Abstract
Introduction
A small bowel gastrointestinal stromal tumor (GIST) is a rare neoplasm of the gastrointestinal tract. The manifestation of bleeding is a diagnostic challenge and could present as a life-threatening situation that needs urgent intervention.
Presentation of case
64-year-old woman consulted for episodes of melena and anemia. The upper and lower endoscopies were not diagnostic. Capsule endoscopy (CE) revealed a probable jejunal hemangioma, however double-balloon enteroscopy and magnetic resonance imaging (MRI) did not show any intestinal nodule but MRI show a pelvic mass apparently related to the uterus confirmed by a gynecologist. Even so, the patient returned with melena, and a contrast-enhanced computed tomography (CT) scan again identified a pelvic mass, highlighting that its vascularization drained into the superior mesenteric territory and seemed to invade the jejunum, with active bleeding, suspicious for jejunal GIST. A laparotomy was performed to remove the jejunal mass. Histopathology and immunohistochemical studies confirmed the diagnosis.
Discussion
Bleeding is a common symptom in small bowel GISTs but its diagnoses could be difficult because its location. In most cases, gastroscopy and colonoscopy are not useful and CE or imaging studies are necessary to find the cause of bleeding. Moreover, it has recently proved that bleeding is a prognostic risk factor because it is related to tumor rupture and tumor invasion of blood vessels.
Conclusion
In this case, bleeding caused by small bowel GIST was misdiagnosed in endoscopic procedures and the clinical management was delayed. CT angiography was the most effective investigation to detect the source of bleeding.
Keywords: Gastrointestinal stromal tumor, Obscure GI bleeding, Capsule endoscopy, CT angiography
Highlights
-
•
Small bowel gastrointestinal stromal tumor is a very rare cause of bleeding.
-
•
Any small bowel submucosal lesion could be misdiagnosed in capsule endoscopy.
-
•
Imaging studies can give details about vascularization and detect active bleeding.
-
•
CT angiography could identify the source of bleeding and guide the management.
-
•
Bleeding is also relevant in GIST as a prognostic factor.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the GI tract [1]. Although previous studies have shown that up to 30 % of patients are asymptomatic, GISTs may be identified clinically because of symptoms including gastrointestinal (GI) bleeding, and abdominal pain due to tumor-induced mass effect [2]. The diagnosis of bleeding small intestine GISTs is challenging due to difficult approach by conventional endoscopy. Computed tomography (CT) angiography could help in demonstrating the tumor as well as the presence of active hemorrhage. We present a case in this scenario in a tertiary hospital. This case has been reported in line with SCARE criteria [3].
2. Presentation of case
A 64-year-old woman, with high blood pressure and diabetes, presented to our emergency department by ambulance in October of 2020 after syncopal episode. The patient denied vomiting, hematochezia/melena or weight loss. On examination, she was afebrile with a blood pressure of 134/76 mmHg and heart rate of 103 bpm. Her abdominal examination was unremarkable and digital rectal examination revealed the presence of melena. Initial investigations revealed a drop of hemoglobin from previous 13.9 to 5.5 g/dl. She was promptly resuscitated with fluid therapy and blood transfusion, and an early upper endoscopy was performed. This procedure did not reveal any lesion that could cause bleeding. Patient was admitted in the gastroenterology ward. Colonoscopy was also normal. During hospitalization, stools were normal and hemoglobin remains stable after blood and iron transfusion and patient was discharged to continue study on outpatient clinic.
