Abstract
Background
In the I CARE study, colon cancer patients were randomly assigned to receive follow-up care from either a general practitioner (GP) or a surgeon. Here, we address a secondary outcome, namely, detection of recurrences and effect on time to detection of transferring care from surgeon to GP.
Methods
Pattern, stage, and treatment of recurrences were described after 3 years. Time to event was defined as date of surgery, until date of recurrence or last follow-up, with death as competing event. Effects on time to recurrence and death were estimated as hazard ratios (HRs) using Cox regression. Restricted mean survival times were estimated.
Results
Of 303 patients, 141 were randomly assigned to the GP and 162 to the surgeon. Patients were male (67%) with a mean age of 68.0 (8.4) years. During follow-up, 46 recurrences were detected; 18 (13%) in the GP vs 28 (17%) in the surgeon group. Most recurrences were detected via abnormal follow-up tests (74%) and treated with curative intent (59%). Hazard ratio for recurrence was 0.75 (95% confidence interval [CI] = 0.41 to 1.36) in GP vs surgeon group. Patients in the GP group remained in the disease-free state slightly longer (2.76 vs 2.71 years). Of the patients, 38 died during follow-up; 15 (11%) in the GP vs 23 (14%) in the surgeon group. Of these, 21 (55%) deaths were related to colon cancer. There were no differences in overall deaths between the groups (HR = 0.76, 95% CI = 0.39 to 1.46).
Conclusion
Follow-up provided by GPs vs surgeons leads to similar detection of recurrences. Also, no differences in mortality were found.
Colon cancer is a common disease worldwide (1). In 2021, there were more than 9000 patients diagnosed with colon cancer in the Netherlands (2). In turn, this is leading to a large number of patients who require survivorship care after they have been treated with curative intent. Survivorship care consists of several components, including follow-up (monitoring and detection of recurrences) and aftercare and rehabilitation (3).
In the Netherlands, survivorship care is provided by a surgeon, whereas general practitioners (GPs) do not play a formal role. However, in practice, GPs often provide support to patients in terms of aftercare and rehabilitation (4). It has therefore been suggested that GPs could play a greater role in survivorship care (5,6). GPs are familiar not only with patients’ medical history but also with their social context, which may help personalize care to the individual needs (7). This has led to the initiation of the Improving Care After colon canceR treatment in the Netherlands, personalized care to Enhance quality of life (I CARE) study in 2015 (8). In this randomized study, colon cancer patients were allocated to receive survivorship care by a GP vs care by a surgeon. Within the first year after surgery, care by the GP did not improve quality of life recovery, the primary outcome of the study (9). Other outcomes are therefore important to consider.
Follow-up is aimed at the surveillance of recurrences, either locally recurrent disease, and also metastases and new colorectal malignancies. Follow-up of colon cancer consists of routine check-ups for 5 years after treatment, including blood tests, imaging, and colonoscopy (10). To date, limited studies have looked at the effects of follow-up provided by a GP on outcomes such as the detection of recurrences and mortality (11). The effects on these outcomes remain uncertain and highlight the need for larger, randomized trials (12,13). Here, we report a secondary outcome of the I CARE study. We assessed the detection of recurrences and the effect on time to detection of recurrences and overall death of transferring follow-up care from surgeon to GP.
Methods
Study design and setting
The ongoing I CARE study is a multicenter 2 x 2 factorial randomized controlled trial comparing GP- to surgeon-led (usual) care after colon cancer treatment, with or without access to a supporting e-Health application called Oncokompas. The study is conducted in 8 hospitals in the Netherlands. The study includes 303 patients who were curatively treated for stage I-III colon cancer and were randomly assigned after finishing primary treatment. GPs were provided with a summarized care plan, which includes general information about the follow-up schedule and instructions on what to do if a recurrence is suspected (Supplementary Materials, available online). The recommended follow-up schedules were identical for patients in the GP- and surgeon-led groups. The primary outcome is quality of life. The full study protocol has been published in 2015 (8). The trial is registered in the Netherlands Trial Register; NTR4860.
Outcomes
We assessed the detection of recurrences and the effect on time to detection of recurrences of transferring follow-up care from the surgeon to the GP. We evaluated the pattern and stage of recurrences and whether the recurrences were treated with curative intent. Additionally, we explored mortality in both trial arms. Despite the factorial design of the study, Oncokompas was not included in the analyses because the uptake by patients was low and did not allow a reliable evaluation (9).
