Highlights:
-
•
Intraplacental choriocarcinoma exists on the spectrum of gestational trophoblastic disease.
-
•
Because patients are often asymptomatic, incidence is likely underestimated.
-
•
Formal guidelines on diagnostic evaluation and surveillance protocols are needed.
Keywords: Choriocarcinoma, Intraplacental, Trophoblastic disease, Gestational trophoblastic neoplasia
Abstract
Intraplacental choriocarcinoma (IC), or choriocarcinoma in situ, is a rare disease on the gestational trophoblastic disease (GTD) spectrum, with <100 case reports available in the literature. We propose that many patients with IC are likely to be missed as the majority of patients do not present with metastases. Currently, there are no standardized protocols in existence for postpartum monitoring of these patients. We present a case of IC identified in the term placenta of a 21-year-old who delivered by primary cesarean due to concern for fetal intolerance of labor. Subsequently, we review the recommendations available on postpartum monitoring of this likely under-diagnosed condition.
1. Introduction
Intraplacental choriocarcinoma (IC), also known as choriocarcinoma in situ, is a rare form of gestational trophoblastic disease (GTD), accounting for 0.04% of all cases (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Kanehira et al., 2013). Given the rarity of the condition and the paucity of literature about it, IC has been treated as distinct from gestational choriocarcinoma (GC). While presentation of IC, if symptomatic, is similar to that of GC, diagnostic criteria are different (Table 1).
Table 1.
Comparison of presentation and diagnosis of intraplacental choriocarcinoma and gestational choriocarcinoma.
| Intraplacental choriocarcinoma | Gestational choriocarcinoma | |
|---|---|---|
| Presentation | Routine evaluation of placenta after delivery Evaluation of placenta after non-reassuring fetal status or stillbirth Evaluation of products of conception after pregnancy termination or early pregnancy loss Gross placental lesion noted after delivery Vaginal bleeding during pregnancy Symptoms of metastases (respiratory, neurologic) |
Evaluation of pregnancy tissue, including molar gestation and early pregnancy loss Vaginal bleeding or bleeding from metastases Other symptoms of metastases (respiratory, neurologic) Persistently elevated hCG |
| Diagnosis | Histopathologic diagnosis | Clinical diagnosis, including - Histopathology - Rise or plateau of hCG - Symptoms or radiologic evidence of metastases |
Histologically, IC is characterized by a proliferation of cytotrophoblasts and syncytiotrophoblasts, often with extensive necrosis due to their lack of intrinsic blood supply. Synonymously, choriocarcinoma in situ has been used to describe neoplastic trophoblast proliferations that are restricted to the placenta without metastases (Trask et al., 1994). Disease presentation can range from asymptomatic with incidental findings of IC on placental pathology to metastatic maternal and infantile disease (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Kanehira et al., 2013, Trask et al., 1994, Medeiros et al., 2008, Liu and Guo, 2006, Christopherson et al., 1992, Duleba et al., 1992, Sauvestre, 2014, Aonahata et al., 1998, Jacques et al., 1998, Lee and Cho, 2019, Sebire et al., 2005). Most cases present with no evidence of disseminated disease and are monitored with serial beta human chorionic gonadotropin (hCG) levels (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Jacques et al., 1998). If metastases are identified, the prognosis is poor unless both the patient and the newborn, if also affected, are treated with multi-agent chemotherapy, most commonly EMA/CO (etoposide, methotrexate, actinomycin-D, cyclophosphamide, and vincristine) (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Liu and Guo, 2006, Christopherson et al., 1992, Duleba et al., 1992, Sauvestre, 2014).
