Highlights
-
•
White matter mapping in brain tumor patients using directionally encoded color (DEC)
-
•
Diffusion tensor vs. advanced DEC maps from post-tractography track density imaging.
-
•
Expert raters prefer advanced DEC maps for neurosurgical planning.
-
•
These maps could help guide neurosurgical planning for brain tumor resections.
-
•
Clinically typical dMRI acquisition parameters allow for generalizability of results.
Keywords: Track density imaging, Directionally encoded color maps, Brain tumor, Neurosurgical planning
Abstract
Background
Diffusion magnetic resonance imaging white matter tractography, an increasingly popular preoperative planning modality used for pre-surgical planning in brain tumor patients, is employed with the goal of maximizing tumor resection while sparing postoperative neurological function. Clinical translation of white matter tractography has been limited by several shortcomings of standard diffusion tensor imaging (DTI), including poor modeling of fibers crossing through regions of peritumoral edema and low spatial resolution for typical clinical diffusion MRI (dMRI) sequences. Track density imaging (TDI) is a post-tractography technique that uses the number of tractography streamlines and their long-range continuity to map the white matter connections of the brain with enhanced image resolution relative to the acquired dMRI data, potentially offering improved white matter visualization in patients with brain tumors. The aim of this study was to assess the utility of TDI-based white matter maps in a neurosurgical planning context compared to the current clinical standard of DTI-based white matter maps.
Methods
Fourteen consecutive brain tumor patients from a single institution were retrospectively selected for the study. Each patient underwent 3-Tesla dMRI scanning with 30 gradient directions and a b-value of 1000 s/mm2. For each patient, two directionally encoded color (DEC) maps were produced as follows. DTI-based DEC-fractional anisotropy maps (DEC-FA) were generated on the scanner, while DEC-track density images (DEC-TDI) were generated using constrained spherical deconvolution based tractography. The potential clinical utility of each map was assessed by five practicing neurosurgeons, who rated the maps according to four clinical utility statements regarding different clinical aspects of pre-surgical planning. The neurosurgeons rated each map according to their agreement with four clinical utility statements regarding if the map 1 identified clinically relevant tracts, (2) helped establish a goal resection margin, (3) influenced a planned surgical route, and (4) was useful overall. Cumulative link mixed effect modeling and analysis of variance were performed to test the primary effect of map type (DEC-TDI vs. DEC-FA) on rater score. Pairwise comparisons using estimated marginal means were then calculated to determine the magnitude and directionality of differences in rater scores by map type.
Results
A majority of rater responses agreed with the four clinical utility statements, indicating that neurosurgeons found both DEC maps to be useful. Across all four investigated clinical utility statements, the DEC map type significantly influenced rater score. Rater scores were significantly higher for DEC-TDI maps compared to DEC-FA maps. The largest effect size in rater scores in favor of DEC-TDI maps was observed for clinical utility statement 2, which assessed establishing a goal resection margin.
Conclusion
We observed a significant neurosurgeon preference for DEC-TDI maps, indicating their potential utility for neurosurgical planning.
1. Introduction
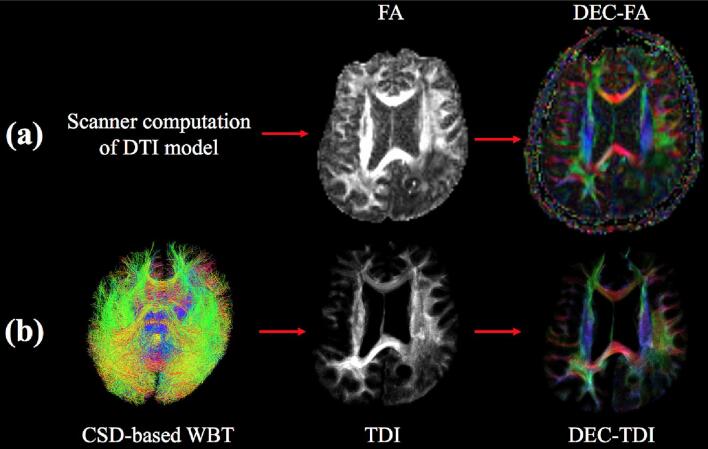
Diffusion magnetic resonance imaging (dMRI) detects the naturally occurring diffusion of water in the body as a means to infer the underlying tissue structure (Le Bihan and Johansen-Berg, 2012). In oncological brain surgery, the surgeon must balance two important factors: maximizing resection of the tumor and minimizing damage of surrounding critical brain tissue (Essayed et al., 2017). Through preoperative dMRI imaging and tractography, neurosurgeons can identify the location of the tumor relative to eloquent white matter tracts that must be preserved (Becker et al., 2020, Essayed et al., 2017, Henderson et al., 2020, Panesar et al., 2019, Yang et al., 2021). dMRI is often visualized clinically using directionally encoded color (DEC) maps (Pajevic and Pierpaoli, 2000) (Fig. 1). DEC maps employ a standardized color scheme (blue = superior-inferior, red = left–right, green = anterior-posterior) to represent the orientation of the white matter fiber tracts being visualized. Preoperative DEC map visualization may aid in assessing the type and degree of white matter tract involvement in brain tumor patients including whether tracts have been infiltrated, displaced or destroyed by the tumor (Field et al., 2005, Field et al., 2004, Schneider et al., 2021, Schonberg et al., 2006, Witwer et al., 2002, Young, 2007).
Fig. 1.
Overview of (a) DEC-FA and (b) DEC-TDI map creation with representative visualizations shown for brain tumor patient 1. CSD = constrained spherical deconvolution, DEC = directionally encoded color, DTI = diffusion tensor imaging, FA = fractional anisotropy, TDI = track density imaging, WBT = whole brain tractography.
Traditional DEC maps are based on the diffusion tensor imaging (DTI) model, where the major eigenvector of the tensor model represents the primary white matter fiber orientation of the tissue (Basser et al., 1994). In these traditional DEC maps, the brightness of each voxel reflects the local fractional anisotropy (FA), a measure of the variation in diffusion direction (O’Donnell and Westin, 2011). For clarity, in the rest of this report we will refer to these traditional DEC maps as DEC-FA maps.1 Because DEC-FA maps are automatically produced by the MRI scanner from clinical DTI sequences, they provide a convenient and rapid method for visualizing white matter tracts.
