Abstract
Huntington’s disease (HD) is a neurodegenerative disease caused by a CAG repeat expansion in the Huntingtin (HTT) gene. The resulting polyglutamine (polyQ) tract alters the function of the HTT protein. Although HTT is expressed in different tissues, the medium-spiny projection neurons (MSNs) in the striatum are particularly vulnerable in HD. Thus, we sought to define the proteome of human HD patient–derived MSNs. We differentiated HD72-induced pluripotent stem cells and isogenic controls into MSNs and carried out quantitative proteomic analysis. Using data-dependent acquisitions with FAIMS for label-free quantification on the Orbitrap Lumos mass spectrometer, we identified 6323 proteins with at least two unique peptides. Of these, 901 proteins were altered significantly more in the HD72-MSNs than in isogenic controls. Functional enrichment analysis of upregulated proteins demonstrated extracellular matrix and DNA signaling (DNA replication pathway, double-strand break repair, G1/S transition) with the highest significance. Conversely, processes associated with the downregulated proteins included neurogenesis-axogenesis, the brain-derived neurotrophic factor–signaling pathway, Ephrin-A:EphA pathway, regulation of synaptic plasticity, triglyceride homeostasis cholesterol, plasmid lipoprotein particle immune response, interferon-γ signaling, immune system major histocompatibility complex, lipid metabolism, and cellular response to stimulus. Moreover, proteins involved in the formation and maintenance of axons, dendrites, and synapses (e.g., septin protein members) were dysregulated in HD72-MSNs. Importantly, lipid metabolism pathways were altered, and using quantitative image analysis, we found that lipid droplets accumulated in the HD72-MSN, suggesting a deficit in the turnover of lipids possibly through lipophagy. Our proteomics analysis of HD72-MSNs identified relevant pathways that are altered in MSNs and confirm current and new therapeutic targets for HD.
Keywords: neurodegeneration, Huntington’s disease, induced pluripotent stem cells, medium spiny neurons, quantitative proteomics, data-independent acquisitions, data-dependent acquisitions, ion mobility
Graphical Abstract
Huntington's disease MSN model reveals alterations in lipid droplets.
Highlights
-
•
Proteome of human Huntington’ disease medium spiny neurons are defined.
-
•
Pathways are extracellular matrix, double-strand break repair, and lipid metabolism.
-
•
Septins, APOE, and minichromosome maintenance (MCM)s are dysregulated.
-
•
Lipid droplets accumulate due to increased lipid uptake and deficits in lipophagy.
In Brief
The proteome of the medium spiny neurons of human Huntington’s disease from isogenic HD iPSCs is defined and potential therapeutic targets or biomarkers are defined.
Huntington’s disease (HD) is a rare progressive monogenic neurological disorder caused by a trinucleotide repeat expansion in exon-1 of the Huntingtin gene (HTT) (1, 2, 3). The clinical hallmark of HD is a chorea that coexists with cognitive decline and emotional disturbances (4). There is no cure, and no treatment alters the course of this devastating disease. HD phenotypes are linked to the expression of mutant HTT protein (mHTT) that harbors expanded glutamine stretches (over 38) in the N-terminal region. HD features neuronal degeneration in the brain, and medium-spiny projection neurons (MSNs) within the striatum are particularly vulnerable (5).
While substantial progress has been made towards elucidating how the CAG repeats within mHTT lead to the clinical outcomes in HD, our understanding of the mechanisms underlying the motor deficits and striatal degeneration is incomplete (6). Those mechanisms likely occur in parallel. For example, mHTT has altered localization, conformation, and protein interactions (7, 8, 9, 10, 11). Proteolysis of mHTT generates N-terminal fragments containing the polyQ expansion, which is found in HD human brain and mouse models (9, 12, 13, 14, 15, 16, 17, 18). The cleaved forms of the protein are found in multiple cellular compartments, including the nucleus, and cause aberrant interactions of mHTT with key partners, such as transcription factors, autophagy, and mitochondrial proteins, and thus lead to neuronal death in the striatum and cortex (19, 20, 21). Deciphering how the mHTT alters the proteome is critical to understanding HD molecular mechanisms, and few studies have focused on defining the proteomes of human HD patient–derived neurons.
Recent progress in mass spectrometry (MS)-based proteomics has allowed significant improvements in proteome resolution, sensitivity, and depth coverage of a given biological system (22, 23). Previous studies used quantitative MS-based proteomics to measure relative changes in the protein abundances in human postmortem HD frontal cortex and identified signaling pathways that are dysregulated in HD, including Rho-mediated, actin cytoskeleton and integrin signaling, mitochondrial dysfunction, and axonal guidance (24). Comprehensive quantitative proteomics applied to investigate spatiotemporal mechanisms of mHTT in R6/2 HD mice characterized the insoluble proteome during disease progression and highlighted extensive dysregulation in brain regions vulnerable to HD (25). A recent review highlights the proteomics carried out in HD and some of the critical gaps in the field, including the generation of robust human HD cell type–specific proteome data sets (26).
Disease modeling in human induced pluripotent stem cells (iPSCs) allows central nervous system (CNS)-relevant cells to be generated in vitro and molecular defects to be identified that contribute to polyQ-expansion disorders (27, 28, 29, 30). In our previous work, we used human patient–derived HD-iPSCs (72CAG/19CAG, HD72) and genetically corrected the cells to a normal repeat length (21CAG/19CAG, C116), thus creating an isogenic control (31). Our group showed that HD phenotypes manifest in differentiated neural stem cells (NSCs), not in iPSCs (32, 33). Further, our transcriptomic analysis of isogenic HD72-NSCs suggested that HD is linked to developmental impairments that prevent the proper generation of MSNs and subsequent loss of MSN identity (27, 31, 32, 34, 35). So far, quantitative proteomics of iPSCs modeling HD focused on undifferentiated stem cells or unrestricted neuronal populations (36, 37). In contrast, the proteome in directed HD72-MSNs derived from iPSCs has not been explored.
To define the proteomic signature in isogenic HD72-MSNs and to determine how mHTT leads to neurotoxicity in MSNs, we performed comprehensive quantitative proteomics by LC-tandem mass spectrometry (LC-MS/MS) using complementary approaches. First, we compared triplicates of HD72-MSNs and isogenic controls in which the CAG expansion was genetically corrected to a normal repeat length. We used an unbiased discovery workflow combining a modern high-field asymmetric-waveform ion mobility spectrometry (FAIMS) device (38) with an Orbitrap Lumos mass spectrometer operating in data-dependent acquisition (DDA) mode for the identification and MS1-based label-free quantification (LFQ) of significantly changing protein candidates (Discovery). Subsequently, we acquired the same samples by data-independent acquisition (DIA) on a TripleTOF 6600 mass spectrometer for further quantification and validation (MS2-based Quantification, DIA) (39). Our proteomic workflow is summarized in Figure 1. Findings on altered triglyceride homeostasis were followed up using quantitative confocal microscopy image analysis relevant to autophagy. This pathway was found in both the FAIMS-DDA MS and DIA workflow.
Fig. 1.
Schematic representation of HD, isogenic HD-MSN, and proteomics workflow.Upper left, HD is a monogenic disease caused by a CAG (coding for glutamine) expansion in the HTT gene. The striatum, depicted in the upper left panel, is heavily affected in HD. The inhibitory medium spiny neurons are lost during disease progression. Upper right, total proteins were isolated from C116-MSN and HD72-MSN cultures (in triplicates). Bottom, samples were digested and subjected to a comprehensive quantitative proteomic analysis with deep coverage with FAIMS ion mobility separation coupled to data-dependent acquisition mode on an Orbitrap Lumos mass spectrometer for label-free quantification. Subsequently, significantly changed proteins were validated with an independent quantitative approach collecting data-independent acquisitions on a TripleTOF 6600 mass spectrometer. After protein identification and quantification, bioinformatic analyses were used to identify molecular pathways and networks relevant in HD. FAIMS, high-field asymmetric-waveform ion mobility spectrometry; HD, Huntington’s disease; MSN, medium spiny projection neuron.
Experimental Procedures
Human iPSC-Derived NSC Cultures
C116 and HD72 iPSCs were maintained in mTeSR1 (STEMCELL Technology, 05850) medium before the differentiation. To induce iPSCs toward a neuroepithelial fate, we used a monolayer differentiation approach with modifications. Briefly, iPSCs were manually cleaned by removing any colonies with spontaneous differentiation. To initiate differentiation (day 0), iPSCs were passaged with 1 mg/ml collagenase [(Type IV, Thermo Fisher Scientific, 17104019) in Gibco KnockOut DMEM/F-12 medium (Thermo Fisher Scientific, 12660012)] for 35 min at 37 °C. The colonies were gently detached by scraping, and the cell aggregates were triturated by pipetting 2 to 3 times with a 2-ml pipette to yield a uniform suspension of aggregates and avoiding creating a single-cell suspension. The cell aggregates were transferred onto a Matrigel (1 ml, 50 μg, Corning, CB-40234)-coated plate containing mTeSR1 and incubated at 37 °C. For neural induction (day 2), SMAD signaling was inhibited by adding SB431542 (10 μM, Tocris, 1614) and LDN-193189 (1 μM, Tocris, 6053) in mTeSR1 during the medium changing, thus promoting neuroectodermal differentiation and suppressing mesoderm and endoderm fates (40). From day 4, the mTeSR1 medium was changed every day in the presence of SB431542 (10 μM, Tocris, 1614) and LDN-193189 (1 μM, Tocris, 6053). From day 8, the colonies became organized and increased in size. The center of colonies became dense and compact, and the peripheral regions presented elongated cells. At day 10, the colonies were first cleaned to remove peripheral regions, and then the dense center regions were manually picked by scraping after a collagenase treatment of 25 min at 37 °C, to avoid over-collagenase. Cell aggregates from the center regions were transferred at low density to minimize merging, into a 10-cm Matrigel (1 ml, 50 μg, Corning, CB-40234)-coated plate containing N2B27 medium [(DMEM/F12, Gibco, Thermo Fisher Scientific, 11320-033) supplemented with 1× N2 (Thermo Fisher Scientific, 17502001), 1× B27 (Thermo Fisher Scientific, 17504001), 1× GlutaMAX (Thermo Fisher Scientific, 35050061), 1× Non-Essential Amino Acids (Thermo Fisher Scientific, 11140050), 25 ng/ml β-fibroblast growth factor (FGF, Peprotech, 100-18B), and 100 U/ml penicillin/streptomycin (P/S, Thermo Fisher Scientific, 15140122)]. The cells were cultured in the presence of 25 ng/ml activin A (PeproTech, AF-120-14E) to induce regional patterning toward a lateral ganglionic eminence identity. The N2B27 medium was changed every 2 days in the presence of activin A and β-FGF. At day 12, neuroepithelial differentiation became apparent with the formation of small neural rosettes showing a columnar shape that further organized and increased in size at day 14 in forming neural tube-like structures with a central lumen and three-dimensional growth. Thereafter, the neural rosette structures were mechanically selected by separating the island from the surrounding cells with a needle to minimize contamination with nonneural cells. The isolated rosettes were triturated by pipetting 8 to 10 times with 1000-μL pipette tip. At least 15 to 20 neural rosettes/well were plated in a Matrigel-coated P12-well plate in the presence of Neural Proliferation Medium [Neurobasal medium (Thermo Fisher Scientific, 21103049), B27-supplement 1× (Thermo Fisher Scientific, 17504001), GlutaMAX 1× (Thermo Fisher Scientific, 35050061), and 10 ng/ml leukemia inhibitory factor (PeproTech, 300-05), 100 U/ml P/S] supplemented with 25 ng/ml β-FGF and 25 ng/ml activin A. The resulting NSCs were passaged when cell cultures became confluent. The passaging cells were moved gradually from P6, P12 wells to 6-cm plates with the cells plated at a high density. Nestin, SOX1, SOX2, and PAX6 staining of NSCs validated the cell type.
MSN Differentiation
Activin A (25 ng/ml, PeproTech, AF-120-14E)–generated C116 and HD72 NSCs were used to prepare MSNs. Nunc six-well plates were treated with poly-D-lysine hydrobromide (1 ml, 100 μg/ml by Sigma Aldrich, P6407) and incubated (37 °C and 5% CO2) overnight (ON). Corning cell culture grade water, 25-055-CVC (1 ml), was used to wash plates, and the plates were dried for 1 h. Next, the plates were treated with Matrigel (1 ml, 50 μg, Corning, CB-40234) ON in a 37 °C incubator. MSNs were prepared according to Kemp et al. (30). Synaptojuice A medium (2 ml) was used for seeding NSCs (1 × 106 per well). Synaptojuice A was prepared with 10× synaptojuice A supplement (5 ml), advanced DMEM/F12 medium (44.1 ml, Gibco, 12634010), penicillin/streptomycin (P/S) (450 μl, Invitrogen, 15140122), and 100× Glutamax (450 μl, Invitrogen, 35050079). Synaptojuice A supplement (10×) contains advanced DMEM/F12 medium (38 ml, Thermo Fisher Scientific, 12634010), MACS NeuroBrew-21 with retinoic acid with final concentrations noted (10 ml, MACS Miltenyi Biotec, 130-093-566), PD0332991 (20 μM, Tocris Bioscience, 4786), DAPT (100 μM, Tocris Bioscience, 2634), human brain-derived neurotrophic factor (BDNF, 100 ng/ml, MACS Miltenyi Biotec, 130-096-286), LM22A4 (5 μM, Tocris Biotec, 4607), forskolin (100 μM, Tocris Bioscience, 1099), CHIR 99021 (30 μM, Tocris Bioscience, 1099), gamma aminobutyric acid (GABA, 3 mM, Tocris Bioscience, 0344), CaCl2 (1.8 mM, Tocris Bioscience, 3148), ascorbic acid (2 mM, Tocris Bioscience, 4055). Medium was passed through a 0.22-μm filter. Cells were treated with synaptojuice A (2 ml) for 7 days, performing half-medium changes every other day. On day 8, full-medium changes were completed, and then, the cells were treated with synaptojuice B (2 ml) for the next 7 days. Synaptojuice B was prepared with 10× synaptojuice B (5 ml) supplement and basal medium (45 ml). Basal medium contains advanced DMEM/F12 medium (22.5 ml, Thermo Fisher Scientific, 12634010), P/S (450 μl, Invitrogen, 15140122), and 100× Glutamax (450 μl, Invitrogen, 35050079) and Neurobasal A Medium (22.5 ml, Gibco, 10888022), P/S (450 μl, Invitrogen, 15140122), and 100× Glutamax (450 μl, Invitrogen, 35050079). Synaptojuice B supplement contains advanced DMEM/F12 medium (19.7 ml, Thermo Fisher Scientific, 12634010), Neurobasal A medium (19.7 ml, Gibco, 10888022), MACS NeuroBrew-21 with retinoic (10 ml, MACS Miltenyi Biotec, 130-093-566), PD0332991 (100 μl, 20 μM, Tocris Bioscience, 4786), human BDNF (50 μl, 100 ng/ml, MACS Miltenyi Biotec, 130-096-286), LM22A4 (25 μl, 5 μM, Tocris Biotec, 4607), CHIR 99021 (250 μl, 30 μM, Tocris Bioscience, 1099), GABA (500 μl, 3 mM, Tocris Bioscience, 0344), CaCl2 (370 μl, 1.8 mM, Tocris Bioscience, 3148), and ascorbic acid (100 μl, 2 mM, Tocris Bioscience, 4055). Synaptojuice B medium was filtered through a 0.22-μm filter. Cells were treated with synaptojuice B (2 ml) for 7 days, and half-medium changes were performed until day 14.
MSNs Treatments With IFN-γ
For the interferon-gamma (IFN-γ) experiments, prepatterned activin A–treated NSCs from C116 and HD72 were plated at 90,000 cells per well in an eight-well chamber slide for MSN differentiation. After synaptojuice A and B treatment, MSNs were stimulated for 48 h with IFN-γ (PeproTech, 300-02-100UG) at different concentrations: 10, 50, 100, and 200 ng/ml. Nontreated MSNs were used as control. For each treatment, a duplicate was performed.
