Abstract
We explored the role of urokinase and tissue-type plasminogen activators (uPA and tPA), as well as the uPA receptor (uPAR; CD87) in mouse severe malaria (SM), using genetically deficient (−/−) mice. The mortality resulting from Plasmodium berghei ANKA infection was delayed in uPA−/− and uPAR−/− mice but was similar to that of the wild type (+/+) in tPA−/− mice. Parasitemia levels were similar in uPA−/−, uPAR−/−, and +/+ mice. Production of tumor necrosis factor, as judged from the plasma level and the mRNA levels in brain and lung, was markedly increased by infection in both +/+ and uPAR−/− mice. Breakdown of the blood-brain barrier, as evidenced by the leakage of Evans Blue, was similar in +/+ and uPAR−/− mice. SM was associated with a profound thrombocytopenia, which was attenuated in uPA−/− and uPAR−/− mice. Administration of aprotinin, a plasmin antagonist, also delayed mortality and attenuated thrombocytopenia. Platelet trapping in cerebral venules or alveolar capillaries was evident in +/+ mice but absent in uPAR−/− mice. In contrast, macrophage sequestration in cerebral venules or alveolar capillaries was evident in both +/+ and uPAR−/− mice. Polymorphonuclear leukocyte sequestration in alveolar capillaries was similar in +/+ and uPAR−/− mice. These results demonstrate that the uPAR deficiency attenuates the severity of SM, probably by its important role in platelet kinetics and trapping. These results therefore suggest that platelet sequestration contributes to the pathogenesis of SM.
In mice, infection by Plasmodium bergei ANKA leads, in susceptible mouse strains, to a lethal syndrome, commonly known as cerebral or severe malaria (SM), in which mice die 7 to 9 days after infection in a state of coma associated with neurological manifestations; (51; reviewed in reference 49). Prominent in this syndrome are a breakdown of the blood-brain barrier, microhemorrhages, and sequestration of macrophages in the cortical venules (6, 12, 46). In addition, there is a sequestration of macrophages, polymorphonuclear neutrophils (PMNs), parasitized red blood cells (pRBC), and platelets in other organs, notably the lung (12, 46). Hence, since this syndrome is not limited to the brain, it is also referred to as SM. Various studies using antibodies, recombinant cytokines or knockout mice have shown that the secretion of tumor necrosis factor (TNF) is an important effector of the mortality of SM (15, 20, 43).
In humans, acute phase of malaria infection can also result in severe complications, leading eventually to death, with symptoms ranging from respiratory distress up to coma (reviewed in references 49 and 50). Especially in children, Plasmodium falciparum infection can manifest itself as a coma, associated with neurological dysfunctions resembling ANKA infection in susceptible mice. The pathogenesis of coma is poorly understood and might be different in humans and mice since in humans cerebral dysfunction is associated with the sequestration of RBC or pRBC within the brain capillaries, while during ANKA-induced coma in mice, pRBC sequestration is not evident in the brain but is important in the lungs (7, 12, 28). Coma occurring in mice is generally attributed to the sequestration of macrophages within the cortical venules, an alteration also observed during human SM (36). The sequestration of pRBC within the microcirculation might be critical for the severity of the disease in either species since they might release a glycosylphosphatidylinositol toxin (44) or, alternatively, they might obstruct the capillary circulation (28). Breakdown of the blood-brain barrier is evident during SM, but its pathogenesis or role in the coma in either species is not established. Microhemorrhages have also been reported in the brain and elsewhere in both human and mouse SM (46). SM, even with predominant neurological symptoms, is a systemic disease, with associated dysfunctions of the major organs. Involvement of the lung is evident during “cerebral malaria” in humans as in mice and is associated with the sequestration of macrophages, neutrophils, platelets, and pRBC in the alveolar capillaries (5, 7, 10, 12, 28). Thrombocytopenia, due to a reduced platelet life span is common to SM in both mice and humans and is not associated with coagulopathy (21, 45, 49). Finally, the essential role of TNF in the mortality appears to be similar in both species since a strong correlation has been documented between TNF production and the lethality of malaria in humans (22, 31).
TNF production is induced by an immune response elicited by the presence of the parasite in the blood (19). Indeed, depletion of CD4 T lymphocytes or administration of cyclosporin prevents the acute mortality of ANKA infection in mice, apparently by decreasing TNF production (17, 19, 49). TNF might be responsible for some of the manifestations of SM such as hypoglycemia and the increase of the expression of adhesion molecules. Indeed, TNF induces an upregulation of adhesion molecules on endothelia, notably of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) (26, 27), which might increase the adhesion of leukocytes and other cells and thereby disturb the microcirculation in the brain and other organs. This pathogenic hypothesis is supported by the delayed mortality seen in mice treated with anti-CD11a monoclonal antibody (MAb; LFA-1, a β2-integrin determinant) or in ICAM-1-deficient mice (11, 12, 18).
β2 integrin is expressed on leukocytes and is a ligand for ICAM-1, which is widely distributed. Recent evidence indicates that the function of β2 and other integrins is modulated by the urokinase plasminogen activator receptor (uPAR; CD87) (reviewed in reference 4). The uPAR is a 55-kDa glycosylphosphatidylinositol (GPI)-linked surface receptor known to bind urokinase plasminogen activator (uPA), plasminogen activator inhibitor 1 (PAI-1), and vitronectin. Binding of uPA or PAI-1 to uPAR is believed to influence the conformation of the uPAR and its interaction with the integrin. Thus, the binding of uPA to the uPAR might, on one hand, increase the generation of plasmin on the cell surface and, on the other hand, increase the affinity or function of the β2 integrin. In vivo, the traffic of PMNs in the mouse peritoneum has been shown to be delayed in uPAR−/− mice (32).
