Abstract
The formation of adherent multilayered biofilms embedded into a glycocalyx represents an essential factor in the pathogenesis of Staphylococcus epidermidis biomaterial-related infections. Using biofilm-producing S. epidermidis 1457 and transposon Tn917 carried on plasmid pTV1ts, we isolated nine isogenic biofilm-negative transposon mutants. Transduction by S. epidermidis phage 71 was used to prove the genetic linkage of transposon insertions and altered phenotypes. Mapping of the different transposon insertions by Southern hybridization and pulsed-field gel electrophoresis indicated that these were inserted in four unlinked genetic loci. According to their phenotypes, including quantitative differences in biofilm production in different growth media, in the amount of the polysaccharide intercellular adhesin (PIA) produced, in the hemagglutination titers, and in the altered colony morphology, the mutants could be separated into four phenotypic classes corresponding with the genetic classes. Synthesis of PIA was not detectable with class I and II mutants, whereas the amount of PIA produced reflected the residual degree of biofilm production of class III and IV mutants in different growth media. Chromosomal DNA flanking the transposon insertions of five class I mutants was cloned and sequenced, and the insertions were mapped to different locations of icaADBC, representing the synthetic genes for PIA. Expression of icaADBC from a xylose-dependent promoter in the different isogenic mutant classes reconstituted biofilm production in all mutants. In a Northern blot analysis no icaADBC-specific transcripts were observed in RNA isolated from mutants of classes II, III, and IV. Apparently, in addition to icaADBC, three other gene loci have a direct or indirect regulatory influence on expression of the synthetic genes for PIA on the level of transcription.
Today, coagulase-negative staphylococci, mostly strains of Staphylococcus epidermidis, represent the most frequent causes of nosocomial sepsis and are the most prominent organisms responsible for infections of implanted medical devices (1, 30, 38, 40, 41). In vitro, a proportion of S. epidermidis strains produce a macroscopically visible, adherent biofilm on test tubes or tissue culture plates, with a morphology in scanning electron micrographs very similar to that of infected intravascular catheters (3, 4, 14). This phenotype is now regularly referred to as biofilm formation, whereas the somewhat ambiguous term “slime production” was used earlier (13, 15, 16).
The molecular mechanisms leading to biofilm formation of S. epidermidis have attracted considerable interest in recent years. Biofilm formation may be divided into two phases. First, a complex process involving multiple physicochemical, protein, and polysaccharide factors leads to primary attachment of bacterial cells to a polymer surface (9, 11, 15, 16, 26–28, 39, 44). In the second phase, the attached bacteria proliferate and accumulate in a multilayered biofilm. Using transposon mutagenesis our group identified a linear homoglycan composed primarily of N-acetylglucosamine in β-1,6-glycosidic linkages containing deacetylated amino groups and succinate and phosphate substituents (17, 19). This polysaccharide is referred to as polysaccharide intercellular adhesin (PIA), which is functional in cell-to-cell adhesion and is essential for biofilm accumulation of most clinical S. epidermidis strains (17–19, 21, 22). In addition, PIA is essential for hemagglutination of erythrocytes by S. epidermidis (5, 20, 29, 31). The gene products of the icaADBC locus of S. epidermidis have enzymatic activity, which leads to synthesis of PIA in vivo and in vitro (6, 10, 20). Recently, it was demonstrated using a well-characterized isogenic biofilm-negative transposon mutant 1457-M10 in two relevant animal foreign-body infection models, that a functional icaADBC locus and the ability to produce PIA is essential for the pathogenesis of S. epidermidis biomaterial-related infections (19, 25, 32, 33).
In the present study we extend our genetic analysis of the mechanisms of S. epidermidis biofilm formation. Our results indicate that several independent genetic loci are essential for PIA synthesis and biofilm formation by S. epidermidis.
(Part of this work will appear in the doctoral theses of H.R., J.R., M.N., and J.K.-M.K., Universitäts-Krankenhaus Eppendorf, Hamburg, Germany.)
MATERIALS AND METHODS
Bacterial strains.
The biofilm-producing S. epidermidis 1457, its variant cured of an endogenous plasmid, S. epidermidis 1457c, and biofilm-producing S. epidermidis 9142, as well as the isogenic biofilm-negative mutants M10 and M11 and the biofilm-negative transductant 1457-M10, have been described (20, 21, 25). Staphylococcus carnosus containing the recombinant plasmids pCN27 (10) or pTXicaADBC (6), which contains the cloned icaADBC locus under control of its own or a xylose-inducible promoter, were kindly provided by Friedrich Götz (University of Tübingen, Tübingen, Germany). S. aureus WBG4883 carrying the conjugative plasmid pWBG636 was kindly provided by W. B. Grubb (Curtin University of Technology, Perth, Australia) (42, 43). Escherichia coli MC1061 (kindly provided by J. A. Gutierrez, Department of Oral Biology, University of Florida, Gainesville) was used as a host for cloning Tn917 insertion sites in plasmid pBluescript II SK (7). The relevant plasmids and antibiotic resistance markers of these strains are listed in Table 1.
TABLE 1.
Strains and plasmids
| Species or strain | Plasmid | Antibiotic resistance(s) | Properties | Reference(s) |
|---|---|---|---|---|
| 1457 | p1457 | Biofilm positive | 21, 25 | |
| 1457c | pTV1ts | Eryr Cmr | Biofilm positive | 25 |
| 9142-M10 | Eryr Cipror | Biofilm negative | 20 | |
| 1457-M10 | Eryr | Biofilm negative | 19 | |
| M10 | Eryr Cipror Gmr | Biofilm negative | 19 | |
| M11 | Eryr Cipror Gmr | Biofilm negative | 19 | |
| E. coli MC1061 | Smr | Expression of Tn917 Eryr | 7 | |
| S. aureus WBG4881 | pWBG636 | Gmr | Conjugative mobilization of plasmids | 43 |
| E. coli | pBluescript II SK | Ampr | Cloning vector for E. coli | 20 |
| S. carnosus | pCN27 | Cmr | icaADBC cloned in pCA44 | 10 |
| S. carnosus | pTXicaADBC | Tetr | icaADBC under control of xylose-inducible promoter | 6 |
Transposon mutagenesis.
