Abstract
Background
The role of ovulation in epithelial ovarian cancer (EOC) is supported by the consistent protective effects of parity and oral contraceptive use. Whether these factors protect through anovulation alone remains unclear. We explored the association between lifetime ovulatory years (LOY) and EOC.
Methods
LOY was calculated using 12 algorithms. Odds ratios (ORs) and 95% confidence intervals (CIs) estimated the association between LOY or LOY components and EOC among 26 204 control participants and 21 267 case patients from 25 studies. To assess whether LOY components act through ovulation suppression alone, we compared beta coefficients obtained from regression models with expected estimates assuming 1 year of ovulation suppression has the same effect regardless of source.
Results
LOY was associated with increased EOC risk (OR per year increase = 1.014, 95% CI = 1.009 to 1.020 to OR per year increase = 1.044, 95% CI = 1.041 to 1.048). Individual LOY components, except age at menarche, also associated with EOC. The estimated model coefficient for oral contraceptive use and pregnancies were 4.45 times and 12- to 15-fold greater than expected, respectively. LOY was associated with high-grade serous, low-grade serous, endometrioid, and clear cell histotypes (ORs per year increase = 1.054, 1.040, 1.065, and 1.098, respectively) but not mucinous tumors. Estimated coefficients of LOY components were close to expected estimates for high-grade serous but larger than expected for low-grade serous, endometrioid, and clear cell histotypes.
Conclusions
LOY is positively associated with nonmucinous EOC. Differences between estimated and expected model coefficients for LOY components suggest factors beyond ovulation underlie the associations between LOY components and EOC in general and for non-HGSOC.
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy. The consistent protective effects of oral contraceptives (OC) (1-3), bearing children (3,4), and breastfeeding (5), which all suppress ovulation, suggest that ovulation may play a key role in disease origin (6). In support of this hypothesis, lifetime ovulatory years (LOY) have been associated with increased EOC risk (2,7-14). However, differences in how studies define LOY and categorize exposure make it challenging to quantify the LOY-EOC relationship (15). Moreover, it remains unclear whether the mechanism whereby LOY components exert their impacts is through ovulation suppression alone or other means (7).
Although EOC is considered a set of diseases defined by histologic subtypes (histotypes), the relationship between LOY and EOC histotypes remains understudied. Although LOY might be associated with specific EOC subtypes (2,10-14), no individual study has had a large enough sample size to undertake a detailed histotype-specific analysis to evaluate the actual vs expected effects of individual LOY components to assess whether the mechanism of action of these components is solely by ovulation suppression.
To investigate the effects of LOY and its components on EOC, we pooled data from 25 case-control studies from the Ovarian Cancer Association Consortium (OCAC). Our goals were to 1) quantify the LOY-EOC association overall and for individual histotypes, 2) assess the impact of LOY definition on the LOY-EOC relationship, and 3) determine whether the relationship between LOY components and EOC is beyond ovulation suppression.
Methods
Study population
This study included 25 case-control studies (see Table 1) (16-42) from OCAC (43). Participants provided informed consent for original studies, whose protocols were approved by their respective institutional review boards.
Table 1.
Characteristics of the 25 case-control studies from the Ovarian Cancer Association Consortium, conducted in Asia, Australia, Europe, and North America from 1989 to present and included in the lifetime ovulatory years (LOY) analyses
| Study | Region | Study name | Study period | Case type | Method of data collection | Age, mean (SD), y | Control participants, No. (%) | Case patients, No. (%) |
|---|---|---|---|---|---|---|---|---|
| AUS (16) | Australia | Australian Ovarian Cancer Study/Australian Cancer Study | 2002-2006 | Population-based | Self-completed questionnaire | 56.88 (12.28) | 1506 (43.2) | 1984 (56.8) |
| BAV (17) | Germany | Bavarian Ovarian Cancer Cases and Controls | 2002-2006 | Hospital or Clinic-based | Interview | 57.31 (13.77) | 629 (47.9) | 684 (52.1) |
| CON (18) | USA | Connecticut Ovarian Cancer Study | 1998-2003 | Population-based | Interview | 55.27 (11.04) | 551 (52.6) | 497 (47.4) |
| DOV (19) | USA | Diseases of the Ovary and their Evaluation | 2002-2009 | Population-based | Interview | 55.78 (9.26) | 1849 (54.2) | 1562 (45.8) |
| GER (20) | Germany | German Ovarian Cancer Study | 1993-1996 | Population-based | Self-completed questionnaire | 55.07 (12.24) | 533 (67.4) | 258 (32.6) |
| HAW (21) | USA | Hawaii Ovarian Cancer Case-Control Study | 1993-2008 | Population-based | Interview | 54.98 (14.28) | 1103 (55.2) | 895 (44.8) |
| HOP (22) | USA | Hormones and Ovarian cancer PrEdiction | 2003-2009 | Population-based | Interview | 58.66 (12.52) | 1802 (68.3) | 836 (31.7) |
| JPN (23) | Japan | Hospital-based Research Program at Aichi Cancer Center | 2001-2005 | Hospital or Clinic-based | Interview | 52.36 (11.17) | 233 (60.5) | 152 (39.5) |
| MAY (24) | USA | Mayo Clinic Ovarian Cancer Case-Control Study | 1999-2018 | Hospital or Clinic-based | Interview | 60.51 (13.58) | 2299 (55.5) | 1846 (44.5) |
| MCC (25)a | Australia | Melbourne Collaborative Cohort Study | 1990-2008 | Defined cohort | Self-completed questionnaire | 64.07 (9.62) | 471 (73.1) | 173 (26.9) |
| NCO (26) | USA | North Carolina Ovarian Cancer Study | 1999-2008 | Population-based | Interview | 55.28 (11.53) | 1085 (47.6) | 1195 (42.4) |
| NEC (27) | USA | New England Case Control Study | 1992-2003 | Population-based | Interview | 53.54 (12.35) | 2100 (50.0) | 2075 (49.7) |
| NJO (28) | USA | New Jersey Ovarian Cancer Study | 2002-2008 | Population-based | Interview | 61.48 (11.60) | 458 (65.9) | 237 (34.1) |
| NTH (29,30) | Netherlands | Nijmegen Ovarian Cancer Study | 1997-2008 | Population-based | Self-completed questionnaire | 55.90 (10.79) | 600 (69.4) | 265 (30.6) |
| OVA | Canada | Ovarian Cancer in Alberta and British Columbia | 2002-2012 | Population-based | Self-completed questionnaire 2002-2004; interview 2004-2012 | 56.81 (10.62) | 2698 (62.2) | 1637 (37.8) |
| POL (31) | Poland | Polish Ovarian Cancer Case Control Study | 2000-2003 | Population-based | Interview | 55.70 (10.62) | 1128 (79.3) | 294 (20.7) |
| SON (32) | Canada | Southern Ontario Ovarian Cancer Study | 1989-1993 | Population-based | Interview | 56.86 (11.97) | 564 (55.6) | 450 (44.4) |
| STA (33) | USA | Family Registry for Ovarian Cancer AND Genetic Epidemiology of Ovarian Cancer | 1997-2001 | Population-based | Interview | 47.77 (10.07) | 567 (46.0) | 665 (54.0) |
| SWH (34) | China | Shanghai Women’s Health Study | 1996-present | Defined cohort | Interview | 53.36 (9.70) | 986 (86.6) | 152 (13.4) |
| TBO (35) | USA | Tampa Bay Ovarian Cancer Study | 2000-present | Population-based | Interview | 60.53 (10.85) | 205 (41.8) | 285 (58.2) |
| TOR (36) | Canada | Familial Ovarian Tumour Study (FOTS) AND Health Watch (HW) | 1995-1999 and 2000-2003 | Population-based | Interview | 56.62 (12.77) | 322 (21.6) | 1167 (78.4) |
| UCI (37) | USA | University California Irvine Ovarian Study | 1993-2005 | Population-based | Interview | 54.29 (13.17) | 614 (49.1) | 636 (50.9) |
| UKO (38) | UK | United Kingdom Ovarian cancer Population Study | 2006-2010 | Hospital or Clinic-based | Interview | 63.06 (8.93) | 1182 (58.5) | 839 (41.5) |
| USC (39-41) | USA | Los Angeles County Case-Control Studies of Ovarian Cancer | 1992-2009 | Population-based | Interview | 55.07 (12.41) | 2595 (52.2) | 2380 (47.8) |
| VTL (42)a | USA | VITamins And Lifestyle Cohort Study | 2000-2010 | Defined cohort | Self-completed questionnaire | 68.19 (7.62) | 124 (54.6) | 103 (45.4) |
| Total | 56.55 (12.20) | 26204 (55.2) | 21267 (44.8) |
Employed a nested-case control study design within a cohort study.