However, the DTI model, which only represents a single tract orientation per voxel, has a limited capability to resolve areas of crossing fiber tracts (Farquharson et al., 2013, Jeurissen et al., 2013). Another well described limitation of DTI is its less robust modeling of fiber orientation in regions containing peritumoral edema in the setting of brain lesions (Essayed et al., 2017, Gong et al., 2018, Zhang et al., 2013). Finally, the spatial resolution of clinical DTI may be insufficient to enable visualization of fine details of white matter structure (Calamante et al., 2010). Higher-order multi-fiber models, such as constrained spherical deconvolution (CSD) (Tournier et al., 2007), potentially overcome many limitations of DTI-based methods, including the modeling of crossing fibers and improved tracking through edema (Becker et al., 2020, Chen et al., 2015, Essayed et al., 2017, Gong et al., 2018, Henderson et al., 2020, Mormina et al., 2015).
Track Density Imaging (TDI) is a technique that leverages dMRI tractography to create a white matter map with enhanced resolution relative to the acquired dMRI data (Calamante et al., 2013, Calamante et al., 2010). TDI maps enable high-resolution visualization of the track density, defined as the number of tractography streamlines passing through each image voxel. It has been suggested that high-resolution TDI maps may confer additional anatomical detail to neurosurgeons planning brain tumor resections (Calamante et al., 2010, Wei et al., 2017). The TDI framework can be used to create high-resolution DEC maps, where color represents the average direction of all tractography streamlines passing through a given voxel, and brightness is determined by the local track density (Calamante et al., 2010). We refer to these maps as DEC-TDI (Fig. 1). These maps benefit from white matter tractography based on a higher-order multi-fiber model, which has known advantages for neurosurgical planning (Becker et al., 2020, Essayed et al., 2017, Farquharson et al., 2013, Henderson et al., 2020, Mormina et al., 2015). Therefore, DEC-TDI may offer improved white matter visualization in patients with brain tumors.
The main aim of this study was to assess the potential of DEC-TDI as a tool for neuro-oncology surgical planning. We compared the potential clinical utility of DEC-TDI maps versus scanner default DEC-FA maps according to neurosurgeons’ expert rating. We developed four clinical utility statements to rate the maps. Five practicing neurosurgeons participated in the expert rating process. We found rater scores were significantly higher for DEC-TDI maps than DEC-FA maps on data from fourteen consecutive brain tumor patients. Overall, we observed a general neurosurgeon preference for DEC-TDI maps, indicating their potential utility for neurosurgical planning.
2. Methods
2.1. Subjects
Sixteen consecutive brain tumor patients treated by a single neurosurgeon [AJG] with clinical MRI scans collected from 8/22/2019–7/28/2020 at Brigham and Women’s Hospital in Boston, MA were identified. One patient was excluded due to excessive motion artifacts and another was excluded due to a processing error in the tumor segmentation map, leaving fourteen (eight male, six female; average age, 55.6 years, age range 23–73 years) for inclusion in this study (Table 1). Tumor grade breakdown of the included study sample was: one grade II, one grade III, seven grade IV, one unclear grade, and four metastatic disease. Handedness was determined according to the Edinburgh Handedness Inventory for all patients except for BTP 2, whose handedness is self-reported. The study was approved by the Partners Healthcare Institutional Review Board, and written informed consent was obtained from all subjects prior to participation.
Table 1.
Patient characteristics. Peritumoral edema is categorized as extensive (spanning > 50% of a single lobe or multiple lobes), limited (spanning < 50% of a single lobe), or none. BTP = brain tumor patient, GBM = glioblastoma multiforme, WHO = World Health Organization.
| Patient | Sex | Age (y) |
Handedness | Pathology Diagnosis | WHO Grade | Tumor Location | Tumor diameter (mm) | Peritumoral edema (none, limited, extensive) |
|---|---|---|---|---|---|---|---|---|
| BTP 1 | M | 73 | Right | Metastasis | N/A | Left parietal | 22.3 | Extensive |
| BTP 2 | M | 69 | Right | Metastasis | N/A | Left frontal | 11.9 | Extensive |
| BTP 3 | M | 68 | Left | Oligodendro-glioma | III | Left frontal | 49.1 | Limited |
| BTP 4 | F | 39 | Right | GBM | IV | Left temporal | 14.4 | Limited |
| BTP 5 | F | 23 | Right | GBM | IV | Left frontal | 30.8 | Extensive |
| BTP 6 | M | 53 | Right | GBM | IV | Left temporal | 44.2 | Limited |
| BTP 7 | M | 63 | Right | GBM | IV | Left frontal | 18.0 | Extensive |
| BTP 8 | M | 47 | Right | Astrocytoma | Unclear | Right temporal | 62.9 | None |
| BTP 9 | M | 67 | Left | GBM | IV | Right frontal | 24.7 | Limited |
| BTP 10 | F | 65 | Right | Metastasis | N/A | Left frontal | 17.8 | Limited |
| BTP 11 | F | 66 | Right | GBM | IV | Right frontal | 11.0 | Limited |
| BTP 12 | F | 32 | Right | Astrocytoma | II | Left temporal | 38.5 | None |
| BTP 13 | M | 53 | Ambidex- trous |
GBM | IV | Right temporal | 69.6 | Extensive |
| BTP 14 | F | 53 | Ambidex- trous |
Metastasis | N/A | Left frontal | 54.3 | Extensive |
2.2. Data acquisition and DEC-FA map generation
Preoperative structural and diffusion images were acquired using Siemens 3T scanners (Siemens Trio and Verio, Siemens Healthcare, Erlangen, Germany). Diffusion-weighted images (DWI) were acquired using an echo planar imaging (EPI) sequence (30 gradient directions, 6 baseline (b = 0) images, b = 1000 s/mm2, TR = 3200 ms, TE = 58 ms, flip angle = 90°, voxel size = 2.0 mm isotropic). DEC-FA DICOM files were output directly by the MRI scanner and were computed from the DWI data using diffusion tensor modeling. T1-weighted scans (TR = 1900–2000 ms, TE = 252–340 ms, flip angle = 9–15°, voxel size = 1.0 mm isotropic) and T2-weighted scans (TR = 2000 ms, TE = 232 ms, flip angle = 120°, voxel size = 1.0 mm isotropic) were acquired as clinically indicated for each patient.