Cell Immunofluorescence of Human MSNs
Cells were fixed using 4% paraformaldehyde (Sigma, 158127) in 0.1 M PBS, pH 7.4 (Corning, 21-040-CV) for 30 min. After three washes in cold PBS, cells were permeabilized and blocked for 1 h at room temperature (RT) using 0.1% Triton X-100 (Thermo Fisher Scientific, 28313) and 4% normal donkey serum (Jackson Immuno Research, 017-000-121) in PBS. Primary antibodies were added in the presence of blocking buffer ON at 4 °C. Secondary antibodies (1:500) were added after three PBS washes in blocking buffer at RT for 1 h. The following primary antibodies were used for the immunofluorescence studies: rabbit anti-DARPP-32 (Santa Cruz, sc-271111, 1:100), rabbit anti-MAP2 (Millipore, AB5622, 1:100), rabbit anti-Nestin (Abcam, ab92391, 1:00), mouse anti-MHC-class-II (Abcam, ab55152, 1:100), rabbit anti-Cleaved Caspase-3 (CellSignal, 9661, 1:100), and mouse anti-HTT (Millipore, MAB2166, 1:100). The secondary antibodies were donkey anti-rabbit, anti-mouse IgG conjugated with Alexa-546 (Invitrogen, A10040 and A10036) or Alexa-647 (Invitrogen, A-31573 and A-31571). Images were acquired using a Biotek Cytation 5 microscope and were prepared using Fiji software (ImageJ, https://fiji.sc/).
Protein Extraction for Proteomic Analysis
Triplicate samples of cultured C116-MSNs and HD72-MSNs were washed three times with cold PBS 1×, pH 7.4 (Corning, 21-040-CV), and total proteins were isolated using 300 μl of cold mammalian protein extracting reagent (Thermo Fisher Scientific, 78501) containing protease inhibitor cocktail (cOmplete, Mini Protease Inhibitor Cocktail, Roche, 11836170001). The cell lysate was harvested by scraping and transferred directly into a cold 1.5-ml tube and stored at −80 °C.
Proteomic Sample Preparation
Chemicals
Acetonitrile (AH015) and water (AH365) were from Burdick & Jackson. Iodoacetamide (I1149), DT (D9779), formic acid (94318-50ML-F), and triethylammonium bicarbonate buffer 1.0 M, pH 8.5 (T7408) were from Sigma Aldrich; urea (29700) was from Thermo Fisher Scientific, sequencing grade trypsin (V5113) was from Promega, and HLB Oasis SPE cartridges (186003908) were from Waters.
Protein Precipitation, Digestion, and Desalting
Protein samples were precipitated with a ProteoExtract Protein Precipitation Kit (539180) from MilliporeSigma as per the manufacturer’s protocol. Samples were resuspended in 50 mM triethylammonium bicarbonate. Total protein concentration was determined with a BCA kit (23227) from Thermo Fisher Scientific. Aliquots of each sample containing ∼100 μg of protein were brought to equal volumes with 50 mM triethylammonium bicarbonate buffer at pH 8. The mixtures were reduced with 20 mM DTT (37 °C for 1 h) and then alkylated with 40 mM iodoacetamide (30 min at RT in the dark). Samples were diluted 10-fold with 50 mM triethylammonium bicarbonate buffer at pH 8 and incubated ON at 37 °C with sequencing grade trypsin (Promega) at a 1:50 enzyme:substrate ratio (wt:wt). Peptide supernatants were collected and desalted with Oasis HLB 30-mg Sorbent Cartridges (Waters; 186003908), concentrated, and resuspended in a solution containing mass spectrometric “Hyper Reaction Monitoring” retention time peptide standards (Biognosys; Kit-3003) and 0.2% formic acid in water.
Mass Spectrometric Analysis
Orbitrap Lumos FAIMS DDA MS Analysis
Triplicate samples from corrected C116-MSNs and HD-MSNs were analyzed by reverse-phase HPLC-ESI-MS/MS on the EASY-nLC 1200 system and analytical column (Thermo EASYspray 50 cm × 75 μm ID, PepMap C18 2 μm, 100 Å) coupled to the Orbitrap Lumos mass spectrometer (Thermo Fisher Scientific) with an EASY-Spray source. Column temperature was set to 50 °C. Mobile phase A was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in 80% acetonitrile and 19.9% water. Flow rate was set at 300 nl/min, and a two-stage gradient was used for each sample: (1) 7 to 30% mobile phase B over 125 min and (2) 30 to 45% mobile phase B over 40 min. For each sample, 2 μg of peptides were injected onto the column. All samples were analyzed by DDA. For DDA analysis, full MS scans were performed over m/z 380 to 1580 with the Orbitrap analyzer operating at 240,000 resolution and automatic gain control = 400,000 ions with FAIMS settings enabled at three compensation voltages (CVs): −50 V, −65 V, −85 V. Each of the selected CVs was applied to sequential survey scans and MS/MS cycles (1 s per CV). Survey scans were followed by MS2 scans of the most intense precursor ions for 1 s. MS2 scans were performed by 0.7 m/z isolation with the quadrupole, normalized higher-energy collisional dissociation collision energy of 35%. Dynamic exclusion was set to 30 s, mass tolerance to 10 ppm, and intensity threshold to 5000. Maximum injection time was set to 30 ms, AGC target was set to 10,000 ions, charge states +1 or >+8 were excluded, and the advanced peak determination was toggled on.
TripleTOF 6600 DIA MS Analysis
Samples were analyzed by reverse-phase HPLC-ESI-MS/MS using the Eksigent Ultra Plus nano-LC 2D HPLC system combined with a cHiPLC system directly connected to an orthogonal quadrupole time-of-flight TripleTOF 6600 mass spectrometer (SCIEX). Typically, mass resolution in precursor scans was approximately 45,000, and fragment ion resolution was approximately 15,000 in “high sensitivity” product ion scan mode. After injection, peptide mixtures were transferred onto a C18 pre-column chip (200 μm × 6 mm ChromXP C18-CL chip, 3 μm, 300 Å; SCIEX) and washed at 2 μl/min for 10 min with the loading solvent (H2O/0.1% formic acid) for desalting. Peptides were transferred to the 75 μm × 15 cm ChromXP C18-CL chip, 3 μm, 300 Å (SCIEX) and eluted at 300 nl/min with a 3-h gradient using aqueous and acetonitrile solvent buffers. All samples were analyzed by DIA, specifically using variable window DIA acquisitions (41). In these DIA acquisitions, 64 windows of variable width (5–90 m/z) were passed in incremental steps over the full mass range (m/z 400–1250) with an overlap of 1 m/z. The cycle time of 3.2 s included a 250-ms precursor ion scan, followed by acquisition of 64 DIA MS/MS segments, each with a 45-ms accumulation time (see supplemental Table S1 for the window isolation scheme). The variable windows were determined according to the complexity of the typical MS1 ion current observed within a certain m/z range using a SCIEX “variable window calculator” algorithm (more narrow windows were chosen in “busy” m/z ranges and wide windows in m/z ranges with few eluting precursor ions) (42). DIA tandem mass spectra produce complex MS/MS spectra, which are a composite of all the analytes within each selected Q1 m/z window.
Data Processing
For FAIMS DDA experiments, data analysis was performed with Proteome Discoverer version 2.3.0.523 (Thermo Fisher Scientific). The database search was performed using SEQUEST HT (Thermo Fisher Scientific), and parameters were as follows: SwissProt human protein database (20,417 entries, 09 April, 2019), trypsin enzyme digestion allowing two missed cleavages, 10-ppm precursor ion mass tolerance, and 0.6-Da fragment ion mass tolerance. Dynamic modifications were methionine oxidation (+15.995 Da) and N-terminal protein acetylation (+42.011 Da), and a static modification was defined as cysteine carbamidomethylation (+57.021 Da). Identifications were filtered to 1% false discovery rate (FDR) (peptide spectrum match, peptide, and protein levels) with Percolator (43). LFQ was performed within Proteome Discoverer using razor and unique peptides, and chromatographic alignment was enabled (maximum 10-min retention time shift and 10-ppm mass tolerance). Abundance was normalized to the total peptide amount and scaled on control average. Modified peptides were excluded from quantification, and peptide quantities were summed for protein abundances. Statistical analysis was performed using ProStaR software suite (44). Proteins with less than two unique peptides and proteins with more than three missing values across all conditions were removed. Data were log2-transformed, and missing values were replaced by the 2.5 percentile value for the partially observed values and missing on the entire condition. Pairwise protein statistics were performed using a Limma t test, and an absolute log2(fold-change) threshold set at 0.58. Sliding linear model method (45) was applied to adjust p-values for multiple testing, and significantly altered proteins were sorted out using a p-value threshold that guarantees a FDR at 1.04%.
For DIA quantification, all collected data were processed in Spectronaut (version 14.2.200619.47784) using Biognosys factory settings. Briefly, calibration was set to nonlinear indexed retention time calibration with precision indexed retention time selected. DIA data were matched against a panhuman library that provides quantitative DIA assays for 10,316 human proteins (46) and supplemented with scrambled decoys (library size fraction of 0.1), using dynamic mass tolerances and dynamic extraction windows. The DIA/SWATH data were processed for relative quantification, comparing peptide peak areas from various different time points during the cell cycle. For the DIA/SWATH MS2 data sets, quantification was based on extracted ion chromatograms (XICs) of 3 to 6 MS/MS fragment ions, typically y- and b-ions, matching to specific peptides present in the spectral library. Interference correction was enabled on MS1 and MS2 levels. Precursor and protein identifications were filtered to 1% FDR, estimated using the mProphet algorithm (47). Quantification was normalized to local total ion chromatogram. Statistical comparison of relative protein changes was performed with paired t-tests, and p-values were corrected for multiple testing, specifically applying group wise testing corrections using the Storey method (48). Finally, proteins identified with less than two unique peptides were excluded from the assay. The quantification significance level was as follows: q-value less than 0.05 and absolute Log2(fold-change) greater than 0.58 when comparing HD72-MSNs versus C116-MSNs.
Data Accession
Raw data and complete MS data sets have been uploaded to the Center for Computational Mass Spectrometry, to the MassIVE repository at UCSD, and can be downloaded using the following link:https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=3a14708986c7468197598b328d1db750 (MassIVE ID number: MSV000088650; ProteomeXchange ID: PXD030786).
Western Blot Analysis
C116 and HD72-MSNs were harvested in mammalian protein extracting reagent (150 μl, Thermo Fisher Scientific, 78501) mixed with a protease inhibitor cocktail (1 tablet/10 ml, Roche, 11836170001). Cells were then processed further via sonification using 5 s of pulsing, 5 s of rest for five rounds at 40 mA. Samples were then spun down at 14,000 rpm at 4 °C for 20 min and quantified using a bicinchoninic acid assay (Thermo Fisher Scientific, 23227). Protein lysates of 10 to 20 μg were added in with DTT (1 μl) and LDS NuPAGE buffer (6 μl). Proteins were boiled at 95 °C for 10 min. Running conditions used were 4 to 12% Bis-Tris gel in 5% MOPS Running Buffer (Invitrogen, NP0001) at 200 V for 1 h. The transfer conditions used were as follows: a 0.45-μm polyvinylidene fluoride membrane transferred in 5% transfer buffer (Invitrogen, NP00061) at 20 mA for 840 min. Primary mouse monoclonal antibody Septin-2 (Proteintech, 60075-1-Ig, 1:20,000), rabbit polyclonal antibody Septin-9 (Proteintech, 10769-1-AP, 1:500), mouse monoclonal IGFBP7 antibody (Santa Cruz Biotechnology, sc-365293, 1:100), and rabbit polyclonal antibody HMGCR (Sigma-Aldrich, SAB4200529, 1:100) were incubated at 4 °C.
MSNs Treatment With APOE3 and Lipid Metabolism Quantification
Prepatterned activin A–treated NSCs from C116 and HD72 were plated at 90,000 cells per well in eight-well chamber slide (Falcon, 354108) for MSNs differentiation using synaptojuice A and B medium. MSNs cultured in serum withdrawal were treated for 48 h with APOE3 (PeproTech, 350-02-500UG) at 312 ng/ml. Nontreated cells were used as control. For each treatment, a duplicate was performed. The cells were fixed using 4% paraformaldehyde (Sigma, 158127) in 0.1 M PBS, pH 7.4 (Corning, 21-040-CV) for 30 min. To identify the lipid droplets, the fixed MSNs were stained with Nile Red (Thermo Fisher Scientific, N1142), a lipophilic dye at a dilution of 1/1000 in PBS for 30 min. To quantify the lipid droplets, confocal microscopy with online spectral fingerprinting on a Zeiss LSM 980 laser scanning confocal microscope was used. The fluorescence emission peak of Nile Red shifts yellow to red, based on increasing polarity of the bound lipid (49). Using automated spectral component extraction, we observed a shorter wavelength punctate (peak at 593 nm) and a longer wavelength (peak at 611 nm) diffuse fluorescence of Nile Red using excitation at 514 nm. Using the “Count cellular foci with secondary cell segmentation” pipeline in Image Analyst MKII (Image Analyst Software), images taken using a Plan-Apochromat 63×/1.40 Oil lens were segmented using DAPI-stained nuclei as seeds and finding cell boundaries using the diffuse, longer wavelength fluorescence of Nile Red. Then, the punctate shorter wavelength foci were counted per cellular area. For analysis of intensities, images recorded with a Plan-Apochromat 20×/0.8 lens and the "Basic fluorescence histometry using nuclear markers (1–3 labels, basic)" pipline. For analysis of colocalization of neutral lipid vesicles with p62, LC3, and LAMP1, Nile Red–stained cultures were permeabilized, and immunofluorescence staining was performed with mouse p62 (SQSTM1) (Abcam, ab56416, 1:100), rabbit anti-LC3 (Novus, Nb100-2331, 1:100), mouse anti-LAMP1 (Abcam, ab25630, 1:100). The secondary antibodies were donkey anti-rabbit, anti-mouse IgG conjugated with Alexa-488 (Invitrogen, A21206 and A21202). The fluorescence spectrum of Alexa-488 was measured in single-probe–stained cultures and then was used for recording using online spectral fingerprinting in the presence of DAPI and Nile Red staining. Image capture was performed in an unbiased manner by setting up positions for recording in bright-field preview scans. In permeabilized cells, the long-wavelength component of the Nile Red did not outline the cells, therefore cell area masks were calculated using Labkit trainable segmentation (50) from the background immunofluorescence. The short-wavelength, neutral lipid component of Nile Red fluorescence and the immunofluorescence spots were identified by image segmentation in Image Analyst MKII and gated with the cell area masks. To amplify punctate fluorescence, local background was removed using rolling ball background subtraction for immunofluorescence. Representative images were smoothed using Wiener filtering. The identified puncta (image segments) were used for measuring size, density and intensities of lipid droplets, immunofluorescence puncta, and counting droplets that colocalized with the immunostaining using a modification of the “Fluorescence and absorbance histometry using nuclear and secondary whole-cell segmentation (1–4 labels - advanced background options)” pipeline and data processing in Microsoft Excel. All images were analyzed using the same pipeline and using global intensity scaling for segmentation. Statistical analysis was performed with Graphpad Prism (Graphpad Software Inc) using an unpaired t test with Welch's correction or a two-way ANOVA with Sidak’s multiple comparison as indicated. Bar graphs represent the mean ± SEM.