Given that most patients do not present with symptoms of metastasis and placental pathology is not done routinely, the actual prevalence of IC is likely higher than currently estimated. Furthermore, diagnosis of IC requires methodical review by a specialized pathologist; lesions that can appear to be simple infarcts are often not examined meticulously, which could lead to missed cases of IC (Kanehira et al., 2013, Duleba et al., 1992, Jacques et al., 1998, Lee and Cho, 2019, Sebire et al., 2005). Survival patterns are overall reassuring for patients affected by IC, but with there being a possibility of undiagnosed cases of IC, the actual morbidity and mortality of the disease cannot be estimated. Herein, we present the case of an asymptomatic 21-year-old patient in whom IC was discovered after cesarean delivery for non-reassuring fetal heart tracing remote from delivery. We hope to add to the existing body of literature with this case report and discuss the available recommendations for diagnostic evaluation and surveillance of this rare disease, as well as address the implications of routine placental pathologic analysis as a means of reducing morbidity and mortality from this rare disease.
2. Presentation
2.1 Initial presentation and management
A 21-year-old gravida 1 para 0–0–0–0 at 38 weeks and 4 days gestational age presented to the Labor and Delivery triage unit with pre-labor rupture of membranes and was admitted for induction of labor. Her pregnancy was complicated by polyhydramnios, group B streptococcus colonization, and obesity with a BMI of 32 kg/m2. The placenta was noted to be normal in appearance at her anatomic scan with no further mentions in subsequent ultrasounds. She had no other known medical, surgical, or family history. Her labor induction was performed with intravenous oxytocin. About 12 hours into her labor course, she had an intermittent category II fetal heart tracing that responded well to intrauterine resuscitation. Approximately 24 hours into her induction, a category II tracing was again noted with prolonged late decelerations not responsive to resuscitation, and the decision was made to proceed with primary cesarean delivery. She underwent an uncomplicated primary low transverse cesarean section at 38 weeks and 5 days. There was delivery of a viable male infant weighing 3108 g with 1- and 5-minute APGAR scores of 8 and 9, respectively. The placenta was sent for pathologic review due to the non-reassuring fetal heart tracing necessitating cesarean delivery. She had an unremarkable postoperative course and was discharged home on postoperative day 2.
2.2. Pathology and disposition
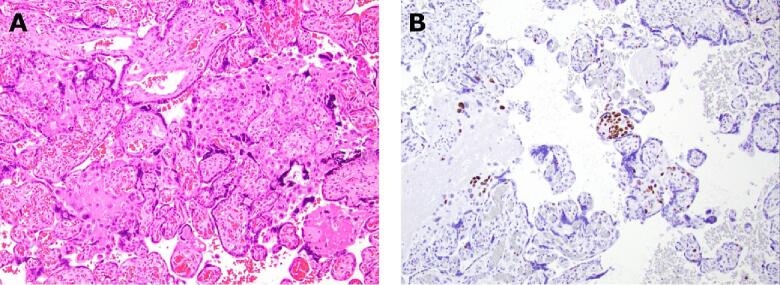
Gross examination of the placenta (465 g, 25th to 50th percentile) on first review showed no obvious focal lesions. Histopathologic analysis found maternal vascular malperfusion lesions consistent with early infarctions, fetal vascular malperfusion lesions, chorangiosis, acute subchorionitis, and atypical extravillous and villous trophoblasts suspicious for choriocarcinoma in situ (Fig. 1A). Immunohistochemical (IHC) staining with Ki-67, a marker for cell proliferation, showed positive staining in more than 80% of these atypical extravillous and villous trophoblasts (Fig. 1B). The cells of interest were negative when stained with inhibin. These sections of the placenta were reviewed by two outside pathologists who supported the concern for IC and recommended further sampling of the placenta. Extensive additional sections were taken; however, no additional foci of atypical cells were identified. She was referred to Gynecologic Oncology and the departmental multidisciplinary tumor board recommended active surveillance with serial beta hCGs, completed monthly. To this point, all her beta hCG values have remained <1mIU/mL.
Fig. 1.
Histopathological findings from the placenta. (A), Hematoxylin and eosin (H&E) shows the isolated focus of atypical trophoblasts within multiple villi, as well as pushing out into the extravillous spaces (10 × ). (B), Ki-67 staining in more than 80% of the atypical trophoblasts (10 × ).