2.3. DWI data preprocessing
We applied a minimal preprocessing pipeline that has been applied in several of our clinical studies (Chen et al., 2016, Chen et al., 2015, Gong et al., 2018, O’Donnell et al., 2017). DEC-FA and DWI DICOM data were converted to NIFTI format using the Diffusion-weighted DICOM Import (DCM2niixGUI) module (Li et al., 2016) in 3D Slicer (https://www.slicer.org) (Fedorov et al., 2012) via the SlicerDMRI project (https://dmri.slicer.org) (Norton et al., 2017, Zhang et al., 2020b). Binary brain masks were automatically generated in 3D Slicer using the Diffusion Brain Masking module. MP-PCA denoising was applied to the NIFTI DWI data using the MRTrix3 software package (J-D Tournier, Brain Research Institute, Melbourne, Australia; https://github.com/MRtrix3/mrtrix3) (Tournier et al., 2019, Veraart et al., 2016). This step is recommended to reduce the contribution of noise to anatomical details seen in TDI maps (Dhollander et al., 2014, Dhollander et al., 2012). We applied DTIPrep (https://www.nitrc.org/projects/dtiprep) (Oguz et al., 2014) to perform motion and eddy current distortion correction of the denoised DWI data.
2.4. Tractography
Whole brain single tissue probabilistic constrained spherical deconvolution (CSD)-based tractography without anatomical constraints was performed for each patient DWI dataset in MRTrix using the second order integration over fiber orientation distributions (iFOD2) algorithm (Tournier et al., 2007, Tournier et al., 2010). Tractography streamlines were randomly seeded within the binary brain masks generated for each patient. Tractography parameters were chosen to be consistent with previous TDI studies (Calamante et al., 2010, Calamante et al., 2011, Calamante et al., 2012), as follows. We used a sufficiently large number of streamlines (2 million) to achieve a nearly continuous white matter representation for TDI (Calamante et al., 2010, Calamante et al., 2011). The tractography step size (0.3 mm) was set smaller than the TDI grid size (0.5 mm) to enable TDI to exploit subvoxel tractography information (Calamante et al., 2010). The maximum harmonic order was set to 6 based on the number of DWI gradient directions (Tournier et al., 2009). The remaining tractography parameters were set to default values for consistency with TDI studies (Calamante et al., 2010, Calamante et al., 2011, Calamante et al., 2012) and recent CSD-based glioma patient tractography studies (Fekonja et al., 2020, Schult et al., 2019, Sheng et al., 2021), as follows: maximum branching angle = 45°, 4 FOD samples per step, minimum fiber length = 10 mm (5 × voxel size), maximum fiber length = 200 mm (100 × voxel size), fiber orientation distribution (FOD) amplitude threshold for seeding/stopping = 0.1.
2.5. DEC-TDI map generation
CSD-derived DEC-TDI maps were calculated in MRtrix at 0.5 mm isotropic grid size using each patient’s baseline b = 0 image as a template for consistent track display in diffusion space (Calamante et al., 2010). The grid size determines the image resolution of the DEC-TDI map, and a grid size of 0.5 mm is consistent with the TDI literature (Barajas et al., 2013, Calamante et al., 2010, Dhollander et al., 2014, Woodworth et al., 2015, Ziegler et al., 2014).
2.6. Tumor segmentation and creation of visualizations for expert rating
For visualization, each patient’s T1- and T2-weighted images, DEC-FA and DEC-TDI maps were rigidly aligned using the General Registration (BRAINS) and Transforms modules in 3D Slicer. A physician with significant surgical planning experience [PJ] segmented brain tumors in 3D Slicer with reference to preoperative T1- and/or T2-weighted brain MR images with and without gadolinium contrast. Tumor diameters were then measured in axial slices using the Annotations module in 3D Slicer. For each patient, the single axial slice with the largest tumor diameter was selected for visualization for expert rating. If a patient had multiple lesions, the diameters of the individual lesions were summed to determine the appropriate axial slice. Then screenshots of the selected axial slices were captured (Fig. 2) including T1- and T2- weighted images, DEC-FA, and DEC-TDI maps. For anatomical reference, tumor outlines were overlaid on all images. For consistent comparison across DEC maps, the brightness and contrast was tuned to provide a similar image intensity: DEC-FA maps were viewed at a window level of 100 and window width of 200, while DEC-TDI maps were viewed at a window level of 75 and a window width of 150.
Fig. 2.
Example visualizations from two patients. (a) BTP 4, 39-year old female with glioblastoma, (b) BTP 12, 32-year old female with astrocytoma. Shown from left to right: single axial slices of the T1-weighted image, T2-weighted image, DEC-FA map, and DEC-TDI map. Tumor outlines are provided in pink on each image for reference. DEC = directionally encoded color, FA = fractional anisotropy, T1 = T1-weighted image, T2 = T2-weighted image, TDI = track density imaging. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.7. Clinical utility criteria
We defined criteria for an effective pre-surgical planning white matter visualization with the input of two practicing neurosurgeons [PJ, AG]. The neurosurgeons recommended assessing the ability of each map to help demonstrate clinically relevant peritumoral white matter tracts, to establish a goal margin of resection, and to influence the planned surgical route, as well as to assess the overall usefulness of each map. Therefore, we developed the following four clinical utility statements for evaluation of DEC maps:
-
1.
This DEC map demonstrates clinically relevant tracts in the peritumoral region (i.e. areas of T1/T2 signal change).
-
2.
The resolution of this DEC map is helpful in establishing a goal margin of resection.
-
3.
This DEC map would influence my planned surgical route.
-
4.
Overall, this DEC map is useful.
2.8. Expert rating
Five MDs [EFT, AB, PJ, WIE, DB] with extensive neurosurgical training performed expert rating to assess how well each DEC map met the four clinical utility criteria described above. To ensure that raters were blinded to map type and patient, the 28 DEC maps (two per patient) were presented in a fully randomized order. Rating was performed using a survey in Qualtrics (Qualtrics, Provo, UT) following similar past methodology for expert rating of visualizations (Franke et al., 2021, Tie et al., 2014). The survey displayed images of the selected axial image slice (as described in Section 2.6) of a DEC-FA or DEC-TDI map, alongside the corresponding T1-weighted and T2-weighted images. (A visualization of all patient DEC maps as presented to the raters is provided in Supplementary Fig. 1.) After viewing each DEC map, the expert raters were asked to rate the degree to which they agreed with the four clinical utility statements described in Section 2.7. The expert responses followed a 5-point Likert scale (Likert, 1932): strongly disagree (1), somewhat disagree (2), neither agree nor disagree (3), somewhat agree (4), strongly agree (5). Each expert response was therefore recorded as a “rater score” between 1 and 5.
