Abstract
BACKGROUND
Preeclampsia affects between 2% and 5% of pregnant people in North America. First-trimester preeclampsia screening based on the Fetal Medicine Foundation risk calculation algorithm combined with treatment of high-risk patients with aspirin effectively reduces the incidence of preterm preeclampsia more than the currently used risk factor–based screening. However, the impact of such screening on patient satisfaction and maternal anxiety is unknown.
OBJECTIVE
This study aimed to assess the impact of first-trimester prediction and prevention of preterm preeclampsia on patient satisfaction and anxiety.
STUDY DESIGN
Consenting pregnant patients participating in a local first-trimester (11–13+6 weeks) preterm preeclampsia screening and prevention implementation study1 were contacted 6 weeks postpartum to complete an online patient satisfaction survey, designed to assess their satisfaction with the screening program and their levels of trait anxiety (using an abbreviated version of the State-Trait Anxiety Inventory [STAIT-5]). In addition to assessing overall patient satisfaction, the level of patient satisfaction was stratified and compared according to levels of patient risk for preterm preeclampsia.
RESULTS
Between June 2021 and December 2021, surveys were emailed to 765 participants. The response rate was 47.80% (358/765). Overall, 93% of participants reported high levels of satisfaction with preterm preeclampsia screening (70%–100%), and 98% stated that they would recommend the screening to all pregnant patients. With respect to levels of satisfaction with the program's support in reducing feelings of worry and anxiety, 87.9% of the total sample reported high satisfaction (70%–100%). The level of clinically significant symptoms of anxiety did not differ significantly between low- and high-risk groups (8% vs 10.8%, respectively).
CONCLUSION
Overall, first-trimester preeclampsia screening was associated with high patient satisfaction and did not lead to differences in patient anxiety between those with high- and low-risk screen results.
AJOG Global Reports at a Glance.
Why was this study conducted?
This study aimed to assess the impact of first-trimester preterm preeclampsia screening and prevention on patient satisfaction and anxiety levels.
Key findings
The implementation of a program for first-trimester screening and prevention of preterm preeclampsia was met with high levels of patient satisfaction and did not lead to differences in anxiety levels while accounting for preeclampsia risk.
What does this add to what is known?
This study highlights the importance of patient-oriented care and patient mental health when considering the implementation of a new program.
Introduction
Preeclampsia, a multisystem hypertensive disorder of pregnancy affecting 2% to 5% of pregnancies in North America,2 is one of the leading causes of maternal and perinatal morbidity and mortality.3, 4, 5 Several screening and prevention models have been proposed to reduce the impact of preeclampsia and improve maternal and perinatal outcomes. Of these, the Fetal Medicine Foundation (FMF) preterm preeclampsia screening algorithm, which incorporates biochemical, biophysical, and clinical variables, has been shown to be more accurate in predicting preeclampsia than conventional methods, and can be reliably applied at the time of an 11- to 13+6-week ultrasound.6,7 When combined with treatment (150-mg aspirin daily, initiated before 16 weeks’ gestation), a large multicenter randomized trial (ASPRE) showed a significant reduction in the incidence of preterm preeclampsia among the treated group.8,9 This approach has been validated in several studies10,11 and endorsed by local and international bodies. Despite this, systematic preeclampsia screening is not a part of routine antenatal care in Canada. One of the potential barriers to implementation is the psychological impact on the patient.12 Although some pregnant patients may feel comforted by additional screening, others may find it burdensome and even distressing. It is thus important to understand the impact of such screening on the patient experience. The objectives of this study were to assess: (1) patient satisfaction with the first-trimester preterm preeclampsia screen, and (2) symptoms of anxiety following the preeclampsia screen.
Materials and Methods
This study included participants in a local first-trimester preeclampsia screening and prevention implementation study (the IMPRESS study1) who consented to be contacted 6 weeks postpartum to complete an online satisfaction survey about their experience with preeclampsia screening.