Transposon mutagenesis was carried out at the nonpermissive temperature of plasmid pTV1ts using S. epidermidis 1457c(pTV1ts) as described previously (19, 25).
Phage transduction.
Phage transduction using S. epidermidis phages 48 or 71, kindly provided by V. T. Rosdahl, Statens Seruminstitut, Copenhagen, Denmark, was performed as described previously (25). For transduction of chromosomal markers the phage lysates were UV irradiated as described elsewhere (19, 25).
Mobilization of pTXicaADBC into S. epidermidis by coconjugation and transduction.
S. carnosus carrying plasmid pTXicaADBC and S. aureus WBG4883 were mated on membrane filters as described earlier (19). Donor and recipient strains were grown in brain heart infusion (BHI) broth (Oxoid, Basingstoke, England) overnight at 37°C with shaking, and 3 ml of recipient and 1 ml of donor cultures were filtered onto 0.45-μm (pore-size) nitrocellulose filters. These were incubated on BHI agar at 37°C for 20 h. Bacterial growth was plated on peptone-yeast (PY) agar (1.0% peptone, 0.5% yeast extract, 0.5% NaCl, 0.1% glucose, 1.5% agar; pH 7.5) while selecting for pTXicaADBC (tetracycline, 10 μg/ml) and for pWBG636 (gentamicin, 8 μg/ml) at 37°C. Transconjugants were purified on selective PY agar plates and were mated with S. epidermidis 9142 on membrane filters. Bacteria were plated on PY agar selecting for pTXicaADBC with tetracycline (10 μg/ml) and for S. epidermidis 9142 with 2 μg of ciprofloxacin per ml at 37°C. A transconjugant clone with the expected resistance pattern was used to move pTXicaADBC into different recipients using S. epidermidis phages 48 and 71 (25).
PFGE, DNA isolation, and Southern blot hybridization.
Pulsed-field gel electrophoresis (PFGE) was performed essentially as described using SmaI for cleavage of DNA and a CHEF-DR II system (Bio-Rad, Munich, Germany) for the analysis of resulting fragments (36). Phage lambda DNA concatemers were used as molecular weight markers. Chromosomal and plasmid DNA was isolated as previously described (19). DNA was digested with restriction enzymes under conditions suggested by the manufacturer (Pharmacia, Freiburg, Germany). DNA fragments were separated on 0.5% agarose gels in Tris-borate buffer (34). Alkaline capillary blotting and hybridization with [32P]dCTP-labeled probes were performed as described previously (19). The blots were exposed to Kodak X-Omat X-ray films.
Cloning of Tn917 containing DNA fragments of transposon mutants.
Tn917-containing restriction fragments were cloned directly in E. coli MC1061 using pBluescript II SK (Stratagene, La Jolla, Calif.) as a vector and selecting ampicillin-resistant (100 μg/ml) transformants. Positive clones were transferred using soft velvet to erythromycin-containing Luria-Bertani agar plates (300 μg/ml) directly selecting for inserts expressing the erythromycin resistance gene of Tn917 (7). Sequences of the transposon insertion sites of the mutants were obtained using oligonucleotides 5′-GGC CTT GAA ACA TTG GTT TAG TGG G-3′ and 5′-CTC ACA ATA GAG AGA TGT CAC CG-3′ complementary to the 5′- and 3′-junctions of Tn917 (37). DNA sequence analysis was performed using the Sequenase version 2.0 kit (U.S. Biochemicals) as described earlier (20).
Isolation of RNA and Northern blot analysis.
An overnight culture of the respective strains was diluted 1:100 into fresh, prewarmed tryptic soy broth (Oxoid) TSBOxoid and then grown into the mid-exponential-growth phase (optical density at 600 nm [OD600] of ∼3.0). Total bacterial RNA was isolated by the FastPrep system (Bio 101, Vista, Calif.) as described earlier (2). Then, 10 μg of total RNA was analyzed on a 1% agarose-formaldehyde gel in MOPS (morpholinepropanesulfonic acid) running buffer (20 mM MOPS, 5 mM sodium acetate, 1 mM EDTA; pH 7.0). RNA was blotted onto Zeta-Probe blotting membranes (Bio-Rad, Munich, Germany) and fixed to the membrane by baking at 80°C for 30 min. Hybridization with a 32P-labeled icaC-specific (5′-GAA ATA GCC ATA CCA TTG TCC-3′) oligonucleotide was performed at 52°C overnight in hybridization buffer containing 7% sodium dodecyl sulfate (SDS), 20 mM sodium phosphate (pH 7.0), 10× Denhardt solution, 5× SSC (standard saline citrate), 10% dextran sulfate, and 100 μg of denatured herring sperm DNA per ml. The membranes were then washed at 52°C for 30 min in 5% SDS, 20 mM sodium phosphate (pH 7.0), 10× Denhardt solution, and 3× SSC, followed by 1 h in 1× SSC–1% SDS. The membranes were exposed to Kodak X-Omat X-ray films.
Adherence assay for measurement of biofilm production by S. epidermidis.
Biofilm production by S. epidermidis strains grown in Trypticase soy broth (TSBBBL; Becton Dickinson, Cockeysville, Md.) and TSBOxoid (Oxoi, Basingstoke, England), respectively, was determined with a semiquantitative adherence assay using 96-well tissue culture plates (Nunc, Roskilde, Denmark) as described previously (4, 21).
Preparation of bacterial extracts and quantitation of PIA concentration.
Bacterial extracts of S. epidermidis strains grown in TSBBBL or TSBOxoid on plastic tissue culture plates were prepared by sonication (21). The concentration of PIA in bacterial extracts was determined by a specific coagglutination assay (19–21).