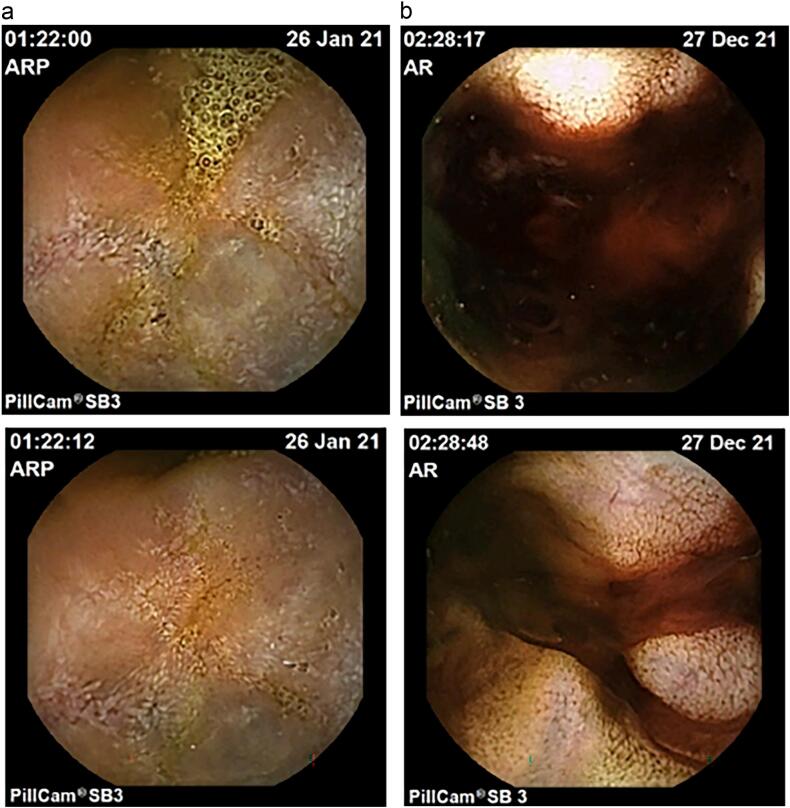
Subsequently, capsule endoscopy (CE) in January 2021 revealed probable jejunal hemangioma (Fig. 1a). Then, double-balloon enteroscopy did not reach the jejunal lesion and magnetic resonance image (MRI) did not show any intestinal nodule but pelvic mass was found apparently related to the uterus (Fig. 2). Patient was sent to Gynecology, that confirmed the presence of 8-cm subserous myoma by transvaginal ultrasound.
Fig. 1.
Capsule endoscopy studies during follow-up. (A) In January 2021 CE showed dubious hemangioma in jejunum. (B) In December 2021 CE revealed same lesion with adherent clot and blood nearly.
Fig. 2.
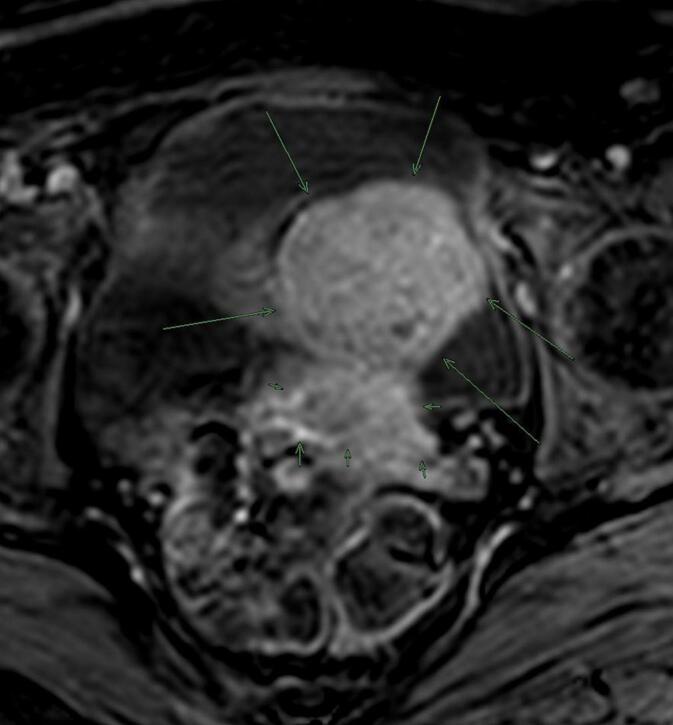
MRI found a pelvic mass (long arrows) probably depending on the uterus (short arrows).
