Abstract
Background
Assessments of air leaks are usually performed subjectively, precluding the use of air leaks as an evaluation factor. We aimed to identify objective parameters as predictive factors for prolonged air leak (PAL) and air leak cessation (ALC) from air flow data produced by a digital drainage system.
Methods
Flow data records of 352 patients who underwent lung lobectomy were reviewed, and flow data at designated intervals (1, 2, and 3 hours postoperatively [POH] and 3 times a day thereafter [0600, 1300, 1900]) were extracted. ALC was defined by flow less than 20 mL/min over 12 hours, and PAL was defined as ALC after 5 days. Cumulative incidence curves were obtained using Kaplan-Meier estimates of time to ALC. Cox regression analysis was performed to determine the effects of variables on the rate of ALC.
Results
The incidence of PAL was 18.2% (64/352). Receiver operating characteristic curve analysis showed cut-off values of 180 mL/min for the flow at 3 POH and 73.3 mL/min for the flow on postoperative day 1; the sensitivity and specificity of these values were 88.9% and 82.5%, respectively. The rates of ALC by Kaplan-Meier analysis were 56.8% at 48 POH and 65.6% at 72 POH. Multivariate Cox regression analysis revealed that the flow at 3 POH (≤80 mL/min), operation time (≤220 minutes), and right middle lobectomy independently predicted ALC.
Conclusion
Air flow measured by a digital drainage system is a useful predictor of PAL and ALC and may help optimize the hospital course.
Keywords: Prolonged air leak, Digital drainage system, Lobectomy
Introduction
Although most patients with postoperative air leaks usually recover after a couple of days, some patients show prolonged air leak (PAL) that continues for more than 5 days. The prediction of air leak cessation (ALC) and, by extension, PAL can enable early, selective postoperative management of patients, which may influence the cost of treatment [1-3] and decrease the risk of other complications, including empyema [1,3,4]. With the increasing adoption of lung resection surgery, prediction of the postoperative hospital course and optimization of the hospital stay are becoming increasingly important for both surgeons and patients.
Conventionally, air leak grading by examiners is based on subjective assessments of air bubbles, raising questions about the reliability of this approach and interobserver agreement. However, a digital drainage system can enable quantitative measurements of air leaks without interobserver discrepancies [5,6]. Thus, we sought to determine whether measurement of the amount of air leaks could be a more direct and accurate approach for predicting ALC.
The purpose of this study was to extract flow data over the postoperative time course as an objective parameter by using a digital drainage system and to determine its correlation of air flow with ALC and its potential as a predictor of PAL and ALC.
Methods
Patients and data collection
The data of 489 patients who underwent lung resection surgery and had flow records for a digital drainage system (Thopaz; Medela, Baar, Switzerland) between January 2013 and June 2017 were reviewed. Patients who received lung segmentectomy or wedge resection and those with insufficient flow records were excluded from this study. We also excluded cases involving the placement of 2 or more chest tubes, the use of mechanical ventilation, and the implementation of pleurodesis within 5 days. Finally, 352 patients who underwent lung lobectomy and had daily flow records were enrolled in the study.
All hospital records were retrieved, and the following data were investigated: demographic characteristics, operative details, flow records, and length of hospital stay. Operative details were recorded, including intraoperative sealant use and intraoperative adhesiolysis. The flow data at designated intervals were extracted from medical records. The start of the flow evaluation (=0 postoperative hours [POH]) was the time when the patient entered the surgical intensive care unit or recovery room. The stat flow in the digital drainage system at 1, 2, and 3 POH on the operation day and recordings obtained 3 times a day (06:00, 13:00, and 19:00) on the subsequent postoperative days were recorded. On the basis of previous reports [7-13], an air leak was defined by an air flow of 20 mL/min or more in Thopaz, and ALC was defined as flow less than 20 mL/min for the past 12 hours with no spike on the flow graph. PAL was defined as the persistence of an air leak for 5 or more days. This study was reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital, and the requirement for informed consent was waived (eIRB no., KC22RASI0760).