The IMPRESS study was conducted through the local first-trimester, 1-stop aneuploidy screening program (the Early Risk Assessment [ERA] Program, www.earlyriskassessment.ca). The program uses the perinatal software Astraia (NEXUS / ASTRAIA GmbH, Ismaning, Germany), which contains the FMF-approved first-trimester aneuploidy and preeclampsia risk-calculation algorithms, allowing preterm preeclampsia risk calculation to be performed at the same time as the aneuploidy screen.
Patients referred for first-trimester combined screening (FTS) were eligible for preeclampsia screening if they were pregnant with a singleton, live fetus between 11 and 13+6 weeks’ gestation, and consented to preterm preeclampsia screening concurrently with their aneuploidy screen and follow-up of pregnancy outcome. Exclusion criteria included contraindications (ie, bleeding disorder such as von Willebrand disease and peptic ulceration) or known sensitivity to aspirin, multiple pregnancy, fetal demise, or major anomaly. Approval for the study was obtained from the local research ethics board (REB19-0359).
Clinic model and patient flow
Patients referred for FTS are provided with information about FTS and preeclampsia screening (IMPRESS study) through the ERA website and when they arrive at the clinic. It should be noted that although preeclampsia screening was added to the existing aneuploidy screening program in the current study, they do not need to be completed together. Patients are advised to undergo phlebotomy 3 to 5 days before their appointment for measurement of aneuploidy biochemical markers (free beta-human chorionic gonadotropin [fbHCG] and pregnancy-associated plasma protein-A [PAPP-A]). Placental growth factor (PlGF) was also measured in patients consenting to preterm preeclampsia screening. Following one-on-one counseling with a study nurse, consenting participants provided the additional maternal factors (MF) required for preeclampsia risk calculation. Participants then completed their nuchal translucency ultrasound scan, which included measurements of the uterine artery Doppler pulsatility indices (UTADPI). They were then escorted to a private cubicle to record their mean arterial blood pressure (MAP) measurements. These variables (MF, UTADPI, MAP) and the biochemical variables (PlGF) were used to calculate an individualized risk for preterm preeclampsia, which was generated at the same time as their aneuploidy risk (based on nuchal translucency, nasal bone, fbHCG, and PAPP-A). All staff (nurses, maternal–fetal medicine [MFM)] physicians, and ultrasound technologists) were FMF-certified in FTS and preeclampsia screening, and complied with ongoing quality assurance and audit.
Following the ASPRE trial protocol, low risk was defined as ≤1 in 100, whereas high risk was defined as >1 in 100.8 Results of both the FTS and preterm preeclampsia risk calculation were made available to the participants in the form of a report. If participants were deemed to be at high risk for preterm preeclampsia, the report also contained recommendations for the participant's primary healthcare provider for initiation of low-dose aspirin. The aspirin dosage for this study was 162 mg daily at bedtime because the ASPRE trial aspirin formulation of 150 mg is not available in Canada.11
Posttest counseling was provided to participants by the study nurse and MFM physicians, defined as one-on-one review of the combined FTS and preeclampsia-risk calculation report. The time spent in the clinic for patients receiving FTS and preeclampsia screening was approximately 1 hour.
The online satisfaction and anxiety survey
Patients who participated in the IMPRESS study who had a live birth and consented to the online survey were contacted approximately 6 weeks following their delivery to assess their satisfaction and anxiety with preeclampsia screening. Participation in the online survey was optional. The consent form for the current preeclampsia study was embedded within the online Qualtrics survey. No compensation was offered for the completion of the survey.
Patient satisfaction
Patient satisfaction with the preeclampsia screening was assessed using statements about the quality of counseling, care, efficiency, and overall ease of understanding in the Qualtrics survey. A 1-to-10 Likert scale was used, with higher scores being indicative of higher levels of satisfaction. Specifically, scores of 1 or 2 represented very low levels of satisfaction, 3 or 4 represented low levels of satisfaction, 5 or 6 represented moderate levels of satisfaction, 7 or 8 represented high levels of satisfaction, and 9 or 10 represented very high levels of satisfaction. This classification system was based on a similar type of study that also assessed patient satisfaction using a 5-point Likert scale.13 A 10-point Likert scale was used because we wanted to be more conservative with our estimates of patient satisfaction.14 An open comment form was also included. The Supplemental Materials contain the questionnaire provided to participants.
