Abstract
Background
Frankincense, a resin derived from trees of the Boswellia genus, has been used as an incense and a type of herbal medicine for treating inflammatory diseases such arthritis, chronic bowel illness, and asthma. While endometriosis is a well-known inflammatory gynecological illness caused by the ectopic attachment and development of uterine tissue over the menstrual cycle, the impact of frankincense on this illness is poorly understood. The purpose of this study was to explore the effects of frankincense on endometriosis.
Methods
We used a network pharmacological assessment, in vitro and in vivo investigations with a human endometriotic cell line as well as a syngeneic uterine transfer mouse model. High-performance liquid chromatographic analysis was used to compare water-extracted frankincense (Fr) to its reference compounds and validate the sample.
Results
A network pharmacological analysis suggested a positive effect of Fr on endometriosis. Fr relieved endometriosis by reducing ectopic endometrial adherence and development, according to both in vivo and in vitro models. We suggested that the ER stress/p53-apoptosis and chemokine-migration/adhesion pathways underlie Fr's anti-endometriotic action using RNA sequencing and bioinformatic analysis.
Conclusion
This study revealed the potential effect of Fr on endometriosis using an experimental investigation. Fr may have the potential to be an effective and safe treatment for endometriosis.
Keyword: Adhesion, Apoptosis, Endometriosis, Frankincense
1. Introduction
A common gynecological illness known as endometriosis occurs when endometrial tissue grows outside of the uterus.1 It develops inflammation, bleeding, visceral discomfort, fibrosis, and infertility in response to hormonal cues like the eutopic endometrium.2,3 Several multistep mechanisms, such as endometrial cell migration, adhesion, proliferation, and invasion, are crucial for the pathogenesis of endometriosis even if its pathophysiology is not entirely understood.4, 5, 6 An initial step in the development of an endometriotic lesion is the adhesive implantation of migrating endometrial tissue, which is controlled by adhesion molecules.7,8 In addition, a dysregulated apoptotic pathway also generally upregulates the growth of ectopic endometrial tissue.9,10 As a result, apoptosis induction and adhesion reduction are seen as attractive new therapeutic targets.11, 12, 13, 14
Since ancient times, frankincense, a resin derived from trees of the Boswellia genus, has been used as an incense and a type of herbal medicine.15 Three principal species of frankincense are used for collection: B. sacra Flueck. from East Africa, the Middle East, and China; B. frereana Birdw. from Somalia; and B. serrata Roxb. ex Colebr. from India.15, 16, 17 It is currently recognized that B. carteri Birdw., which were widely used in China, is a synonym for B. sacra.16,17 Frankincense has been used to treat inflammatory diseases such arthritis, chronic bowel illness, and asthma, despite species differences.15,18
A recent systematic review found that the chemical components in Boswellia species and their anti-inflammatory and anti-cancer effects occur through a variety of mechanisms, including reducing oxidative stress, controlling immune cells, modifying cell cycle, and preventing angiogenesis, invasion, and metastasis.19 Due to its ability to increase blood flow and reduce discomfort,15 frankincense is also used in herbal formulations that are typically combined with myrrh or ginger to treat a number of gynecological disorders, such as dysmenorrhea and menstrual bleeding.20,21 However, to the best of our knowledge, there isn't any direct experimental proof that frankincense has an inhibitory effect on the etiology of endometriosis.
In this study, we investigated the potential anti-endometriosis effects of frankincense using bioinformatic analyses of network pharmacology and RNA-sequencing data, as well as in vitro and in vivo experiments. As a result, we discovered that frankincense could prevent the development of endometriosis by decreasing adhesive implantation and increasing apoptotic cell death. For the development of novel pharmaceutical treatments, we thus propose frankincense as a potential anti-endometriosis agent.
2. Methods
2.1. Preparation of frankincense extracts
Frankincense water-extract (Fr) was purchased from the Korea Plant Extract Bank at the Korea Research Institute of Bioscience and Biotechnology (Chungju, Korea). The original plant, B. sacra, were cultivated in China and the lot number is CW04–036. The extract was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, Mo), and immediately diluted with culture media or corn oil immediately before use in vitro experiments or animal study, respectively.
2.2. High performance liquid chromatography (HPLC) analysis of Fr extract
Standard compounds, including β-boswellic acid (97.1% purity), 3-acetyl-11-keto-beta-boswellic acid, and 11-keto-beta-boswellic acid (100% purity), were provided by Cayman Chemical (Ann Arbor, MI) as standard chemicals according to previous studies.22,23 For usage as a standard stock solution, the compounds were dissolved in methanol at a concentration of 500 μg/ml. This solution was then diluted further for analysis. The Fr was filtered through a 0.45 μm syringe filter (Merck Millipore, Burlington, MA) after being dissolved in methanol and sonicated using ultrasound for 20 min. An Agilent 1200 series system (Agilent Technologies, Santa Clara, CA) with a C18 reverse phase column (Capcell Pak C18 UG120 4.6 × 250 mm, 5 μm; Shiseido, Tokyo, Japan) maintained at 30 °C was used for the HPLC study. For all three substances, the mobile phase was acetonitrile (95%): 0.5% acetic acid (5%). Beta-boswellic acid was determined from samples (20 L) using an ultraviolet detector at 205 nm and 11-keto-beta-boswellic acid and 3-acetyl-11-keto-beta-boswellic acid at 250 nm, respectively.
2.3. Network pharmacologic analysis
The databases of the traditional Chinese medicine integrative database (TCMID, http://bidd.group/TCMID/)24 and DisGeNet (https://www.disgenet.org/)25 were used to gather the possible target genes for Fr and endometriosis, respectively. The 81 genes of targets of Fr were analyzed for finding out potential pathways by Cytoscape (version 3.9.1, https://cytoscape.org/) with the aid of the JEPPETO plugin.26 Visualization was performed by constructing a scatter plot with XD-score and q value as axes. XD-score > 0.5 and q value < 0.25 was used as the cutoff criterion. A Venn diagram was created to display the 38 genes that are shared by the prospective targets of Fr and endometriosis. Cytoscape with the STRING application27 was used for analyzing the protein-protein interaction network of the 38 common targets.
2.4. Cell culture
Human pleural mesothelial Met-5A cells and immortalized human endometriotic epithelial 12Z cells were obtained from American Type Culture Collection (#CRL-9444; Manassas, VA) and Applied Biological Materials (#T0764; Richmond, Canada), respectively. Platinum A cells were provided by Cell Biolabs Inc. (RV-102; San Diego, CA). Met-5A cells or Platinum A cells were maintained in M199 (Welgene, Daegu, Korea) or DMEM (Welgene), respectively, with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA) and 1% penicillin/streptomycin supplemented. 12Z cells were maintained in Precoat T75 flask (Applied Biological Materials) with DMEM/F12 (Welgene) containing 10% non-inactivated charcoal-stripped FBS and 1% penicillin. All cell lines were grown at 37 °C in a humid environment with 5% CO2.