After that, patient came to emergency department in December 2021 with melena and anemia. At that time, an additional upper endoscopy was performed and showed two Forrest III small antral ulcers without any recent hemostatic sign. During the hospitalization, patient remained hemodynamically stable, but hemoglobin was dropping and therefore patient needed blood transfusion almost every day. CE was repeated and revealed the same lesion in jejunum with adherent clot and the presence of blood near the lesion (Fig. 1b). Thus, CT angiography was performed. Pelvic mass was again found but notably its vascularization drained to the superior mesenteric territory and it seemed to invade jejunum, with active bleeding through jejunum (Fig. 3). In light of this situation, jejunal GIST with active bleeding was suspect. Few hours later, midline laparotomy was performed. Intraoperative findings revealed an exophytic lesion measuring 10 cm approximately 2 m proximal to the ileocecal valve (Fig. 4). Small bowel resection and side-to-side anastomosis were performed. She recovered uneventfully and was discharged.
Fig. 3.
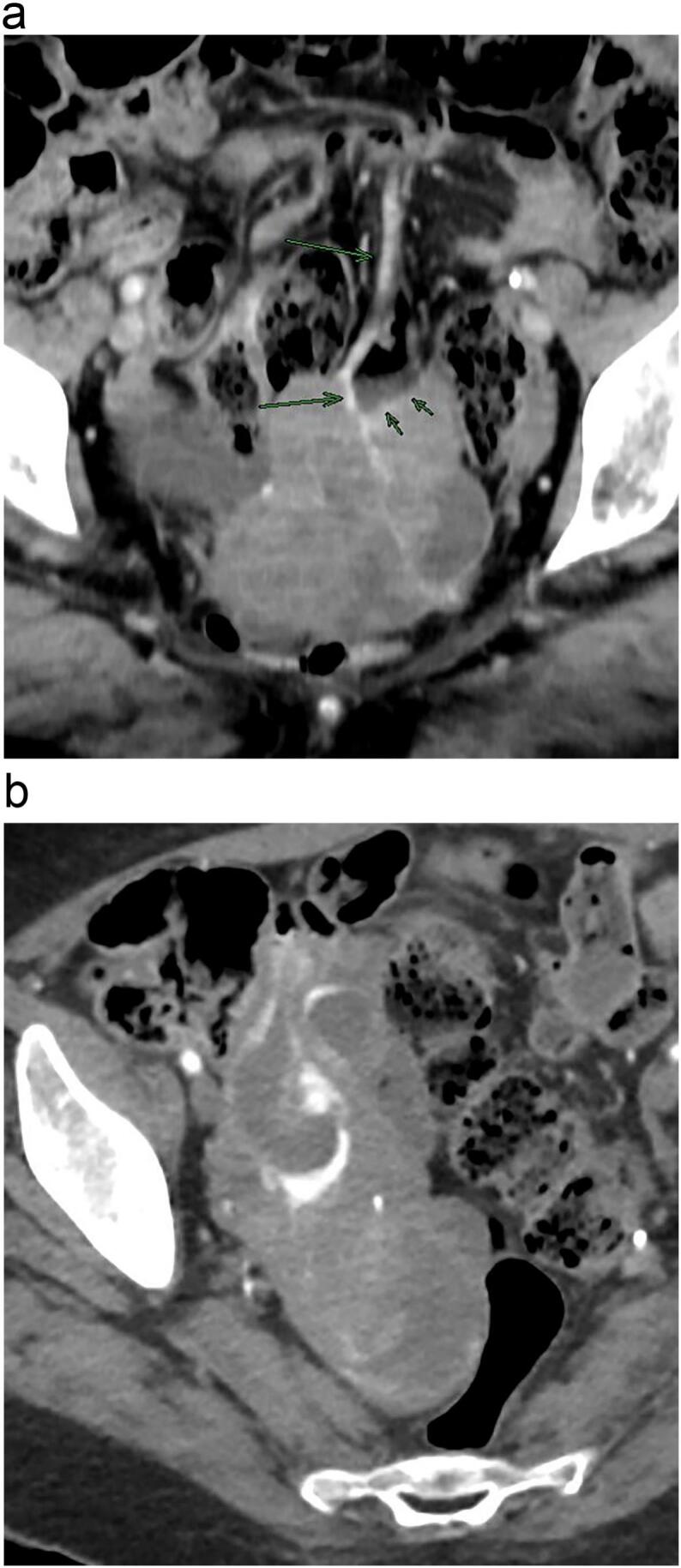
CT angiography. (A) Pelvic mass with mesenteric venous drainage (long arrows); it seemed to invade jejunum (short arrows). (B) Active bleeding through jejunum.