Symptoms of anxiety
Trait anxiety was measured using an abbreviated version of the State-Trait Anxiety Inventory (STAIT-5),15 which demonstrates excellent reliability and high levels of internal consistency.15 Clinically significant symptoms of anxiety are indicated by a score of ≥13.5.15 Anxiety symptoms were also approximated through the Qualtrics survey. Of note, the following statement was included: “Participating in the IMPRESS study, and having the preeclampsia screening test for preterm preeclampsia provided a sense of relief and decreased my levels of anxiety.” Participants were prompted to grade their agreement with the statement using a 10-point Likert scale. A score of 1 or 2 represented very low levels of agreement, 3 or 4 represented low levels of agreement, 5 or 6 represented moderate levels of agreement, 7 or 8 represented high levels of agreement, and 9 or 10 represented very high levels of agreement.
Statistical analysis
Descriptive statistics were used to evaluate the levels of patient satisfaction and levels of trait anxiety following preterm preeclampsia screening/intervention using frequencies (percentages). An analysis of variance was run to determine whether there were differences based on demographic characteristics between completers and noncompleters of the current survey. Descriptive statistics were also used to determine the overall demographic and clinical characteristics of the study cohort. Specifically, incidence (and variances) of preterm/term preeclampsia were calculated in low- and high-risk women. Subgroup analyses were also completed to compare any demographic or clinical differences that existed between the high- and low-risk groups. Potential differences between the high- and low-risk groups were further explored through independent t-tests and chi-square tests. Data analyses were completed using a completer sample.
Results
A total of 765 participants were sent an email invite through Qualtrics to complete a survey based on their participation in preeclampsia screening/IMPRESS study during their pregnancy. In total, 358 of the eligible participants responded to the survey (response rate=46.80%).
The demographics of the patients who completed the survey are described in Table 1. Overall, 47.6% of the participants were aged between 30 and 35 years, 48% were primiparous, and 14.0% screened as high-risk for preterm preeclampsia. Most reported being married or in a common-law relationship (96.3%), were White/of European descent (77.9%), had completed education to the level of a bachelor's degree or higher (63.8%), and reported a household income between $100,000 and $149,000 annually (Table 1).
Table 1.
Patient demographics and clinical characteristics
| Demographic variables | All (n=358) | High-risk (n=50) | Low-risk (n=297) |
|---|---|---|---|
| High-risk for preterm preeclampsia, n (%) | 50 (14.4%) | ||
| Age (y), n (%) | |||
| 18-25 | 12 (3.4) | 2 (4) | 10 (3.4) |
| 25-30 | 79 (22.6) | 9 (18) | 70 (23.6) |
