Highlights
-
•
Meningiomas with larger treated gross tumour volume (GTV) have poorer progression-free survival rates following primary fractionated radiotherapy.
-
•
Optic nerve sheath and cavernous sinus meningiomas are well controlled with primary fractionated radiotherapy.
-
•
Larger meningioma GTV is associated with higher rates of grades 3 and 4 adverse events.
Keywords: Radiotherapy, Meningioma, Fractionated, Primary, Intracranial
Abstract
Background
Surgery is the primary treatment for most meningiomas. However, primary fractionated radiotherapy (fRT) remains an option for patients with larger meningiomas in challenging anatomic locations or patients at prohibitively high surgical risk. Outcome prediction for these patients is uncertain and cannot be guided by histopathology without available tumor tissue from surgery. Therefore, we aimed to assess the clinical factors that contribute to treatment failure in a large cohort of meningiomas consecutively treated with fRT as primary therapy, with the goal of identifying predictors of response.
Methods
Patients treated with primary fRT for intracranial meningiomas from 1998 to 2017 were reviewed. Those who received primary surgical resection, radiosurgery, previous fRT, or had <6 months of clinical follow-up were excluded. We applied logistic regression and Cox regression modeling to ascertain key predictors of treatment failure, progression-free survival (PFS), and adverse events (AE) following fRT.
Results
Our cohort included 137 meningiomas, 21 of which progressed after fRT (median PFS 3.45 years). Progressive meningiomas had a larger median gross tumor volume (GTV) compared to those that remained stable (19.1 cm3 vs 9.6 cm3, p = 2.86 × 10−2). GTV > 11.27 cm3 was independently predictive of progression and larger GTV was associated with higher risk of significant (grades 3/4) AE following fRT. Cavernous sinus and optic nerve sheath meningiomas had overall excellent outcomes post-fRT.
Conclusions
We present a large cohort of meningiomas treated with primary fRT and find GTV and anatomic location to be key predictors of outcome, adding to the complex treatment considerations for this heterogeneous disease.
Introduction
Meningiomas are the most common primary brain tumor in adults with surgery as the mainstay of therapy for symptomatic cases [1], [2], [3], [4]. However, some meningioma patients may not be surgical candidates due to tumor location (encasing critical neurovascular structures), medical comorbidities, or patient preference. These patients are generally recommended either stereotactic radiosurgery (SRS) or fractionated radiotherapy (fRT) as first-line treatment instead. While SRS provides good local control for most small (<3 cm) meningiomas, larger tumors, particularly in close proximity to radiosensitive structures (such as the optic apparatus) have been associated with higher rates of treatment failure and cranial nerve deficits following treatment [5], [6], [7], [8], [9], [10]. For these patients, fRT is often the only available treatment option, and without the benefit of tumor tissue for histopathologic or molecular analyses, prognostication is exceptionally challenging. Contemporary studies on primary fRT for meningiomas have been largely limited to smaller case series in specific anatomic locations [11], [12], [13], [14], [15], [16]. Therefore, there remains a critical knowledge gap on the clinical covariates that could predict a meningioma’s response to primary fRT. To address this, we analyzed our institutional data over the past decade of implementing primary fRT for a diverse group of meningiomas to identify clinical predictors of outcome that may help guide future treatment decisions for this rare but important patient population.
Methods
Patient selection and tumour characteristics
We reviewed the radiation oncology databases for meningioma patients treated at the University Health Network (Toronto, ON) between November 1, 1998 to December 30, 2017. Patients were included if they underwent fractionated radiation therapy, (daily fraction (Fr) size of 3 Gy or less for >5 Fr) Patients were excluded if they underwent surgical resection (including surgical biopsy), previous RT to their meningioma (including previous SRS or fRT), had a confirmed or suspected radiation induced meningioma, clinical diagnosis of neurofibromatosis type 2 (NF2) or meningiomatosis, could not complete all RT fractions, or had follow-up <6 months after RT completion. Clinical information collected included age, biological sex, previous RT to the meningioma, tumor location, new or progressive symptoms prior to diagnosis, documented radiographic progression prior to RT, number of Fr received, total dose, date of RT initiation and completion (first and last Fr), gross tumor volume (GTV), clinical target volume (CTV), adverse events (AEs), date of radiographic recurrence, salvage therapy, and date of last follow-up. This study was approved by the University Health Network Research Ethics Board (CAPCR ID 18-5820).
Radiotherapy dose, technique, and adverse events
All patients undergoing fRT received both a planning computed tomography (CT) and magnetic resonance imaging (MRI) scan with T1-weighted gadolinium and T2-weighted thin slice image sequences. During treatment, patients were immobilized with either a thermoplastic frame or stereotactic relocatable frame. Clinical target volume expansions were 0-mm for unresected/presumed World Health Organization (WHO) grade 1 lesions, and 5 mm for optic nerve sheath (ONS) meningiomas. A 3-mm planning target volume with daily cone beam image guided radiotherapy (IGRT) was used. All patients were treated with step-and-shoot intensity-modulated radiation therapy (iMRT) or volumetric modulated arc therapy (VMAT). Conventional fractionation was used for most cases to a total dose of 50 Gy/25 Fr or 54 Gy/30 Fr. Hypofractionation (40 Gy/15 Fr) was considered for older, frail patients, while dose escalation to 60 Gy/30 Fr were used for patients with rapidly progressive meningiomas following presentation/diagnosis. Patients were monitored for AEs during each Fr and following treatment. AE grading were done in accordance with the Common Terminology Criteria for Adverse Events (CTCAE version 5.0) definitions by two independent observers (APL, JZW). AEs related to fRT were defined as the development of new symptoms, including neurological symptoms, that were not pre-existing prior to fRT or the progression of existing neurological symptoms without radiographic evidence of tumour progression. Where there were discrepancies between grading, discussion was initiated between the two observers until a consensus could be agreed upon.