Study variables and LOY calculation
OCAC’s harmonized core data provided LOY component variables: age at last menstrual period (LMP) before diagnosis (case participants) or interview (control participants); age at menarche; number of pregnancies; number of full-term births; and total durations of pregnancy, breastfeeding, and OC use.
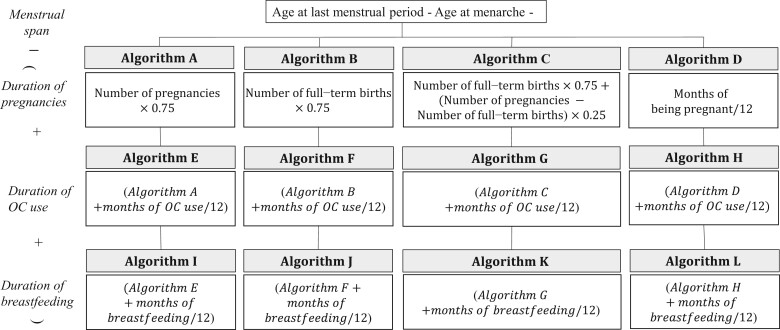
LOY was calculated with 12 algorithms (Supplementary Table 1, available online) (8) using the formula: where “menstrual span” was calculated from age at LMP minus age at menarche. The algorithms were divided into 4 classes based on how “years of anovulation” was defined (see Figure 1).
Figure 1.
Flowchart for algorithms to calculate lifetime ovulatory years. OC = oral contraceptive.
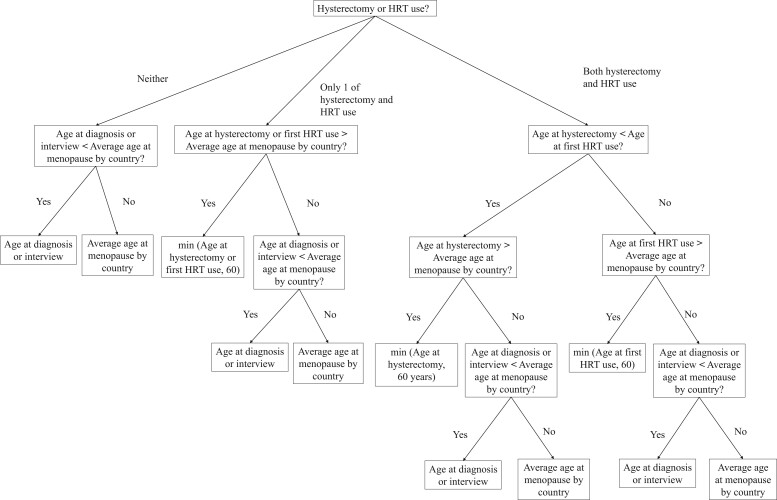
Seven studies recorded age at LMP (cases = 6881 [32.4% of total]; controls = 8316 [31.7% of total]). For the remaining studies, we imputed age at LMP (see Figure 2) (44) and assessed the imputation algorithm by comparing actual vs imputed age at LMP for the 7 sites (Supplementary Table 2, available online). Sites with 50% or more missing values in any LOY component except age at LMP were excluded from algorithms using those components (Supplementary Table 3, available online) (45,46).
Figure 2.
Flowchart for imputation of age at last menstrual period (LMP). HRT = hormone replacement therapy.
Variables considered a priori as potential confounders included age at diagnosis (cases) or interview (controls), self-reported race (Asian, Black, Other, Unknown, White, where Other was defined by each participating study as not Asian, Black, or White), education, body mass index (BMI) 1 year to 5 years prior, family history of ovarian or breast cancer in a first-degree relative, smoking status, history of endometriosis, and tubal ligation.
Statistical analyses
Assessment of study heterogeneity
We used random effects meta-analysis to assess interstudy LOY-EOC heterogeneity. Because we observed no substantive heterogeneity (Supplementary Figure 1, available online), we used the pooled data set adjusted for study site for all analyses.
Correlations between LOY values among algorithms and between LOY and LOY components
We used Pearson correlation to assess pairwise correlations of LOY calculated among algorithms limiting analyses to observations with complete data for each algorithm in the pairwise comparison. Pearson correlation was also used to assess the correlations of individual components with LOY calculated by each algorithm.
Estimation of LOY-EOC association
Multivariable logistic regression was used to estimate odds ratios (ORs) with 95% confidence intervals (CIs) for the association between LOY and EOC overall and by histotype. Models were adjusted for study site, age at diagnosis or interview, race, education, BMI, smoking status, and family history. Inclusion of tubal ligation and endometriosis in models did not alter any findings; thus, tubal ligation and endometriosis were omitted from final models. Because OCAC only recorded total months of breastfeeding across all live births and not months per breastfeeding episode, to account for return of ovulation once food is introduced typically at 6 months, we performed sensitivity analyses replacing breastfeeding duration with either 1) number of live births times the average duration of breastfeeding per live birth if the average duration was less than 6 months or 2) number of live births times 6 months if the average duration was 6 months or greater. Similar sensitivity analyses were performed for algorithms containing a term for breastfeeding duration (algorithms I-L). Sensitivity analyses were performed with multiple imputation by chained equation to assess the effect of missing values on LOY-EOC associations (47) including the same covariates as main models. Nested imputations were done for number of pregnancies, number of full-term births, duration of breastfeeding, and duration of OC use using the binary variables of ever pregnant, ever breastfed, and OC use, respectively. Imputations were done 5 times with auxiliary variables defined as Pearson correlation larger than 0.4 (48). Sensitivity analyses also examined limiting models to population-based studies and using only observations with complete data for all variables.
To assess the relationship between LOY and EOC histotypes, we present results using algorithm K because this algorithm most closely reflects lifetime ovulatory years accounting for OC use, pregnancy type, and breastfeeding.
Prior studies suggest that the relationship between LOY and EOC may not be linear (49); thus, we constructed models using LOY and log(LOY). Because log(LOY) did not improve model fit when included with LOY and models with LOY alone provided a better fit than those with log(LOY) alone, we report only analyses using LOY.
Estimation of EOC risk related to LOY components: observed vs expected estimates
The association of each LOY component and EOC risk overall and separately for each histotype was estimated using multivariable logistic regression adjusted for study site, age at diagnosis (case patients) or interview (control participants), race, education, BMI 1 to 5 years prior to diagnosis (case patients) or interview (control participants), smoking status, family history, and other LOY components.
To assess whether each component acts through ovulation suppression alone, we compared expected beta coefficient with actual estimates obtained from regression models (7). Based on the incessant ovulation hypothesis, 1 year of ovulation suppression should have the same effect on the log odds of EOC regardless of origin. Thus, if we assign one as the expected beta coefficient for age at LMP per year (indicating that a 1-year increase in LMP, which would increase LOY by 1, would increase the log odds by 1), then the expected beta coefficient for age at menarche per year would be −1 because each additional year increase would decrease LOY by 1 year and hence decrease the log odds by 1. Similarly, the expected beta coefficients for OC use per year, number of incomplete pregnancies (assumed to be 3 months or 0.25 years), number of full-term births (assumed to be 9 months or 0.75 years), and breastfeeding per year would be −1, −0.25, −0.75, and −1, respectively.
We then computed the relative coefficients, defined as the actual coefficients from regression models divided by the actual coefficient of age at LMP. This set the relative coefficient for age at LMP to 1, just as in the expected model. This enabled us to compare the relative coefficient estimates with their expected counterparts. To assess the statistical significance of individual components, χ2 statistics and P values were obtained from the likelihood-ratio test for the removal of each component from the full model. Sensitivity analyses examined limiting models to population-based studies and using only observations with complete data for all variables.
All statistical tests were 2-sided and performed in Stata/SE version 16.1 (StataCorp, College Station, TX, USA).
Results
Study population
Among the 25 studies, there were 26 204 control participants and 21 267 case patients (Table 2). Compared with controls, cases were more likely to have a family history of breast or ovarian cancer and a history of endometriosis, be hysterectomized, and be obese or overweight. Controls were more likely to have never smoked, be premenopausal, and have had a tubal ligation. Cases reported a shorter total duration of OC use and breastfeeding and fewer total pregnancies.
Table 2.