Hemagglutination.
Hemagglutination was assessed as described previously (20, 29). Bacteria were grown in TSBBBL in plastic tissue culture dishes for 22 h at 37°C (20). The medium was aspirated, and cells were scraped from the surface into 12 ml of phosphate-buffered saline. After passage through a 23-gauge needle, the bacterial suspension was adjusted to an OD578 of 1.0. The hemagglutination assay was performed with 96-well (U-shaped) microtiter plates (Greiner, Nürtingen, Germany) using sheep erythrocytes (Sigma, Deisenhofen, Germany) as described earlier (20, 29).
RESULTS
Isolation of biofilm-negative transposon mutants.
Using the biofilm-producing S. epidermidis 1457c cured of a cryptic plasmid of S. epidermidis 1457 and the temperature-sensitive plasmid pTV1ts, nine transposon mutants displaying altered biofilm production were isolated (25). For all mutants, genetic linkage of the observed phenotypic changes and the respective transposon insertions was demonstrated by transduction using S. epidermidis phage 71. Similar phenotypes and hybridization patterns using a Tn917-specific probe were detected with all mutants and their respective transductants (data not shown).
Genetic analysis of transposon insertion sites. (i) Characterization of isogenic biofilm-negative mutants by Southern blot hybridization and PFGE.
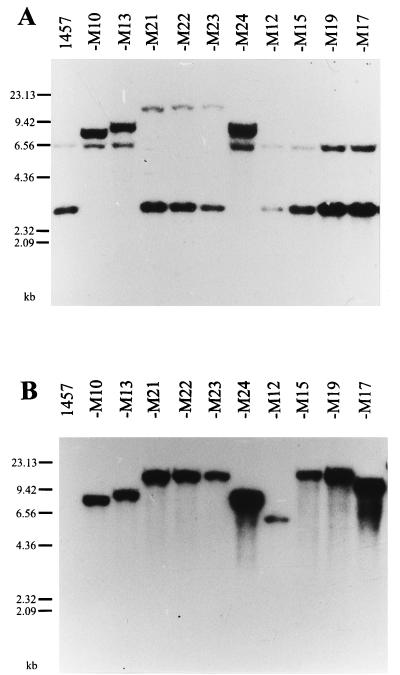
Southern blot hybridization of EcoRI-cleaved chromosomal DNA of the mutants using the icaADBC-specific insert of plasmid pCN27 as a probe revealed single insertions of Tn917 into the ica-specific 3-kb fragment of S. epidermidis 1457 in mutants M13 and M24, whereas in mutants M21, M22, and M23 Tn917 inserted into the 6.4-kb EcoRI fragment specific for the icaADBC locus (Fig. 1A). In contrast, in mutants M12, M15, M17, and M19 Tn917 insertions were apparently not related to the icaADBC locus, since no fragment size alterations of the two ica-specific EcoRI fragments were observed (Fig. 1A).
FIG. 1.
Chromosomal DNA of the wild-type biofilm-producing S. epidermidis 1457 and the isogenic biofilm-negative Tn917 mutants M10, M13, and M21 to M24 (class I), M12 (class II), M15 and M19 (class III), and M17 (class IV) was digested with EcoRI. Fragments were separated by agarose gel electrophoresis. Autoradiograms of a Southern blot hybridized with the icaADBC-specific XbaI-HindIII fragment of plasmid pCN27 (A) or a Tn917-specific probe (B) are shown. The discrepant intensities in panel A of the 6.4- and 3.0-kb fragments and their respective variants containing Tn917 insertions probably result from the fact that more than two-thirds of the icaADBC-specific probe used covered the smaller fragment, whereas the remainder hybridized to the larger fragment.
Hybridization with a Tn917-specific probe revealed that all mutants contained single insertions of Tn917 (Fig. 1B). Two identical 14.5-kb EcoRI-fragments were labeled in mutants M15 and M19, suggesting that the insertion sites of these mutants could be closely linked. Using chromosomal DNA of mutants M12 and M17, insertion of Tn917 into EcoRI fragments of 6.0 and 9.8 kb was detected.
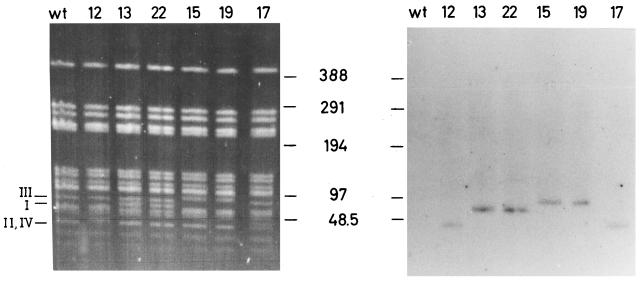
Analysis of the insertion sites of the mutants by PFGE revealed insertion of Tn917 in a 60-kb SmaI fragment with mutants M13 and M22 (class I), a 40-kb SmaI fragment with mutant M12 (class II), a 97-kb SmaI fragment with mutants M15 and M19 (class III), and a 40-kb SmaI fragment for mutant M17 (class IV; Fig. 2). Mobility differences of fragments resulting from Tn917 insertions can be observed by comparison with neighboring lanes with mutants of different classes in the ethidium bromide-stained gel, as indicated in the left panel of Fig. 2. In the case of mutants M12 (class II) and M17 (class IV), insertions occurred in two different fragments of approximately 40 kb, which are not separated in the wild type and mutants of classes I and III, resulting in a relatively intense band. Tn917 insertion in mutant M12 (class II) leads to decreased mobility of that double band, probably by insertion into the smaller of the two comigrating fragments. In mutant M17 (class IV) a distinct double band is observed, probably resulting from insertion of Tn917 into the larger of the two comigrating fragments. These studies indicate that in mutants M13 and M21 through M24, the insertion of Tn917 occurred within or near the icaADBC locus, whereas the transposon insertions of the other four mutants represent gene loci, which influence biofilm formation of S. epidermidis differently from the icaADBC locus.