2.5. Cell viability
Cell viability was examined using 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich). The stated concentrations of Fr were applied to 12Z cells that had been seeded into 96 wells. Using a Spectramax M2 spectrofluorometer (Molecular Devices, San Jose, CA), absorbance at 450 nm was measured to determine the vitality of the cells. The dose of 50% growth inhibition (GI50) was calculated with the assistance of GraphPad Prism software (GraphPad, San Diego, CA).
2.6. Animal care
Female C57BL/6 mice aged five weeks were acquired from Orient Bio (Seongnam, Korea), and housed in an animal facility managed by Pusan National University's Institute for Laboratory Animals. The animal facility was regularly kept at a room temperature of 22 ± 2 °C, a humidity level of 50–60%, and a day/night cycle of 12 hrs. Inhaling CO2 or isoflurane was applied for anesthesia or sacrifice, respectively. The Institutional Animal Care and Use Committee of Pusan National University granted its approval to all experimental methods (Pusan, Republic of Korea; PNU-2020–2679).
2.7. Endometriosis mouse models
Based on a recently published version of our protocol, endometriosis was induced.28 Briefly, female C57BL/6 mice that were six weeks old had their ovaries removed and then recovered. Isoflurane inhalation was used for anesthesia. Mice were subcutaneously injected every week with 100 mg/kg of β-estradiol (Santa Cruz Biotechnology; Dallas, TX) fourteen days after ovariectomy. The uteri of donor mice were extracted and chopped using Gentle Max (Miltenyi Biotec, Bergisch Gladbach, Germany) before being inoculated intraperitoneally into recipient mice at a 1:1 ratio. After one day, the mice were given Fr orally 5 days a week, and 100 mg/kg β-estradiol was subcutaneously injected once a week for 3 weeks. Based on GI50 of Fr in 12Z cells, the doses of Fr given to mice were estimated. Two dosages, such as 0.3 mg/kg/day and 1.5 mg/kg/day, were lower than the usual dose, 1.321 g/kg/day, that was derived from the general human equivalent dose (HED) (0.2 g/kg/day) of Fr using the FDA-provided formula presented below.29
The mice were dissected under CO2 inhalation 1 day following the final injection of β-estradiol, and the number and weight of endometriotic lesions were assessed. There were five mice per group.
2.8. Apoptosis analysis
Apoptosis of the cells was examined propidium iodide (PI)/Annexin V (BD Biosciences, Franklin Lakes, NJ) techniques. 12Z cells were plated into 6 wells for PI/Annexin V detection. Fluorescence-activated cell sorting (FACS) analysis using Attune X (Thermo Fisher Scientific) was used to assess the proportion of PI/Annexin V positive cells at excitation (Ex) 494/emission (Em) 525 nm for Annexin V and Ex 535/Em 617 nm for PI. The FlowJo application was used for the analysis of FACS data (BD Bioscience).
2.9. Immunoblot analysis
Immunoblot analysis was used to investigate cell apoptotic signal proteins. RIPA buffer (Thermo Fisher Scientific) containing a protease inhibitor cocktail (Roche, Basel, Switzerland) was used to extract total proteins, and the concentration of proteins were then measured using the Bradford assay. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were separated and then transferred to a polyvinylidene fluoride membrane (Amersham Bioscience, Uppsala, Sweden). The membranes were incubated with primary antibody, including anti-human poly (ADP-ribose) polymerase (PARP, #9542 s; Cell Signalling, Danvers, MA), caspases-3 (#9665 s; Cell Signalling), caspase-9 (#9508 s; Cell Signalling), bax (NB100–56095; Novus Biologicals, Centennial, CO), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-32233; Santa Cruz), at 4 °C overnight. After vigorous washing with tris-buffered saline, the horseradish peroxidase-conjugated secondary antibodies, such as anti-rabbit IgG or anti-rat IgG (Invitrogen, Waltham, MA), were applied to the membranes at room temperature for 1 hrs. The specific bands were developed using the ImageQuant LAS 4000 chemiluminescence imaging equipment (GE Healthcare, Chicago, IL) and a western blot detection kit from Bio-Rad (Hercules, CA).
2.10. Plasmid transfection and retroviral infection in Met-5A cells
Using a retroviral packaging technique, pMX mCherry(N)-IRES plasmid DNA was transfected into Met-5A cells to produce red fluorescent cell lines. Our team member Professor Bae (Kosin University, Korea) developed the plasmid DNA designated as pMX mCherry(N)-IRES. Briefly, retroviral packaging was carried out by transfecting Platinum A with linear polyethylenimine (Polysciences, Warrington, PA) and retroviral vector. The media was replaced with new media a day after transfection, and transfected Platinum A cells were grown for the following 48 hrs. The virus particles in the cultured media were then collected and purified through a 0.45 μm syringe filter. Then, Met-5A cells were grown in medium containing viruses and polybrene (5 μg/ml; Sigma-Aldrich). Blasticidin (10 μg/ml; Sigma-Aldrich) was used to select transduced cells for 3 days.
2.11. Cell adhesion assay
A cell adhesion experiment was performed in accordance with a prior study.30 Met-5A cells were grown in 6-well plates, while 12Z cells were seeded in 100 mm cell culture dishes. The next day, 12Z cells were incubated with prescribed dosage of Fr for 24 hrs and were labeled with chloromethylfluorescein diacetate (CMFDA; Invitrogen) for 15 min at 37 °C. After being washed with phosphate-buffered saline (PBS), CMFDA-labeled 12Z cells were then carefully transferred onto a monolayer of Met-5A cells. For the purpose of removing non-adhered 12Z cells, cells were gently shaken at 20 rpm for 90 min at 37 °C. Following this, attached 12Z cells were observed under a fluorescent microscope (200x magnification; Axio Imager M1, Carl Zeiss, Oberkochen, Germany), and four randomly selected areas' numbers of adherent 12Z cells were determined for statistical analysis.
2.12. RNA-Sequencing
The 12Z cells were cultured at a density of 5 × 105/well in 6-well plates and treated with Fr (60 μg/ml) or vehicle (DMSO) for 12 h. Three wells of each treatment group were employed for RNA sequencing, and all sequencing processes were performed using the services of DNAlink Inc. (Seoul, South Korea). The quality of total RNA was verified using 2100 Expert Bioanalyzer with RNA 6000 Nano kit (Agilent Inc., Santa Clara, CA, USA) for ensuring the RNA integrity number of all samples > 7.0.
2.13. Gene set enrichment analysis (GSEA)
The GSEA was carried out using GSEA software from the Broad Institute (version 4.3.0, available at http://www.gsea-msigdb.org/gsea/downloads.jsp) as described previously.31,32 Analysis of the biological processes of DEGs was done using hallmark, Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Ontology (GO). Cytoscape was used to display the results of the network analysis of the GO gene with values of p < 0.001 and q < 0.5.