Characteristics of ovarian cancer cases and controls included in the lifetime ovulatory years (LOY) analyses
| Variables | Control participants, | Case patients, |
|---|---|---|
| (n = 26 204) | (n = 21 267) | |
| Age, mean (SD), y | 56.51 (12.06) | 56.59 (12.36) |
| Race, No. (%)a | ||
| Asian | 2019 (7.7) | 1227 (5.8) |
| Black | 566 (2.2) | 460 (2.2) |
| Other | 775 (3.0) | 692 (3.3) |
| Unknown | 258 (1.0) | 203 (1.0) |
| White | 22 586 (86.2) | 18 685 (87.9) |
| Education, No. (%) | ||
| Less than high school | 2857 (10.9) | 2512 (11.8) |
| Completed high school | 6508 (24.8) | 5309 (25.0) |
| Completed some college | 5573 (21.3) | 4849 (22.8) |
| Completed college or university bachelor degree | 4727 (18.0) | 3344 (15.7) |
| Completed graduate or professorial degree | 3139 (12.0) | 2271 (10.7) |
| Unknown | 3400 (13.0) | 2982 (14.0) |
| BMI at age 18 y, No. (%) | ||
| <18.5 kg/m2 | 2637 (10.1) | 2008 (9.4) |
| 18.5-24.9 kg/m2 | 10 697 (40.8) | 8809 (41.4) |
| 25-29.9 kg/m2 | 992 (3.8) | 1002 (4.7) |
| ≥30 kg/m2 | 310 (1.2) | 353 (1.7) |
| Unknown | 11 568 (44.2) | 9095 (42.8) |
| BMI 1 or 5 years prior, No. (%) | ||
| <18.5 kg/m2 | 286 (1.1) | 274 (1.3) |
| 18.5-24.9 kg/m2 | 7472 (28.5) | 5672 (26.7) |
| 25-29.9 kg/m2 | 4541 (17.3) | 3570 (16.8) |
| ≥30 kg/m2 | 3074 (11.7) | 3021 (14.2) |
| Unknown | 10 831 (41.3) | 8730 (41.1) |
| Smoking status, No. (%) | ||
| Never smoker | 13 311 (50.8) | 10 106 (47.5) |
| Former smoker | 2900 (11.1) | 2682 (12.6) |
| Current smoker | 7449 (28.4) | 5930 (27.9) |
| Unknown | 2544 (9.7) | 2549 (12.0) |
| Family history of breast or ovarian cancer in first-degree relative, No. (%) | ||
| No | 16 038 (61.2) | 11 574 (54.4) |
| Yes | 1569 (6.0) | 1808 (8.5) |
| Unknown | 8597 (32.8) | 7885 (37.1) |
| Tubal ligation, No. (%) | ||
| No | 16 351 (62.4) | 15 035 (70.7) |
| Yes | 5138 (19.6) | 3345 (15.7) |
| Unknown | 4715 (18.0) | 2887 (13.6) |
| Menopausal status, No. (%) | ||
| Pre/perimenopausal | 8206 (31.3) | 5775 (27.2) |
| Postmenopausal | 16 749 (63.9) | 14 422 (67.8) |
| Unknown | 1249 (4.8) | 1070 (5.0) |
| Endometriosis, No. (%) | ||
| No | 18 294 (69.8) | 15 128 (71.1) |
| Yes | 1291 (4.9) | 1615 (7.6) |
| Unknown | 6619 (25.3) | 4524 (21.3) |
| Hysterectomy prediagnosis (cases) or interview (controls), No. (%) | ||
| No | 20 969 (80.0) | 14 562 (68.5) |
| Yes | 4004 (15.3) | 5008 (23.6) |
| Unknown | 1231 (4.7) | 1697 (8.0) |
| Hormone replacement therapy, No. (%) | ||
| No | 15 547 (59.3) | 13 097 (61.6) |
| Yes | 7472 (28.5) | 5921 (27.8) |
| Unknown | 3185 (12.2) | 2249 (10.6) |
| Components of lifetime ovulatory years | ||
| Age at last menstrual period before diagnosis or interview, No. (%) | 26 204 (100.0) | 21 267 (100.0) |
| Mean (SD), y | 48.77 (6.03) | 48.84 (6.4) |
| Age at menarche, No. (%) | 25 255 (96.4) | 20 101 (94.5) |
| Mean (SD), y | 12.91 (1.7) | 12.79 (1.6) |
| Duration of oral contraceptive use, No. (%) | 24 948 (95.2) | 19 762 (92.9) |
| Mean (SD), mo | 52.12 (71.3) | 37.42 (59.3) |
| No. of pregnancies, regardless of outcome, No. (%) | 25 429 (97.0) | 20 429 (96.1) |
| Mean (SD) | 2.75 (1.8) | 2.40 (1.9) |
| Total number of months of being pregnant, regardless of outcome(s), No. (%) | 14 438 (55.1) | 12 195 (57.3) |
| Mean No. (SD) | 21.42 (22.3) | 16.39 (17.6) |
| Total number of full-term births, No. (%) | 22 835 (87.1) | 18 304 (86.1) |
| Mean No. (SD) | 2.13 (1.5) | 1.85 (1.6) |
| Total months of breastfeeding, No. (%) | 18 578 (70.1) | 13 619 (64.0) |
| Mean (SD), mo | 9.52 (14.4) | 6.86 (13.1) |
| Behavior and histotypes, No. (%) | ||
| Invasive | — | 17 465 (82.1) |
| High-grade serous | — | 7492 (71.8) |
| Low-grade serous | — | 513 (4.9) |
| Serous (unknown grade) | — | 2418 (23.2) |
| Endometrioid | — | 2536 (14.5) |
| Mucinous | — | 1134 (6.5) |
| Clear cell | — | 1310 (7.5) |
| Mixed | — | 566 (3.2) |
| Others | — | 1496 (8.6) |
| Low malignant potential (borderline tumors) | — | 3602 (16.9) |
| Unknown behavior | — | 200 (0.9) |
Race was self-reported by participants and provided to the OCAC Core. The category “other” refers to lack of self-identification as Asian, Black, or White. BMI = body mass index; OCAC = Ovarian Cancer Association Consortium.
LOY estimations and correlations
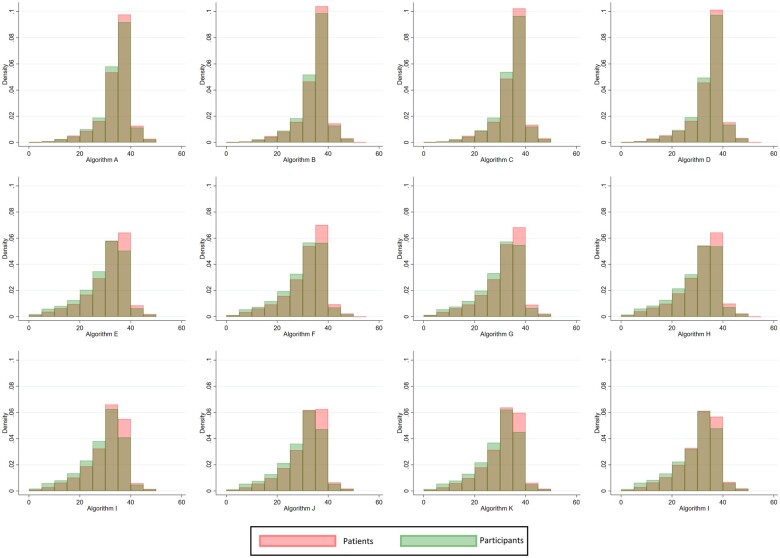
Among the 12 algorithms, median LOY ranged from 31.67 (interquartile range [IQR] = 25.50-35.20) to 35.75 (IQR = 32.50-37.50) years (Figure 3; Supplementary Table 4, available online). Pairwise LOY correlations ranged from 0.75 between the algorithms in the first class (inclusive of pregnancies only) and the third class (inclusive of pregnancies, OC use, and breastfeeding) to at least 0.99 for correlations within the same class (Supplementary Table 5, available online). Correlations between individual components and LOY are presented in Supplementary Table 6 (available online). As algorithm complexity increased, correlations between age at LMP and LOY decreased. OC duration was moderately negatively correlated with LOY (rho range: −0.68 to −0.69); correlations between the other components and LOY were low.
Figure 3.
Distribution of lifetime ovulatory years calculated from 12 different algorithms.
