Abstract
Intestinal integrity losses have been identified as a main driver for poor performance in broilers. The oral administration of markers such as iohexol is a major asset for measuring intestinal permeability (IP) alterations. The aim of the current study was to evaluate oral iohexol administration and serum levels as a quantitative measure for IP in Ross 308 broilers and to identify possible associations with histologic measurements. A total of 40, day-old broiler chickens were randomly divided into 4 groups of 10 broilers and a coccidiosis model was used to induce IP. Three challenge groups received a mixture of different field strains and concentrations of Eimeria acervulina and Eimeria maxima at d 16, and 1 group operated as an uninfected control group. On d 20, 5 birds per group were orally administered the permeability marker iohexol at a dose of 64.7 mg/kg body weight and blood was taken 60 min after the oral gavage. On d 21 these 5 birds per group were euthanized. On d 21, 5 other birds per group were given iohexol where after blood was taken. These birds were euthanized on d 22. During necropsy, birds were scored for coccidiosis lesions and a duodenal segment was taken for histology. The Eimeria challenge had a significant impact on the villus length, crypt depth, villus-to-crypt ratio and CD3+ T-lymphocytes area percentage. Challenged birds had a significant higher concentration of serum iohexol on both sampling days, as compared to the uninfected controls. A significant correlation could be found between the serum iohexol concentration and the histologic parameters (villus length, crypt depth and villus-to-crypt ratio) on the first sampling day. This suggests that iohexol may be used as a gut permeability marker in broilers under Eimeria challenge.
Key words: broiler, iohexol, coccidiosis, intestinal permeability, gut health
INTRODUCTION
The digestive system is a complex organ with multiple functions including the absorption and digestion of nutrients, excretion of waste products and protection against potential harmful substances (Hornbuckle et al., 2008; Salvo-Romero et al., 2015). A functional gut barrier plays a vital role in fulfilling these principal functions. The permeability of the gut barrier is strictly regulated by multiple factors and determines which molecules can cross the barrier and enter the bloodstream (Camilleri, 2019). Damage to the epithelial barrier can cause an increase in intestinal permeability (IP) which directly leads to the passage of intraluminal macromolecules, xenobiotics and pathogens into the bloodstream (Hornbuckle et al., 2008). An increased IP is extensively investigated in human and veterinary medicine. Multiple human gastrointestinal and extra-intestinal disorders such as inflammatory bowel disease, allergies or metabolic diseases are associated with alterations of the IP (May et al., 1993; Gerova et al., 2011; Bischoff et al., 2014; Vivinus-Nébot et al., 2014; González-González et al., 2018). In broilers, IP has a direct association with performance. Several stressors, including pathogens, specific dietary factors or fasting have been used to elevate IP in experimental animal models (Gilani et al., 2018b; Gilani et al., 2021; Liu et al., 2021).
A major asset in experimental models is a reliable test that is able to measure the gut integrity of the birds. The evaluation of the IP in broilers is particularly performed using molecules or probes that either or not pass the intestinal barrier via the paracellular pathway, that are orally administered and quantified in the blood (Schoultz and Keita, 2020; Gilani et al., 2021). Every probe has its own benefits and disadvantages. Chromium-ethylenediaminetetraacetic acid as well as the saccharides lactulose, mannitol and rhamnose are frequently used in humans and animals (Frias et al., 2012). The radioactivity of Chromium-ethylenediaminetetraacetic acid and the bacterial fermentation of sugars have resulted in the introduction of other probes (Hall and Batt, 1996; Klenner et al., 2009; Bischoff et al., 2014). Nowadays, fluorescein isothiocyanate-dextran (FITC-d) is the most broadly used IP marker in broiler experiments (Gilani et al., 2021). The large molecular size of FITC-d (4-kDa) makes sure it does not pass the gut epithelium under normal circumstances (Gilani et al., 2017). Multiple gut challenge experiments have demonstrated that FITC-d can cross a disrupted gut barrier via the paracellular pathway (Latorre et al., 2018; Barekatain et al., 2019; Zanu et al., 2020). However, the interpretation of FITC-d results need to be done with caution. Liu et al. (2021) described a broad range of factors that can influence the FITC-d concentrations, including the protocol (e.g., gavage dose, fasting period) and the quantification procedure. Recently, iohexol has been introduced as a marker for IP in birds such as domestic pigeons, laying hens, cockatiels, and falcons (Wilhelm et al., 2020). It is used as a contrast medium in radiographic imaging and is considered to be a safe, inert and water-soluble marker (Frias et al., 2012). Iohexol is not being metabolized but completely eliminated by renal excretion (glomerular filtration) and has a high stability in biological samples (Lee et al., 2006; Wilhelm et al., 2020; Ortín-Piqueras et al., 2021). Because of these advantages, the potential of iohexol as a permeability marker has been assessed in human medicine. Iohexol is not only an excellent marker for measuring the glomerular filtration rate in humans and animals (Langlois, 2008; Gasthuys et al., 2019), but has also proved to be a successful IP marker in gut challenge models (Frias et al., 2012). Moreover, several gastrointestinal diseases have been associated with an elevated iohexol serum concentration after oral administration in humans and rodent models (Halme et al., 2000; Andersen et al., 2001; Gerova et al., 2011; Ortín-Piqueras et al., 2021). In birds, iohexol has shown potential as a permeability marker under healthy conditions (Wilhelm et al., 2020). As far as we know, the iohexol marker has not been used in broilers with gut permeability alterations.