FIG. 2.
Chromosomal DNA of biofilm-producing S. epidermidis 1457 (wt) and transposon mutants M12 (class II), M13 and M22 (class I), M15 and M19 (class III), and M17 (class IV) were cleaved by SmaI. Fragments were separated by PFGE. (Left panel) Ethidium bromide-stained gel. Fragments with altered mobility due to Tn917 insertions in the different mutant classes are indicated. (Right panel) Autoradiogram after hybridization of the Southern blot with a probe specific for Tn917.
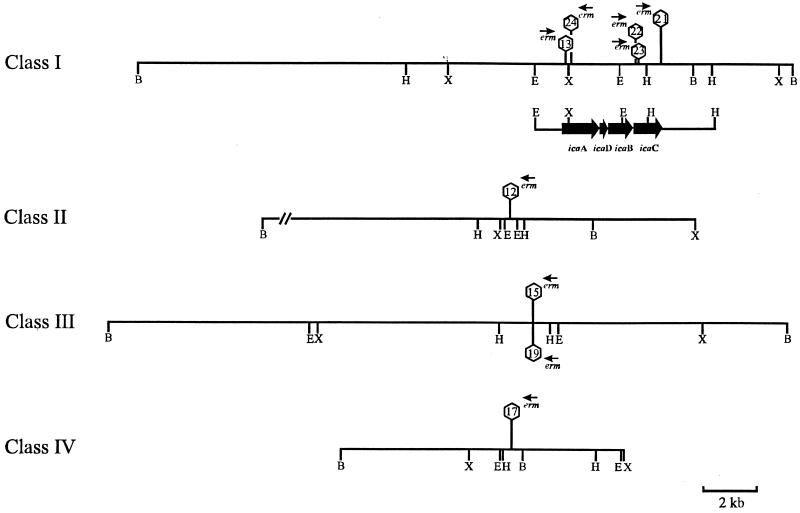
(ii) Mapping of the different transposon insertion sites.
Using ica-specific probes and two AvaI fragments corresponding to the 5′ (erm) and the 3′ junctions of Tn917, preliminary mapping of the transposon insertions of the different mutant classes was performed using restriction enzymes XbaI, HindIII, and BglII, which all have cleavage sites within the Tn917 sequence (Fig. 3). Apparently, Tn917 inserted at different locations of the icaADBC locus with the identical transcriptional direction in mutants M13, M21, M22, and M23, but not with mutant M24, where Tn917 inserted in a direction opposite to the transcriptional direction of icaADBC. Mapping of the mutants M12, M15, M19, and M17 confirmed the close linkage of Tn917 insertions in mutants M15 and M19. Due to the different restriction fragment patterns observed by mapping of mutants M12 and M17, linkage of the transposon insertions of these two mutants is highly unlikely, as already indicated from the PFGE results. Apparently, in addition to Tn917 insertions in the icaADBC locus, three additional classes of unlinked insertions of Tn917 into the chromosome of S. epidermidis 1457 lead to a biofilm-negative phenotype.
FIG. 3.
Preliminary genetic map of transposon insertions of the different mutant classes. H, HindIII; X, XbaI; B, BglII; E, EcoRI. erm indicates the direction of transcription of the erm gene of Tn917.
(iii) Identification of Tn917 insertion sites of class I biofilm-negative mutants by nucleotide sequencing.
The chromosomal EcoRI fragments of the biofilm-negative S. epidermidis transposon mutants M13 and M24 containing the entire transposon Tn917 were cloned in E. coli MC1061. For mutants M22 and M23 chromosomal EcoRI-SalI fragments and for mutant M21 a HindIII-SalI fragment containing the 5′ end of Tn917, including the erm gene, were cloned in E. coli. Sequence analysis revealed that mutants M13 and M24 had their insertions in the coding sequence of the icaA gene of the icaADBC locus, which contains synthetic genes for PIA (Table 2). In contrast, in the biofilm-negative mutants M21, M22, and M23, Tn917 was inserted at different sites within the coding sequence of icaC (Table 2).
TABLE 2.
Nucleotide sequences flanking Tn917 insertions of class I biofilm-negative S. epidermidis transposon mutants
| Mutant | Host strain | Gene inactivated | Insertion site sequence (position)a |
|---|---|---|---|
| M13 | 1457 | icaA | ATT TTT TTA TTA (85) Tn917TAT TAA AGA AAA |
| M24 | 1457 | icaA | Tn917 (331) ATA GAG GTA AAG |
| M22 | 1457 | icaC | ATT TTT ATT TTC (154) Tn917 |
| M23 | 1457 | icaC | ATT GTT CAA TAG (443) Tn917 |
| M21 | 1457 | icaC | GTT TTA TAT ATT (1001) Tn917 |
The position of the transposon insertions is indicated relative to the ATG start codon of the icaA gene or the icaC gene of the icaADBC gene cluster of S. epidermidis RP62A. The bold type indicates the 5-bp duplication typically occurring at Tn917 insertion sites.
Phenotypic characterization of the transposon mutants. (i) Biofilm production.
According to their differential degree of biofilm production in TSBOxoid and TSBBBL and their colony morphology, the isogenic transposon mutants could be assigned to four phenotypic classes, which clearly correspond to the genetic classes defined by the different insertion sites of Tn917. All mutants were completely biofilm negative in TSBOxoid. In TSBBBL all class I mutants were completely biofilm negative with the exception of mutant M21, in which Tn917 inserted at the extreme end of icaC, apparently resulting in a low residual enzymatic activity. Mutant M12 (class II) was also completely biofilm negative in TSBBBL. Mutants M15 and M19 (class III) displayed quantitatively reduced biofilm production in TSBBBL, which often was near the cutoff value of the adherence assay, whereas M17 (class IV) nearly reached wild-type levels of biofilm production in TSBBBL (Table 3).
TABLE 3.