Estimation of LOY-EOC association
Odds ratios for LOY per year increase across the 12 algorithms ranged from 1.014 (95% CI = 1.009 to 1.020) to 1.044 (95% CI = 1.041 to 1.048) (Table 3). Associations with LOY calculated from the third class of algorithms (inclusive of pregnancies, OC use, and breastfeeding) were not changed when months of breastfeeding were truncated at 6 for participants reporting more than 6 months per birth (data not shown). LOY associations remain unchanged when adjusting models in the first class of algorithms (which included only pregnancies) for OC and breastfeeding duration, as well as when adjusting the second class of algorithms (which included pregnancies and OC duration) for breastfeeding duration (data not shown). Sensitivity analyses with multiple imputations of missing values did not alter LOY-EOC associations (Table 3). Sensitivity analyses limited to population-based studies and those limited to observations with complete data also did not alter the LOY-EOC association (data not shown).
Table 3.
Odds ratio for ovarian cancer per lifetime ovulatory year using complete data and full data with imputation
| Lifetime ovulatory years algorithm | Main analysesa (complete data only) |
Sensitivity analysesa (includes imputed data) | ||
|---|---|---|---|---|
| Control participants | Case patients | Odds ratiob (95% CI) | Odds ratiob (95% CI) | |
| First class of algorithms—anovulation due to pregnancy | ||||
| Algorithm A | 25 081 | 20 046 | 1.018 (1.013 to 1.022) | 1.015 (1.011 to 1.020) |
| Algorithm B | 22 519 | 18 013 | 1.014 (1.009 to 1.020) | 1.012 (1.007 to 1.017) |
| Algorithm C | 22 509 | 18 003 | 1.016 (1.011 to 1.021) | 1.014 (1.009 to 1.019) |
| Algorithm Dc | 13 617 | 10 689 | 1.016 (1.010 to 1.023) | 1.009 (1.003 to 1.016) |
| Second class of algorithms—anovulation due to pregnancy and OC use | ||||
| Algorithm E | 24 480 | 19 323 | 1.044 (1.041 to 1.048) | 1.043 (1.039 to 1.046) |
| Algorithm Fd | 22 316 | 17 772 | 1.043 (1.039 to 1.046) | 1.042 (1.039 to 1.046) |
| Algorithm Gd | 22 306 | 17 762 | 1.043 (1.040 to 1.047) | 1.043 (1.039 to 1.047) |
| Algorithm Hc,d | 13 515 | 10 576 | 1.043 (1.039 to 1.048) | 1.041 (1.036 to 1.045) |
| Third class of algorithms—anovulation due to pregnancy, OC use, and breastfeeding | ||||
| Algorithm Ie | 14 900 | 11 829 | 1.041 (1.036 to 1.045) | 1.047 (1.043 to 1.051) |
| Algorithm Jf | 14 902 | 11 339 | 1.041 (1.036 to 1.045) | 1.046 (1.042 to 1.050) |
| Algorithm Kf | 14 900 | 11 329 | 1.041 (1.036 to 1.046) | 1.046 (1.042 to 1.050) |
| Algorithm L | 8473 | 6498 | 1.040 (1.034 to 1.046) | 1.047 (1.042 to 1.052) |
Main analyses included participants without missing values in any component for LOY calculation; sensitivity analyses included all participants with imputation. CI = confidence interval; MCC = Melbourne Collaborative Cohort Study; NTC = Nijmegen Ovarian Cancer Study; OC = oral contraceptive; TBO = Tampa Bay Ovarian Cancer Study.
Adjusted for study site, age, self-reported race (Asian, Black, Other [as defined by participants as not being Asian, Black, or White], Unknown, White), education (less than high school, completed high school, completed some college, completed college or university bachelor degree, completed graduate or professorial degree, unknown), body mass index 1 or 5 years prior (underweight, normal, overweight, obese, unknown), smoking status (never, former, current, unknown), and family history (yes, no, unknown).
TBO was excluded from the sensitivity analyses because of limited numbers within site to impute missing values.
MCC was excluded from the sensitivity analyses because of limited numbers within site to impute missing values.
NTH was excluded from the sensitivity analyses because of failure to converge on observed data.
NTH was excluded from the sensitivity analyses because of limited numbers within site to impute missing values.
Estimation of EOC risk related to LOY components: observed vs expected estimates
Individual components in LOY, except for age at menarche, were associated with EOC (Table 4). There were substantial deviations between relative estimated coefficients and expected estimates for each component. The estimated coefficient of OC use per year was 4.45 times larger than expected, and estimates for pregnancies were 11- to 15-fold greater than expected regardless of pregnancy type. Estimated coefficient of breastfeeding per year was −13.45, instead of the expected −1. Results were similar when truncating breastfeeding at 6 months per full-term birth, when limiting analyses to population-based studies and when limiting analyses to observations with complete data (data not shown).
Table 4.
Odds ratios, expected beta coefficients, and normalized beta coefficients for ovarian cancer by individual components of lifetime ovulatory years
| Lifetime ovulatory years component | OR (95% CI)a | Expected estimate of coefficient | Normalized coefficientb | P for removal of component from model |
|---|---|---|---|---|
| Age at last menstrual period before diagnosis or interview | ||||
| Per year | 1.011 (1.004 to 1.019) | 1 (defined) | 1 | .004 |
| Age at menarche | ||||
| Per year | 1.002 (0.985 to 1.018) | −1 | 0.13 | .86 |
| Duration of oral contraceptive use, y | ||||
| Per year | 0.950 (0.945 to 0.956) | −1 | −4.45 | <.001 |
| No. of incomplete pregnancies | ||||
| Per pregnancy | 0.968 (0.944 to 0.992) | −0.25 | −2.89 | .009 |
| Number of full-term births | ||||
| Per pregnancy | 0.877 (0.857 to 0.897) | −0.75 | −11.53 | <.001 |
| Total years of breastfeeding | ||||
| Per year | 0.858 (0.816 to 0.901) | −1.0 | −13.45 | <.001 |
Adjusted for study site, age, self-reported race (Asian, Black, Other [as defined by participants as not being Asian, Black or White], Unknown, White), education (less than high school, completed high school, completed some college, completed college or university bachelor degree, completed graduate or professorial degree, unknown), body mass index 1 or 5 years prior (underweight, normal, overweight, obese, unknown), smoking status (never, former, current, unknown), family history (yes, no, unknown), and other components of lifetime ovulatory cycles in the model. CI = confidence interval; OR = odds ratio.
Normalized to the beta coefficient of age at last menstrual period.
Histotype-specific estimation for LOY and individual components: observed vs expected estimates
LOY was associated with invasive high-grade serous (HGSOC; OR per year = 1.054, 95% CI = 1.048 to 1.061), low-grade serous (LGSOC; OR = 1.040, 95% CI = 1.019 to 1.061), endometrioid (OR = 1.065, 95% CI = 1.053 to 1.076), and clear cell (OR = 1.098, 95% CI = 1.079 to 1.117) but not mucinous EOC (OR = 1.006, 95% CI = 0.992 to 1.019) (Table 5). Except for breastfeeding, estimated coefficients of LOY components were close to expected for HGSOC. In contrast, estimated coefficients of individual components, except for age at menarche, were larger than the expected for LGSOC, endometrioid, and clear cell cancers.
Table 5.
