in-office-based-settings. Accessed February 16, 2023.
- Australian Medical Association. (2021). Vision for Australia's health. Retrieved from https://www.ama.com.au/vision-for-australias-health. Accessed February 16, 2023.
- Bardwell G., Wood E., Brar R. Fentanyl assisted treatment: A possible role in the opioid overdose epidemic? Substance Abuse Treatment, Prevention, and Policy. 2019;14(1):50. doi: 10.1186/s13011-019-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. The global diversion of pharmaceutical drugs. Addiction. 2010;105(9):1531–1537. doi: 10.1111/j.1360-0443.2010.03014.x. [DOI] [PubMed] [Google Scholar]
- Bouck Z., Scheim A.I., Gomes T., Ling V., Caudarella A., Werb D. Evaluating interventions to facilitate opioid agonist treatment access among people who inject drugs in Toronto, Ontario during COVID-19 pandemic restrictions. International Journal of Drug Policy. 2022;104 doi: 10.1016/j.drugpo.2022.103680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia Centre on Substance Use. (2020a). Risk mitigation in the context of dual public health emergencies, version 1.5.https://www.bccsu.ca/wp-content/uploads/2020/04/Risk-Mitigation-in-the-Context-of-Dual-Public-Health-Emergencies-v1.5.pdf.
- British Columbia Centre on Substance Use. (2020b). Information for opioid agonist treatment prescribers and pharmacists (March 31, 2020). https://www.bccsu.ca/wp-content/uploads/2020/03/COVID-19-Bulletin-Mar-31-2020.pdf.
- British Columbia Centre on Substance Use. (2021). Clinical bulletin: Benzodiazepines and opioids. https://www.bccsu.ca/wp-content/uploads/2021/06/Bulletin-Benzos-and-Opioids.pdf.
- British Columbia Centre on Substance Use . BCCSU; 2023. Prescribed safer supply protocols: Fentanyl patch.https://www.bccsu.ca/clinical-care-guidance/prescribed-safer-supply/fentanyl-patch/ Retrieved from. Accessed February 16, 2023. [Google Scholar]
- Brothers T.D., Bonn M. Patient-centred care in opioid agonist treatment could improve outcomes. CMAJ : Canadian Medical Association Journal. 2019;191(17):E460–E461. doi: 10.1503/cmaj.190430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers T.D., Leaman M., Bonn M., Lewer D., Atkinson J., Fraser J., Gillis A., Gniewek M., Hawker L., Hayman H., Jorna P., Martell D., O'Donnell T., Rivers-Bowerman H., Genge L. Evaluation of an emergency safe supply drugs and managed alcohol program in COVID-19 isolation hotel shelters for people experiencing homelessness. Drug and Alcohol Dependence. 2022;235 doi: 10.1016/j.drugalcdep.2022.109440. (ebs, 7513587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADTH. (2019, April). Grey matters: A practical tool for searching health-related grey literature | CADTH. https://www.cadth.ca/grey-matters-practical-tool-searching-health-related-grey-literature-0.
- Canadian Medical Association Achieving patient-centred collaborative care. CMA Policybase. https://policybase.cma.ca/viewer?file=%2Fmedia%2FPolicyPDF%2FPD08-02.pdf#page=1
- Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Current Opinion in Psychiatry. 2021;34(4):344. doi: 10.1097/YCO.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin C.J., Garside R., Wainwright M., Munthe-Kaas H., Glenton C., Bohren M.A., Carlsen B., Tunçalp Ö., Noyes J., Booth A., Rashidian A., Flottorp S., Lewin S. Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 4: How to assess coherence. Implementation Science : IS. 2018;13(Suppl 1) doi: 10.1186/s13012-017-0691-8. 13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corace K., Suschinsky K., Wyman J., Leece P., Cragg S., Konefal S., Pana P., Barrass S., Porath A., Hutton B. Evaluating how has care been affected by the Ontario COVID-19 Opioid Agonist Treatment Guidance: Patients’ and prescribers’ experiences with changes in unsupervised dosing. International Journal of Drug Policy. 2022;102 doi: 10.1016/j.drugpo.2021.103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme. (2018). CASP qualitative checklist. https://casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018_fillable_form.pdf.