2.9. Statistical analysis
Our primary goal was to compare the DEC map types (DEC-FA or DEC-TDI) by assessing how map type impacted rater score for each clinical utility statement. The data were modeled using a two-way repeated ordinal regression with a cumulative link mixed effect model (CLMM) (McCullagh, 1980) following the methodology detailed by Mangiafico (2016). This model was chosen for its simplicity and efficacy in evaluating the individual effects of several variables (i.e., expert rater, map type, and interaction between expert rater and map type) on an ordinal dependent variable (i.e. rater scores via a 5-point Likert scale) (McCullagh, 1980). To account for individual differences across patients, a patient blocking variable was added to the models; likelihood ratio tests (Satorra and Saris, 1985) were conducted to assess the goodness of fit of its inclusion. The hypothesis that map type influenced rater score was tested using a type II analysis of deviance (ANODE) based on Wald χ2 tests (Christensen, 2018, Scheffé, 1999). In a post-hoc analysis, the effect of map type (DEC-FA vs. DEC-TDI) on rater score was measured using an estimated marginal means (EMM) analysis (Lenth and Love, 2018). Significance thresholds were set at α = 0.05. Analyses were performed in R, a statistical software program (R Core Team, 2020), using the “clmm,” “car,” “emmeans,” and “ggplot2” packages (Christensen, 2018, Kahle and Wickham, 2013, Lenth and Love, 2018).
3. Results
3.1. Data acquisition time
DWI data acquisition time was approximately 2–3 min per subject. Average tractography runtime across included subjects was 81.1 ± 12.1 min. DEC-TDI maps were generated in less than one minute following tractography.
3.2. Summary of rater responses
Fig. 3 shows aggregate rater score data (across the five neurosurgeons and fourteen patients). Expert raters found both maps to be useful overall: it can be observed that a majority of rater responses are shown in green (indicating “somewhat agree” or “strongly agree”) for both map types. Furthermore, based on the larger number of green responses for DEC-TDI, it is apparent that there is a general preference among the raters for the DEC-TDI maps (see Section 3.4 for statistical analyses).
Fig. 3.
Visual summary of rater scores. Stacked bar chart visualization of overall rater score results separated by map type (top: DEC-FA, bottom: DEC-TDI) for each of the four clinical utility statements (1. Identifies clinically relevant tracts, 2. Helps establish goal resection margin, 3. Influences planned surgical route, 4. Overall, is useful). Bars are labeled with the percentage of ratings receiving each score. DEC = directionally encoded color, FA = fractional anisotropy, TDI = track density imaging.
3.3. Visualization of selected patient data
Example cases were selected to illustrate neurosurgeon preferences for a particular DEC map. Fig. 4 shows one example patient (BTP 1) where the DEC-TDI map was preferred over the DEC-FA map with the largest difference in raw rater scores. The largest rater score difference in favor of DEC-TDI was observed for clinical utility statement 2, “The resolution of this DEC map is helpful in establishing a goal margin of resection.” This indicates that visualization of the tumor margin was the most important reason raters preferred this DEC-TDI map. Fig. 4 also shows the only patient (BTP 10) where the DEC-FA map was generally preferred over the DEC-TDI map. The largest rater score difference in favor of DEC-FA was observed for clinical utility statement 1, “This DEC map demonstrates clinically relevant tracts in the peritumoral region (i.e. areas of T1/T2 signal change).” This indicates that in this case, the visualization of apparent fiber tracts was a driving factor behind the rater preference for the DEC-FA map.
Fig. 4.
DEC maps illustrating expert rater preferences. DEC maps from BTP 1 ((a) full axial view, (b) zoomed-in peritumoral view), whose DEC-TDI map was consistently preferred over the DEC-FA map. DEC maps from BTP 10 ((c) full axial view, (d) zoomed-in peritumoral view), whose DEC-FA map was slightly preferred over the DEC-TDI map. Red boxes in rows (a) and (c) indicate the preferred map for each patient. Shown from left to right: single axial slices of the T1-weighted image, T2-weighted image, DEC-FA map, and DEC-TDI map. Tumor outlines are provided in pink on each image for reference. DEC = directionally encoded color, FA = fractional anisotropy, T1 = T1-weighted image, T2 = T2-weighted image, TDI = track density imaging. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Statistical analyses
For all four clinical utility statements, the cumulative model including patient as a random blocking variable significantly outperformed the model not including a blocking variable (Supplementary Table 1). Therefore, the cumulative model including the blocking variable, patient, was used for the following analyses.
The analysis of deviance results for the variables included in the cumulative model are reported in Table 2. Across the four clinical utility statements, the primary explanatory variable, map type, had a statistically significant effect on rater score. This finding indicates that map type significantly influenced rater score across all four investigated clinical utility statements. Both secondary explanatory variables, rater and map/rater interaction, also reached statistical significance across all clinical utility statements, indicating that rater and the interaction between map type and rater also influenced rater score.
Table 2.
Analysis of deviance results. A significant result indicates a significant contribution of a given explanatory variable on rater score. Map type was the primary explanatory variable of interest. χ2 = chi-squared statistic, df = degrees of freedom, N = number of data points for each statistical test.
| Clinical Utility Statements | Explanatory Variables |
|||||
|---|---|---|---|---|---|---|
| Map type |
Rater |
Map type/Rater Interaction |
||||
| χ2 (df = 1, N = 140) | p-value | χ2 (df = 4, N = 140) | p-value | χ2 (df = 4, N = 140) | p-value | |
| 1. Identifies clinically relevant tracts | 12.9 | 0.00033 | 12.6 | 0.013 | 23.5 | 0.00010 |
| 2. Helps establish goal resection margin | 55.5 | <0.0001 | 26.6 | <0.0001 | 41.8 | <0.0001 |
| 3. Influences planned surgical route | 10.6 | 0.0011 | 11.8 | 0.019 | 17.7 | 0.0014 |
| 4. Overall, is useful | 38.6 | <0.0001 | 29.3 | <0.0001 | 18.6 | 0.00094 |
The results of the estimated marginal means (EMM) analysis are plotted in Fig. 5. The EMM contrast (defined as the difference between the estimated marginal mean rater score for DEC-TDI and DEC-FA maps) was statistically significant in all analyses, showing a general expert preference for DEC-TDI maps across all clinical utility statements.
Fig. 5.
Plot of estimated marginal mean (EMM) rater scores for the four clinical utility statements. Error bars indicate the 95% confidence interval of the EMM. Significant differences between map types are indicated with stars (*** = p ≤ 0.001, **** = p ≤ 0.0001). DEC = directionally encoded color, FA = fractional anisotropy, TDI = track density imaging.