Perioperative management
All lung resections in this study were conducted by a single surgeon (S.W.S.), and a mechanical stapling device was used to separate interlobar fissures and cut the bronchus. If an air leak was observed before chest wall closure, we tried to stop the leak by suturing, sealants, and buttressing materials. A 20F or 24F chest tube was placed before chest wall closure and connected to the Thopaz device. Intrapleural pressure was set at -15 cmH2O. The chest tube was removed when the air leak stopped and the drainage fluid volume was less than 200 mL per day.
Statistical analysis
Receiver operating characteristic (ROC) curves were used to define cut-off values to transform the numerical flow variable into a binary categorical variable. The time from lung lobectomy (0 POH) to the achievement of ALC was determined from medical records. The Kaplan-Meier estimate of time to ALC and the percentages of ALC at specific time points were calculated. Cumulative incidence curves were obtained using fitted Cox models adjusted for covariates. Statistical analyses were performed using the R computing environment (2008; R Development Core Team, Vienna, Austria).
Univariate and multivariate regression analyses were performed using Cox models to determine the effects of variables on the rates of ALC. The variables used in multivariate analysis were those with a p-value of <0.2 in the univariate analysis. Maximally selected log-rank statistics were used for estimating the cut-off points of continuous predictors, which were suitable for discrimination between groups of patients with respect to overall survival (censored) time. A p-value of <0.05 was considered statistically significant.
Results
Patient characteristics
In total, 489 consecutive patients who underwent lung operations were placed on the digital drainage system. Among them, 352 patients met the eligibility criteria of this study. The characteristics of these patients are summarized in Table 1. This group included 168 men (47.7%) and had a median age of 64 years (range, 22–85 years). Sixteen patients (4.5%) had chronic obstructive pulmonary disease. Intraoperative pleural adhesions were found in 10 patients (2.8%), and a medical sealant was used intraoperatively in 167 patients (47.4%). The median time to postoperative ALC was 39 hours (interquartile range [IQR], 18.0–89.0 hours), and the median time to chest tube removal was 3 postoperative days (POD) (IQR, 2.0–6.5 days). The incidence of PAL was 18.2% (64/352). Most of the operations were video-assisted thoracoscopic (VATS) lobectomies for primary lung cancer.
Table 1.
Characteristics of patients with or without PAL
| Characteristic | All patients (N=352) | Non-PAL group (n=288) | PAL group (n=64) | p-value |
|---|---|---|---|---|
| Age (yr) | 64 (57–72) | 64 (58–72) | 60.5 (57–72) | 0.287 |
| Sex (male) | 168 (47.7) | 132 (45.8) | 36 (56.2) | 0.170 |
| COPD | 16 (4.5) | 11 (3.8) | 5 (7.8) | 0.291 |
| Diabetes mellitus | 53 (15.1) | 44 (15.3) | 9 (14.1) | 0.958 |
| Smoking | ||||
| Never-smoker | 210 (59.7) | 179 (62.2) | 31 (48.4) | 0.046 |
| Ex-smoker | 87 (24.7) | 70 (24.3) | 17 (26.6) | |
| Current smoker | 55 (15.6) | 39 (13.5) | 16 (25.0) | |
| Laterality, right | 233 (66.2) | 180 (62.5) | 53 (82.8) | 0.003 |
| Lobe of resection | ||||
| Upper (RUL, LUL) | 178 (51.9) | 131 (46.6) | 47 (75.8) | <0.001 |
| Middle (RML) | 39 (11.4) | 39 (13.9) | 0 | |
| Lower (RLL, LLL) | 126 (36.7) | 111 (39.5) | 15 (24.2) | |
| Surgical approach (VATS) | 329 (93.5) | 271 (94.1) | 58 (90.6) | 0.461 |
| Intraoperative | ||||
| Pleural adhesiolysis | 10 (2.8) | 8 (2.8) | 2 (3.1) | 1.000 |
| Use of surgical sealant | 167 (47.4) | 137 (47.6) | 30 (46.9) | 1.000 |
| Operation time (min) | 145 (120–180) | 145 (120–175) | 147 (115.5–199.5) | 0.355 |
| Time to ALC (hr) | 39 (18–89) | 26 (17–65.5) | 193 (161.5–240.5) | <0.001 |
Values are presented as number (%) for categorical variables and median (interquartile range) for continuous variables.