- Cunningham C.O., Khalid L., Deng Y., Torres-Lockhart K., Masyukova M., Thomas S., Zhang C., Lu T. A comparison of office-based buprenorphine treatment outcomes in bronx community clinics before versus during the COVID-19 pandemic. Journal of Substance Abuse Treatment. 2022;135 doi: 10.1016/j.jsat.2021.108641. 108641-108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Departament de Salut . Barcelona: Departament de Salut; 2020. Coronavirus SARS-CoV-2: Recomanacions per reduir el risc de contagi de la COVID-19 per a centres d'atenció i seguiment de les drogodependències (CAS)https://scientiasalut.gencat.cat/handle/11351/4799 March 12. Retrieved from. Accessed February 15, 2023. [Google Scholar]
- Department of Health and Social Care . GOV.UK.; 2021. [Withdrawn] COVID-19: Guidance for commissioners and providers of services for people who use drugs or alcohol.https://www.gov.uk/government/publications/covid-19-guidance-for-commissioners-and-providers-of-services-for-people-who-use-drugs-or-alcohol/covid-19-guidance-for-commissioners-and-providers-of-services-for-people-who-use-drugs-or-alcohol Retrieved from. . Accessed on February 16, 2023. [Google Scholar]
- Deering D.E.A., Sheridan J., Sellman J.D., Adamson S.J., Pooley S., Robertson R., Henderson C. Consumer and treatment provider perspectives on reducing barriers to opioid substitution treatment and improving treatment attractiveness. Addictive Behaviors. 2011;36(6):636–642. doi: 10.1016/j.addbeh.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Doernberg M., Krawczyk N., Agus D., Fingerhood M. Demystifying buprenorphine misuse: Has fear of diversion gotten in the way of addressing the opioid crisis? Substance Abuse. 2019;40(2):148–153. doi: 10.1080/08897077.2019.1572052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezie C., Badolato R., Rockas M., Nafiz R., Sands B., Wolkin A., Farahmand P. COVID 19 and the opioid epidemic: An analysis of clinical outcomes during COVID 19. Substance Abuse. 2022;16 doi: 10.1177/11782218221085590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoudian A., Baldacchino A., Clark N., Gerra G., Ekhtiari H., Dom G., Mokri A., Sadeghi M., Nematollahi P., Demasi M., Schütz C.G., Hash-emian S.M., Tabarsi P., Galea-Singer S., Carrà G., Clausen T., Kouimtsidis C., Tolomeo S., Radfar S.R., Razaghi E.M. COVID-19 and substance use disorders: Recommendations to a comprehensive healthcare response. An International Society of Addiction Medicine Practice and Policy Interest Group position paper. Basic and Clinical Neuroscience. 2020;11(2):133–150. doi: 10.32598/bcn.11.covid19.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid M.S., Rahman D.L., Islam D.S., Chowdhury E.I. Take home dose of methadone: New arena for OST adherence during COVID-19 in Bangladesh. [Poster exhibition abstract] Journal of the International Aids Society. 2022;25 150-150. [Google Scholar]
- Ferri M., Davoli M., Perucci C.A. Heroin maintenance for chronic heroin-dependent individuals. The Cochrane Database of Systematic Reviews. 2011;12 doi: 10.1002/14651858.CD003410.pub4. CD003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J., Strang J., Gossop M., Farrell M., Griffiths P. Diversion of prescribed drugs by drug users in treatment: Analysis of the UK market and new data from London. Addiction. 2000;95(3):393–406. doi: 10.1046/j.1360-0443.2000.95339310.x. [DOI] [PubMed] [Google Scholar]