4. Discussion
In this study, we have investigated the potential application of traditional and advanced DEC maps to aid neurosurgical planning in brain tumor patients. To our knowledge, this study is the first to specifically assess the utility of DEC-TDI for neurosurgical planning in brain tumor patients. We found that five neurosurgeon expert raters broadly preferred DEC-TDI maps over traditional DTI-based DEC-FA maps in a cohort of 14 brain tumor patients. This study provides evidence that DEC-TDI maps may help neurosurgeons identify important peritumoral tracts, decide on a goal resection margin, and plan a surgical route. To assess why DEC-TDI maps were generally preferred by neurosurgeon raters, we consider the interpretation of clinical utility statements 1 and 2, which assessed specific components of neurosurgical planning. The largest effect size in rater scores in favor of DEC-TDI maps was observed for statement 2, “The resolution of this DEC map is helpful in establishing a goal margin of resection” (Fig. 5). This result suggests that the increased image resolution achievable with DEC-TDI (Calamante et al., 2010) has potential clinical utility to improve visualization of the tumor margin and that DEC-TDI maps could potentially assist neurosurgeons as they establish a planned resection margin. This potential role of DEC-TDI aligns with well-established clinical guidelines that affirm the importance of a precise resection margin that both removes tumor while sparing healthy tissue for patient morbidity and mortality (Nabors et al., 2020) and the potentially impactful role of white matter visualization techniques in determining a resection margin (Dimou et al., 2013, Romano et al., 2009). Rater scores also strongly favored DEC-TDI maps for clinical utility statement 1, “This DEC map demonstrates clinically relevant tracts in the peritumoral region (i.e. areas of T1/T2 signal change)” (Fig. 5). Visualization and identification of white matter tracts and their relationship to the brain tumor is a well-recognized goal of white matter visualization methods for use in presurgical planning (Essayed et al., 2017, Yeh et al., 2021), further supporting the potential role of DEC-TDI maps in this clinical context. Finally, rater scores for clinical utility statements 3 and 4, which were designed to judge the overall influence of each DEC map on a planned surgical route and the overall utility of each DEC map, also significantly favored DEC-TDI, supporting its overall potential to help neurosurgeons plan function-preserving brain tumor resections in conjunction with currently employed imaging techniques. While DEC-TDI was preferred, raters found both DEC maps to be useful, with a majority of “somewhat agree” or “strongly agree” responses for both map types. This result is in agreement with existing studies supporting the utility of preoperative DEC-FA visualization in patients (Field et al., 2005, Field et al., 2004, Schneider et al., 2021, Schonberg et al., 2006, Witwer et al., 2002, Young, 2007). Our findings may generalize to a wide patient population across institutions, given the diversity of brain tumor diagnoses and grades included in the study as well as the clinically typical dMRI data used.
5. Limitations & future directions
Our study has several limitations. To make expert rating feasible for neurosurgeons, the study design restricted visualization to a single axial slice of each DEC map. However, in clinical practice, access to the full DEC map could allow for improved tract visualization in relation to the tumor, including in different orientations. A second limitation to the study design is that the DEC-TDI maps enabled visualization only within the brain mask (where tractography was performed), while the DEC-FA maps included all anatomy that was scanned, such as the brain, cerebrospinal fluid, and skull. Our goal was to preserve the default nature of the DEC-FA maps, presenting them to expert raters as they are produced by the scanner without additional processing. The corresponding difference in visual presentation of the maps may have biased raters toward the brain extracted DEC-TDI maps, while providing raters with a way to to identify the map type despite the random, blind presentation of maps. In terms of patient selection, we selected a cohort of consecutive patients for this study with the goal of studying multiple different tumor histopathologic diagnoses, locations, sizes, and degree of edema without any a priori judgment of which cases would be most suited for DEC visualization. A number of patient- and tumor-specific factors therefore impact the potential clinical utility of DEC maps. First, for tumors located near the cortical surface (e.g. BTP 2, 3, 4, 7, 10, and 13), there is relatively less contrast visible in the DEC maps. Similarly, diffusion anisotropy is lower in areas of peritumoral edema, which leads to reduced brightness and contrast in DEC-FA maps and can limit fiber tracking (Essayed et al., 2017, Gong et al., 2018, Zhang et al., 2013), therefore reducing DEC-TDI contrast. Furthermore, DEC-TDI may be most useful in cases where tractography is particularly useful. For these reasons, DEC maps may offer minimal added value to the preoperative plan in some cases. In this work, we performed limited dMRI preprocessing in line with clinical practice and to reduce potential differences between the input data to DEC-TDI and that used for DEC-FA on the scanner. We performed MP-PCA denoising to reduce the known sensitivity of TDI-based visualization to noise (Dhollander et al., 2014, Dhollander et al., 2012), but future work may improve results of DEC-TDI by employing additional DWI preprocessing steps such as Gibbs-ringing correction, EPI distortion correction, and bias field inhomogeneity correction. In addition, all patients received diffusion MRI scanning as clinically indicated. Patient selection was not determined by the lateralization or localization of specific behavioral, cognitive, or neurological functions or clinical functional presentation. Therefore, the importance of DEC visualization in surgical planning with regard to specific behavioral, cognitive, or neurological functions (such as language function) was not investigated in this study and is of interest for future work. Furthermore, we acknowledge that our overall goal is a clinically motivated comparison of a newer method of white matter visualization (DEC-TDI) versus a traditional method (DEC-FA), rather than a technical comparison of these methods. More research is needed to assess which underlying technical factors (e.g. DTI vs CSD modeling, the usage of tractography to produce the DEC-TDI map, and differences in pre-processing on and off the scanner) may be driving expert preferences. The comparison of other types of DEC map, such as that derived from an orientation distribution function (ODF) field to create a directionally encoded color fiber orientation distribution map (DEC-FOD) (Dhollander et al., 2015), is of interest in the future. Finally, this initial study focused on evaluation of retrospective patient DEC maps. In order to move beyond the hypothetical setting of this study to clinical practice, further prospective study is needed to assess the clinical outcomes of brain tumor patients whose preoperative plan includes DEC-TDI.