In the present report, we took advantage of mice genetically deficient in the uPA or uPAR genes to explore the contribution of this system to the mortality and sequestration of various cell types induced by ANKA infection. The results indicate that the severity of malaria is attenuated in uPA- and uPAR-deficient mice.
MATERIALS AND METHODS
Mice.
uPA−/−, tPA−/−, and uPAR−/− genetically deficient mice (uPAR−/−), isolated on the C57BL/6 × 129 background (3, 8), were obtained from Carmeliet (Leuven, Belgium) and bred in our animal house. Wild-type (+/+) controls were either C57BL/6J or (C57BL/6 × 129)F1.
P. berghei ANKA infection.
The ANKA strain has been passaged in rodents for decades (1). Mice were injected intraperitoneally (i.p.) with 106 pRBC as described previously (19). More recently, mice were also infected by an intravenous (i.v.) injection of 5 × 104 pRBC. This latter type of infection, which bypasses the peritoneal macrophages, results in a parasitemia and mortality similar to the i.p. infection but with less variability.
Treatment with aprotinin.
Aprotinin was purchased from Sigma Chemical (St. Louis, Mo.), and 500 μg was injected i.v.
Blood collection.
Blood (0.015 ml) was isolated from the retroorbital plexus of ethrane-anesthetized mice by using heparinized capillaries and then diluted in EDTA (1%, final) in accordance with the Swiss national guidelines. Blood elements were counted in a cell counter (Casy 1; Schärfe, Reutlingen, Germany). To count the parasitemia, a smear was prepared, dried, fixed in methanol, and stained with May-Grünwald Giemsa.
Light and electron microscopy.
Mice were sacrificed via an i.p. injection of pentobarbital sodium (Nembutal). The aorta was cut, the thorax opened, and the lung was fixed by intratracheal instillation of glutaraldehyde and processed for embedding in Epon. A section was taken in the left lobe, across the hilus. Thin sections were prepared from two blocks taken from the parenchyma per mouse. Thin sections were examined with a Philips 400 electron microscope at 60 kV. Cells within alveolar capillaries were examined, and the RBC, platelets, and PMNs were counted. RBC were used as a neutral indicator of blood stasis, and sequestration was evaluated by determining the platelet/RBC and PMN/RBC ratios.
Assessment of vascular leak.
Mice (three wild type and three mutant) were injected i.v. with 0.2 ml of 1% Evans Blue on day 6, shortly before the death of the wild-type mice. After 1 h, mice were sacrificed, and the staining of brain sections was assessed as an indicator of increased capillary permeability. The vascular leak was also assessed by the injection of labeled fibrinogen (see below).
Fibrinogen half-life and distribution.
Human 125I-labeled fibrinogen was purchased from Amersham (Little Chalfont, United Kingdom). Ca. 105 cpm were injected i.v. To evaluate fibrinogen turnover, 0.05 ml of blood was withdrawn form the retroorbital sinus in a calibrated and heparinized capillary and then counted in a gamma counter (Packard).
Platelet isolation and 51Cr labeling.
Donor mice were pretreated with heparin (5 U, given intramuscularly), and blood was withdrawn from the retroorbital plexus, using heparinized capillaries, and collected in EDTA (1% final concentration in NaCl [pH 7.0]) and separated by differential centrifugations (30). The preparation was examined in a hemocytometer to evaluate leukocyte or RBC contaminants, which were always below 10−4. Platelets (4 × 109 to 10 × 109) were incubated at room temperature in ACD, supplemented with mouse plasma (1%) and with 51Cr (0.1 mCi; The Radiochemical Center, Amersham, United Kingdom) for 2 h. Platelets were then washed in ACD and counted. In different experiments, the labeling obtained ranged from 0.2 to 1 cpm/platelet, depending upon the age of the 51Cr batch. Platelets were diluted with NaCl in order to inject (i.v.) 5 × 104 to 10 × 104 cpm in 0.1 ml in the retroorbital sinus of the recipient mice.
Evaluation of TNF production.
TNF mRNA levels were evaluated on Northern blots prepared from the lung, brain, and spleen RNA as described previously (37). Quantification of TNF mRNA was performed by PhosphorImager analysis (Molecular Dynamics, Inc., Sunnywale, Calif.), and the amount was normalized to the 18S RNA level (1). The TNF serum level was assayed by using the InnoBasics 10m TNF ELISA kit (Innogenetics), which detects both free and receptor-bound TNF with a sensitivity of 12 pg/ml.
Immunohistochemistry.
Frozen tissue sections were immunostained as described elsewhere. Briefly, 5-μm sections were incubated overnight at 4°C with a rat MAb directed against murine GPIIb/IIIa (CD41, CD61, and MWReg30 immunoglobulin G1 [IgG1]; Pharmingen, San Diego, Calif.) (34). After a washing, the sections were incubated 1 h at room temperature with biotinylated goat anti-rat IgG (Southern Biotechnology, Bioreba, Reinach, Switzerland) followed by the addition of horseradish peroxidase-avidin. A color reaction was obtained with the addition of AEC substrate-chromogen (Dako, Zug, Switzerland). Samples were analyzed on a Zeiss Axiophot microscope using SAMBA quantitative image analysis software (Faculty of Medicine, Université de la Méditerranée). Results were expressed as arbitrary units (AU) of stained surface per square millimeter of lumen surface, as determined by planimetry. Leukocytes were counted as nucleated elements (stained with hemalun) within an identified blood vessel.
Statistical evaluation.
Significance analysis of survival curves was determined by using the Fisher's exact test. Groups of values were compared using the nonparametric Mann-Whitney U test (29).
RESULTS
Effect of uPA, tPA, and uPAR on the mortality associated with SM.