2.14. Differentially expressed gene (DEG) and Heatmap analysis
The R program DESeq2 (version 4.1.1, available at https://www.r-project.org/) was used to perform the transcriptome analysis as described previously.32, 33 Genes having p-values and Log2|FoldChange| (Log|FC|) values under and above 0.05 and 1, respectively, were deemed to be DEGs. Morpheus, a flexible matrix visualization and analysis program from the Broad Institute (https://software.broadinstitute.org/morpheus/), was used to create a heatmap of 88 discovered DEGs (50 up- and 37 down-regulated), which are engaged in the endometriosis-related pathways using GSEA analyses.
2.15. Statistical analysis
The mean and SEM are used to express values. For comparisons between two distinct groups, a two-tailed Student's t-test was employed, and for comparisons between multiple groups, a one-way analysis of variance followed by a Tukey's post-hoc test was utilized. These were completed with the use of GraphPad Prism software.
3. Results
3.1. Standardization of frankincense water-extract (Fr)
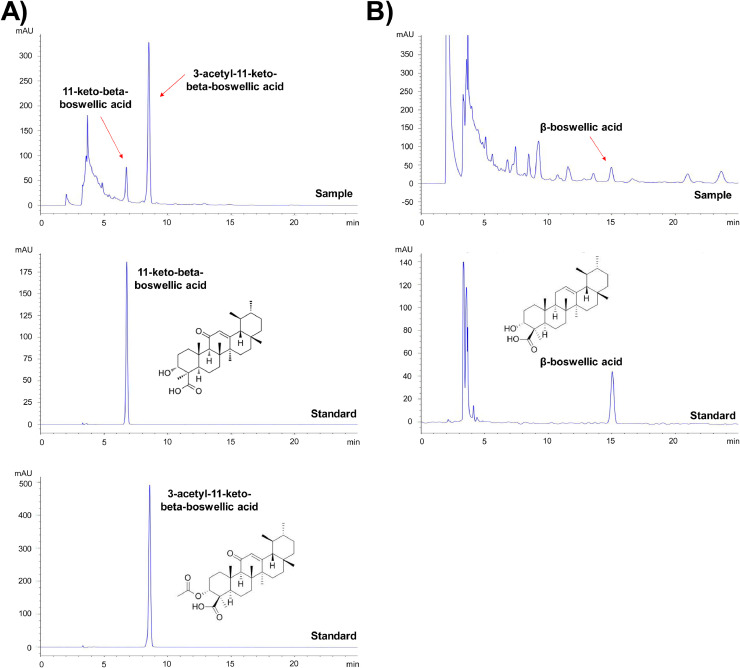
Using high-performance liquid chromatography with UV detection, the Fr used in this study was examined for validation and quantification. Boswellic acid derivatives, such as β-boswellic acid (97.1% purity), 3-acetyl-11-keto-beta-boswellic acid, and 11-keto-beta-boswellic acid (100% purity), were utilized according to earlier studies [22, 23]. As shown in Fig. 1, Fr was well separated within 20 min, and we estimated concentrations by the retention times of the peaks with those of three boswellic acid-derived standard solutions. To evaluate the reproducibility of the extraction efficiency, HPLC analysis was repeated three times using same samples. According to the data shown in Table 1, there was 9.225 ± 0.151 mg, 47.191 ± 0.162 mg, and 20.789 ± 0.148 mg of 11-keto-beta-boswellic acid, 3-acetyl-11-keto-beta-boswellic acid, and β-boswellic acid in 1 g of Fr were, respectively (Table 1).
Fig. 1.
Fr was validated by high performance liquid chromatography. Fr was subjected to 20 min of ultrasound sonication before being passed through a 0.45 μm syringe filter. Beta-boswellic acid, 3-acetyl-11-keto-beta-boswellic acid, and 11-keto-beta-boswellic acid were employed as reference materials. The samples were injected with 20 μL and examined using an ultraviolet detector at 205 nm for β-boswellic acid and 3-acetyl-11-keto-beta-boswellic acid (A), and at 250 nm for 11-keto-beta-boswellic acid and (B).
Table 1.
The concentration of each compound within Fr.
| Compounds | Retention time (min) | Concentration (mg/g) |
|---|---|---|
| 11-keto-beta-boswellic acid | 6.785 | 9.225 ± 0.151 |
| 3-acetyl-11-keto-beta-boswellic acid | 8.587 | 47.191 ± 0.162 |
| β-boswellic acid | 15.054 | 20.789 ± 0.148 |
3.2. Network pharmacological predicted Fr as a potential anti-endometriotic agent
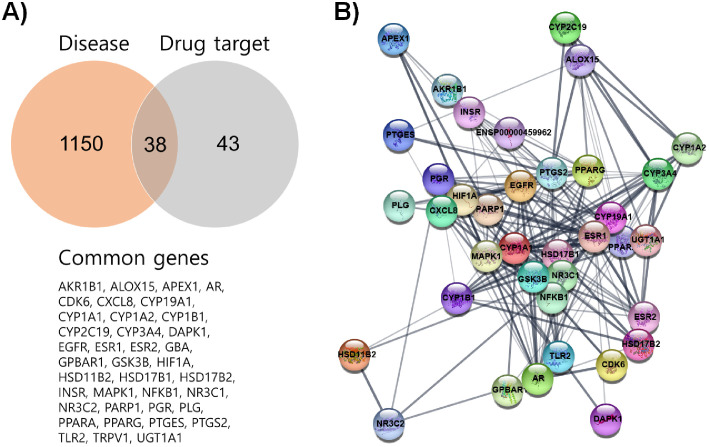
The list of potential Fr targets obtained from the TCMID (Supplementary Table S1) was employed in the network analysis of the JEPPTO plugin on the Cytoscape software. The findings showed that steroid hormone, inflammatory, migratory, adhesion, angiogenesis, and autophagic pathways were significantly altered, which were linked to endometriosis34, 35 (Supplementary Fig. S1 and Supplementary Table S2-S5). Thus, we hypothesized that endometriosis could be a potential target disease of Fr. The notion was tested by comparing the Fr targets to endometriosis-related genes from the DisGeNet database (listed in Supplementary Table S6). There were 38 genes identified as shared targets between endometriosis and Fr (Fig. 2A). With the exception of GBA, 37 of them have robust protein-protein interactions (PPI). Higher PPI levels were seen in the genes AR, CXCL8, CYP1A1, CYP1A2, CYP3A4, EGFR, ESR2, GSK3B, HIF1A, HSD17B2, MAPK1, NFKB, NR3C1, PGR, PPARG, PTGS2, and UGT1A1 (Fig. 2B).
Fig. 2.
The potential therapeutic effect of Fr on endometriosis is presented via network pharmacological analysis. The DisGeNet database was used to compile the list of endometriosis-related genes. A Venn diagram analysis was performed utilizing endometriosis-related genes and Fr targets. 38 common genes were listed (A). Cytoscape with the STRING plugin was used to show the protein-protein interaction network of the 38 common genes (B).