The objective of this study was to evaluate iohexol as a permeability marker in broilers infected with Eimeria species that induces coccidiosis. We evaluated associations between iohexol serum concentrations and gut morphologic parameters (villus length and crypt depth) in a coccidiosis trial.
MATERIALS AND METHODS
Ethical Statement
The experiment was approved by the ethical committee of Poulpharm Bvba, Izegem, Belgium (coccidiosis trial; I22151-EC).
Study Design and Sampling
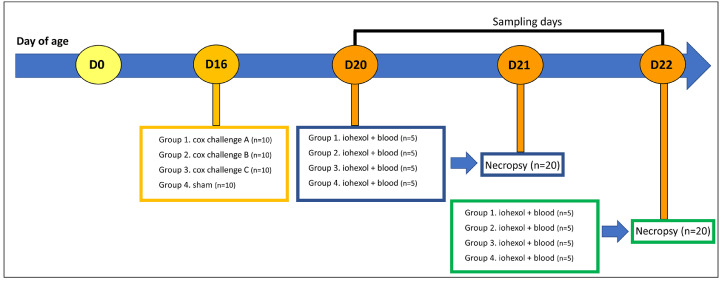
A total of 40 one-day-old mixed sex Ross 308 broiler chickens were purchased from a commercial hatchery (Vervaeke-Belavi, Tielt, Belgium) and randomly allocated into 4 groups of 10 broilers and fed a diet ad libitum (Research Diets Services B.V., Wijk bij Duurstede, the Netherlands). At d 16 the animals from treatment group 1 received intracrop 95,436 oocysts of Eimeria acervulina field strain Hungary (A) and 54,912 oocysts of Eimeria maxima field strain Hungary (A). Treatment group 2 received intracrop 70,810 oocysts of E. acervulina field strain Netherlands (B) and 43,380 oocysts of E. maxima field strain Netherlands (B). Treatment group 3 received intracrop 146,080 oocysts of E. acervulina field strain Poland (C) and 39,840 oocysts of E. maxima field strain Poland (C). One group of 10 animals operated as uninfected control group and received a sham inoculation (distilled water, VWR, Belgium). On d 20, 5 birds per group were given the permeability marker iohexol (Omnipaque 350, GE Healthcare AS, Oslo, Norway) by oral gavage (intracrop) at a dose of 64.7 mg/kg body weight. Sixty minutes after the gavage blood was taken in order to determine serum iohexol levels. These birds were euthanized by cervical dislocation preceded by concussion on d 21. On d 21, 5 other birds per group were administered iohexol where after blood was taken. These birds were euthanized on d 22. The protocol of the study design is summarized schematically in Figure 1.
Figure 1.
Timeline of the procedure of the challenge model. A total of 40 one-day-old mixed sex Ross 308 broiler chickens were purchased and randomly divided into 4 groups of 10 broilers at the age of 14 d. Three treatment groups (each group n = 10) were challenged at d 16 with a mix of Eimeria species (E. acervulina and E. maxima), and 1 group operated as an uninfected control group. A necropsy was performed of 5 selected birds per group at 5 (D21) and 6 d (D22) after challenge. The iohexol gavage and blood sampling was done the day prior to the euthanasia of the selected animals (D20 & D21).
The broilers received feed and water ad libitum before the oral gavage of iohexol, which resulted into full crops during the administration. Iohexol was diluted in Hanks' Balanced Salt Solution (HBSS, Life Technologies Europe B.V., Merelbeke, Belgium). The individual BW was recorded and used to determine the exact volume of iohexol for each bird at a dose of 64.7 mg/kg. The product was given orally via a 1 mL syringe into the crop. Blood samples were collected 1 h later from the basilic vein by direct venipuncture and collected in Eppendorf tubes. Blood was kept at room temperature for about 30 min and centrifuged (1,500 × g, 15 min) to separate the serum. The serum samples were stored at -20°C and analyzed between 21 and 37 d after collection. At necropsy, the animals were scored for coccidiosis lesions using the Johnson and Reid method (Johnson and Reid, 1970). The total mean lesion score (TMLS) for coccidiosis was determined as the sum of the average of the individual mean lesion scores for E. acervulina and E. maxima. The individual mean lesion scores per Eimeria species were calculated as the average of the individual scores of the broilers. Each Eimeria species was scored from 0 (no lesions) to 4 (severe lesions). The duodenal loop was collected in 4% formaldehyde for histologic examination.
Iohexol Quantification in Serum
Serum concentrations of iohexol were determined with ultra‐high performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS). This method was fully validated for iohexol measurements in broiler chicken plasma (Stroobant et al., 2020).