- Frank D., Mateu-Gelabert P., Perlman D.C., Walters S.M., Curran L., Guarino H. “It's like 'liquid handcuffs”: The effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm Reduction Journal. 2021;18(1):88. doi: 10.1186/s12954-021-00535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. A chance to do it better: Methadone maintenance treatment in the age of Covid-19. Journal of Substance Abuse Treatment. 2021;123 doi: 10.1016/j.jsat.2020.108246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Kitchen S.A., Men S., Campbell T.J., Bozinoff N., Tadrous M., Antoniou T., Wyman J., Werb D., Munro C., Gomes T. Impact of the COVID-19 pandemic on the prevalence of opioid agonist therapy discontinuation in Ontario, Canada: A population-based time series analysis. Drug and Alcohol Dependence. 2022;236(7513587) doi: 10.1016/j.drugalcdep.2022.109459. ebs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glegg S., McCrae K., Kolla G., Touesnard N., Turnbull J., Brothers T.D., Brar R., Sutherland C., Le Foll B., Sereda A., Goyer M.-E., Rai N., Bernstein S., Fairbairn N. COVID just kind of opened a can of whoop-ass”: The rapid growth of safer supply prescribing during the pandemic documented through an environmental scan of addiction and harm reduction services in Canada. International Journal of Drug Policy. 2022;106(9014759) doi: 10.1016/j.drugpo.2022.103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenton C., Carlsen B., Lewin S., Munthe-Kaas H., Colvin C.J., Tunçalp Ö., Bohren M.A., Noyes J., Booth A., Garside R., Rashidian A., Flottorp S., Wainwright M. Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 5: How to assess adequacy of data. Implementation Science : IS. 2018;13(Suppl 1) doi: 10.1186/s13012-017-0692-7. 14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg S.M., Perry C., Watt S., Bingham B., Braschel M., Shannon K. Violence, policing, and systemic racism as structural barriers to substance use treatment amongst women sex workers who use drugs: Findings of a community-based cohort in Vancouver, Canada (2010-2019) Drug and Alcohol Dependence. 2022;237 doi: 10.1016/j.drugalcdep.2022.109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsamt L.A., Rosenblum A., Appel P., Paris P., Nazia N. The impact of COVID-19 on opioid treatment programs in the United States. Drug and Alcohol Dependence. 2021;228(7513587) doi: 10.1016/j.drugalcdep.2021.109049. ebs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T., Campbell T.J., Kitchen S.A., Garg R., Bozinoff N., Men S., Tadrous M., Munro C., Antoniou T., Werb D., Wyman J. Association between increased dispensing of opioid agonist therapy take-home doses and opioid overdose and treatment interruption and discontinuation. JAMA : The Journal of the American Medical Association. 2022;327(9):846–855. doi: 10.1001/jama.2022.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu, P. (2020, September 1). Letter from the Minister of Health regarding treatment and safer supply [Education and awareness]. Retrieved from https://www.canada.ca/en/health-canada/services/substance-use/minister-letter-treatment-safer-supply.html. Accessed February 15, 2023.
- Harris M., Rhodes T. Methadone diversion as a protective strategy: The harm reduction potential of ‘generous constraints. International Journal of Drug Policy. 2013;24(6):e43–e50. doi: 10.1016/j.drugpo.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Hatch-Maillette M.A., Peavy K.M., Tsui J.I., Banta-Green C.J., Woolworth S., Grekin P. Re-thinking patient stability for methadone in opioid treatment programs during a global pandemic: Provider perspectives. Journal of Substance Abuse Treatment. 2021;124(kai, 8500909) doi: 10.1016/j.jsat.2020.108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. (2021). Safer supply: Prescribed medications as a safer alternative to toxic illegal drugs [Service description]. Retrieved from https://www.canada.ca/en/health-canada/services/opioids/responding-canada-opioid-crisis/safer-supply.html. Accessed February 15, 2023.
