Abstract
Monitoring specific secretory immunoglobulin A (IgA) responses in the intestines after mucosal immunization or infection is impeded by the fact that sampling of small intestinal secretions requires invasive methods not feasible for routine diagnostics. Since IgA plasma cells generated after intragastric immunization are known to populate remote mucosal sites as well, secretory IgA responses at other mucosal surfaces may correlate to those in the intestines and could serve as proxy measures for IgA secretion in the gut. To evaluate the practicability of this approach, mice were immunized intragastrically with 0.2, 2, and 20 mg of ovalbumin plus 10 μg of cholera toxin, and the antigen-specific local secretory IgA responses in duodenal, ileal, jejunal, rectal, and vaginal secretions, saliva, urine, and feces, as well as serum IgG and IgA responses were analyzed by enzyme-linked immunosorbent assay. Correlation analysis revealed significant relationships between serum IgG and IgA, urinary IgA, salivary IgA, and secretory IgA in duodenal, jejunal, ileal, and rectal secretions for the 0.2-mg but not for the 20-mg ovalbumin dose. Fecal samples were poor predictors for intestinal antiovalbumin IgA responses, and no correlations could be established for cholera toxin, neither between local anti-cholera toxin levels nor to the antiovalbumin responses. Thus, specific IgA in serum, saliva, or urine can serve as a predictor of the release of specific IgA at intestinal surfaces after intragastric immunization, but the lack of correlations for high ovalbumin doses and for cholera toxin indicates a strong dependency on antigen type and dosage for these relationships.
Secretory immunoglobulin A (sIgA) is considered a cornerstone of the immunological defense mechanisms that protect mucosal surfaces. sIgA is secreted in gram amounts per day across the mucosal surfaces in humans (14, 20) and has been shown to confer protection against a number of bacterial and viral pathogens, such as Vibrio cholerae (33), Salmonella enterica serovar Typhimurium (21), respiratory syncytial virus (32), rotavirus (28), and influenza virus (27).
The recognition of sIgA as a powerful means to protect against enteric pathogens led to considerable interest in the development of mucosal vaccines in recent years. However, the induction of sIgA responses is an onerous task. In addition to being delivered via the mucosal route, the antigen should be formulated so that it is taken up by M cells, a specialized epithelial cell type located in the epithelium over the organized mucosa-associated lymphoid tissue (11, 22). Alternatively, ADP-ribosylating toxins like cholera toxin (CT) must be coadministered as mucosal adjuvants (4).
Beyond that, any vaccination requires some measurement of efficacy, such as the titer of specific IgA responses in local secretions. Unfortunately, analysis of antibody responses in the gut is complicated by the fact that sampling of intestinal secretions requires invasive methods which are not practicable for routine diagnostics. To overcome this problem, specific sIgA in fecal samples has been used as a substitute for directly sampled intestinal specimens (3, 12, 16), but the validity of this approach has been questioned (7). Intestinal lavage techniques have been proposed as an alternative technique (1, 5, 7, 24), but again sampling must be carried out under the supervision of a physician and may be too labor- and cost-intensive for routine diagnostic purposes.
Since IgA plasma cells generated after oral immunization are known to populate remote mucosal sites as well (19, 31), it seems conceivable that specific sIgA responses at other mucosal surfaces may closely correlate to those in the intestine and thus could serve as predictors for sIgA secretion in the gut after oral immunization. Seeking alternative measures for sIgA status at small and large intestinal surfaces, we carried out a comprehensive intragastric immunization study in mice using the model antigen ovalbumin (OVA) plus CT adjuvant and analyzed the specific IgA content in excretions, serum, and mucosal secretions from various sites in search for diagnostically important relationships between the IgA responses. When highly sensitive detection systems were used, specific antibody responses against both ovalbumin and CT were readily detectable in humoral samples, secretions, and excretions, but strong correlations could be established only between urinary, salivary, and serum IgA levels and IgA from intestinal surfaces for the lowest dose of OVA.
MATERIALS AND METHODS
Animals.
Female BALB/c mice were obtained from Charles River Wiga (Sulzfeld, Germany). The animals had been reared and were kept on a chicken egg protein-free rodent chow (Altromin 1324; Altromin, Lage, Germany) throughout the study. They were 8 weeks of age at the beginning of the immunization experiments.
Materials and reagents.
Animal feeding needles (20 gauge by 1.5 in. [ca. 4 cm]) were obtained from Popper & Sons (New Hyde Park, N.Y.), UniWick filters (25-mm long, 2.5-mm diameter) from Polyfiltronics (Rockland, Mass.) and glass applicators (10-cm long, 4-mm outer diameter, 2.5-mm inner diameter, smoothed and bevelled at one end) were custom-made by Glasgerätebau Ochs (Bovenden-Lenglern, Germany). High-binding polystyrene enzyme-radioimmunoassay microtiter plates were from Corning Costar (Bodenheim, Germany).
Methoxyfluorane (Metofane) was from Pitmann-Moore (Mundelein, Ill.); 1,1,1-tribromoethanol (avertin) and tert-amyl alcohol were obtained from Aldrich (Steinheim, Germany). Azide-free CT was purchased from List Biological Laboratories (Campbell, Calif., via Quadratech, Epsom, U.K.). OVA and leupeptin hydrogensulfate were from Calbiochem-Novabiochem (Bad Soden, Germany). OVA (lot B11706) displayed a single band after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue R staining (5 μg/lane) and showed a single peak corresponding to a purity of ≥97% after high-pressure liquid chromatography on a TSK gel G3000SW column with 0.1 M Na2HPO4 (pH 7.0), 100 mM NaCl, and 0.1% (wt/vol) SDS as the mobile phase. 4-(2-Aminoethyl)benzenesulfonylfluoride hydrochloride (AEBSF hydrochloride) was from Merck (Darmstadt, Germany). Aprotinin, bestatin hydrochloride, pilocarpine hydrochloride, and the mouse anti-chicken egg albumin IgG1 monoclonal antibody (clone OVA-14) were purchased from Sigma (Deisenhofen, Germany). Peroxidase-labeled, heavy-chain-specific goat anti-mouse IgA and IgG, unlabeled heavy-chain-specific goat anti-mouse IgA and IgG, and purified mouse IgA were obtained from Southern Biotechnology Associates (Birmingham, Ala., via Biozol, Eching, Germany). Purified mouse IgG was from Serotec (Oxford, U.K., via Biozol).
Antigen preparation and intragastric immunizations.