Pathway Analysis and Network Visualization
Pathway enrichment analysis was performed using g:Profiler with parameters set to Homo sapiens, custom background (all proteins identified in FAIMS DDA acquisitions: supplemental Table S6), Benjamini-Hochberg FDR, and threshold at 0.05. The gene sets included for the pathway enrichment analyses were obtained from Gene Ontology (GO) database (GOBP_AllPathways), updated February 01, 2020 (http://download.baderlab.org/EM_Genesets/). Enrichment results are available in supplemental Tables S7 and S8. The pathway analysis and network visualization were carried out by using Cytoscape 3.7.2 and Cytoscape Enrichment Map application [version 3.2.1 of Enrichment Map software (51) with the following parameters: analysis type = generic/gProfiler, p-value cutoff = 1, FDR Q-value cutoff = 0.05, and similarity between gene sets was filtered by Jaccard plus overlap combined (coefficient: 0.375)]. The network was manually rearranged to improve layout, and clusters of nodes were automatically annotated using the AutoAnnotate Cytoscape App to highlight the prevalent biological functions among a set of related gene sets. Data were analyzed using QIAGEN Ingenuity Pathway Analysis.
Comparison with Multiple Data sets and Drug Prediction
Enrichment analysis for GO biological processes with differentially expressed proteins (FDR < 0.05, logFC > 0.58) was done utilizing the R package clusterProfiler. Drug prediction was done utilizing the LINCS L1000 characteristic direction signatures search engine (https://maayanlab.cloud/L1000CDS2/#/index) with upregulated and downregulated proteins as input (52).
Experimental Design and Statistical Rationale
In this study, we used human patient–derived HD-iPSCs (72CAG/19CAG, HD72) and genetically corrected the cells to a normal repeat length (21CAG/19CAG, C116), thus creating an isogenic control (31) that was then differentiated into MSNs. Proteomic experiments were conducted with iPSC-derived cultured C116-MSNs (n = 3) and HD72-MSNs (n = 3). “Hyper Reaction Monitoring” retention time peptide standards (Biognosys; Kit-3003) were spiked into the samples before LC-MS/MS analysis in DDA and DIA modes. First, as a discovery step, DDA acquisitions were performed on an Orbitrap Lumos mass spectrometer coupled to a FAIMS device. Identification and MS1 XIC-based LFQ were performed with Proteome Discoverer, as detailed above. To determine altered protein groups in HD72-MSNs versus C116-MSNs significantly, pairwise comparison was performed with ProStaR software suite (44) using a Limma t test and correcting the p-values for multiple testing using the sliding linear model method (45). Then, as a validation step, the same samples were acquired in DIA mode on a TripleTOF 6600 mass spectrometer, and one DIA cycle (3.2 s) was composed of the acquisition of one MS1 scan, followed by the acquisition of 64 variable windows (5–90 m/z) covering the full mass range (m/z 400–1250) with an overlap of 1 m/z. DIA data were processed in Spectronaut v14 using a peptide-centric approach and a panhuman library containing 149,066 precursors and 10,316 human proteins (46) to retrieve MS2 XIC-based quantification information, as described above, and significantly altered protein groups in HD72-MSNs versus C116-MSNs were obtained using a paired t test followed by p-value correction for multiple testing using the Storey method (48).
Results
Generation and Characterization of MSNs From HD-iPSCs
The striatum is dramatically impacted in HD. MSNs, GABAergic inhibitory neurons, are one of the main cell types lost from this region and represent 90% of the striatal neuronal population. To model HD, we used human patient–derived HD-iPSCs (72CAG/19CAG, HD72) that were genetically corrected to a normal repeat length (21CAG/19CAG, C116), thus creating an isogenic control (31). Then, both cell types were differentiated into MSN-like neurons by a method that mimics the major brain developmental stages for this neuronal type: neural induction, regional patterning toward a lateral ganglionic eminence identity in presence of activin A, and terminal differentiation (30, 31, 34, 53, 54) (Fig. 2A). Using immunocytochemistry, we found that the cultures were positive for the MSN marker DARPP-32 and neuronal marker MAP2 (Fig. 2B). HD72-MSNs showed less DARPP-32 (p ≤ 0.01) and MAP2 (p ≤ 0.001) than in C116-MSNs (Fig. 2C). This result is consistent with the expression of these markers in postmortem HD striatum and in mouse models of HD (55, 56, 57).
Fig. 2.
Generation and characterization of iPSC-derived MSNs.A, schematic of steps illustrating the generation of neural stem cells and differentiated MSNs. The method used to differentiate iPSCs into MSNs mimics the major brain developmental stages, including neural induction, regional patterning toward an LGE identity in presence of Activin A, and terminal differentiation. The images were acquired using the bright field from the Biotek, on 10× and 20× magnifications with scale bars of 500 and 200 μm, respectively. B, C116-MSN and HD72-MSN were immunostained after differentiation into MSNs with DARPP-32 (green) and MAP2 (red). Scale bars represent 50 μm. C, expression levels of DARPP-32 and MAP2 were determined using Biotek and Image J analysis. Unpaired t test with Welch's correction ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001. Four micrographs were captured for each genotype. For quantification, a minimum of N = 300 nucleus (DAPI positive) were used. DAPI, 4′,6-diamidino-2-phenylindole; HD, Huntington’s disease; iPSC, induced pluripotent stem cell; LGE, lateral ganglionic eminence; MSN, medium spiny projection neuron.
Proteomic Analysis of HD-MSNs With FAIMS-DDA MS on an Orbitrap Lumos System
The HD72- and C116-MSNs were grown in parallel with three replicates for each genotype and subjected to the proteomic workflow in Figure 1. Intracellular proteins were extracted, digested, and subjected to a comprehensive quantitative proteomic analysis by LC-MS/MS using a combined approach: protein discovery and LFQ using FAIMS-DDA MS on an Orbitrap Lumos system, followed by protein candidate validation using DIA MS on a TripleTOF 6600 system, and data and bioinformatic analysis (Fig. 1 and supplemental Table S1).
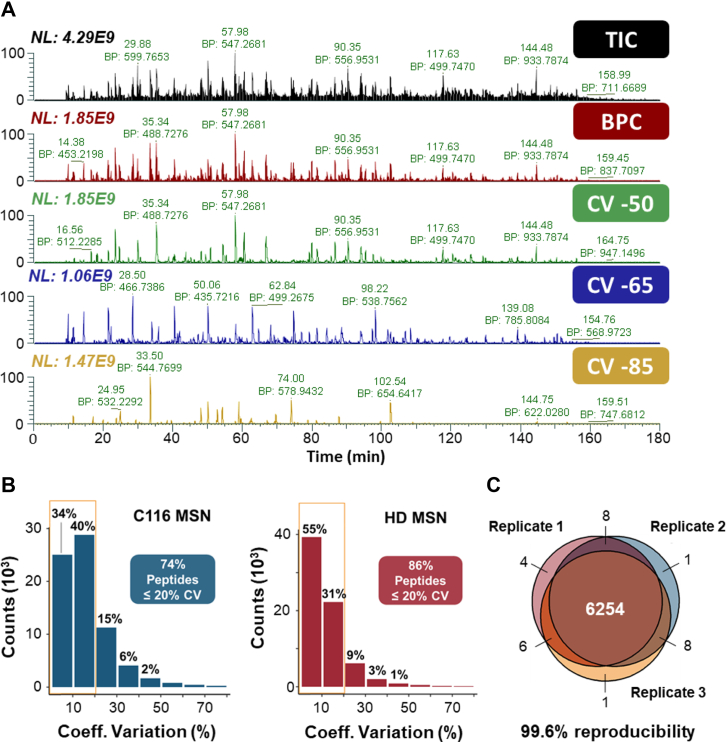
First, we coupled an additional gas-phase separation using FAIMS ion mobility to DDA acquisitions and specifically applied three internal CV steps, −50 V, −65 V, and −85 V. The gas-phase separation protocol reduces the complexity of the ion population entering the mass spectrometer, which provides deeper MS/MS sampling and proteome coverage (38, 58, 59) (Fig. 3A). This process allowed us to identify 6323 unique protein groups (≥2 unique peptides, FDR ≤ 0.01, supplemental Table S2), among which 6294 protein groups were quantifiable by LFQ algorithms in Proteome Discoverer (Table 1 and supplemental Table S3), providing a comprehensive and deep proteomic dataset for the HD72-MSNs. Assessing the LFQ-MS1–based protein quantification reproducibility within each experimental condition, using three biological replicates of isogenic C116-MSNs and HD72-MSNs, revealed that the coefficient of variation for peptide peak areas was under 20% for 74% of all peptides of the C116-MSN group and 86% of all peptides of the HD72-MSN group (Fig. 3B). Reproducibility of protein group identifications is displayed for three biological replicates of HD72-MSNs (Fig. 3C). FAIMS-DDA quantification details for all replicates are shown in supplemental Table S3. Of the 6294 quantifiable protein groups (using FAIMS-DDA), when comparing HD72-MSNs to C116-MSNs, 901 proteins were significantly changed (supplemental Table S4B): 443 proteins were upregulated, 458 were downregulated (FDR set at 1% and absolute Log2(fold-change) ≥ 0.58), and 5393 were unchanged (Fig. 4A and Table 1).
Fig. 3.
Deep proteome coverage using FAIMS gas-phase separation with DDA: Performance of the FAIMS-DDA MS workflow.A, total ion chromatogram (TIC) and base peak chromatogram (BPC), followed by BPC with the three differential coefficients of variation (CVs) (−50 V, −65 V, and −85 V) for a 2 μg C116-corrected MSN sample injection on a FAIMS-Orbitrap Lumos system operating in DDA mode. B, CVs of peptides quantified in three biological replicates of C116-corrected MSNs and HD-MSNs. C, reproducibility of protein groups identified in three biological replicates of HD-MSNs. DDA, data-dependent acquisition; FAIMS, high-field asymmetric-waveform ion mobility spectrometry; HD, Huntington’s disease; MS, mass spectrometry; MSN, medium spiny projection neuron.
Table 1.
Label-free quantification overview (DDA-LFQ and DIA)
| LC-MS/MS system | FAIMS-Orbitrap Fusion Lumos Tribrid | TripleTOF 6600 |
|---|---|---|
| Acquisition (Quantification) | FAIMS-DDA MS for label-free quantification at the MS1 level | DIA MS for label-free quantification at the MS2 level |
| Protein groups quantified (≥2 peptides, 1% FDR) | 6294 | 3106 |
| Upregulated protein groups HD-MSN versus C116-MSN | 443 (1% FDR and logFC absolute value >0.58) | 96 (q-value <0.05 and logFC absolute value >0.58) |
| Downregulated protein groups HD-MSN versus C116-MSN | 458 (1% FDR and logFC absolute value >0.58) | 66 (q-value <0.05 and logFC absolute value >0.58) |
Abbreviations: DDA, data-dependent acquisition; DIA, data-independent acquisition; FDR, false discovery rate; LFQ, label-free quantification; logFC, log2 fold change.
Fig. 4.
Differential analysis of the proteome of isogenic C116- and HD72-MSNs by FAIMS-DDA MS.A, summary of the proteins quantified and significantly altered using FAIMS gas-phase separation with DDA. B, heat map illustrating the abundance of the proteins of the C116- and HD72-MSNs identified by FAIMS DDA MS with at least two peptides (FDR ≤ 0.01). The heat map represents more precisely the values of MS peak area for n = 3 C116-MSNs and n = 3 HD72-MSNs. C, heat map illustrating the top 50 statistically significant altered proteins in the HD72- versus C116-MSNs using FAIMS-DDA MS. D, volcano plot illustrating the proteins differentially expressed when comparing HD72- versus C116-MSNs (significant proteins: FDR at 1% and log2 fold-change absolute value >0.58). DDA, data-dependent acquisition; FAIMS, high-field asymmetric-waveform ion mobility spectrometry; FDR, false discovery rate; HD, Huntington’s disease; MS, mass spectrometry; MSN, medium spiny projection neuron.
Proteomic Analysis of HD-MSNs With DIA-MS
To further validate these protein candidates for HD72-MSNs, we used a comprehensive quantitative methodology, DIA-MS, in which fragment ions (MS2) were quantified with accurate relative quantification results. This approach for C116 and HD72-MSN provided protein quantification for 3106 protein groups with very high reproducibility (supplemental Fig. S1) with at least two peptides identified (supplemental Table S5). In fact, 72% and 80% of the identified precursor ions presented a coefficient of variation below 20% for C116-MSNs and HD72-MSNs, respectively (supplemental Fig. S1). Out of the 3106 quantifiable protein groups, 162 protein groups were significantly altered in HD72-MSNs versus C116-MSNs using DIA-MS (q-value ≤ 0.05 and absolute Log2(ratio) ≥ 0.58) (supplemental Table S5B). Among those, a panel of 129 protein groups identified in the FAIMS DDA discovery study was thus validated by the highly quantitative DIA-MS strategy (supplemental Fig. S2A and supplemental Table S4, B and C). More precisely, 54 protein groups were significantly downregulated, and 75 protein groups were significantly upregulated in both FAIMS DDA and DIA-MS data sets, when comparing HD72-MSNs to C116-MSNs (supplemental Fig. S2, B and C). A total of 292 proteins were common between the significant FAIMS DDA proteins and all measured DIA-MS proteins. Two hundred sixty six proteins agree or trend in the same direction as the FAIMS DDA (see correlation plots supplemental Fig. S2D). The correlation plot shows 91% of the proteins trend in the same direction. Pathways that had proteins in both data sets were further validated as described below (SEPTs, apolipoprotein E (APOE), and minichromosome maintenance (MCM)).
Visualization of the Proteomic Analysis of HD-MSNs With FAIMS-DDA MS
Using hierarchical clustering of protein abundances, we evaluated the variation in C116-MSNs and HD72-MSNs. Heat map representation of the proteomics showed distinct clustering of the two sample groups that depended on the polyQ-repeat length, with HD72-MSN samples being clearly delineated from C116-MSNs (Fig. 4B and supplemental Table S3). This is consistent with previous studies that found distinct phenotypes for HD and corrected NSCs (32).
To visualize the clustering, quality, and significantly altered proteins in HD72-MSNs and C116-MSNs, a heat map is shown in Figure 4C for the top 50 proteins with the highest statistical significance. The biological function, cellular component, molecular function, KEGG, Reactome, and WIKI pathways of each protein are summarized in supplemental Table S2. The analysis of significantly altered proteins is depicted in the volcano plot showing the estimated Log2(fold-change) versus −Log10(p-value) for each protein, with significantly differentially regulated proteins having a p-value that guarantees a 1% FDR and an absolute Log2(fold-change) value above 0.58 (Fig. 4D, Table 1 and supplemental Table S4). The FAIMS-DDA MS workflow applied for the discovery step resulted in 901 significantly changed protein candidates (Table 1), and 129 of these proteins were additionally validated by DIA MS with confidence as significantly changing (Table 1).
Newly discovered and previously implicated proteins in HD are shown in the heat map and volcano plots (Fig. 4, C and D). One of the top upregulated proteins was insulin-like growth factor-binding protein 7 (IGFBP7). IGFBP7 is released by senescent cells, and cellular senescence is a pathway we previously identified as activated in HD-MSNs with a multitude of relevant markers, including IGFBP7 mRNA (60). IGFBPs are biomarkers for multiple diseases, and their expression causes neurodegeneration (61, 62, 63, 64, 65, 66, 67). Western blot analysis further validated the increased levels of IGFBP7 in HD72-MSNs (supplemental Fig. S3). OCIAD2, another top upregulated protein, is implicated in Parkinson’s disease (PD) and Alzheimer’s disease (AD) and activates STAT3 (68, 69). GPX7 (glutathione peroxidase) is upregulated in HD72-MSNs. GPX activity is increased in HD patient blood (40, 70), and GPX7 (related family member, GPX6) is neuroprotective when overexpressed in HD yeast, Drosophila, and mouse models (71, 72). Proteins involved in lipid metabolism, such as APOE, are downregulated and will be discussed further below (Fig. 4D). The levels of the HTT were detected in our studies, but the levels were not changed (supplemental Table S3) between the two genotypes.