Data collection and processing
Data were recorded using Castor Electronic Data Capture (14). At inclusion, baseline characteristics (such as age, sex, tumor stage, and adjuvant chemotherapy treatment) were collected from the hospital electronic medical records. Adherence to protocol was monitored at regular intervals. If a patient was receiving care from the GP, a research assistant (ES) contacted the general practice to acquire follow-up data and referrals back to the surgeon (with the corresponding date and reason). If follow-up data was missing, the GP was advised to perform follow-up as per schedule. In case of a recurrence, additional data were collected from the hospital records, which included the date of diagnosis of recurrence, how the tumor was diagnosed (on the basis of symptoms or abnormal follow-up test results), the tumor location, and its treatment (either curative or palliative). Metastasis and new malignancies of the colon and rectum were considered part of the recurrences and also recorded in detail. In case of a death during follow-up, the date and cause of death were recorded. An independent data and safety monitoring board was informed about the adherence to protocol, recurrences, deaths, and other potential adverse effects at regular intervals (8). The last date of an on-site visit was considered the last date of follow-up.
Statistical analysis
Because the majority of colon cancer recurrences occur within the first 2 to 3 years after curative treatment (15-17), the analyses were performed after all patients had finished their 3-year follow-up period. Because recruitment took longer than expected (18), some patients had already finished their 4- or 5-year follow-up period. For the analyses, all available follow-up data were used.
All primary analyses were done according to the intention-to-treat principle. Descriptive statistics were used for the comparison of baseline characteristics. Time to (first) event was defined as the index date (date of surgery) until the date of recurrence (defined as the start of recurrence-related symptoms or abnormal follow-up test results), death, or last date of follow-up. Cumulative incidences of recurrences and deaths were charted using the cumulative incidence function curve (based on the Aalen-Johansen estimator) (19). To quantify differences in recurrence rates between the 2 groups, their cause-specific hazard functions were compared in the presence of a competing event (death) and expressed as a hazard ratio (HR). For this, an extended Cox regression model based on counting processes was used to also handle delayed entries of the individual patients (ie, random assignment and allocation took place after surgery). The proportional hazard assumption was checked by examining the Shoenfeld residuals. Variables that had a relevant effect on entry time (P ≤ .05) were included in the multivariable models. To help interpret and quantify the effect of GP-led care on time to recurrence, restricted mean survival time (RMST) was reported as a supplement to the hazard ratio (20,21). RMST provides a summary measure of the patients’ survival event profiles over time. RMST was calculated over a fixed period of 3 years and illustrated the mean number of years in each of the 3 health states, namely, disease-free, recurrence, and death; 95% bias-corrected and accelerated confidence intervals (CIs) for RMST health states were calculated using 3000 bootstrap samples.
Because the patients could transfer to the surgeon or to the GP at any point in time after random assignment, per-protocol analyses were done. These analyses aimed to reflect the true observed survival prospects (taking into account any transfers between trial arms). Lastly, the rate of follow-up testing was explored. A (mixed) Poisson regression model was used to estimate the number of follow-up tests per patient over a maximum of 3 years. Rate ratios (RR) and 95% confidence intervals were calculated for all follow-up tests combined, as well as for the individual tests (carcinoembryonic antigen [CEA], imaging, and colonoscopy). Statistical tests were 2-sided, and the statistical significance level was set at a P value equal to .05. All analyses were performed using SPSS (version 26.0.0.1) and R (version 4.0.3).
Ethics
The study protocol was approved by the medical ethics committee of the Academic Medical Centre (Amsterdam, The Netherlands) (MEC 2014_332). The study was conducted according to the principles of Good Clinical Practice. Written informed consent for data collection was obtained from all participants.