Odds ratios, expected beta coefficients, and normalized beta coefficients for ovarian cancer histotypes by individual components of lifetime ovulatory years
| Component of lifetime ovulatory years | Expected estimate of coefficient | Low malignant potential | Invasive high-grade serous | Invasive low-grade serous | Invasive endometrioid | Invasive mucinous | Invasive clear cell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 2014) |
(n = 4139) |
(n = 282) |
(n = 1322) |
(n = 602) |
(n = 547) |
||||||||
| ORa (95% CI) | β̂b | ORa (95% CI) | β̂b | ORa (95% CI) | β̂b | ORa (95% CI) | β̂b | ORa (95% CI) | β̂c | ORa (95% CI) | β̂b | ||
| Lifetime ovulatory yearsc,d | |||||||||||||
| Per year | 1.015 (1.007 to 1.024) | — | 1.054 (1.048 to 1.061) | — | 1.040 (1.019 to 1.061) | — | 1.065 (1.053 to 1.076) | — | 1.006 (0.992 to 1.019) | — | 1.098 (1.079 to 1.117) | — | |
| Age at last menstrual period before diagnosis or interview | |||||||||||||
| Per year | 1 | 0.981 (0.967 to 0.995) | 1 | 1.056 (1.044 to 1.069) | 1 | 1.010 (0.976 to 1.044) | 1 | 1.031 (1.013 to 1.049) | 1 | 0.977 (0.955 to 1.000) | 1 | 1.086 (1.057 to 1.117) | 1 |
| Age at menarche | |||||||||||||
| Per year | −1 | 1.027 (0.995 to 1.059) | −1.360 | 1.000 (0.977 to 1.023) | −0.008 | 0.961 (0.891 to 1.037) | −4.157 | 1.003 (0.967 to 1.041) | 0.093 | 1.076 (1.023 to 1.132) | −3.208 | 0.948 (0.867 to 1.002) | −0.643 |
| Duration of oral contraceptive use, y | |||||||||||||
| Per year | −1 | 0.973 (0.962 to 0.983) | 1.441 | 0.948 (0.940 to 0.956) | −0.975 | 0.953 (0.929 to 0.978) | −5.042 | 0.928 (0.914 to 0.942) | −2.472 | 0.973 (0.955 to 0.991) | 1.186 | 0.925 (0.904 to 0.947) | −0.942 |
| No. of incomplete pregnancies | |||||||||||||
| Per pregnancy | −0.25 | 1.00 (0.96 to 1.04) | 0.082 | 0.99 (0.95 to 1.02) | −0.245 | 0.89 (0.78 to 1.01) | −12.484 | 0.93 (0.87 to 0.98) | −2.533 | 0.94 (0.87 to 1.03) | 2.516 | 0.85 (0.77 to 0.94) | −1.924 |
| Total number of full-term births | |||||||||||||
| Per pregnancy | −0.75 | 0.82 (0.79 to 0.86) | 10.085 | 0.94 (0.91 to 0.97) | −1.170 | 0.92 (0.82 to 1.02) | −9.356 | 0.74 (0.70 to 0.78) | −9.884 | 0.88 (0.82 to 0.95) | 5.417 | 0.63 (0.57 to 0.69) | −5.583 |
| Total years of breastfeeding | |||||||||||||
| Per year | −1 | 0.963 (0.867 to 1.069) | 1.970 | 0.827 (0.771 to 0.886) | −3.467 | 0.808 (0.627 to 1.041) | −22.533 | 0.830 (0.727 to 0.948) | −6.120 | 1.010 (0.858 to 1.190) | −0.455 | 0.893 (0.723 to 1.103) | −1.370 |
Adjusted for study site, age, self-reported race (Asian, Black, Other [as defined by participants as not being Asian, Black or White], Unknown, White), education (less than high school, completed high school, completed some college, completed college or university bachelor degree, completed graduate or professorial degree, unknown), body mass index 1 or 5 years prior (underweight, normal, overweight, obese, unknown), smoking status (never, former, current, unknown), family history (yes, no, unknown), and other components of lifetime ovulatory cycles in the model. β = estimated coefficient; CI = confidence interval; OC = oral contraceptive; OR = odds ratio.
Normalized to the beta coefficient of age at last menstrual period.
Adjusted for study site, age, self-reported race (Asian, Black, Other [as defined by participants as not being Asian, Black, or White], Unknown, White), education (less than high school, completed high school, completed some college, completed college or university bachelor degree, completed graduate or professorial degree, unknown), body mass index 1 or 5 years prior (underweight, normal, overweight, obese, unknown), smoking status (never, former, current, unknown), and family history (yes, no, unknown).
Using algorithm K with complete data: (age at last menstrual period—age at menarche) – years of OC use—(0.25*number of incomplete pregnancies)—(0.75*number of full-term births) – years of breastfeeding. This algorithm was chosen because it most closely accounts for expected ovulation suppression due to pregnancies, OC use, and breastfeeding.
Discussion
Pooling data from 25 case-control studies, we show a positive association between LOY and EOC, with each year of ovulation associated with a 4% increase in risk. We also found a positive association between LOY and HGSOC, LGSOC, endometrioid, and clear cell EOC but not with mucinous tumors. These LOY-EOC associations were not altered when using different algorithms to compute LOY or when imputing missing data. We further found that LOY components, except age at menarche, were associated with EOC, with the magnitude of these associations varying substantially from expectation if their mechanism of action was solely ovulation suppression. There was also notable heterogeneity in these component-specific findings among EOC histotypes. Together, these data suggest that reproductive factors comprising LOY exert their effects through means beyond ovulation suppression, and those relationships vary by EOC subtype.
Most prior studies report a positive relationship between LOY and EOC (2,7-14,50-63). Differences in LOY definitions among studies make it challenging to compare specific findings across studies. In the present study, we defined LOY from available harmonized data using 12 algorithms. Like the Polish Cancer study (8) (1 of the 25 studies in this analysis), we found a high correlation for LOY among algorithms, although point estimates varied depending on the algorithm. When assessing overall EOC per 1-year increase in LOY, estimates ranged from 1.01 to 1.04, which is similar to estimates reported by the US Nurses’ Health Study (1976-2006) and Nurses’ Health Study II (1989-2005) (OR = 1.07, 95% CI = 1.05 to 1.08) (10). Although it is reassuring that our results are similar to previous work, because each study used different LOY algorithms and units of presentation (eg, quartiles, ovulatory cycles) (15), a direct comparison of estimated magnitudes is not possible. A standardized definition of LOY would facilitate cross-study comparisons and allow for more robust interstudy analyses. Our findings confirm that among algorithms that account for menstrual span, number of pregnancies, total duration of OC use, and total duration of breastfeeding, point estimates for the LOY-EOC relationship are similar. Defining LOY using these factors would facilitate interstudy analyses.
We report differences in the association of LOY with EOC subtypes. We report a positive association between LOY and HGSOC and LGSOC. Whereas previous studies have reported a positive association between LOY and risk of serous tumors (2,10-15), only the Ovarian Cancer Cohort Consortium (OC3) (14) reported results separately for HGSOC, also finding a positive association. Separating serous EOC analyses is important because HGSOC and LGSOC are distinct diseases (64,65). Also consistent with most (10-14) but not all previous studies (2,15), we found positive associations between LOY and clear cell and endometrioid but not mucinous tumors. These results are consistent with epidemiologic evidence that suggests a different risk-factor profile for mucinous EOC (3,66).
Results regarding the associations between LOY components and EOC appeared consistent with previous studies (7,8,10,12,58,62). Beyond considering statistical significance, our study also compared the magnitudes of each component’s effect on EOC risk and found the actual magnitudes varied substantially from expectation (7). Based on the incessant ovulation hypothesis (6), women with the same LOY should have the same estimated risk if ovulation is the only etiologic mechanism underlying the relationship between the components of LOY and EOC. However, consistent with 2 case-control studies (7,62), we show that pregnancy, OC use, and breastfeeding are associated with stronger protective effects than would be expected based on ovulation suppression alone. Moreover, the protection from 1 year of pregnancy, whether complete or incomplete, was substantially greater than that of 1 year of OC use (7). Together, these data imply that mechanisms beyond ovulation suppression, such as hormonal alterations (67,68) or inflammation (69), contribute to the LOY-EOC association. They further imply differences in the mechanisms whereby individual LOY components impact EOC risk, especially for non-HGSOC subtypes, suggesting that a model of EOC risk incorporating just LOY and not its component parts would be insufficient in fully capturing the effects of exposure to LOY components.
Our results indicate heterogeneity in the associations between LOY components and histotype-specific risk. Notably, except for breastfeeding, the estimated coefficients for HGSOC were close to expected if only ovulation suppression underlies the component-HGSOC relationship. This suggests that ovulation may be the primary etiologic mechanism for HGSOC; however, because HGSOC is believed to arise in the fimbriated end of the fallopian tube and not the ovary (70-72), ovulation effects must extend beyond ovarian surface epithelium trauma, as originally proposed by Fathalla (6). Notably, during ovulation, fallopian tube fimbria come in close proximity to the site of ovulation, directly exposing the fimbria to ovarian follicular fluid. In vitro studies show that normal fallopian tube epithelia exposed to follicular fluid aspirates develop TP53 mutations, a hallmark of HGSOC (73). Moreover, follicular fluid has both mutagenic and tumorigenic effects facilitating the full transformation process for developing HGSOC from the fallopian tube (74-77). Thus, follicular fluid may be the link between greater number of ovulations and HGSOC.
In contrast to HGSOC, factors beyond ovulation suppression underlie the link between LOY and other histotypes. For LGSOC, endometrioid and clear cell histotypes, we found that actual coefficient estimates were substantially larger than expected for OC use, pregnancies, and breastfeeding. This suggests that other mechanisms, such as increased progestin exposure (78), may play a role in the protective effects of these factors.