Meningioma volumetric measurement
Baseline meningioma GTV were determined for each dataset based on segmentation of T1W postcontrast images by the treating radiation oncologist and neurosurgeon on a slice-by-slice basis to be used for fRT treatment planning. GTV encompassed the entirety of the enhancing lesion and any visible dural tail when present on MRI.
Clinical Follow-Up and recurrence
Following fRT, patients were monitored for tumor recurrence with serial MRI scans at intervals of 6 months-1 year. Radiographic progression following the index fRT treatment was defined as any increase in tumor volume, enhancement, or nodularity compared to the prior MRI identified by the reporting neuroradiologist and corroborated by the treating clinicians (radiation oncologist or neurosurgeon). All recurrences were also verified by the authors when images were available [5]. Only in-field or marginal progression were classified as true progression, excluding de novo meningiomas or progressive meningiomas outside the fRT treatment field. In cases of minimal increase in size or equivocal change, progression was confirmed by either the persistence of this change or further increase in size on subsequent imaging. Date of progression was denoted as the earliest date when this change was detectable. Progression-free survival (PFS) was calculated from the last date of fRT treatment to the date of progression/recurrence on imaging (as per above) if this occurred, date of last follow-up (if no progression was seen or censored if lost-to-follow-up), or date of death (if documented). Salvage therapy was defined as treatment offered upon radiographic progression after the index fRT treatment.
Statistical analysis
All statistical analyses were performed using R (version 4.0.3). PFS estimates and comparisons were calculated using the Kaplan-Meier method. Welch’s two-sample t-test and two-sample test for equality of proportions with continuity correction were used to compare baseline characteristics. Cox proportional hazards models were used to evaluate clinical covariates hypothesized to contribute to PFS post RT. The proportional hazards assumption was tested by plotting the scaled Schoenfeld residuals against time and obtaining the p-value through both the Schoenfeld individual test and global Schoenfeld test. All covariates used in the univariate analysis that were not collinear with one another were carried over to the multivariate analysis as per best statistical practices [6]. Multivariable logistic regression was fit to evaluate clinical covariates hypothesized to be associated with adverse events post fRT. A p-value of 0.05 was set as the threshold below which statistical significance was determined unless otherwise specified. Determination of optimal cut-points to dichotomize continuous variables for predicting the outcome of interest were performed using the cutpointr package by maximizing the Youden index (sum of the sensitivity and specificity – 1) [7].
Results
Study cohort
A total of 137 meningiomas in 136 patients were included for analysis after review of 1832 patients. We excluded: 972 patients that did not receive RT, 364 patients that had surgical resection or biopsy as primary therapy, 326 patients that received Gamma Knife SRS, 3 patients that had previous fRT, 15 patients with radiation induced meningiomas, 5 patients with suspected or documented NF2, and 12 patients with spinal meningiomas. All patients received an initial neurosurgical consult. The subsequent decision of SRS versus fRT were made by the treating radiation oncologist and equivocal cases were reviewed at our multidisciplinary tumour boards (MDT).
Baseline characteristics of patients are outlined in Table 1. Most primary fRT-treated meningiomas were in the cavernous sinus (CS) (27%), petrous/petroclival region (19%), or optic nerve sheath (ONS) (15%; Table 1). Median duration of follow-up after fRT completion was 7.2 years (95% CI 6.4–8.0). Most meningiomas that progressed post-fRT did so within 5 years of treatment completion (N = 15/21, 71%). Nearly all patients (N = 129, 94%) received conventional fractional with at a total RT dose of 50 Gy/25 Fr (N = 76, 56%) or 54 Gy/30 Fr (N = 53, 38%). Only 5 patients received hypofractionated RT at 40 Gy/15 Fr (4%) and 3 patients were given dose escalation to 60 Gy/30 Fr (2%, Table 1). All 5 patients that received the hypofractionated 40 Gy/15 Fr treatment plan were over the age of 80 (median 87, range 81–92) and were determined to be more medically frail.
Table 1.