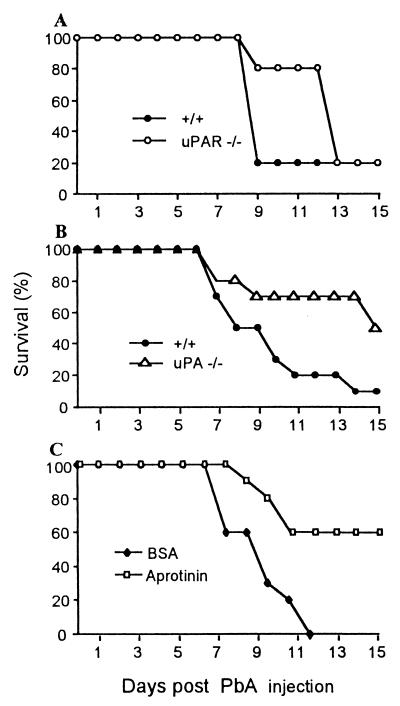
Infection of +/+ mice resulted in an acute mortality, reaching frequently 90%, by 8 to 9 days after injection as reported previously (15). Mortality was significantly delayed in uPA−/− and in uPAR−/− mice (Fig. 1). In tPA−/− mice, mortality was similar to that observed in +/+ recipients (not shown). These results indicate that the uPA-uPAR system contributes to mortality induced by the malarial infection. These genetic deficiencies had no influence on the mortality observed 15 days after infection, which is due to a severe anemia.
FIG. 1.
Survival of P. berghei ANKA (PbA)-infected mice. (A) Survival of +/+ and uPAR−/− mice after infection. Results were pooled from three experiments (n = 20, P < 0.04). (B) Survival of +/+ and uPA−/− mice after infection (n = 10, P < 0.05). (C) Survival of infected +/+ mice treated from day 6 with aprotinin or BSA as a control at 500 μg/day (difference between the groups: n = 10, P < 0.05).
Effect of uPAR on parasitemia and thrombocytopenia associated with SM.
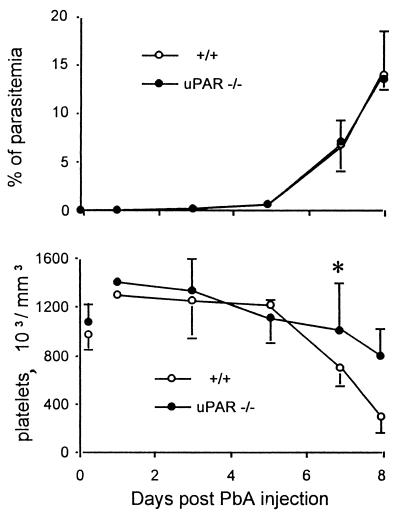
Parasitemia was similar in +/+, uPAR−/− (Fig. 2) and uPA−/− mice (not shown), suggesting that this system has no major influence on the early diffusion of the parasite and also that parasitemia is not closely related to mortality. Infection of +/+ mice induced a thrombocytopenia which became profound when mice died, ca. day 7 to 8 days after infection (Fig. 2). In uPAR mice, thrombocytopenia was markedly attenuated (Fig. 2). Thrombocytopenia was also significantly (P < 0.05) attenuated in uPA−/− mice; on day 8, the platelet counts were 6.5 (±2.4) × 105 and 9.5 (±1.7) × 105/μl for infected +/+ and uPA−/− mice, respectively (mean ± the standard deviation [SD]; n = 7).
FIG. 2.
Parasitemia and platelet counts in P. berghei ANKA (PbA)-infected +/+ and uPAR−/− mice. (Top) Mean percentages of pRBC observed in three experiments involving 15 mice. (Bottom) Mean platelet counts observed in an experiment involving eight mice. On day 8, there were only two survivors in infected +/+ mice. ∗, P < 0.05. The vertical bars indicate the SD values.
Effect of aprotinin on mortality and thrombocytopenia associated with SM.
uPA and uPAR are likely to contribute to enhance the activity of plasmin, a protease of broad specificity. Plasmin can be neutralized by the naturally occurring antagonist aprotinin (42). The administration of aprotinin, started on day 6, also decreased the mortality associated with the P. berghei ANKA infection (Fig. 1C). ANKA infection-induced thrombocytopenia was also significantly (P < 0.05) attenuated by aprotinin; the platelet counts were of 5.1 (±1.8) × 105 and 7.2 (±1.5) × 105/μl for bovine serum albumin (BSA)- and aprotinin-treated mice, respectively (n = 10).
Effect of malarial infection and uPAR on platelet kinetics.
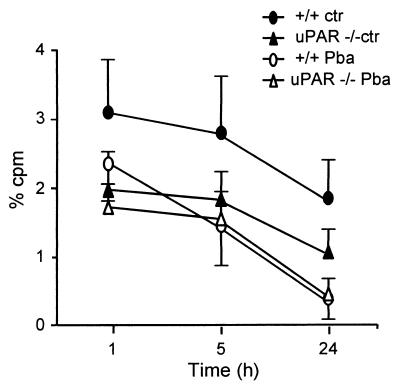
The thrombocytopenia induced by ANKA infection is due in a large part to a decrease in platelet survival (21). We transferred labeled platelets from +/+ or uPAR−/− donors to identical recipients, either normal or infected, and investigated their survival (Fig. 3). In +/+ recipients, the survival of +/+ platelets was markedly reduced by the infection, as reported previously (21). In contrast, the survival of uPAR−/− platelets in a uPAR−/− host was only slightly reduced by the infection. However, the platelet life span in noninfected mice was markedly decreased in UPAR−/− mice, an observation described in more detail elsewhere (38). Comparison between infected +/+ and uPAR−/− mice showed only a slight reduction in life span with platelets from uPAR−/− mice, indicating that the infection in uPAR−/− mice does not decrease platelet life span much more than is already the case in noninfected uPAR−/− mice. This raises the possibility that ANKA infection and uPAR deficiency decrease platelet life span by similar, nonadditive mechanisms.