Other study limitations relate to challenges of clinical implementation of DEC-TDI. While DEC-TDI benefits from the advantages of tractography, it also suffers from limitations of tractography including false positive streamlines and challenges tracing through peritumoral edema that can lead to false negatives (Essayed et al., 2017, Henderson et al., 2020, Yang et al., 2021), as well as potentially cumbersome computational run time. Furthermore, the clinically standard data employed in this project is not ideally suited for CSD modeling, which benefits from data with b-values of 3000 s/mm2 and over 45 gradient directions to better model crossing fibers while reducing the effect of noise. DEC-TDI maps derived from tractography that suffers from false positives or false negatives could increase the likelihood of a suboptimal surgical approach. While the DEC-FA maps were automatically generated on the scanner, in our study the average time to generate a DEC-TDI map (including tractography and map generation) was over an hour per patient on a modern Linux computer workstation. In addition, while our study employed recommended and default parameters for tractography and DEC-TDI generation, further study may be needed to optimize and standardize these parameters for generalization across different brain tumor patients and dMRI sequences.
In future clinical applications, DEC-TDI visualization may be useful to aid in virtual dissection and visualization of specific white matter tracts adjacent to the tumor. In conjunction with automated tractography segmentation techniques described previously by our group (O’Donnell et al., 2017, Zhang et al., 2020a), clinically relevant tracts can be clearly visualized and anatomically defined, providing neurosurgeons with an improved presurgical visualization of the important peritumoral white matter tracts that will likely influence the resection strategy.
6. Conclusion
Our study demonstrates that preoperative planning white matter visualization using DEC-TDI maps is preferred by expert neurosurgeon raters over the current clinical standard, DEC-FA maps. DEC-TDI may have a potential role in conjunction with currently employed imaging techniques to help neurosurgeons plan function-preserving brain tumor resections. Further study is needed for methodology validation and clinical translation.
CRediT authorship contribution statement
Jared J. Sullivan: Conceptualization, Investigation, Formal analysis, Visualization, Writing – original draft. Leo R. Zekelman: Formal analysis, Writing – original draft. Fan Zhang: Methodology, Investigation, Writing – review & editing. Parikshit Juvekar: Visualization, Investigation. Erickson F. Torio: Investigation. Adomas Bunevicius: Investigation. Walid I. Essayed: Investigation. Dhiego Bastos: Investigation. Jianzhong He: Data curation. Laura Rigolo: . Alexandra J. Golby: Supervision, Conceptualization, Investigation, Writing – review & editing. Lauren J. O'Donnell: Supervision, Conceptualization, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This work is supported in part by the following grants: National Institutes of Health R01MH125860, R01MH119222, R01MH074794, P41EB015902, R01NS125781, R01NS125307, , and P41EB028741, as well as the Jennifer Ward Oppenheimer Cancer Research Initiative and the Brigham and Women’s Hospital Radiology Research Pilot Grant.
Footnotes
We note that DEC-FA maps are also referred to as FA color maps (Young, 2007), color by orientation maps (Norton et al., 2017), color-coded maps (Schneider et al., 2021), color-coded FA maps (Voltoline and Wu, 2021), and other similar names.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103412.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- Barajas R.F., Jr, Hess C.P., Phillips J.J., Von Morze C.J., Yu J.P., Chang S.M., Nelson S.J., McDermott M.W., Berger M.S., Cha S. Super-resolution track density imaging of glioblastoma: histopathologic correlation. AJNR Am. J. Neuroradiol. 2013;34:1319–1325. doi: 10.3174/ajnr.A3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994 doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Scherer M., Neher P., Jungk C., Jesser J., Pflüger I., Brinster R., Bendszus M., Bruckner T., Maier-Hein K., Unterberg A. Going beyond diffusion tensor imaging tractography in eloquent glioma surgery-high-resolution fiber tractography: Q-ball or constrained spherical deconvolution? World Neurosurg. 2020;134:e596–e609. doi: 10.1016/j.wneu.2019.10.138. [DOI] [PubMed] [Google Scholar]
- Calamante F., Tournier J.-D., Jackson G.D., Connelly A. Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage. 2010;53:1233–1243. doi: 10.1016/j.neuroimage.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Calamante F., Tournier J.-D., Heidemann R.M., Anwander A., Jackson G.D., Connelly A. Track density imaging (TDI): validation of super resolution property. Neuroimage. 2011;56:1259–1266. doi: 10.1016/j.neuroimage.2011.02.059. [DOI] [PubMed] [Google Scholar]
- Calamante F., Tournier J.-D., Smith R.E., Connelly A. A generalised framework for super-resolution track-weighted imaging. Neuroimage. 2012;59:2494–2503. doi: 10.1016/j.neuroimage.2011.08.099. [DOI] [PubMed] [Google Scholar]
- Calamante F., Oh S.-H., Tournier J.-D., Park S.-Y., Son Y.-D., Chung J.-Y., Chi J.-G., Jackson G.D., Park C.-W., Kim Y.-B., Connelly A., Cho Z.-H. Super-resolution track-density imaging of thalamic substructures: comparison with high-resolution anatomical magnetic resonance imaging at 7.0T. Hum. Brain Mapp. 2013;34:2538–2548. doi: 10.1002/hbm.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Tie Y., Olubiyi O., Rigolo L., Mehrtash A., Norton I., Pasternak O., Rathi Y., Golby A.J., O’Donnell L.J. Reconstruction of the arcuate fasciculus for surgical planning in the setting of peritumoral edema using two-tensor unscented Kalman filter tractography. NeuroImage: Clinical. 2015;7:815–822. doi: 10.1016/j.nicl.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Tie Y., Olubiyi O., Zhang F., Mehrtash A., Rigolo L., Kahali P., Norton I., Pasternak O., Rathi Y., Golby A.J., O’Donnell L.J. Corticospinal tract modeling for neurosurgical planning by tracking through regions of peritumoral edema and crossing fibers using two-tensor unscented Kalman filter tractography. Int. J. Comput. Assist. Radiol. Surg. 2016;11:1475–1486. doi: 10.1007/s11548-015-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, R.H.B., 2018. Cumulative link models for ordinal regression with the R package ordinal [WWW Document]. URL https://cran.r-project.org/web/packages/ordinal/vignettes/clm_article.pdf (accessed 4.2.21).
- Dhollander T., Emsell L., Van Hecke W., Maes F., Sunaert S., Suetens P. Track orientation density imaging (TODI) and track orientation distribution (TOD) based tractography. Neuroimage. 2014;94:312–336. doi: 10.1016/j.neuroimage.2013.12.047. [DOI] [PubMed] [Google Scholar]
- Dhollander, T., Emsell, L., Hecke, W., Maes, F., Sunaert, S., Suetens, P., 2012. Track-density imaging & noise: when super-resolution quality does not yield accuracy.