Results
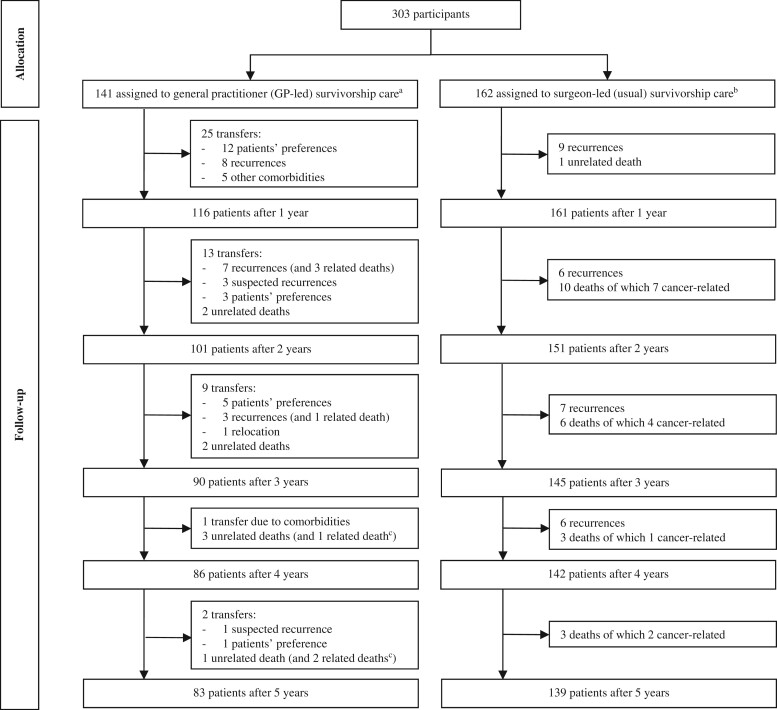
Of the 303 participants, 141 were randomly assigned to receive care from the GP and 162 to receive (usual) care from the surgeon (Table 1). The small difference between trial arm sizes was the result of 50 patients dropping out shortly after random assignment (18). Briefly, the study population had a mean age of 68.0 (8.4) years and included more males (203 [67%] of 303 patients were male; 100 [33%] were female). Tumor stages were distributed in a similar fashion, though stage I tumors were seen relatively more often in the GP group (42% vs 33%; P = .10). Adjuvant chemotherapy was given to 68 (22%) patients. Only 13 (4%) patients had a stoma. Median time to inclusion was 3.5 (interquartile range = 1.8-6.0) months. During follow-up, 50 patients transferred from the GP back to the surgeon (Figure 1 shows the trial profile). Transfers were due to (suspected) recurrences (n = 22), patients’ preferences (n = 21), other comorbidities that required specialist care (n = 6), and relocation of the patient (n = 1).
Table 1.
Baseline characteristics of the participantsa
| Characteristics | Care by a general practitioner (n = 141) | Care by a surgeon (n = 162) | P |
|---|---|---|---|
| Sociodemographic | |||
| Age, mean (SD), y | 67.9 (8.3) | 68.2 (8.4) | .83 |
| Sex, No. (%) | |||
| Females | 43 (30) | 57 (35) | |
| Male | 98 (70) | 105 (65) | .39 |
| Living situation, together, No. (%) | 107 (76) | 120 (74) | .89 |
| Educational attainment, No. (%) | .17 | ||
| Primary or non | 14 (10) | 13 (8) | |
| Secondary | 28 (20) | 40 (25) | |
| Vocational education | 75 (53) | 71 (44) | |
| University | 12 (9) | 24 (15) | |
| Missing | 12 (9) | 14 (9) | |
| Randomly assigned to Oncokompas, No. (%) | 68 (48) | 83 (51) | .60 |
| Clinical and pathological | |||
| Comorbidities, No. (%) | .21 | ||
| 0-1 | 63 (45) | 84 (52) | |
| ≥2 | 78 (55) | 78 (48) | |
| Cancer diagnosis via, No. (%) | .45 | ||
| Population screening | 74 (53) | 78 (48) | |
| Clinical course | 67 (48) | 84 (52) | |
| Tumor stage, No. (%); | .10 | ||
| I | 59 (42) | 54 (33) | |
| II | 50 (36) | 54 (33) | |
| III | 32 (23) | 54 (33) | |
| Stoma, No. (%) | 6 (4) | 7 (4) | .98 |
| Chemotherapy, No. (%) | 27 (19) | 41 (25) | .26 |
| Time to inclusion, median (IQR), mo | 3.6 (1.8-5.9) | 3.5 (1.8-6.1) | .97 |
IQR = interquartile range.
Figure 1.
Trial profile. aPatients could transfer from GP back to the surgeon at any point in time for any reason. No patients were lost to follow-up or withdrew their consent during follow-up. bPatients received usual care after colon cancer treatment, so there were no transfers from the trial arm. cThese patients had already transferred back to the surgeon in the previous year.
Recurrences
A total of 46 recurrences were detected during follow-up (Table 2). There were 18 (13%) recurrences of 141 patients in the GP group and 28 (17%) of 162 patients in the surgeon group. In 74% of the cases, the recurrences were detected via abnormal follow-up test results (n = 34), in particular the imaging result (n = 21) and the CEA blood test (n = 12). In 22% of cases, the recurrences were detected because of symptoms (n = 10), such as (abdominal) pain (n = 5), weight loss (n = 2), and changes in stool (n = 1). Most of the recurrences occurred in the liver (n = 15), gastrointestinal tract (n = 10), or lungs (n = 6). Recurrences also often occurred at multiple sites (n = 10). Most patients were treated with curative intent after detection of the recurrence (27 [59%] of 46). Patients in the GP group were more often treated with curative intent for recurrences (67% vs 54%), though this was based on a small number of observations.