Functional Enrichment and Protein Network Analysis Reveal Molecular Hallmarks of HD
Functional enrichment studies (51, 73, 74) with the significantly altered proteins in HD72-MSNs revealed molecular dysregulation in several pathways (supplemental Fig. S4). We applied the upregulated and downregulated protein lists from FAIMS-DDA MS proteomic workflow to g:Profiler with custom background proteins (supplemental Table S6) (75). The most significant-enriched GO biological process terms, upregulated in HD72-MSNs, include pathways related to the extracellular matrix (ECM) (e.g., Integrin-Laminin signaling, TGF-beta regulation of ECM, epithelial-mesenchymal transition (EMT) activation, activation of matrix metalloproteinases), cardiovascular system, angiogenesis, TAp63 pathway, DNA replication, senescence, organism development, regulation of cell migration and locomotion, aminoglycan glycosaminoglycan proteoglycan, organism development, regulation of cell migration and locomotion, growth factor stimulus, and fatty acid processes (supplemental Table S7). Conversely, processes associated with the downregulated proteins include neurogenesis-axogenesis and, more specifically, the BDNF signaling pathway, Ephrin-A:EphA pathway, regulation of synaptic plasticity, triglyceride homeostasis cholesterol, plasmid lipoprotein particle immune response, INF-γ signaling, immune system major histocompatibility complex (MHC), triglyceride homeostasis, lipid metabolism, lymphocyte proliferation, and cellular response to stimulus (supplemental Fig. S4). Pathways involved in organism development and regulation of cell migration and locomotion, and regulation of biological and homeostatic processes were both upregulated and downregulated. Our proteomic analysis demonstrated that pathways related to cardiomyopathy, cardiovascular, and angiogenesis are upregulated in HD72-MSN consistent with peripheral effects of HD (supplemental Fig. S4 and supplemental Table S7) (76, 77).
The canonical pathways from a complementary analysis using Ingenuity Pathway Analysis (IPA) are shown in supplemental Fig. S5. The top pathways include hepatic fibrosis, semaphorin neuronal repulsive signaling pathway, regulation of cellular mechanics of calpain protease, IL-4 signaling, axonal guidance signaling, caveolar-mediated endocytosis signaling, SNARE signaling, estrogen receptor, RHO GTPase signaling, CLEAR signaling, and many more that have been implicated in HD.
Reactome Functional Interaction Network for Isogenic HD-MSNs Upregulated Proteins
Next, we used functional interaction analysis to define clusters of proteins that are closely connected to each other with ReactomeFIViz, a reactome functional interaction network (78). We identified an interconnected network of the 443 upregulated proteins (Fig. 5 and supplemental Table S8A). There were 11 clusters for the upregulated proteins with ECM and DNA signaling (DNA replication pathway, double-strand break repair, G1/S transition) having the highest significance (Fig. 5, A and B). We describe each cluster below, along with its correlation with functional enrichment and the relevant HD pathogenic mechanisms.
Fig. 5.
De novo subnetwork construction and clustering using proteins differentially upregulated when comparing HD72-MSNs to C116-MSNs.A, networks of genes were constructed using 443 upregulated proteins in HD72-MSNs as determined using FAIMS-DDA MS. The functional network and clustering were performed using the Reactome Functional Interaction Network (ReactomeFIViz). Nodes in the network correspond to genes, and edges correspond to interactions. Shaded ovals represent clusters of genes sharing common enriched biological functions. B, classification of clusters based on false discovery rates. C, quantitative proteomics reveals MCM3, MCM4, MCM6, and MCM6 are expressed more highly in HD72-MSNs than C116-MSNs. Unpaired t test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001. DDA, data-dependent acquisition; FAIMS, high-field asymmetric-waveform ion mobility spectrometry; HD, Huntington’s disease; MCM, minichromosome maintenance; MS, mass spectrometry; MSN, medium spiny projection neuron.
Clusters 0, 1, 4, 6 – ECM Organization, Replicative Senescence
The upregulated interaction network confirmed the dysregulation of ECM-regulated pathways in HD72-MSNs. The Cluster 0 protein network was associated with ECM organization, disassembly, and tissue development, highlighting the role of matrix metalloproteinases in HD72-MSNs: CD44, MMP2, MMP14, and TIMP2 (Fig. 5A and supplemental Table S8A). We previously described the role of MMP14 and TIMPs in HD (79, 80).Cluster 1 network identified key proteins involved in ECM-organization, collagen, and integrin signaling: ITGA6, ITGB4, ITGB3, COL1A1, COL4A1, COL4A2, COL5A2, CAV1, FLN, and TNC (Fig. 5A). Dysregulation of ECM components impairs the formation and maintenance of neural circuitry and increases risk for several neurological pathologies, such as HD, AD, and autism spectrum disorder (81).
In clusters 4 and 6, we also identified proteins involved in cellular senescence, including CDKN1A (p16), SERPINE1, and IGFBP7. Recent studies in mouse models of AD and PD suggest cellular senescence is important in disease progression and pathogenesis (82, 83, 84, 85, 86).
Clusters 2, 3 – Muscle Contraction, Netrin Signaling, and Angiogenesis
Interestingly, clusters 2 and 3, Netrin/SEMA1 signaling, Ras protein, Erbb2 signaling, and angiogenesis were novel dysregulated pathways in HD72-MSN that were not identified in pathway enrichment analysis (Fig. 5A). IPA analysis also identified these pathways, and the networks are shown in supplemental Fig. S4.
Cluster 5 – Septin Signaling Pathways in HD
Notably, three members of the septin protein family, SEPT2, SEPT6, and SEPT9, were present in cluster 5. Septin family members were found in the FAIMS DDA and DIA-MS data sets, when comparing HD72-MSNs to C116-MSNs (supplemental Fig. S2, B and C). Septin proteins participate in various physiological processes, such as cytoskeleton regulation, cell division, membrane trafficking, neuronal formation, and maintenance. Septin dysregulation is associated with diverse diseases, including cancer, infection, and neurological disorders (87, 88). Using quantitative proteomics, we found that SEPT2, SEPT3, SEPT4, SEPT5, and SEPT9 are dysregulated in HD MSN (Fig. 6A). Further, Western blot analysis on HD72- and C116-MSNs provide validation of the proteomic results. Both SEPT2 and SEPT9 were upregulated in HD72, compared to C116-MSNs (Fig. 6, B and C, p ≤ 0.05, p ≤ 0.01). Importantly, SEPT9 and phosphoinositides regulate lysosome localization, their association with lipid droplets, and lipid droplet growth (89, 90). This is relevant to Cluster 8 that identifies dysregulation of lipid metabolism, including triglyceride and cholesterol pathways in the HD72-MSNs.
Fig. 6.
SEPTIN family members are dysregulated in HD-MSNs.A, quantitative proteomics reveals SEPT2, SEPT3, SEPT4, SEPT5, and SEPT9 are dysregulated in HD-MSNs. B, Western blot analysis shows that SEPT2 and SEPT9 were upregulated in HD72-MSNs, compared to C116-MSNs. ∗indicates nonspecific band. C, quantification of SEPT2 and SEPT9 levels, normalized to vinculin. Unpaired t test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001, ns, not significant. HD, Huntington’s disease; MSN, medium spiny projection neuron.
Cluster 7 – Glycosaminoglycan Biosynthetic Process and Wnt Signaling
Cluster 7 identifies glycosaminoglycan biosynthetic process and Wnt signaling. The Wnt signaling is altered in human and mouse models of HD and may be an early event in the pathogenesis of HD (91, 92, 93). The ECM, particularly, the sulfated glycosaminoglycan component, is structurally and functionally altered in AD (94).
Clusters 8, 9 – Oxidation Reduction Process and Fructose Metabolic Process
Clusters 8 and 9 highlight that proteins associated with fatty acid oxidation and fructose metabolic processes were largely upregulated in the HD-MSNs. Fatty acid metabolism is dysregulated in HD and linked to reduction of active sterol regulatory element responsive protein 2 (SREBP-2) (95, 96, 97). Fructose metabolism is linked to levels of uric acid, and levels of uric acid in biofluids are lower in HD patients than controls (98).
Cluster 10 – DNA Signaling is a Top Enriched Pathway in HD MSNs and Implicates MCM Proteins
Cluster 10 is a prereplicative complex assembly involved in nuclear cell-cycle DNA replication. Genome-wide association studies suggest genes involved in DNA damage-repair mechanisms are modifiers of the age of onset of HD (99). Further, HTT acts as a stress-response protein to modulate DNA damage. We identified the MCM proteins 2 to 7 as a top dysregulated pathway. The MCM complex regulates DNA replication, cell-cycle, and DNA damage responses, and so, it is likely an important signaling pathway for HD (100, 101, 102). Cluster 10 showed MCM3, 4, 5, and 6 as dysregulated pathways in HD-MSNs (Fig. 5, A and B). Analysis of individual MS peaks confirmed that levels of MCM3, 4, 5, and 6 are significantly greater in HD-MSNs than C116-MSNs (Fig. 5C). MCM family members were found in the FAIMS DDA and DIA-MS data sets, when comparing HD72-MSNs to C116-MSNs (supplemental Fig. S2, B and C).
Reactome Functional Interaction Network for Isogenic HD-MSNs Downregulated Proteins
We identified an interconnected network of the 458 downregulated proteins (FAIMS-DDA MS) (Fig. 7 and supplemental Table S8B) using ReactomeFIViz (78). The downregulated-related clusters were associated with immune system-related pathways, axon guidance signaling, MAPK cascade, calcium modulation, and the Wnt signaling pathway (Fig. 7A). Correspondingly, for downregulated pathways, antigen processing, and presentation, interferon-gamma signaling and ephrin receptor signaling were the most significant (Fig. 7B). We describe each cluster below, including its correlation with functional enrichment and relevance to HD pathogenic mechanisms.
Fig. 7.
De novo subnetwork construction and clustering using proteins differentially downregulated when comparing HD72-MSNs to C116-MSNs.A, networks of genes were constructed using 458 downregulated genes in HD72-MSNs as determined using FAIMS-DDA MS. The functional network and clustering were performed using the Reactome Functional Interaction Network (ReactomeFIViz). Nodes in the network correspond to genes, and edges correspond to interactions. Shaded ovals represent clusters of genes sharing common enriched biological functions. B, classification of clusters based on false discovery rate. DDA, data-dependent acquisition; FAIMS, high-field asymmetric-waveform ion mobility spectrometry; HD, Huntington’s disease; MS, mass spectrometry; MSN, medium spiny projection neuron.
Clusters 0 – Vascular Endothelial Growth Factor Receptor, Integrin, and Fc Gamma Receptor Signaling Pathways
Alterations in vascular endothelial growth factor and vascular endothelial growth factor receptor are common in several triplet repeat diseases, and modulation of vascular endothelial growth factor receptor is neuroprotective in HD. Depletion of this growth factor may contribute to the HD-MSN phenotype. The Fc gamma receptors are generally thought to be expressed in immune cells but are also expressed in neurons where they may mediate excitatory pathways and therefore are downregulated in HD-MSNs.
Clusters 1, 2 – Axonal Guidance Through Ephrin Signaling and the Stathmin Pathway
Previous transcriptomic studies of HD-derived NSCs identified altered pathways related to neuronal development, axonogenesis, and axonal guidance (32, 33). Our proteomic analysis of HD-MSNs is consistent with the hypothesis that mHTT prevents the proper development and maintenance of MSNs by downregulating processes related to CNS development (Fig. 7) (103). Furthermore, the functional interactions analysis identified the downregulation of axonal guidance pathway by impairing ephrin proteins, including EPHA5, EPHA7, EPHB2, and EPHA3 (Fig. 7, A and B). We also found that stathmin-1 (STMN1), a protein belonging to the stathmin family, is downregulated in HD-MSNs (Fig. 7A, Cluster 2). Dysregulation of STMN1 occurs in neurological disorders, including AD (104), amyotrophic lateral sclerosis (105), and spinal muscular atrophy (106).
Clusters 3, 8 – Dysregulation of APOE Signaling and Lipid Metabolism in HD72-MSNs
Pathway enrichment analysis revealed a dysregulation of lipid metabolism, including triglyceride and cholesterol pathways in the HD72-MSNs, when compared to the corrected C116-MSNs (Fig. 7). This pathway is particularly interesting as APOE and related lipid metabolism enzymes were found in both the FAIMS DDA and DIA-MS data sets, when comparing HD72-MSNs to C116-MSNs (supplemental Fig. S2, B and C). Correspondingly, APOE expression was downregulated in HD-MSNs and likely modulates the lipid metabolism alterations in HD72-MSNs (Fig. 7A). Further, cluster 3 links HD to altered inositol phosphate metabolism and phosphatidylinositol metabolic processes. Numerous studies link HD to alterations in lipid metabolism, and IPA analysis suggested APOE is a top upstream regulator (107, 108, 109). In addition, several of the lipid metabolism proteins significantly altered in HD-MSNs are regulators of lipid droplet formation (110). These include monoacylglycerol lipase, lipid droplet–associated hydrolase, diacylglycerol O-acyltransferase, low density lipoprotein receptor, NPC intracellular cholesterol transporter 2, and sodium-coupled neutral amino acid transporter.
To quantify for lipid-rich components, including lipid droplets, we used a lipophilic dye Nile Red and discriminated for neutral lipid and phospholipid (Fig. 8, A and B). In serum-withdrawal culture, we found that HD72-MSNs had significantly higher levels of lipid droplets (Fig. 8C) and a trend towards increases in neutral lipids and phospholipids (supplemental Fig. S6), compared to C116-MSNs. Treatment with APOE3 increased the numbers of lipid droplets in HD72 more than in C116-MSNs (Fig. 8, B and C). This suggests HD72-MSNs respond and modify lipid metabolism distinctly from control cells. In addition, we showed that HD72-MSNs have lower levels of APOE than C116-MSNs (Fig. 8, D and E). APOE promotes cholesterol phospholipid efflux via ABCA1 and ABC subfamily G member 1 and uptake (via low density lipoprotein receptor (LDLR) and LRP1). Correspondingly, our unbiased proteomics revealed a significant increase in LDLR and lower levels of ABCA1 (supplemental Fig. S7). Interestingly, the relative expression of HMG-CoA reductase, a key regulator of sterol synthesis, was unchanged as measured by Western blot analysis (supplemental Fig. S8). These data indicate that HD72-MSNs have dysregulated levels of endogenous APOE, in association with the ability to accumulate a significant number of lipid droplets.
Fig. 8.
HD-MSN lipid metabolism and its modulation by APOE3. C116-MSNs and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/ml APOE3. Nontreated (NT) cells were used as controls. The cells were stained with Nile Red, a lipophilic dye. Micrographs were cropped and saturated for presentation. A and B, representative confocal images for lipid droplets. Scale bar represents 10 μm. C, quantification of the density of the lipid droplets in normal and APOE3-treated conditions (sum of the neutral and phospholipid Nile Red staining). Two-way ANOVA comparing the overall effect of genotype (∗∗∗p ≤ 0.001) and using Sidak’s multiple comparison for the effect of APOE in each genotype (∗p ≤ 0.05). D, Western blot analysis of APOE in C116-MSNs and HD72-MSNs. E, quantification of APOE levels normalized to vinculin. Unpaired t test with Welch's correction, ∗∗∗∗p ≤ 0.0001. APOE, apolipoprotein E; HD, Huntington’s disease; MSN, medium spiny projection neuron.