FIG. 3.
Effect of P. berghei ANKA (PbA) infection on platelet survival. 51Cr-labeled platelets from +/+ or uPAR−/− donors were injected into +/+ or uPAR−/− recipients that were either normal or ANKA infected 7 days earlier. Results are the mean (± the SD; n = 7) cpm in 0.05 ml of blood, expressed as the percent injected cpm at various times after platelet injection. The +/+ mice were significantly (P < 0.05) different from all other groups at all time points; the uPAR−/− controls were different from uPAR−/− mice infected at 24 h; the +/+ infected mice were different from the uPAR−/− mice infected at 1 h.
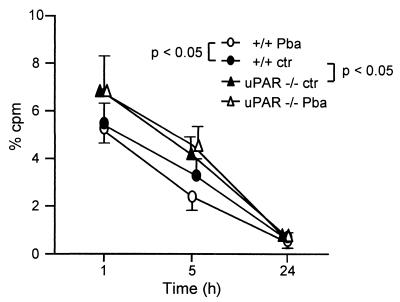
Effect of malarial infection and uPAR on fibrinogen clearance.
We examined the role of the uPAR in the clearance of labeled fibrinogen in normal or infected mice. Malarial infection slightly, but significantly, reduced the survival of labeled fibrinogen in +/+ mice, while in uPAR−/− mice the survival of fibrinogen was not modified by infection (Fig. 4). In noninfected mice the clearance of fibrinogen was significantly prolonged in uPAR−/− mice compared to +/+ mice (Fig. 4).
FIG. 4.
Survival of radiolabeled fibrinogen. 125I-labeled fibrinogen was injected into normal mice or into mice infected with P. berghei ANKA (PbA) 7 days previously. Results are the mean (± sd; n = 7) cpm/0.05 ml of blood and are expressed as the percent injected cpm. Groups significantly (P < 0.05) different at one or more time points are indicated.
The distribution of labeled fibrinogen was also examined in mice sacrificed on day 7 after infection and 24 h after fibrinogen injection. Infection increased the counts per minute (cpm) in liver, spleen, lungs, and brain in +/+ mice and, to a lesser extent, in uPAR−/− mice (not shown).
Effect of uPAR and malarial infection on spleen and lung weight.
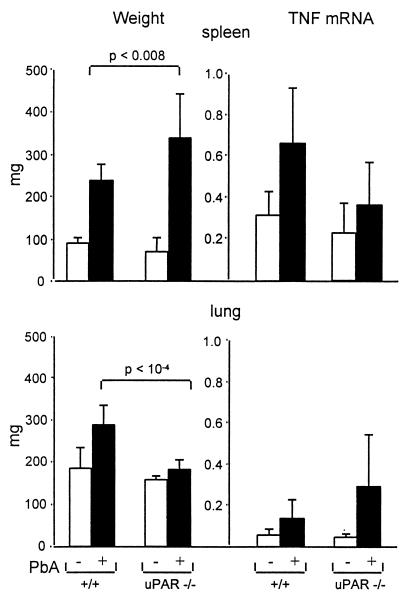
Malarial infection led to a massive lymphoproliferative syndrome, resulting in a two- to threefold increase in the size of the lymphoid organs after 7 days. As seen in Fig. 5, the increase was more pronounced in uPAR−/− mice than in +/+ mice, indicating that the attenuation of the disease in uPAR−/− mice is not due to a decrease of lymphoproliferation. The lung weight was significantly increased by infection in +/+ mice but not in uPAR−/− mice, suggesting that uPAR deficiency attenuates the pulmonary component of SM.
FIG. 5.
Weight and TNF mRNA levels of the lung and spleen. Organs were collected on day 8 after infection. (Left) Weights of the spleen (top) and lung (bottom). (Right) TNF mRNA levels expressed in AU as evaluated on Northern blots made with the spleen and lung RNA. The results are mean (+SD; n = 5) values. PbA, P. berghei ANKA.
Effect of uPAR on the TNF mRNA levels.
The TNF mRNA levels were examined in lung, brain, and spleen tissue from normal or infected +/+ and uPAR−/− mice. As seen in Fig. 5, the mRNAs levels were markedly increased by the P. berghei ANKA infection in the lung and spleen, without a significant difference between infected +/+ and uPAR−/− mice. The values are expressed as the amount of TNF mRNA per microgram of RNA and do not take into account the increase in size of the organs, i.e., they underestimate the amount of mRNA/organ or mRNA/mouse. In the brain, the levels were about fivefold lower than in the spleen, and there was no difference between +/+ and uPAR−/− animals (not shown).
Effect of uPAR on the breakdown of the blood-brain and blood-lung barriers (Evans Blue).
SM was associated with an increase of the leakage of Evans Blue, which was especially evident in the brain and lung. Under these present conditions, we did not detect a marked difference between infected +/+ and uPAR−/− mice (data not shown). Localization of labeled fibrinogen was also examined (see above), and localization was similar in infected +/+ and uPAR−/− mice (not shown).
Effect of uPAR on cell sequestration in alveolar capillaries.
