Abstract
Prostate cancer (PCa) is generally considered a disease of older men; however, about 10% of new diagnoses in the US occur in men ≤ 55 years old. Socioeconomic status (SES) has been shown to influence survival in patients with PCa; however, the impact of SES on men with early-onset PCa remains undescribed. Using the National Cancer Database, we identified adult men ≤ 55 years of age with a diagnosis of prostatic adenocarcinoma between 2004-2018. Descriptive statistics were used to characterize differences among different SES groups. Kaplan-Meier (KM) and Cox regression analyses were used to assess the effect of SES on overall survival (OS). A total of 112,563 young patients with PCa with a median follow-up of 79.0 months were identified. Compared to high SES patients, low SES patients were more likely to be African American (42.4% vs. 8.6%; P<0.001), Hispanic (9.5% vs. 2.7%; P<0.001), and uninsured (5.2% vs. 1.1%; P<0.001); they were also more likely to live in a rural area (3.2% vs. 0.1%; P<0.001) and have stage IV disease (5.5% vs. 3.1%; P<0.001). KM analysis showed that a decreasing SES was directly associated with lower rates of OS (log-rank test P<0.001). On multivariable analysis, SES was found to have a negative effect on OS (low SES vs. high SES; hazard ratio [HR] 1.54; 95% confidence interval [CI] 1.41-1.68; P<0.001). In patients with early-onset PCa, SES was associated with lower OS. SES may be considered when implementing programs to improve the management of patients with early-onset PCa.
Keywords: Prostate cancer, social determinants of health, outcomes
Introduction
Despite being considered a disease of older men, 10% of new PCa diagnoses occur in men ≤ 55 years old (defined as early-onset PCa) [1]. During the last few decades, men ≤ 55 years old have experienced the greatest increase in PCa incidence [2]. In addition, men diagnosed with early-onset PCa have been shown to have worse 5- and 10-year survival rates compared to men 56-80 years old [1]. This is expected as patients with early-onset PCa tend to present with high-risk and advanced-stage disease [3].
Important biological differences may exist in early-onset PCa compared to late-onset PCa. Early-onset PCa has shown to have a more significant genetic component supporting the idea that a clinical subtype may exist in men with early-onset PCa. Rare genetic variants with low penetrance are potential candidates and are poorly identified in published studies. Men with early-onset PCa are more likely to have higher cause-specific mortality than others and are at higher risk for disease progression due to their extended life expectancy [31].
In early-onset PCa, patients with fast growing tumors may be entirely missed by screening as the timeframe from onset to developing symptoms is short. Additionally, the effect of length bias is specifically pronounced in early-onset PCa with its shortened latency period. Therefore, rapidly growing tumors in young men with brief window for detection would be associated with worst prognosis and advanced disease. Shortened sojourn time for PCa in young patients suggest that the most aggressive tumors will occur more commonly in early-onset PCa [32].
Although biology is most likely the culprit for differences in clinicopathologic features among early-onset PCa patients, sociodemographic factors may also play a role. In the US, young uninsured adults are more likely to be men, belong to a racial or ethnic minority, and have lower family income [4]. Despite the expanded access to health insurance coverage provided by the Patient Protection and Affordable Care Act (ACA), select young adults may forego buying health care coverage due to higher premiums, highlighting the fact that household income is closely linked to insurance status [5,6]. Thus, it is critically important to explore the relationship between sociodemographic factors and outcomes in patients with early-onset PCa.
To optimize cancer screening, the impact of not only biological factors but also socioeconomic characteristics must be considered. There is various evidence in the literature showing that lower socioeconomic status (SES) is associated with poor health and increased mortality. Men with low SES may have reduced health literacy and awareness decreasing the need to seek medical attention. However, men with high SES have an increased incidence in PCa that may be attributed to different behavior towards their health and screening. In addition, the role of poor nutrition and environmental risk factor exposure in disease progression and mortality among men with low SES increases the probability of developing early-onset PCa. Therefore, men with low SES could be a high-priority group for PCa screening at a younger age compared to the standard of care.