Baseline characteristics of all fRT treated meningiomas.
| Primary fRT cohort (n = 137) | ||||
|---|---|---|---|---|
| Total | Progression post-fRT | Stable post-fRT | P-value1 | |
| Gender | 137 | 21 | 116 | |
| Male | 39 (28%) | 7 (33%) | 32 (28%) | 0.784 |
| Female | 98 (72%) | 14 (67%) | 84 (72%) | |
| Age at Diagnosis (median [range]) | 58 (16–92) | 58 (47–92) | 59 (16–88) | 0.288 |
| Clinical/radiographic progression pre-fRT | ||||
| Yes | 110 (80%) | 16 (76%) | 94 (81%) | 0.830 |
| No | 27 (20%) | 5 (24%) | 22 (19%) | |
| Location | ||||
| Supratentorial (ST) | 38 (27%) | 8 (38%) | 30 (26%) | 0.375 |
| Anterior fossa | 6 (4%) | 1 (5%) | 5 (4%) | 1 |
| Convexity | 4 (3%) | 1 (5%) | 3 (3%) | 1 |
| Middle fossa | 17 (12%) | 2 (9%) | 15 (13%) | 0.939 |
| Parasagittal/parafalcine | 7 (5%) | 4 (19%) | 3 (3%) | 8.95 × 10−3* |
| Sphenoorbital/Orbital | 4 (3%) | 0 | 4 (3%) | 0.873 |
| Infratentorial (IT) | 42 (31%) | 13 (62%) | 31 (26%) | 3.47 × 10−3* |
| Petroclival/petrous | 25 (19%) | 5 (24%) | 20 (17%) | 0.682 |
| Other posterior fossa | 17 (12%) | 6 (29%) | 11 (9%) | 3.73 × 10−2* |
| Cavernous sinus (CS) | 37 (27%) | 2 (9%) | 35 (30%) | 9.03 × 10−2 |
| Optic nerve sheath (ONS) | 20 (15%) | 0 | 20 (14%) | 8.49 × 10−2 |
| fRT | ||||
| RT technique | ||||
| iMRT | 125 (91%) | 19 (90%) | 106 (91%) | 1 |
| VMAT | 12 (9%) | 2 (10%) | 10 (9%) | 1 |
| GTV in cm3 (median [IQR]) | 11.7 (5.09–29.1) | 19.1 (13.6–47.8) | 9.59 (4.46–24.1) | 2.86 × 10−2* |
| # of patients in each fRT treatment plan | ||||
| 40 Gy/15 Fr | 5 (4%) | 1 (5%) | 4 (3%) | 1 |
| 50 Gy/25 Fr | 76 (56%) | 11 (52%) | 70 (57%) | 0.659 |
| 54 Gy/30 Fr | 53 (38%) | 7 (33%) | 47 (39%) | 0.705 |
| 60 Gy/30 Fr | 3 (2%) | 2 (10%) | 1 (1%) | 0.0919 |
| Salvage therapy | ||||
| Surgery | 4 (3%) | 4 (19%) | ||
| WHO 1 | 3 | 3 | ||
| WHO 2 | 1 | 1 | ||
| Repeat fRT | 5 (3%) | 5 (23%) | ||
| Palliation | 6 (6%) | 6 (29%) | ||
| Observation | 6 (4%) | 6 (29%) | ||
P-value from Welch 2-sample T-test for continuous variables or Chi-square test of proportions for categorical variables; RT- radiotherapy; Gy- gray; Fr- fraction; GTV- gross tumour volume; WHO- World Health Organization; fRT- fractionated radiotherapy; SRS- stereotactic radiosurgery; iMRT- intensity-modulated radiation therapy; VMAT- volumetric modulated arc therapy; *p < 0.05.
Primary fRT provides durable control for most intracranial meningiomas
Median 3-, 5-, 8-, and 10-year PFS rates post-fRT were 0.923 (95% CI 0.878–0.970), 0.879 (95% CI 0.823–0.938), 0.866 (95% CI 0.805–0.930), and 0.768 (95% CI 0.668–0.883) respectively. More than half of the progressive meningiomas were infratentorial in location (62%; Table 1). Four meningiomas (3%) were resected surgically after progression with 3 cases being classified as WHO grade 1 meningiomas, and 1 case as WHO grade 2 (initially treated with dose escalation to 60 Gy/30 Fr initially due to rapid progression following presentation). Five patients received salvage/palliative re-irradiation upon recurrence. An additional 6 patients were palliated without any salvage therapies due to poor functional status, advanced age, or significant medical comorbidities precluding further treatment. Six patients continued to be observed but in shorter interval follow-up after MDT review as radiographic progression was minimal and they remained asymptomatic. Of the 21 patients that progressed, 15 (71%) met the Response Assessment in Neuro-Oncology (RANO) criteria for definitive progressive disease (PD) by virtue of a ≥ 25% increase in the product of the maximal perpendicular diameters of the tumor on MRI compared to their baseline image (Supplementary Fig. 1). Nonetheless, all of the progressive meningiomas in our study (including those that did not meet these RANO criteria) were significantly larger in volume pre-treatment (median GTV 19.1 cm3, interquartile range (IQR) 13.6–47.8 cm3) compared to meningiomas that remained stable after fRT (GTV 9.59 cm3, IQR 4.46–24.1 cm3, p = 2.86 × 10−2, Welch two sample t-test, Table 1).