Table 2.
Recurrences and deaths
| Outcome | Care by a general practitioner (n = 141) | Care by a surgeon (n = 162) | Total participants (n = 303) |
|---|---|---|---|
| Recurrences, No. (%) | 18 (13) | 28 (17) | 46 (15) |
| Diagnosis via abnormal follow-up test results, No. | 14 | 20 | 34 |
| Imaging | 8 | 13 | 21 |
| Carcinoembryonic antigen | 5 | 7 | 12 |
| Colonoscopy | 1 | NA | 1 |
| Diagnosis via symptoms, No. | 4 | 6 | 10 |
| (Abdominal) pain | 2 | 3 | 5 |
| Weight loss | 1 | 1 | 2 |
| Changes in stool | 1 | NA | 1 |
| Othera | 2 | 3 | 5 |
| Diagnosis via other route, No.b | NA | 2 | 2 |
| Localization of recurrence, No. | |||
| Liver | 8 | 7 | 15 |
| Gastrointestinal tract and/or peritoneum | 3 | 7 | 10 |
| Lungs | 2 | 4 | 6 |
| Lymph nodes | 1 | 2 | 3 |
| Multiple sites (stage IV) | 3 | 7 | 10 |
| Otherc | 1 | 1 | 2 |
| Treatment of recurrence, No. | |||
| With curative intent | 12 | 15 | 27 |
| Palliative (or no treatment) | 6 | 13 | 19 |
| Deaths, No. (%) | 15 (11) | 23 (14) | 38 (13) |
| Reason of death, No. | |||
| Related to colon cancer | 7 | 14 | 21 |
| Unrelatedd | 8 | 9 | 17 |
Patients may experience multiple symptoms. Other symptoms include coughing (n = 2), dyspnea (n = 1), clavicle lump (n = 1), and symptomatic abdominal wall hernia (n = 1).
Recurrence was found after a positive fecal occult blood test (n = 1) and as an incidental finding on a chest computed tomography after trauma (n = 1).
Other localizations include bone metastases (n = 1) and abdominal wall (n = 1).
Unrelated causes of death include second primary cancers (n = 11), cardiac failure (n = 2), stroke (n = 1), complications after trauma (n = 1), progressive parkinsonism (n = 1), and COVID-19 (n = 1).
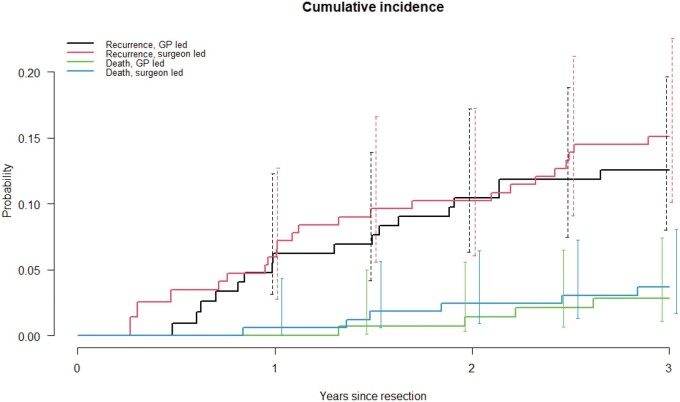
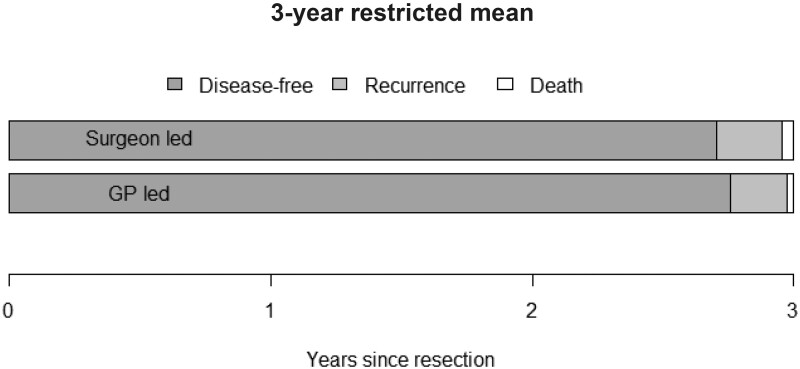
Cumulative incidence curves (and 95% CIs) for recurrences are shown in Figure 2, demonstrating no important differences between the 2 groups. The hazard ratio for recurrences was 0.75 (95% CI = 0.41 to 1.36) in the GP compared with surgeon group (Table 3), indicating fewer detections of recurrences in the same time period, though not statistically significantly different from the surgeon. Multivariable Cox regression and per-protocol analyses showed similar results. The RMST further illustrated this result. Patients in the GP group remained in the disease-free state slightly longer compared with patients in the surgeon group (2.76 vs 2.71 years), meaning that the detection of recurrences in the GP group took somewhat longer (Table 3 and Figure 3). In the exploratory analyses, differences were seen in rate of follow-up testing. Overall, the rate of follow-up testing was higher in the surgeon vs GP group (RR = 1.15, 95% CI = 1.07 to 1.24). Patients in the surgeon group were more likely to have more CEA tests (RR = 1.19, 95% CI = 1.05 to 1.36) and imaging tests (RR = 1.20, 95% CI = 1.03 to 1.40). There were no stastically significant differences in the number of colonoscopies (RR = 0.92, 95% CI = 0.72 to 1.17).