At the beginning of the immunization study, the total amounts of OVA antigen and CT adjuvant required for all immunizations were dissolved in sterile 3% (wt/vol) sodium bicarbonate or reconstituted in sterile water, snap-frozen in liquid N2, and stored in single-use aliquots at −70°C. Immediately before use, the respective antigen and adjuvant aliquots were warmed to 37°C and mixed by gentle rocking.
Five groups of six mice were immunized on days 0, 21, 35, and 49 via the intragastric route with the following antigen mixtures: 0 mg of OVA plus 0 μg of CT, 0 mg of OVA plus 10 μg of CT, 0.2 mg of OVA plus 10 μg of CT, 2 mg of OVA plus 10 μg of CT, and 20 mg of OVA plus 10 μg of CT, each in 300 μl of 3% (wt/vol) sodium bicarbonate. Immunization was performed under light metofane anesthesia using ball-tipped disposable animal feeding needles which were checked carefully for the absence of blood after each gavage. The animals were deprived of food for 1 h before and 2 h after intubation. The health status of the animals was checked routinely by visual inspection, and their growth and weight gain were monitored throughout the study.
Sample collection and extraction.
Feces were sampled 2 days before the first and 10 days after each immunization. The animals were separated individually into wire mesh cages equipped with metal trays underneath, and 20 to 30 freshly voided, urine-free fecal pellets were sampled over 3 to 8 h. Urine (50 to 500 μl) was sampled 10 days after the last immunization from the metal trays along with the fecal samples. All samples were kept on ice throughout the entire sampling procedure before they were snap-frozen in liquid N2 and stored at −70°C.
Blood samples were drawn 1 day before the first and 11 days after each immunization by retroorbital bleed under metofane or avertin anesthesia. Sera were snap-frozen in liquid N2 and stored at −70°C.
Eleven days after the last immunization, local secretions were sampled from each animal using the filter wick method of Haneberg et al. (13). The animal was anesthetized by intraperitoneal injection of 250 μl of avertin solution, which was prepared by dissolving avertin 5:3 (wt/vol) in tert-amyl alcohol and diluting this stock 1:80 in warmed (37°C) Dulbecco's phosphate-buffered saline (D-PBS [pH 7.3]: 2.7 mM KCl, 1.5 mM KH2PO4, 136 mM NaCl, 8.1 mM Na2HPO4) immediately before use. Fluid secretion at mucosal surfaces was induced by intraperitoneal injection of 50 μl of 1-mg/ml pilocarpine in D-PBS. Saliva was collected by placing two preweighed, 12-mm-long UniWick filters into the cheek pouches for 5 to 10 min. Meanwhile, 10 μl of D-PBS containing a protease inhibitor mixture (154 nM aprotinin, 10 μM leupeptin hydrogensulfate, 200 μM AEBSF hydrochloride, 6 μM bestatin hydrochloride) was instilled into the vagina using a blunt-ended micropipettor tip before a preweighed, 6.25-mm-long filter wick was applied with a glass applicator and allowed to soak for 5 to 10 min, during which time blood was sampled. The animal was killed by cervical dislocation, the small intestine was dissected and placed on an ice-cold glass plate, and the lumen was flushed with cold D-PBS containing the protease inhibitor mixture described above. Preweighed, 25-mm-long filter wicks were inserted into the intestine using a glass applicator and allowed to absorb the local secretions for 5 to 10 min. Meanwhile, a preweighed, 25-mm-long filter wick was inserted into the rectum by use of a glass applicator and allowed to absorb the colorectal secretions for 5 to 10 min. All wicks were checked visually for the absence of blood and/or urine, weighed, snap-frozen in liquid N2, and stored at −70°C.
Extraction of immunoglobulins from feces and filter wicks was performed as previously described (13). Fecal pellets were lyophilized, weighed, and homogenized in 15 μl of cold extraction buffer (D-PBS containing 5% [wt/vol] nonfat dry milk and the protease inhibitor mixture) per mg of dry feces at 0°C for 30 min. After another 20-min incubation on an end-to-end mixer (1 to 2 rpm) at 4°C, the solids were separated by 10 min of centrifugation at 16,000 × g at 4°C, the extraction was repeated with 10 μl of fresh cold extraction buffer per mg of dry solids, and the extracts were combined. For extraction of wicks, 10 μl of extraction buffer per mg of secretion collected, but at least 300 μl for salivary, vaginal, and rectal filters and 500 μl for small-intestinal filters, was added, and the secretions were eluted from the wicks by incubation for 30 min at 0°C with occasional mixing. For maximum recovery of liquids, the buffer-soaked filter wicks were transferred to a fresh, perforated microcentrifuge tube, which was placed in another centrifuge tube and spun dry by 1 to 2 min of centrifugation at 10,000 × g. The extracts were cleared by a final centrifugation step at 1,000 × g for 30 s. The fecal and filter extracts were snap-frozen in liquid N2 and stored at −70°C.
Quantitation of anti-OVA and anti-CT immunoglobulins.
Microtiter plates were coated with 75 μl of either OVA (5 μg/ml) in 50 mM sodium acetate buffer (pH 5.0) or CT (5 μg/ml) in 10 mM sodium phosphate buffer (pH 7.0)–10 mM NaCl per well overnight at 4°C. Plates were washed three times with 350 μl of PBST (D-PBS containing 0.05% [vol/vol] Tween-20) per well, and nonspecific binding sites were blocked with 250 μl of PBS-Blotto (D-PBS containing 5% [wt/vol] nonfat dry milk) per well for 5 h at room temperature. Plates were washed four times with PBST before 75 μl of serially diluted sera, anti-OVA standard (mouse anti-chicken OVA IgG monoclonal antibody OVA-14; starting dilution, 5.2 ng/ml), or fecal or filter extracts in PBS-Blotto was applied per well, and the plates were incubated overnight at 4°C.
The plates were again washed four times with PBST, 75 μl of horseradish peroxidase-labeled goat anti-mouse IgG or IgA, both diluted 1:2,000 in PBS-Blotto, was applied per well, and the plates were incubated for 90 min at room temperature. Plates were washed again six times with PBST, and color was developed at room temperature in the dark by adding 75 μl of a highly sensitive two-component tetramethylbenzidine substrate reagent which contains 1 mM 3,3′,5,5′-tetramethylbenzidine and 3 mM H2O2 in 200 mM potassium citrate buffer (pH 4.0) (9) per well. The reaction was terminated after 30 min by addition of 125 μl of 1 M sulfuric acid per well, and the plates were read at 450 nm on an Emax precision microtiter plate reader (Molecular Devices, Sunnyvale, Calif.).
Quantitation of total IgA.