Cavernous sinus and optic nerve sheath meningiomas are well controlled with fRT
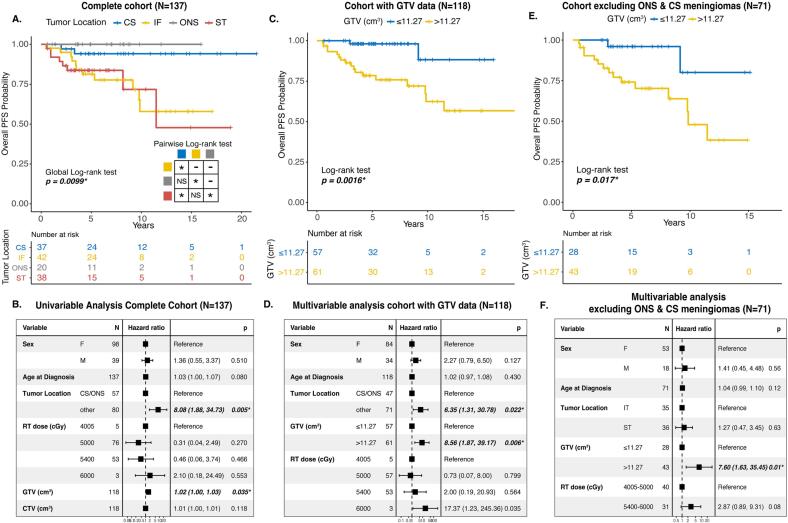
Due to a lack of histopathologic data for most meningiomas treated with primary fRT, tumor location often takes on a central role for treatment planning and prognostication. KM survival analysis showed that CS and ONS meningiomas had significantly better PFS rates post-fRT compared to other supratentorial (ST) or infratentorial (IF) meningiomas (Fig. 1A). There was no significant difference in the PFS rates of CS meningiomas compared to ONS meningiomas (pairwise Log-rank test, p = 0.299), and similarly between ST and IT meningiomas (pairwise Log-rank test, p = 0.909). When CS and ONS meningiomas were grouped together versus meningiomas in all other locations, no significant association was found between RT dose and anatomic location (p = 0.2669, Pearson’s Chi-squared test). When we fit a univariable Cox proportional hazards model, tumor location other than CS/ONS location was significantly associated with poorer PFS post-fRT (HR 8.08, 95% CI 1.88–34.73, p = 0.005; Fig. 1B). Progression in the IT meningioma group was driven by CPA (N = 4), and petrous/petroclival meningiomas (N = 5). In the ST meningioma group, half of all irradiated parasagittal/parafalcine meningiomas (N = 4) progressed post-fRT, all of which were either invading or occluding part of the superior sagittal sinus. The invaded sinus disease was included in the treatment fields of all these meningiomas with a PTV:CTV (cm3) ratio ranging from 1.69 to 2.98. None of the parasagittal/parafalcine meningiomas that remained stable following fRT had definitive venous sinus invasion.
Fig. 1.
(Colour figure) A. Kaplan-Meier (KM) survival curve of PFS post-fRT for meningiomas stratified by location. B. Forest plot of results from univariable Cox proportional hazards model in the complete cohort of fRT-treated meningiomas. C. KM survival curve of all meningiomas with available volumetric treatment data (GTV) stratified based on a GTV cut-off that optimizes the Youden index. D. Forest plot of results from the multivariable Cox proportional hazards model of meningiomas with volumetric data from all tumor locations. E. KM survival curve of meningiomas with volumetric data excluding those in optic nerve sheath (ONS) or cavernous sinus (CS) locations. F. Forest plot of results from multivariable Cox proportional hazards model of meningiomas with volumetric data excluding ONS and CS meningiomas. PFS- progression-free survival; CS- cavernous sinus; IF- infratentorial; ONS- optic nerve sheath; ST- supratentorial; RT- radiotherapy; cGy- centigray; GTV- gross tumor volume; CTV- clinical target volume. *p < 0.05 where group sizes are sufficiently large to draw statistical conclusions.
The effect of GTV on meningioma progression post-fRT
Due to the observed volumetric differences in the meningiomas that failed fRT vs those that remained stable after treatment, we wanted to ascertain whether increasing GTV could be associated with PFS post-fRT. Overall, we had granular, volumetric fRT planning data on 118 meningiomas (86%). Increasing GTV as a continuous variable was significantly associated with poorer PFS on univariable analysis, but the effect size was small and challenging to interpret from a practical standpoint (Fig. 1B). Therefore, to determine if instead there was a volumetric cut-off that could be used to dichotomize outcomes, analogous to a critical tumor volume above which treatment failure would be more common, the point on the ROC that maximized the Youden index was identified as 11.27 cm3 (Supplementary Fig. 2). KM survival analysis based on this dichotomization showed that meningiomas with a GTV > 11.27 cm3 had significantly poorer PFS post-fRT compared to their smaller counterparts (p = 0.0016, Log-rank test, Fig. 1C). When we fit a multivariable Cox proportional hazards model with this GTV cut-off, when controlling for other clinical covariates, we found that a larger GTV > 11.27 cm3 was independently associated with poorer PFS (HR 8.56, 95% CI 1.87–39.17, p = 0.006; Fig. 1D). Another factor associated with poorer PFS post-fRT was non CS/ONS tumor location (N = 71). A total RT dose of 60 Gy appeared to be associated with worse outcomes, however this was a rare treatment dose assigned to only a small number of patients (N = 3), all of whom had rapid progression of their meningiomas even before treatment, and therefore meaningful conclusions could not be drawn for these cases. There was no statistically significant interaction between total RT dose given or tumor location and our GTV cut-off (p = 0.759, 0.258 respectively, Likelihood ratio test). However, as CS/ONS meningiomas are usually small and may confound our results, we also performed a subgroup analysis with only non-CS/ONS meningiomas. As there were only 2 meningiomas in this subgroup that received a total RT dose of 60 Gy, we dichotomized total RT dose into a 40–50 Gy and 54–60 Gy group. KM survival analysis showed that using the same GTV cut-off, larger non-CS/ONS meningiomas (>11.27 cm3) still had significantly poorer PFS post-fRT (Fig. 1E). Multivariable Cox proportional hazards analysis again demonstrated that even within this subgroup of meningiomas (excluding CS/ONS meningiomas), having a tumor that exceeded this volumetric cut-off (GTV > 11.27 cm3) was independently associated with poorer PFS post-fRT (HR 7.60, 95% CI 1.63–35.45, p = 0.01, Fig. 1F). Sensitivity analyses excluding ONS meningiomas, and meningiomas treated with non-conventional fRT regimens (40 or 60 Gy total) did not meaningfully change these findings, nor did these results change when we defined tumor progression based on the RANO criteria above (Supplementary Figure 4).