3.3. Fr reduced the viability of 12Z cells
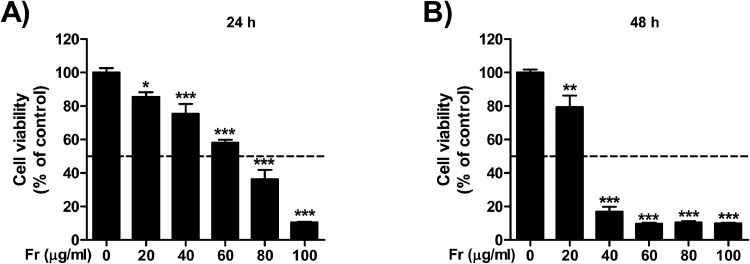
To first confirm the plausibility of an anti-endometriotic effect, we used an in vitro colorimetric cell viability assay. Fr was treated for 24 and 48 h at the stated concentrations (0, 20, 40, 60, 80, and 100 g/mL). The results indicated that Fr treatment decreased the viability of 12Z cells in a dose-dependent manner (Fig. 3A-B). The GI50 for 24 and 48 h is 78.46 μg/ml and 26.34 μg/ml, respectively. In the subsequent experiments, including the animal research, the apoptosis analysis, and the adhesion assay, we used the dose as a reference.
Fig. 3.
Fr reduced human endometriotic cell proliferation. Fr was dissolved in DMSO and diluted in DMEM-F12 medium. Indicated concentrations of Fr was treated to human endometriotic epithelial (12Z) cells. After 24 h (A) and 48 h (B), the cell viability was analyzed by measuring at a wavelength of 450 nm using MTT. Data shown as mean ± SEM of quintuplicated and are representative of three independent experiment. Statistical analysis was conducted using a Dunnet one-way ANOVA (*; p < 0.05, **; p < 0.01, ***; p < 0.001).
3.4. Fr decreased the number and weight of mouse endometriotic lesion
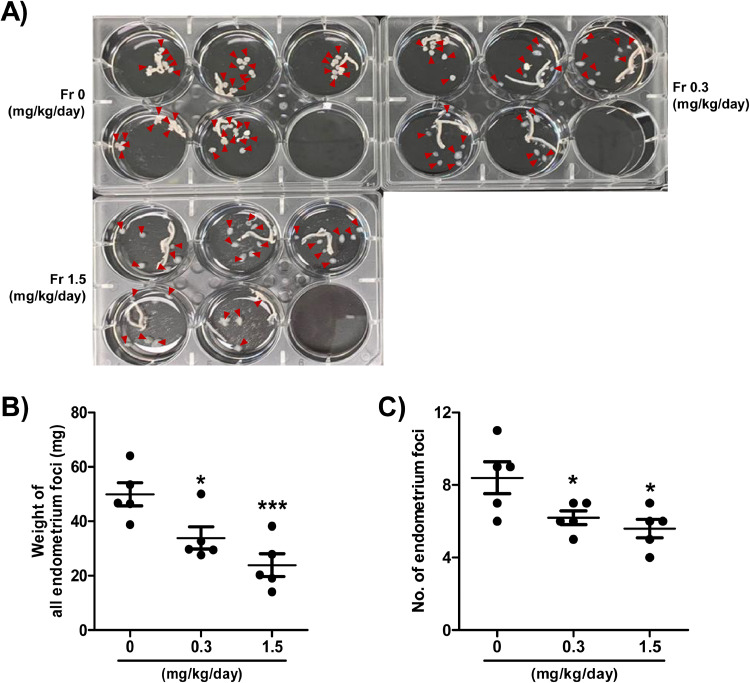
To validate the in vivo effectiveness of Fr in endometriosis, we applied the endometriosis mouse model using transfer of syngeneic uterus. After experimentally inducing endometriosis, Fr (0.3 or 1.5 mg/kg/day) was administered orally five times per week. We examined the quantity and weight of cyst-like ectopic lesions, i.e. endometrial foci, adherent to intestinal or abdominal tissues three weeks after Fr administration. When compared to the vehicle, Fr significantly reduced the number and volume of ectopic endometrial foci (Fig. 4). Under microscopic examination of H&E-stained sections of kidney and liver tissues, the amounts of Fr employed in the in vivo experiment were determined to be nontoxic (Supplementary Fig. S2). These findings suggest that administering Fr orally prevented the adhesion or development of ectopic endometriotic tissues.
Fig. 4.
Fr reduces the size and number of murine ectopic endometrial lesions. The pictures of the ectopic endometrial tissues and uterus. Red arrows indicate endometrial tissues (A). The weight and number of ectopic endometrial tissues (B-C). Data are expressed as mean ± SEM. Statistical analysis was conducted using a t-test (*; p < 0.05, ***; p < 0.001).
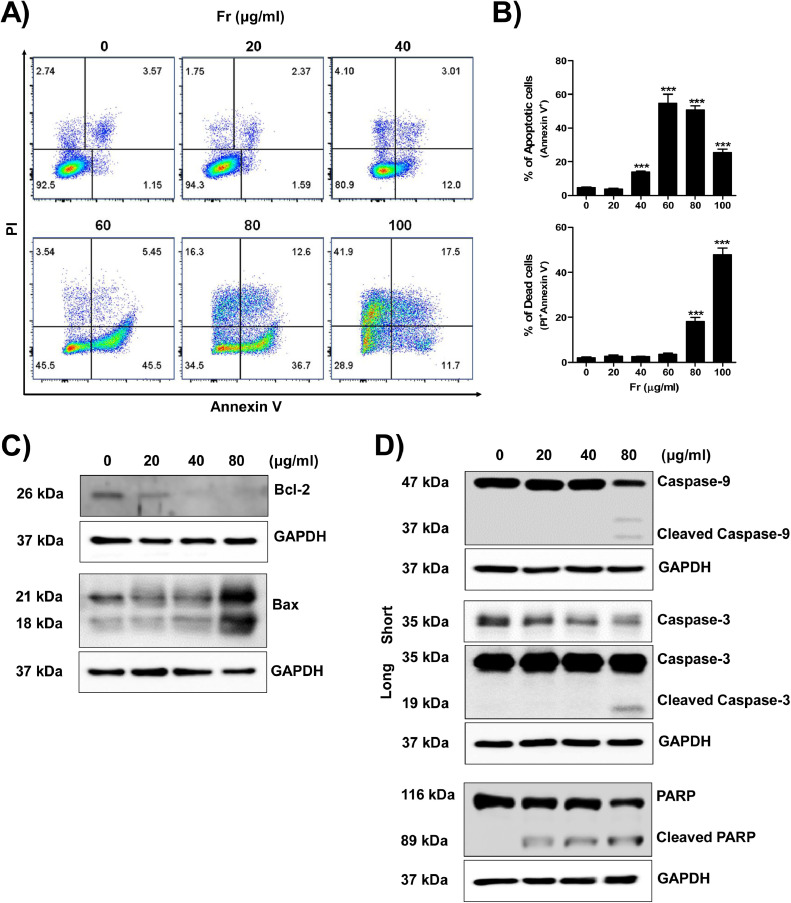
3.5. Fr induced apoptosis in 12Z cells
We investigated the possibility of apoptotic cell death to identify the mechanism explaining the reduced viability of 12Z cells treated with Fr. The PI/Annexin V apoptosis assay demonstrated that Fr dose-dependently promoted apoptosis of 12Z cells, similar to the cell viability analyses. At a concentration of 40 μg/ml, apoptotic cells (Annexin V+), including necroptic and early apoptotic cells, were first noticed. At an increase of 80 μg/mL, these apoptotic cells were noticeably more prevalent. More than 50% of the dead cells (PI+) were seen at a concentration of 100 μg/ml. (Fig. 5A and B). Following that, we evaluated the expression of proteins implicated in intracellular apoptotic signaling in 12Z cells. As illustrated in Fig. 5C, the Fr treatment decreased the expression of the bcl-2 protein while increasing the expression of the bax protein. Fr also regulated the conversion of caspases-3, −9, and PARP from pro- to cleaved active-form (Fig. 5D). These findings suggest that Fr induces apoptosis in 12Z cells by activating intracellular apoptosis pathways and modulating the bcl2/bax balance.