3. Discussion
Herein is a case of IC identified in the placenta of a primigravida who had a cesarean section for non-reassuring fetal heart tracing. The placenta was grossly normal in appearance, but microscopic examination revealed a single focus of abnormal trophoblasts. The extension and infiltration of these abnormal trophoblasts from the villi into the extravillous space raised concern for IC over isolated atypical trophoblasts that have been previously described within intervillous trophoblastic islands. Choriocarcinoma has been associated with a Ki-67 index of greater than 50%, while atypical trophoblasts isolated to intervillous trophoblastic islands have been associated with Ki-67 indices of <10% (Shih and Kurman, 1998). In order to characterize the atypical trophoblasts in this case, a Ki-67 IHC stain was applied to determine a proliferation index. In this case, more than 80% of the atypical cells stained with Ki-67, indicating a high rate of cellular proliferation consistent with a diagnosis of choriocarcinoma. Beta hCG has been monitored monthly since delivery and has remained <1 IU/mL, with no clinical evidence of disease progression noted.
The characteristics of cases presented in the literature are heterogeneous; while most case reports present uncomplicated term pregnancies, others describe intrauterine fetal demise both preterm and at term, patients with history of molar pregnancy, and several multi-fetal gestations (Jiao et al., 2016, Caldas et al., 2017, Christopherson et al., 1992, Duleba et al., 1992, Jacques et al., 1998). The indication for placental pathology assessment of term placentas was most commonly for non-reassuring fetal status intrapartum. If there was a visible lesion on gross examination of the placenta, the most common appearance was that of an infarct or hemorrhage (Jiao et al., 2016, Lele et al., 1999, Kanehira et al., 2013, Lee and Cho, 2019, Sebire et al., 2005). Given that most patients are asymptomatic and appear to have disease resolution without intervention or apparent impact on future pregnancies, it seems likely that if placental pathology were done routinely, more cases of IC may be identified (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Trask et al., 1994, Jacques et al., 1998). Thus, it stands to reason that the current estimated prevalence of IC based on existing literature is an underestimate.
The clinical significance of the underdiagnosis of IC is unclear; without more data, the actual morbidity and mortality attributable to this rare disease are unknown. Further, the impact of IC on future fertility and pregnancy outcomes cannot be fully elucidated with the current paucity of information. Several case reports have presented instances of IC discovered after stillbirth – the nature of this possible association is unclear with scant numbers of patient cases available for analysis (Jiao et al., 2016, Trask et al., 1994). With the paucity of available information, no firm statement can be made on surveillance for subsequent pregnancies, such as with fetal non-stress tests or frequency thereof. Other case reports have presented patients with personal history of molar pregnancies (Duleba et al., 1992, Jacques et al., 1998); studying why multiple expressions of GTD would occur in one patient could possibly provide clues to better understanding why certain patients are at higher risk for developing GTD. And with regard to imaging, there is little information available about prenatal diagnosis of IC by ultrasound; to our knowledge there are only a couple of reports of an identifiable placental lesion being ultimately diagnosed as IC, so firm statements cannot yet be made on the appropriate prenatal radiologic diagnosis and evaluation of IC (Jiao et al., 2016). The most obvious way to detect more cases of IC would be to send every placenta for Pathology review. However, this could strain already overburdened Pathology departments and thus create delays in diagnosis. Further, the histologic diagnosis of IC can be challenging (Medeiros et al., 2008, Sauvestre, 2014), and the impact of possible false diagnoses could lead to undue psychological burden on patients and interventions that could be costly or even toxic, if chemotherapy were given. The possible costs to patients and the medical system as a whole with universal placental pathology are unknown and could be significant.