- Hov, L., Mosdøl, A., Ding, Y., Strømme, H., & Vist, G. E. (2016). Unsupervised intake of medicines for individuals in opioid maintenance. Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH). [PubMed]
- Hunter S.B., Dopp A.R., Ober A.J., Uscher-Pines L. Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: A qualitative study. Journal of Substance Abuse Treatment. 2021;124(kai, 8500909) doi: 10.1016/j.jsat.2021.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Quality of Health Care in America . National Academies Press (US); 2001. Crossing the quality chasm: A new health system for the 21st century.http://www.ncbi.nlm.nih.gov/books/NBK222274/ [PubMed] [Google Scholar]
- Jin H., Marshall B.D.L., Degenhardt L., Strang J., Hickman M., Fiellin D.A., Ali R., Bruneau J., Larney S. Global opioid agonist treatment: A review of clinical practices by country. Addiction. 2020;115(12):2243–2254. doi: 10.1111/add.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: The importance of patients’ attitudes and norms. Journal of Substance Abuse Treatment. 2015;54:50–55. doi: 10.1016/j.jsat.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Joseph G., Torres-Lockhart K., Stein M.R., Mund P.A., Nahvi S. Reimagining patient-centered care in opioid treatment programs: Lessons from the Bronx during COVID-19. Journal of Substance Abuse Treatment. 2021;122 doi: 10.1016/j.jsat.2020.108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas J., Hamilton M.-A., Gorfinkel L., Adam A., Cullen W., Wood E. Retention in opioid agonist treatment: A rapid review and meta-analysis comparing observational studies and randomized controlled trials. Systematic Reviews. 2021;10(1):216. doi: 10.1186/s13643-021-01764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind T., Hesse M. Patient-centred care—Perhaps the future of substance abuse treatment. Addiction. 2017;112(3):465–466. doi: 10.1111/add.13673. [DOI] [PubMed] [Google Scholar]
- Krawczyk N., Fingerhood M.I., Agus D. Lessons from COVID 19: Are we finally ready to make opioid treatment accessible? Journal of Substance Abuse Treatment. 2020;117 doi: 10.1016/j.jsat.2020.108074. 108074-108074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Rivera B.D., Levin E., Dooling B.C.E. Synthesizing evidence on the impacts of COVID-19 regulatory changes on methadone treatment for opioid use disorder: Implications for policy. The Lancet Public Health. 2022;8(3) doi: 10.1016/S2468-2667(23)00023-3. e238-e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Maniates H., Hulsey E., Smith J.S., DiDomenico E., Stuart E.A., Saloner B., Bandara S. Shifting medication treatment practices in the COVID-19 pandemic: A statewide survey of Pennsylvania opioid treatment programs. Journal of Addiction Medicine. 2022;16(6):645–652. doi: 10.1097/ADM.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, V., Sankey, C., Wyman, J., & Zhang, M. (2020). COVID-19 opioid agonist treatment guidance (March 22, 2020). https://www.camh.ca/-/media/files/covid-19-modifications-to-opioid-agonist-treatment-delivery-pdf.pdf.
- Larance B., Degenhardt L., Lintzeris N., Winstock A., Mattick R. Definitions related to the use of pharmaceutical opioids: Extramedical use, diversion, non-adherence and aberrant medication-related behaviours. Drug and Alcohol Review. 2011;30(3):236–245. doi: 10.1111/j.1465-3362.2010.00283.x. [DOI] [PubMed] [Google Scholar]
- Larney S., Peacock A., Leung J., Colledge S., Hickman M., Vickerman P., Grebely J., Dumchev K.V., Griffiths P., Hines L., Cunningham E.B., Mattick R.P., Lynskey M., Marsden J., Strang J., Degenhardt L. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: A systematic review. The Lancet Global Health. 2017;5(12):e1208–e1220. doi: 10.1016/S2214-109X(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander X.A., Pytell J.D., Stoller K.B., Korthuis P.T., Chander G. COVID-19-related policy changes for methadone take-home dosing: A multistate survey of opioid treatment program leadership. Substance Abuse. 2022;43(1):633–639. doi: 10.1080/08897077.2021.1986768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin S., Booth A., Glenton C., Munthe-Kaas H., Rashidian A., Wainwright M., Bohren M.A., Tunçalp Ö., Colvin C.J., Garside R., Carlsen B., Langlois E.V., Noyes J. Applying GRADE-CERQual to qualitative evidence synthesis findings: Introduction to the series. Implementation Science. 2018;13(1):2. doi: 10.1186/s13012-017-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin S., Bohren M.A., Rashidian A., Munthe-Kaas H., Glenton C., Colvin C.J., Garside R., Noyes J., Booth A., Tunçalp Ö., Wainwright M., Flottorp S., Tucker J.D., Carlsen B. Applying GRADE-CERQual to qualitative evidence synthesis findings: paper 2: how to make an overall CERQual assessment of confidence and create a Summary of Qualitative Findings table. Implementation Science. 2018;13(S1):10. doi: 10.1186/s13012-017-0689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N., Deacon R.M., Hayes V., Cowan T., Mills L., Parvaresh L., Harvey Dodds L., Jansen L., Dojcinovic R., Leung M.C., Demirkol A., Finch T., Mammen K. Opioid agonist treatment and patient outcomes during the COVID -19 pandemic in south east Sydney, Australia. Drug and Alcohol Review. 2022;41(5):1009–1019. doi: 10.1111/dar.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris, N., Hayes, D. V., FAChAM, F., Arunogiri, D. S., & FAChAM, A. D. (2020). Interim guidance for the delivery of medication assisted treatment of opioid dependence in response to COVID-19: A national response. Retrieved from https://www.racp.edu.au/docs/default-source/news-and-events/covid-19/interim-guidance-delivery-of-medication-assisted-treatment-of-opiod-dependence-covid-19.pdf?sfvrsn=e36eeb1a_4. Accessed on February 16, 2023.