It is well established that sociodemographic factors, such as income, education, and social support, influence both the incidence and survival rates of PCa [7]. However, the degree of impact that SES has on disease features and outcomes among patients with early-onset PCa has not been well defined. Herein, we used the National Cancer Database (NCDB) to evaluate the association between SES and early-onset PCa outcomes.
Patients and methods
Data acquisition
Data from the 2004 to 2018 NCDB was obtained after Institutional Review Board approval as an exempt study. The NCDB is a national cancer registry database sponsored by the American College of Surgeons and the American Cancer Society. Data is captured from more than 1500 hospitals in the US, accounting for almost 34 million records, and representing approximately 70% of all new cancer cases in the US. The Participant User Files (PUF) contain de-identified data compliant with the Health Insurance Portability and Accountability Act (HIPAA) [8].
Study population
From 1,742,973 PCa patients captured in the NCDB during 2004-2018, we included those aged 18-55 years with the diagnosis of prostatic adenocarcinoma (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] code 8140). We excluded patients with unknown race, ethnicity, insurance status, income quartile, education level, facility type, distance to hospital, follow-up information, NCDB analytic stage, and area of residence. Patients with missing pathologic confirmation of PCa or those with a secondary malignancy were also excluded. A summary of the inclusion and exclusion criteria can be found in Figure 1.
Figure 1.
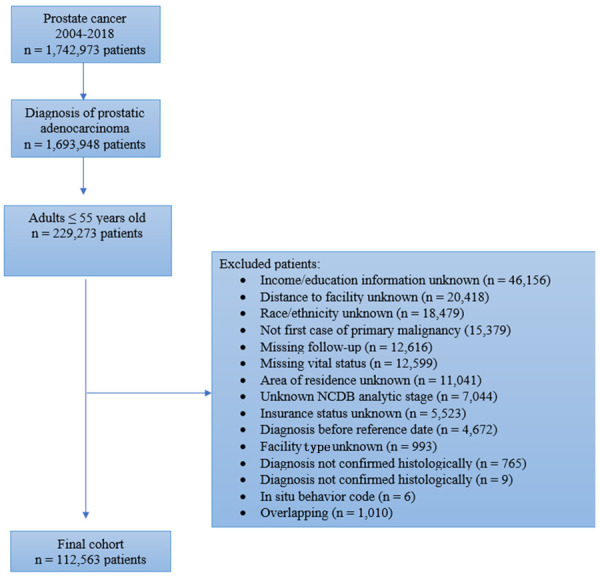
Flowchart for patient selection. NCDB, National Cancer Database.
Study variables
We evaluated the following variables: age, race, ethnicity, insurance type, pathologic TNM stage, American Joint Committee on Cancer (AJCC) stage group, Charlson-Deyo comorbidity index (CDCI), population density of the patient’s county of residence (metropolitan [>20,000 population in the metro area], urban [>2500 to >20,000 population adjacent to the metro area], or rural [<2500 population]), distance to facility, and facility type (community [>100 to ≤ 500 newly diagnosed cancer cases per year], comprehensive community [>500 newly diagnosed cancer cases per year], academic [>500 newly diagnosed cancer cases per year in addition to providing postgraduate medical education], or integrated network cancer program [a joint venture with multiple facilities, at least 1 hospital, no minimum for newly diagnosed cancer cases per year]).
To establish the impact of SES, the quartile assignments of median income and education level were combined to create a composite SES measure. Income and education level, as specified by the NCDB, were determined by matching each patient’s ZIP code at the time of diagnosis with data derived from the 2016 American Community Survey on median household income and the percentage of people aged ≥ 25 years old who had not earned a high school diploma, respectively. Income quartiles were defined as: <$40,227 (Q1), $40,227 - $50,353 (Q2), $50,354 - $63,332 (Q3), and ≥ $63,333 (Q4). Likewise, education quartiles were defined as proportion of men without high school diploma: ≥ 17.6% (Q1), 10.9% - 17.5% (Q2), 6.3% - 10.8% (Q3), and <6.3% (Q4) [9]. The quartile assignments (Q1, Q2, Q3, Q4) of the income and education measures were added together to form four composite SES categories: 2-3= Low SES; 4-5= Mid-Low SES; 6-7= Mid-High SES; and 8= High SES.