Jiao et al. have provided recommendations for the management of IC, proposing indications for histological placental examination and suggested guidelines for surveillance and treatment (Jiao et al., 2016). They suggest that placental pathology should be obtained for patients with history of vaginal bleeding in pregnancy or respiratory or neurologic symptoms, gross abnormality of the placenta on routine inspection, adverse fetal or neonatal outcomes, and history of previous GTD. After a diagnosis of IC, they recommend whole-body imaging to investigate metastatic disease, infant urinary hCG, and lifelong serum hCG surveillance. Chemotherapy is advised for patients with metastases or elevated hCG and is stratified between single-agent therapy with methotrexate and multi-agent therapy with EMA/CO based on World Health Organization (WHO) score greater or less than 7. On review of the available literature, the frequency of serum hCG measurement for postpartum surveillance has not directly been commented on; several case reports mention monitoring serum hCG after delivery until normalization, but it is not clear how long or how frequently labs were drawn. And while Jiao et al. recommend lifelong hCG surveillance, they do not make a specific recommendation for frequency (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Jacques et al., 1998). Similarly, there is insufficient information to guide surveillance of neonates if an initial BhCG is <1; further study is indicated to better characterize appropriate surveillance in neonates without evidence of disease.
The available literature on IC consistently reports elevated hCG levels in patients identified to have metastases (Jiao et al., 2016, Caldas et al., 2017, Lele et al., 1999, Liu and Guo, 2006, Sauvestre, 2014). This calls into question the recommendation for routine post-diagnostic evaluation with whole body imaging and lifelong hCG surveillance; with IC’s good prognosis and the predictable nature of its associated metastases, serial serum hCG measurement alone could be a reasonable alternative to a more extensive diagnostic evaluation. If hCG is monitored after diagnosis and found to be normal (<1 IU/mL), perhaps patients could be spared radiation exposure, cost of imaging, and interruption to life of additional testing appointments, with a reasonable presumption of absence of metastases. This manner of approach would relieve some of the burden of the disease on both the patients and the health care system in general and would be easier to facilitate for patients with limited access to healthcare. In the same vein, proposing a duration of time for hCG surveillance after diagnosis of IC, rather than lifelong surveillance, would decrease health-care related costs, burdens, and interruptions to patients’ lives. Given IC’s good prognosis and the likelihood that cases are going undiagnosed without an obvious impact on maternal morbidity and mortality, further data should be collected to illuminate the most appropriate diagnostic and surveillance protocol, as well as to evaluate if postpartum hCG surveillance is warranted at all once the hCG level has returned to normal.
4. Conclusion
IC is a rare manifestation of GTD and carries an excellent prognosis if metastatic disease is treated promptly with modern multi-agent chemotherapy. Most of the limited information about this disease is drawn from case reports; accordingly, the true nature and impact of this disease on maternal morbidity and mortality is unknown. There is currently no consensus on which patients should be screened for IC and on the appropriate management and surveillance. With the excellent prognosis of IC and the unlikelihood of metastases without elevated hCG, we suggest that surveillance and initial diagnostic evaluation may not need to be as intensive as previously thought. However, more research is needed to better guide recommendations on which patients should be screened, how to evaluate and monitor patients given the diagnosis, and what interventions, if any, should occur in subsequent pregnancies.
Author contributions:
LM conception, manuscript writing, editing. NZ manuscript writing, editing. KJ manuscript writing, pathology review. LM pathology review. AS conception, manuscript editing, supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
"Written informed consent was obtained from the patient for publication of this case report and accompanying images. IRB exemption was received from the University of Rochester Research Subjects Review Board. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request".