Figure 2.
Cumulative incidence curves (Aalen-Johansen) for recurrences and deaths according to the intention-to-treat principle. 95% confidence intervals are provided at several points in time (t = 1, 1.5, 2, 2.5, and 3 years of follow-up).
Table 3.
Survival analysis
| Outcome | Care by a general practitioner vs surgeon |
|---|---|
| HR (95% CI) | |
| Recurrences | |
| Intention-to-treat | |
| Univariable | 0.75 (0.41 to 1.36) |
| Multivariablea | 0.78 (0.43 to 1.43) |
| Per-protocol | |
| Univariable | 0.68 (0.36 to 1.26) |
| Multivariablea | 0.71 (0.38 to 1.34) |
| Deaths | |
| Intention-to-treat | |
| Univariable | 0.76 (0.39 to 1.46) |
| Multivariablea | 0.70 (0.36 to 1.35) |
| Per-protocol | |
| Univariable | 0.53 (0.25 to 1.12) |
| Multivariablea | 0.49 (0.23 to 1.04) |
| Restricted mean survival time in each health state, y | |
| Disease-free | 2.76 (2.68 to 2.85) vs 2.71 (2.62 to 2.83) |
| Recurrence | 0.21 (0.13 to 0.29) vs 0.25 (0.13 to 0.33) |
| Death | 0.03 (0.00 to 0.04) vs 0.04 (0.00 to 0.07) |
The following variables were included in multivariable analyses treatment with adjuvant chemotherapy and presence of comorbidities. CI = confidence interval; HR = hazard ratio.
Figure 3.
Restricted mean duration in each health state over a period of 3 years.
Deaths
There were 38 deaths during follow-up of which 15 (11%) were in the GP group and 23 (14%) in the surgeon group (Table 2). In total, 21 (55%) of 38 deaths were related to colon cancer, and 17 (45%) deaths were unrelated. Unrelated deaths were mostly due to second primary cancers, unrelated to colon cancer (n = 11). The hazard ratio for overall death was 0.76 (95% CI = 0.39 to 1.46), indicating fewer deaths in the GP compared with surgeon group, though this was not statistically significant. Similar results were seen in the multivariable Cox regression and per-protocol analyses.
Discussion
In this randomized controlled trial, follow-up by a GP led to a similar detection of recurrences as follow-up by a surgeon (HR = 0.75, 95% CI = 0.41 to 1.36), with similar pattern, stage, and treatment of recurrences. Recurrences in the GP group were more often treated with curative intent (67% vs 54%), but the number of observations was limited. Mortality also did not differ between the 2 groups (HR = 0.76, 95% CI = 0.41 to 1.36).