Phenotypic properties of biofilm-negative S. epidermidis transposon mutants
| Strain | Classa | Expression on TSBBBLb
|
Expression on TSBOxoidb
|
Hemagglutinationc (reciprocal titer) | ||
|---|---|---|---|---|---|---|
| Biofilm (OD570) | PIA (reciprocal titer) | Biofilm (OD570) | PIA (reciprocal titer) | |||
| 1457 | wt | 2.489 | 1,024 | 1.246 | 256 | 512 |
| 1457c | wt | 1.582 | 1,024 | 1.326 | 128 | 512 |
| M13 | I | 0.053 | 0 | 0.016 | 0 | Neg |
| M21 | I | 0.155 | 8 | 0.031 | 0 | 4 |
| M22 | I | 0.044 | 0 | 0.027 | 0 | Neg |
| M23 | I | 0.047 | 0 | 0.021 | 0 | Neg |
| M24 | I | 0.060 | 0 | 0.045 | 0 | Neg |
| M12 | II | 0.020 | 0 | 0.013 | 0 | Neg |
| M15 | III | 0.217 | 32 | 0.030 | 0 | 16 |
| M19 | III | 0.228 | 32 | 0.033 | 0 | 16 |
| M17 | IV | 1.596 | 256 | 0.039 | 16 | 16 |
wt, wild type.
For the biofilm data, strains were grown in the respective medium in 96-well tissue culture plates, the plates were washed with phosphate-buffered saline, cells were fixed with Bouin's fixative, and adherent bacterial biofilms were stained with Gentian violet. Biofilm-producing strains were arbitrarily defined to have a mean OD570 of >0.1. The PIA expression was determined by coagglutination of extracts of strains grown in the respective medium. A coagglutination reagent specific for PIA was prepared by sensitizing S. aureus Cowan I with PIA-specific absorbed antiserum as described in Materials and Methods.
Hemagglutination was assessed as described in Materials and Methods. Neg, negative.
(ii) PIA production.
All mutants of class I did not detectably produce PIA in TSBOxoid and TSBBBL, except for mutant M21, which corresponds to the residual biofilm production of M21 in TSBBBL (Table 3). Mutant M12 (class II) also did not produce any PIA in either medium. Class III mutants did not produce PIA in TSBOxoid but had 32-fold-reduced PIA titers compared to the wild type in TSBBBL. The class IV mutant (M17) produced eightfold- and fourfold-reduced PIA titers in TSBOxoid and TSBBBL, respectively (Table 3).
(iii) Hemagglutination.
PIA was identified as the hemagglutinin of S. epidermidis (5, 20). In TSBBBL the isogenic transposon mutants of class I were all hemagglutination negative, with the exception of mutant M21, which also displayed residual PIA synthesis (Table 3). Mutant M12 (class II) was also completely hemagglutination negative (Table 3). Mutants M15 and M19 (class III) displayed reduced hemagglutination titers proportional to their reduced synthesis of PIA (Table 3). In contrast, the hemagglutination titers displayed by mutant M17 (class IV) were significantly lower, as would have been expected from the amount of PIA synthesized by this mutant (Table 3).
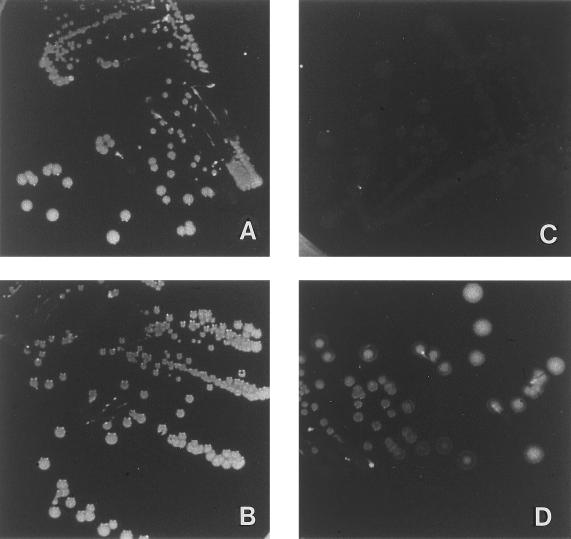
(iv) Changes of colony morphology.
Class II and class III mutants displayed gray to translucent colonies on blood agar compared to the white colonies of the wild-type strain and the mutants of classes I and IV (Fig. 4).
FIG. 4.
Colonial morphology of biofilm-producing S. epidermidis 1457 (A), mutant M13 (class I) (B), M12 (class II) (C), and M15 (class III) (D) on blood agar plates.
(v) Expression of icaADBC using a xylose-inducible promoter in biofilm-negative mutants of different classes.
Using the conjugative plasmid pWBG636 the recombinant plasmid pTXicaADBC containing the icaADBC locus cloned under the control of a xylose-inducible promoter (6) was mobilized from S. carnosus carrying pTXicaADBC into the biofilm-producing S. epidermidis 9142. Strain 9142(pTXicaADBC) with the expected antibiotic resistance markers was used as a donor for the production of transducing phage 48 lysates. Using this lysate the isogenic biofilm-negative transposon mutants M13 (class I), M12 (class II), M15 (class III), and M17 (class IV) were transduced with plasmid pTXicaADBC. All transductants had the expected antibiotic resistance profile (data not shown). Using TSBOxoid as the growth medium, mutant M13 containing a Tn917 insertion at nucleotide 85 of the icaA gene was complemented to a biofilm-producing phenotype in the presence of 4% xylose, whereas in the absence of xylose this strain displayed a completely biofilm-negative phenotype (Table 4). Similarly, in TSBOxoid lacking xylose the transductants of the isogenic mutants of classes II, III, and IV were completely biofilm negative. In contrast, in the presence of 4% xylose all of these mutants expressed a strongly biofilm-producing phenotype, indicating that the genetic defect resulting from the Tn917 insertions in these different mutant classes can be complemented, when icaADBC is expressed from an independent promoter (Table 4). Apparently, the mutations of the isogenic mutants of classes II, III, and IV directly or indirectly suppress expression of icaADBC from its own promoter.