Outcome
The primary outcome from our analysis was overall survival (OS), which was defined as the number of months from the date of PCa diagnosis to the date of death or last reported follow-up. The NCDB does not collect data on cancer-specific survival or recurrence.
Statistical analysis
Continuous variables are presented as the median and interquartile range (IQR) with SES group differences evaluated using the Kruskal-Wallis test. Categorical variables are reported as frequency and percentage, evaluated using the chi-square test. Unadjusted differences in OS between SES groups were evaluated using the Kaplan-Meier method with the log-rank test. A multivariable Cox regression model was used to study SES group differences in OS after adjusting for clinical, patient, and facility covariates. All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) version 28.0 (IBM Corp, Armonk, NY, USA) and R version 4.1.0, with significance defined as two-tailed P<0.05 for all tests. For R, we used the Rcommander package and EZR PlugIn [10].
Results
The study cohort was compromised of 112,563 patients with early-onset PCa (Figure 1). Median follow-up for the cohort was 79.0 months. Compared to high SES patients, low SES patients were more likely to be African American (42.4% vs. 8.6%; P<0.001), Hispanic (9.5% vs. 2.7%; P<0.001), and uninsured (5.2% vs. 1.1%; P<0.001); they were also more likely to live in a rural area (3.2% vs. 0.1%; P<0.001) and have stage IV disease (5.5% vs. 3.1%; P<0.001). In addition, low SES patients were less likely to receive care at integrated network cancer programs, and less likely to undergo radical prostatectomy (RP) (Table 1). On KM analysis, a decreasing SES was directly associated with lower rates of OS (log-rank test P<0.001) (Figure 2).
Table 1.
Patient and tumor characteristics by socioeconomic status groups
| Characteristic | Total | Low SES | Mid-low SES | Mid-high SES | High SES | P-value |
|---|---|---|---|---|---|---|
| n (%) | 112563 | 21343 (19.0%) | 27260 (24.2%) | 34989 (31.1%) | 28971 (25.7%) | |
| Age (median [interquartile range]) | 52.00 [50.00, 54.00] | 52.00 [50.00, 54.00] | 52.00 [50.00, 54.00] | 52.00 [50.00, 54.00] | 52.00 [50.00, 54.00] | 0.684 |
| Race (%) | ||||||
| Caucasian | 86756 (77.1) | 11771 (55.2) | 20742 (76.1) | 28558 (81.6) | 25685 (88.7) | <0.001 |
| African American | 22881 (20.3) | 9049 (42.4) | 5872 (21.5) | 5468 (15.6) | 2492 (8.6) | |