Although we did not find any association between LOY and mucinous EOC, we report associations for several LOY components. Thus, factors other than ovulation may be driving mucinous carcinogenesis. Moreover, the relationship between LOY components and mucinous disease varied from that of other histotypes. Together, these observations suggest that factors underlying the relationship between exposures and EOC vary based on histotype and confirm the unique origin of mucinous cancers (79,80).
The major strength of our work was pooling 25 case-control studies, allowing us to estimate more precisely the LOY-EOC association overall and by histotype. The large data set also enabled comparison of different LOY definitions and their impact on the LOY-EOC relationship. For LOY components, the sample size enabled us to separate the effects of ovulation suppression from other potential etiologic mechanisms. The range of studies from 4 continents and 9 countries supports the generalizability of our findings.
Despite these strengths, there are several limitations. Because all but 2 studies (25,42) employed a retrospective case-control design, recall and selection bias are always a concern. Regardless of study design limitations, our estimates were consistent with previous prospective studies, including the US Nurses’ Health Study and US Nurses’ Health Study II studies (10) and the OC3 pooled analysis of prospective studies (14). We made some assumptions about LOY components that may impact results. If age at LMP was unknown, we imputed it using an algorithm based on average age at menopause by country, age at first hormone replacement therapy use, or age at hysterectomy. We compared the observed and imputed age at LMP from 7 sites, conducted sensitivity analyses using LOY calculated from the imputed value for those sites, and noted no differences in observed associations. To prevent overestimating the duration of anovulation from breastfeeding, we repeated analyses capping women at 6 months of breastfeeding per live birth. Results were unchanged.
In conclusion, increasing LOY is associated with increased EOC risk, as well as the risk of HGSOC, LGSOC, endometrioid, and clear cell histotypes. Although point estimates varied slightly, the association between LOY and EOC was not altered when LOY was calculated in different ways using core components. Our study also indicated heterogeneity in the expected estimated coefficients of each LOY component on histotype-specific EOC. Together, our findings suggest that ovulation suppression is not the sole mechanism whereby reproductive factors affect EOC overall and for non-HGSOC histotypes. Identifying these mechanisms and understanding their individual and joint roles can provide deeper insight into disease etiology and potential risk-reducing approaches.
Supplementary Material
Contributor Information
Zhuxuan Fu, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Maria Mori Brooks, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA; Department of Biostatistics, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Sarah Irvin, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Susan Jordan, The School of Public Health, The University of Queensland, Brisbane, Queensland, Australia.
Katja K H Aben, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Netherlands Comprehensive Cancer Organisation, Utrecht, The Netherlands.
Hoda Anton-Culver, Department of Medicine, Genetic Epidemiology Research Institute, University of California Irvine, Irvine, CA, USA.
Elisa V Bandera, Cancer Prevention and Control Program, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA.
Matthias W Beckmann, Department of Gynecology and Obstetrics, Comprehensive Cancer Center Erlangen-European Metropolitan Region of Nuremberg (EMN), Friedrich-Alexander University Erlangen-Nuremberg, University Hospital Erlangen, Erlangen, Germany.
Andrew Berchuck, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, USA.
Angela Brooks-Wilson, Canada’s Michael Smith Genome Sciences Centre, BC Cancer, Vancouver, BC, Canada.
Jenny Chang-Claude, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Cancer Epidemiology Group, University Cancer Center Hamburg (UCCH), University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Linda S Cook, Epidemiology, School of Public Health, University of Colorado, Aurora, CO, USA; Community Health Sciences, University of Calgary, Calgary, AB, Canada.
Daniel W Cramer, Department of Obstetrics and Gynecology, Obstetrics and Gynecology Epidemiology Center, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Kara L Cushing-Haugen, Program in Epidemiology, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Jennifer A Doherty, Department of Population Health Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Arif B Ekici, Institute of Human Genetics, Comprehensive Cancer Center Erlangen-EMN, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg (FAU), Erlangen, Germany.
Peter A Fasching, Department of Gynecology and Obstetrics, Comprehensive Cancer Center Erlangen-European Metropolitan Region of Nuremberg (EMN), Friedrich-Alexander University Erlangen-Nuremberg, University Hospital Erlangen, Erlangen, Germany.
Renée T Fortner, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Simon A Gayther, Center for Bioinformatics and Functional Genomics and the Cedars Sinai Genomics Core, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Aleksandra Gentry-Maharaj, MRC Clinical Trials Unit, Institute of Clinical Trials & Methodology, University College London, London, UK.
Graham G Giles, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Victoria, Australia.
Ellen L Goode, Department of Quantitative Health Sciences, Division of Epidemiology, Mayo Clinic, Rochester, MN, USA.
Marc T Goodman, Cancer Prevention and Control Program, Cedars-Sinai Cancer, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Holly R Harris, Program in Epidemiology, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
Alexander Hein, Department of Gynecology and Obstetrics, Comprehensive Cancer Center Erlangen-European Metropolitan Region of Nuremberg (EMN), Friedrich-Alexander University Erlangen-Nuremberg, University Hospital Erlangen, Erlangen, Germany.
Rudolf Kaaks, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Lambertus A Kiemeney, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Martin Köbel, Department of Pathology and Laboratory Medicine, University of Calgary, Foothills Medical Center, Calgary, AB, Canada.
Joanne Kotsopoulos, Women’s College Research Institute, Women’s College Hospital, University of Toronto, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Nhu D Le, Cancer Control Research, BC Cancer, Vancouver, BC, Canada.
Alice W Lee, Department of Health Science, California State University, Fullerton, Fullerton, CA, USA.
Keitaro Matsuo, Division of Cancer Epidemiology and Prevention, Aichi Cancer Center Research Institute, Nagoya, Japan; Division of Cancer Epidemiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Valerie McGuire, Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA.
John R McLaughlin, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Usha Menon, MRC Clinical Trials Unit, Institute of Clinical Trials & Methodology, University College London, London, UK.
Roger L Milne, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Victoria, Australia.
Kirsten B Moysich, Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY, USA.
Celeste Leigh Pearce, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Malcolm C Pike, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA; Department of Population Health and Public Health Sciences, Keck School of Medicine, University of Southern California Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Bo Qin, Cancer Prevention and Control Program, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA.
Susan J Ramus, School of Clinical Medicine, University of New South Wales Medicine and Health, University of New South Wales Sydney, Sydney, New South Wales, Australia; Adult Cancer Program, Lowy Cancer Research Centre, University of New South Wales Sydney, Sydney, New South Wales, Australia.
Marjorie J Riggan, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, USA.
Joseph H Rothstein, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Joellen M Schildkraut, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Weiva Sieh, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Rebecca Sutphen, Epidemiology Center, College of Medicine, University of South Florida, Tampa, FL, USA.
Kathryn L Terry, Department of Obstetrics and Gynecology, Obstetrics and Gynecology Epidemiology Center, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Pamela J Thompson, Samuel Oschin Comprehensive Cancer Institute, Cancer Prevention and Genetics Program, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Linda Titus, Muskie School of Public Policy, Public Health, Portland, ME, USA.
Anne M van Altena, Department of Obstetrics and Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Emily White, Department of Epidemiology, University of Washington, Seattle, WA, USA; Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Alice S Whittemore, Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA; Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA, USA.
Anna H Wu, Department of Population Health and Public Health Sciences, Keck School of Medicine, University of Southern California Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Wei Zheng, Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, USA.
Argyrios Ziogas, Department of Medicine, Genetic Epidemiology Research Institute, University of California Irvine, Irvine, CA, USA.
Sarah E Taylor, Division of Gynecologic Oncology, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Lu Tang, Department of Biostatistics, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Thomas Songer, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Nicolas Wentzensen, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Penelope M Webb, The School of Public Health, The University of Queensland, Brisbane, Queensland, Australia.
AOCS Group, Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Center, Melbourne, Victoria, Australia; Centre for Cancer Research, The Westmead Institute for Medical Research, Sydney, New South Wales, Australia.
Harvey A Risch, Chronic Disease Epidemiology, Yale School of Public Health, New Haven, CT, USA.
Francesmary Modugno, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA; Division of Gynecologic Oncology, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Women’s Cancer Research Center, Magee-Womens Research Institute and Hillman Cancer Center, Pittsburgh, PA, USA.
Funding
The Ovarian Cancer Association Consortium is funded by generous contributions from its research investigators and through anonymous donations. The Ovarian Cancer Association Consortium was funded by a grant from the Ovarian Cancer Research Fund.