Fig. 5.
Fr activates the apoptosis-death signaling pathway in endometriotic cells. For analysis of frequency of apoptotic or dead cell populations, 12Z cells were incubated with the increasing doses of Fr for 24 h, and then the percentage of Annexin+ or PI+ cells were measured at excitation (Ex) 494/emission (Em) 525 nm for Annexin V and Ex 535/Em 617 nm for PI (A, B). Mitochondria-relative apoptosis molecules, Bax and Bcl-2 (C) and the cleavage forms of caspase −3 and −9, and PARP, main intracellular apoptosis signaling proteins (D), were detected 24 hrs after Fr treatment. GAPDH was used as control, and the specific molecular weight is represented on the left.
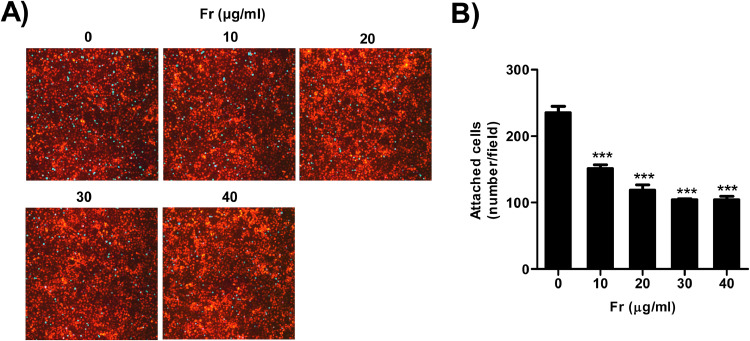
3.6. Fr prevented 12Z from attaching to Met-5A cells
According to the amount of endometriotic foci in an animal investigation, we speculated that Fr could prevent endometriotic tissue from adhering to abdominal tissues. Thus, using Met-5A and 12Z cells, we conducted an in vitro adhesion assay to test the potential. As seen in Fig. 6, Fr prevented transferred 12Z cells (green) from adhering to the Met-5A cells underneath them (red). In a dose-dependent way, there was a considerable decrease in the amount of 12Z cells adhering to Met-5A cells. The amount of attached 12Z cells was decreased to 50.49 percent of control with 20 μg/ml of Fr-treatment. Overall, the findings suggest that Fr's potential function in limiting the initial stage of endometriosis by regulating the endometrial-mesothelial adhesion.
Fig. 6.
Fr decreased the ability of endometrial cells to adhere to mesothelial cells. 12Z cells were stained with CMFDA after 24 h of Fr treatment and then gently transferred onto a red fluorescent Met-5A cell monolayer. After 90 min with gentle shaking at 20 rpm, unbound 12Z cells were removed, and cells were then visualized using a fluorescent microscope (200 × magnification) (A). The number of 12Z cells bound to Met-5A cells was manually counted in four randomly chosen areas for statistical analysis (B). Data are representative of one of five independent experiments and values are expressed in mean ± SEM. Statistical analysis was conducted using a Dunnet one-way ANOVA (***; p < 0.001).
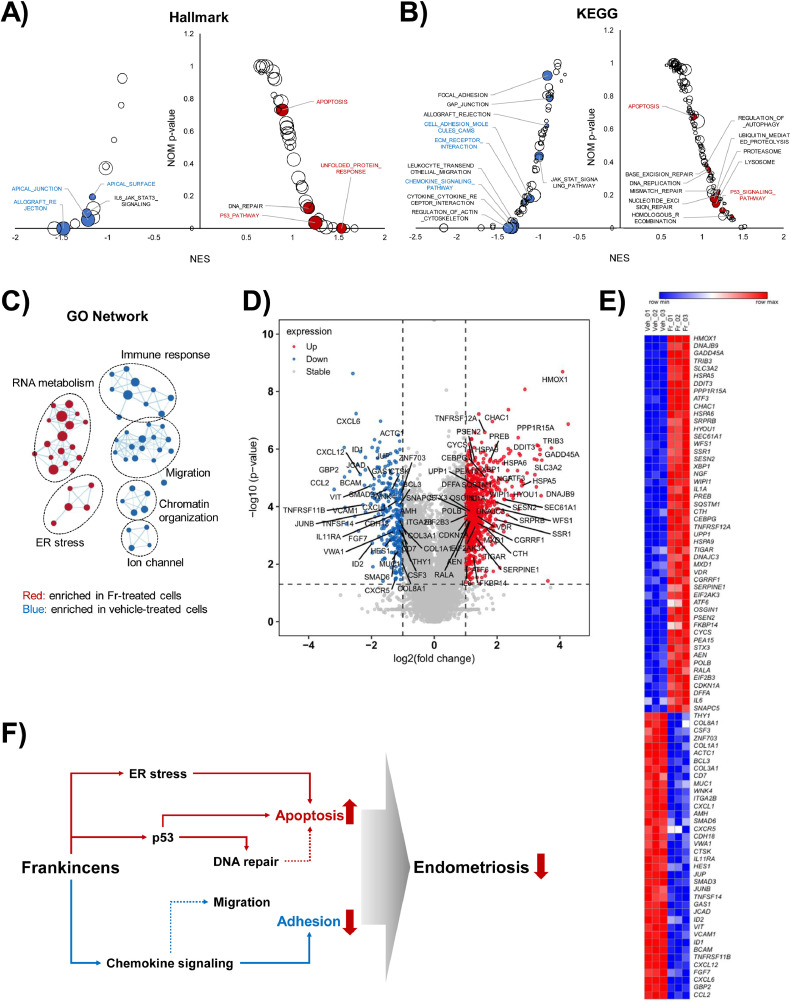
3.7. RNA sequencing identified potential mechanisms for Fr's anti-endometriotic activity
We employed RNA sequencing to investigate how Fr regulates cell death and adhesion at the gene expression level. According to a GSEA analysis using KEGG and hallmark, p53 and apoptosis pathways were increased whereas chemokine signaling and cellular adhesion pathways were downregulated (Fig. 7A, B, and Supplementary Fig. S3). Proteolytic activities such as unfolded protein response (UPR), proteasome, lysosome, and autophagy were elevated. Additionally, the network analysis of the GO analysis revealed downregulated immune response and migration pathways, and elevated ER stress signature (Fig. 7C). The mapping of KEGG pathways, including p53, apoptosis, chemokine signaling, ECM-receptor, and cell adhesion molecule, was shown in Supplementary Fig. S4. According to DEG analysis, 949 genes were considerably elevated, whereas 284 genes were significantly downregulated (Fig. 7D). The 88 genes (38 downregulated and 50 upregulated) were chosen based on their involvement in the pathways depicted in Fig. 7D and Supplementary Fig. S3. shows the symbols of such chosen genes separately, and heatmap analysis was used to summarize the relative expression levels of 88 genes (Fig. 7E).