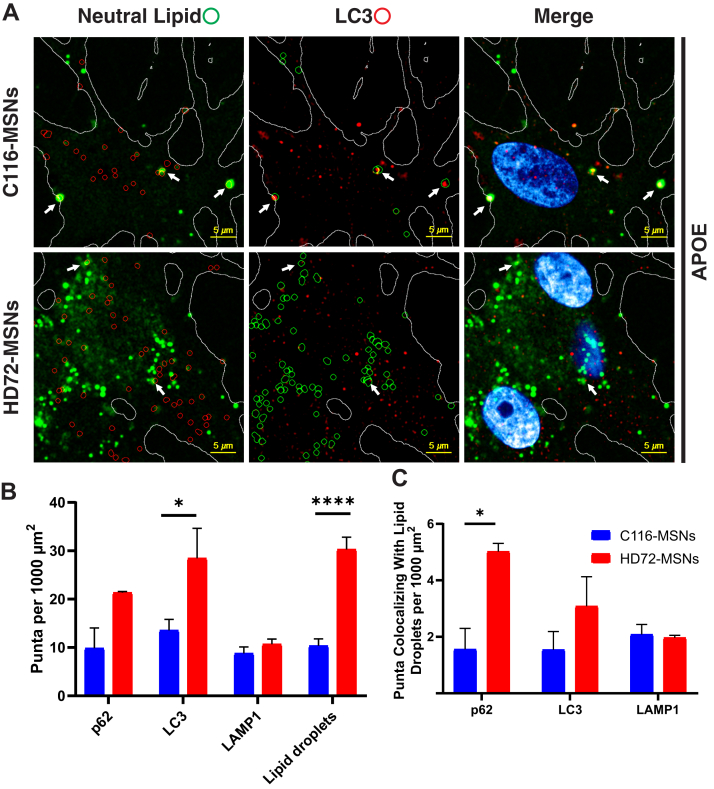
We previously reported a deficit in autophagic flux in our isogenic HD iPSCs model and, therefore, evaluated how markers of autophagy localized with the lipid droplets in the presence of APOE (111). We quantified the lipid droplets that colabel with autophagy markers (p62, LC3, LAMP1) using a combination of Nile Red staining and immunofluorescence (Fig. 9A and supplemental Fig. S9). As expected, the number of LC3 puncta were increased in HD MSNs, compared to C116 (Fig. 9A). The number of autophagosomes colocalizing with lipid droplets was higher for p62 and trending higher for LC3 in HD-MSNs (Fig. 9B). This indicates that marking lipid droplets for autophagy by p62 responds to the increased lipid droplet load. However, autophagy is insufficient to clear lipid droplets perhaps due to increased lipid uptake due to the higher levels of LDLR.
Fig. 9.
Autophagy in the HD-MSN. C116-MSNs and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/ml APOE3. A, cells were stained with Nile Red, a lipophilic dye, and only the short-wavelength, neutral lipid bound fluorescence is shown in representative confocal images (green). In addition, cultures were immunostained for LC3 (red) or p62 and LAMP1 (supplemental Fig. S14). Lipid droplets (indicated by green circles in the other channel) and immunofluorescence puncta (indicated by red circles) were quantified for colabeling (arrows) and density. The white outlines show the boundary of the cells. Micrographs were cropped, smoothed, and saturated for presentation. B, quantification of lipid droplet and immunofluorescence puncta density for p62, LC3, LAMP1, or lipid droplet. C, counts of colabeled lipid droplets normalized to cell surface area. Two-way ANOVA with Sidak’s multiple comparison comparing HD72-MSNs with C116-MSNs, ∗p ≤ 0.05, ∗∗∗∗p ≤ 0.0001. Image quantification was done on total 80 unbiased view fields capturing total 458 DAPI-stained nuclei, a representative of two culture replicates. DAPI, 4′,6-diamidino-2-phenylindole; HD, Huntington’s disease; MSN, medium spiny projection neuron.
Interestingly, APOE3-treated, serum-starved HD72-MSNs have a significantly smaller fraction of the more abundant neutral lipid droplets colabeled with the autophagy markers p62, LC3, and LAMP1 than in C116-MSNs in the same conditions (supplemental Fig. S10). In other words, the few lipid droplets found in the C116-MSNs had relatively more colabeling with the autophagosome markers as a percentage overall. Notably, the average per spot intensities of p62, LC3, and LAMP1 labeling were not different between HD72-MSNs and C116-MSNs, and lipid droplets in HD72-MSNs were slightly smaller than in C116-MSNs but the intensity of individual lipid droplets did not differ (supplemental Fig. S9).
Cluster 4 – TGFβ, SMAD, and TCF12 Signaling Pathways
Cluster 4 identifies TGFβ2 and SMAD1 proteins as dysregulated in HD72-MSNs (Fig. 7A), consistent with previous studies in HD-NSCs (32, 33).
Clusters 5, 7 – Downregulation of HLA- and CNS-Related Proteins in HD-MSNs
Intriguingly, pathway enrichment and functional interaction analysis illustrated that CNS-related pathways are downregulated in HD72-MSNs along with the immune system and IFN-γ responses (Fig. 7). Although the CNS was considered to be immunologically inert, the MHC class I is expressed in mouse brain and neurons (112, 113, 114, 115). Recently, using a single-cell transcriptomic approach, Darmanis et al. (116) showed that MHC-I genes are expressed in a subset of neurons in the human adult brain. Therefore, our results show the expression of MHC components and the IFN-γ response are dysregulated in HD72-MSNs. The functional interactions analysis of downregulated proteins within cluster 0 identified key proteins involved in these pathways: HLA-DPA1, HLA-DPB1, HLA-DMB, HLA-DRA, HLA-DRB1, HLA-DRB4, HLA-DRB3, and HLA-DQB1 (Fig. 7 and supplemental Table S8B). Immune system and IFN-γ responses were downregulated in HD72-MSNs, and this raises the intriguing possibility that neurons use this innate immunity signaling system to regulate neuronal differentiation and neuronal subtype selection during development. Interestingly, MHC-II and DARPP-32 levels were lower in HD72- than C116-MSNs (supplemental Fig. S11).
Clusters 6, 9 – Sodium and Potassium Ion Homeostasis and Proteoglycan Pathways
Cluster 6 was enriched with proteins related to sodium and potassium ion transport across the plasma membrane, including ATP1A3 and ATP1B1. ATP1A3 is highly expressed in the CNS and critical during brain development by modulating osmotic equilibrium and membrane potential (117). Interestingly, in cluster 9, we found that chondroitin sulfate proteins, such as VCAN and BCAN, are downregulated in HD-MSNs. Those proteins may illustrate the function of proteoglycan during important processes of neurodevelopment, such as neuronal migration, differentiation, and maturation (118).
Cluster 11 – Transcriptional Initiation, Elongation, and Termination from the RNA Polymerase 1 Promoter
Altered RNA polymerase I activity is consistent with studies showing ribosomal transcription is regulated by PGC-1 alpha, and this process is impaired in HD (119). HTT is also part of a transcription-coupled DNA complex formed by RNA polymerase II subunit A, basic transcription factors, PNKP, ATXN3, DNA ligase 3, CREB protein (CBP, histone acetyltransferase), and this complex identifies lesions in the template DNA strand and mediates their repair during transcriptional elongation. mHTT likely disrupts RNA polymerase I activity in a similar complex (120).
Comparison of HD-MSNs Proteomic Data Set to Human and Mouse HD Proteome and Modifier Data Sets
We compared our proteomics data set to published proteomics of postmortem HD cortex and knock-in HD mouse model Q175 striatum (supplemental Fig. S12A) (24, 103). Enrichment analysis of downregulated proteins showed dysregulation in all three data sets for the regulation of small GTPase-mediated signal transduction, regulation of neurotransmitter levels, regulation of neuronal synaptic plasticity, regulation of GTPase activity, regulation of cell morphogenesis involved in differentiation, regulation of axonogenesis, neurotransmitter transport, neuron projection guidance, negative regulation of neuron projection development, negative regulation of cell projection organization, axonogenesis, and axon guidance. Enrichment analysis of upregulated proteins showed alterations in the regulation of GTPase-mediated signal transduction, regulation of cell morphogenesis, and axonogenesis. Comparison of differentially expressed proteins in the postmortem cortex of HD patients (24) and those in MSN showed an overlap of 50 proteins. In contrast, compared to the Q175 striatal proteome (103), a well-established HD model, there was an overlap of 19 proteins. Analysis with Enrichr indicates our data set overlaps with transcriptomics of HD grade 3 caudate nucleus GSE3790 with a p-value of 2.0e-27 with 147 genes in common.
Drug Signature of HD-MSNs
We utilized the L1000CDS2 website to identify possible drugs that are predicted to reverse the proteomic signature of HD72-MSNs into C116-MSNs (supplemental Fig. S12B) (121). Trichostatin A, Scriptaid, and Vorinostat were among the top hits identified. They belong to the classes of histone deacetylase inhibitors that are beneficial in HD models (122, 123). An extensive list of drugs identified can be found in supplemental Table S9.
HTT Protein Interaction Network Overlap
We also compared our HD72-MSN proteome with known HTT protein interactors. This revealed over 50 known HTT protein interactors that are altered in the HD MSN proteome (supplemental Fig. S13B). Hub proteins that are highly enriched in our proteomic data set include YWHAB, GRIN2B, ITGB1, amyloid precursor protein, and DLG4; these are proteins implicated in the pathogenesis of HD.
Discussion
Using quantitative proteomic analysis by LC-MS/MS with FAIMS for protein discovery (DDA-MS), we provide a comprehensive coverage of the MSN proteome with 6294 quantifiable proteins identified. Of these proteins, we found ∼14% of the identified proteins had altered expression in HD72-MSNs, compared to isogenic control C116-MSNs. HTT has numerous cellular functions, and the polyQ expansion in the protein would be expected to disrupt multiple pathways involved in neuronal homeostasis. Because these studies were carried out on human neurons derived from HD patient iPSCs, the data may prove useful for further understanding the biology and therapeutic targets in HD MSNs. The comparison of our proteomics data set to published proteomics of postmortem HD cortex (24) showed a strong correlation for downregulated proteins identifying small GTPase-mediated signal transduction, regulation of neurotransmitter levels, regulation of neuronal synaptic plasticity, regulation of GTPase activity, regulation of cell morphogenesis involved in differentiation, regulation of axonogenesis, neurotransmitter transport, neuron projection guidance, negative regulation of neuron projection development, negative regulation of cell projection organization, axonogenesis, and axon guidance.
Our data set has a robust enrichment for processes involved in the brain function, including neurogenesis-axogenesis, the BDNF-signaling pathway, Ephrin-A:EphA pathway, regulation of synaptic plasticity, axonal guidance signaling, caveolar-mediated endocytosis signaling, SNARE signaling, and RHO GTPase signaling. Septin family members were particularly interesting as the polyQ expansion in HTT dysregulated multiple family members SEPT2, 3, 4, 5, and 9. Septin family members form highly organized presynaptic and postsynaptic supramolecular structures and regulate synaptic transmission. Misregulation of human septins has been linked to AD and PD (124). SEPT5 is a substrate for ubiquitin ligase Parkin, and the PD loss of function mutations in Parkin leads to accumulation of SEPT5 and dopamine-dependent neurodegeneration. Correspondingly, SEPT4 KO mice exhibit reduced dopaminergic neurotransmission. Interestingly, SEPT9 modulates cargo entry into dendrites by regulating the motility of two distinct kinesin motors (125). In HD MSNs, SEPT2 and SEPT9 had increased expression, whereas SEPT3, 4, and 5 were downregulated in HD MSNs.
A highly enriched pathway found in HD-MSNs is the dysregulation of ECM. Normal HTT has a role in the construction and regulation of the ECM, and its absence results in disruption of ECM components (126). Our results support the hypothesis that the abnormal polyQ expansion within the mHTT affects the ECM components that ensure the integrity of MSNs in terms of neuronal identity, architecture, and ability to interact with neighboring cells. This hypothesis is consistent with the detection of EMT pathways in the upregulated proteins (Fig. 6 and supplemental Table S7). EMT is a critical cellular process in embryonic development that enables epithelial cells to acquire the properties of mesenchymal cells. ECM proteins are important in maintaining epithelial integrity, in addition to initiating and regulating the EMT (127). HD is characterized by impairment of specification and maturation of MSNs (128). mHTT may impair age-dependent maintenance of striatal MSN identity gene expression (103). Our proteomic analysis showed a dysregulation of ECM-related pathways with the increased EMT-related proteins, which may indicate the inability of HD72-MSNs to acquire and maintain a neuronal signature. Notably, we found that CNS-related pathways were downregulated in HD72-MSNs, including neurogenesis, axonogenesis, axon guidance, regulation of axonal synapse activity, dopamine, and glutamine metabolic process (Fig. 7 and supplemental Table S7).
One of the top statistically significantly enriched pathways was DNA signaling (DNA replication pathway, double-strand break repair, G1/S transition). This fits with numerous studies in the field suggesting an increase in DNA damage in HD, defects in DNA repair mechanisms, and a multitude of genes that enhance or prevent CAG expansion modifying the age of onset of HD (129, 130). We identified the MCM proteins 2 to 7 as a top dysregulated pathway. The MCM complex regulates DNA replication, cell-cycle, and DNA damage responses, and so, it is likely an important signaling pathway for HD (100, 101, 102). The IPA pathway of DNA replication, recombination, repair, and DNA metabolism for the HD72-MSN proteome is shown in supplemental Fig. S14.
One of the signaling pathways identified in our analysis is cellular senescence. We previously showed the development of senescence features in human HD NSCs and MSNs (60). p16INK4a promotes cellular senescence in these human HD cells. FOXO3 is a major cell survival factor that represses cell senescence, opposing p16INK4a expression via the FOXO3 repression of the transcriptional modulator ETS2. In our current study, we find the genes CDKN1A (p21), IGFBP7, HMGA1, and SERPINE1 are part of the cellular senescence pathway activated in HD72-MSNs. Senescent cells also have a senescence-associated secretory phenotype (SASP). Interestingly, when we compare our data set to the recently defined “SASP Atlas”, a database of the secretomes of senescent cells (131), we found the following SASP proteins elevated in HD-MSNs: FLNC, AHNAK, CD44, HSPA1B, HSPA1A, TNC, TIMP2, TKT, EMILIN1, COL6A1, HSPG2, TPM2, TAGLN2, PLEC, MMP2, PKM, HMGA1, ALDOC, CALD1, PSAP, YWHAE, and MIF.
Lipid droplets accumulate during aging, inflammation, oxidative stress, and in neurological diseases (amyotrophic lateral sclerosis, AD, PD). For AD, the interaction of glial cell lipid droplets and neurons play a role in neurodegeneration (132, 133, 134). The roles of lipid droplets in neurological diseases and the brain are not completely understood (135). Guided by pathway analysis of the HD-72 MSNs proteomics identifying lipid metabolism and genes involved in lipid droplet formation, we found an increase in lipid droplets in HD72-MSNs. Our results indicate that HD72-MSNs have dysregulated levels of endogenous APOE, in association with the ability to accumulate a significant number of lipid droplets. Our work and that of others suggest that HD autophagy impairment comes from a deficit in lysosomal processing reflecting changes in p62, LC3 levels, flux, and cargo loading (110, 111, 136). Interestingly, SEPT9, which is increased in HD-MSNs, is directly linked to lipid droplet biology. SEPT9 and phosphoinositides regulate lysosome localization, their association with lipid droplets, and lipid droplet growth (89, 90). In Figure 10, we present a model summarizing our findings that includes increased levels of LDLR, lower levels of ABCA1, increased lipid droplet formation, possible insufficient lipophagy, and dysregulation of SEPT9, a key regulator of lipid droplet biology.
Fig. 10.
Schematic summarizing the altered triglyceride homeostasis, lipophagy, and lipid droplet formation in HD-MSNs. Image was made with BioRender. HD, Huntington’s disease; MSN, medium spiny projection neuron.
Our quantitative unbiased proteomics analysis of HD-MSNs provides a comprehensive understanding of the proteins altered in human HD-MSNs derived from patient iPSCs. We identified signaling pathways not dysregulated in proteomic of human HD neurons, including MHC class proteins, IFN-γ, cellular senescence, ApoE signaling/lipid metabolism, and regulation of cellular response to heat in neurons. Since many of the proteins discovered in our study have been identified in related neurological diseases, our findings will likely accelerate the identification of new biomarkers for HD.