Serum samples (100 μL) were diluted with 100 μL of Milli-Q water and spiked with 25 μL of the internal standard iohexol-d5 (100 µg/mL), followed by addition of 15 µL of 100% trifluoroacetic acid. After 10 sec of vortexing, the samples were centrifuged (13,000 rpm, 15 min). The supernatant was transferred to an autosampler vial and 5 μL was injected in the UHPLC-MS/MS instrument (Quattro Premier XE, Waters, Milford, MA). Matrix-matched calibration curve and quality control samples were prepared by spiking blank serum with a known iohexol concentration. The lowest Limit of Quantification for iohexol was 0.25 µg/mL.
Morphometrical Evaluation
The duodenal loops were fixated in 4% formaldehyde for 24 h, dehydrated in xylene and embedded in paraffin. Sections of 4 µm were cut using a microtome (HM360, Thermo Scientific, Waltham, MA) and processed as described by (De Maesschalck et al., 2015). After staining with haematoxylin and eosin, morphologic parameters were assessed using standard light microscopy. Villus length, measured from the crypt–villus junction to the villus tip, and crypt depth, measured from the junction to the base, in the duodenum were determined by random measurement of 10 villi per section at 5x magnification using a Leica DM LB2 microscope equipped with a camera and a computer based image analysis program, LAS V4.1 (Leica Application Suite V4, Wetzlar, Germany).
Immunohistochemistry
Antigen retrieval was performed on 4 µm duodenal sections with a pressure cooker in citrate buffer (10 mM, pH 6). Slides were rinsed with washing buffer (Dako kit, K4011, Glostrup, Denmark) and blocked with peroxidase reagent (Dako, S2023) for 5 min. Slides were rinsed with distilled water and Dako washing buffer before incubation with anti-CD3 primary antibodies (Dako CD3, A0452) for 30 min at room temperature diluted 1:100 in antibody diluent (Dako, S3022). After rinsing again with washing buffer, slides were incubated with labelled polymer-HRP anti-rabbit (Envision+ System-HRP, K4011) for 30 min at room temperature. Before adding di-amino-benzidine (DAB+) substrate and DAB+ chromogen (Dako kit, K4011) for 5 min, slides were rinsed 2 times with washing buffer. To stop the staining, the slides were rinsed with distilled water, dehydrated using the Shandon Varistain-Gemini Automated Slide Stainer and counterstained with hematoxylin for 10 s. The slides were analyzed with a Leica DM LB2 microscope and a computer based image analysis program LAS V4.1 (Leica Application Suite V4, Germany) to measure CD3+ cells in a total area of 3.5 ± 0.5 mm2 which represents the area of approximately 10 to 12 villi.
Statistical Analysis
Data analysis was performed with the statistical software R 4.1.3 in R studio (R Core Team, 2021). The normality of the quantitative variables was tested with the Shapiro-Wilk's method and histograms. The data (necropsy and iohexol gavage) were analyzed per sampling day.
Multiple linear regression was used to compare the control and the challenge group with each other, taking into account the different type of strains (A, B, C) in the challenge group. A linear model was fitted for each parameter (TMLS, gut histopathology parameters, BW and iohexol concentration) using the linear model function in the R package stats (R Core Team, 2021). The fixed effects of the multiple linear regression model were the treatment group (control or challenge) and the strain (A, B, C).
The associations between the histologic parameters and the iohexol concentration (µg/mL) was evaluated with multiple linear regression (R Core Team, 2021). Associations were either made between the iohexol concentration (D20) and histologic parameters (D21) or between the iohexol concentration (D21) and histologic parameters (D22), in order to determine significant associations and make predictions between parameters. The response variable (Y) in the model was the iohexol concentration (µg/mL). The different types of strain (A, B, C) and each histologic parameter were added as independent variables. Only the data of the challenge group was included for making the associations between the gut histology parameters and iohexol concentration.
Interactions were tested between the independent variables and the results were interpreted with the function joint.test (Lenth, 2022), which corresponds to an ANOVA type III test. A statistical result with a P value of ≤ 0.05 was considered to be significant and the asterisks illustrate the significance level of the multiple linear regression model *P ≤ 0.05, ⁎⁎P ≤ 0.01, ⁎⁎⁎P ≤ 0.001 in the figures.
RESULTS
Challenge Effect on the Gut Health Parameters
The effect of the Eimeria challenge on the different parameters (TMLS, gut histopathology parameters, body weight and iohexol concentration) on both sampling days (i.e., iohexol gavage (D20) and necropsy (D21); iohexol gavage (D21) and necropsy (D22)) is presented in Figure 2, Figure 3, Figure 4, Figure 5.
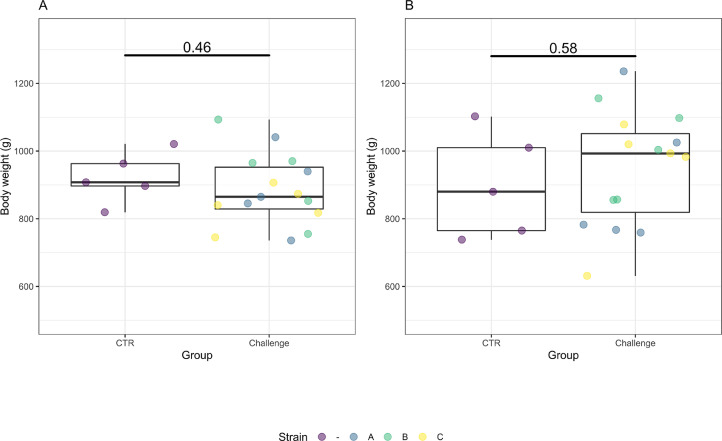
Figure 2.