Significant adverse events following primary fRT were rare but associated with larger meningiomas
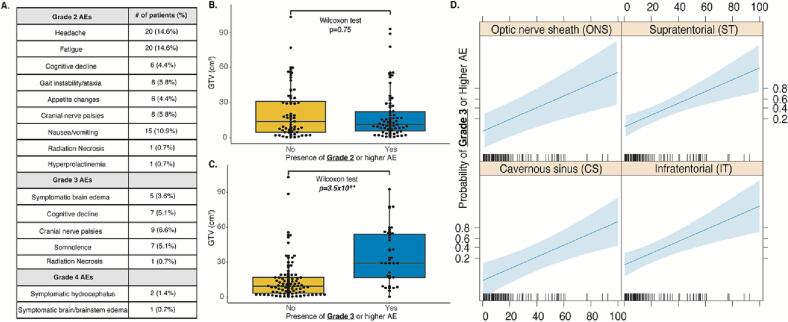
Reported early AEs excluding alopecia are listed in Fig. 2A. Late AEs (>5 years) including pituitary failure were inconsistently reported and therefore not included. Only 1 patient with a cavernous sinus meningioma developed hyperprolactinemia (diagnosed due to amenorrhea) within a year of treatment with 54 Gy/30 Fr and required treatment with cabergoline. All 3 patients that received a total RT dose of 60 Gy developed grade 3 brain edema requiring dexamethasone. The incidence of radiation necrosis was rare, with clear radiographic evidence demonstrated in only 2 patients (1.5%, one grade 2 event, one grade 3 event). Overall, grade 4 AEs were rare (N = 3), with 2 patients developing symptomatic hydrocephalus requiring a ventriculoperitoneal shunt (CPA, sphenoid wing locations), and 1 patient with a CPA meningioma developing severe, symptomatic brainstem edema following fRT requiring protracted hospitalization and escalating dexamethasone. There were no grade 5 AEs.
Fig. 2.
A. List of reported adverse events following fRT graded by the CTCAE v5.0 criteria. B. GTV of meningioma patients with and without a grade 2 or higher AE. C. GTV of meningioma patients with and without a grade 3 or higher AE. D. Predicted probability plots of a grade 3 or higher AE against pre-fRT GTV based on the logistic regression model stratified by tumour location, adjusted for patient age. Blue shading represents the 95% CI. AEs- adverse events; GTV- gross tumor volume; ONS- optic nerve sheath, ST- supratentorial; CS- cavernous sinus; IT- infratentorial. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For logistic regression modeling, our outcome was the presence of any grade 3 or 4 AEs, as these are considered significant enough to impair patients’ activities of daily living. Covariates were the same as in the above regression models. In this model, the only independent predictor of significant AEs was larger GTV as a continuous variable (OR 1.71, 95% CI 1.27–2.30, p = 3.90 × 10−4, Table 2). There was no significant difference in GTV between patients with and without grade 2 AEs (median GTV 18.78 cm3 vs 21.27 cm3, p = 0.75, Wilcoxon test, Fig. 2A). However, patients with grade 3 or higher AEs had significantly larger meningiomas compared to those without grade 3 or higher AEs (median GTV 35.34 cm3 vs 14.49 cm3 p = 3.5 × 10−6, Wilcoxon test, Fig. 2B). The plotted predicted probability function based on our logistic regression modeling suggests there is an increased probability of a grade 3 or higher AE with increasing GTV when all other covariates are fixed (Fig. 2C).
Table 2.
Logistic regression modeling predicting significant (CTCAE grade 3 or 4) AEs.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age at Diagnosis | 1.12 | 0.52–2.46 | 0.762 |
| Sex (M:F) | 2.22 | 0.75–6.56 | 0.150 |
| Radiotherapy | |||
| Total dose (cGy) | |||
| 4005:5000 | 10.60 | 0.60–186.5 | 0.107 |
| 5400:5000 | 2.09 | 0.69–5.55 | 0.166 |
| 6000:5000 | 11.35 | 0.50–256.8 | 0.127 |
| Tumour Location (vs ONS) | |||
| CS | 0.41 | 0.048–3.56 | 0.417 |
| IF | 1.58 | 0.22–11.48 | 0.650 |
| ST | 1.69 | 0.24–11.87 | 0.598 |
| GTV (cm3) | |||
| Per 10 cm3 increase in GTV | 1.71 | 1.27–2.30 | 3.90 × 10−4 |
*p < 0.05 Wald test; RT- radiotherapy; Gy- gray; GTV- gross tumour volume; WHO- World Health Organization; fRT- fractionated radiotherapy.