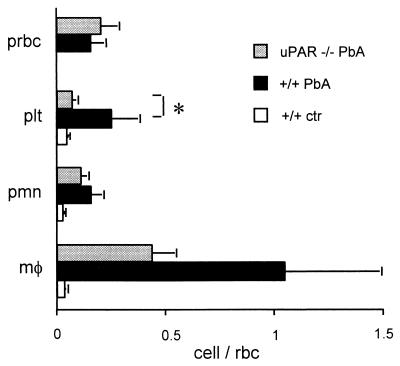
An involvement of the lung was made evident by the lung weight, which was significantly increased by the infection in +/+ mice (Fig. 5). As seen by light microscopy, the infection resulted in a thickening of the alveolar septa as reported previously (46), which was evident in both infected +/+ and uPAR−/− mice (data not shown). The variety of the cells trapped was examined in more detail by semiquantitative electron microscopy (Fig. 6). Infection increased the number of macrophages, PMNs, pRBC, and platelets within the alveolar capillaries. In uPAR−/− mice, platelet trapping was markedly decreased, while PMN and macrophage sequestration was slightly, but not significantly, decreased. The percentage of pRBC in the alveolar capillaries was increased by ca. 25%, compared to 5 to 10% in the orbital sinus (Fig. 2 and 6), suggesting that the adherence of pRBC in the alveolar capillaries is also increased. This pRBC sequestration was similar in +/+ and uPAR−/− mice (Fig. 6).
FIG. 6.
Cells trapping in the alveolar capillaries. Results are the mean (+SD) numbers of cells seen in alveolar capillaries from five mice, which were sacrificed on day 7 after infection. Cells were counted under an electron microscope and are expressed as a ratio with RBC used as an indicator of blood stasis. prbc, pRBC; plt, platelets; pmn, PMNs; mφ, macrophages; rbc, RBC. Cells were identified by electron microscopy, and the results are the mean (+SD) of a ratio with RBC (n = 5). ∗, P < 0.05. PbA, P. berghei ANKA.
Platelet sequestration in the brain venules: evaluation by immunohistochemistry.
In the brain, SM induced a sequestration of macrophages in the cortical venules, which was evident by light microscopy on sections stained with hematoxylin and eosin. Macrophage sequestration was seen in both +/+ and uPAR−/− infected mice.
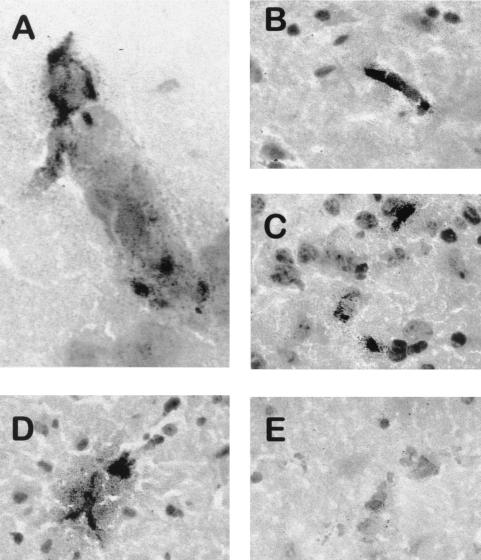
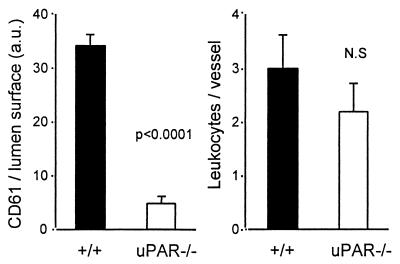
Frozen brain sections were stained with a rat anti-mouse GPIIb/IIIa MAb to evaluate the extent of platelet sequestration. In +/+ mice, various patterns of platelet accumulation were observed in brain venules (Fig. 7). Platelets appeared between sequestered leukocytes (Fig. 7A), in a formation filling the lumen (Fig. 7B). Platelets were also found to obstruct the vascular lumen in the absence of leukocytes (Fig. 7C). In addition, a fine granular pattern of GPIIb/IIIa staining was observed in perivascular areas (Fig. 7D). In sharp contrast, platelets were not detectable in ANKA-infected uPAR−/− mice (Fig. 7E). These data were analyzed by quantitative image analysis. As shown in Fig. 8, the intensity of GPIIb/IIIa staining normalized for the surface of individual vessels was significantly lower in uPAR−/− than in +/+ mice (4.3 ± 1.2 versus 33.3 ± 3.7 AU, respectively; n = 37 vessels analyzed in four distinct mice; Mann-Whitney, P < 0.0001). This staining pattern of brain vessels from noninfected mice was similar to those of infected uPAR−/− mice (data not shown). In contrast, sequestration of mononuclear leukocytes was not significantly reduced in infected uPAR−/− mice compared to +/+ mice (Fig. 8).
FIG. 7.
Identification of platelets in the cerebral venules of +/+ mice but not of uPAR−/− mice. Brains from P. berghei ANKA-infected +/+ mice (A to D) and uPAR−/− mice (E) were isolated and frozen on day 7 of infection. Sections were stained with anti-GPIIb/IIIa MAb. (A) Venule with sequestered macrophages and platelets stained in red. (B and C) Platelet material is evident in blood vessels of various sizes. (D) Immunoreactive material has diffused from a blood vessel. (E) A blood vessel contains macrophages but no platelets. Magnifications: A, ×1,000; B to E, ×400.
FIG. 8.
Amount of GPIIb/IIIa (CD61)-positive elements and leukocytes in the cortical venules detected by image analysis. The results are the mean + the SD (n = 4) of the values observed. a.u., AU.
DISCUSSION
In the present report we provide evidence that uPA, in relation to the uPAR (CD87), contributes to the mortality of P. berghei ANKA infection, probably by its influence on platelet sequestration.