References
- Aonahata M., Masuzawa Y., Tsutsui Y. A case of intraplacental choriocarcinoma associated with placental hemangioma. Pathol. Int. 1998;48(11):897–901. doi: 10.1111/j.1440-1827.1998.tb03858. [DOI] [PubMed] [Google Scholar]
- Caldas R.F., Oliveira P., Rodrigues C., Reis I., Scigliano H., Nogueira R., Araujo C., Ferreira S. Intraplacental choriocarcinoma: rare or underdiagnosed? Report of 2 cases after an incomplete miscarriage and a preterm spontaneous vaginal delivery. Case Rep. Med. 2017;2017 doi: 10.1155/2017/7892980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson W.A., Kanbour A., Szulman A.E. Choriocarcinoma in a term placenta with maternal metastases. Gynecol. Oncol. 1992;46(2):239–245. doi: 10.1016/0090-8258(92)90264-j. [DOI] [PubMed] [Google Scholar]
- Duleba A.J., Miller D., Taylor G., Effer S. Expectant management of choriocarcinoma limited to placenta. Gynecol. Oncol. 1992;44(3):277–280. doi: 10.1016/0090-8258(92)90057-p. [DOI] [PubMed] [Google Scholar]
- Jacques S.M., Qureshi F., Doss B.J., Munkarah A. Intraplacental choriocarcinoma associated with viable pregnancy: pathologic features and implications for the mother and infant. Pediatr. Dev. Pathol. 1998;1(5):380–387. doi: 10.1007/s100249900052. [DOI] [PubMed] [Google Scholar]
- Jiao L., Ghorani E., Sebire N.J., Seckl M.J. Intraplacental choriocarcinoma: systematic review and management guidance. Gynecol. Oncol. 2016;141(3):624–631. doi: 10.1016/j.ygyno.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Kanehira K., Starostik P., Kasznica J., Khoury T. Primary intraplacental gestational choriocarcinoma: histologic and genetic analyses. Int. J. Gynecol. Pathol. 2013;32(1):71–75. doi: 10.1097/PGP.0b013e3182566552. [DOI] [PubMed] [Google Scholar]
- Lee E., Cho H. A case of intraplacental choriocarcinoma with pulmonary metastasis. Case Rep. Oncol. 2019;12(3):802–806. doi: 10.1159/000503816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele S.M., Crowder S.E., Grafe M.R. Asymptomatic intraplacental choriocarcinoma diagnosed on routine placental examination. J. Perinatol. 1999;19(3):244–247. doi: 10.1038/sj.jp.7200140. [DOI] [PubMed] [Google Scholar]
- Liu J., Guo L. Intraplacental choriocarcinoma in a term placenta with both maternal and infantile metastases: a case report and review of the literature. Gynecol. Oncol. 2006;103(3):1147–1151. doi: 10.1016/j.ygyno.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Medeiros F., Callahan M.J., Elvin J.A., Dorfman D.M., Berkowitz R.S., Quade B.J. Intraplacental choriocarcinoma arising in a second trimester placenta with partial hydatidiform mole. Int. J. Gynecol. Pathol. 2008;27(2):247–251. doi: 10.1097/PGP.0b013e3181577dc8. [DOI] [PubMed] [Google Scholar]
- Sauvestre F., et al. Incidental intraplacental gestational choriocarcinoma on a full-term placenta. Ann. Pathol. 2014;34(2):119–123. doi: 10.1016/j.annpat.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Sebire N.J., Lindsay I., Fisher R.A., Seckl M.J. Intraplacental choriocarcinoma: experience from a tertiary referral center and relationship with infantile choriocarcinoma. Fetal Pediatr. Pathol. 2005;24(1):21–29. doi: 10.1080/15227950590961180. [DOI] [PubMed] [Google Scholar]
- Shih I.M., Kurman R.J. Ki-67 labeling index in the differential diagnosis of exaggerated placental site, placental site trophoblastic tumor, and choriocarcinoma: a double immunohistochemical staining technique using Ki-67 and Mel-CAM antibodies. Hum. Pathol. 1998;29(1):27–33. doi: 10.1016/s0046-8177(98)90386-0. [DOI] [PubMed] [Google Scholar]
- Trask C., Lage J.M., Roberts D.J. A second case of “chorangiocarcinoma” presenting in a term asymptomatic twin pregnancy: choriocarcinoma in situ with associated villous vascular proliferation. Int. J. Gynecol. Pathol. 1994;13(1):87–91. doi: 10.1097/00004347-199401000-00011. [DOI] [PubMed] [Google Scholar]