Discussion
The role of meningioma tumour volume on treatment considerations
While SRS has emerged as a viable alternative to surgery for some meningioma patients, it is well known that for SRS, an optimal tumor size/volume threshold should be respected to in order to achieve favorable treatment outcomes [5], [7], [17], [18]. Similar to SRS, our study found a higher GTV is associated with poorer tumor control rates and higher rates of significant AEs following primary fRT. Whether our specific GTV cut-off is generalizable across other cohorts remains to be determined. Nevertheless, in the absence of tumor tissue and resultant molecular or histopathologic data, the findings presented in this study may prove valuable in guiding patient selection for treatment, providing counseling, and offering prognostic insights for this unique subset of meningioma patients who undergo fRT as their primary therapeutic approach.
The role of RT dose and treatment technique on meningioma progression
The majority of meningiomas treated in our study received 50 Gy/25 Fr or 54 Gy/30 Fr (129/137, 94%) with only a minority receiving a hypofractionated regimen (40 Gy/15 Fr) or dose escalation (60 Gy/30 Fr). While hypofractionation has been found to be an effective strategy for some meningiomas post-operatively, our cohort did not include a sufficient number of patients to perform a matched analysis comparing this to conventional fractionation [19], [20], [21]. Dose escalation (to 60 Gy and beyond) has also been investigated but mostly in smaller studies and in the context of treating higher grade meningiomas (WHO grades 2 and 3), including those with subtotal resections [22], [23], [24], [25]. At our institution, WHO grade 2 or 3 meningiomas receive 60 Gy/30 Fr with an optional boost to any residual enhancing mass with a 3 mm margin (PTV) of 6 Gy/3 Fr for a total of 66 Gy/33 Fr. Although the patients treated with 60 Gy/30 Fr (N = 3) in this study did not have diagnostic tissue for grading, their meningiomas demonstrated rapid progression prior to treatment, suggesting they were likely to be higher grade to begin with (salvage resection confirmed one of these cases was a WHO grade 2 meningioma). All 3 of these patients also developed grade 3 cerebral edema requiring dexamethasone. Regardless of the higher treatment dose, 2/3 of these patients had in-field progression, likely reflecting the fact that many biologically aggressive meningiomas are refractory to RT. Further work is needed in order to uncover these markers of radiation resistance and to determine the optimal fractionation for meningioma patients as both adjuvant and primary therapy. Two landmark phase 3 clinical trials randomizing WHO grade 2 meningioma patients to adjuvant RT versus observation (ROAM/EORTC-1308 and NRG-BN003) following gross total resection will have significant translational components that will significantly improve our understanding of biomarkers of RT responsiveness in meningiomas [26], [27], [28], [29].
The role of meningioma location on treatment considerations
Concordant with previous studies, we found that CS and ONS meningiomas were optimally controlled with primary fRT [5], [7], [12], [30], [31]. Large CPA meningiomas however may be at higher risk for recurrence and AEs post-fRT, due to their close proximity to the brainstem and their larger size, excluding them from SRS eligibility. Parasagittal/parafalcine meningiomas with venous sinus invasion also had higher recurrence rates following fRT despite the inclusion of the sinus disease in treatment plans, concordant with outcomes following surgery [32], [33], [34], [35]. Although skull base meningiomas have been found to be more biologically benign compared to their non-skull base counterparts, whether the differences in outcome we see here could be attributed to inherent differences in the radiation responsiveness of these meningiomas (CS/ONS vs CPA, parafalcine/parasagittal) as opposed to other factors such as dosing, treatment planning, and tumor size remain to be determined through molecular studies.
Limitations and future directions
This study is limited by its retrospective nature and data arising from a single center which may limit its generalizability. Late adverse events of RT including pituitary failure were also likely underreported due to a lack of routine hormonal testing post-procedure. Cognitive decline may be underreported given that previous studies on the RT-treated meningioma patients from our institution who were referred for formal cognitive testing demonstrated a substantial proportion of patients experiencing global cognitive impairment (68%) with 48% unable to return to work after [36], [37]. We also defined tumor progression based on in-field radiographic recurrences that were confirmed by a neuroradiologist instead of using a standardized volumetric cut-off [38]. However, analysis using the RANO criteria for definitive progressive disease did not appreciably change our results or our conclusions, although these criteria were originally defined for prospective studies and for PFS after surgery, not after primary fRT. Based on our findings however, a prospective study comparing primary SRS versus fRT may be justifiable but should include patients within a predetermined GTV range for meningiomas in anatomic locations that are eligible for both treatment modalities and include formal cognitive testing in follow-up. Furthermore, tissue sampling of meningiomas that fail RT (SRS, primary or adjuvant fRT), for both retrospective and prospective cases from the aforementioned ROAM/EORTC-1308 and NRG-BN003 trials is critical and will provide important insights into the mechanisms of RT resistance in meningiomas.
Conclusions
We present a large retrospective analysis of meningiomas treated with primary fRT. We find meningioma GTV and location to be key outcome predictors for this rare and challenging subpopulation of patients that may not otherwise be treatable with surgery or SRS.
Funding
Justin Z. Wang was funded by the Vanier Scholarship from the Canadian Institutes for Health Research and the Neurosurgery Research Educational Fund & Southeastern Brain Tumor Foundation Research Fellowship.