Box plots representing the body weight of the birds in control and challenge groups on d 20 (A) and d 21 (B). The bars of the boxplot represent the minimum value, 75th percentile, median, 25th percentile and the maximum value. Data from each challenge group is illustrated in different colors (blue = A = Hungary field strains, green = B = Netherlands field strains, yellow = C = Poland field strains). No significant differences were observed between the control and challenge group on both sampling days (P > 0.05).
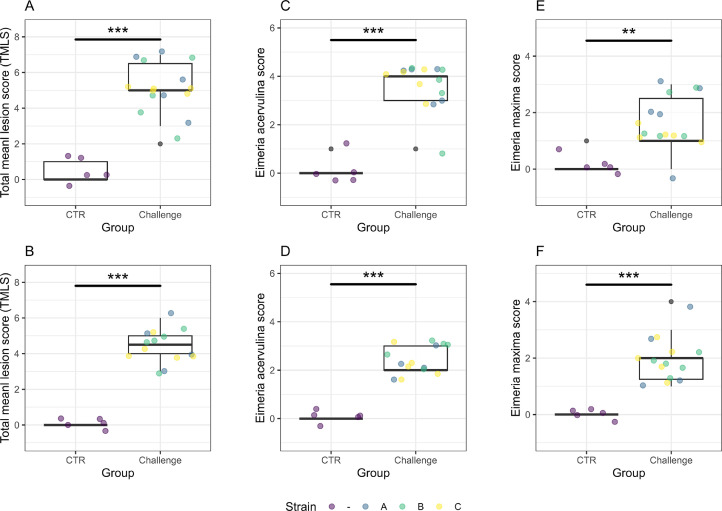
Figure 3.
Box plots of the coccidiosis lesion scores on d 21 (A, C, E) and d 22 (B, D, F). The data of the total mean lesion score (A, B), E. acervulina lesion score (C, D) and E. maxima lesion score (E, F) are presented and the strains (A, B, C) of the challenge group are illustrated in different colors (blue = A = Hungary field strains, green = B = Netherlands field strains, yellow = C = Poland field strains). The total mean lesion score (TLMS), E. acervulina lesion score and E. maxima lesion score is significantly higher in the challenge groups compared to the control group on both sampling days.
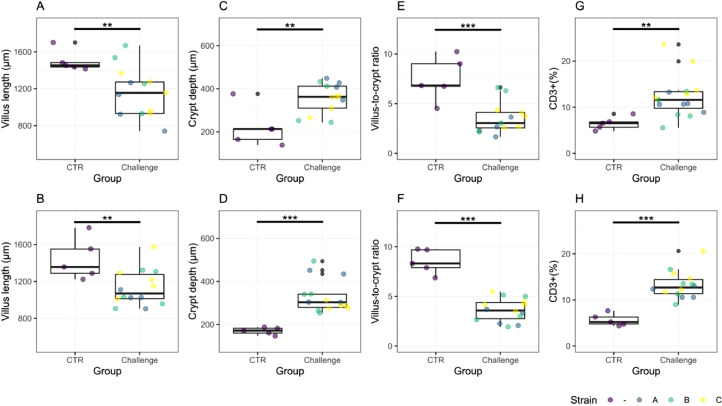
Figure 4.
Box plots of the intestinal morphometrical measurements (villus length, crypt depth, villus-to-crypt ratio) and gut inflammation (CD3+ T-lymphocytes area percentage) on d 21 (A, C, E, H) and d 22 (B, D, F, G). The strains (A, B, C) of the challenge group are illustrated in different colors (blue = A = Hungary field strains, green = B = Netherlands field strains, yellow = C = Poland field strains). Gut histomorphology (villus length, crypt depth, villus-to-crypt ratio) and intestinal inflammation (CD3+) is significantly different between the control and challenge groups on both sampling days (P < 0.05).
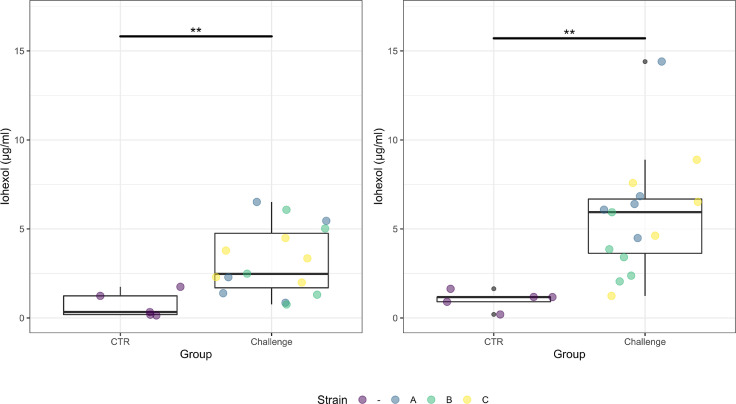
Figure 5.