Fig. 7.
RNA-sequencing analysis suggests possible mode of action (MoA) underlying anti-endometriosis effect of Fr. The 12Z cells were treated with Fr (70 μg/ml) for 12 h. The RNA-sequencing dataset supplied by commercial service was applied to GSEA. The results from hallmark and KEGG analysis were visualized by bubble plot. The major pathways related with endometriosis was indicated by red (upregulated) or blue (downregulated) characters (A, B). The data from GO analysis was described by Cytoscape with enrichment map visualization. The cutoff value was set as p > 0.001 and q > 0.5 (C). The DEG analysis visualized as log2 fold change and -log10 p-value. Genes that were noticeably up- or down-regulated were denoted by red or blue characters, respectively. The genes participating in endometriosis-related pathways, as shown in the A and B panels, were indicated by a symbol for each gene (D). Heatmap analysis was used to display the relative expression levels of genes connected to endometriosis-related pathways (E). A graphical scheme was used to outline the potential mechanisms of action (MoA) underlying the relieving effects of Fr on endometriosis (F).
4. Discussion
Several frankincense-containing herbal remedies, including Bushen Huoxue prescription, Qu Yi Kang, and Qing Yi decoction, have been used to treat endometriosis.36,37 In addition, due to its anti-proliferative, anti-nociceptive, and anti-inflammatory activities, frankincense was already proposed as a possible herbal therapy for treating endometriosis.38 However, there is no concrete evidence to support the review paper's claim that frankincense has an anti-endometriotic effect. In this investigation, we validated the possibility by employing network pharmacologic analysis using a list of probable target genes from TCMID and several signaling pathways associated to steroid hormone, inflammation, migration, and cell-cell adhesion, were discovered. These pathways are recognized as having a close connection to the pathogenesis of endometriosis.34,39,40 Additionally, GO biological process analysis established the vascular endothelial growth factor and autophagy pathways, which are emerging pathways for the development of endometriosis.41, 42, 43
The in vivo experiments employing syngeneic uterine transfer mice model showed that Fr effectively reduced the number and weight of ectopic endometriosis foci. Based on the findings, we hypothesized that Fr might decrease the attachment of injected uterine cells to peritoneal myometrium and subsequent proliferation of the ectopic endometrial cells. Increased adhesion and resistance to apoptosis are thought to be crucial stages in the development of endometriosis and promising therapeutic targets.8,13,44 In vitro experiments using a FACS-based apoptosis assay, western blot analysis, and cell-cell adhesion assay were used to conform the possibilities. In multiple previous studies, frankincense was identified as a therapeutic herb that promoted apoptosis and prevented tumor migration in a number of cancer types, particularly estrogen-dependent breast cancer.45, 46, 47 Despite differences in cell types, the prior publications agree with our observations.
Herbal medications are generally recognized to have multi-activity due to the multi-component nature of its constituent phytochemicals and secondary metabolites.48,49 As a consequence, it is challenging to understand the mode of action (MoA) that drives the effects of herbal medications. Several prior studies used bioinformatic analysis based on omics data, including transcriptome, to address the issue.31,50,51 Frankincense contains a wide range of phytochemicals, including volatile oil, polysaccharides, monoterpenes, diterpenes, and lipophilic pentacyclic triterpene acids.19,22 In order to understand the molecular mechanisms behind the pro-apoptotic and anti-adhesive effects of Fr, we also used the transcriptome and bioinformatic analyses in this study. Based on the results, we hypothesized that the anti-adhesive activity of Fr may be related to cytokine and chemokine signaling. Additionally, the p53-DNA damage response and ER stress-mediated UPR may serve as a model for its pro-apoptotic activity (Fig. 7F). However, more extensive investigation is required to identify the main active ingredients and assess potential MoA of Fr pathways.
Among the numerous constituent compounds in frankincense, boswellic acids and triterpenoids are well-known main constituents.52 Although we have not examined each substance's activity, based on previous research, 3-acetyl-11-keto-β-boswellic acid is expected to be the most effective endometriosis inhibitor. There are three main reasons why 3-acetyl-11-keto-β-boswellic acid is the most effective ingredient. To begin, in vitro experiments with tumor cells show that 3-acetyl-11-keto-β-boswellic acid and 11-keto-β-boswellic acid initiate an apoptotic cascade by increasing the activity of caspase-8, −9, −3, and PARP cleavage. However, β-boswellic acid has no effect on the induction of cell apoptosis.53 Beta-boswellic acids, in particular, act as an apoptosis inhibitor in normal cells but induce apoptosis in cancer cells. Furthermore, 3-acetyl-11-keto-β-boswellic acid has antitumor effects in vivo by inhibiting cell growth, proliferation, and migration/invasion potential.54,55 Finally, in HPLC analysis, the content of 3-acetyl-11-keto-β-boswellic acid in Fr is the highest of the three types of boswellic acids (Table 1).
In an animal investigation, mice with endometriosis were given two different dosages of Fr that were calculated from an in vitro cytotoxic assay. The doses are substantially lower than those predicted by the typical human use of Fr. Moreover, histological examination of the liver and kidney at the levels used in the animal study revealed no abnormal morphological alterations. A number of previous researches carried on the other species, such as rat, dog, horse, and human, have shown that general human ingestion is safe across the board.56, 57, 58 According to these publications and our results, Fr may be a favorable candidate for endometriosis treatment in terms of safety.
In conclusion, here we firstly revealed the potential effect of Fr on endometriosis using an experimental investigation. The results from both in vivo and in vitro models showed that Fr relieved endometriosis by decreasing ectopic endometrial adherence and development. Using RNA sequencing and bioinformatic analysis, we proposed the ER stress/p53-apoptosis and chemokine-migration/adhesion pathways as the mechanisms underlying the anti-endometriotic action of Fr. As a result, we recommend Fr, a water-extract of frankincense, as an efficient and safe option for treating endometriosis.
CRediT authorship contribution statement
Min Kyoung Cho: Methodology, Investigation, Validation, Formal analysis, Writing original draft, Writing review & editing. Jung-Sook Jin: Methodology, Investigation, Writing review & editing. Yunju Jo: Formal analysis, Writing review & editing. Jung Ho Han: Investigation, Writing review & editing. Su Shin: Investigation, Writing review & editing. Sung-Jin Bae: Methodology, Writing review & editing. Dongryeol Ryu: Formal analysis, Writing review & editing. Jongkil Joo: Writing review & editing. Jang-Kyung Park: Conceptualization, Funding acquisition, Writing review & editing. Ki-Tae Ha: Conceptualization, Funding acquisition, Writing review & editing, Supervision.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (NRF-2019R1G1A1100022) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HF20C0055).