Fig. 4.
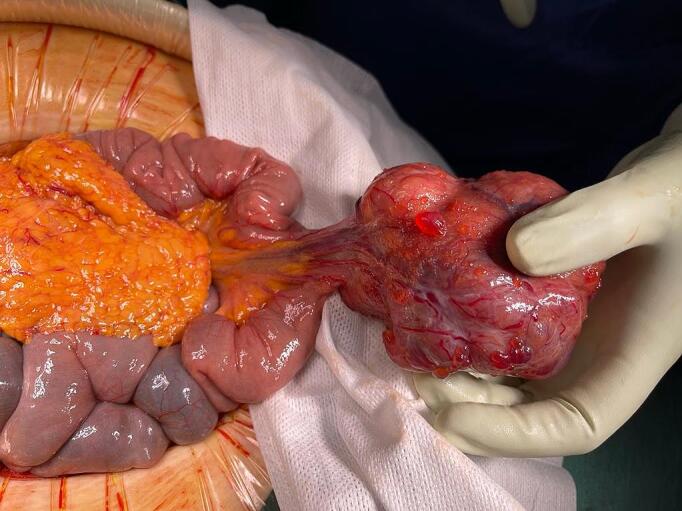
Surgical piece of mass excised from the jejunum with its vascular pedicle.
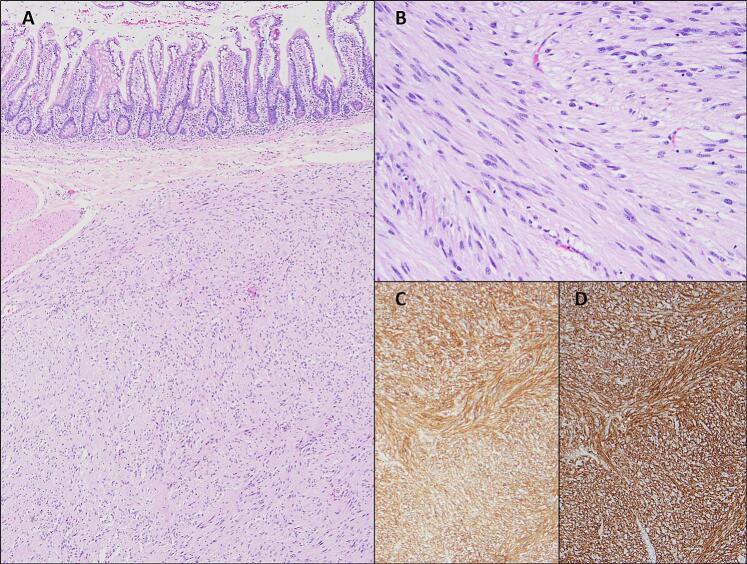
The histopathological report (Fig. 5) confirmed the diagnosis of GIST arising from the jejunum (immunohistochemistry staining positive for cKIT). The long axis measured 80 mm, with a mitosis index of 1 per 50 high power field (HPF), with no evidence of tumor rupture. The final TNM stage was IB (T3 N0 M0), and classified as intermediary risk. Mutational study was performed and KIT exon 11 mutation was detected. No mutations were identified in PDGFRA gen. Close surveillance is undergoing.
Fig. 5.
Histopathology results. (A) Submucosal spindle cell proliferation with diffuse sheets or vague storiform arrangements of bland tumor cells. (B) At higher magnification, there is no nuclear pleomorphism, mitosis or necrosis. (C) KIT (CD117) immunohistochemistry shows intense and diffuse positivity (cytoplasmatic and membrane-associated). (D) Immunohistochemistry for DOG1 (ANO1) is also a sensitive and specific marker and may be helpful in KIT-negative GISTs.
3. Discussion
GISTs are malignant mesenchymal tumors that originate from the interstitial cells of Cajal and contain spindle cells. They are rare tumors with significant variations in reported incidence (around 8 cases per million per year) due to different diagnostic criteria over the decades besides the fact that the smallest GISTs normally are not diagnosed histologically [2], [4].