Funding for individual studies:
AUS: The Australian Ovarian Cancer Study was supported by the U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729), National Health & Medical Research Council of Australia (199600, 400413 and 400281), Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania and Cancer Foundation of Western Australia (Multi-State Applications 191, 211 and 182). The Australian Ovarian Cancer Study gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Foundation; BAV: Funds of the University of Erlangen-Nuremberg; CON: United States National Institutes of Health (R01-CA063678, R01-CA074850; R01-CA080742); DOV: United States National Institutes of Health R01-CA112523 and R01-CA87538; GER: German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research (01 GB 9401) and the German Cancer Research Center (DKFZ); HAW: United States National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP: University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award (F. Modugno), Department of Defense (DAMD17-02-1-0669, OC20085) and United States National Cancer Institute (R21-CA267050, K07-CA080668, R01-CA95023, MO1-RR000056). Funding was also provided by the United States National Science Foundation (DGE-2217399-Modugno). The views expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation; JPN: Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare; MAY: United States National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; MCC: Cohort recruitment was funded by VicHealth and Cancer Council Victoria. Additional support from the Australian National Health and Medical Research Council grants 209057, 396414, and 1074383 and infrastructure support provided by Cancer Council Victoria; NCO: United States National Institutes of Health (R01-CA76016) and the Department of Defense (DAMD17-02-1-0666); NEC: United States National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10-1-02802; NJO: United States National Cancer Institute (NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, and P30-CA072720) and the Cancer Institute of New Jersey; NTH: Radboud University Medical Centre; OVA: This work was supported by Canadian Institutes of Health Research grant (MOP-86727) and by United States National Institute of Health R01CA160669-01A1; POL: Intramural Research Program of the National Cancer Institute; SON: National Health Research and Development Program, Health Canada, grant 6613-1415-53; STA: United States National Institutes of Health grants U01 CA71966 and U01 CA69417; SWH: United States National Institutes of Health grant R37-CA070867 and UM1-CA182910; TBO: United States National Institutes of Health (R01-CA106414-A2), American Cancer Society (CRTG-00-196-01-CCE), Department of Defense (DAMD17-98-1-8659), Celma Mastry Ovarian Cancer Foundation; TOR: United States National Institutes of Health grants R01 CA063678 and R01 CA063682; UCI: United States National Institutes of Health R01-CA058860 and the Lon V Smith Foundation grant LVS-39420; UKO: The UKOPS study was funded by The Eve Appeal (The Oak Foundation) with contribution to author’s salary through Medical Research Council core funding MC_UU_00004/01 and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. UM and AGM are supported by salary contributions through Medical Research Council core funding (MC_UU_00004/01); USC: United States National Institutes of Health P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403, and California Cancer Research Program (00-01389 V-20170, 2II0200); VTL: United States National Institutes of Health K05-CA154337.
Notes
Role of the funder: Provision of funding only. Funders had no role in the execution of this study.
Disclosures: Elisa Bandera is currently a member of an Advisory Board for the Pfizer Clinical Trial Diversity Initiative. Usha Menon held personal shares in Abcodia between April 1, 2011, and October 30, 2021. Penelope Webb received grant funding for an unrelated study of ovarian cancer and a speaker’s honorarium from AstraZeneca. No other authors reported any disclosures. Harvey A. Risch, a JNCI Associate Editor and co-author on this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: ZF: Conceptualization, Methodology, Investigation, Writing—Original Draft, Writing—Review and Editing. MMB: Conceptualization, Methodology, Investigation, Writing—Review and Editing. SI, SJ, NW, PMW: Writing—Original Draft, Writing—Review and Editing, Data Curation. HAR: Conceptualization, Methodology, Investigation, Writing—Review and Editing, Data Curation. FM: Conceptualization, Methodology, Investigation, Supervision, Writing—Original Draft, Writing—Review and Editing, Funding Acquisition, Resources, Data Curation. KKHA, HAC, EVB, MWB, AB, ABW, JCC, LSC, DWC, KLCH, JAD, ABE, PAF, RTF, SAG, AGM, GGG, ELG, MTG, AOCSG, HRH, AH, RK, LAK, MK, JK, NDL, AWL, KM, VM, JRM, UM, RLM, KBM, CLP, MCP, BOQ, SJR, MJR, JHR, JMS, WS, RS, KLT, PJT, LT, AMVA, EW, ASW, AHW, WZ, AZ, SET, LT, TS: Writing—Review and Editing, Data Curation.
Acknowledgements: We thank all the study participants who contributed to this study and all the researchers, clinicians, technical and administrative staff who have made this work possible.
Acknowledgements for individual studies:
AUS: We acknowledge the cooperation of the participating institutions in Australia, and the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete Study Group can be found at www.aocstudy.org. CON: The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. This study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data; MCC: Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database; NJO: We thank all participants and research staff at the Rutgers Cancer Institute of New Jersey, the New Jersey State Cancer Registry, and Memorial Sloan-Kettering Cancer Center; SWH: We thank the participants and the research staff of the Shanghai Women’s Health Study for making this study possible.
Data Availability
The data generated in this study are not publicly available due to restrictions of some included studies’ informed consent. The corresponding author will facilitate access to data through existing data request processes for OCAC.
References
- 1. Wu AH, Pearce CL, Lee AW, et al. Timing of births and oral contraceptive use influences ovarian cancer risk. Int J Cancer. 2017;141(12):2392-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soegaard M, Jensen A, Hogdall E, et al. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1160-1166. [DOI] [PubMed] [Google Scholar]
- 3. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sung HK, Ma SH, Choi JY, et al. The effect of breastfeeding duration and parity on the risk of epithelial ovarian cancer: a systematic review and meta-analysis. J Prev Med Public Health. 2016;49(6):349-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babic A, Sasamoto N, Rosner BA, et al. Association between breastfeeding and ovarian cancer risk. JAMA Oncol. 2020;6(6):e200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. [DOI] [PubMed] [Google Scholar]
- 7. Risch HA, Weiss NS, Lyon JL, Daling JR, Liff JM.. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117(2):128-139. [DOI] [PubMed] [Google Scholar]
- 8. Yang HP, Murphy KR, Pfeiffer RM, et al. Lifetime number of ovulatory cycles and risks of ovarian and endometrial cancer among postmenopausal women. Am J Epidemiol. 2016;183(9):800-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu ML, Whittemore AS, Paffenbarger RS Jr, et al. Personal and environmental characteristics related to epithelial ovarian cancer. I. Reproductive and menstrual events and oral contraceptive use. Am J Epidemiol. 1988;128(6):1216-1227. [DOI] [PubMed] [Google Scholar]
- 10. Gates MA, Rosner BA, Hecht JL, Tworoger SS.. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171(1):45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW.. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(1):30-35. [DOI] [PubMed] [Google Scholar]
- 12. Tung KH, Goodman MT, Wu AH, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003;158(7):629-638. [DOI] [PubMed] [Google Scholar]
- 13. Peres LC, Moorman PG, Alberg AJ, et al. Lifetime number of ovulatory cycles and epithelial ovarian cancer risk in African American women. Cancer Causes Control. 2017;28(5):405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trabert B, Tworoger SS, O’Brien KM, et al. ; for the Ovarian Cancer Cohort Consortium (OC3). The risk of ovarian cancer increases with an increase in the lifetime number of ovulatory cycles: an analysis from the Ovarian Cancer Cohort Consortium (OC3). Cancer Res. 2020;80(5):1210-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu Z, Taylor S, Modugno F.. Lifetime ovulations and epithelial ovarian cancer risk and survival: a systematic review and meta-analysis. Gynecol Oncol. 2022;165(3):650-663. [DOI] [PubMed] [Google Scholar]
- 16. Merritt MA, Green AC, Nagle CM, Webb PM; for the Australian Ovarian Cancer Study Group. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170-176. [DOI] [PubMed] [Google Scholar]
- 17. Song H, Ramus SJ, Tyrer J, et al. ; for the Ovarian Cancer Association Consortium. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41(9):996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Risch HA, Bale AE, Beck PA, Zheng W.. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1738-1741. [DOI] [PubMed] [Google Scholar]
- 19. Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA.. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23(12):1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Royar J, Becher H, Chang-Claude J.. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95(6):370-374. [DOI] [PubMed] [Google Scholar]