The uPA-uPAR deficiency, as well as the treatment with aprotinin, might modify several systems implicated in the generation of the lethal syndrome, such as the immune response, the parasitemia, the generation of plasmin, and cell adhesion. There is no indication that uPAR deficiency decreased the immune response. The increase in the size of the lymphoid organs, induced by the infection, was not decreased in uPAR−/− mice (and was, in fact, significantly increased [see Fig. 5]), and the increase in TNF production was similar in +/+ and uPAR−/− mice. The treatment with aprotinin, a plasmin inhibitor, which was effective when given immediately before the syndrome, also argues that uPA-uPAR is involved in the effector and not in the sensitization phase of the disease. A study in vitro has raised the possibility that plasmodia might use uPA to infect RBC (41), but in the present study no important differences in parasitemia appeared among +/+, uPA−/−, and uPAR−/− mice that would support such a possibility. Activation of the uPA-uPAR system might increase the generation of plasmin and, consequently, proteolysis within the plasma. As judged from the plasmatic fibrinogen, uPAR deficiency slightly increased, and malaria slightly decreased, the fibrinogen half-life. These observations are in accord with previous reports indicating that a coagulopathy is unlikely to be an important pathogenic mechanism in SM (45) and that the uPA-uPAR system is therefore unlikely to act at this level. Thus, these results favor the possibility that the main effect of the uPA-uPAR is exerted on cell adhesion rather than on extracellular proteolysis. This interpretation is also consistent with the fact that tPA deficiency, which is not believed to influence adhesion, had no effect on mortality.
Mortality in SM in both humans and mice is associated with a breakdown of the blood-brain barrier, but whether this is really an important factor in the lethality is controversial (6). This increase in vascular permeability is not closely correlated with macrophage sequestration in the brain venules, since treatment with anti-CD11a MAb or PMN depletion attenuate the breakdown of the blood-brain barrier without decreasing macrophage sequestration in the brain (46). The uPA-uPAR system had little influence on the breakdown of the blood-brain barrier, suggesting that it is only one of the factors which contributes to lethality. TNF might be responsible for the increase in the vascular permeability, and the fact that uPAR−/− mice did not show a decrease in TNF mRNA levels is in accord with this possibility.
The uPA-uPAR system has been reported to modulate the function of β2 and other integrins (47) and, consequently, the adhesion of leukocytes, i.e., PMNs and macrophages. A critical role of β2 integrin in ANKA-induced mortality was demonstrated by the lifesaving effect of anti-CD11a MAb (11, 18), apparently by decreasing PMN sequestration in the lung (46). The most obvious effect of mouse P. berghei ANKA infection is to increase the sequestration of macrophages, which are apparently activated by the phagocytosis of malarial pigment, in venules and alveolar capillaries. In the brain and lung, uPAR did not increase, or increased only slightly, macrophage trapping. PMN trapping is evident in the alveolar capillaries, and this was not decreased in uPAR-deficient mice. In mouse SM, sequestration of pRBC is important in the alveolar capillaries and was decreased in ICAM-1-deficient mice (12) but not in uPAR-deficient mice. Thus, uPAR deficiency had only a moderate or insignificant influence on the sequestration of PMNs, macrophages, or pRBC in the lung or brain. In another study uPAR deficiency has been shown to moderately decrease PMN transmigration in the mouse peritoneum (32).
By far, the most profound effect of uPAR deficiency was exerted on platelet kinetics. Mouse or human SM is associated with thrombocytopenia, which appears relatively shortly before death (reviewed in reference 49). Thrombocytopenia was markedly attenuated in uPA−/− and uPAR−/− mice, as well as in aprotinin-treated mice and, as suggested by Fig. 1 and 2, the extent of thrombocytopenia is a reliable indicator of the forthcoming death. Since one of the effects of TNF is to produce thrombocytopenia and platelet trapping (48), this interpretation is consistent with evidence in both humans and mice indicating that the lethality of malaria is correlated with TNF production (15, 16, 31). Interestingly, malarial infection or TNF injection did induce thrombocytopenia without coagulopathy (45).
Thrombocytopenia is in large part due to a reduced life span of platelets, resulting from a sequestration in various organs, such as the brain, lung, and spleen (14). During SM, platelets appear to bind either to the activated endothelium or as a cosequestration with macrophages, both of which are evident in brain venules or alveolar capillaries and without fibrin deposition. Since platelets are known to disintegrate quickly once activated, it is likely that the semiquantitative evaluations performed by either electron microscopy or immunohistochemistry underestimate the dynamic process of platelet sequestration. A process of platelet disintegration is suggested in the brain by the diffusion of the immunoreactive material outside the blood vessels. In primates, localization of platelet in brain venules, detected by staining with an anti-GPIIb/IIIa MAb, has also been reported during SM (13). Studies of the lung and brain demonstrate that platelet trapping does not occur in uPAR−/− mice. Since uPAR is widely distributed, interpretation of these results might be complex. In a recent study, we presented evidence that uPAR is detectable on platelets and that labeled platelets from uPAR−/− mice, injected into +/+ recipients, are unable to respond to an injection of TNF, suggesting that it is indeed the platelet uPAR which is important for their localization in response to TNF (30). Since uPAR deficiency does not affect hemostasis or coagulation (8), these findings further emphasize the selective effect of uPAR on platelet kinetics and also suggest that platelet trapping, but not coagulation, contributes to the pathogenesis of SM.
In various models of diseases, platelets have been reported to either attenuate or aggravate inflammation (24, 30). The role of platelets in inflammation (i.e., in the absence of hemostasis) is, however, still difficult to delineate. This is in part because platelet depletion experiments, which have been extensively used in the past, elicit hemorrhages which prevent the study of inflammation. Platelets, because of their rich content in mediators, such as transforming growth factor β, interleukin-1, chemokines, BDNF, PAI-1, CD40L, etc. (23, 25, 35, 40), can modulate the function (including the state of activation and survival) of a wide variety of cells implicated in SM, notably, endothelial cells, leukocytes, and neurones. Especially intriguing is the mechanism by which platelets might deliver their content. There are suggestions that this might happen either via microparticles released in circulation (2) or by a process of membrane fusion with the target cells, which has been suggested by morphological observations (33, 39). Thus, in the present study, we observed both direct platelet-endothelium interactions, suggesting that platelets might activate endothelial cells (or promote their apoptosis), or platelet-macrophage interactions, raising the possibility that platelets contribute to the activation or adhesion of circulating leukocytes. In support of this latter possibility, platelet-dependent lymphocyte trapping has been recently reported (9). However, in infected uPAR−/− mice, macrophage sequestration was to a large extent preserved in spite of markedly reduced platelet sequestration.