| 30-35 | 166 (47.6) | 16 (32) | 148 (49.8) |
| 36-45 | 92 (26.4) | 23 (46) | 69 (23.2) |
| Race or ethnic group, n (%) | |||
| White/European descent | 272 (77.9) | 28 (56) | 244 (82.2) |
| Black/African descent | 5 (1.4) | 2 (4) | 3 (1) |
| South Asian (ie, Indian, Pakistani, Afghanistani) | 11 (3.2) | 1 (2) | 9 (3) |
| East/Southeast Asian (ie, Chinese, Korean, Filipino, Vietnamese) | 36 (10.3) | 12 (24) | 23 (7.7) |
| Indigenous (ie, First Nations, Métis, Inuit) | 6 (1.7) | 3 (6) | 3 (1) |
| Middle Eastern and North African (ie, Iranian, Egyptian) | 4 (1.1) | 1 (2) | 3 (1) |
| Latino (ie, Bolivian, Mexican, Salvadoran, Costa Rican) | 5 (1.4) | 1 (2) | 4 (1.3) |
| Other | 6 (1.7) | 2 (4) | 4 (1.3) |
| Prefer not to answer | 4 (1.1) | / | 4 (1.3) |
| Marital status, n (%) | |||
| Single | 10 (2.9) | 4 (8) | 6 (2) |
| Married/living with partner | 336 (96.3) | 45 (90.4) | 279 (97.3) |
| Other | 3 (0.9) | 1 (2) | 2 (0.7) |
| Level of education, n (%) | |||
| Less than high school | 3 (0.9) | / | 3 (1) |
| High school | 23 (6.6) | 3 (6) | 19 (6.4) |
| Diploma | 93 (26.9) | 16 (32) | 77 (25.9) |
| Bachelor's degree | 160 (46.2) | 22 (44) | 138 (46.5) |
| Postgraduate degree | 61 (17.6) | 8 (16) | 53 (17.8) |
| Other | 6 (1.7) | 1 (2) | 5 (1.7) |
| Household income, n (%) | |||
| <$29,000 | 14 (4.1) | / | 14 (4.8) |
| $30,000-$39,000 | 11 (3.2) | 3 (6) | 8 (2.7) |
| $40,000-$49,000 | 19 (5.6) | 6 (12) | 13 (4.5) |
| $50,000-$69,000 | 25 (7.3) | 5 (10) | 20 (13) |
| $70,000-$99,999 | 48 (14.1) | 10 (20) | 38 (13) |
| $100,000-$149,000 | 98 (28.7) | 15 (30) | 83 (28.4) |
| $150,000-$200,000 | 82 (24) | 7 (14) | 75 (25.7) |
| >$200,000 | 44 (12.9) | 3 (6) | 41 (14) |
| Number of children before current pregnancy, n (%) | |||
| 0 | 167 (48) | 25 (50) | 142 (47.8) |
| 1 | 133 (38.2) | 19 (38) | 114 (38.4) |
| 2 | 31 (8.9) | 5 (10) | 36 (8.8) |
| >3 | 17 (4.9) | 1 (2) | 15 (5.1) |
Silang. First-trimester preeclampsia screening and prevention. Am J Obstet Gynecol Glob Rep 2023.
Participants who did not complete the Qualtrics survey were more likely to be younger in age, non-White (Black and South Asian specifically), parous with children, and to have conceived spontaneously.
Patient satisfaction
Overall, patient satisfaction with preeclampsia screening was high, with 93% reporting high levels of satisfaction, and 98% indicating that they would recommend preeclampsia screening to all pregnant individuals. The median score for overall satisfaction with preeclampsia screening was noted to be 10 (interquartile range [IQR], 8–10). Participants also noted high levels of satisfaction with prescreen counseling, where the median of participant satisfaction was a 10 (IQR, 8–10). Furthermore, participants also noted that preeclampsia screening was informative and increased their knowledge and understanding of preeclampsia (median, 10; IQR, 8–10) (Table 2). Table 3 shows the satisfaction levels stratified by level of risk for preterm preeclampsia.
Table 2.