| Other | 2926 (2.6) | 523 (2.5) | 646 (2.4) | 963 (2.8) | 794 (2.7) | |
| Hispanic ethnicity (%) | ||||||
| Yes | 5910 (5.3) | 2020 (9.5) | 1650 (6.1) | 1472 (4.2) | 768 (2.7) | <0.001 |
| No | 106653 (94.7) | 19323 (90.5) | 25610 (93.9) | 33517 (95.8) | 28203 (97.3) | |
| Insurance status (%) | ||||||
| Not Insured | 2756 (2.4) | 1113 (5.2) | 728 (2.7) | 584 (1.7) | 331 (1.1) | <0.001 |
| Medicaid | 4451 (4.0) | 2000 (9.4) | 1199 (4.4) | 896 (2.6) | 356 (1.2) | |
| Medicare | 4873 (4.3) | 1895 (8.9) | 1343 (4.9) | 1119 (3.2) | 516 (1.8) | |
| Other Government | 2396 (2.1) | 487 (2.3) | 707 (2.6) | 786 (2.2) | 416 (1.4) | |
| Private Insurance/Managed Care | 98087 (87.1) | 15848 (74.3) | 23283 (85.4) | 31604 (90.3) | 27352 (94.4) | |
| Facility type (%) | ||||||
| Academic/Research Program | 48658 (43.2) | 9190 (43.1) | 10551 (38.7) | 14864 (42.5) | 14053 (48.5) | <0.001 |
| Comprehensive Community Cancer Program | 37786 (33.6) | 7650 (35.8) | 10101 (37.1) | 11920 (34.1) | 8115 (28.0) | |
| Integrated Network Cancer Program | 21349 (19.0) | 3501 (16.4) | 5068 (18.6) | 6716 (19.2) | 6064 (20.9) | |
| Community Cancer Program | 4770 (4.2) | 1002 (4.7) | 1540 (5.6) | 1489 (4.3) | 739 (2.6) | |
| Distance to hospital (miles) | 12.9 [6, 30] | 11.20 [4.40, 38.60] | 14.80 [6.00, 37.30] | 13.40 [6.40, 27.90] | 12.00 [6.80, 22.40] | <0.001 |
| Area of residence (%) | ||||||
| Rural | 1570 (1.4) | 686 (3.2) | 514 (1.9) | 329 (0.9) | 41 (0.1) | <0.001 |
| Urban | 46665 (41.5) | 11143 (52.2) | 14849 (54.5) | 13912 (39.8) | 6761 (23.3) | |
| Metro | 64328 (57.1) | 9514 (44.6) | 11897 (43.6) | 20748 (59.3) | 22169 (76.5) | |
| Charlson-Deyo Comorbidity Index (%) | ||||||
| 0 | 98720 (87.7) | 17850 (83.6) | 23701 (86.9) | 30917 (88.4) | 26252 (90.6) | <0.001 |
| 1 | 11829 (10.5) | 2860 (13.4) | 3022 (11.1) | 3521 (10.1) | 2426 (8.4) | |
| ≥ 2 | 2014 (1.8) | 633 (3.0) | 537 (2.0) | 551 (1.6) | 293 (1.0) | |
| Surgical treatment (%) | ||||||
| Radical prostatectomy | 92746 (82.4) | 16477 (77.2) | 22103 (81.1) | 29316 (83.8) | 24850 (85.8) | <0.001 |
| No surgery | 17142 (15.2) | 4175 (19.6) | 4441 (16.3) | 4937 (14.1) | 3589 (12.4) | |
| Other treatment | 2522 (2.2) | 667 (3.1) | 671 (2.5) | 687 (2.0) | 497 (1.7) | |
| Unknown | 153 (0.1) | 24 (0.1) | 45 (0.2) | 49 (0.1) | 35 (0.1) | |
| Analytic stage group (%) | ||||||
| Stage I | 18328 (16.3) | 3465 (16.2) | 4542 (16.7) | 5572 (15.9) | 4749 (16.4) | <0.001 |