Microtiter plates were coated with 75 μl of 5-ng/ml unlabeled goat anti-mouse IgA in D-PBS per well overnight at 4°C. The plates were washed, blocked, and washed again as described above before 75 μl of serially diluted IgA standard (purified mouse IgA; starting dilution, 16 μg/ml), sera, or fecal or filter extracts in PBS-Blotto was applied per well, and the plates were processed as described above using horseradish peroxidase-labeled goat anti-mouse IgA as the secondary antibody.
Determination of cross-reactivities of class-specific anti-mouse immunoglobulin detection reagents.
Microtiter plates were coated with 75 μl of 7-μg/ml unlabeled goat anti-mouse IgA or goat-anti mouse IgG in D-PBS per well overnight at 4°C. The plates were washed, blocked, and washed again as described above before 75 μl of serially diluted IgA or IgG standard (purified mouse IgA or IgG; starting dilution, 6.4 μg/ml) in PBS-Blotto was applied per well, and the plates were processed as described above. Both anti-mouse IgA- and anti-mouse IgG-horseradish peroxidase conjugate were reacted with captured IgG as well as captured IgA.
Data analysis and statistics.
Specific antibody responses were expressed as endpoint titers, being the reciprocal of the highest dilution that gave a reading above the cutoff. The cutoff was defined as the upper limit of a 99.5% confidence interval above the mean control level and was calculated by t statistics (10), e.g., for five mock-immunized control animals and a 99.5% confidence interval, the cutoff is calculated as meancontrols + 5.0 × SDcontrols where SD is the standard deviation. Titers were transformed logarithmically [log (titer+1)] for calculation of group means and standard errors of the means (SEM) or used directly for correlation analysis. Total IgA amounts were determined on the basis of four-parameter curve fit approximations of IgA standard titration curves using the readouts of the unknown samples at the steepest slopes of their titration curves (SOFTmax Pro v1.0; Molecular Devices). For endpoint titers standardized on total IgA contents (relative endpoint titers), endpoint titers were divided by the total IgA concentration of the respective undiluted sample.
Secondary antibody cross-reactivity was defined as the detection limit obtained for the immunoglobulin recognized by cross-reactivity divided by that obtained for the specifically recognized immunoglobulin.
Assessment of plasma leakage into mucosal samples was carried out using IgG as a plasma marker, taking into account the respective secondary-antibody cross-reactivities (see Appendix for details). Based on these considerations, equation 1 describes the relationship between the relative amount of plasma-borne IgA in a fecal or filter wick-collected mucosal sample (% IgAtrans) and the serum IgG and IgA titers (TiterIgGser, TiterIgAser), the IgG and IgA titers of the respective mucosal sample (TiterIgGmuc, TiterIgAmuc), and the cross-reactivities of the anti-mouse IgG antibody with mouse IgA (XrαIgG) and anti-mouse IgA with mouse IgG (XrαIgA):
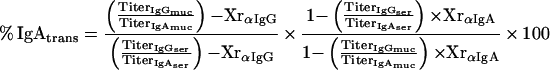 |
1 |
Equation 2 describes the relationship between the relative amount of plasma which leaked into a filter wick-collected secretion (% Volumetrans) and the serum IgG and IgA titers (TiterIgGser, TiterIgAser), the IgG and IgA titers of the filter-sampled mucosal secretion (TiterIgGmuc, TiterIgAmuc), and the cross-reactivity of the anti-mouse IgG antibody with mouse IgA (XrαIgG):
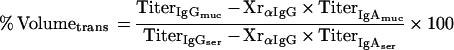 |
2 |
Multiple (between-group) comparisons were performed by one-way analysis of variance (ANOVA) using Fisher's protected least-significant difference test at a 5% level of significance. Fisher's r-to-z transformation of correlation coefficients was used to obtain the P values in correlation analysis. Results of statistical analyses were considered significant only if P was <0.05. All calculations and statistical analyses were carried out using the Statview 4.5 program package (Abacus Concepts, Berkeley, Calif.).
RESULTS
Development of highly sensitive immunoassays for quantitation of anti-OVA and anti-CT immunoglobulins.
In order to detect even minute amounts of immunoglobulins in diluted samples such as urine and saliva, we established highly sensitive enzyme-linked immunosorbent assay (ELISA) systems for antibodies against OVA and CT. For coating the microtiter plates, best results were obtained with OVA at 5 μg/ml in 50 mM sodium acetate (pH 5.0) and CT at 5 μg/ml in 10 mM sodium phosphate (pH 7.0)–10 mM sodium chloride. Under these conditions, assay sensitivity could be increased by a factor of 50 for detection of anti-OVA and by a factor of 2 for detection of anti-CT immunoglobulins, compared to standard ELISA coating conditions (2 μg of antigen per ml in sodium carbonate-bicarbonate [pH 9.6]) (6, 13). Assay sensitivity was further improved by using horseradish peroxidase-labeled secondary reagents in combination with a novel tetramethylbenzidine-based colorimetric ELISA substrate system which we developed for this purpose (9). Using both optimized coating conditions and the novel substrate system, a detection limit for a monoclonal anti-OVA IgG1 of 24 pg/ml was achieved, which corresponds to a concentration of 160 fM or 12 attomol of specific immunoglobulin per well.
Lack of correlations between humoral and fecal immune responses over repeated intragastric immunizations with OVA and CT.
To assess whether fecal and serum immunoglobulin responses may correlate after intragastric immunization, a typical immunization study with priming and several booster immunizations was performed using the model antigen OVA and CT adjuvant. Groups of six BALB/c mice were gavaged on days 0, 21, 35, and 49 with 0.2, 2, or 20 mg of OVA plus 10 μg of CT or with 10 μg of CT alone. This immunization procedure had no apparent impact on the health status and the thriving of the animals, as assayed by visual inspection and determination of body mass gain (data not shown). Feces and blood samples were collected 10 to 11 days after each immunization and tested for their content of specific IgA and IgG against OVA and CT.