TABLE 4.
Complementation of biofilm formation in different biofilm-negative mutant classes by icaADBC expressed by a xylose-dependent promoter
| Strain (class)a | Biofilm formation (OD570)b on TSBOxoid:
|
|
|---|---|---|
| Without xylose | With 4% xylose | |
| 1457 (wt) | 2.10 | 2.30 |
| M13 (I) | 0.03 | 0.02 |
| M13/pTXicaADBC | 0.04 | 2.20 |
| M12 (II) | 0.01 | 0.02 |
| M12/pTXicaADBC | 0.01 | 1.03 |
| M15 (III) | 0.02 | 0.02 |
| M15/pTXicaADBC | 0.05 | 1.06 |
| M17 (IV) | 0.07 | 0.06 |
| M17/pTXicaADBC | 0.09 | 2.50 |
wt, wild type.
Biofilm formation was assessed as described in Materials and Methods using TSBOxoid supplemented or not supplemented with 4% xylose as indicated.
(vi) Transcription of icaADBC in biofilm-negative mutants of different classes.
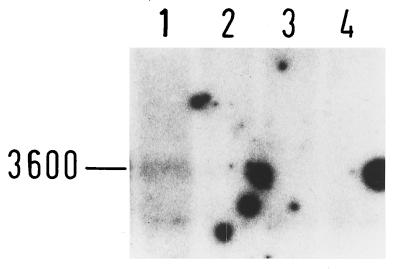
To directly evaluate the effect of the different Tn917 insertions of the biofilm-negative mutants of classes II, III, and IV on transcription of the icaADBC locus, RNA was prepared in mid-exponential-growth phase from mutants M12, M15, and M17 and from wild-type S. epidermidis 1457. An icaADBC-specific transcript (ca. 3.6 kb) was detected in RNA prepared from the biofilm-producing S. epidermidis 1457 grown in TSBOxoid. In contrast, an icaADBC-specific transcript was not detected with any of the mutants M12 (class II), M15 (class III), or M17 (class IV) (Fig. 5).
FIG. 5.
Transcription of icaADBC in biofilm-negative class II, III, and IV mutants. Total RNA was extracted from S. epidermidis 1457 (lane 1) and mutants M12 (class II; lane 2), M15 (class III; lane 3), and M17 (class IV; lane 4) grown into the mid-exponential-growth phase in TSBOxoid. An icaC-specific oligonucleotide probe was used in Northern hybridization, and an autoradiogram is shown.
DISCUSSION
In the present study we have isolated nine isogenic biofilm-negative Tn917 insertion mutants with Tn917 insertions in four unlinked chromosomal loci. The resulting transposon mutants can be separated into four genetic classes according to the preliminary chromosomal mapping of their respective Tn917 insertions. These genetic classes are reflected by phenotypic differences observed with the mutants, which include differences in the quantity of biofilm formation and PIA production in TSBBBL or TSBOxoid and altered colony morphology (Table 3, Fig. 4).
The five mutants of class I have Tn917 insertions within the coding region of the icaADBC locus. Mutants M13 and M24 inactivate icaA by insertion at nucleotides 85 and 331 of the icaA gene, closely resembling the isogenic mutants M10 and M11 with insertions at nucleotides 87 and 1031 of the icaA gene (19, 20). In mutants M22 and M23 the icaC gene is inactivated at nucleotides 154 and 443 of the icaC gene. All of these insertions lead to complete inactivation of PIA synthesis. In contrast, the insertion of mutant M21 is located at nucleotide 1001 of the icaC gene, only 67 nucleotides from the stop codon of icaC, allowing residual enzymatic activity of the icaADBC operon leading to low-level PIA synthesis and biofilm formation in TSBBBL. These observations indicate that a correct sequence of the last 21 amino acids of IcaC is essential for full functional activity for PIA synthesis.
No Tn917 insertions in icaB or icaD were noted in our study. This could simply result from the lack of insertion sites for Tn917 in these genes. However, this could also mean that these two genes are not essential for PIA synthesis and biofilm formation. This could be anticipated for icaB since in an in vitro-reconstituted reaction the synthesis of PIA clearly depended on the presence of IcaA, IcaD, and IcaC, but not on IcaB (6). In addition, it was reported that expression of icaADC without icaB led to cell clustering of S. carnosus as in S. carnosus expressing icaADBC and synthesizing PIA (6). In contrast to these observations, it was reported that the insertion of insertion sequence IS256 into icaB and a frame shift mutation of icaB led to a biofilm-negative phenotype (10, 45).
We isolated four additional isogenic biofilm-negative transposon mutants with insertions unlinked to the icaADBC locus. These insertions can be separated into genetic classes II, III, and IV, which correspond also with phenotypic differences of these mutants. There are several possible mechanisms which could lead to impaired PIA synthesis and biofilm formation of these mutants.
First, these gene loci could be necessary for the synthesis of an essential precursor for PIA synthesis. Since PIA is a homoglycan synthesized primarily of N-acetylglucosamine (17), this is unlikely as this sugar is an essential constituent of the peptidoglycan and therefore necessary for bacterial cell proliferation. However, PIA contains additional modifications, like the deacetylation of the amino groups, and the transfer of phosphate and succinate groups (17) and inactivation of the respective gene loci of mutants of class II to IV could interfere with these modifications. Second, some of these mutations could interfere with the transport of PIA outside the cell and therefore inhibit synthesis of the polysaccharide. Third, the respective gene loci could interfere with the expression of the icaADBC locus at the level of transcription.