| Stage II | 73399 (65.2) | 13613 (63.8) | 17475 (64.1) | 23036 (65.8) | 19275 (66.5) | |
| Stage III | 16213 (14.4) | 3091 (14.5) | 4038 (14.8) | 5043 (14.4) | 4041 (13.9) | |
| Stage IV | 4623 (4.1) | 1174 (5.5) | 1205 (4.4) | 1338 (3.8) | 906 (3.1) | |
| pT (%) | ||||||
| 0 | 86 (0.1) | 16 (0.1) | 22 (0.1) | 25 (0.1) | 23 (0.1) | <0.001 |
| 1 | 686 (0.6) | 185 (0.9) | 168 (0.6) | 197 (0.6) | 136 (0.5) | |
| 2 | 69872 (62.1) | 12330 (57.8) | 16519 (60.6) | 22111 (63.2) | 18912 (65.3) | |
| 3 | 17832 (15.8) | 3490 (16.4) | 4422 (16.2) | 5508 (15.7) | 4412 (15.2) | |
| 4 | 442 (0.4) | 103 (0.5) | 116 (0.4) | 139 (0.4) | 84 (0.3) | |
| X | 11935 (10.6) | 2371 (11.1) | 2930 (10.7) | 3687 (10.5) | 2947 (10.2) | |
| Missing | 11710 (10.4) | 2848 (13.3) | 3083 (11.3) | 3322 (9.5) | 2457 (8.5) | |
| pN (%) | ||||||
| 0 | 70169 (62.3) | 12826 (60.1) | 16556 (60.7) | 22087 (63.1) | 18700 (64.5) | <0.001 |
| 1 | 2628 (2.3) | 561 (2.6) | 654 (2.4) | 790 (2.3) | 623 (2.2) | |
| X | 26072 (23.2) | 4766 (22.3) | 6490 (23.8) | 8135 (23.3) | 6681 (23.1) | |
| Missing | 13694 (12.2) | 3190 (14.9) | 3560 (13.1) | 3977 (11.4) | 2967 (10.2) | |
| pM (%) | ||||||
| 0 | 30123 (26.8) | 5332 (25.0) | 7152 (26.2) | 9424 (26.9) | 8215 (28.4) | <0.001 |
| 1 | 997 (0.9) | 271 (1.3) | 285 (1.0) | 290 (0.8) | 151 (0.5) | |
| X | 19077 (16.9) | 3437 (16.1) | 4666 (17.1) | 6102 (17.4) | 4872 (16.8) | |
| Missing | 62366 (55.4) | 12303 (57.6) | 15157 (55.6) | 19173 (54.8) | 15733 (54.3) | |
| Lymphovascular invasion (%) | ||||||
| Present/Identified | 3837 (5.4) | 779 (5.5) | 885 (5.1) | 1204 (5.5) | 969 (5.3) | <0.001 |
| Absent/Not identified | 50696 (70.9) | 9544 (67.7) | 12205 (70.3) | 15675 (71.5) | 13272 (73.0) | |
| Unknown | 17012 (23.8) | 3771 (26.8) | 4263 (24.6) | 5048 (23.0) | 3930 (21.6) | |
| Surgical margins (%) | ||||||
| Residual tumor | 20411 (18.1) | 3945 (18.5) | 5134 (18.8) | 6375 (18.2) | 4957 (17.1) | <0.001 |
| No residual tumor | 73169 (65.0) | 12770 (59.8) | 17218 (63.2) | 23137 (66.1) | 20044 (69.2) | |
| No primary site surgery | 17142 (15.2) | 4175 (19.6) | 4441 (16.3) | 4937 (14.1) | 3589 (12.4) | |
| Margins not evaluable | 912 (0.8) | 249 (1.2) | 243 (0.9) | 252 (0.7) | 168 (0.6) | |
| Unknown or not applicable | 929 (0.8) | 204 (1.0) | 224 (0.8) | 288 (0.8) | 213 S0.7) |
Figure 2.
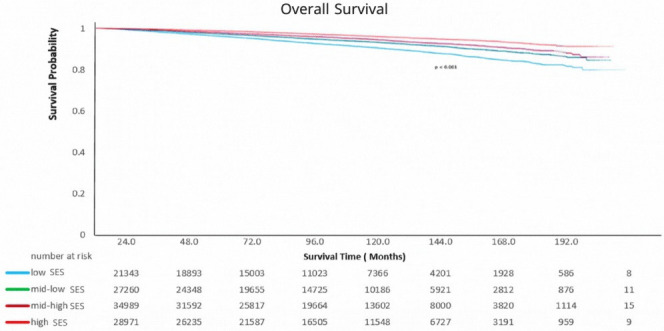
Kaplan-Meier curve for overall survival stratified by socioeconomic status (SES).