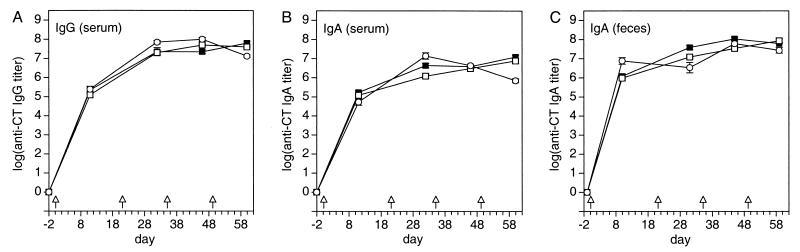
Anti-CT serum IgG and IgA as well as fecal IgA were already detectable after the first immunization and reached a plateau after the second immunization, with titers between 106 and 108 (Fig. 1). The general shape of the immune response curves was identical in all groups for serum as well as for feces, and the between-group differences were less than fourfold on average (ratios of geometric group means of anti-CT Ig [all samplings]: serum IgG, 2.2-fold; serum IgA, 3.1-fold; fecal IgA, 3.1-fold; range, 1.1- to 17-fold). The within-group variation was also very low (arithmetic mean CV ± SD of log anti-CT Ig [all groups, samplings, samples, and immunoglobulin types]: 4.2% ± 2.2%; range, 0 to 10.3%). Despite the like course of all titration curves, no statistically significant correlation between anti-CT serum IgG and anti-CT fecal IgA of individual animals could be established over the entire study. Only anti-CT serum IgA and anti-CT fecal IgA correlated for some groups at some time points in an inconsistent manner.
FIG. 1.
Time course of the anti-CT IgA and IgG responses in serum and feces after priming and three booster immunizations with various doses of OVA plus CT adjuvant. Solid squares, 0.2 mg of OVA plus 10 μg of CT; open squares, 20 mg of OVA plus 10 μg of CT; open circles, 0 mg of OVA plus 10 μg of CT. Arrows indicate time points of immunization. Values represent means ± SEM of samples from six mice. Absolute A450 readings of samples from mock-immunized animals used to calculate the endpoints and buffer-for-sample readouts were 0.042 ± 0.003 and 0.041 ± 0.002 (mean ± SD of 38 microplates), respectively.
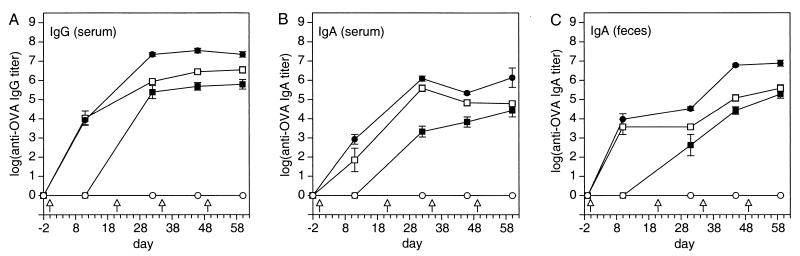
The anti-OVA antibody immune responses showed antigen dose-dependent differences in onset, strength, and time course (Fig. 2). The 2-mg and 20-mg OVA doses induced serum and fecal IgG and IgA responses after a single immunization, while the 0.2-mg OVA dose required a booster immunization before IgG or IgA responses were detectable. From then on, differences in the strength of the anti-OVA immune responses were surprisingly well reflected by the differences in antigen dosage when comparing the responses to the 0.2-mg and the 20-mg OVA dose. The 100-fold-higher antigen dose resulted on average in 83-fold-higher group mean titers (ratios of geometric group means of anti-OVA Ig of the 0.2-mg and the 20-mg OVA doses [all samplings]: serum IgG, 64-fold; serum IgA, 98-fold; fecal IgA, 90-fold; range, 32 to 573-fold) (Fig. 2). Animals immunized with 2 mg of OVA showed an intermediate behavior depending on sample, time point, and immunoglobulin analyzed. In general, the immune responses of this group behaved similar to that of the 20-mg OVA group at the beginning of the study and shifted towards that of the 0.2-mg OVA group at the end of the experiment. The within-group variations were also antigen dose-dependent, being consistently highest for the low and lowest for the high OVA dose (arithmetic mean CV ± SD of log anti-OVA Ig [all samplings, samples, and immunoglobulin types after the first booster immunization]: 0.2 mg of OVA: 18.1% ± 13.2%; range, 8.8% to 51.4%; 2 mg of OVA: 6.5% ± 2.1%; range, 2.8 to 8.4%; 20 mg of OVA: 3.9% ± 1.3%; range, 2.0 to 5.5%). As for the anti-CT responses, statistically significant correlations between anti-OVA serum IgA and IgG and anti-OVA fecal IgA responses of individual animals occurred for some groups at some time points in an inconsistent manner. Likewise, no consistent correlations between anti-CT and anti-OVA immunoglobulin responses in serum and feces could be detected over the entire course of the immunization study.
FIG. 2.
Time course of the anti-OVA IgA and IgG responses in serum and feces after priming and three booster immunizations with various doses of OVA plus 10 μg of CT adjuvant. Solid squares, 0.2 mg of OVA; open squares, 2 mg of OVA; solid circles, 20 mg of OVA; open circles, 0 mg of OVA. Arrows indicate time points of immunization. Values represent means ± SEM of samples from six mice. Absolute A450 readings of samples from mock-immunized animals used to calculate the endpoints and buffer-for-sample readouts were 0.045 ± 0.002 and 0.044 ± 0.002 (mean ± SD of 40 microplates), respectively.
Distribution of the antibody immune responses at different mucosal effector sites after intragastric immunization with OVA and CT.
To perform a cross-comparison of the local IgA responses, samples of local secretions in the gut together with saliva and vaginal washes were collected after killing the animals 11 days after the last immunization. Urine had been sampled along with feces no more than 24 h earlier. On the basis of these samples, distribution profiles for the local IgA responses against OVA and CT were established.
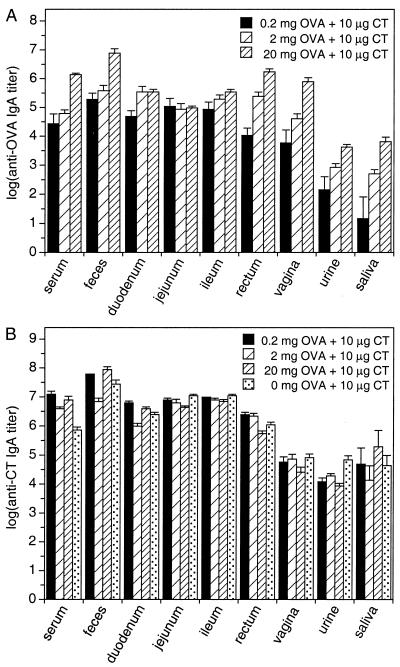
First, we juxtaposed the absolute endpoint titers of the specific IgA (Fig. 3). For the local anti-OVA IgA responses, a clear site and dose dependency stood out. Compared sitewise, the greatest differences were found for fecal versus salivary or urinary responses, with ratios exceeding 10,000. Compared groupwise, an almost linear relation between antigen dosage and the resulting group mean IgA responses was observed for all sites except the small intestine (ratios of geometric group means of anti-OVA IgA [serum, urine, saliva, feces, and vaginal and rectal secretion samples]: 20-mg to 0.2-mg dose: 91-fold; range, 30- to 442-fold; 20-mg to 2-mg dose: 13-fold; range, 5.0- to 23-fold; 2-mg to 0.2-mg dose: 7.2-fold; range, 2.0- to 34-fold). Analogous to the time course study, the within-group variation declined with increasing antigen dosage (arithmetic mean CV ± SD of log anti-OVA Ig [all sites and immunoglobulin types after the third booster immunization]: 0.2 mg of OVA: 32.6% ± 45.1%; range, 10.0 to 156%; 2 mg of OVA: 8.6% ± 2.3%; range, 6.3 to 13.5%; 20 mg of OVA: 5.0% ± 2.2%; range, 2.0 to 10.2%) (Fig. 3A).