- Dhollander T., Smith R.E., Tournier J.-D., Connelly A. 23rd International Society of Magnetic Resonance in Medicine. unknown. 2015. Time to move on: an FOD-based DEC map to replace DTI’s trademark DEC FA. [Google Scholar]
- Dimou S., Battisti R.A., Hermens D.F., Lagopoulos J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg. Rev. 2013;36:205–214. doi: 10.1007/s10143-012-0436-8. discussion 214. [DOI] [PubMed] [Google Scholar]
- Essayed W.I., Zhang F., Unadkat P., Cosgrove G.R., Golby A.J., O’Donnell L.J. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. Neuroimage Clin. 2017;15:659–672. doi: 10.1016/j.nicl.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson S., Tournier J.-D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G.D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J. Neurosurg. 2013;118:1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Fedorov A., Beichel R., Kalpathy-Cramer J., Finet J., Fillion-Robin J.-C., Pujol S., Bauer C., Jennings D., Fennessy F., Sonka M., Buatti J., Aylward S., Miller J.V., Pieper S., Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekonja L.S., Wang Z., Aydogan D.B., Roine T., Engelhardt M., Dreyer F.R., Vajkoczy P., Picht T. Detecting corticospinal tract impairment in tumor patients with fiber density and tensor-based metrics. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.622358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.S., Alexander A.L., Wu Y.-C., Hasan K.M., Witwer B., Badie B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J. Magn. Reson. Imaging. 2004;20:555–562. doi: 10.1002/jmri.20169. [DOI] [PubMed] [Google Scholar]
- Field A.S., Wu Y.-C., Alexander A.L. Principal diffusion direction in peritumoral fiber tracts: Color map patterns and directional statistics. Ann. N. Y. Acad. Sci. 2005;1064:193–201. doi: 10.1196/annals.1340.037. [DOI] [PubMed] [Google Scholar]
- Franke L., Weidele D.K.I., Zhang F., Cetin-Karayumak S., Pieper S., O’Donnell L.J., Rathi Y., Haehn D. 2021 IEEE 14th Pacific Visualization Symposium (PacificVis) 2021. FiberStars: visual comparison of diffusion tractography data between multiple subjects; pp. 116–125. [DOI] [Google Scholar]
- Gong S., Zhang F., Norton I., Essayed W.I., Unadkat P., Rigolo L., Pasternak O., Rathi Y., Hou L., Golby A.J., O’Donnell L.J. Free water modeling of peritumoral edema using multi-fiber tractography: Application to tracking the arcuate fasciculus for neurosurgical planning. PLoS One. 2018;13:e0197056. doi: 10.1371/journal.pone.0197056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F., Abdullah K.G., Verma R., Brem S. Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential. Neurosurg. Focus. 2020;48:E6. doi: 10.3171/2019.11.FOCUS19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.-D., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013;34:2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle D., Wickham H. Ggmap: Spatial visualization with ggplot2. R J. 2013;5:144. doi: 10.32614/rj-2013-014. [DOI] [Google Scholar]
- Le Bihan D., Johansen-Berg H. Diffusion MRI at 25: exploring brain tissue structure and function. Neuroimage. 2012;61:324–341. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, Singmann, Love, Buerkner, Herve, 2018. Emmeans: Estimated marginal means, aka least-squares means. R package version.
- Li X., Morgan P.S., Ashburner J., Smith J., Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Likert R. A technique for the measurement of attitudes. Arch. Psychol. 1932;22:140. [Google Scholar]
- Mangiafico, S.S. 2016. Summary and Analysis of Extension Program Evaluation in R, version 1.20.01. rcompanion.org/handbook/. (Pdf version: rcompanion.org/documents/RHandbookProgramEvaluation.pdf.).
- McCullagh P. Regression models for ordinal data. J. R. Stat. Soc. 1980;42:109–127. doi: 10.1111/j.2517-6161.1980.tb01109.x. [DOI] [Google Scholar]
- Mormina E., Longo M., Arrigo A., Alafaci C., Tomasello F., Calamuneri A., Marino S., Gaeta M., Vinci S.L., Granata F. MRI tractography of corticospinal tract and arcuate fasciculus in high-grade gliomas performed by constrained spherical deconvolution: qualitative and quantitative analysis. AJNR Am. J. Neuroradiol. 2015;36:1853–1858. doi: 10.3174/ajnr.A4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors L.B., Portnow J., Ahluwalia M., Baehring J., Brem H., Brem S., Butowski N., Campian J.L., Clark S.W., Fabiano A.J., Forsyth P., Hattangadi-Gluth J., Holdhoff M., Horbinski C., Junck L., Kaley T., Kumthekar P., Loeffler J.S., Mrugala M.M., Nagpal S., Pandey M., Parney I., Peters K., Puduvalli V.K., Robins I., Rockhill J., Rusthoven C., Shonka N., Shrieve D.C., Swinnen L.J., Weiss S., Wen P.Y., Willmarth N.E., Bergman M.A., Darlow S.D. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020;18:1537–1570. doi: 10.6004/jnccn.2020.0052. [DOI] [PubMed] [Google Scholar]
- Norton I., Essayed W.I., Zhang F., Pujol S., Yarmarkovich A., Golby A.J., Kindlmann G., Wassermann D., Estepar R.S.J., Rathi Y., Pieper S., Kikinis R., Johnson H.J., Westin C.-F., O’Donnell L.J. SlicerDMRI: open source diffusion MRI software for brain cancer research. Cancer Res. 2017;77:e101–e103. doi: 10.1158/0008-5472.CAN-17-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell, L.J., Westin, C.-F., 2011. An introduction to diffusion tensor image analysis. Neurosurg. Clin. N. Am. 22, 185–96, viii. https://doi.org/10.1016/j.nec.2010.12.004. [DOI] [PMC free article] [PubMed]
- O’Donnell L.J., Suter Y., Rigolo L., Kahali P., Zhang F., Norton I., Albi A., Olubiyi O., Meola A., Essayed W.I., Unadkat P., Ciris P.A., Wells W.M., 3rd, Rathi Y., Westin C.-F., Golby A.J. Automated white matter fiber tract identification in patients with brain tumors. Neuroimage Clin. 2017;13:138–153. doi: 10.1016/j.nicl.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz I., Farzinfar M., Matsui J., Budin F., Liu Z., Gerig G., Johnson H.J., Styner M. DTIPrep: quality control of diffusion-weighted images. Front. Neuroinform. 2014;8:4. doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S., Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn. Reson. Med. 2000;43:921. doi: 10.1002/1522-2594(200006)43:6<921::aid-mrm23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Panesar S.S., Abhinav K., Yeh F.-C., Jacquesson T., Collins M., Fernandez-Miranda J. Tractography for surgical neuro-oncology planning: towards a gold standard. Neurotherapeutics. 2019;16:36–51. doi: 10.1007/s13311-018-00697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing.