PAL, prolonged air leak; COPD, chronic obstructive pulmonary disease; RUL, right upper lobe; LUL, left upper lobe; RML, right middle lobe; RLL, right lower lobe; LLL, left lower lobe; VATS, video-assisted thoracoscopic surgery; ALC, air leak cessation.
Air flow at each time point
Air flow over the postoperative period was compared among patients showing early ALC, late ALC, and PAL (Table 2). While 75% of the patients in the early and late ALC groups had flow rates below 25 and 460 mL/min at 3 POH, respectively, patients in the PAL group had a median flow over 500 mL/min at 3 POH (IQR, 210–1,150 mL/min). However, the flow declined sequentially in all groups and showed approximately 50% reduction between 3 POH and 1 POD (Fig. 1). Nevertheless, the flow in the PAL group was significantly greater than that in the early and late ALC groups at each time point. The median flow in the early ALC group was 0 mL/min even at 3 POH, while the median flow in the late ALC group was below 100 mL/min early at 1 POD. In contrast, the median flow in the PAL group did not decrease to 100 mL/min even at 3 POD.
Table 2.
Comparison of air flow between patient groups
| Variable | Early ALC(time to ALC: 1D, 2D) | Late ALC(time to ALC: 3D–5D) | PAL(time to ALC: ≥6D) | p-value |
|---|---|---|---|---|
| No. of patients | 200 | 88 | 64 | |
| Air flow (mL/min) | ||||
| 3 POHa) | 0 (0–25) | 210 (70–460) | 550 (210–1,150) | <0.001 |
| 1 PODb) (average) | 0 (0–6.7) | 83 (33–193) | 380 (202–825) | <0.001 |
| 2 PODb) (average) | 0 (0–0) | 33 (10–77) | 197 (72–412) | <0.001 |
| 3 PODb) (average) | 6 (0–20) | 107 (37–258) | <0.001 |
Values are presented as median (interquartile range).
ALC, air leak cessation; PAL, prolonged air leak; POH, postoperative hour; POD, postoperative day.
a)Statistic value. b)Average value of 3 time points (06:00, 13:00, 19:00).
Fig. 1.
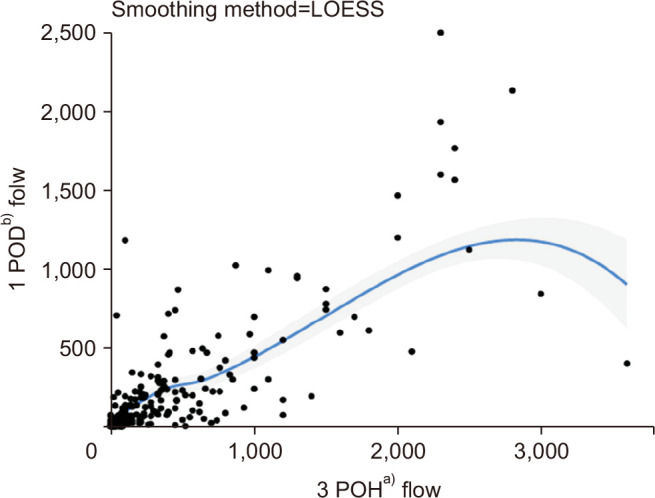
Scatter plot for 2 continuous variables (3-postoperative hour [POH] flowa) vs. 1-postoperative day [POD] flowb)) in all patients and the 2 variables’ regression line with locally estimated scatterplot smoothing (LOESS) locally weighted polynomial regression with a confidence level of 95%. The flow declined with time and showed approximately 50% reduction between these 2 time points. a)Statistic value. b)Average value of 3 time points (06:00, 13:00, 19:00).
Prediction of prolonged air leak on the basis of the flow scale
An ROC curve analysis was performed to evaluate the cut-off values of predictors. The area under the curve (AUC) value for the combination of flow at 3 POH and 1 POD was 0.911 (95% confidence interval, 0.864–0.958) (Fig. 2). If the cut-off value was set at 180 mL/min for 3-POH flow and 73.3 mL/min for 1-POD flow on the basis of the ROC curve, the sensitivity and specificity were 88.9% and 82.5%, respectively. These results indicated that the flow at 3 POH and 1 POD together were promising predictors for PAL. On the basis of these cut-off values, the PAL rates were as follows: flow at 3 POH >180 mL/min or 1 POD >73.3 mL/min: 45% (59/131); flow at 3 POH >180 mL/min and 1 POD >73.3 mL/min: 53% (48/91) (Table 3).