After adjusting for age, race, ethnicity, travel distance, comorbidity burden (as per the CDCI), area of residence, facility type, insurance status, stage group, and surgical treatment on multivariable Cox regression, SES was found to have a negative effect on OS (low SES vs. high SES; hazard ratio [HR] 1.54; 95% confidence interval [CI] 1.41-1.68; P<0.001). Other predictors of mortality included: age (HR per year increase 1.02; 95% CI 1.01-1.03; P<0.001), stage group (stage IV vs. stage I; HR 17.65; 95% CI 15.84-19.65; P<0.001), comorbidity burden (CDCI ≥ 2 vs. CDCI=0; HR 1.89; 95% CI 1.66-2.15; P<0.001), area of residence (rural vs. metropolitan; HR 1.24; 95% CI 1.03-1.50; P=0.026), and insurance status (no insurance vs. private insurance; HR 2.10 95% CI 1.88-2.33; P<0.001) (Table 2).
Table 2.
Multivariable Cox proportional hazard model for overall mortality
| Parameter | Hazard ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Socioeconomic status | ||||
| High | Reference | |||
| Mid-high | 1.23 | 1.14 | 1.33 | <0.001 |
| Mid-low | 1.36 | 1.25 | 1.48 | <0.001 |
| Low | 1.54 | 1.41 | 1.68 | <0.001 |
| Age (per year increase) | 1.02 | 1.01 | 1.03 | <0.001 |
| Race | ||||
| Caucasian | Reference | |||
| Black | 0.94 | 0.88 | 1.00 | 0.050 |
| Other | 0.81 | 0.68 | 0.97 | 0.024 |
| Hispanic ethnicity | ||||
| No | Reference | |||
| Yes | 0.61 | 0.54 | 0.70 | <0.001 |
| Insurance status | ||||
| Private | Reference | |||
| Not insured | 2.10 | 1.88 | 2.33 | <0.001 |
| Medicaid | 2.25 | 2.05 | 2.47 | <0.001 |
| Medicare | 2.38 | 2.18 | 2.60 | <0.001 |
| Other government | 1.20 | 0.99 | 1.45 | 0.066 |
| Facility type | ||||
| Academic/Research | Reference | |||
| Comprehensive community | 1.17 | 1.10 | 1.25 | <0.001 |
| Integrated cancer network | 1.07 | 1.00 | 1.16 | 0.053 |
| Community | 1.23 | 1.10 | 1.38 | <0.001 |
| Distance to hospital (per mile increase) | 1.00 | 1.00 | 1.00 | 0.009 |
| Area of residence | ||||
| Metropolitan | Reference | |||
| Urban | 1.05 | 0.99 | 1.11 | 0.1 |
| Rural | 1.24 | 1.03 | 1.50 | 0.026 |
| Charlson-Deyo Comorbidity Index | ||||
| 0 | Reference | |||
| 1 | 1.55 | 1.44 | 1.67 | <0.001 |
| ≥ 2 | 1.89 | 1.66 | 2.15 | <0.001 |
| Surgical treatment | ||||
| No surgery | Reference | |||
| Radical prostatectomy | 0.28 | 0.26 | 0.30 | <0.001 |
| Other treatment | 0.88 | 0.78 | 1.00 | 0.055 |
| Unknown | 1.03 | 0.46 | 2.30 | 0.9 |
| Analytic stage group | ||||
| I | Reference | |||
| II | 1.82 | 1.64 | 2.03 | <0.001 |
| III | 4.62 | 4.10 | 5.21 | <0.001 |
| IV | 17.65 | 15.84 | 19.65 | <0.001 |
Discussion
PCa mortality among men ≤ 55 years old is low but has been increasing during the past few decades [1,2]. This could be in part explained by the recommendations of the European and North American urological associations regarding PSA screening in men aged over 55 years. Because screening for PCa is variable and unclear in men less than 55 years age, early onset PCa in younger men may remain undetected until later disease stage. In addition, men with early-onset PCa are more likely to be symptomatic at the time of the diagnosis which could predict worse disease compared to the standard of care PCa patients identified merely based on PSA screening-prompted prostate needle biopsy according to current clinical guidelines and protocols [11-14].