Data Availability
Raw data and complete MS data sets have been uploaded to the Center for Computational Mass Spectrometry, to the MassIVE repository at UCSD, and can be downloaded using the following link:https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=3a14708986c7468197598b328d1db750 (MassIVE ID number: MSV000088650; ProteomeXchange ID: PXD030786).
[Note to the reviewers: To access the data repository MassIVE (UCSD) for MS data, please use: Username: MSV000088650_reviewer; Password: winter].
Supplemental data
This article contains supplemental data (24, 103, 121, 137, 138).
Conflict of interest
A. A. G. has a financial interest in Image Analyst Software. All other authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
B. S. would like to thank Thermo Scientific for scientific and technological support and expertise. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Gary Howard for editing manuscript and John Carroll for graphics.
Funding and additional information
This work was supported by the National Institutes of Health awards: K99 AG065484 (PI: Basisty), S10 OD028654 (PI: Schilling), and 1S10 OD016281 and R01-NS100529 (PI: Ellerby). Support was also provided by “The Taube Family Program in Regenerative Medicine Genome Editing for Huntington's Disease” to L. M. E. K.-T. T. was supported by a fellowship from the Collaborative Center for X-linked Dystonia Parkinsonism (CCXDP). We would like to thank Agencia Nacional de Investigación y Desarrollo (ANID) and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), No. 1180186 and 1191538 and Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias (FONDAP) program 15150012 to CH and Post-doctoral fellowship FONDECYT No. 3200718 to MM.
Author contributions
K.-T. T., C. G. A., J. B., A. A. G., N. B., S. S., J. R., A. L.-R., S. N., A. L., E. B., M. M., C. W., A. H., B. S., and L. M. E. methodology; K.-T. T., C. G. A., J. B., A. A. G., B. S., and L. M. E. validation; K.-T. T., C. G. A., J. B., A. A. G., N. B., S. S., J. R., A. L.-R., S. N., A. L., E. B., M. M., C. W., A. H., B. S., and L. M. E. formal analysis; K.-T. T., C. G. A., J. B., A. A. G., N. B., S. S., J. R., A. L.-R., S. N., A. L., E. B., M. M., C. W., A. H., B. S., and L. M. E. investigation; K.-T. T., C. G. A., J. B., A. A. G., B. S., and L. M. E. resources; K.-T. T., B. S., and L. M. E. writing–original draft; K.-T. T., C. G. A., J. B., A. A. G., B. S., and L. M. E. visualization; K.-T. T., C. G. A., J. B., A. A. G., C. H., S. D. M., B. S., and L. M. E. supervision; K.-T. T., B. S., and L. M. E. funding acquisition; C. G. A. and S. S. software; L. M. E., J. B. and K.-T. T. writing–review and editing; B. S. and L. M. E. project administration.
Contributor Information
Birgit Schilling, Email: bschilling@buckinstitute.org.
Lisa M. Ellerby, Email: lellerby@buckinstitute.org.
Supplemental Data
DIA window isolation scheme of the DIA MS acquisition method.
Protein, peptide and peptide spectrum match identification results obtained from the FAIMS-DDA MS data set.
Protein quantification and statistical analysis results obtained from the FAIMS-DDA MS data set.
Comparison and validation of the significantly changing protein candidates obtained from the FAIMS-DDA MS data set with the significantly changing proteins obtained from the DIA MS data set.
Protein quantification and statistical analysis results obtained from the DIA MS data set.
Custom background of proteins.
Pathway enrichment analysis of the upregulated and downregulated proteins from the FAIMS-DDA MS proteomic analysis.
AReactome functional interaction network analysis of the upregulated proteins in HD72-MSN to define clusters of proteins that are closely connected.
8BReactome functional interaction network analysis of the downregulated proteins in HD72-MSN to define clusters of proteins that are closely connected.
9Predicted drugs that modify HD signature.
Supplemental Figure S1. Performances of the TripleTOF 6600 DIA MS workflow. DIA data were processed using the pan-human spectral library (46).A, coefficients of variation (CV) of precursors identified in three biological replicates of C116-corrected MSN and HD-MSN. 72% and 80% of the identified precursor ions presented a coefficient of variation below 20% in C116-corrected MSNs and HD-MSNs, respectively. 42% and 54% of the identified precursor ions presented a coefficient of variation below 10% in C116-corrected MSN and HD MSN, respectively. B, number of missing values obtained for each precursor ion in the dataset. 15,531 of a total of 31,183 precursor ions were identified and quantified in all six samples of the dataset (complete profile).
Supplemental Figure S2. Validation of the protein groups altered in HD72-MSN versus C116-MSN. Venn diagrams showing the overlap of A, all significantly altered, B, all down-regulated, and C, all up-regulated protein groups obtained by DDA-MS as discovery step and by DIA-MS as validation step, when comparing HD72-MSNs vs C116-MSNs. Common protein groups are listed in supplemental Table S4C. D, scatter plot of log2 fold change of all shared proteins between DIA and DDA. E, scatter plot of log2 fold change of all shared significant proteins (FDR < 0.05, absolute log2 fold change > 0.58) between DIA and DDA. F, scatter plot of log2 fold change of all significant proteins (FDR < 0.05, absolute log2 fold change > 0.58) in DDA dataset that are also found in the DIA dataset.
Supplemental Figure S3. Insulin-like growth factor-binding protein 7 levels are increased in HD72-MSNs.A, Western blot analysis of IGFBP7 in C116-MSNs and HD72-MSNs. B, Quantification of IGFBP7 protein levels normalized to vinculin. Unpaired t-test with Welch's correction ∗p ≤ 0.05.
Supplemental Figure S4. Functional enrichment map of significantly altered proteins in HD72-MSNs. Enrichment results from g:Profiler were mapped as a network of gene sets (nodes) related by mutual overlap (edges) in Cytoscape using its EnrichmentMap. Red color represents gene sets upregulated in HD72-MSNs, whereas blue represents gene sets downregulated in HD72-MSNs. Node size is proportional to the gene-set size, and edge thickness represents the number of overlapping genes between sets. Functional enrichment map was generated with proteins quantified by FAIMS-DDA MS.
Supplemental Figure S5. IPA analysis of the differentially expressed proteins in HD72-MSNs compared to controls.
Supplemental Figure S6. HD-MSN lipid metabolism and its modulation by APOE3. C116- and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/mL APOE3. Non-treated cells were used as controls. The cells were stained with Nile Red, a lipophilic dye. A and B, quantifications of the phospholipid (red) and neutral lipid (green) intensity in normal and APOE3-treated conditions, as illustrated from Figure 8. Unpaired t-test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ns, not significant. Image quantification was done on total 13 unbiased, low-resolution view fields capturing total 599 DAPI-stained nuclei.
Supplemental Figure S7. HD-MSN lipid metabolism and its modulation. Quantitative proteomics reveals LDLR, CYP51A1 and DHCR24 are expressed more highly in HD72-MSNs than C116-MSNs. Quantitative proteomics reveals ABCA1 and HMGCL are expressed less in HD72-MSNs than C116-MSNs. Unpaired t-test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Supplemental Figure S8. HMG-CoA reductase levels are unchanged. 3-Hydroxy-3-methylglutaryl coenzyme A levels are similar in HD72-MSNs. compared to C116-MSNs. A, Western blot analysis of HMG-CoA reductase in C116-MSNs and HD72-MSNs. B, Quantification of HMG-CoA protein levels normalized to vinculin. Unpaired t-test with Welch's correction ns.
Supplemental Figure S9. Quantification of p61, LC3 and LAMP1 in HD MSNs with APOE. C116-MSNs and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/mL APOE3. A, cells were stained with Nile Red, a lipophilic dye, and only the short-wavelength, neutral lipid bound fluorescence is shown in representative confocal images (green). In addition, cultures were immunostained for p62 or LAMP1 (red). Lipid droplets (indicated by green circles in the other channel) and immunofluorescence puncta (indicated by red circles). Select co-labeling is indicated by arrows. The white outlines show the boundary of the cells. Micrographs were cropped, Wiener smoothed and saturated for presentation. Matching intensity scaling was used in all Nile Red and in all immunofluorescence channels, here and in Figure 9.
Supplemental Figure S10. Quantification of p61, LC3 and LAMP1 in HD MSNs with APOE. Immunofluorescence puncta were intensified by rolling ball local background subtraction before smoothing.A–C, quantification of lipid droplet and immunofluorescence spot density, fluorescence intensity (F.U.) and size for p62, LC3 or LAMP1, corresponding to panel A and Figure 9. B–D, two-way ANOVA with Sidak’s multiple comparison comparing HD72-MSNs with C116-MSNs, ∗p ≤ 0.05, ∗∗∗p ≤ 0.001. Including Figure 9A, images were selected and cropped from 80 unbiased view fields capturing total 458 DAPI-stained nuclei, representing the quantified data.
Supplemental Figure S11. Modulation of MHC and IFN-γ rescues HD cellular phenotypes. C116-MSNs and HD72-MSNs were treated for 48 h with IFN-γ at different concentrations. Non-treated cells were used as control. Representative immunofluorescence images showing that the expressions of DARPP-32 and MHC-II (A and B) were modulated after IFN-γ treatment. C and D, quantifications of the expression levels of each marker based on the analysis of pixel intensity were carried using the Biotek and Image J. Scale bar: 200 μm. One-way ANOVA for multiple comparisons (Tukey’s) ∗∗∗∗p < 0.0001.
Supplemental Figure S12. Comparison of HD proteomics data sets.A, comparison of dysregulated GO Biological Process (BP) terms among MSNs from human cortex of HD patients (24) and the Q175 mouse model of HD (103). B, the signature of differentially expressed proteins in MSNs was used as input on the L1000CDS2 (121) website to identify drugs that would modify the proteomic signature from HD to corrected. The top drugs identified are shown and are known modifiers of HD. The heatmap on the top shows the proteomic signature of HD and how it is predicted to be affected by each of the drugs.
Supplemental Figure S13. HD protein expression overlaps with HTT interacting proteins. The overlapping network between significantly altered proteins from FAIMS-DDA MS and the HTT protein interactome identified with A, yeast two-hybrid method (137) or B, Weighted Correlation Network Analysis (138) or C, in vitro affinity pull-down assays using mouse protein (7) or D, in vitro affinity pull-down assays using human protein (7). E, The overlapping network between significantly altered proteins as determined by DIA MS and HTT proteomic interactome identified with Weighted Correlation Network Analysis (138).
Supplemental Figure S14. IPA analysis of the differentially expressed proteins in HD72-MSNs compared to controls showing the DNA signaling network.
References
- 1.La Spada A.R., Taylor J.P. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 2010;11:247–258. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 3.Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol. Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh R., Tabrizi S.J. Clinical features of Huntington's disease. Adv. Exp. Med. Biol. 2018;1049:1–28. doi: 10.1007/978-3-319-71779-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Rub U., Seidel K., Heinsen H., Vonsattel J.P., den Dunnen W.F., Korf H.W. Huntington's disease (HD): the neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol. 2016;26:726–740. doi: 10.1111/bpa.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saudou F., Humbert S. The biology of huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Kaltenbach L.S., Romero E., Becklin R.R., Chettier R., Bell R., Phansalkar A., et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3 doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landles C., Milton R.E., Ali N., Flomen R., Flower M., Schindler F., et al. Subcellular localization and formation of huntingtin aggregates correlates with symptom onset and progression in a Huntington's disease model. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien R., DeGiacomo F., Holcomb J., Bonner A., Ring K.L., Zhang N., et al. Integration-independent transgenic Huntington disease fragment mouse models reveal distinct phenotypes and life span in vivo. J. Biol. Chem. 2015;290:19287–19306. doi: 10.1074/jbc.M114.623561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailus B.J., Scheeler S., Simons J., Sanchez M.A., Tshilenge K.T., Creus-Muncunill J., et al. Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels. Autophagy. 2021;17:4119–4140. doi: 10.1080/15548627.2021.1904489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Peskett T.R., Landles C., Warner J.B., Sathasivam K., Smith E.J., et al. Correlative light and electron microscopy suggests that mutant huntingtin dysregulates the endolysosomal pathway in presymptomatic Huntington's disease. Acta Neuropathol. Commun. 2021;9:70. doi: 10.1186/s40478-021-01172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gafni J., Cong X., Chen S.F., Gibson B.W., Ellerby L.M. Calpain-1 cleaves and activates caspase-7. J. Biol. Chem. 2009;284:25441–25449. doi: 10.1074/jbc.M109.038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gafni J., Hermel E., Young J.E., Wellington C.L., Hayden M.R., Ellerby L.M. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J. Biol. Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- 14.Gafni J., Papanikolaou T., Degiacomo F., Holcomb J., Chen S., Menalled L., et al. Caspase-6 activity in a BACHD mouse modulates steady-state levels of mutant huntingtin protein but is not necessary for production of a 586 amino acid proteolytic fragment. J. Neurosci. 2012;32:7454–7465. doi: 10.1523/JNEUROSCI.6379-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schilling B., Gafni J., Torcassi C., Cong X., Row R.H., LaFevre-Bernt M.A., et al. Huntingtin phosphorylation sites mapped by mass spectrometry. Modulation of cleavage and toxicity. J. Biol. Chem. 2006;281:23686–23697. doi: 10.1074/jbc.M513507200. [DOI] [PubMed] [Google Scholar]
- 16.Wellington C.L., Ellerby L.M., Gutekunst C.A., Rogers D., Warby S., Graham R.K., et al. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington's disease. J. Neurosci. 2002;22:7862–7872. doi: 10.1523/JNEUROSCI.22-18-07862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellington C.L., Ellerby L.M., Hackam A.S., Margolis R.L., Trifiro M.A., Singaraja R., et al. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 1998;273:9158–9167. doi: 10.1074/jbc.273.15.9158. [DOI] [PubMed] [Google Scholar]
- 18.Wellington C.L., Singaraja R., Ellerby L., Savill J., Roy S., Leavitt B., et al. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J. Biol. Chem. 2000;275:19831–19838. doi: 10.1074/jbc.M001475200. [DOI] [PubMed] [Google Scholar]
- 19.Graham R.K., Deng Y., Slow E.J., Haigh B., Bissada N., Lu G., et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Benn C.L., Landles C., Li H., Strand A.D., Woodman B., Sathasivam K., et al. Contribution of nuclear and extranuclear polyQ to neurological phenotypes in mouse models of Huntington's disease. Hum. Mol. Genet. 2005;14:3065–3078. doi: 10.1093/hmg/ddi340. [DOI] [PubMed] [Google Scholar]
- 21.Ross C.A., Tabrizi S.J. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 22.Aebersold R., Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016;537:347–355. doi: 10.1038/nature19949. [DOI] [PubMed] [Google Scholar]