Overall sample satisfaction
| Item | All | High-risk | Low-risk |
|---|---|---|---|
| Satisfaction with counseling before FTS Plus, median (IQR) | 10 (8–10) | ||
| Satisfaction with counseling before FTS Plus, n (%) 7/10 | 284 (84.8) | 36 (73.5) | 248 (86.7) |
| Satisfaction with ultrasound follow-up appointments, median (IQR) | 10 (8–10) | ||
| Satisfaction with ultrasound follow-up appointments, n (%) 7/10 | 269 (89.4) | 37 (84.1) | 232 (90.3) |
| Satisfaction with support to overcome worry/anxiety, median (IQR) | 10 (8–10) | 9 (7–10) | 10 (8–10) |
| Satisfaction with support to overcome worry/anxiety, n (%) 7/10 | 263 (87.9) | 38 (84.4) | 225 (88.6) |
| Best source of support following FTS Plus, n (%) | |||
| Support from healthcare provider | 83 (27.6) | 15 (33.3) | 68 (26.6) |
| Support from ultrasound clinic team | 40 (13.3) | 1 (2.2) | 39 (15.2) |
| Ultrasound appointment for health of fetus | 149 (49.5) | 24 (53.3) | 125 (48.8) |
| The resources online | 15 (5) | 2 (4.4) | 13 (5.1) |
| Other | 14 (4.7) | 3 (6.7) | 11 (4.3) |
| Overall satisfaction with the FTS Plus, median (IQR) | 10 (9–10) | 10 (7.5–10) | 10 (9–10) |
| Overall satisfaction with the FTS Plus, n (%) 7/10 | 280 (93.0) | 38 (84.4) | 242 (94.5) |
| Should all pregnant people be offered FTS Plus?, n (%) | 302 (98.4) | 43 (95.6) | 259 (98.9) |
| FTS Plus increased awareness and knowledge, median (IQR) | 10 (8–10) | 9 (7–10) | 10 (8–10) |
| FTS Plus increased awareness and knowledge, n (%) 7/10 | 252 (83.4) | 37 (82.2) | 215 (83.7) |
| FTS Plus provided sense of relief and decreased anxiety, median (IQR) | 9.5 (7–10) | 8 (6–10) | 10 (8–10) |
| FTS Plus provided sense of relief and decreased anxiety, n (%) 7/10 | 248 (83.8) | 31 (70.5) | 217 (86.1) |
FTS, first-trimester screening; IQR, interquartile range.
Silang. First-trimester preeclampsia screening and prevention. Am J Obstet Gynecol Glob Rep 2023.
Table 3.
Patient satisfaction within the low-risk and high-risk preeclampsia sample
| High-risk variables | Low-risk (n=297) | High-risk (n=50) |
|---|---|---|
| Satisfaction with counseling received (≥7), n (%) | 248 (86.7) | 34 (73.9) |
| Counseling received was very informative (≥7), n (%) | 236 (85.2) | 34 (72.3) |
| Care received was quick and efficient (≥7), n (%) | 254 (91.4) | 41 (87.2) |
| Counseling received was easy to understand (≥7), n (%) | 251 (90.9) | 36 (78.3) |
| Staff was kind and accommodating (≥7), n (%) | 266 (95.7) | 41 (87.2) |
| My questions about PE risk were properly addressed (≥7), n (%) | 258 (94.5) | 35 (74.5) |
| Counseling about prevention of PE (aspirin) was informative (≥7), n (%) | — | 34 (91.9) |
| Care received about prevention of PE (aspirin) was quick and efficient (≥7), n (%) | — | 37 (82.2) |
| Counseling about prevention of PE (aspirin) was easy to understand (≥7), n (%) | — | 35 (77.8) |
PE, preeclampsia.
Silang. First-trimester preeclampsia screening and prevention. Am J Obstet Gynecol Glob Rep 2023.
When directly comparing levels of overall satisfaction with the preterm preeclampsia screening test, results from an independent t test revealed a significant difference in levels of satisfaction between groups, with the low-risk group noting higher levels of satisfaction (mean difference, −.681; t[51.42], −2.31; P=.025).
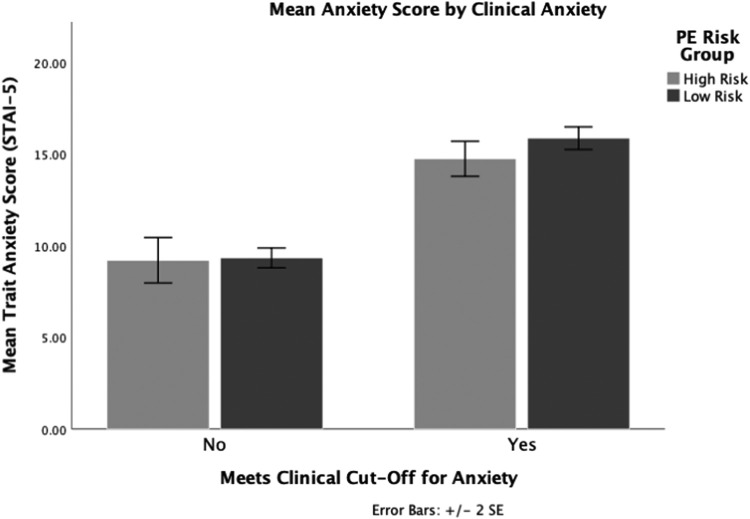
Patient anxiety
In the overall sample, there was no significant difference in levels of clinical anxiety between the low- (8%) and high-risk group (10.8%) (X2 [1, N=154]=.541; P=.462) (Figure). Furthermore, in response to the statement, “Preeclampsia screening provided a sense of relief and decreased anxiety,” 83.8% of participants reported high levels of agreement (median, 9.5; IQR, 7–10). When stratified by risk level, both groups reported high levels of agreement for preeclampsia screening's ability to decrease anxiety, although the high-risk group had a lower level of agreement (median, 8; IQR, 6–10) in comparison with the low-risk group (median, 10; IQR, 8–10).