To differentiate between these possibilities, we expressed icaADBC from a xylose-inducible promoter by transfer of the recombinant plasmid pTXicaADBC into mutants of classes I (M13), II (M12), III (M15), and IV (M17). All mutants containing the recombinant plasmid still had a biofilm-negative phenotype in the absence of xylose. However, in the presence of xylose the icaA insertion mutant M13 was complemented to a biofilm-producing phenotype as expected, indicating that the recombinant icaADBC locus of pTXicaADBC was still functional after the genetic manipulations of conjugative mobilization and transduction. Interestingly, in the presence of xylose the mutants M12, M15, and M17 containing pTXicaADBC produced biofilm. This clearly rules out the possibility that the respective gene loci of the mutants of classes II to IV encode functions necessary as precursors for PIA synthesis or are responsible for the transport of the polysaccharide. Since expression of icaADBC in trans from an independent promoter led to biofilm formation in these mutants, the inactivated genes apparently influence expression of icaADBC in a regulatory way by either directly or indirectly modulating transcription of icaADBC. This was confirmed directly since transcription of icaADBC was not detected in mutants M12, M15, and M17 compared to the wild-type S. epidermidis 1457 in the mid-exponential-growth phase. Apparently, functional activity of all of the inactivated genes in these mutants is essential for biofilm formation in TSBOxoid whereas in TSBBBL the inactivated gene of mutant M17 seems to be dispensable for biofilm formation and PIA synthesis. In contrast to the lack of biofilm formation in TSBOxoid, it was observed over time that, although biofilm formation of mutant M17 in different lots of TSBBBL varied between reaching an optical density similar to that of the wild type and being significantly reduced, a biofilm always formed. At present it is unknown which specific constituents of these complex media are relevant for the differential expression of biofilm formation by the different isogenic mutants.
Several other biofilm-negative mutants have been described previously. Tn917 mutant Mut1 is impaired in primary attachment due to a deletion in the gene of the major autolysin atlE of S. epidermidis (9). Since this mutant still produces PIA in quantities similar to that of the wild-type strain O-47, mutant Mut1 can be easily differentiated from the mutants isolated in the present study (5, 8).
Mutant M7, obtained by chemical mutagenesis of S. epidermidis RP62A, is impaired in the accumulative phase of biofilm production (35). This mutant does not produce a 140-kDa accumulation associated protein (AAP) believed to be responsible for the observed defect in accumulation (12). However, since the mutant still produces PIA in quantities similar to those with the wild type (12) (D. Mack and C. de Grahl, unpublished results), it seems highly unlikely that AAP has a regulatory effect on the expression of icaADBC and that it is functionally related to mutants described in this study. In addition, AAP appears in the protein fraction secreted from the cells (12). It is therefore difficult to assume how AAP could directly influence expression of icaADBC.
Mutant M187-sn3 is a biofilm-negative transposon mutant, which is impaired in the synthesis of a polysaccharide referred to as capsular polysaccharide/adhesin (PS/A) (24). Analysis of purified PS/A revealed a composition of 54% hexoses, 20% amino sugars, and 10% uronic acids (39). As specific sugars, galactose (22%), glucosamine (15%), and galactosamine (5%) were detected (39). Recently, the composition of PS/A from S. epidermidis RP62A and M187 grown in a chemically defined medium was reevaluated. There was an almost identical reactivity of anti-PS/A and anti-PIA antisera with several well-characterized S. epidermidis strains, including the PS/A-producing S. epidermidis M187 and the PS/A-negative transposon mutant M187-sn3, the PIA-producing S. epidermidis 1457, and the isogenic PIA-negative mutant 1457-M11, as well as the recombinant S. carnosus containing the cloned icaADBC locus (23). These data suggest that PS/A and PIA are structurally related or even identical. Those authors reported that PS/A is a polysaccharide of high molecular mass composed of β-1,6-linked glucosamine residues with a high degree of substitution with succinate and acetate and that its production is dependent on the icaADBC locus (23). Since detailed data on the structural analysis of PS/A produced by S. epidermidis have not yet been reported, it remains to be determined whether PS/A is identical to polysaccharide II of PIA or whether it represents an additional variant of PIA. The genes inactivated by the transposon insertion in mutant M187-sn3 have not yet been identified; however, they are different from icaADBC (23) (D. Mack and H. Rohde, unpublished results). Its phenotype is very similar to class I and II mutants; however, no altered colony morphology was observed, as is typical for mutant M12, indicating that its phenotype most probably results from a different insertional gene inactivation. It is relevant to note that a similar altered colony morphology was also observed with other independent wild-type strains in which the Tn917 insertion of mutant M12 was transferred by transduction (D. Mack and A. Sabottke, unpublished results).
Our results indicate that expression of icaADBC and PIA synthesis is tightly regulated in S. epidermidis. Cure of biomaterial-related S. epidermidis infections typically requires removal of the infected biomaterial and complete debridement of the infected tissue, since antibiotic therapy regularly fails when the foreign material remains in situ (1). It is believed that the resistance of an established biomaterial-related S. epidermidis infection to therapy is related to the biofilm mode of growth of the bacteria. Indeed, Rupp and collaborators recently demonstrated by using the isogenic icaA insertion mutant 1457-M10 that a functional icaADBC locus and the ability for PIA synthesis and biofilm formation are essential virulence factors of S. epidermidis in a subcutaneous mouse catheter infection model and a rat central venous catheter infection model (32, 33). Further characterization of the regulatory mechanisms governing the expression of PIA and biofilm formation on a molecular level is of major importance, because it is reasonable to speculate that interference with these regulatory mechanisms may lead to improved methods of prevention and therapy of biomaterial-related S. epidermidis infections.
ACKNOWLEDGMENTS
We thank Rainer Laufs for his continuous support. The kind gift of plasmids pCN27 and pTXicaADBC by Friedrich Götz, Molekulare Genetik, University of Tübingen, Tübingen, Germany, is gratefully acknowledged. We thank Vibeke T. Rosdahl, Statens Serum Institute, Copenhagen, Denmark, for providing phages and propagating strains. For bacterial strains and plasmids, we thank W. B. Grubb, Curtin University of Technology, Perth, Australia; and J. A. Gutierrez, Department of Oral Biology, University of Florida, Gainesville. The photographic work of C. Schlüter is acknowledged.