FIG. 3.
Specific IgA responses against OVA and CT in serum and local secretions after priming and three booster immunizations with various doses of OVA plus CT adjuvant. (A) Absolute anti-OVA endpoint titers. (B) Absolute anti-CT endpoint titers. Values represent means ± SEM of samples from six mice. Absolute A450 readings of samples from mock-immunized animals used to calculate the endpoints and buffer-for-sample readouts were 0.044 ± 0.003 and 0.043 ± 0.003 (anti-OVA ELISAs, mean ± SD of 30 microplates), respectively, and 0.042 ± 0.003 and 0.041 ± 0.002 (anti-CT ELISAs, mean ± SD of 30 microplates), respectively.
For CT, similar differences between individual sites but less pronounced between-group variations (ratios of geometric group means of anti-CT Ig [all sites and immunoglobulin types after the third booster immunization]: 2.7-fold; range, 1.1- to 17-fold) were observed, and the within-group variations were low as well (arithmetic mean CV ± SD of log anti-CT Ig [all sites and immunoglobulin types after the third booster immunization]: 4.5% ± 3.1%; range, 0 to 12.0%) (Fig. 3B).
Since several reports stressed the importance of normalizing the specific IgA responses on the total IgA release of the respective site (e.g., reference 8), we also determined the relative anti-OVA and anti-CT IgA titers by standardizing the absolute titers on the total IgA content of the samples. The results are summarized in Fig. 4.
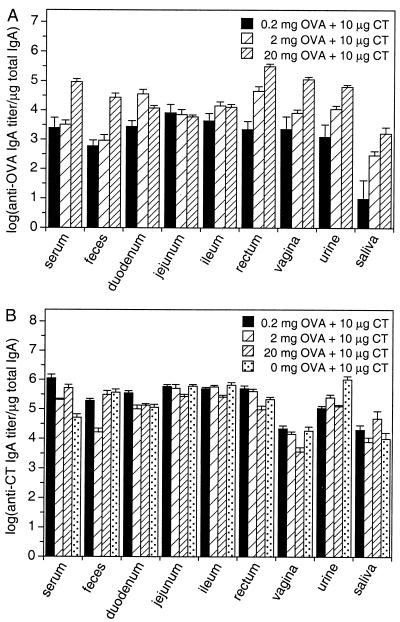
FIG. 4.
Specific IgA responses against OVA and CT in serum and local secretions after priming and three booster immunizations with various doses of OVA plus CT adjuvant, normalized to total IgA. (A) Anti-OVA endpoint titers normalized to the total IgA content of the respective samples. (B) Anti-CT endpoint titers normalized to the total IgA content of the respective samples. Values represent means ± SEM of samples from six mice.
With respect to the between- and within-group variations for given sites, absolute and relative titers were not dramatically different from each other, i.e., the antigen dose dependency of the serum, fecal, rectal, vaginal, and urinary anti-OVA IgA responses persisted (ratios of geometric group means of relative anti-OVA IgA [serum, urine, saliva, feces, and vaginal and rectal secretion samples]: 20-mg to 0.2-mg dose: 68-fold; range, 38- to 168-fold; 20-mg to 2-mg dose: 12-fold; range, 5.6- to 30-fold; 2-mg to 0.2-mg dose: 5.8-fold; range, 1.3- to 30-fold), as did the dose-dependent decrease of the within group variation for increasing antigen doses (arithmetic mean CV ± SD of log relative anti-OVA Ig [all sites and immunoglobulin types after the third booster immunization]: 0.2 mg of OVA: 37.0% ± 44.9%; range, 14.1 to 155%; 2 mg of OVA: 10.1% ± 3.3%; range, 6.9 to 16.1%; 20 mg of OVA: 6.2% ± 3.9%; range, 3.4 to 15.8%). However, the site-to-site differences in local IgA responses were considerably reduced, especially for the 0.2-mg OVA dose, where all samples except saliva showed no statistically significant differences (no statistically significant differences between mean relative anti-OVA IgA titers at different sites [one-way ANOVA, P ≥ 0.35]) (Fig. 4A).
For the anti-CT responses (Fig. 4B), a similar trend was observed, with reduced between-site differences and low between-group (ratios of geometric group means of anti-CT Ig [all sites and immunoglobulin types after the third booster immunization]: 2.7-fold; range, 1.0- to 22-fold) and within-group (arithmetic mean CV ± SD of log anti-CT Ig [all sites and immunoglobulin types after the third booster immunization]: 5.2% ± 2.8%; range, 1.9 to 13.4%) variations.
Antigen type and dose dependency of correlations between specific antibody responses at different mucosal sites.
On the basis of the local anti-OVA and anti-CT IgA responses, a comprehensive correlation analysis was carried out in which the anti-OVA and anti-CT immunoglobulin responses were compared.
When comparing anti-OVA with anti-CT immunoglobulin responses for given effector sites, no statistically significant correlation whatsoever was observed for relative titers (lack of correlation between anti-OVA and anti-CT relative IgA responses for given sites: P ≥ 0.07, Fisher's r-to-z transformation). For absolute titers, some positive correlations were detected in an inconsistent manner for serum IgG and urinary, salivary, and vaginal IgA but not for intestinal IgA (rate of correlation between anti-OVA and anti-CT responses for given sites: 17% of all possible correlations were significant; P < 0.05, Fisher's r-to-z transformation). Thus, the mucosal antibody immune responses against CT and OVA appear not to be synchronized over all mucosal effector sites after intragastric immunization with OVA and CT.
Anti-CT responses at different effector sites were mainly unrelated as well. Less than 7% of all possible relationships were statistically significant, no matter whether relative or absolute titers were subjected to correlation analysis (Table 1). As in all previous comparisons, the few correlations which could be established did not occur in a consistent manner throughout the different groups. The lack of correlations was apparently not caused by the bystander antigen OVA, since the animals immunized with CT alone displayed a similarly low rate of significant correlations (Table 1).