In both mice and humans, numerous pathogenic mechanisms have been associated with the mortality of SM, raising the possibility that different types of pathogenic events might contribute to the severity of the disease. Thus, the present results indicate that, without excluding other pathogenic processes, the uPA-uPAR system is a contributor to the severity of malaria, probably by its selective influence on platelet kinetics and trapping. Platelet sequestration in brain or elsewhere may therefore be one of the factors contributing to the gravity of the acute phase of malarial infection.
ACKNOWLEDGMENTS
This work was supported by a grant 32-47284.96 from the Swiss National Science Foundation.
We are grateful to D. Männel (Regensburg, Germany) for providing the anti-GPIIb/IIIa MAb MWReg30 and to C. Allasia and H. Lepidi (Marseille, France) for their help in computerized image analysis.
REFERENCES
- 1.Barazzone C, Horowitz S, Donati Y, Vesin C, Rochat A, Rodriguez I, Piguet P F. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998;19:573–581. doi: 10.1165/ajrcmb.19.4.3173. [DOI] [PubMed] [Google Scholar]
- 2.Barry O P, Pratico D, Lawson J A, Fitzgerald G A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Investig. 1997;99:2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugge T H, Suh T T, Flick M J, Daugherty C C, Romer J, Solberg H, Ellis V, Dano K, Degen J L. The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J Biol Chem. 1995;270:16886–16894. doi: 10.1074/jbc.270.28.16886. [DOI] [PubMed] [Google Scholar]
- 4.Chapman H A. Plasminogen activators, integrins and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 5.Charoenpan P, Indraprasit S, Kiatboonsri S, Suvachttanont O, Tanomsup S. Pulmonary oedema in severe falciparum malaria. Hemodynamic study and clinicophysiologic correlation. Chest. 1990;97:1190–1197. doi: 10.1378/chest.97.5.1190. [DOI] [PubMed] [Google Scholar]
- 6.Clark I A, Macmicking J D, Gray K M, Rockett K A, Cowden W B. Malaria mimicry with tumor necrosis factor. Contrasts between species of murine malaria and Plasmodium falciparum. Am J Pathol. 1992;140:325–336. [PMC free article] [PubMed] [Google Scholar]
- 7.Coquelin F, Boulard Y, Mora-Silvera E, Richard F, Chabaud A G, Landau I. Final stage maturation of the erythrocytic schizonts of rodent Plasmodium in the lungs. C R Acad Sci (III) 1999;322:55–62. doi: 10.1016/s0764-4469(99)80017-1. [DOI] [PubMed] [Google Scholar]
- 8.Dewerchin M, Van Nuffelen A, Wallays G, Bouché A, Moons L, Carmeliet P. Generation and characterization of urokinase receptor-deficient mice. J Clin Investig. 1996;97:870–878. doi: 10.1172/JCI118489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diacovo T G, Catalina M D, Siegelman M H, von Andrian U H. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte M I S, Corbett C E P, Boulos M, Neto A. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985;34:31–35. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- 11.Falanga P B, Butcher E C. Late treatment with anti-LFA-1(CD11a) antibody prevents cerebral malaria in a mouse model. Eur J Immunol. 1991;21:2259–2263. doi: 10.1002/eji.1830210938. [DOI] [PubMed] [Google Scholar]
- 12.Favre N, Willimann K, Ryffel B, Weiss N, Imhof B A, Rudin W, Lucas R, Piguet P F. Role of ICAM-1 in the development of murine cerebral malaria. Microbes Infect. 1999;1:961–968. doi: 10.1016/s1286-4579(99)80513-9. [DOI] [PubMed] [Google Scholar]
- 13.Fujioka H, Millet P, Maeno Y, Nakazawa S, Ito Y, Howard R J, Collins W E, Aikawa M. A nonhuman primate model for human cerebral malaria: rhesus monkeys experimentally infected with Plasmodium fragile. Exp Parasitol. 1994;78:371–376. doi: 10.1006/expr.1994.1040. [DOI] [PubMed] [Google Scholar]
- 14.Grau G E, Tacchini-Cottier F, Vesin C, Milon G, Lou J N, Piguet P F, Juillard P. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cyto Net. 1993;4:415–419. [PubMed] [Google Scholar]
- 15.Grau G E, Fajardo L F, Piguet P F, Allet B, Lambert P H, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 16.Grau G E, Taylor T, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P H. Tumor necrosis factor and disease severity in children with falciparum malaria. New Engl J Med. 1989;24:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 17.Grau G E, Gretener D, Lambert P H. Prevention of murine cerebral malaria by low-dose cyclosporin A. Immunology. 1987;61:521–525. [PMC free article] [PubMed] [Google Scholar]
- 18.Grau G E, Pointaire P, Piguet P F, Vesin C, Rosen H, Stamenkovic I, Takei F, Vassalli P. Late administration of monoclonal antibody to leukocyte function antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991;21:2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- 19.Grau G E, Piguet P F, Engers H D, Louis J A, Vassalli P, Lambert P H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986;137:2348–2354. [PubMed] [Google Scholar]
- 20.Grau G E, Heremans H, Piguet P F, Pointaire P, Lambert P H, Billiau A, Vassalli P. Monoclonal antibody against interferon-gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grau G E, Piguet P F, Gretener D, Vesin C, Lambert P H. Immunopathology of thrombocytopenia in experimental malaria. Immunology. 1988;65:501–506. [PMC free article] [PubMed] [Google Scholar]