GISTs appear in the small intestine up to 36 %, being the second most frequent location after the stomach [5]. The estimated mean age at diagnosis is in the sixth decade [6] with a wide range of symptoms depending on site, size and growth pattern. It has been reported that patients with GI bleeding constitute approximately 23–33% [1], [9] especially when the GIST is located in the small intestine due to narrower intestinal wall [7]. Besides, when the tumor volume is large, it is more likely to develop ulcers or break through the intestinal cavity. In other studies, the microvessel density and vascular endothelial growth factor were higher in tumors with tumor diameter ≥ 5 cm [8].
Most gastric GISTs are detected as submucosal endoscopic lesions during ordinary upper endoscopy as a nonspecific smooth nodule covered with normal mucosa. Given that optical endoscopic examination provides insufficient information, endoscopic ultrasound (EUS) is a key test for differential diagnosis. In large nodules (> 2 cm), EUS-guided fine needle aspiration is recommended to obtain a conclusive diagnose by immunohistochemical analysis [9].
In cases of bleeding GIST, upper and lower GI endoscopy remained the first line investigation. However, small intestinal location is a diagnostic challenge as this region is inaccessible by conventional endoscopy. Facing that situation, CE and enteroscopy play a predominant role in the diagnosis and treatment of these lesions. However, in some cases the lesions are not visible or are not accessible and other imaging modalities such as CT- or MR- enterography are essential for diagnosis. In addition, these studies provide additional information about the cause of the bleeding, which helps to guide the appropriate intervention.
GIST on CT shows the characteristic of a well-defined soft tissue of relatively low density, which is homogenous. However, it is difficult to differentiate GIST of the small bowel and rectosigmoid or gynecological lesions based on imaging studies.
In fact, the role of image test in small bowel bleeding is increasingly established. In patients with obscure hemorrhage, comparison of CE with angiography demonstrated that immediate CE has superior yield, although long-term outcomes, including rebleeding and hospitalization rates, and deaths, did not differ between two groups [10]. Visceral arteriography can detect bleeding in the upper GI tract at rates as low as 0.5 ml/min and it also has the ability to define in greater detail the vascular anatomy which can help to find the origin of the lesions [11] such as the case that has been exposed in this review. Recently, a small series of cases has been published showing the usefulness of CT for the management of acute massive over bleeding due to jejunal GIST [12].
Pathologically, CD117 expression is the GIST main feature (only 5 % of GISTs are CD117-negative). The mitotic count and the mutational analysis have predictive value for sensitivity to molecular-targeted therapy (PDGFRA D842V mutations are associated to lack of sensitivity to imatinib) [13].
Standard treatment of localized GISTs is complete surgical excision of the lesion, with no dissection of clinically negative lymph nodes. Pre-operative imatinib should be considered for those tumors where R0 surgery with no expected major sequelae is not feasible [9].
After surgery, risk assessment should be taken into account. A widely used risk classification was proposed by the Armed Forces Institute of Pathology, which incorporates the primary mitotic count, tumor size and tumor site and the presence or absence of tumor capsule rupture [14], [15].
However, recent research demonstrates GI bleeding is of great significance to the prognosis of GIST [16] due to increases the risk of recurrence and affects the overall survival rate in a retrospective analysis [17]. Authors suggest that the causes of GI bleeding in patients with GIST are tumor rupture and tumor invasion of mucosal or submucosal blood vessels, thus favoring tumor dissemination, tumor recurrence and worse overall survival. Additionally, in a recent meta-analysis, authors found that mitotic index >5 per 50 HPF can significantly increase the risk of GI bleeding in GIST [18]. Moreover, patients with GI bleeding usually need emergency care, including transfusion support requirements, urgent endoscopic techniques and even emergency surgery, that also may contribute to worse prognosis.
4. Conclusion
Jejunal GIST is a very rare cause of small intestinal bleeding. Moreover, it could be part of a difficult differential diagnosis in women with abdominopelvic mass. In patients with GI bleeding with negative upper and lower endoscopies, a small intestinal source should be suspected, and CT may be useful in the diagnosis, especially in emergency situations. In addition, it also can show the precise location of bleeding, and guide subsequent management. Early diagnosis of bleeding GIST is of great importance because it has a worse prognosis and lower overall survival.
Consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer reviewed.
Ethical approval
This case report was approved by the Hospital del Mar Clinical Research and Ethics Committee in 2023 (reference number: 2023/10881).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Author contribution
Maria González-Vivó: Conceptualization, Writing- Original draft preparation. Ander Zugazaga: Visualization, Writing- Reviewing and Editing. Josep Maria Dedeu Cusco: Visualization, Writing- Reviewing and Editing. Dolores Naranjo-Hans: Visualization, Writing- Reviewing and Editing. Anna Casajoana: Supervision, Writing- Reviewing and Editing. Laura Carot: Conceptualization, Writing- Original draft preparation.
Guarantor
Maria Gonzalez-Vivo
Laura Carot
Research registration number
This case report was not registered in any place because it is not “First in Man”.
Conflict of interest statement
None.
Acknowledgements
We are grateful to all of those with whom we have had the pleasure to work during this and other related projects.
References
- 1.Scarpa M., et al. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. Journal of Surgical Oncology. 2008;98 doi: 10.1002/jso.21120. Preprint at. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B., et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era - a population-based study in western Sweden. Cancer. 2005;103 doi: 10.1002/cncr.20862. Preprint at. [DOI] [PubMed] [Google Scholar]
- 3.Agha R.A., et al. “The SCARE guidelines: Consensus-based surgical case report guidelines” [Int. J. Surg. 34 (2016) 180–186]((2016) 34 (180–186)(S174391911630303X)(10.1016/j.ijsu.2016.08.014)) International Journal of Surgery. 2016;36 doi: 10.1016/j.ijsu.2016.11.021. Preprint at. [DOI] [PubMed] [Google Scholar]
- 4.van der Graaf W.T.A., et al. Nationwide trends in the incidence and outcome of patients with gastrointestinal stromal tumour in the imatinib era. Br. J. Surg. 2018;105 doi: 10.1002/bjs.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graadt van Roggen J.F., van Velthuysen M.L.F., Hogendoorn P.C.W. The histopathological differential diagnosis of gastrointestinal stromal tumours. Journal of Clinical Pathology. 2001;54 doi: 10.1136/jcp.54.2.96. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran T., Davila J.A., El-Serag H.B. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am. J. Gastroenterol. 2005;100 doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson N.S.S., et al. How to perform gastrointestinal ultrasound: Anatomy and normal findings. World Journal of Gastroenterology. 2017;23 doi: 10.3748/wjg.v23.i38.6931. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imamura M., et al. Prognostic significance of angiogenesis in gastrointestinal stromal tumor. Mod. Pathol. 2007;20 doi: 10.1038/modpathol.3800767. [DOI] [PubMed] [Google Scholar]
- 9.Casali P.G., et al. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33 doi: 10.1016/j.annonc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Leung W.K., et al. Capsule endoscopy or angiography in patients with acute overt obscure gastrointestinal bleeding: a prospective randomized study with long-term follow-up. Am. J. Gastroenterol. 2012;107 doi: 10.1038/ajg.2012.212. [DOI] [PubMed] [Google Scholar]
- 11.Geffroy Y., et al. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31 doi: 10.1148/rg.313105206. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraj S.S., et al. Diagnostic and therapeutic challenges in the Management of Acute Massive Overt Bleeding of jejunal gastrointestinal stromal tumours: case series. J. Gastrointest Cancer. 2022 doi: 10.1007/s12029-021-00650-w. [DOI] [PubMed] [Google Scholar]
- 13.Joensuu H., et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2016.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M., Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006;23 doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Joensuu H., et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13 doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 16.Joensuu H., et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. European Journal of Cancer. 2015;51 doi: 10.1016/j.ejca.2015.05.009. Preprint at. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Li Y., Dong M., Kong F., Dong Q. Gastrointestinal bleeding is an independent risk factor for poor prognosis in GIST patients. Biomed. Res. Int. 2017;2017 doi: 10.1155/2017/7152406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan X., et al. Prognostic value of bleeding in gastrointestinal stromal tumors: a meta-analysis. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/15330338211034259. [DOI] [PMC free article] [PubMed] [Google Scholar]