- Romano A., D’Andrea G., Minniti G., Mastronardi L., Ferrante L., Fantozzi L.M., Bozzao A. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur. Radiol. 2009;19:2798–2808. doi: 10.1007/s00330-009-1483-6. [DOI] [PubMed] [Google Scholar]
- Satorra A., Saris W.E. Power of the likelihood ratio test in covariance structure analysis. Psychometrika. 1985;50:83–90. doi: 10.1007/BF02294150. [DOI] [Google Scholar]
- Scheffé H. John Wiley & Sons; 1999. The Analysis of Variance. [Google Scholar]
- Schneider J.R., Raval A.B., Black K., Schulder M. Diffusion tensor imaging color-coded maps: An alternative to tractography. Stereotact. Funct. Neurosurg. 2021;1–10 doi: 10.1159/000512092. [DOI] [PubMed] [Google Scholar]
- Schonberg T., Pianka P., Hendler T., Pasternak O., Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006;30:1100–1111. doi: 10.1016/j.neuroimage.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Schult T., Hauser T.-K., Klose U., Hurth H., Ehricke H.-H. Fiber visualization for preoperative glioma assessment: Tractography versus local connectivity mapping. PLoS One. 2019;14:e0226153. doi: 10.1371/journal.pone.0226153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z., Yu J., Chen Z., Sun Y., Bu X., Wang M., Sarica C., Hernesniemi J., Nelson B.J., Zemmar A., Avecillas-Chasin J.M. Constrained-spherical deconvolution tractography in the evaluation of the corticospinal tract in glioma surgery. Front Surg. 2021;8 doi: 10.3389/fsurg.2021.646465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y., Rigolo L., Norton I.H., Huang R.Y., Wu W., Orringer D., Mukundan S., Jr, Golby A.J. Defining language networks from resting-state fMRI for surgical planning–a feasibility study. Hum. Brain Mapp. 2014;35:1018–1030. doi: 10.1002/hbm.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.-D., Calamante F., Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tournier, J.D., Calamante, F., Connelly, A., 2009. How many diffusion gradient directions are required for HARDI, in: Proceedings of the International Society for Magnetic Resonance in Medicine. p. 358.
- Tournier, J.D., Calamante, F., Connelly, A., 2010. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions 18.
- Tournier J.-D., Smith R., Raffelt D., Tabbara R., Dhollander T., Pietsch M., Christiaens D., Jeurissen B., Yeh C.-H., Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- Veraart J., Novikov D.S., Christiaens D., Ades-Aron B., Sijbers J., Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltoline R., Wu S.-T. Multimodal visualization of complementary color-coded FA map and tensor glyphs for interactive tractography ROI seeding. Comput. Graph. 2021;96:24–35. doi: 10.1016/j.cag.2021.03.001. [DOI] [Google Scholar]
- Wei P.-H., Cong F., Chen G., Li M.-C., Yu X.-G., Bao Y.-H. Neuronavigation based on track density image extracted from deterministic high-definition fiber tractography. World Neurosurg. 2017;98:880.e9–880.e15. doi: 10.1016/j.wneu.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Witwer B.P., Moftakhar R., Hasan K.M., Deshmukh P., Haughton V., Field A., Arfanakis K., Noyes J., Moritz C.H., Meyerand M.E., Rowley H.A., Alexander A.L., Badie B. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J. Neurosurg. 2002;97:568–575. doi: 10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]
- Woodworth, D., Mayer, E., Leu, K., Ashe-McNalley, C., Naliboff, B.D., Labus, J.S., Tillisch, K., Kutch, J.J., Farmer, M.A., Apkarian, A.V., Johnson, K.A., Mackey, S.C., Ness, T.J., Landis, J.R., Deutsch, G., Harris, R.E., Clauw, D.J., Mullins, C., Ellingson, B.M., MAPP Research Network, 2015. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS One 10, e0140250. https://doi.org/10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed]
- Yang J.-Y.-M., Yeh C.-H., Poupon C., Calamante F. Diffusion MRI tractography for neurosurgery: the basics, current state, technical reliability and challenges. Phys. Med. Biol. 2021;66 doi: 10.1088/1361-6560/ac0d90. [DOI] [PubMed] [Google Scholar]
- Yeh F.-C., Irimia A., de Bastos D.C., A., Golby, A.J. Tractography methods and findings in brain tumors and traumatic brain injury. Neuroimage. 2021;245 doi: 10.1016/j.neuroimage.2021.118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, G.S., 2007. Advanced MRI of adult brain tumors. Neurol. Clin. 25, 947–73, viii. https://doi.org/10.1016/j.ncl.2007.07.010. [DOI] [PubMed]
- Zhang F., Cetin Karayumak S., Hoffmann N., Rathi Y., Golby A.J., O’Donnell L.J. Deep white matter analysis (DeepWMA): Fast and consistent tractography segmentation. Med. Image Anal. 2020;65 doi: 10.1016/j.media.2020.101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Wang, Y., Lu, T., Qiu, B., Tang, Y., Ou, S., Tie, X., Sun, C., Xu, K., Wang, Y., 2013. Differences between generalized q-sampling imaging and diffusion tensor imaging in the preoperative visualization of the nerve fiber tracts within peritumoral edema in brain. Neurosurgery 73, 1044–53; discussion 1053. https://doi.org/10.1227/NEU.0000000000000146. [DOI] [PubMed]
- Zhang F., Noh T., Juvekar P., Frisken S.F., Rigolo L., Norton I., Kapur T., Pujol S., Wells W., 3rd, Yarmarkovich A., Kindlmann G., Wassermann D., Estepar S.J.R., Rathi Y., Kikinis R., Johnson H.J., Westin C.-F., Pieper S., Golby A.J., O’Donnell L.J. SlicerDMRI: diffusion MRI and tractography research software for brain cancer surgery planning and visualization. JCO Clin. Cancer Inform. 2020;4:299–309. doi: 10.1200/CCI.19.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E., Rouillard M., André E., Coolen T., Stender J., Balteau E., Phillips C., Garraux G. Mapping track density changes in nigrostriatal and extranigral pathways in Parkinson’s disease. Neuroimage. 2014;99:498–508. doi: 10.1016/j.neuroimage.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.