- 21. Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME.. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15(4):1055-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB.. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23(2):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamajima N, Matsuo K, Saito T, et al. Gene-environment interactions and polymorphism studies of cancer risk in the Hospital-based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC-II). Asian Pac J Cancer Prev. 2001;2(2):99-107. [PubMed] [Google Scholar]
- 24. Kelemen LE, Sellers TA, Schildkraut JM, et al. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68(7):2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giles GG, English DR.. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156(3):69-70. [PubMed] [Google Scholar]
- 26. Schildkraut JM, Iversen ES, Wilson MA, et al. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5(4):e10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW.. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65(13):5974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH.. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Altena AM, van Aarle S, Kiemeney LA, Hoogerbrugge N, Massuger LF, de Hullu JA.. Adequacy of family history taking in ovarian cancer patients: a population-based study. Fam Cancer. 2012;11(3):343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M.. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72(5):632-637. [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Closas M, Brinton LA, Lissowska J, et al. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC Cancer. 2007;7(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Risch HA, Marrett LD, Howe GR.. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140(7):585-597. [DOI] [PubMed] [Google Scholar]
- 33. McGuire V, Felberg A, Mills M, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160(7):613-618. [DOI] [PubMed] [Google Scholar]
- 34. Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123-1131. [DOI] [PubMed] [Google Scholar]
- 35. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer-Am Cancer Soc. 2005;104(12):2807-2816. [DOI] [PubMed] [Google Scholar]
- 36. Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353-357. [DOI] [PubMed] [Google Scholar]
- 37. Ziogas A, Gildea M, Cohen P, et al. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(1):103-111. [PubMed] [Google Scholar]
- 38. Balogun N, Gentry-Maharaj A, Wozniak EL, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. J Clin Epidemiol. 2011;64(5):525-530. [DOI] [PubMed] [Google Scholar]
- 39. Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH.. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82(1):186-195. [DOI] [PubMed] [Google Scholar]
- 40. Ness RB, Cramer DW, Goodman MT, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155(3):217-224. [DOI] [PubMed] [Google Scholar]
- 41. Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC.. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124(6):1409-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White E, Patterson RE, Kristal AR, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159(1):83-93. [DOI] [PubMed] [Google Scholar]
- 43. Ramus SJ, Vierkant RA, Johnatty SE, et al. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123(2):380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosner BA, Colditz GA.. Age at menopause: imputing age at menopause for women with a hysterectomy with application to risk of postmenopausal breast cancer. Ann Epidemiol. 2011;21(6):450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clavel J, Merceron G, Escarguel G.. Missing data estimation in morphometrics: how much is too much? Syst Biol. 2014;63(2):203-218. [DOI] [PubMed] [Google Scholar]
- 46. McNeish D. Missing data methods for arbitrary missingness with small samples. J Appl Stat. 2017;44(1):24-39. [Google Scholar]
- 47. Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227-241. [Google Scholar]
- 48. Enders CK. Applied Missing Data Analysis. New York City: Guilford Press; 2010. [Google Scholar]
- 49. Pike MC. Age-related factors in cancers of the breast, ovary, and endometrium. Journal of Chronic Diseases. 1987;40(S2):59S-69S. [DOI] [PubMed] [Google Scholar]
- 50. Hildreth NG, Kelsey JL, LiVolsi VA, et al. An epidemiologic study of epithelial carcinoma of the ovary. Am J Epidemiol. 1981;114(3):398-405. [DOI] [PubMed] [Google Scholar]
- 51. La Vecchia C, Franceschi S, Gallus G, Decarli A, Liberati A, Tognoni G.. Incessant ovulation and ovarian cancer: a critical approach. Int J Epidemiol. 1983;12(2):161-164. [DOI] [PubMed] [Google Scholar]
- 52. Whittemore AS, Harris R, Itnyre J, Halpern J.. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. I. Methods. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136(10):1175-1183. [DOI] [PubMed] [Google Scholar]
- 53. Whittemore AS, Wu ML, Paffenbarger RS Jr, et al. Epithelial ovarian cancer and the ability to conceive. Cancer Res. 1989;49(14):4047-4052. [PubMed] [Google Scholar]
- 54. Bernal A, Méndez-Moran L, Fajardo-Gutiérrez A, González-Lira G, Escudero P, Ortiz H.. Univariate and multivariate analysis of risk factors for ovarian cancer: case-control study, Mexico City. Arch Med Res. 1995;26(3):245-249. [PubMed] [Google Scholar]
- 55. Schildkraut JM, Bastos E, Berchuck A.. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1997;89(13):932-938. [DOI] [PubMed] [Google Scholar]
- 56. Schildkraut JM, Moorman PG, Bland AE, et al. Cyclin E overexpression in epithelial ovarian cancer characterizes an etiologic subgroup. Cancer Epidemiol Biomarkers Prev. 2008;17(3):585-593. [DOI] [PubMed] [Google Scholar]
- 57. Webb PM, Green A, Cummings MC, Purdie DM, Walsh MD, Chenevix-Trench G.. Relationship between number of ovulatory cycles and accumulation of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1998;90(22):1729-1734. [DOI] [PubMed] [Google Scholar]
- 58. Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Vine MF, Berchuck A.. Ovulation and ovarian cancer: a comparison of two methods for calculating lifetime ovulatory cycles (United States). Cancer Causes Control. 2002;13(9):807-811. [DOI] [PubMed] [Google Scholar]
- 59. Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC.. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104(2):228-232. [DOI] [PubMed] [Google Scholar]
- 60. Rosner BA, Colditz GA, Webb PM, Hankinson SE.. Mathematical models of ovarian cancer incidence. Epidemiology. 2005;16(4):508-515. [DOI] [PubMed] [Google Scholar]
- 61. Tung KH, Wilkens LR, Wu AH, et al. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am J Epidemiol. 2005;161(4):321-329. [DOI] [PubMed] [Google Scholar]
- 62. Pelucchi C, Galeone C, Talamini R, et al. Lifetime ovulatory cycles and ovarian cancer risk in 2 Italian case-control studies. Am J Obstet Gynecol. 2007;196(1):83.e1-83.e7. [DOI] [PubMed] [Google Scholar]
- 63. Le DC, Kubo T, Fujino Y, et al. Reproductive factors in relation to ovarian cancer: a case-control study in Northern Vietnam. Contraception. 2012;86(5):494-499. [DOI] [PubMed] [Google Scholar]
- 64. Furuya M. Ovarian cancer stroma: pathophysiology and the roles in cancer development. Cancers (Basel) .2012;4(3):701-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L.. Molecular characterization of epithelial ovarian cancer: implications for diagnosis and treatment. Int J Mol Sci. 2016;17(12):2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Risch HA, Marrett LD, Jain M, Howe GR.. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144(4):363-372. [DOI] [PubMed] [Google Scholar]
- 67. Cramer DW, Welch WR.. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71(4):717-721. [PubMed] [Google Scholar]
- 68. Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90(23):1774-1786. [DOI] [PubMed] [Google Scholar]
- 69. Ness RB, Cottreau C.. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459-1467. [DOI] [PubMed] [Google Scholar]
- 70. Kim J, Park EY, Kim O, et al. Cell origins of high-grade serous ovarian cancer. Cancers (Basel). 2018;10(11):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kurman RJ, Shih IM.. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salvador S, Gilks B, Köbel M, Huntsman D, Rosen B, Miller D.. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19(1):58-64. [DOI] [PubMed] [Google Scholar]
- 73. Bahar-Shany K, Brand H, Sapoznik S, et al. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecol Oncol. 2014;132(2):322-327. [DOI] [PubMed] [Google Scholar]
- 74. Huang HS, Chu SC, Hsu CF, et al. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: Initiation of fimbria carcinogenesis. Carcinogenesis. 2015;36(11):1419-1428. [DOI] [PubMed] [Google Scholar]
- 75. Huang HS, Hsu CF, Chu SC, et al. Haemoglobin in pelvic fluid rescues fallopian tube epithelial cells from reactive oxygen species stress and apoptosis. J Pathol. 2016;240(4):484-494. [DOI] [PubMed] [Google Scholar]
- 76. Hsu CF, Huang HS, Chen PC, Ding DC, Chu TY.. IGF-axis confers transformation and regeneration of fallopian tube fimbria epithelium upon ovulation. EBioMedicine. 2019;41:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hsu CF, Chen PC, Seenan V, Ding DC, Chu TY.. Ovulatory follicular fluid facilitates the full transformation process for the development of high-grade serous carcinoma. Cancers (Basel). 2021;13(3):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rodriguez GC, Walmer DK, Cline M, et al. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5(5):271-276. [DOI] [PubMed] [Google Scholar]
- 79. Babaier A, Ghatage P.. Mucinous cancer of the ovary: overview and current status. Diagnostics (Basel) .2020;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Purdie DM, Webb PM, Siskind V, Bain CJ, Green AC.. The different etiologies of mucinous and nonmucinous epithelial ovarian cancers. Gynecol Oncol. 2003;88(1 pt 2):S145-S148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to restrictions of some included studies’ informed consent. The corresponding author will facilitate access to data through existing data request processes for OCAC.