- Livingston J.D., Milne T., Fang M.L., Amari E. The effectiveness of interventions for reducing stigma related to substance use disorders: A systematic review. Addiction. 2012;107(1):39–50. doi: 10.1111/j.1360-0443.2011.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. The stigmatization of problem drug users: A narrative literature review. Drugs: Education, Prevention & Policy. 2013;20(2):85–95. doi: 10.3109/09687637.2012.743506. [DOI] [Google Scholar]
- Madden A., Lea T., Bath N., Winstock A.R. Satisfaction guaranteed? What clients on methadone and buprenorphine think about their treatment. Drug and Alcohol Review. 2008;27(6):671–678. doi: 10.1080/09595230801935706. [DOI] [PubMed] [Google Scholar]
- Madden E.F., Christian B.T., Lagisetty P.A., Ray B.R., Sulzer S.H. Treatment provider perceptions of take-home methadone regulation before and during COVID-19. Drug and Alcohol Dependence. 2021;228(7513587) doi: 10.1016/j.drugalcdep.2021.109100. ebs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand K., Beaumont S., Westfall J., MacDonald S., Harrison S., Marsh D.C., Schechter M.T., Oviedo-Joekes E. Conceptualizing patient-centered care for substance use disorder treatment: Findings from a systematic scoping review. Substance Abuse Treatment, Prevention, and Policy. 2019;14(1):37. doi: 10.1186/s13011-019-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall T., Hancock M., Kinnard E.N., Olson K., Abba-Aji A., Rittenbach K., Stea J.N., Tanguay R., Vohra S. Treatment options and shared decision-making in the treatment of opioid use disorder: A scoping review. Journal of Substance Abuse Treatment. 2022;135 doi: 10.1016/j.jsat.2021.108646. [DOI] [PubMed] [Google Scholar]
- Matsuzaka S., Knapp M. Anti-racism and substance use treatment: Addiction does not discriminate, but do we? Journal of Ethnicity in Substance Abuse. 2020;19(4):567–593. doi: 10.1080/15332640.2018.1548323. [DOI] [PubMed] [Google Scholar]
- May T., Dawes J., Fancourt D., Burton A. A qualitative study exploring the impact of the COVID-19 pandemic on People Who Inject Drugs (PWID) and drug service provision in the UK: PWID and service provider perspectives. International Journal of Drug Policy. 2022;106(9014759) doi: 10.1016/j.drugpo.2022.103752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae K., Glegg S., Goyer M.-E., Le Foll B., Brar R., Sutherland C., Fairbairn N. The changing landscape of pharmaceutical alternatives to the unregulated drug supply during COVID-19. Harm Reduction Journal. 2022;19(1):77. doi: 10.1186/s12954-022-00657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J.O., Rhodes L.A., Deyo R.A., Marlatt G.A., Bradley K.A. Mutual mistrust in the medical care of drug users. Journal of General Internal Medicine. 2002;17(5):327–333. doi: 10.1046/j.1525-1497.2002.10625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteliuk A., Galvez de Leon S.J., Madden L.M., Pykalo I., Fomenko T., Filippovych M., Farnum S.O., Dvoryak S., Islam Z.M., Altice F.L. Rapid transitional response to the covid-19 pandemic by opioid agonist treatment programs in Ukraine. Journal of Substance Abuse Treatment. 2020;121 doi: 10.1016/j.jsat.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales K.B., Park J.N., Glick J.L., Rouhani S., Green T.C., Sherman S.G. Preference for drugs containing fentanyl from a cross-sectional survey of people who use illicit opioids in three United States cities. Drug and Alcohol Dependence. 2019;204 doi: 10.1016/j.drugalcdep.2019.107547. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas H., Bohren M.A., Glenton C., Lewin S., Noyes J., Tunçalp Ö., Booth A., Garside R., Colvin C.J., Wainwright M., Rashidian A., Flottorp S., Carlsen B. Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 3: How to assess methodological limitations. Implementation Science : IS. 2018;13(Suppl 1) doi: 10.1186/s13012-017-0690-9. 9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Safer Supply Community of Practice . 2022. Reframing diversion for health care providers: Frequently asked questions.https://www.nss-aps.ca/sites/default/files/resources/ReframingDiversionForHealthCareProviders.pdf [Google Scholar]
- Notley C., Holland R., Maskrey V., Nagar J., Kouimtsidis C. Regaining control: The patient experience of supervised compared with unsupervised consumption in opiate substitution treatment. Drug and Alcohol Review. 2014;33(1):64–70. doi: 10.1111/dar.12079. [DOI] [PubMed] [Google Scholar]
- Noyes J., Booth A., Cargo M., Flemming K., Harden A., Harris J., Garside R., Hannes K., Pantoja T., Thomas J. In: Cochrane handbook for systematic reviews of interventions, version 6.3 (updated February 2022) Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane; 2022. Chapter 21: Qualitative evidence.www.training.cochrane.org/handbook Eds. Available at. [Google Scholar]
- Noyes J., Booth A., Flemming K., Garside R., Harden A., Lewin S., Pantoja T., Hannes K., Cargo M., Thomas J. Cochrane Qualitative and Implementation Methods Group guidance series—paper 3: Methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. Journal of Clinical Epidemiology. 2018;97:49–58. doi: 10.1016/j.jclinepi.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Noyes J., Booth A., Lewin S., Carlsen B., Glenton C., Colvin C.J., Garside R., Bohren M.A., Rashidian A., Wainwright M., Tunςalp Ö., Chandler J., Flottorp S., Pantoja T., Tucker J.D., Munthe-Kaas H. Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 6: How to assess relevance of the data. Implementation Science : IS. 2018;13(Suppl 1) doi: 10.1186/s13012-017-0693-6. 4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olding M., Ivsins A., Mayer S., Betsos A., Boyd J., Sutherland C., Culbertson C., Kerr T., McNeil R. A low-barrier and comprehensive community-based harm-reduction site in Vancouver, Canada. American Journal of Public Health. 2020;110(6):833–835. doi: 10.2105/AJPH.2020.305612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E., Guh D., Brissette S., Marchand K., MacDonald S., Lock K. Hydromorphone compared with diacetylmorphine for long-term opioid dependence: A randomized clinical trial. JAMA Psychiatry. 2016;73(5):447–455. doi: 10.1001/jamapsychiatry.2016.0109. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S.…Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Systematic Reviews. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.(Ethan), Mosley J.E., Grogan C.M., Pollack H.A., Humphreys K., D'Aunno T., Friedmann P.D. Patient-centered care's relationship with substance use disorder treatment utilization. Journal of Substance Abuse Treatment. 2020;118 doi: 10.1016/j.jsat.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L., Minozzi S., Reed J., Vickerman P., Hagan H., French C., Jordan A., Degenhardt L., Hope V., Hutchinson S., Maher L., Palmateer N., Taylor A., Bruneau J., Hickman M. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: Findings from a Cochrane Review and meta-analysis. Addiction. 2018;113(3):545–563. doi: 10.1111/add.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QSR International Pty Ltd. (2020). NVivo Release 1 (released in March 2020). https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home.