This work was supported in part by a grant of the Deutsche Forschungsgemeinschaft to D.M.
REFERENCES
- 1.Bisno A L, Waldvogel F A. Infections associated with indwelling medical devices. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 2.Cheung A L, Eberhardt K J, Fischetti V A. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 3.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fey P D, Ulphani J S, Götz F, Heilmann C, Mack D, Rupp M E. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J Infect Dis. 1999;179:1561–1564. doi: 10.1086/314762. [DOI] [PubMed] [Google Scholar]
- 6.Gerke C, Kraft A, Süssmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilmann C, Götz F. Further characterization of Staphylococcus epidermidis transposon mutants deficient in primary attachment or intercellular adhesion. Zentbl Bakteriol. 1998;287:69–83. doi: 10.1016/s0934-8840(98)80149-7. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain M, Wilcox M H, White P J. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev. 1993;10:191–207. doi: 10.1111/j.1574-6968.1993.tb05867.x. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M, Wilcox M H, White P J, Faulkner M K, Spencer R C. Importance of medium and atmosphere type to both slime production and adherence by coagulase-negative staphylococci. J Hosp Infect. 1992;20:173–184. doi: 10.1016/0195-6701(92)90085-z. [DOI] [PubMed] [Google Scholar]
- 15.Mack D. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J Hosp Infect. 1999;43(Suppl.):S113–S125. doi: 10.1016/s0195-6701(99)90074-9. [DOI] [PubMed] [Google Scholar]
- 16.Mack D, Bartscht K, Dobinsky S, Horstkotte M A, Kiel K, Knobloch J K M, Schäfer P. Staphylococcal factors involved in adhesion and biofilm formation on biomaterials. In: An Y H, Friedman R J, editors. Handbook for studying bacterial adhesion: principles, methods, and applications. Totowa, N.J: Humana Press; 2000. pp. 307–330. [Google Scholar]
- 17.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 19.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack D, Riedewald J, Rohde H, Magnus T, Feucht H H, Elsner H A, Laufs R, Rupp M E. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect Immun. 1999;67:1004–1008. doi: 10.1128/iai.67.2.1004-1008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack D, Siemssen N, Laufs R. Identification of a cell cluster associated antigen specific for plastic-adherent Staphylococcus epidermidis which is functional related to intercellular adhesion. Zentbl Bakteriol Suppl. 1994;26:411–413. [Google Scholar]
- 23.McKenney D, Hübner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller E, Hübner J, Gutierrez N, Takeda S, Goldmann D A, Pier G B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993;61:551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedelmann M, Sabottke A, Laufs R, Mack D. Generalized transduction for genetic linkage analysis and transfer of transposon insertions in different Staphylococcus epidermidis strains. Zentbl Bakteriol. 1998;287:85–92. doi: 10.1016/s0934-8840(98)80151-5. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson M, Frykberg L, Flock J I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual A, Fleer A, Westerdaal N A, Verhoef J. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur J Clin Microbiol. 1986;5:518–522. doi: 10.1007/BF02017694. [DOI] [PubMed] [Google Scholar]
- 28.Pei L, Palma M, Nilsson M, Guss B, Flock J I. Functional studies of a fibrinogen binding protein from Staphylococcus epidermidis. Infect Immun. 1999;67:4525–4530. doi: 10.1128/iai.67.9.4525-4530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupp M E, Archer G L. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect Immun. 1992;60:4322–4327. doi: 10.1128/iai.60.10.4322-4327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 31.Rupp M E, Sloot N, Meyer H G, Han J, Gatermann S. Characterization of the hemagglutinin of Staphylococcus epidermidis. J Infect Dis. 1995;172:1509–1518. doi: 10.1093/infdis/172.6.1509. [DOI] [PubMed] [Google Scholar]
- 32.Rupp M E, Ulphani J S, Fey P D, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67:2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupp M E, Ulphani J S, Fey P D, Mack D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect Immun. 1999;67:2656–2659. doi: 10.1128/iai.67.5.2656-2659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schumacher-Perdreau F, Heilmann C, Peters G, Götz F, Pulverer G. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol Lett. 1994;117:71–78. doi: 10.1111/j.1574-6968.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzkopf A, Karch H, Schmidt H, Lenz W, Heesemann J. Phenotypical and genotypical characterization of epidemic clumping factor-negative, oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:2281–2285. doi: 10.1128/jcm.31.9.2281-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw J H, Clewell D B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B- resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985;164:782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thylefors J D, Harbarth S, Pittet D. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect Control Hosp Epidemiol. 1998;19:581–589. doi: 10.1086/647878. [DOI] [PubMed] [Google Scholar]
- 39.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. . (Erratum, 158:268.) [DOI] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. Public Health Service: National nosocomial infectious surveillance (NNIS) report. Data summary from October 1986–April 1996, issued May 1996. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services. Public Health Service: National nosocomial infectious surveillance (NNIS) report. Data summary from October 1986–April 1997, issued May 1997. Am J Infect Control. 1997;24:477–487. [PubMed] [Google Scholar]
- 42.Udo E E, Grubb W B. A new class of conjugative plasmid in Staphylococcus aureus. J Med Microbiol. 1990;31:207–212. doi: 10.1099/00222615-31-3-207. [DOI] [PubMed] [Google Scholar]
- 43.Udo E E, Grubb W B. Conjugal transfer of plasmid pWBG637 from Staphylococcus aureus to Staphylococcus epidermidis and Streptococcus faecalis. FEMS Microbiol Lett. 1990;60:183–187. doi: 10.1016/0378-1097(90)90369-2. [DOI] [PubMed] [Google Scholar]
- 44.Veenstra G J, Cremers F F, van Dijk H, Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J Bacteriol. 1996;178:537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziebuhr W, Krimmer V, Rachid S, Lössner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]