Ethical statement
This research was approved by the guidelines of Institutional Animal Care and Use Ethical Committee of Pusan National University (Pusan, Republic of Korea; PNU-2020–2679).
Data availability
The GEO datasets was uploaded privately on the GEO homepage, and the accession number is GSE217151. All data generated during the current study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary information on methods, supplementary tables, and supplementary figures associated with this article can be found, in the online version, at doi:10.1016/j.imr.2023.100947.
Contributor Information
Jang-Kyung Park, Email: vivat314@pusan.ac.kr.
Ki-Tae Ha, Email: hagis@pusan.ac.kr.
Appendix. Supplementary materials
References
- 1.Wang Y., Nicholes K., Shih I.M. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol. 2020;15:71–95. doi: 10.1146/annurev-pathmechdis-012419-032654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–852. doi: 10.1016/S0140-6736(21)00389-5. [DOI] [PubMed] [Google Scholar]
- 3.Arafah M., Rashid S., Akhtar M. Endometriosis: a comprehensive review. Adv Anat Pathol. 2021;28(1):30–43. doi: 10.1097/PAP.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 4.Chapron C., Marcellin L., Borghese B., Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 5.Bora G., Yaba A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J Obstet Gynaecol Res. 2021;47(5):1610–1623. doi: 10.1111/jog.14710. [DOI] [PubMed] [Google Scholar]
- 6.Samimi M., Pourhanifeh M.H., Mehdizadehkashi A., Eftekhar T., Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: basic science and new insights based on gene expression. J Cell Physiol. 2019;234(11):19384–19392. doi: 10.1002/jcp.28666. [DOI] [PubMed] [Google Scholar]
- 7.Adachi M., Nasu K., Tsuno A., Yuge A., Kawano Y., Narahara H. Attachment to extracellular matrices is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):85–88. doi: 10.1016/j.ejogrb.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Rutherford E.J., Hill A.D.K., Hopkins A.M. Adhesion in Physiological, Benign and Malignant Proliferative States of the Endometrium: microenvironment and the Clinical Big Picture. Cells. 2018;7(5) doi: 10.3390/cells7050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delbandi A.A., Mahmoudi M., Shervin A., Heidari S., Kolahdouz-Mohammadi R., Zarnani A.H. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health. 2020;20(1):3. doi: 10.1186/s12905-019-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beliard A., Noel A., Foidart J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82(1):80–85. doi: 10.1016/j.fertnstert.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 11.Sugihara K., Kobayashi Y., Suzuki A., Tamura N., Motamedchaboki K., Huang C.T., et al. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nat Commun. 2014;5:4478. doi: 10.1038/ncomms5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uegaki T., Taniguchi F., Nakamura K., Osaki M., Okada F., Yamamoto O., et al. Inhibitor of apoptosis proteins (IAPs) may be effective therapeutic targets for treating endometriosis. Hum Reprod. 2015;30(1):149–158. doi: 10.1093/humrep/deu288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero S., Evangelisti G., Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018;19(10):1109–1125. doi: 10.1080/14656566.2018.1494154. [DOI] [PubMed] [Google Scholar]
- 14.Wei C., Pan Y., Zhang Y., Dai Y., Jiang L., Shi L., et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell Death Dis. 2020;11(9):755. doi: 10.1038/s41419-020-02956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M. Frankincense (ru xiang; boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med. 2013;3(4):221–226. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathe C., Connan J., Archier P., Mouton M., Vieillescazes C. Analysis of frankincense in archaeological samples by gas chromatography-mass spectrometry. Ann Chim. 2007;97(7):433–445. doi: 10.1002/adic.200790029. [DOI] [PubMed] [Google Scholar]
- 17.Thulin M., Warfa A.M. The Frankincense Trees (Boswellia spp., Burseraceae) of Northern Somalia and Southern Arabia. Kew Bulletin. 1987;42(3):487–500. [Google Scholar]
- 18.Abdel-Tawab M., Werz O., Schubert-Zsilavecz M. Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet. 2011;50(6):349–369. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Efferth T., Oesch F. Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities. Semin Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Eshaghian R., Mazaheri M., Ghanadian M., Rouholamin S., Feizi A., Babaeian M. The effect of frankincense (Boswellia serrata, oleoresin) and ginger (Zingiber officinale, rhizoma) on heavy menstrual bleeding: a randomized, placebo-controlled, clinical trial. Complement Ther Med. 2019;42:42–47. doi: 10.1016/j.ctim.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Su S., Hua Y., Wang Y., Gu W., Zhou W., Duan J.A., et al. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J Ethnopharmacol. 2012;139(2):649–656. doi: 10.1016/j.jep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Shah S.A., Rathod I.S., Suhagia B.N., Pandya S.S., Parmar V.K. A simple high-performance liquid chromatographic method for the estimation of boswellic acids from the market formulations containing Boswellia serrata extract. J Chromatogr Sci. 2008;46(8):735–738. doi: 10.1093/chromsci/46.8.735. [DOI] [PubMed] [Google Scholar]
- 23.Miscioscia E., Shmalberg J., Scott K.C. Measurement of 3-acetyl-11-keto-beta-boswellic acid and 11-keto-beta-boswellic acid in Boswellia serrata Supplements Administered to Dogs. BMC Vet Res. 2019;15(1):270. doi: 10.1186/s12917-019-2021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue R.C., Fang Z., Zhang M.X., Yi Z.H., Wen C.P., Shi T.L. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 2013;41(D1):D1089–D1D95. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinero J., Ramirez-Anguita J.M., Sauch-Pitarch J., Ronzano F., Centeno E., Sanz F., et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48(D1):D845–DD55. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winterhalter C., Widera P., Krasnogor N. JEPETTO: a Cytoscape plugin for gene set enrichment and topological analysis based on interaction networks. Bioinformatics. 2014;30(7):1029–1030. doi: 10.1093/bioinformatics/btt732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–DD68. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho M.K., Jin L., Han J.H., Jin J.S., Cheon S.Y., Shin S., et al. Water-Extracted Prunella vulgaris Alleviates Endometriosis by Reducing Aerobic Glycolysis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.872810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi H.J., Chung T.W., Choi H.J., Han J.H., Choi J.H., Kim C.H., et al. Increased alpha2-6 sialylation of endometrial cells contributes to the development of endometriosis. Exp Mol Med. 2018;50(12):1–12. doi: 10.1038/s12276-018-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim B.Y., Lim H.S., Kim Y.J., Sohn E., Kim Y.H., Koo I., et al. Similarity of therapeutic networks induced by a multi-component herbal remedy, Ukgansan, in neurovascular unit cells. Sci Rep. 2020;10(1):2658. doi: 10.1038/s41598-020-59537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E.Y., Jin B.R., Chung T.W., Bae S.J., Park H., Ryu D., et al. 6-sialyllactose ameliorates dihydrotestosterone-induced benign prostatic hyperplasia through suppressing VEGF-mediated angiogenesis. BMB Rep. 2019;52(9):560–565. doi: 10.5483/BMBRep.2019.52.9.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J., Lee H., Jin E.J., Jo Y., Kang B.E., Ryu D., et al. A Microfluidic Device to Fabricate One-Step Cell Bead-Laden Hydrogel Struts for Tissue Engineering. Small. 2022;18(1) doi: 10.1002/smll.202106487. [DOI] [PubMed] [Google Scholar]