In general, cancer patients with low SES have poor survival outcomes compared to those with high SES [15]. Factors affecting social differences in cancer diagnosis, treatment, and prognosis remain incompletely understood, but have been linked to disease characteristics, patient’s factors, and health care access and quality [16]. Advanced cancer stage and pathologic features at the time of the diagnosis are associated with poor outcomes, and are often hypothesized as being related to health disparities affecting survival outcomes [17].
In our study, low SES was assessed by income and education level. A study by Watson et al., including 2194 men with PCa, showed that men living in low SES neighborhoods as defined by lower income and lower educational levels were less likely to receive definitive treatment [18]. Tomic et al. found that men with lower income were less likely to receive definitive treatment for intermediate, high risk, and very high-risk PCa, and more likely to have positive surgical margins at prostatectomy. Moreover, low SES patients with very low-risk PCa are less likely to be offered active surveillance for their disease compared with men at higher SES levels [19]. This is concordant with our data, which showed that younger men with low SES are more likely to present with advanced-stage disease at the time of diagnosis and less likely to receive definitive treatment in the form of RP.
Men with a low SES also tend to have a higher comorbidity burden. These men usually have poor general health and lifestyle risk factors including physical inactivity and smoking, along with a higher incidence of obesity and metabolic syndrome which limit the options for definitive treatment. In the current study, we showed that patients with low SES are more likely to have a higher comorbidity burden, and a higher comorbidity burden was associated with worse OS.
It has been postulated that African American men carry a higher incidence and mortality rate for PCa compared to other racial or ethnic groups [20-22]. Compared to Caucasian Americans, African American men tend to be younger, have a more advanced disease stage at the time of diagnosis, and are more likely to have metastatic disease with a lower OS when treated for PCa [21,23-27]. In our cohort, when adjusted for available potential confounders, African American race was not associated with worse OS. A recent study by Wen et al. concluded that African Americans diagnosed with localized high-grade PCa who underwent RP have a 51% higher overall mortality rate compared to Caucasian American patients, however adjusting for education level, income, and insurance status, the disparities in mortality rates dropped to 30%. Adjusting for comorbid conditions and nonclinical parameters the overall mortality disparity decreased to 19% [28].
Quality of cancer care is another key factor influencing outcomes in cancer patients. In our cohort, we showed that the mortality rate among patients with early-onset PCa was higher among those not insured, those treated at community cancer centers, and those who lived in rural areas. All these factors potentially impede young men diagnosed with PCa with from receiving prompt curative treatment at high-quality centers compared with men in higher SES [19,29,30].
Although our study addresses the mortality rate in a large population of young men diagnosed with PCa, the current study is not without limitations. The retrospective nature of our cohort may lead to indication and selection biases. In the current study, we defined low SES based on educational level and income; however, the use of those factors as indicators of SES does not cover all aspects of SES concerning health. We were unable to assess the effect of other factors such as lifestyle habits, health awareness, health beliefs, and health behavior. Also, we were only able to assess the OS among young men with PCa; however, we are not able to assess PCa-specific survival or recurrence due to the absence of such information in the NCDB.
Conclusions
Socioeconomic inequities among men diagnosed with early-onset PCa affect OS. SES in men with early-onset prostate cancer could be considered in prognostic algorithms and implemented in management programs to improve overall outcomes among these men.
Disclosure of conflict of interest
None.