TABLE 1.
Dose- and antigen-dependent occurrence of correlations between antibody immune responses in serum and local secretions after mucosal immunization
| OVA dose (mg)a | Rate (%) of significant site-to-site correlationsb among:
|
|||
|---|---|---|---|---|
| Absolute antibody titers against:
|
Relative antibody titers against:
|
|||
| OVA | CT | OVA | CT | |
| 0 | NA | 6.7 (3/45) | NA | 4.4 (2/45) |
| 0.2 | 62.2 (28/45) | 6.7 (3/45) | 55.6 (25/45) | 2.2 (1/45) |
| 2 | 20.0 (9/45) | 6.7 (3/45) | 8.9 (4/45) | 4.4 (2/45) |
| 20 | 2.2 (1/45) | 2.2 (1/45) | 4.4 (2/45) | 4.4 (2/45) |
CT (10 μg) was administered to all groups.
The rate of significant site-to-site correlations is the percentage of significant correlations (P < 0.05, Fisher's r-to-z transformation) among all possible correlations between the samples analyzed. Absolute and relative titers of the following samples were used in the correlation analyses: serum IgA, salivary IgA, urinary IgA, fecal IgA, duodenal IgA, jejunal IgA, ileal IgA, rectal IgA, and vaginal IgA. For serum IgG, absolute titers were used in all calculations. Actual numbers of correlations and the number of all possible permutations are given in parentheses. NA, not applicable.
When comparing the anti-OVA responses at different effector sites, the occurrence of correlations showed a clear antigen dose dependency. The rate of significant correlations between local anti-OVA immunoglobulin responses increased from 2% for the 20-mg OVA dose to over 60% for the 0.2-mg OVA dose when comparing absolute titers (Table 1). Similar results were obtained for relative titers. Most importantly, only for the 0.2-mg OVA dose could correlations between intestinal anti-IgA responses and those of saliva, urine, or serum be established, for both absolute and relative titers. When using this antigen dose, specific salivary and urinary IgA as well as serum IgA and IgG turned out to be excellent predictors of the specific IgA content in small and large intestinal secretions (Table 2). The correlation coefficients ranged from 1.0 to 0.82. In this context it seems noteworthy that the quality of correlations was independent of titer ratios, e.g., good correlations could be established between salivary IgA responses and those in other secretions despite the fact that absolute and relative anti-OVA IgA titers in saliva were up to 8,000-fold lower than those at other sites.
TABLE 2.
Dose dependency of the predictive power of salivary, urinary, and serum antibodies for the antibody responses at remote mucosal surfacesa
| Antibody response | OVA dose (mg)
|
Antibody response | OVA dose (mg)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2
|
2
|
20
|
0.2
|
2
|
20
|
|||||||||
| Titer ratiob | rc | Titer ratio | r | Titer ratio | r | Titer ratio | r | Titer ratio | r | Titer ratio | r | |||
| Absolute salivary IgA in relation to absolute: | Relative salivary IgA in relation to relative: | |||||||||||||
| Urinary IgA | 9.6 × 10−2 | 1.00** | — | −0.03 | — | −0.05 | Urinary IgA | 7.0 × 10−3 | 0.84* | — | 0.74 | — | 0.61 | |
| Serum IgA | 5.1 × 10−4 | 0.97** | — | 0.01 | — | 0.39 | Serum IgA | 3.6 × 10−3 | 0.93* | — | 0.67 | — | 0.79 | |
| Serum IgG | 2.2 × 10−5 | 0.98** | — | −0.41 | — | 0.57 | Serum IgGd | 1.4 × 10−5 | 1.00** | — | −0.56 | — | 0.06 | |
| Duodenal IgA | 2.8 × 10−4 | 0.99** | — | −0.61 | — | −0.23 | Duodenal IgA | 3.2 × 10−3 | 0.99** | — | 0.09 | — | 0.05 | |
| Jejunal IgA | 1.3 × 10−4 | 0.95* | — | −0.61 | — | 0.62 | Jejunal IgA | 1.1 × 10−3 | 0.92* | — | 0.27 | 2.7 × 10−1 | 0.92* | |
| Ileal IgA | 1.6 × 10−4 | 0.97** | — | −0.25 | — | −0.03 | Ileal IgA | 2.0 × 10−3 | 0.97** | — | 0.44 | — | 0.66 | |
| Rectal IgA | 1.3 × 10−3 | 0.97** | — | −0.15 | — | 0.26 | Rectal IgA | 4.0 × 10−3 | 0.92* | — | 0.69 | — | 0.69 | |
| Vaginal IgA | — | 0.24 | — | 0.02 | — | 0.50 | Vaginal IgA | — | 0.36 | — | 0.49 | — | 0.29 | |
| Fecal IgA | — | 0.54 | — | 0.01 | — | 0.23 | Fecal IgA | — | 0.52 | — | 0.23 | — | 0.69 | |
| Absolute urinary IgA in relation to absolute: | Relative urinary IgA in relation to relative: | |||||||||||||
| Salivary IgA | 1.0 × 101 | 1.00** | — | −0.03 | — | −0.05 | Salivary IgA | 1.4 × 102 | 0.84* | — | 0.74 | — | 0.61 | |
| Serum IgA | 5.3 × 10−3 | 0.97** | 1.4 × 10−2 | 0.95* | — | 0.00 | Serum IgA | 5.1 × 10−1 | 0.97** | — | 0.69 | — | 0.27 | |
| Serum IgG | 2.3 × 10−4 | 0.97** | — | 0.34 | — | 0.34 | Serum IgGd | 2.0 × 10−3 | 0.87* | — | −0.01 | — | 0.34 | |
| Duodenal IgA | 2.9 × 10−3 | 0.99** | — | 0.53 | — | −0.30 | Duodenal IgA | 4.5 × 10−1 | 0.84* | — | 0.13 | — | 0.24 | |
| Jejunal IgA | 1.3 × 10−3 | 0.95* | — | 0.53 | — | −0.35 | Jejunal IgA | — | 0.59 | — | 0.40 | — | 0.48 | |
| Ileal IgA | 1.7 × 10−3 | 0.98** | 4.4 × 10−3 | 0.96** | — | 0.60 | Ileal IgA | 2.9 × 10−1 | 0.88* | — | 0.71 | — | 0.25 | |
| Rectal IgA | 1.3 × 10−2 | 0.98** | — | 0.75 | — | 0.41 | Rectal IgA | — | 0.59 | — | 0.74 | — | 0.55 | |