Box plots showing the iohexol serum concentrations on d 20 (A) and d 21 (B). The strains (A, B, C) of the challenge group are illustrated in different colors (blue = A = Hungary field strains, green = B = Netherlands field strains, yellow = C = Poland field strains). The iohexol concentration was significantly higher in the challenge groups compared to the control group on d 20 (P = 0.0188) and d 21 (P = 0.0031).
Individual body weight. There was no significant effect of the Eimeria challenge on the body weight on d 20 (P = 0.461) and d 21 (P = 0.578) (Figure 2).
Coccidiosis lesion scores. The TMLS was significantly higher in the challenge group compared to the control group on d 21 (P < 0.001) and 22 (P < 0.001) (Figure 3). The scores for E. acervulina (P < 0.001 for d 21 and 22) and for E. maxima (P = 0.005 for d 21, P < 0.001 for d 22) were significantly different between the control and challenge groups on both sampling days.
Histopathology. The Eimeria challenge affected the gut morphology and caused an increase of CD3+ T-cell lymphocytes (CD3+ %) in the wall of the small intestine (Figure 4). On d 21 and 22, the challenge groups had a significant lower villus length (D21: P = 0.013, D22: P = 0.007), higher crypt depth (D21: P = 0.004, D22: P = 0.001), lower villus-to-crypt ratio (D21: P = 0.001, D22: P < 0.001) and a higher CD3+ % (D21: P = 0.003, D22: P < 0.001) compared to the control group.
Iohexol concentration. The concentration of the IP marker iohexol was significantly higher in the serum of challenged birds compared to the control birds on d 20 (P = 0.019) and on d 21 (P = 0.003) (Figure 5).
Association Between the Iohexol Concentrations and the Gut Histologic Parameters
The association between the serum iohexol concentrations and the gut histologic parameters (CD3+ %, villus length, crypt depth and the villus-to-crypt ratio) was evaluated with a multiple linear regression model (Figure 6).
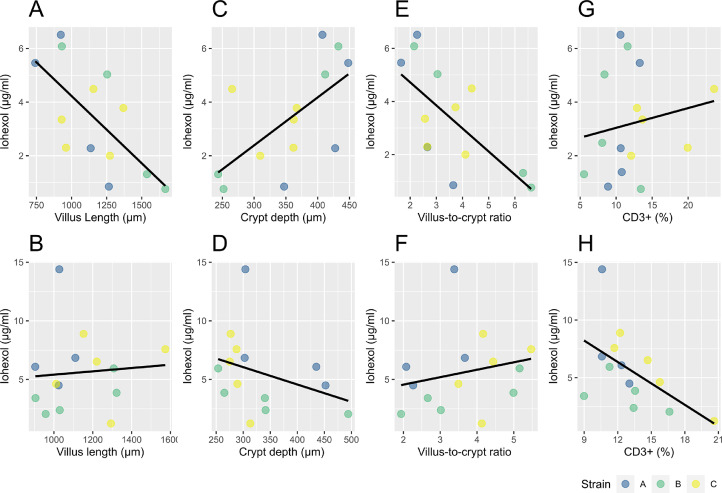
Figure 6.
Multiple linear regression results presented in scatterplots for the iohexol serum concentrations of d 20 and the gut histologic parameters of d 21 (A, C, E, G). The bottom row (B, D, F, H) presents the association between the iohexol serum concentrations (d 21) and the gut histology (d 22). The strains (A, B, C) of the challenge groups are illustrated in different colors (blue = A = Hungary field strains, green = B = Netherlands field strains, yellow = C = Poland field strains) and a regression line illustrates the linear association between the gut histology (CD3+ (%), villus length, crypt depth and the villus-to-crypt ratio) and iohexol concentration. A significant association is observed between the iohexol concentration on d 20 and the gut morphology (villus length, crypt depth, villus-to-crypt ratio) on d 21 (A, C, E), but not between the iohexol gavage on d 21 and the gut histology on d 22 (B, D, F). The CD3+ (%) on d 22 is only linked with the iohexol concentration on d 21 (H).
On d 21, a significant negative association (P = 0.009) was obtained between the serum iohexol concentration (measured on d 20) and the villus length. The iohexol concentration was also significantly associated with the crypt depth (P = 0.027) and had a borderline significant association (P = 0.057) with the villus-to-crypt ratio. The CD3+ % did not correlate with the iohexol concentrations on d 21.
For d 22, no significant associations were obtained between the serum iohexol concentrations (measured on d 21) and the villus length (P = 0.474) and the villus-to-crypt ratio (P = 0.118). Only the crypt depth had a borderline significant association (P = 0.055) with the serum iohexol concentration. The CD3+ % was shown to be significant negatively associated with the serum iohexol concentration (P = 0.021).