References
- 1.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11:317–23. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
- 3.Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: an emerging young adult and older adolescent challenge. Cancer. 2020;126:46–57. doi: 10.1002/cncr.32498. [DOI] [PubMed] [Google Scholar]
- 4.Cohen RA, Terlizzi EP, Martinez ME, Cha AE. Health insurance coverage: early release of estimates from the National Health Interview Survey, 2020. National Center for Health Statistics. 2021 August [Google Scholar]
- 5.Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, Hu JC, Martin NE, Trinh QD, Alexander BM, Nguyen PL. Cancer-specific outcomes among young adults without health insurance. J. Clin. Oncol. 2014;32:2025–30. doi: 10.1200/JCO.2013.54.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monaghan M. The affordable care act and implications for young adult health. Transl Behav Med. 2014;4:170–4. doi: 10.1007/s13142-013-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020;8:49–54. doi: 10.1016/j.prnil.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Surgeons (ACS) National Cancer Database - Getting Started with the 2018 PUF Data. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/2018_puf_getting_started.ashx. Accessed December 12, 2021.
- 9.American College of Surgeons (ACS) National Cancer Database Participant User File - 2018 Data Dictionary. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary.ashx. Accessed December 12, 2021.
- 10.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huben R, Natarajan N, Pontes E, Mettlin C, Smart CR, Murphy GP. Carcinoma of prostate in men less than fifty years old. Data from American College of Surgeons’ National Survey. Urology. 1982;20:585–8. doi: 10.1016/0090-4295(82)90304-1. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JM, Kemp IW, Stein GJ. Cancer of the prostate. Do younger men have a poorer survival rate? Br J Urol. 1984;56:391–6. doi: 10.1111/j.1464-410x.1984.tb05828.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DE, Lanieri JP Jr, Ayala AG. Prostatic adenocarcinoma occurring in men under 50 years of age. J Surg Oncol. 1972;4:207–16. doi: 10.1002/jso.2930040305. [DOI] [PubMed] [Google Scholar]
- 14.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2009;115:2863–71. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 16.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, Yin X Patterns of Care Study Group. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113:582–91. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst. 2001;93:705–9. doi: 10.1093/jnci/93.9.705. [DOI] [PubMed] [Google Scholar]
- 18.Watson M, Grande D, Radhakrishnan A, Mitra N, Ward KR, Pollack CE. Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethn Dis. 2017;27:201–208. doi: 10.18865/ed.27.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomic K, Ventimiglia E, Robinson D, Häggström C, Lambe M, Stattin P. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population-based study. Int J Cancer. 2018;142:2478–2484. doi: 10.1002/ijc.31272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz NM. Cancer survivorship research: state of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–32. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 21.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 22.Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, Penberthy L. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992-2013. Cancer Epidemiol Biomarkers Prev. 2017;26:632–641. doi: 10.1158/1055-9965.EPI-16-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellizzi KM, Mustian KM, Palesh OG, Diefenbach M. Cancer survivorship and aging: moving the science forward. Cancer. 2008;113(Suppl):3530–9. doi: 10.1002/cncr.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985–97. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92:1451–9. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001;50:222–32. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lubeck DP, Kim H, Grossfeld G, Ray P, Penson DF, Flanders SC, Carroll PR. Health related quality of life differences between black and white men with prostate cancer: data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2001;166:2281–5. [PubMed] [Google Scholar]
- 28.Wen W, Luckenbaugh AN, Bayley CE, Penson DF, Shu XO. Racial disparities in mortality for patients with prostate cancer after radical prostatectomy. Cancer. 2021;127:1517–1528. doi: 10.1002/cncr.33152. [DOI] [PubMed] [Google Scholar]
- 29.Krupski TL, Kwan L, Afifi AA, Litwin MS. Geographic and socioeconomic variation in the treatment of prostate cancer. J. Clin. Oncol. 2005;23:7881–8. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 30.Lyratzopoulos G, Barbiere JM, Greenberg DC, Wright KA, Neal DE. Population based time trends and socioeconomic variation in use of radiotherapy and radical surgery for prostate cancer in a UK region: continuous survey. BMJ. 2010;340:c1928. doi: 10.1136/bmj.c1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11:317–23. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, Hamdy F, Neal DE. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer. 2009;100:1198–204. doi: 10.1038/sj.bjc.6604973. [DOI] [PMC free article] [PubMed] [Google Scholar]