| Vaginal IgA | — | 0.23 | 2.1 × 10−2 | 0.86* | — | 0.14 | Vaginal IgA | — | 0.80 | — | 0.59 | — | 0.28 | |
| Fecal IgA | — | 0.57 | 2.2 × 10−3 | 0.94* | — | 0.23 | Fecal IgA | 2.1 | 0.82* | — | 0.70 | — | 0.27 | |
| Absolute serum IgA in relation to absolute: | Relative serum IgA in relation to relative: | |||||||||||||
| Salivary IgA | 2.0 × 103 | 0.97** | — | 0.01 | — | 0.39 | Salivary IgA | 2.8 × 102 | 0.93* | — | 0.67 | — | 0.79 | |
| Urinary IgA | 1.9 × 102 | 0.97** | 7.2 × 101 | 0.95* | — | 0.00 | Urinary IgA | 2.0 | 0.97** | — | 0.69 | — | 0.27 | |
| Serum IgG | 4.4 × 10−2 | 0.99** | — | 0.25 | — | −0.18 | Serum IgGd | 4.0 × 10−3 | 0.95* | — | 0.02 | — | −0.15 | |
| Duodenal IgA | 5.6 × 10−1 | 0.95* | — | 0.66 | — | −0.20 | Duodenal IgA | 8.9 × 10−1 | 0.94* | — | 0.50 | — | −0.17 | |
| Jejunal IgA | 2.5 × 10−1 | 0.89* | — | 0.66 | — | 0.63 | Jejunal IgA | — | 0.76 | — | 0.80 | — | 0.73 | |
| Ileal IgA | 3.2 × 10−1 | 0.95* | 3.2 × 10−1 | 0.86* | — | 0.60 | Ileal IgA | 5.7 × 10−1 | 0.97** | 2.3 × 10−1 | 0.85* | — | 0.45 | |
| Rectal IgA | 2.5 | 0.95* | — | 0.78 | — | 0.31 | Rectal IgA | — | 0.76 | 7.2 × 10−2 | 0.97** | — | 0.71 | |
| Vaginal IgA | — | 0.45 | — | 0.79 | — | −0.12 | Vaginal IgA | — | 0.63 | — | 0.59 | — | 0.37 | |
| Fecal IgA | — | 0.67 | 1.6 × 10−1 | 0.95* | — | 0.43 | Fecal IgA | — | 0.76 | — | 0.69 | — | 0.25 | |
| Absolute serum IgG in relation to absolute: | Absolute serum IgG in relation to relative: | |||||||||||||
| Salivary IgA | 4.5 × 104 | 0.98** | — | −0.41 | — | 0.57 | Salivary IgA | 7.0 × 104 | 1.00** | — | −0.56 | — | 0.06 | |
| Urinary IgA | 4.3 × 103 | 0.97** | — | 0.34 | — | 0.34 | Urinary IgA | 4.9 × 102 | 0.87** | — | −0.01 | — | 0.34 | |
| Serum IgA | 2.3 × 101 | 0.99** | — | 0.25 | — | −0.18 | Serum IgA | 2.5 × 102 | 0.95* | — | 0.02 | — | −0.15 | |
| Duodenal IgA | 1.3 × 101 | 0.95* | — | 0.13 | — | −0.67 | Duodenal IgA | 2.2 × 102 | 0.99** | — | −0.14 | — | −0.69 | |
| Jejunal IgA | 5.7 | 0.88* | — | 0.13 | — | −0.29 | Jejunal IgA | 7.5 × 101 | 0.89* | — | 0.14 | — | 0.17 | |
| Ileal IgA | 7.1 | 0.93* | — | 0.53 | — | −0.30 | Ileal IgA | 1.4 × 102 | 0.97** | — | 0.45 | — | −0.44 | |
| Rectal IgA | 5.7 × 101 | 0.93* | — | 0.31 | — | −0.22 | Rectal IgA | 2.8 × 102 | 0.90* | — | 0.09 | — | −0.33 | |
| Vaginal IgA | — | 0.45 | — | 0.65 | 2.8 × 101 | 0.93* | Vaginal IgA | — | 0.42 | — | 0.11 | — | 0.80 | |
| Fecal IgA | — | 0.62 | — | 0.49 | — | −0.34 | Fecal IgA | — | 0.56 | — | 0.40 | — | −0.12 | |
Linear body fluid-to-site correlations of anti-OVA antibody titers after four intragastric immunizations with OVA (amounts indicated) plus CT adjuvant. Significant correlations between anti-OVA responses are highlighted by bold characters.
Titer ratios were calculated from the respective geometric group means (six samples per group). The numbers show the mean antibody titer of the respective body fluid divided by the mean antibody titer of the mucosal site or body fluid indicated in the table. —, no titer ratios were calculated for nonsignificant correlations.
r, Pearson's correlation coefficient. Asterisks indicate the significance of the correlations determined after Fisher's r-to-z transformation of the correlation coefficient: ∗, P < 0.05; ∗∗, P < 0.001.
Absolute serum IgG titers were used for this analysis.
Taken together, the occurrence of correlations between local antibody immune responses after intragastric immunization seems not to be a general phenomenon but rather to depend on the antigen type and dosage used for immunization.
Contribution of plasma transudate to immunoglobulins in feces and local secretions.
In order to rule out an artifactual cause for correlations between serum and filter wick-sampled secretions due to physical damage to the intestinal epithelium by the filter wick fabric or capillary suction, we wanted to compare the naturally occurring plasma leak into feces with that observed after sampling with UniWick filters. Assuming a nonselective effusion of plasma at sites of leakage, any plasma protein marker, such as albumin or IgG, allows the determination of the amount of plasma that leaked into a mucosal sample. We used IgG as a marker and determined the anti-OVA IgG and IgA titers in serum, feces, and jejunal secretions for three randomly selected animals from each immunization group. The relative contribution of plasma transudate to the sampled volume of intestinal secretions as well as to the specific IgA contents in feces and intestinal secretions was computed with equations 1 and 2. The results are summarized in Table 3 and show that plasma leakage contributes very little to the specific mucosal IgA responses. Highest leakage was observed for the 20-mg OVA dose group, for which no correlations between serum and jejunal IgA existed. We conclude that the correlations between immunoglobulins in serum and filter wick-sampled secretions are not caused by plasma transudate.
TABLE 3.