Fig. 2.
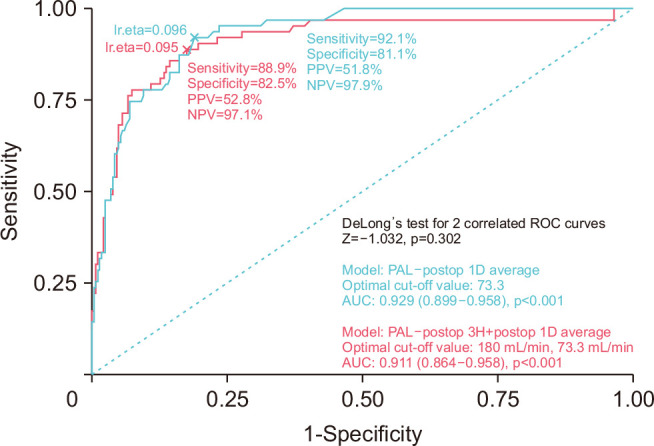
Receiver operating characteristic (ROC) curve representing the prediction of prolonged air leak (PAL) using 3-postoperative hour (POH) flowa) and 1-postoperative day (POD) flowb). The cut-off values were set at 180 mL/min for postoperative 3 hours and 73.3 mL/min for 1 POD based on the ROC curve with a sensitivity of 88.9% and a specificity of 82.5%. AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; Postop, postoperative; lr.eta, logistic regression estimation. a)Statistic value. b)Average value of 3 time points (06:00, 13:00, 19:00).
Table 3.
Prolonged air leak rates in each category by 2 cut-off values
| Flow (mL/min) | 3 POHa) ≤180 | 3 POHa) >180 |
|---|---|---|
| 1 PODb) ≤73.3 | 5/221 (2.3) | 1/18 (5.6) |
| 1 PODb) >73.3 | 10/22 (45.5) | 48/91 (52.7) |
Values are presented as number (%).
POH, postoperative hour; POD, postoperative day.
a)>Statistic value. >b)>Average value of 3 time points (06:00, 13:00, 19:00).
Prediction of air leak cessation by time-dependent covariates
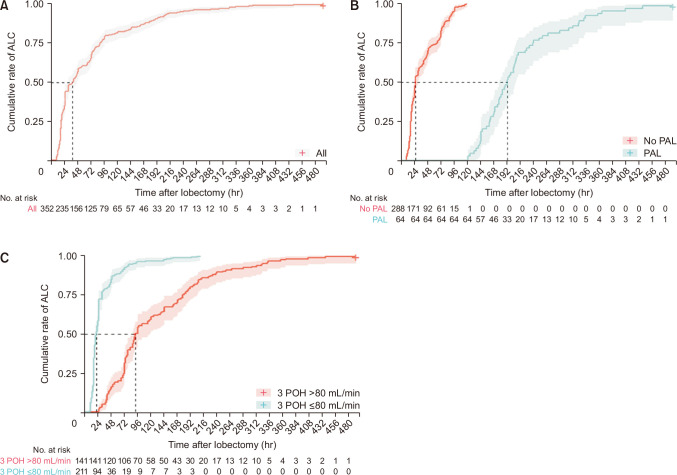
The rates of ALC were 56.8% at 48 POH and 65.6% at 72 POH by Kaplan-Meier analysis (Fig. 3A). The percentage of patients with ALC increased with each consecutive hour in the postoperative period. PAL significantly prolonged the median time to ALC (25 hours versus 189 hours, p<0.001) (Fig. 3B). The “maxstat” tool in the R package was used to identify the cut-off 3-POH flow value that provided the best separation of the cumulative incidence of ALC into 2 groups. For the overall failure time, the estimated cut-off point was 80 mL/min (the maximum of the log-rank statistic M was 3.1772, and the p-value was <0.0001). By 48 and 72 hours, respectively, only 17% and 27% of patients with high flow at 3 POH showed ALC, in comparison with 84% and 92% of patients with low flow at 3 POH (Fig. 3C).
Fig. 3.