- 23.Mann M., Kulak N.A., Nagaraj N., Cox J. The coming age of complete, accurate, and ubiquitous proteomes. Mol. Cell. 2013;49:583–590. doi: 10.1016/j.molcel.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Ratovitski T., Chaerkady R., Kammers K., Stewart J.C., Zavala A., Pletnikova O., et al. Quantitative proteomic analysis reveals similarities between Huntington's disease (HD) and Huntington's disease-like 2 (HDL2) human brains. J. Proteome Res. 2016;15:3266–3283. doi: 10.1021/acs.jproteome.6b00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosp F., Gutierrez-Angel S., Schaefer M.H., Cox J., Meissner F., Hipp M.S., et al. Spatiotemporal proteomic profiling of Huntington's disease inclusions reveals widespread loss of protein function. Cell Rep. 2017;21:2291–2303. doi: 10.1016/j.celrep.2017.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeley C., Kegel-Gleason K.B. Taming the Huntington's disease proteome: what have we learned? J. Huntingtons Dis. 2021;10:239–257. doi: 10.3233/JHD-200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naphade S., Tshilenge K.T., Ellerby L.M. Modeling polyglutamine expansion diseases with induced pluripotent stem cells. Neurotherapeutics. 2019;16:979–998. doi: 10.1007/s13311-019-00810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubry L., Bugi A., Lefort N., Rousseau F., Peschanski M., Perrier A.L. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Zhang S.C. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell. Mol. Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp P.J., Rushton D.J., Yarova P.L., Schnell C., Geater C., Hancock J.M., et al. Improving and accelerating the differentiation and functional maturation of human stem cell-derived neurons: role of extracellular calcium and GABA. J. Physiol. 2016;594:6583–6594. doi: 10.1113/JP270655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An M.C., Zhang N., Scott G., Montoro D., Wittkop T., Mooney S., et al. Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ring K.L., An M.C., Zhang N., O'Brien R.N., Ramos E.M., Gao F., et al. Genomic analysis reveals disruption of striatal neuronal development and therapeutic targets in human Huntington's disease neural stem cells. Stem Cell Rep. 2015;5:1023–1038. doi: 10.1016/j.stemcr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HD iPSC Consortium Developmental alterations in Huntington's disease neural cells and pharmacological rescue in cells and mice. Nat. Neurosci. 2017;20:648–660. doi: 10.1038/nn.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N., An M.C., Montoro D., Ellerby L.M. Characterization of human Huntington's disease cell model from induced pluripotent stem cells. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N., Bailus B.J., Ring K.L., Ellerby L.M. iPSC-based drug screening for Huntington's disease. Brain Res. 2016;1638:42–56. doi: 10.1016/j.brainres.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chae J.I., Kim D.W., Lee N., Jeon Y.J., Jeon I., Kwon J., et al. Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington's disease patient. Biochem. J. 2012;446:359–371. doi: 10.1042/BJ20111495. [DOI] [PubMed] [Google Scholar]
- 37.McQuade L.R., Balachandran A., Scott H.A., Khaira S., Baker M.S., Schmidt U. Proteomics of Huntington's disease-affected human embryonic stem cells reveals an evolving pathology involving mitochondrial dysfunction and metabolic disturbances. J. Proteome Res. 2014;13:5648–5659. doi: 10.1021/pr500649m. [DOI] [PubMed] [Google Scholar]
- 38.Hebert A.S., Prasad S., Belford M.W., Bailey D.J., McAlister G.C., Abbatiello S.E., et al. Comprehensive single-shot proteomics with FAIMS on a hybrid orbitrap mass spectrometer. Anal. Chem. 2018;90:9529–9537. doi: 10.1021/acs.analchem.8b02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillet L.C., Navarro P., Tate S., Rost H., Selevsek N., Reiter L., et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz Munoz S., Engel M., Balez R., Do-Ha D., Cabral-da-Silva M.C. A simple differentiation protocol for generation of induced pluripotent stem cell-derived basal forebrain-like cholinergic neurons for Alzheimer's disease and frontotemporal dementia disease modeling. Cells. 2020;9 doi: 10.3390/cells9092018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins B.C., Hunter C.L., Liu Y., Schilling B., Rosenberger G., Bader S.L., et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 2017;8:291. doi: 10.1038/s41467-017-00249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schilling B., Gibson B.W., Hunter C.L. Generation of high-quality SWATH((R)) acquisition data for label-free quantitative proteomics studies using TripleTOF((R)) mass spectrometers. Methods Mol. Biol. 2017;1550:223–233. doi: 10.1007/978-1-4939-6747-6_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kall L., Canterbury J.D., Weston J., Noble W.S., MacCoss M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 2007;4:923–925. doi: 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- 44.Wieczorek S., Combes F., Lazar C., Giai Gianetto Q., Gatto L., Dorffer A., et al. DAPAR & ProStaR: software to perform statistical analyses in quantitative discovery proteomics. Bioinformatics. 2017;33:135–136. doi: 10.1093/bioinformatics/btw580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H.Q., Tuominen L.K., Tsai C.J. Slim: a sliding linear model for estimating the proportion of true null hypotheses in datasets with dependence structures. Bioinformatics. 2011;27:225–231. doi: 10.1093/bioinformatics/btq650. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberger G., Koh C.C., Guo T., Rost H.L., Kouvonen P., Collins B.C., et al. A repository of assays to quantify 10,000 human proteins by SWATH-MS. Sci. Data. 2014;1:140031. doi: 10.1038/sdata.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiter L., Rinner O., Picotti P., Huttenhain R., Beck M., Brusniak M.Y., et al. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat. Methods. 2011;8:430–435. doi: 10.1038/nmeth.1584. [DOI] [PubMed] [Google Scholar]
- 48.Burger T. Gentle introduction to the statistical foundations of false discovery rate in quantitative proteomics. J. Proteome Res. 2018;17:12–22. doi: 10.1021/acs.jproteome.7b00170. [DOI] [PubMed] [Google Scholar]
- 49.Diaz G., Melis M., Batetta B., Angius F., Falchi A.M. Hydrophobic characterization of intracellular lipids in situ by nile red red/yellow emission ratio. Micron. 2008;39:819–824. doi: 10.1016/j.micron.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Arzt M., Deschamps J., Schmied C., Pietzsch T., Schmidt D., Tomancak P., et al. Labkit: labeling and segmentation toolkit for big image data. Front. Comput. Sci. 2022;4:777728. [Google Scholar]
- 51.Merico D., Isserlin R., Stueker O., Emili A., Bader G.D. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeon J., Xie Z., Evangelista J.E., Wojciechowicz M.L., Clarke D.J.B., Ma’ayan, A. Transforming L1000 profiles to RNA-seq-like profiles with deep learning. BMC Bioinformatics. 2022;13 doi: 10.1186/s12859-022-04895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telezhkin V., Schnell C., Yarova P., Yung S., Cope E., Hughes A., et al. Forced cell cycle exit and modulation of GABAA, CREB, and GSK3beta signaling promote functional maturation of induced pluripotent stem cell-derived neurons. Am. J. Physiol. Cell Physiol. 2016;310:C520–C541. doi: 10.1152/ajpcell.00166.2015. [DOI] [PubMed] [Google Scholar]
- 54.Arber C., Precious S.V., Cambray S., Risner-Janiczek J.R., Kelly C., Noakes Z., et al. Activin a directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bibb J.A., Yan Z., Svenningsson P., Snyder G.L., Pieribone V.A., Horiuchi A., et al. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seeman P., Niznik H.B., Guan H.C., Booth G., Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc. Natl. Acad. Sci. U. S. A. 1989;86:10156–10160. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dellen A., Welch J., Dixon R.M., Cordery P., York D., Styles P., et al. N-Acetylaspartate and DARPP-32 levels decrease in the corpus striatum of Huntington's disease mice. Neuroreport. 2000;11:3751–3757. doi: 10.1097/00001756-200011270-00032. [DOI] [PubMed] [Google Scholar]
- 58.Bekker-Jensen D.B., Martinez-Val A., Steigerwald S., Ruther P., Fort K.L., Arrey T.N., et al. A compact quadrupole-orbitrap mass spectrometer with FAIMS interface improves proteome coverage in short LC gradients. Mol. Cell. Proteomics. 2020;19:716–729. doi: 10.1074/mcp.TIR119.001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweppe D.K., Rusin S.F., Gygi S.P., Paulo J.A. Optimized workflow for multiplexed phosphorylation analysis of TMT-labeled peptides using high-field asymmetric waveform ion mobility spectrometry. J. Proteome Res. 2020;19:554–560. doi: 10.1021/acs.jproteome.9b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voisin J., Farina F., Naphade S., Fontaine M., Tshilenge K.T., Galicia Aguirre C., et al. FOXO3 targets are reprogrammed as Huntington's disease neural cells and striatal neurons face senescence with p16(INK4a) increase. Aging Cell. 2020;19 doi: 10.1111/acel.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonham L.W., Geier E.G., Steele N.Z.R., Holland D., Miller B.L., Dale A.M., et al. Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer's disease pathology and shows differential expression in transgenic mice. Front. Neurosci. 2018;12:476. doi: 10.3389/fnins.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lane E.M., Hohman T.J., Jefferson A.L., Alzheimer's Disease Neuroimaging Initiative Insulin-like growth factor binding protein-2 interactions with Alzheimer's disease biomarkers. Brain Imaging Behav. 2017;11:1779–1786. doi: 10.1007/s11682-016-9636-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parvaneh Tafreshi A., Talebi F., Ghorbani S., Bernard C., Noorbakhsh F. Altered expression of IGF-I system in neurons of the inflamed spinal cord during acute experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2017;525:3072–3082. doi: 10.1002/cne.24263. [DOI] [PubMed] [Google Scholar]
- 64.Simon C.M., Rauskolb S., Gunnersen J.M., Holtmann B., Drepper C., Dombert B., et al. Dysregulated IGFBP5 expression causes axon degeneration and motoneuron loss in diabetic neuropathy. Acta Neuropathol. 2015;130:373–387. doi: 10.1007/s00401-015-1446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hetmanczyk-Sawicka K., Iwanicka-Nowicka R., Fogtman A., Ciesla J., Wlodarski P., Zyzynska-Granica B., et al. Changes in global gene expression indicate disordered autophagy, apoptosis and inflammatory processes and downregulation of cytoskeletal signalling and neuronal development in patients with Niemann-Pick C disease. Neurogenetics. 2020;21:105–119. doi: 10.1007/s10048-019-00600-6. [DOI] [PubMed] [Google Scholar]
- 66.Januzzi J.L., Jr., Packer M., Claggett B., Liu J., Shah A.M., Zile M.R., et al. IGFBP7 (insulin-like growth factor-binding protein-7) and neprilysin inhibition in patients with heart failure. Circ. Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005133. [DOI] [PubMed] [Google Scholar]
- 67.Xie Y., Ankawi G., Yang B., Garzotto F., Passannante A., Breglia A., et al. Tissue inhibitor metalloproteinase-2 (TIMP-2) ∗ IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019;95:1486–1493. doi: 10.1016/j.kint.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Han J., Jung S., Jang J., Kam T.I., Choi H., Kim B.J., et al. OCIAD2 activates gamma-secretase to enhance amyloid beta production by interacting with nicastrin. Cell. Mol. Life Sci. 2014;71:2561–2576. doi: 10.1007/s00018-013-1515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha S., Bheemsetty V.A., Inamdar M.S. A double helical motif in OCIAD2 is essential for its localization, interactions and STAT3 activation. Sci. Rep. 2018;8:7362. doi: 10.1038/s41598-018-25667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang Q., Lui H., Shi X.J., Cheng Y. Blood oxidative stress marker aberrations in patients with Huntington's disease: a meta-analysis study. Oxid. Med. Cell Longev. 2020 doi: 10.1155/2020/9187195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mason R.P., Casu M., Butler N., Breda C., Campesan S., Clapp J., et al. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nat. Genet. 2013;45:1249–1254. doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shema R., Kulicke R., Cowley G.S., Stein R., Root D.E., Heiman M. Synthetic lethal screening in the mammalian central nervous system identifies Gpx6 as a modulator of Huntington's disease. Proc. Natl. Acad. Sci. U. S. A. 2015;112:268–272. doi: 10.1073/pnas.1417231112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., et al. Pathway enrichment analysis and visualization of omics data using g:profiler, GSEA, cytoscape and enrichmentmap. Nat. Protoc. 2019;14:482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creixell P., Reimand J., Haider S., Wu G., Shibata T., Vazquez M., et al. Pathway and network analysis of cancer genomes. Nat. Methods. 2015;12:615–621. doi: 10.1038/nmeth.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Burg J.M., Bjorkqvist M., Brundin P. Beyond the brain: widespread pathology in Huntington's disease. Lancet Neurol. 2009;8:765–774. doi: 10.1016/S1474-4422(09)70178-4. [DOI] [PubMed] [Google Scholar]
- 77.Mielcarek M. Huntington's disease is a multi-system disorder. Rare Dis. 2015;3 doi: 10.1080/21675511.2015.1058464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haw R., Loney F., Ong E., He Y., Wu G. Perform pathway enrichment analysis using ReactomeFIViz. Methods Mol. Biol. 2020;2074:165–179. doi: 10.1007/978-1-4939-9873-9_13. [DOI] [PubMed] [Google Scholar]
- 79.Miller J.P., Holcomb J., Al-Ramahi I., de Haro M., Gafni J., Zhang N., et al. Matrix metalloproteinases are modifiers of huntingtin proteolysis and toxicity in Huntington's disease. Neuron. 2010;67:199–212. doi: 10.1016/j.neuron.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naphade S., Embusch A., Madushani K.L., Ring K.L., Ellerby L.M. Altered expression of matrix metalloproteinases and their endogenous inhibitors in a human isogenic stem cell model of Huntington's disease. Front. Neurosci. 2017;11:736. doi: 10.3389/fnins.2017.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X., Reddy D.S. Integrins as receptor targets for neurological disorders. Pharmacol. Ther. 2012;134:68–81. doi: 10.1016/j.pharmthera.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bussian T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M., Baker D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chinta S.J., Woods G., Demaria M., Rane A., Zou Y., McQuade A., et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson's disease. Cell Rep. 2018;22:930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mendelsohn A.R., Larrick J.W. Cellular senescence as the key intermediate in tau-mediated neurodegeneration. Rejuvenation Res. 2018;21:572–579. doi: 10.1089/rej.2018.2155. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., Chen C., Hua S., Liao H., Wang M., Xiong Y., et al. An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer's disease. Diabetes Res. Clin. Pract. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 87.Dolat L., Hu Q., Spiliotis E.T. Septin functions in organ system physiology and pathology. Biol. Chem. 2014;395:123–141. doi: 10.1515/hsz-2013-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mostowy S., Cossart P. Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 89.Akil A., Peng J., Omrane M., Gondeau C., Desterke C., Marin M., et al. Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat. Commun. 2016;7:12203. doi: 10.1038/ncomms12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song P.X., Peng J., Omrane M., Cai T.T., Samuel D., Gassama-Diagne A. Septin 9 and phosphoinositides regulate lysosome localization and their association with lipid droplets. iScience. 2022;25:104288. doi: 10.1016/j.isci.2022.104288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galli S., Stancheva S.H., Dufor T., Gibb A.J., Salinas P.C. Striatal synapse degeneration and dysfunction are reversed by reactivation of Wnt signaling, Front. Synaptic Neurosci. 2021;13 doi: 10.3389/fnsyn.2021.670467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith-Geater C., Hernandez S.J., Lim R.G., Adam M., Wu J., Stocksdale J.T., et al. Aberrant development corrected in adult-onset Huntington's disease iPSC-derived neuronal cultures via WNT signaling modulation. Stem Cell Rep. 2020;14:406–419. doi: 10.1016/j.stemcr.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tourette C., Farina F., Vazquez-Manrique R.P., Orfila A.M., Voisin J., Hernandez S., et al. The Wnt receptor Ryk reduces neuronal and cell survival capacity by repressing FOXO activity during the early phases of mutant huntingtin pathogenicity. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huynh M.B., Ouidja M.O., Chantepie S., Carpentier G., Maiza A., Zhang G., et al. Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Block R.C., Dorsey E.R., Beck C.A., Brenna J.T., Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. J. Clin. Lipidol. 2010;4:17–23. doi: 10.1016/j.jacl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Pardo A., Monyror J., Morales L.C., Kadam V., Lingrell S., Maglione V., et al. Mutant huntingtin interacts with the sterol regulatory element-binding proteins and impairs their nuclear import. Hum. Mol. Genet. 2020;29:418–431. doi: 10.1093/hmg/ddz298. [DOI] [PubMed] [Google Scholar]