- Radfar S.R., De Jong C.A.J., Farhoudian A., Ebrahimi M., Rafei P., Vahidi M., Yunesian M., Kouimtsidis C., Arunogiri S., Massah O., Deylamizadeh A., Brady K.T., Busse A., ISAM-PPIG Global Survey Consortium. Potenza M.N., Ekhtiari H., Baldacchino A.M., Abagiu A.O., Abouna F.D.N.…Zonoozi A.K. Reorganization of substance use treatment and harm reduction services during the COVID-19 pandemic: A global survey. Frontiers in Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.639393. https://www.frontiersin.org/articles/10.3389/fpsyt.2021.639393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J., Wright N., Somaini L., Roncero C., Maremmani I., McKeganey N., Littlewood R., Krajci P., Alho H., D'Agnone O. The impact of misuse and diversion of opioid substitution treatment medicines: Evidence review and expert consensus. European Addiction Research. 2016;22(2):99–106. doi: 10.1159/000438988. [DOI] [PubMed] [Google Scholar]
- Ritter A., Di Natale R. The relationship between take-away methadone policies and methadone diversion. Drug and Alcohol Review. 2005;24(4):347–352. doi: 10.1080/09595230500263939. [DOI] [PubMed] [Google Scholar]
- Roche A., McCabe S., Smyth B.P. Illicit methadone use and abuse in young people accessing treatment for opiate dependence. European Addiction Research. 2008;14(4):219–225. doi: 10.1159/000149631. [DOI] [PubMed] [Google Scholar]
- Russell C., Lange S., Kouyoumdjian F., Butler A., Ali F. Opioid agonist treatment take-home doses (‘carries’): Are current guidelines resulting in low treatment coverage among high-risk populations in Canada and the USA? Harm Reduction Journal. 2022;19(1):89. doi: 10.1186/s12954-022-00671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulle R., Vecchi S., Gowing L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database of Systematic Reviews. 2017;(4):2017. doi: 10.1002/14651858.cd011983.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman-Olivier Z., Albanese M., Nelson S.E., Roland L., Puopolo F., Klinker L., Shaffer H.J. Self-treatment: Illicit buprenorphine use by opioid-dependent treatment seekers. Journal of Substance Abuse Treatment. 2010;39(1):41–50. doi: 10.1016/j.jsat.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Strike C.J., Guta A. Patient-centred care and patient engagement to inform the use of psychosocial interventions with opioid substitution treatment: Another path for Day & Mitcheson to follow. Addiction. 2017;112(8):1338–1339. doi: 10.1111/add.13708. [DOI] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L., Ferri M., Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. British Medical Journal. 2017:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Special Advisory Committee on the Epidemic of Opioid Overdoses . Public Health Agency of Canada; 2022. Apparent opioid and stimulant toxicity deaths: Surveillance of opioid- and stimulant-related harms in Canada.https://health-infobase.canada.ca/src/doc/SRHD/Update_Deaths_2022-09.pdf [Google Scholar]
- SSAM. (2020). Empfehlungen zum Umgang mit der COVID-19-Pandemie von Fachpersonen aus dem Suchtbereich. https://www.ssam-sapp.ch/securedl/sdl-eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJpYXQiOjE2NzQ3NTcyMDcsImV4cCI6MTY3NDg0NzIwNSwidXNlciI6MCwiZ3JvdXBzIjpbMCwtMV0sImZpbGUiOiJmaWxlYWRtaW5cL1NTQU1cL3VzZXJfdXBsb2FkXC9FbXBmZWhsdW5nZW5cL2VtcGZlaGx1bmdlbl96dW1fdW1nYW5nX21pdF9kZXJfY292aWQtMTlfcGFuZGVtaWVfdm9uX2ZhY2hwZXJzb25lbl9hdXNfZGVtX3N1Y2h0YmVyZWljaF8wMl8wNF8yMDIwLnBkZiIsInBhZ2UiOjEzMTF9.JNw_CEfCsX0gXnfYa7LWxruM025MM193dHPhOZ-hdzA/empfehlungen_zum_umgang_mit_der_covid-19_pandemie_von_fachpersonen_aus_dem_suchtbereich_02_04_2020.pdf.
- Substance Abuse and Mental Health Services Administration. (2015). Federal guidelines for opioid treatment programs. https://store.samhsa.gov/sites/default/files/d7/priv/pep15-fedguideotp.pdf.
- Substance Abuse and Mental Health Services Administration. (2020). Methadone take-home flexibilities extension guidance. Retrieved from https://www.samhsa.gov/medications-substance-use-disorders/statutes-regulations-guidelines/methadone-guidance. Accessed January 26, 2023.