- 22.Grau G E, de Kossodo S. Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today. 1994;10:408–409. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 23.Hawrylowicz C M, Howells G L, Feldmann M. Platelet-derived interleukin-1 induces human endothelial adhesion molecules expression and cytokine production. J Exp Med. 1991;174:785–790. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffner J E, Repine J E. Platelets. In: Crystal R G, West J B, Weibel E R, Barnes P J, editors. The lung: scientific foundations. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 947–960. [Google Scholar]
- 25.Henn V, Slupsky J R, Grafe M, Anagnostopoulos I, Forster R, Mueller-Berghaus G, Kroczek R. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 26.Imhof B A, Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- 27.Lucas R, Lou J N, Juillard P, Moore M, Bluethmann H, Grau G E. Respective role of TNF receptors in the development of experimental cerebral malaria. J Neuroimmunol. 1997;72:143–148. doi: 10.1016/s0165-5728(96)00185-3. [DOI] [PubMed] [Google Scholar]
- 28.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 29.Mann H B, Whitney D R. On a test of whether one or two random variables is stochastically larger than the other. Ann Math Statist. 1947;18:50–60. [Google Scholar]
- 30.Maennel D, Grau G E. Role of platelet adhesion in homeostasis and immunopathology. J Clin Pathol Mol Pathol. 1997;50:175–185. doi: 10.1136/mp.50.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire W, Hill V S, Allsopp C E M, Greewood B M, Kwjatkowski D. Variation in the TNF-a promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–511. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 32.May A E, Kanse S M, Lund L R, Gisler R H, Imhof B A, Preissner K T. Urokinase receptor (CD87) regulates leukocyte recruitment via beta-2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura M, Shibazaki M, Nitta Y, Endo Y. Translocation of platelets into Disse spaces and their entry into hepatocytes in response to lipopolysaccharides, interleukin-1 and tumor necrosis factor: the role of Kupffer cells. J Hepatol. 1998;28:991–999. doi: 10.1016/s0168-8278(98)80348-6. [DOI] [PubMed] [Google Scholar]
- 34.Nieswandt B, Echtenacher B, Wachs F P, Schroder J, Gessner J E, Schmidt R E, Grau G E, Mannel D N. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
- 35.Nordenhem A, Wiman B. Plasminogen activator inhibitor-1 (PAI-1) content in platelets from healthy individuals genotyped for the 4G/5G polymorphism in the PAI-1 gene. Scand J Clin Lab Investig. 1997;57:453–461. doi: 10.3109/00365519709084594. [DOI] [PubMed] [Google Scholar]
- 36.Patnaik J K, Das B S, Mishra S K, Mohanty S, Sapathy S K, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- 37.Piguet P F, Collart M A, Grau G E, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piguet, P. F., C. Vesin, C. D. Laperousaz, and A. Rochat. 2000. Role of plasminogen activators and of urokinase receptor (uPAR, CD87) in platelet kinetics in mice. J. Hematol., in press. [DOI] [PubMed]
- 39.Piguet P F, Vesin C, Donati Y, Tacchini-Cottier F, Belin D, Barazzone C. Urokinase-receptor (uPAR, CD87) is a platelet receptor important for kinetics and TNF-induced endothelial adhesion in mice. Circulation. 1999;99:3315–3321. doi: 10.1161/01.cir.99.25.3315. [DOI] [PubMed] [Google Scholar]
- 40.Pliego Rivero F B, Bayatti N, Giannakoulopoulos X, Glover V, Bradford H F, Stern G, Sandler M. Brain-derived neurotrophic factor in human platelets. Biochem Pharmacol. 1997;54:207–209. doi: 10.1016/s0006-2952(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 41.Roggwiller E, Fricaud A C, Blisnick T, Braun Breton C. Host urokinase-type plasminogen activator participates in the release of malaria merozoites from infected erythrocytes. Mol Biochem Parasitol. 1997;86:49–59. doi: 10.1016/s0166-6851(97)02848-x. [DOI] [PubMed] [Google Scholar]
- 42.Royston B D, Royston D, Pearson J D. Aprotinin inhibits platelet adhesion to endothelial cells. Blood Coag Fibrin. 1992;3:737–742. doi: 10.1097/00001721-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Rudin W, Eugster H P, Bordmann G, Bonato J, Muller M, Yamage M, Ryffel B. Resistance to cerebral malaria in tumor necrosis factor-alpha/beta-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol. 1997;150:257–266. [PMC free article] [PubMed] [Google Scholar]
- 44.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senaldi G, Piguet P F. Mortality and platelet depletion occur independently of fibrinogen consumption in murine models of tumor necrosis factor-mediated systemic inflammatory responses. Cytokine. 1998;10:382–389. doi: 10.1006/cyto.1997.0303. [DOI] [PubMed] [Google Scholar]
- 46.Senaldi G, Vesin C, Chang R, Grau G, Piguet P F. An effector role of neutrophils in the pathogenesis of murine severe malaria. Infect Immun. 1994;62:1144–1149. doi: 10.1128/iai.62.4.1144-1149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sitrin R G, Todd III R F, Petty H R, Brock T G, Shollenberger S B, Albrecht E, Gyetko M R. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Investig. 1996;97:1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tacchini-Cottier F, Vesin C, Redard M, Buurman W, Piguet P F. Role of TNF receptors I and II in TNF-induced platelets consumption in mice. J Immunol. 1998;160:6182–6186. [PubMed] [Google Scholar]
- 49.White N J, Ho M. The pathophysiology of malaria. New York, N.Y: Academic Press, Inc.; 1992. pp. 84–173. [Google Scholar]
- 50.World Health Organization Malaria Action Programme. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1986;80:3–50. [PubMed] [Google Scholar]
- 51.Wright D H. The effect of neonatal thymectomy on the survival of golden hamsters infected with Plasmodium berghei. Br J Exp Pathol. 1968;49:379–384. [PMC free article] [PubMed] [Google Scholar]