DISCUSSION
Our study is the first that uses iohexol to evaluate IP in broiler chickens under intestinal challenge. Previous studies have demonstrated the effectiveness of iohexol as a marker for IP in humans and mammals (Klenner et al., 2009; Wilson et al., 2009; Gerova et al., 2011; Frias et al., 2012). More recently an experimental study evaluated iohexol as an IP marker in healthy birds (Wilhelm et al., 2020). In addition, increased serum iohexol concentrations after oral administration could be linked with several gastrointestinal diseases in humans and rodent models (Halme et al., 2000; Andersen et al., 2001; Gerova et al., 2011; Ortín-Piqueras et al., 2021). These conclusions are in line with the present study in which we were able to measure a quantitative difference in IP using iohexol in broiler chickens. We observed a significant higher iohexol concentration in the serum of broilers infected with E. acervulina and E. maxima, indicating an altered IP on both sampling days. This result confirms the effectiveness of iohexol as an IP marker in avian species as described in Wilhelm et al., 2020. In our study, we used the protozoa Eimeria to induce IP. This parasite damages the intestine through developing and multiplying in the epithelial cells of the gut wall, resulting into cell lysis and an elevated gut permeability (Williams, 2005; Dos Santos et al., 2020). A recent study demonstrated the dynamic change of the gut permeability during a mixed Eimeria infection and concluded that IP was significantly increased 5 to 7 d post infection (dpi) for the high-dose treatment (50.000 oocysts of E. maxima, 50.000 oocysts of E.tenella and 250.000 oocysts of E. acervulina), with the highest IP at 5 dpi (Teng et al., 2020). We observed a similar result with a significant higher IP at 4 and 5 dpi (i.e., D20 and D21). However, the experimental setup, different Eimeria species and/or strains, different Eimeria doses and the use of a different IP marker need to be taken in consideration.
The molecular weight of the IP marker matters for passing the gut barrier via paracellular passage (Loehry et al., 1970). The water soluble marker iohexol has a lower molecular weight (821.14 Da) compared to the IP marker FITC-d (MW 4,000 Da) that was used in the study of Teng et al. (2020). Smaller molecules as iohexol can diffuse more rapidly through the gut barrier (Loehry et al., 1970) and could therefore possibly be used to detect small intestinal alterations in an earlier stage of the Eimeria infection compared to large molecular markers, but further research is necessary to support this hypothesis.
For our study, it was important to know whether the Eimeria challenge was successful. We used the TMLS with the individual scores for E. acervulina and E. maxima, gut morphology parameters (villus length, crypt depth, villus-to-crypt ratio), CD3+ % and BW as parameters for the success rate of the coccidiosis trial. As mentioned previously, Eimeria spp. damage the intestinal wall causing gut alterations, induce intestinal inflammation and a BW reduction (Williams, 2005; Dos Santos et al., 2020). The results of the current study are in agreement with previous work showing that birds of the challenge groups have a higher TMLS lesion score, higher mean lesion scores of E. acervulina and E. maxima, villus atrophy and gut inflammation (Williams, 2005; Schneiders et al., 2020; Teng et al., 2020). The BW was the only parameter that was not significantly lower in challenged birds. This is in contradiction with previous performed studies where it was concluded that the challenged birds had a significant lower BW (Nabian et al., 2018; Teng et al., 2020). However, the BW of broilers can be influenced by several other factors (Baracho et al., 2019) and the number of birds in our study was not sufficient to evaluate performance. Moreover, the inoculation dose, type of strain and the duration of the study has an impact on the performance of the broilers as well (Taylor et al., 2022).
The iohexol concentrations of our control animals were lower than the ones measured in layer chickens at 1 mL/kg, corresponding to a dose of 755 mg iohexol/kg BW (Wilhelm et al., 2020). They measured the iohexol concentration at 45, 90, and 180 min after the oral gavage of iohexol. The difference with the control group values in our study might be due to the higher dose administered and /or the fasting period (between 2 and 6 h) before the iohexol gavage in the study of Wilhelm et al. (2020), which generally results in higher concentrations. Several studies observed an increased IP in broilers after a feed restriction period (Kuttappan et al., 2015; Vicuña et al., 2015; Gilani et al., 2018a). In addition, the analysis of iohexol was performed with a different type of analysis, that is, enzyme-linked immunosorbent assay (ELISA). However, a recent study observed a strong correlation between iohexol plasma concentrations measured with ELISA and with rapid high-performance liquid chromatography-ultraviolet detection in canine plasma (Ortin-Piqueras et al., 2018). Moreover, analysis of iohexol with UHPLC-MS/MS has also been performed on blood microsamples, such as volumetric absorptive microsamples using blood volumes as low as 10 µL. This blood microsampling technique offers great potential in humans and animals as less invasive technique, especially in the pediatric population and small animal species such as birds, and/or when repetitive blood sampling is required (Dhondt et al., 2021).
The effect of coccidiosis on the gut morphology and intestinal inflammation has been widely investigated (Allen and Fetterer, 2002; Williams, 2005; Nabian et al., 2018). Alterations of the gut morphology and IP during a coccidiosis trial have previously been evaluated simultaneously (Schneiders et al., 2020; Teng et al., 2020). However, these studies did not focus on linking IP with gut morphology nor with intestinal CD3+T-lymphocyte infiltration. According to our results, IP is significantly linked with gut morphology parameters 5 dpi. Six dpi, the IP had only a significant negative association with crypt depth and gut inflammation (CD3+ %). We cannot give a possible biological explanation for the latter negative link.