CRediT authorship contribution statement
Justin Z. Wang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Alexander P. Landry: Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Farshad Nassiri: Investigation, Project administration, Resources, Supervision, Writing - original draft. Zamir A. Merali: Data curation, Resources, Software, Writing - review & editing. Zeel Patel: Data curation, Investigation, Methodology, Writing - review & editing. Grace Lee: Data curation, Investigation. Lauren Rogers: Data curation, Investigation, Writing - review & editing. Jeffrey A. Zuccato: Data curation, Methodology, Writing - review & editing. Mathew R. Voisin: Data curation. David Munoz: Data curation, Methodology. Derek S. Tsang: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing - review & editing. Normand Laperriere: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing - review & editing. Gelareh Zadeh: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing - original draft, Writing- review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Justin Z. Wang was funded by the Vanier Scholarship from the Canadian Institutes for Health Research (CIHR) and the Neurosurgery Research Educational Fund (NREF) & Southeastern Brain Tumor Foundation Research Fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100631.
Contributor Information
Justin Z. Wang, Email: justinz.wang@mail.utoronto.ca.
Alexander P. Landry, Email: alex.landry@mail.utoronto.ca.
Farshad Nassiri, Email: farshad.nassiri@mail.utoronto.ca.
Zamir A. Merali, Email: zamir.merali@mail.utoronto.ca.
Zeel Patel, Email: zeel.patel@mail.utoronto.ca.
Grace Lee, Email: gracesh.lee@mail.utoronto.ca.
Lauren Rogers, Email: 19lcr@queensu.ca.
Jeffrey A. Zuccato, Email: jeff.zuccato@mail.utoronto.ca.
Mathew R. Voisin, Email: mathew.voisin@mail.utoronto.ca.
David Munoz, Email: David.Munoz@unityhealth.to.
Derek S. Tsang, Email: derek.tsang@rmp.uhn.ca.
Normand Laperriere, Email: Norm.Laperriere@rmp.uhn.ca.
Gelareh Zadeh, Email: gelareh.zadeh@uhn.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wiemels J., Wrensch M., Claus E.B. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin D.D., Lin J.L., Deng X.Y., et al. Trends in intracranial meningioma incidence in the United States, 2004–2015. Cancer Med. 2019;8(14):6458–6467. doi: 10.1002/cam4.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom Q.T., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(12 Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasawara C., Philbrick B.D., Adamson D.C. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. 2021;9(3) doi: 10.3390/biomedicines9030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen-Inbar O., Tata A., Moosa S., Lee C.C., Sheehan J.P. Stereotactic radiosurgery in the treatment of parasellar meningiomas: long-term volumetric evaluation. J Neurosurg. 2018;128(2):362–372. doi: 10.3171/2016.11.JNS161402. [DOI] [PubMed] [Google Scholar]
- 6.McGregor J.M., Sarkar A. Stereotactic radiosurgery and stereotactic radiotherapy in the treatment of skull base meningiomas. Otolaryngol Clin North Am. 2009;42(4):677–688. doi: 10.1016/j.otc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Rueß D., Fritsche F., Grau S., et al. Stereotactic radiosurgery of cavernous sinus meningiomas. J Neurol Surg B Skull Base. 2020;81(2):158–164. doi: 10.1055/s-0039-1683430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Franco R., Borzillo V., Ravo V., et al. Radiosurgery and stereotactic radiotherapy with cyberknife system for meningioma treatment. Neuroradiol J. 2018;31(1):18–26. doi: 10.1177/1971400917744885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bir S.C., Patra D.P., Maiti T.K., Bollam P., Minagar A., Nanda A. Direct comparison of gamma knife radiosurgery and microsurgery for small size meningiomas. World Neurosurg. 2017;101:170–179. doi: 10.1016/j.wneu.2017.01.105. [DOI] [PubMed] [Google Scholar]
- 10.Vera E., Iorgulescu J.B., Raper D.M., et al. A review of stereotactic radiosurgery practice in the management of skull base meningiomas. J Neurol Surg B Skull Base. 2014;75(3):152–158. doi: 10.1055/s-0033-1354747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debus J., Wuendrich M., Pirzkall A., et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19(15):3547–3553. doi: 10.1200/JCO.2001.19.15.3547. [DOI] [PubMed] [Google Scholar]
- 12.Kheir V., Faouzi M., Borruat F.X. Visual outcomes of fractionated radiotherapy in optic nerve sheath meningioma: a retrospective study. Klin Monbl Augenheilkd. 2019;236(4):526–529. doi: 10.1055/a-0828-7335. [DOI] [PubMed] [Google Scholar]
- 13.Meniai-Merzouki F., Bernier-Chastagner V., Geffrelot J., et al. Hypofractionated stereotactic radiotherapy for patients with Intracranial meningiomas: impact of radiotherapy regimen on local control. Sci Rep. 2018;8(1):13666. doi: 10.1038/s41598-018-32124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesser R.L., Knisely J.P., Wang S.L., James B.Y., Kupersmith M.J. Long-term response to fractionated radiotherapy of presumed optic nerve sheath meningioma. Br J Ophthalmol. 2010;94(5):559–563. doi: 10.1136/bjo.2009.167346. [DOI] [PubMed] [Google Scholar]