- 97.Valenza M., Rigamonti D., Goffredo D., Zuccato C., Fenu S., Jamot L., et al. Dysfunction of the cholesterol biosynthetic pathway in Huntington's disease. J. Neurosci. 2005;25:9932–9939. doi: 10.1523/JNEUROSCI.3355-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corey-Bloom J., Haque A., Aboufadel S., Snell C., Fischer R.S., Granger S.W., et al. Uric acid as a potential peripheral biomarker for disease features in Huntington's patients. Front. Neurosci. 2020;14:73. doi: 10.3389/fnins.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Genetic Modifiers of Huntington's Disease Consortium. Electronic address: gusella@helix.mgh.harvard.edu; Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium CAG repeat not polyglutamine length determines timing of Huntington's disease onset. Cell. 2019;178:887–900.e14. doi: 10.1016/j.cell.2019.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drissi R., Chauvin A., McKenna A., Levesque D., Blais-Brochu S., Jean D., et al. Destabilization of the MiniChromosome Maintenance (MCM) complex modulates the cellular response to DNA double strand breaks. Cell Cycle. 2018;17:2593–2609. doi: 10.1080/15384101.2018.1553336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forsburg S.L. Eukaryotic MCM proteins: beyond replication initiation. Microbiol. Mol. Biol. Rev. 2004;68:109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ishimi Y. Regulation of MCM2-7 function. Genes Genet. Syst. 2018;93:125–133. doi: 10.1266/ggs.18-00026. [DOI] [PubMed] [Google Scholar]
- 103.Langfelder P., Cantle J.P., Chatzopoulou D., Wang N., Gao F., Al-Ramahi I., et al. Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. Nat. Neurosci. 2016;19:623–633. doi: 10.1038/nn.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller T., Schrotter A., Loosse C., Pfeiffer K., Theiss C., Kauth M., et al. A ternary complex consisting of AICD, FE65, and TIP60 down-regulates stathmin1. Biochim. Biophys. Acta. 2013;1834:387–394. doi: 10.1016/j.bbapap.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 105.Liedtke W., Leman E.E., Fyffe R.E., Raine C.S., Schubart U.K. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am. J. Pathol. 2002;160:469–480. doi: 10.1016/S0002-9440(10)64866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villalon E., Kline R.A., Smith C.E., Lorson Z.C., Osman E.Y., O'Day S., et al. AAV9-Stathmin1 gene delivery improves disease phenotype in an intermediate mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2019;28:3742–3754. doi: 10.1093/hmg/ddz188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Birolini G., Valenza M., Di Paolo E., Vezzoli E., Talpo F., Maniezzi C., et al. Striatal infusion of cholesterol promotes dose-dependent behavioral benefits and exerts disease-modifying effects in Huntington's disease mice. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hunter M., Demarais N.J., Faull R.L.M., Grey A.C., Curtis M.A. An imaging mass spectrometry atlas of lipids in the human neurologically normal and Huntington's disease caudate nucleus. J. Neurochem. 2021;157:2158–2172. doi: 10.1111/jnc.15325. [DOI] [PubMed] [Google Scholar]
- 109.Kacher R., Mounier C., Caboche J., Betuing S. Altered cholesterol homeostasis in Huntington's disease. Front. Aging Neurosci. 2022;14:797220. doi: 10.3389/fnagi.2022.797220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat. Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bailus B.J., Scheeler S.M., Simons J., Sanchez M.A., Tshilenge K.T., Creus-Muncunill J., et al. Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels. Autophagy. 2021;17:4119–4140. doi: 10.1080/15548627.2021.1904489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corriveau R.A., Huh G.S., Shatz C.J. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 113.Chacon M.A., Boulanger L.M. MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Mol. Cell. Neurosci. 2013;52:117–127. doi: 10.1016/j.mcn.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Shatz C.J. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goddard C.A., Butts D.A., Shatz C.J. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Darmanis S., Sloan S.A., Zhang Y., Enge M., Caneda C., Shuer L.M., et al. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith R.S., Florio M., Akula S.K., Neil J.E., Wang Y., Hill R.S., et al. Early role for a Na(+),K(+)-ATPase (ATP1A3) in brain development. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2023333118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maeda N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 2015;9:98. doi: 10.3389/fnins.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jesse S., Bayer H., Alupei M.C., Zugel M., Mulaw M., Tuorto F., et al. Ribosomal transcription is regulated by PGC-1alpha and disturbed in Huntington's disease. Sci. Rep. 2017;7:8513. doi: 10.1038/s41598-017-09148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao R., Chakraborty A., Geater C., Pradhan S., Gordon K.L., Snowden J., et al. Mutant huntingtin impairs PNKP and ATXN3, disrupting DNA repair and transcription. Elife. 2019;8 doi: 10.7554/eLife.42988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duan Q., Reid S.P., Clark N.R., Wang Z., Fernandez N.F., Rouillard A.D., et al. L1000CDS(2): LINCS L1000 characteristic direction signatures search engine. NPJ Syst. Biol. Appl. 2016;2:16015. doi: 10.1038/npjsba.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dompierre J.P., Godin J.D., Charrin B.C., Cordelieres F.P., King S.J., Humbert S., et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mielcarek M., Benn C.L., Franklin S.A., Smith D.L., Woodman B., Marks P.A., et al. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington's disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neubauer K., Zieger B. The mammalian septin interactome. Front. Cell Dev. Biol. 2017;5:3. doi: 10.3389/fcell.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomez S.B., Yogev S. InterSEPTIN' kinesins in dendrites. Dev. Cell. 2018;46:130–132. doi: 10.1016/j.devcel.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 126.Strehlow A.N., Li J.Z., Myers R.M. Wild-type huntingtin participates in protein trafficking between the Golgi and the extracellular space. Hum. Mol. Genet. 2007;16:391–409. doi: 10.1093/hmg/ddl467. [DOI] [PubMed] [Google Scholar]
- 127.Scott L.E., Weinberg S.H., Lemmon C.A. Mechanochemical signaling of the extracellular matrix in epithelial-mesenchymal transition. Front. Cell Dev. Biol. 2019;7:135. doi: 10.3389/fcell.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Molero A.E., Gokhan S., Gonzalez S., Feig J.L., Alexandre L.C., Mehler M.F. Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21900–21905. doi: 10.1073/pnas.0912171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McAllister B., Donaldson J., Binda C.S., Powell S., Chughtai U., Edwards G., et al. Exome sequencing of individuals with Huntington's disease implicates FAN1 nuclease activity in slowing CAG expansion and disease onset. Nat. Neurosci. 2022;25:446–457. doi: 10.1038/s41593-022-01033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pradhan S., Gao R., Bush K., Zhang N., Wairkar Y.P., Sarkar P.S. Polyglutamine expansion in huntingtin and mechanism of DNA damage repair defects in Huntington's disease. Front. Cell Neurosci. 2022;16:837576. doi: 10.3389/fncel.2022.837576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smolic T., Tavcar P., Horvat A., Cerne U., Haluzan Vasle A., Tratnjek L., et al. Astrocytes in stress accumulate lipid droplets. Glia. 2021;69:1540–1562. doi: 10.1002/glia.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Van Den Brink D.M., Cubizolle A., Chatelain G., Davoust N., Girard V., Johansen S., et al. Physiological and pathological roles of FATP-mediated lipid droplets in drosophila and mice retina. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Farmer B.C., Walsh A.E., Kluemper J.C., Johnson L.A. Lipid droplets in neurodegenerative disorders. Front. Neurosci. 2020;14:742. doi: 10.3389/fnins.2020.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cuervo A.M., Zhang S. Selective autophagy and huntingtin: learning from disease. Cell Cycle. 2015;14:1617–1618. doi: 10.1080/15384101.2015.1039365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tourette C., Li B., Bell R., O'Hare S., Kaltenbach L.S., Mooney S.D., et al. A large scale huntingtin protein interaction network implicates Rho GTPase signaling pathways in Huntington disease. J. Biol. Chem. 2014;289:6709–6726. doi: 10.1074/jbc.M113.523696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shirasaki D.I., Greiner E.R., Al-Ramahi I., Gray M., Boontheung P., Geschwind D.H., et al. Network organization of the huntingtin proteomic interactome in mammalian brain. Neuron. 2012;75:41–57. doi: 10.1016/j.neuron.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DIA window isolation scheme of the DIA MS acquisition method.
Protein, peptide and peptide spectrum match identification results obtained from the FAIMS-DDA MS data set.
Protein quantification and statistical analysis results obtained from the FAIMS-DDA MS data set.
Comparison and validation of the significantly changing protein candidates obtained from the FAIMS-DDA MS data set with the significantly changing proteins obtained from the DIA MS data set.
Protein quantification and statistical analysis results obtained from the DIA MS data set.
Custom background of proteins.
Pathway enrichment analysis of the upregulated and downregulated proteins from the FAIMS-DDA MS proteomic analysis.
AReactome functional interaction network analysis of the upregulated proteins in HD72-MSN to define clusters of proteins that are closely connected.
8BReactome functional interaction network analysis of the downregulated proteins in HD72-MSN to define clusters of proteins that are closely connected.
9Predicted drugs that modify HD signature.
Supplemental Figure S1. Performances of the TripleTOF 6600 DIA MS workflow. DIA data were processed using the pan-human spectral library (46).A, coefficients of variation (CV) of precursors identified in three biological replicates of C116-corrected MSN and HD-MSN. 72% and 80% of the identified precursor ions presented a coefficient of variation below 20% in C116-corrected MSNs and HD-MSNs, respectively. 42% and 54% of the identified precursor ions presented a coefficient of variation below 10% in C116-corrected MSN and HD MSN, respectively. B, number of missing values obtained for each precursor ion in the dataset. 15,531 of a total of 31,183 precursor ions were identified and quantified in all six samples of the dataset (complete profile).
Supplemental Figure S2. Validation of the protein groups altered in HD72-MSN versus C116-MSN. Venn diagrams showing the overlap of A, all significantly altered, B, all down-regulated, and C, all up-regulated protein groups obtained by DDA-MS as discovery step and by DIA-MS as validation step, when comparing HD72-MSNs vs C116-MSNs. Common protein groups are listed in supplemental Table S4C. D, scatter plot of log2 fold change of all shared proteins between DIA and DDA. E, scatter plot of log2 fold change of all shared significant proteins (FDR < 0.05, absolute log2 fold change > 0.58) between DIA and DDA. F, scatter plot of log2 fold change of all significant proteins (FDR < 0.05, absolute log2 fold change > 0.58) in DDA dataset that are also found in the DIA dataset.
Supplemental Figure S3. Insulin-like growth factor-binding protein 7 levels are increased in HD72-MSNs.A, Western blot analysis of IGFBP7 in C116-MSNs and HD72-MSNs. B, Quantification of IGFBP7 protein levels normalized to vinculin. Unpaired t-test with Welch's correction ∗p ≤ 0.05.
Supplemental Figure S4. Functional enrichment map of significantly altered proteins in HD72-MSNs. Enrichment results from g:Profiler were mapped as a network of gene sets (nodes) related by mutual overlap (edges) in Cytoscape using its EnrichmentMap. Red color represents gene sets upregulated in HD72-MSNs, whereas blue represents gene sets downregulated in HD72-MSNs. Node size is proportional to the gene-set size, and edge thickness represents the number of overlapping genes between sets. Functional enrichment map was generated with proteins quantified by FAIMS-DDA MS.
Supplemental Figure S5. IPA analysis of the differentially expressed proteins in HD72-MSNs compared to controls.
Supplemental Figure S6. HD-MSN lipid metabolism and its modulation by APOE3. C116- and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/mL APOE3. Non-treated cells were used as controls. The cells were stained with Nile Red, a lipophilic dye. A and B, quantifications of the phospholipid (red) and neutral lipid (green) intensity in normal and APOE3-treated conditions, as illustrated from Figure 8. Unpaired t-test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ns, not significant. Image quantification was done on total 13 unbiased, low-resolution view fields capturing total 599 DAPI-stained nuclei.
Supplemental Figure S7. HD-MSN lipid metabolism and its modulation. Quantitative proteomics reveals LDLR, CYP51A1 and DHCR24 are expressed more highly in HD72-MSNs than C116-MSNs. Quantitative proteomics reveals ABCA1 and HMGCL are expressed less in HD72-MSNs than C116-MSNs. Unpaired t-test with Welch's correction ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Supplemental Figure S8. HMG-CoA reductase levels are unchanged. 3-Hydroxy-3-methylglutaryl coenzyme A levels are similar in HD72-MSNs. compared to C116-MSNs. A, Western blot analysis of HMG-CoA reductase in C116-MSNs and HD72-MSNs. B, Quantification of HMG-CoA protein levels normalized to vinculin. Unpaired t-test with Welch's correction ns.
Supplemental Figure S9. Quantification of p61, LC3 and LAMP1 in HD MSNs with APOE. C116-MSNs and HD72-MSNs cultured in serum withdrawal were treated for 48 h with 312 ng/mL APOE3. A, cells were stained with Nile Red, a lipophilic dye, and only the short-wavelength, neutral lipid bound fluorescence is shown in representative confocal images (green). In addition, cultures were immunostained for p62 or LAMP1 (red). Lipid droplets (indicated by green circles in the other channel) and immunofluorescence puncta (indicated by red circles). Select co-labeling is indicated by arrows. The white outlines show the boundary of the cells. Micrographs were cropped, Wiener smoothed and saturated for presentation. Matching intensity scaling was used in all Nile Red and in all immunofluorescence channels, here and in Figure 9.
Supplemental Figure S10. Quantification of p61, LC3 and LAMP1 in HD MSNs with APOE. Immunofluorescence puncta were intensified by rolling ball local background subtraction before smoothing.A–C, quantification of lipid droplet and immunofluorescence spot density, fluorescence intensity (F.U.) and size for p62, LC3 or LAMP1, corresponding to panel A and Figure 9. B–D, two-way ANOVA with Sidak’s multiple comparison comparing HD72-MSNs with C116-MSNs, ∗p ≤ 0.05, ∗∗∗p ≤ 0.001. Including Figure 9A, images were selected and cropped from 80 unbiased view fields capturing total 458 DAPI-stained nuclei, representing the quantified data.
Supplemental Figure S11. Modulation of MHC and IFN-γ rescues HD cellular phenotypes. C116-MSNs and HD72-MSNs were treated for 48 h with IFN-γ at different concentrations. Non-treated cells were used as control. Representative immunofluorescence images showing that the expressions of DARPP-32 and MHC-II (A and B) were modulated after IFN-γ treatment. C and D, quantifications of the expression levels of each marker based on the analysis of pixel intensity were carried using the Biotek and Image J. Scale bar: 200 μm. One-way ANOVA for multiple comparisons (Tukey’s) ∗∗∗∗p < 0.0001.
Supplemental Figure S12. Comparison of HD proteomics data sets.A, comparison of dysregulated GO Biological Process (BP) terms among MSNs from human cortex of HD patients (24) and the Q175 mouse model of HD (103). B, the signature of differentially expressed proteins in MSNs was used as input on the L1000CDS2 (121) website to identify drugs that would modify the proteomic signature from HD to corrected. The top drugs identified are shown and are known modifiers of HD. The heatmap on the top shows the proteomic signature of HD and how it is predicted to be affected by each of the drugs.
Supplemental Figure S13. HD protein expression overlaps with HTT interacting proteins. The overlapping network between significantly altered proteins from FAIMS-DDA MS and the HTT protein interactome identified with A, yeast two-hybrid method (137) or B, Weighted Correlation Network Analysis (138) or C, in vitro affinity pull-down assays using mouse protein (7) or D, in vitro affinity pull-down assays using human protein (7). E, The overlapping network between significantly altered proteins as determined by DIA MS and HTT proteomic interactome identified with Weighted Correlation Network Analysis (138).
Supplemental Figure S14. IPA analysis of the differentially expressed proteins in HD72-MSNs compared to controls showing the DNA signaling network.
Data Availability Statement
Raw data and complete MS data sets have been uploaded to the Center for Computational Mass Spectrometry, to the MassIVE repository at UCSD, and can be downloaded using the following link:https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=3a14708986c7468197598b328d1db750 (MassIVE ID number: MSV000088650; ProteomeXchange ID: PXD030786).
[Note to the reviewers: To access the data repository MassIVE (UCSD) for MS data, please use: Username: MSV000088650_reviewer; Password: winter].