(A–C) Cumulative incidence of air leak cessation (ALC) by postoperative hour (POH) (95% confidence interval indicated by the shaded area). PAL, prolonged air leak.
Univariate Cox proportional hazards analysis for ALC showed positive correlations with a 3-POH flow of 80 mL/min or less (p<0.001), operation time of 220 minutes or less (p=0.0004), and right middle lobectomy (p<0.0001). The surgical approach (open versus VATS), whether adhesiolysis was performed, and the use of surgical sealants were not associated with ALC. Multivariate Cox regression analysis for ALC revealed that the 3-POH flow (≤80 mL/min), operation time (≤220 minutes), and right middle lobectomy independently predicted ALC (Table 4).
Table 4.
Analysis of potential predictors of air leak cessation
| Variable | Univariate Cox regression | Multivariate Cox regression | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Unfavorable | Favorable | Hazard ratio | p-value | Hazard ratio | p-value | ||
| 3-POH flow (mL/min) | ≤80 | 5.24 | <0.0001 | 4.90 | <0.0001 | ||
| Operation time (min) | ≤220 | 1.81 | 0.0004 | 1.57 | 0.0298 | ||
| Approach | VATS | 1.60 | 0.349 | 1.03 | 0.9273 | ||
| Adhesiolysis | Yes | 0.66 | 0.1927 | 0.88 | 0.7048 | ||
| Sealant | Yes | 1.03 | 0.7535 | ||||
| Lobe | RML | 2.28 | <0.0001 | 2.40 | <0.0001 | ||
| Ref: lower lobe | Upper lobe | 0.63 | <0.0001 | 0.80 | 0.065 | ||
POH, postoperative hour; VATS, video-assisted thoracoscopic surgery; RML, right middle lobe; ref, reference.
Discussion
The present study evaluated a cohort of patients who underwent lung lobectomy and whose chest tube drain was connected to a digital drainage system. The air flow was followed up daily at designated intervals to determine the timing of ALC and, subsequently, the timing of chest drain removal. This study highlighted the practical application of early postoperative flow data in estimating overall ALC and the occurrence of PAL. The rarity of studies predicting ALC with a cumulative incidence function for time-dependent covariates makes our findings valuable.
PALs are a frequent and bothersome complication after lung resection, and their incidence has been reported to be approximately 10% to 15% post-lobectomy [9,14,15]. Retention of the chest tube for longer-than-expected periods of time may cause distress, anxiety, and pain in patients, and may also result in other cardiopulmonary complications [3,4,9] and empyema [1]. Several studies have attempted to identify risk factors for PAL [14,16-18] and develop a scoring system to stratify the risk of PAL [9], but their results were not consistent and therefore were of limited clinical use. It is well known that the most powerful surrogate of lung recovery is undoubtedly the air leak status itself. However, to our knowledge, previous studies contain limited flow data that could be used in the clinical setting to rapidly stratify the risk of PAL.
Various investigators have evaluated whether grading or quantification of the amount of early postoperative air leak can help predict PAL [17,19,20]. Oh et al. [17] reported that the sum of 6 consecutive values of air leak grades for every 8-hour record on postoperative days 2 and 3 was the most powerful predictor of PAL, presenting a positive predictive value of 75.7% when SUM4to9 ≥16. Goto et al. [16] also reported the importance of quantitative postoperative air flow findings in predicting PAL. They measured the average flow per hour at 0, 12, 24, and 36 hours postoperatively with the digital drainage system and showed that air flow greater than 20 mL/min at 36 POH was a powerful predictor of PAL, with a sensitivity and specificity of 91% and 73%, respectively. The results of our study are consistent with the findings of previous reports. The air flow at 3 POH in the PAL group was significantly higher than that in the non-PAL group in our study. The same results were seen at 1, 2, and 3 POD, when the mean flow over the day was compared. Using ROC curve analysis, our results showed that an air leak of 180 mL/min or greater at 3 POH and a mean air leak of 73.3 mL/min or greater at 1 POD was a risk factor for PAL, with a sensitivity and specificity of 88.9% and 82.5%, respectively.