Few studies have looked at the effects of follow-up care by a GP on the detection of recurrences and mortality, so its impact remains unclear (11-13). Two previous randomized trials have compared follow-up of colon cancer patients by GPs with specialists, but their results are contradictory (22,23). In a Norwegian trial involving 110 patients, the mean time to recurrence detection was shorter in the GP group (35 vs 45 days; P = .46) (22). In an Australian trial however, involving 224 patients, median time to detection was longer in the GP group (9.5 vs 8.0 months; P = .76) (23). There were no differences in time to death between the 2 study groups after 24 months (P = .69). Two other randomized trials have been conducted among early stage (I-III) breast cancer patients in the United Kingdom and Canada (24,25). Both trials showed no statistically significant differences in recurrence detection between GPs vs specialists; 6.8% vs 10.8% in the UK trial and 11.2% vs 13.2% in the Canada trial. This last trial showed no statistically significant difference in deaths either (difference of 0.18%, 95% CI = -2.90% to 3.26%) (25). These results illustrate the difficulty of quantifying effects of follow-up care on survival outcomes. The contradiction may be the result of using measures such as mean and median time to detection, which are highly prone to survival bias. In this study, we have tried to quantify the effects using more sophisticated methods, which can handle this type of bias. Although the trials showed no clear trends in the clinical outcomes, none of the trials were powered to detect any differences in survival outcomes. Such trials would require large numbers of patients, making it an impossible undertaking. For example, colorectal cancer recurrence detection by surgeons is approximately 16% over a 3-year observation period (17). If a 2% difference in recurrence detection between GPs and surgeons was considered relevant, a sample size of approximately 15 000 patients would be required to achieve 90% power with a .05 2-sided significance level (26). The results of the trials, including this one, should therefore be considered altogether.
A previous Cochrane review, which evaluated nonspecialist- vs specialist-led follow-up failed to pool the results of time to detection of recurrences because of limited studies, reporting of results by different estimates, and high variance of the results estimates (12). This study can help strengthen the pooled results and increase the certainty of evidence. To our opinion, it seems plausible that follow-up by the GP leads to at least an equal detection of recurrences compared with follow-up by a surgeon. However, time to detection of recurrences might be slightly longer when follow-up care is provided by the GP, which can have important implications on an individual level. In the exploratory analyses, we observed differences in the rate of follow-up testing between GPs and surgeons. However, because these data were not routinely collected, we could not determine whether follow-up testing was timely or appropriate, and even more so, because the number and timing of follow-up tests have been under debate, and there are already major differences in follow-up practices between surgeons in the Netherlands (27). Despite a potential small delay in time to detection by GPs, it does not seem to affect the overall outcome of the recurrence (ie, pattern, stage, and treatment). This observation also corresponds with the fact that less intensive follow-up after colon cancer probably makes little or no difference to overall survival (12). In 2019, the national follow-up guideline for colon cancer was revised, and it now includes less intensive follow-up compared with when the I CARE study started (10).
Transferring follow-up care from the specialist to the GP seems possible and should be taken into consideration, especially because patients have a need for active GP involvement after cancer (6,28). GPs have an important role in cancer management, and providing them with education and training is helpful and can increase confidence and knowledge and change behavior (29). For the GP to take over completely, it will require additional time, compensation, and reorganization of the infrastructure (30). It will also require a clear understanding of the roles and responsibilities in which the patient and GP need to come to an agreement on who will take the lead in organizing follow-up (31).
This is one of the few randomized trials comparing follow-up by a GP with follow-up by a specialist. In comparison with 2 previous trials among colon cancer patients (22,23), the I CARE study included more patients and longer follow-up time. Even though the study was not powered to detect differences in survival, evidence on this topic is scarce and difficult to attain, highlighting the need of these results. To overcome possible survival bias, we used a comprehensive analysis approach, with death as a competing risk to recurrence and RMST as a supplement to hazard ratios (20,21). This will have helped portray the patients’ survival event profiles over time.
Challenges were faced in the recruitment to the trial, which may have created an unintended selection of the target population (18). Participants were on average younger than nonparticipants and often had limited to no comorbidities. Slightly more stage I tumors were seen in the GP vs surgeon group (42% vs 33%), although this was not statistically significantly different (P = .10). Because stage I tumors have a more favorable prognosis (2), it affects the probability of recurrences and cancer-related deaths. This can also explain why there were slightly more recurrences in the surgeon vs GP group (13% vs 17%). Overall, the number of recurrences and deaths were rather low, which may have hindered the analyses. To account for possible transfers between trial arms, per-protocol analyses were performed. The results all pointed in the same direction, which helped strengthen our conclusions. Even though adherence to protocol was monitored, we did not have direct access to primary care data. A research assistant contacted the general practices at regular intervals, which may have affected adherence to protocol by GPs. Patients might have had more frequent (or fewer) consultations with health-care professionals.
The detection of colon cancer recurrences is similar when follow-up is provided by a GP rather than a surgeon. Time to detection of recurrences might be slightly longer when care is provided by a GP, but this does not seem to affect the outcome of the recurrence. Follow-up by a GP can be considered as an alternative to care by a specialist. However, demonstrating differences in survival outcomes requires a great number of patients and considerable follow-up time, so these results should be pooled together with other studies to increase the certainty of evidence.