Figure.
Levels of trait anxiety by PE risk and clinical cutoffs
PE, preeclampsia; SE, standard error.
Silang. First-trimester preeclampsia screening and prevention. Am J Obstet Gynecol Glob Rep 2023.
For the statement that “Preeclampsia screening provided a sense of relief and decreased anxiety,” participants reported a high level of agreement overall (median, 9.5; IQR, 7–10). With respect to the support provided by preeclampsia screening to overcome worry/anxiety, participants reported high levels of satisfaction overall (median, 10; IQR, 8–10). Table 4 contains further details.
Table 4.
Levels of anxiety and sources of support
| Item | Overall | High risk | Low risk |
|---|---|---|---|
| FTS Plus provided sense of relief and decreased anxiety, median (IQR) | 9.5 (7–10) | 8 (6–10) | 10 (8–10) |
| FTS Plus provided sense of relief and decreased anxiety, n (%) 7/10 | 248 (83.8) | 31 (70.5) | 217 (86.1) |
| Satisfaction with support to overcome worry/anxiety, median (IQR) | 10 (8–10) | 9 (7–10) | 10 (8–10) |
| Satisfaction with support to overcome worry/anxiety, n (%) 7/10 | 263 (87.9) | 38 (84.4) | 225 (88.6) |
| Best source of support following FTS Plus, n (%) | |||
| Support from healthcare provider | 83 (27.6) | 15 (33.3) | 68 (26.6) |
| Support from ultrasound clinic team | 40 (13.3) | 1 (2.2) | 39 (15.2) |
| Ultrasound appointment for health of fetus | 149 (49.5) | 24 (53.3) | 125 (48.8) |
| The resources online | 15 (5) | 2 (4.4) | 13 (5.1) |
| Other | 14 (4.7) | 3 (6.7) | 11 (4.3) |
FTS, first-trimester screening; IQR, interquartile range.
Silang. First-trimester preeclampsia screening and prevention. Am J Obstet Gynecol Glob Rep 2023.
Themes of patient experience
In reference to comments about the preeclampsia screen, a common theme among participants was the perception that the counseling session was brief, and that in retrospect, they would have preferred a more in-depth explanation of preterm preeclampsia and its consequences. Further, the term “counseling” seemed to be misleading to some participants, who might have misconstrued “counseling” to be like therapy as opposed to a conversation reviewing the results of the preeclampsia risk assessment and intervention for prevention.
To better support feelings of worry and/or anxiety, a common theme among participants was the request for more information regarding the health of the fetus from the ultrasound technologist during the ultrasound. Participants also noted how COVID-19 appointment policies precluded the support of a spouse, or another support person during the visit, which was particularly difficult for mitigating anxiety. Additional considerations that were mentioned were follow-up appointments for those who were screened to be high-risk, and more time to allow for the emotional processing of the results in participants who were deemed to be high-risk for preterm preeclampsia.