In addition to proposing a practical and effective method for predicting PAL, this study tangibly confirms our hypothesis that the amount of early postoperative air leakage can predict the timing of ALC in our analysis of time-to-event data. The cases in which the air flow at 3 POH did not reach 80 mL/min tended to show earlier ALC; thus, the air leak ceased by 72 POH in 92% of the patients. This would greatly simplify the selection of patients for preemptive interventions (such as chemical pleurodesis), allowing earlier and safer removal of the chest drain. Based on our findings, patients with a 3-POH flow over 80 mL/min might be considered to be a candidate for early chemical pleurodesis at 3 POD if ALC has not been achieved.
Furthermore, other patient-related or intraoperative variables were positively or negatively associated with ALC according to the Cox proportional hazard model. Middle lobectomy favorably predicted ALC in our cohort. The simplicity of the surgical procedure and the smallest lobe included in the procedure could play a role in controlling air leaks. A shorter operation time also favorably predicted ALC. The operation time for lobectomy depends on the simplicity of the surgical procedure, which is closely associated with the anatomical morphology in terms of the presence or absence of the main fissure and variations in main fissures (incomplete or complete). This is an important aspect in intraoperative planning of lobectomy, since the planned procedure may change to avoid a postoperative air leak once the presence of a variant fissure is noted [21,22]. Moreover, whether adhesiolysis was performed or sealants were used had no impact on ALC. Since the operation time is much longer in cases necessitating adhesiolysis and operations are more likely to be complex in cases requiring sealant usage, the air leaks in those cases were obviously well controlled with sealant usage and by experienced hands.
However, this study had the following potential limitations. The retrospective nature of the study may have resulted in some problems in defining and recording the variables. In particular, since ALC was determined retrospectively from medical records, there may have been some discrepancies between real and defined cessations. In addition, we did not apply strict rules for chest tube removal. The timing of chest tube removal was influenced by the amount of drainage and was also dependent on the physician’s discretion.
In conclusion, this study clarified the importance of evaluation of postoperative air leaks for PAL and ALC prediction after pulmonary lobectomy. The results confirmed that ALC is a predictable condition that should be evaluated based on flow itself and intraoperative factors together. Surgeons are encouraged to apply an objective and preemptive approach to ALC during the postoperative course after lung resection.
Funding Statement
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article information
Author contributions
Conceptualization: KH. Data curation: JY, SWS. Project administration: JY. Visualization: KH. Writing–original draft: JY, KH. Writing–review & editing: JY, KH. Final approval of the manuscript: all authors.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Brunelli A, Xiume F, Al Refai M, Salati M, Marasco R, Sabbatini A. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest. 2006;130:1150–6. doi: 10.1378/chest.130.4.1150. https://doi.org/10.1378/chest.130.4.1150. [DOI] [PubMed] [Google Scholar]
- 2.Irshad K, Feldman LS, Chu VF, Dorval JF, Baslaim G, Morin JE. Causes of increased length of hospitalization on a general thoracic surgery service: a prospective observational study. Can J Surg. 2002;45:264–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Varela G, Jimenez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:329–33. doi: 10.1016/j.ejcts.2004.11.005. https://doi.org/10.1016/j.ejcts.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Okereke I, Murthy SC, Alster JM, Blackstone EH, Rice TW. Characterization and importance of air leak after lobectomy. Ann Thorac Surg. 2005;79:1167–73. doi: 10.1016/j.athoracsur.2004.08.069. https://doi.org/10.1016/j.athoracsur.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 5.Bertolaccini L, Rizzardi G, Filice MJ, Terzi A. 'Six sigma approach': an objective strategy in digital assessment of postoperative air leaks: a prospective randomised study. Eur J Cardiothorac Surg. 2011;39:e128–32. doi: 10.1016/j.ejcts.2010.12.027. https://doi.org/10.1016/j.ejcts.