- 15.Leroy H.-A., Tuleasca C., Reyns N., Levivier M. Radiosurgery and fractionated radiotherapy for cavernous sinus meningioma: a systematic review and meta-analysis. Acta Neurochir. 2018;160(12):2367–2378. doi: 10.1007/s00701-018-3711-9. [DOI] [PubMed] [Google Scholar]
- 16.Milker-Zabel S., Zabel A., Schulz-Ertner D., Schlegel W., Wannenmacher M., Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809–816. doi: 10.1016/j.ijrobp.2004.07.669. [DOI] [PubMed] [Google Scholar]
- 17.Flannery T.J., Kano H., Lunsford L.D., et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. 2010;112(5):957–964. doi: 10.3171/2009.8.JNS09695. [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43(3) doi: 10.1097/00006123-199809000-00001. 405-413; discussion 413. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen E.K., Nguyen T.K., Boldt G., Louie A.V., Bauman G.S. Hypofractionated stereotactic radiotherapy for intracranial meningioma: a systematic review. Neurooncol Pract. 2019;6(5):346–353. doi: 10.1093/nop/npy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen E.K., Pond G.R., Greenspoon J.N., Whitton A.C., Hann C. Hypofractionated stereotactic radiotherapy for the treatment of benign intracranial meningiomas: long-term safety and efficacy. Curr Oncol. 2021;28(5):3683–3691. doi: 10.3390/curroncol28050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarhan N., Abduljabbar L., Laperriere N., et al. Short course hypofractionated radiotherapy for frail or elderly patients with meningioma. Cureus. 2020;12(6) doi: 10.7759/cureus.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.J.B., Lee J., Yoon H.I., et al. Analysis of patterns of failure and appraisal of postoperative radiation field for grade II-III meningioma. J Neurooncol. 2019;144(2):333–341. doi: 10.1007/s11060-019-03232-w. [DOI] [PubMed] [Google Scholar]
- 23.Press R.H., Prabhu R.S., Appin C.L., et al. Outcomes and patterns of failure for grade 2 meningioma treated with reduced-margin intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88(5):1004–1010. doi: 10.1016/j.ijrobp.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Chan A.W., Bernstein K.D., Adams J.A., Parambi R.J., Loeffler J.S. Dose escalation with proton radiation therapy for high-grade meningiomas. Technol Cancer Res Treat. 2012;11(6):607–614. doi: 10.7785/tcrt.2012.500267. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80(2):195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M.D., Javadpour M., Haylock B.J., et al. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519. doi: 10.1186/s13063-015-1040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson M.D., Weber D.C., Haylock B.J., Mallucci C.L., Zakaria R., Javadpour M. Radiotherapy versus observation following surgical resection of atypical meningioma (the ROAM trial) Neuro Oncol. 2014;16(11):1560–1561. doi: 10.1093/neuonc/nou149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming C.W., Parsai S., Suh J.H. A management dilemma: adjuvant radiotherapy after gross total resection of atypical meningioma. Transl Cancer Res. 2019;8(1):1. doi: 10.21037/tcr.2018.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.29. Observation or Radiation Therapy in Treating Patients With Newly Diagnosed Grade II Meningioma That Has Been Completely Removed by Surgery 2018.
- 30.Soldà F., Wharram B., De Ieso P.B., Bonner J., Ashley S., Brada M. Long-term efficacy of fractionated radiotherapy for benign meningiomas. Radiother Oncol. 2013;109(2):330–334. doi: 10.1016/j.radonc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Preusser M., Brastianos P.K., Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. 2018;14(2):106–115. doi: 10.1038/nrneurol.2017.168. [DOI] [PubMed] [Google Scholar]
- 32.Haddad A.F., Young J.S., Kanungo I., et al. WHO grade I meningioma recurrence: identifying high risk patients using histopathological features and the MIB-1 index. Front Oncol. 2020;10:1522. doi: 10.3389/fonc.2020.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han M.-S., Kim Y.-J., Moon K.-S., et al. Lessons from surgical outcome for intracranial meningioma involving major venous sinus. Medicine. 2016;95(35) doi: 10.1097/MD.0000000000004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci. 2001;8(4):8–11. doi: 10.1054/jocn.2001.0868. [DOI] [PubMed] [Google Scholar]
- 35.DiMeco F., Li K.W., Casali C., et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery. 2004;55(6) doi: 10.1227/01.neu.0000143373.74160.f2. 1263-1272; discussion 1272-1264. [DOI] [PubMed] [Google Scholar]
- 36.Sekely A., Tsang D.S., Mabbott D., et al. Radiation dose to circumscribed brain regions and neurocognitive function in patients with meningioma. Neurooncol Pract. 2022;9(3):208–218. doi: 10.1093/nop/npac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekely A., Zakzanis K.K., Mabbott D., et al. Long-term neurocognitive, psychological, and return to work outcomes in meningioma patients. Support Care Cancer. 2022;30(5):3893–3902. doi: 10.1007/s00520-022-06838-5. [DOI] [PubMed] [Google Scholar]
- 38.Nassiri F., Wang J.Z., Au K., et al. Consensus core clinical data elements for meningiomas (v2021.1) Neuro Oncol. 2022;24(5):683–693. doi: 10.1093/neuonc/noab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.