- Suen L.W., Castellanos S., Joshi N., Satterwhite S., Knight K.R. The idea is to help people achieve greater success and liberty”: A qualitative study of expanded methadone take-home access in opioid use disorder treatment. Substance Abuse. 2022;43(1):1143–1150. doi: 10.1080/08897077.2022.2060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Medical Research Methodology. 2008;8(1):45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias S., Grant C.J., Laing R., Arredondo J., Lysyshyn M., Buxton J., Tupper K.W., Wood E., Ti L. Time-series analysis of fentanyl concentration in the unregulated opioid drug supply in a Canadian setting. American Journal of Epidemiology. 2022;191(2):241–247. doi: 10.1093/aje/kwab129. [DOI] [PubMed] [Google Scholar]
- Treitler P.C., Bowden C.F., Lloyd J., Enich M., Nyaku A.N., Crystal S. Perspectives of opioid use disorder treatment providers during COVID-19: Adapting to flexibilities and sustaining reforms. Journal of Substance Abuse Treatment. 2022;132(kai, 8500909) doi: 10.1016/j.jsat.2021.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall M. An emergency response to the opioid overdose crisis in Canada: A regulated opioid distribution program. CMAJ : Canadian Medical Association Journal. 2018;190(2):E35–E36. doi: 10.1503/cmaj.171060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanoski K.A. Need for equity in treatment of substance use among indigenous people in Canada. CMAJ : Canadian Medical Association Journal. 2017;189(44):E1350–E1351. doi: 10.1503/cmaj.171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio S., Ramella R., Drago A., Carraro D., Littlewood R., Somaini L. COVID19 pandemic and people with opioid use disorder: Innovation to reduce risk. Psychiatry Research. 2020;289 doi: 10.1016/j.psychres.2020.113047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veritas Health Innovation. (2021). Covidence systematic review software. www.covidence.org.
- Vicknasingam B., Mohd Salleh N.A., Chooi W.-T., Singh D., Mohd Zaharim N., Kamarulzaman A., Chawarski M.C. COVID-19 impact on healthcare and supportive services for people who use drugs (PWUDs) in Malaysia. Frontiers in Psychiatry. 2021;12(101545006) doi: 10.3389/fpsyt.2021.630730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters S.M., Perlman D.C., Guarino H., Mateu-Gelabert P., Frank D. Lessons from the first wave of COVID-19 for improved medications for opioid use disorder (MOUD) treatment: Benefits of easier access, extended take homes, and new delivery modalities. Substance Use & Misuse. 2022;57(7):1144–1153. doi: 10.1080/10826084.2022.2064509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020, May 18). Overview of public health and social measures in the context of COVID-19. Retrieved from https://apps.who.int/iris/rest/bitstreams/1278127/retrieve. Accessed March 28, 2023.
- World Health Organization. (2021, August 4). Opioid overdose. Retrieved from https://www.who.int/news-room/fact-sheets/detail/opioid-overdose. Accessed January 26, 2023.
- Wyatt J.P., PhD, Suen L.W., MD, MAS, Coe W.H., MD, MPH, Adams Z.M., MA, Gandhi M., MSN, Batchelor H.M., BS, Castellanos S., MA, Joshi N., MS, Satterwhite S., MD, PhD, Perez-Rodríguez R., PsyD, Rodríguez-Guerra E., PhD, Albizu-Garcia C.E., MD, Knight K.R., PhD, Jordan A., MD, PhD, MPH Federal and state regulatory changes to methadone take-home doses: Impact of sociostructural factors. American Journal of Public Health. 2022;112:S143–S146. doi: 10.2105/AJPH.2022.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Kolla G., McCormack D., Campbell T., Leece P., Strike C., Srivastava A., Antoniou T., Bayoumi A.M., Gomes T. Characterizing safer supply prescribing of immediate release hydromorphone for individuals with opioid use disorder across Ontario, Canada. International Journal of Drug Policy. 2022;102(9014759) doi: 10.1016/j.drugpo.2022.103601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Templates of data collection forms, data extracted from included studies, data used in analyses, and other materials used in this review are publicly available and can be obtained from the authors upon reasonable request.