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Varela G, Jimenez MF, Novoa NM, Aranda JL. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg. 2009;35:28–31. doi: 10.1016/j.ejcts.2008.09.005. https://doi.org/10.1016/j.ejcts.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg. 2017;153:690–9. doi: 10.1016/j.jtcvs.2016.10.003. https://doi.org/10.1016/j.jtcvs.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunelli A, Salati M, Pompili C, Refai M, Sabbatini A. Regulated tailored suction vs regulated seal: a prospective randomized trial on air leak duration. Eur J Cardiothorac Surg. 2013;43:899–904. doi: 10.1093/ejcts/ezs518. https://doi.org/10.1093/ejcts/ezs518. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg. 2010;90:204–9. doi: 10.1016/j.athoracsur.2010.02.054. https://doi.org/10.1016/j.athoracsur.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 10.Miller DL, Helms GA, Mayfield WR. Digital drainage system reduces hospitalization after video-assisted thoracoscopic surgery lung resection. Ann Thorac Surg. 2016;102:955–61. doi: 10.1016/j.athoracsur.2016.03.089. https://doi.org/10.1016/j.athoracsur.2016.03.089. [DOI] [PubMed] [Google Scholar]
- 11.Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: computed tomography assessment and risk factors stratification. Surgeon. 2011;9:72–7. doi: 10.1016/j.surge.2010.07.010. https://doi.org/10.1016/j.surge.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Pompili C, Brunelli A, Salati M, Refai M, Sabbatini A. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interact Cardiovasc Thorac Surg. 2011;13:490–3. doi: 10.1510/icvts.2011.280941. https://doi.org/10.1510/icvts.2011.280941. [DOI] [PubMed] [Google Scholar]
- 13.Shoji F, Takamori S, Akamine T, et al. Clinical evaluation and outcomes of digital chest drainage after lung resection. Ann Thorac Cardiovasc Surg. 2016;22:354–8. doi: 10.5761/atcs.oa.16-00179. https://doi.org/10.5761/atcs.oa.16-00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunelli A, Monteverde M, Borri A, Salati M, Marasco RD, Fianchini A. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg. 2004;77:1205–10. doi: 10.1016/j.athoracsur.2003.10.082. https://doi.org/10.1016/j.athoracsur.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 15.Cerfolio RJ, Bass CS, Pask AH, Katholi CR. Predictors and treatment of persistent air leaks. Ann Thorac Surg. 2002;73:1727–31. doi: 10.1016/S0003-4975(02)03531-2. https://doi.org/10.1016/s0003-4975(02)03531-2. [DOI] [PubMed] [Google Scholar]
- 16.Goto M, Aokage K, Sekihara K, et al. Prediction of prolonged air leak after lung resection using continuous log data of flow by digital drainage system. Gen Thorac Cardiovasc Surg. 2019;67:684–9. doi: 10.1007/s11748-019-01073-y. https://doi.org/10.1007/s11748-019-01073-y. [DOI] [PubMed] [Google Scholar]
- 17.Oh SG, Jung Y, Jheon S, et al. Postoperative air leak grading is useful to predict prolonged air leak after pulmonary lobectomy. J Cardiothorac Surg. 2017;12:1. doi: 10.1186/s13019-017-0568-6. https://doi.org/10.1186/s13019-017-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orsini B, Baste JM, Gossot D, et al. Index of prolonged air leak score validation in case of video-assisted thoracoscopic surgery anatomical lung resection: results of a nationwide study based on the French national thoracic database, EPITHOR. Eur J Cardiothorac Surg. 2015;48:608–11. doi: 10.1093/ejcts/ezu505. https://doi.org/10.1093/ejcts/ezu505. [DOI] [PubMed] [Google Scholar]
- 19.Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001;71:1613–7. doi: 10.1016/S0003-4975(01)02474-2. https://doi.org/10.1016/s0003-4975(01)02474-2. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto T, Nishio W, Okada M, Harada H, Uchino K, Tsubota N. Management of air leak after pulmonary resection. Jpn J Thorac Cardiovasc Surg. 2004;52:292–5. doi: 10.1007/s11748-004-0045-8. https://doi.org/10.1007/s11748-004-0045-8. [DOI] [PubMed] [Google Scholar]
- 21.Esomonu UG, Taura MG, Modibbo MH, Egwu AO. Variation in the lobar pattern of the right and left lungs: a case report. Australas Med J. 2013;6:511–4. doi: 10.4066/AMJ.2013.1856. https://doi.org/10.4066/AMJ.2013.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mpolokeng KS, Madolo MY, Louw GJ, Gunston G. Anatomical variations in lung fissures leading to supernumerary lobes in the lungs. Transl Res Anat. 2022;28:100209. doi: 10.1016/j.tria.2022.100209. https://doi.org/10.1016/j.tria.2022.100209. [DOI] [Google Scholar]