Discussion
Principal findings
The primary objectives of this study were to assess the levels of patient satisfaction with a preterm preeclampsia screening and prevention program, and the levels of trait anxiety associated with preeclampsia screening. Results from this study revealed that most patients who participated in preeclampsia screening reported high levels of satisfaction. Furthermore, when directly comparing the levels of trait anxiety between the high-risk and the low-risk group, levels of clinically significant anxiety did not differ (P>.05), which suggests that knowing the results of the screening does not significantly contribute to increased levels of anxiety.
Results
The finding of overall high levels of satisfaction with the preeclampsia screening was unsurprising. The finding that high-risk individuals noted lower levels of satisfaction was also expected given that individuals in the high-risk group are likely to need more emotional support or a longer follow-up after the finding of an increased risk for preterm preeclampsia, as was noted in the open-ended portion of the questionnaire.
Consistent with our understanding that the preterm preeclampsia screen does not contribute to anxiety beyond what is expected during the perinatal period, the level of anxiety found in this study is lower than recent estimates of prenatal anxiety during COVID-19.16
These findings are consistent with similar research that found that conducting a 13-week scan for fetal structural abnormalities did not negatively affect the psychological well-being of pregnant participants.17 Overall, these results provide support for wider implementation of systematic preeclampsia screening, given that an initial concern was that the screening would lead to unnecessary anxiety in a population that is already vulnerable to increased levels of distress. We propose that a contributing factor to these lower levels of anxiety in our center is the 1-stop model of care, where patients receive one-on-one counseling from a healthcare professional before and after receiving their screening results, and additional testing and support are arranged, as indicated before the patient leaves the clinic. This likely helps to alleviate the patients’ worries or concerns regarding their pregnancy and prevents them from feeling disconnected. The results also showed that some patients who screened high-risk felt that they would benefit from more time dedicated to explaining the results, and that providing a social support individual would be beneficial.
Clinical implications
This study shows that an important component of clinical implementation of preterm preeclampsia screening is consideration of the patient's psychosocial needs. Through this study, we have identified what these needs are, and how to better meet them. For example, patients indicated that they would prefer longer appointment times and more in-depth explanations of preterm preeclampsia and the screening components, and that a support person present at the appointment would be beneficial. The high levels of patient satisfaction with the preeclampsia screening confirm that patient anxiety should not be a barrier to further scaling and spreading of population-based preterm preeclampsia screening, which is gaining momentum worldwide. As suggested in a recent paper discussing the ethical issues related to screening for preeclampsia, a multivariable screening approach based on biochemical and biophysical markers to detect high-risk cases for developing preeclampsia in pregnancy should be a standard element within health care.12
Research implications
Future research within this domain should consider further investigating the relationship between the preeclampsia screen and maternal anxiety to replicate the results found in this study. For this research, we relied only on a postmeasure, which hindered our ability to observe how anxiety was able to change relative to the patient's baseline level of anxiety. Another point of interest would be to measure different domains of anxiety; this study focused on trait anxiety, but it would be worthwhile to also explore the use of related anxiety constructs such as state or health anxiety.
Strengths and limitations
This study has several strengths. Primarily, patient-oriented care was prioritized by assessing patient satisfaction. Evaluating patient satisfaction is clinically relevant because satisfied patients are more likely to comply with treatment and take a more active role in their health care.18 Another strength of the study was the inclusion of subgroup analyses that stratified patient responses by the level of risk. These comparisons provided a more holistic understanding of the patient perspective, and will influence future iterations of the preeclampsia screening program. Lastly, the study had a large sample size, which provides greater power to the current analyses and more confidence in our ability to generalize these findings to other populations.
Despite these strengths, this research is not without limitations. Given that this cohort is a part of an implementation study, participants are limited to being from a single community. Furthermore, many of the participants in the study were White, highly educated, and reported higher levels of income. Consequently, our research may not generalize well to individuals of different ethnicities, education levels, areas, and socioeconomic statuses. Relatedly, it is important to consider that this study relied on participants who responded to the survey. Another limitation that is important to note is that the study only assessed trait anxiety following the preeclampsia screening, with no initial baseline measure of anxiety. Thus, we were unable to investigate how levels of anxiety changed within the sample over time. Lastly, given that the survey was administered to participants at 6 weeks postpartum (a year after completing the preeclampsia screening), it is possible that there were other confounding factors that contributed to their levels of anxiety and that preclude isolating the impact of preeclampsia screening on anxiety, raising internal validity concerns.