Supplementary Material
Contributor Information
Julien A M Vos, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health, Research Programme Quality of Care, and Personalized Medicine, Amsterdam, the Netherlands.
Edanur Sert, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Wim B Busschers, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Laura A M Duineveld, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Thijs Wieldraaijer, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Jan Wind, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Sandra C Donkervoort, Department of Surgery, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands.
Marc J P M Govaert, Department of Surgery, Dijklander Hospital, Hoorn, the Netherlands.
Frédérique H Beverdam, Department of Surgery, Franciscus Gasthuis & Vlietland Hospital, Schiedam, the Netherlands.
Anke B Smits, Department of Surgery, St. Antonius Hospital, Nieuwegein, the Netherlands.
Willem A Bemelman, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Gijsbert Heuff, Department of Surgery, Spaarne Gasthuis, Hoofddorp, the Netherlands.
Henk C P M van Weert, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health, Research Programme Quality of Care, and Personalized Medicine, Amsterdam, the Netherlands.
Kristel M van Asselt, Department of General Practice, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health, Research Programme Quality of Care, and Personalized Medicine, Amsterdam, the Netherlands.
I CARE study Group:
Funding
This work was supported by KWF Kankerbestrijding/Stichting Alpe d’HuZes grant number BMA 5954.
Notes
Role of the funder: The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors declare that they have no competing interests.
Authors contribution: Anke B. Smits, MD, PhD (Conceptualization; Data curation; Writing – review & editing), Frédérique H. Beverdam, MD (Conceptualization; Data curation; Writing – review & editing), Marc. J.P.M. Govaert, MD (Conceptualization; Data curation; Writing – review & editing), Willem A. Bemelman, MD, PhD (Conceptualization; Data curation; Writing – review & editing), Kristel M. van Asselt, MD, PhD (Conceptualization; Supervision; Writing – review & editing), Henk C.P.M. van Weert, MD, PhD (Conceptualization; Supervision; Writing – review & editing), Gijsbert Heuff, MD, PhD (Conceptualization; Data curation; Writing – review & editing), Wim B. Busschers, MSc (Conceptualization; Formal analysis; Visualization; Writing – review & editing), Edanur Sert, MSc (Data curation; Project administration; Validation; Writing – review & editing), Julien Vos, MD (Conceptualization; Data curation; Formal analysis; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing), Laura A.M. Duineveld, MD, PhD (Conceptualization; Data curation; Project administration; Validation; Writing – review & editing), Sandra C. Donkervoort, MD, PhD (Conceptualization; Data curation; Writing – review & editing), Jan Wind, MD, PhD (Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing), Thijs Wieldraaijer, MD, PhD (Conceptualization; Data curation; Project administration; Validation; Writing – review & editing).
Acknowledgements: The authors would like to acknowledge and thank all participants and contributing hospitals, in particular the other members of the I CARE study group, namely A. A. W. van Geloven (Tergooi Ziekenhuis, Hilversum) and A. W. H. van de Ven (Flevoziekenhuis, Almere).
In 2015, the I CARE study was initiated as a response to the increasing calls for other and more generalized cancer survivorship care strategies. Representatives of the patient and public were not directly involved in carrying out this research.
Data availability
At the end of study, data can be made available, after anonymization, on request to the corresponding author, taking into account possible national and international legal restrictions.
References
- 1. Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. [DOI] [PubMed] [Google Scholar]
- 2. The Netherlands Cancer Registry. Comprehensive Cancer Centre the Netherlands (IKNL). https://iknl.nl/kankersoorten/darmkanker/registratie. Accessed April 13, 2022.