CONCLUSIONS
In conclusion, we evaluated the nonradioactive contrast medium iohexol as an IP marker under Eimeria challenge. Four and 5 d post infection, the iohexol concentration in serum was significantly higher in broilers infected with E. acervulina and E. maxima. This shows that iohexol can be a successful marker to measure IP alterations in broilers with gastrointestinal disorders, possibly already at an early stage. We were also able to find significant correlations between the iohexol concentration and gut morphology parameters (villus length, crypt depth, villus-to-crypt ratio) on 5 dpi. Iohexol gavage could be one of the alternatives in the evaluation of gut barrier defects.
ACKNOWLEDGMENTS
This work was supported by the Industrial Research Fund (IOF) - Ghent University [F2018/IOF-StepStone/429].
The technical assistance of M. Cherlet and J. Lambrecht in the iohexol analyses was gratefully appreciated. The iohexol serum concentrations were determined using a UHPLC-MS/MS instrument part of the Ghent University MSsmall Expertise Centre for advanced mass spectrometry analysis of small organic molecules.
DISCLOSURES
The authors declare no conflicts of interest
REFERENCES
- Allen P.C., Fetterer R.H. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002;15:58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R., Stordahl A., Aase S., Laerum F. Intestinal permeability of X-Ray contrast media iodixanol and iohexol during bacterial overgrowth of small intestines in rats. Dig. Dis. Sci. 2001;46:208–213. doi: 10.1023/a:1005630429723. [DOI] [PubMed] [Google Scholar]
- Baracho M., Nääs I., Lima N., Cordeiro A.F.S., Moura D. Factors affecting broiler production: a meta-analysis. Rev. Bras. Cienc. Avic. 2019;21 doi: 10.1590/1806-9061-2019-1052. [DOI] [Google Scholar]
- Barekatain R., Chrystal P.V., Howarth G.S., McLaughlan C.J., Gilani S., Nattrass G.S. Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult. Sci. 2019;98:6761–6771. doi: 10.3382/ps/pez393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt L., Croubels S., De Cock P., Dhont E., De Baere S., De Paepe P., Devreese M. Volumetric absorptive microsampling as alternative sampling technique for renal function assessment in the paediatric population using iohexol. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021;1171 doi: 10.1016/j.jchromb.2021.122623. [DOI] [PubMed] [Google Scholar]
- Dos Santos T.S., Teng P.Y., Yadav S., Castro F.L.S., Gould R.L., Craig S.W., Chen C., Fuller A.L., Pazdro R., Sartori J.R., Kim W.K. Effects of inorganic Zn and Cu supplementation on gut health in broiler chickens challenged with Eimeria spp. Front. Vet. Sci. 2020;7:230. doi: 10.3389/fvets.2020.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias R., Strube K., Ternes W., Collado M.C., Spillmann T., Sankari S., Westermarck E. Comparison of 51chromium-labeled ethylenediamine tetra-acetic acid and iohexol as blood markers for intestinal permeability testing in Beagle dogs. Vet. J. 2012;192:123–125. doi: 10.1016/j.tvjl.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Gasthuys E., Montesinos A., Caekebeke N., Devreese M., Baere S., Ardiaca M., Paepe D., Croubels S., Antonissen G. Comparative physiology of glomerular filtration rate by plasma clearance of exogenous creatinine and exo-iohexol in six different avian species. Sci. Rep. 2019;9:19699. doi: 10.1038/s41598-019-56096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerova V.A., Stoynov S.G., Katsarov D.S., Svinarov D.A. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J. Gastroenterol. 2011;17:2211–2215. doi: 10.3748/wjg.v17.i17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani S., Chrystal P.V., Barekatain R. Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 2021;7:801–811. doi: 10.1016/j.aninu.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Kitessa S.M., Tran C.D., Forder R.E.A., Hughes R.J. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2017;101:e237–e245. doi: 10.1111/jpn.12596. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Tran C.D., Barekatain R., Kitessa S.M., Forder R.E.A., Hughes R.J. Reduced fasting periods increase intestinal permeability in chickens. J. Anim. Physiol. Anim. Nutr. 2018;102:e486–e492. doi: 10.1111/jpn.12712. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Tran C.D., Kitessa S.M., Forder R.E.A., Barekatain R., Hughes R.J. Effects of delayed feeding, sodium butyrate and glutamine on intestinal permeability in newly-hatched broiler chickens. J. Appl. Anim. Res. 2018;46:973–976. [Google Scholar]
- González-González M., Díaz-Zepeda C., Eyzaguirre-Velásquez J., González-Arancibia C., Bravo J.A., Julio-Pieper M. Investigating gut permeability in animal models of disease. Front. Physiol. 2018;9:1962. doi: 10.3389/fphys.2018.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.J., Batt R.M. Urinary excretion by dogs of intravenously administered simple sugars. Res. Vet. Sci. 1996;60:280–282. doi: 10.1016/s0034-5288(96)90056-9. [DOI] [PubMed] [Google Scholar]
- Halme L., Turunen U., Tuominen J., Forsström T., Turpeinen U. Comparison of iohexol and lactulose-mannitol tests as markers of disease activity in patients with inflammatory bowel disease. Scand. J. Clin. Lab. Invest. 2000;60:695–701. doi: 10.1080/00365510050216420. [DOI] [PubMed] [Google Scholar]
- Hornbuckle W.E., Simpson K.W., Tennant B.C. In: Clinical Biochemistry of Domestic Animals. (6th ed.) Kaneko J.J., Harvey J.W., Bruss M.L., editors. Academic Press; San Diego, CA: 2008. Gastrointestinal function; pp. 413–457. [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Klenner S., Frias R., Coenen M., Failing K., Hewicker-Trautwein M., Ternes W., Verspohl J., Spillmann T. Estimation of intestinal permeability in healthy dogs using the contrast medium iohexol. Vet. Clin. Pathol. 2009;38:353–360. doi: 10.1111/j.1939-165X.2009.00136.x. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Vicuña E.A., Latorre J.D., Wolfenden A.D., Téllez G.I., Hargis B.M., Bielke L.R. Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front. Vet. Sci. 2015;2:66. doi: 10.3389/fvets.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois V. In: Comprehensive Pediatric Nephrology. Geary D.F., Schaefer F., editors. Mosby; Philadelphia, PA: 2008. Chapter 2—Laboratory evaluation at different ages; pp. 39–54. [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C., Bielke L.R., Hargis B.M., Tellez G. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-Y., Chun M.-R., Kim D.-J., Kim J.W. Determination of iohexol clearance by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;839:124–129. doi: 10.1016/j.jchromb.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Lenth, R.V. 2022. emmeans: estimated marginal means, aka least-squares means. R package version 1.7.3. Accessed Dec. 2022. https://CRAN.R-project.org/package=emmean.