Conclusion
This study assessed the patient experience of a screening and intervention program for preterm preeclampsia. The finding that preeclampsia screening produced high levels of satisfaction among patients and did not increase anxiety above the expected levels within this population provides preliminary evidence for the benefits of widespread implementation of first-trimester preeclampsia screening at a provincial level and potentially national level.
Footnotes
J.A.J. has received research funding for biochemical analytes from PerkinElmer. The remaining authors report no conflict of interest.
The authors confirm that informed patient consent was obtained for this research study.
Cite this article as: Silang KA, Tomfohr-Madsen LM, Maxey C, et al. First-trimester preeclampsia screening and prevention: impact on patient satisfaction and anxiety. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2023.100205.
Appendix. Supplementary materials
References
- 1.Johnson JM, Walsh JD, Okun NB, et al. The Implementation of Preeclampsia Screening and Prevention (IMPRESS) Study. Am J Obstet Gynecol MFM. 2023;5 doi: 10.1016/j.ajogmf.2022.100815. [DOI] [PubMed] [Google Scholar]
- 2.Bezerra Maia E, Holanda Moura S, Marques Lopes L, Murthi P, da Silva Costa F. Prevention of preeclampsia. J Pregnancy. 2012;2012 doi: 10.1155/2012/435090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2000;182:607–611. doi: 10.1067/mob.2000.104224. [DOI] [PubMed] [Google Scholar]
- 5.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1216. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright D, Syngelaki A, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.02.018. 62.e1–10. [DOI] [PubMed] [Google Scholar]
- 7.O'Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214 doi: 10.1016/j.ajog.2015.08.034. 103.e1–12. [DOI] [PubMed] [Google Scholar]
- 8.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 9.Wright D, Rolnik DL, Syngelaki A, et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin on length of stay in the neonatal intensive care unit. Am J Obstet Gynecol. 2018;218 doi: 10.1016/j.ajog.2018.02.014. 612.e1–6. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli E, Seidenari A, Germano C, et al. External validation of a simple risk score based on the ASPRE trial algorithm for preterm pre-eclampsia considering maternal characteristics in nulliparous pregnant women: a multicentre retrospective cohort study. BJOG. 2020;127:1210–1215. doi: 10.1111/1471-0528.16246. [DOI] [PubMed] [Google Scholar]
- 11.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218 doi: 10.1016/j.ajog.2017.11.561. 287–93.e1. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen JM, Hedley PL, Gjerris M, Christiansen M. Ethical issues related to screening for preeclampsia. Bioethics. 2014;28:360–367. doi: 10.1111/j.1467-8519.2012.02005.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu CH, Goyal D, Mittal L, Erdei C. Patient satisfaction with virtual-based prenatal care: implications after the COVID-19 Pandemic. Maternal Child Health J. 2021;25:1735–1743. doi: 10.1007/s10995-021-03211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawes J. Do data characteristics change according to the number of scale points used? An experiment using 5-point, 7-point and 10-point scales. Int J Market Res. 2008;50:61–104. [Google Scholar]
- 15.Zsido AN, Teleki SA, Csokasi K, Rozsa S, Bandi SA. Development of the short version of the spielberger state-trait anxiety inventory. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113223. [DOI] [PubMed] [Google Scholar]
- 16.Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5–13. doi: 10.1016/j.jad.2020.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardi F, Bakker M, Kenkhuis MJ, et al. Psychological outcomes, knowledge and preferences of pregnant women on first-trimester screening for fetal structural abnormalities: a prospective cohort study. PloS one. 2021;16 doi: 10.1371/journal.pone.0245938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilioudi S, Lazakidou A, Tsironi M. Importance of patient satisfaction measurement and electronic surveys: methodology and potential benefits. Int J Health Res Innovation. 2013;1:67–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.