- 3. Hewitt M, Greenfield S, Stovall E, From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 4. Wieldraaijer T, Duineveld LA, van Asselt KM, et al. ; for the ICARE Study Group. Follow-up of colon cancer patients; causes of distress and need for supportive care: results from the ICARE Cohort Study. Eur J Surg Oncol. 2017;43(1):118-125. [DOI] [PubMed] [Google Scholar]
- 5. Emery JD, Shaw K, Williams B, et al. The role of primary care in early detection and follow-up of cancer. Nat Rev Clin Oncol. 2014;11(1):38-48. [DOI] [PubMed] [Google Scholar]
- 6. Meiklejohn JA, Mimery A, Martin JH, et al. The role of the GP in follow-up cancer care: a systematic literature review. J Cancer Surviv. 2016;10(6):990-1011. [DOI] [PubMed] [Google Scholar]
- 7. Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231-1272. [DOI] [PubMed] [Google Scholar]
- 8. Duineveld LA, Wieldraaijer T, van Asselt KM, et al. Improving care after colon cancer treatment in The Netherlands, personalised care to enhance quality of life (I CARE study): study protocol for a randomised controlled trial. Trials. 2015;16(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vos JAM, Duineveld LAM, Wieldraaijer T, et al. ; for the I CARE study group. Effect of general practitioner-led versus surgeon-led colon cancer survivorship care, with or without eHealth support, on quality of life (I CARE): an interim analysis of 1-year results of a randomised, controlled trial. Lancet Oncol. 2021;22(8):1175-1187. [DOI] [PubMed] [Google Scholar]
- 10. Federation of Medical Specialists. National Guideline Colorectal Carcinoma (CRC). https://www.oncoline.nl/colorectaalcarcinoom. Accessed June 10, 2020.
- 11. Vos JAM, Wieldraaijer T, van Weert H, et al. Survivorship care for cancer patients in primary versus secondary care: a systematic review. J Cancer Surviv. 2021;15(1):66-76. doi: 10.1007/s11764-020-00911-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoeg BL, Bidstrup PE, Karlsen RV, et al. Follow-up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database Syst Rev. 2019;2019(11):CD012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jefford M, Howell D, Li Q, et al. Improved models of care for cancer survivors. Lancet. 2022;399(10334):1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castor. Castor Electronic Data Capture 2019. https://www.castoredc.com. Accessed August 27, 2019.
- 15. Guraya SY. Pattern, stage, and time of recurrent colorectal cancer after curative surgery. Clin Colorectal Cancer. 2019;18(2):e223-e228. [DOI] [PubMed] [Google Scholar]
- 16. Yun HR, Lee LJ, Park JH, et al. Local recurrence after curative resection in patients with colon and rectal cancers. Int J Colorectal Dis. 2008;23(11):1081-1087. [DOI] [PubMed] [Google Scholar]
- 17. Qaderi SM, Galjart B, Verhoef C, et al. Disease recurrence after colorectal cancer surgery in the modern era: a population-based study. Int J Colorectal Dis. 2021;36(11):2399-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duineveld LAM, Vos JAM, Wieldraaijer T, et al. ; for the I CARE study group. Recruitment challenges to the I CARE study: a randomised trial on general practitioner-led colon cancer survivorship care. BMJ Open. 2021;11(8):e048985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aalen OO, Borgan Ø, Gjessing S.. Survival and Event History Analysis. A Process Point of View. New York: Springer; 2008. [Google Scholar]
- 20. A’Hern RP. Restricted mean survival time: an obligatory end point for time-to-event analysis in cancer trials? J Clin Oncol. 2016;34(28):3474-3476. [DOI] [PubMed] [Google Scholar]
- 21. Pak K, Uno H, Kim DH, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3(12):1692-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Augestad KM, Norum J, Dehof S, et al. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open. 2013;3(4):e002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer. 2006;94(8):1116-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grunfeld E, Mant D, Yudkin P, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ. 1996;313(7058):665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. 2006;24(6):848-855. [DOI] [PubMed] [Google Scholar]
- 26. Machin D, Campbell M, Fayers P, Pinol A.. Sample Size Tables for Clinical Studies. 2nd ed. Malden, MA: Blackwell Science; 1997. [Google Scholar]
- 27. Qaderi SM, Wijffels NAT, Bremers AJA, et al. Major differences in follow-up practice of patients with colorectal cancer; results of a national survey in the Netherlands. BMC Cancer. 2020;20(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noteboom EA, Perfors IA, May AM, et al. GP involvement after a cancer diagnosis; patients’ call to improve decision support. BJGP Open. 2021;5(1):bjgpopen20X101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan RJ, Agbejule OA, Yates PM, et al. Outcomes of cancer survivorship education and training for primary care providers: a systematic review. J Cancer Surviv. 2022;16(2):279-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vos JAM, de Best R, Duineveld LAM, et al. Delivering colon cancer survivorship care in primary care; a qualitative study on the experiences of general practitioners. BMC Prim Care. 2022;23(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vos JAM, van Miltenburg VE, Beverdam FH, et al. Patients’ experiences with general practitioner-led colon cancer survivorship care; a mixed-methods evaluation at various time points. BJGP. 2022;73(727):e115-e123. doi: 10.3399/BJGP.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
At the end of study, data can be made available, after anonymization, on request to the corresponding author, taking into account possible national and international legal restrictions.