- 34.Klemmt P.A.B., Starzinski-Powitz A. Molecular and Cellular Pathogenesis of Endometriosis. Curr Womens Health Rev. 2018;14(2):106–116. doi: 10.2174/1573404813666170306163448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor R., Stratopoulou C.A., Dolmans M.M. Pathogenesis of endometriosis: new insights into prospective therapies. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan J., Cheng W., Zhai D.X., Zhang D.Y., Yao R.P., Bai L.L., et al. Meta-Analysis of Chinese Traditional Medicine Bushen Huoxue Prescription for Endometriosis Treatment. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/5416423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong S., Zhang Y.H., Liu C.F., Tsui I., Guo Y., Ai B.B., et al. The complementary and alternative medicine for endometriosis: a review of utilization and mechanism. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/146383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wieser F., Cohen M., Gaeddert A., Yu J., Burks-Wicks C., Berga S.L., et al. Evolution of medical treatment for endometriosis: back to the roots? Hum Reprod Update. 2007;13(5):487–499. doi: 10.1093/humupd/dmm015. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Gomez E., Vazquez-Martinez E.R., Reyes-Mayoral C., Cruz-Orozco O.P., Camacho-Arroyo I., Cerbon M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front Endocrinol (Lausanne) 2019;10:935. doi: 10.3389/fendo.2019.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung S.W., Zhang R.Z., Tan Z.Y.R., Chung J.P.W., Zhang T., Wang C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: a review. Med Res Rev. 2021;41(4):2489–2564. doi: 10.1002/med.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siracusa R., D'Amico R., Impellizzeri D., Cordaro M., Peritore A.F., Gugliandolo E., et al. Autophagy and Mitophagy Promotion in a Rat Model of Endometriosis. Int J Mol Sci. 2021;22(10) doi: 10.3390/ijms22105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popli P., Sun A.J., Kommagani R. The Multifaceted Role of Autophagy in Endometrium Homeostasis and Disease. Reprod Sci. 2022;29(4):1054–1067. doi: 10.1007/s43032-021-00587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S., Xin X., Hua T., Shi R., Chi S., Jin Z., et al. Efficacy of anti-VEGF/VEGFR agents on animal models of endometriosis: a systematic review and meta-analysis. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0166658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun D.P., Ding J., Shaheen F., Willey J.C., Rana N., Dmowski W.P. Quantitative expression of apoptosis-regulating genes in endometrium from women with and without endometriosis. Fertil Steril. 2007;87(2):263–268. doi: 10.1016/j.fertnstert.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Khan M.A., Ali R., Parveen R., Najmi A.K., Ahmad S. Pharmacological evidences for cytotoxic and antitumor properties of Boswellic acids from Boswellia serrata. J Ethnopharmacol. 2016;191:315–323. doi: 10.1016/j.jep.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 46.Suhail M.M., Wu W., Cao A., Mondalek F.G., Fung K.M., Shih P.T., et al. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement Altern Med. 2011;11:129. doi: 10.1186/1472-6882-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parr C., Ali A.Y. Boswellia frereana suppresses HGF-mediated breast cancer cell invasion and migration through inhibition of c-Met signalling. J Transl Med. 2018;16(1):281. doi: 10.1186/s12967-018-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Efferth T., Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12(1):122–132. doi: 10.2174/138945011793591626. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari P., Mishra R., Mazumder A., Mazumder R., Singh A. An insight into diverse activities and targets of flavonoids. Curr Drug Targets. 2022 doi: 10.2174/1389450123666220915121236. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Chi X., Pan W., Wang S., Zhang Z., Zhao H., et al. Antidepressant mechanism of classical herbal formula lily bulb and Rehmannia decoction: insights from gene expression profile of medial prefrontal cortex of mice with stress-induced depression-like behavior. Genes Brain Behav. 2020;19(5):e12649. doi: 10.1111/gbb.12649. [DOI] [PubMed] [Google Scholar]
- 51.El-Masry O.S., Goja A., Rateb M., Owaidah A.Y., Alsamman K. RNA sequencing identified novel target genes for Adansonia digitata in breast and colon cancer cells. Sci Prog. 2021;104(3) doi: 10.1177/00368504211032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moussaieff A., Mechoulam R. Boswellia resin: from religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J Pharm Pharmacol. 2009;61(10):1281–1293. doi: 10.1211/jpp/61.10.0003. [DOI] [PubMed] [Google Scholar]
- 53.Liu J.J., Nilsson A., Oredsson S., Badmaev V., Zhao W.Z., Duan R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23(12):2087–2093. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi M., Sung B., Shen Y., Hur K., Link A., Boland C.R., et al. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis. 2012;33(12):2441–2449. doi: 10.1093/carcin/bgs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pengzong Z., Yuanmin L., Xiaoming X., Shang D., Wei X., Zhigang L., et al. Wound Healing Potential of the Standardized Extract of Boswellia serrata on Experimental Diabetic Foot Ulcer via Inhibition of Inflammatory, Angiogenetic and Apoptotic Markers. Planta Med. 2019;85(8):657–669. doi: 10.1055/a-0881-3000. [DOI] [PubMed] [Google Scholar]
- 56.Additives EPo, Products or Substances used in Animal F. Bampidis V., Azimonti G., Bastos M.L., Christensen H., et al. Safety and efficacy of a feed additive consisting of an extract of olibanum from Boswellia serrata Roxb. ex Colebr. for use in dogs and horses (FEFANA asbl) EFSA J. 2022;20(3):e07158. doi: 10.2903/j.efsa.2022.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodda S., Madireddy R.K., Alluri V.K., Golakoti T., Sengupta K. Safety assessment of a novel water-soluble extract of Boswellia serrata gum resin: acute toxicity, 90-day sub-chronic toxicity, Ames' bacterial reverse mutation, and in vivo micronucleus assays. Toxicol Mech Methods. 2022;32(5):362–372. doi: 10.1080/15376516.2021.2012545. [DOI] [PubMed] [Google Scholar]
- 58.Karlapudi V., Sunkara K.B., Konda P.R., Sarma K.V., Rokkam M.P. Efficacy and Safety of Aflapin(R), a Novel Boswellia Serrata extract, in the treatment of osteoarthritis of the knee: a short-term 30-day randomized, double-blind, placebo-controlled clinical study. J Am Nutr Assoc. 2022:1–10. doi: 10.1080/07315724.2021.2014370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GEO datasets was uploaded privately on the GEO homepage, and the accession number is GSE217151. All data generated during the current study are available from the corresponding author upon reasonable request.