- Liu J., Teng P.-Y., Kim W.K., Applegate T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): an indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehry C.A., Axon A.T., Hilton P.J., Hider R.C., Creamer B. Permeability of the small intestine to substances of different molecular weight. Gut. 1970;11:466–470. doi: 10.1136/gut.11.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G.R., Sutherland L.R., Meddings J.B. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology. 1993;104:1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- Nabian S., Arabkhazaeli F., Seifouri P., Farahani A. Morphometric analysis of the intestine in experimental coccidiosis in broilers treated with anticoccidial drugs. Iran J. Parasitol. 2018;13:493–499. [PMC free article] [PubMed] [Google Scholar]
- Ortín-Piqueras V., Freitag T.L., Andersson L.C., Lehtonen S.H., Meri S.K., Spillmann T., Frias R. Urinary excretion of iohexol as a permeability marker in a mouse model of intestinal inflammation: time course, performance and welfare considerations. Animals (Basel) 2021;11:79. doi: 10.3390/ani11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Piqueras V., Spillmann T., Pöytäkangas M., Vaccaro D.E., Sankari S., Frias R. Determination of iohexol in canine plasma - strong correlation between enzyme-linked immunosorbent assay, high-performance liquid chromatography, and neutron activation analysis. Scand. J. Lab. Anim. Sci. 2018;44:1–7. [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Accessed Dec. 2022. https://www.R-project.org/.
- Salvo-Romero E., Alonso-Cotoner C., Pardo-Camacho C., Casado-Bedmar M., Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015;107:686–696. doi: 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- Schneiders G.H., Foutz J.C., Milfort M.C., Ghareeb A.F.A., Sorhue U.G., Richter J.N., Fuller A.L., Williams S.M., Rekaya R., Aggrey S.E. Ontogeny of intestinal permeability in chickens infected with Eimeria maxima: implications for intestinal health. Adv. Parasitol. 2020;6:41–50. [Google Scholar]
- Schoultz I., Keita Å.V. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020;9:1909. doi: 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant L., Croubels S., Dhondt L., Millecam J., De Baere S., Gasthuys E., Morrens J., Antonissen G. Simultaneous measurement of glomerular filtration rate, effective renal plasma flow and tubular secretion in different poultry species by single intravenous bolus of iohexol and para-aminohippuric acid. Animals (Basel) 2020;10:1027. doi: 10.3390/ani10061027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Walk C., Misiura M., Sorbara J.-O.B., Giannenas I., Kyriazakis I. Quantifying the effect of coccidiosis on broiler performance and infection outcomes in the presence and absence of control methods. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.-Y., Yadav S., Castro F.L.d.S., Tompkins Y.H., Fuller A.L., Kim W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M., Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Vivinus-Nébot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R., Filippi J., Saint-Paul M.C., Tulic M.K., Verhasselt V., Hébuterne X., Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- Wilhelm F.R., Krautwald-Junghanns M.-E., Ortín-Piqueras V., Junnila J., Cramer K., Forsgård R.A., Frias R., Spillmann T., Schmidt V. Iohexol-based measurement of intestinal permeability in birds. J. Exot. Pet. Med. 2020;34:18–23. [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wilson K.E., Wilcke J.R., Crisman M.V., Ward D.L., McKenzie H.C., Scarratt W.K. Comparison of serum iohexol clearance and plasma creatinine clearance in clinically normal horses. Am. J. Vet. Res. 2009;70:1545–1550. doi: 10.2460/ajvr.70.12.1545. [DOI] [PubMed] [Google Scholar]
- Zanu H.K., Kheravii S.K., Morgan N.K., Bedford M.R., Swick R.A. Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: Part 2. Inositol phosphate esters hydrolysis, intestinal permeability, hematology, jejunal gene expression and intestinal morphology. Anim. Nutr. 2020;6:488–498. doi: 10.1016/j.aninu.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]