Determination of blood plasma leakage into feces and filter wick-sampled small intestinal secretionsa
| OVA dose (mg) | % Contribution of blood plasma transudate to:
|
||
|---|---|---|---|
| Anti-OVA IgA titersb in:
|
Fluid vol sampled from jejunal surfacec | ||
| Jejunal secretions | Feces | ||
| 0.2 | 1.78 ± 3.55 | 0.84 ± 1.80 | 0.72 ± 0.74 |
| 2 | 1.35 ± 1.25 | 0.86 ± 0.40 | 0.59 ± 0.85 |
| 20 | 5.60 ± 9.53 | 0.89 ± 1.18 | 2.73 ± 3.10 |
| All doses | 2.91 ± 5.51 | 0.86 ± 1.10 | 1.35 ± 1.95 |
Individual anti-OVA endpoint titers from three randomly selected animals of each OVA immunization group or all nine animals together were used to calculate the given data. No significant between-group differences could be detected for either sample or calculation mode (one-way ANOVA, P ≥ 0.364).
Percentage of blood-borne specific IgA in the specific IgA content of the mucosal sample (calculated using equation 1).
Percentage of the fluid volume sampled being blood plasma transudate (calculated using equation 2).
Lack of influence of intragastric immunizations on total IgA release at different mucosal effector sites.
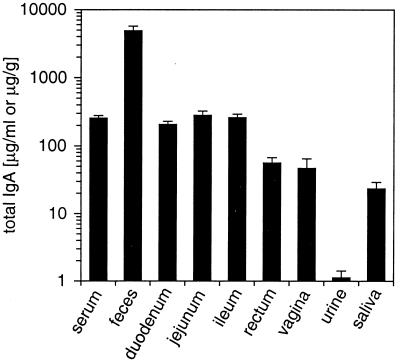
Since the lack of correlations for the 20-mg OVA dosage could be due to an overloading of the IgA transport systems in the mucosal epithelium, we investigated whether the total IgA output might rise upon intragastric immunization in an antigen dose-dependent manner. For normalization, the total IgA contents of sera, secretions, and excretions of immunologically naive, mock-immunized animals were determined (Fig. 5). While the IgA contents of feces and urine differ tremendously, total IgA levels in serum and small intestinal secretions are almost indistinguishable.
FIG. 5.
Total amounts of IgA in serum and local secretions of mock-immunized mice (3% [wt/vol] sodium bicarbonate) after priming and three booster immunizations. IgA contents of fecal samples are given in micrograms per gram (dry weight) and those of all other samples are given in micrograms per milliliter of liquid. Values represent means ± SEM of samples from six mice.
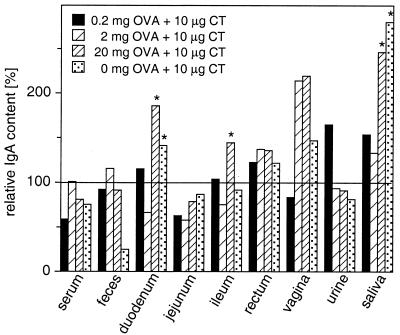
These standard IgA levels were set at 100% and compared to the total IgA output after immunization with different doses of OVA together with CT adjuvant. The results are summarized in Fig. 6 and show that total IgA release at mucosal surfaces is barely affected by intragastric immunization. Total IgA production was in fact lower than the control levels in some immunization groups, and significantly elevated IgA contents were detected in a few samples only. Taken together, these data suggest that intragastric immunization has no or only marginal impact on IgA transport at mucosal surfaces.
FIG. 6.
Relative changes of total IgA in serum and local secretions after priming and three booster immunizations with various doses of OVA plus CT adjuvant. Total IgA contents in serum and local secretions of mock-immunized animals are set at 100%. IgA production significantly higher (asterisk) than that of mock-immunized animals is indicated (one-way ANOVA, Fisher's protected least-significant-difference test, P < 0.05).
DISCUSSION
Direct analysis of the antibody immune status at remote mucosal surfaces requires labor- and cost-intensive methods which are not feasible for routine immune surveys. For that reason, easy-to-sample specimens, such as saliva and feces, were proposed as substitute measures to predict the antibody immune status at less accessible mucosal sites. Unfortunately, there are conflicting reports about the usefulness of this approach (2, 3, 7, 12, 16).
We therefore carried out an intragastric immunization study in mice with different doses of the model antigen OVA and the mucosal adjuvant CT and looked for diagnostically important relationships between immune responses at different effector sites. The antibody contents of the respective samples were expressed as endpoint titers because the endpoint titer technique was shown to more faithfully reflect the actual antibody content of the samples (23), to allow lower detection limits (9), and to be more precise for highly diluted samples (17) than some assays based on standard curves.
Our results demonstrate that strong correlations between specific antibody responses at different effector sites can indeed be established, but the existence of such relationships appears to depend on the type and dose of antigen used, a possible reason for the apparent conflicts between previous reports.
On the practical side, this opens up the possibility of using urine, saliva, and serum samples as indirect measures to predict antibody release in the small intestines after intragastric vaccination. One problem associated with immunoassays based on urine or saliva samples, however, is the high dilution of these specimens. Without optimizing our ELISA system, we would not have been able to detect any anti-OVA IgA responses in urine or saliva after vaccination with the 0.2-mg OVA dose. This might explain the almost complete lack of information about specific IgA responses in urine samples after intragastric immunization or infection. Only one report (18) describes the occurrence of Campylobacter jejuni-specific IgA in feces and urine after naturally acquired infection with C. jejuni, albeit in a qualitative manner only. In light of the fact that modern diagnostic tools like immuno-PCR (29), luminometry (15), and the use of poly-horseradish peroxidase (30) lower the detection limits to almost the single-molecule level, we are confident that monitoring the urinary or salivary IgA response of mucosal vaccinees would be a rapid and cheap method for mass screening. The high relative amount of specific IgA in urine which was not different from that in serum, intestinal secretions, or feces substantiates this concept.
The apparent antigen type and dose dependency of correlations between IgA responses at different sites, however, is a handicap that limits the usefulness of indirect measures for intestinal immune protection. We therefore tried to elucidate the reasons for this behavior. Since higher antigen doses also induced higher anti-OVA immunoglobulin titers, we first speculated that total IgA release at mucosal surfaces increases in an analogous manner and eventually becomes saturated, abolishing correlations between different sites. Yet total IgA release at the mucosal surfaces of immunized animals was, for the most part, not significantly different from that of buffer-treated controls. Obviously, the sIgA transport systems in the gut were still far from being saturated after all intragastric immunizations. This is supported by the observation that we did not see any negative correlations between anti-OVA and anti-CT immune responses in the gut, which would have occurred if both IgA responses competed for an insufficient number of poly(Ig) receptors. Also, the anti-CT responses were uncorrelated even in the absence of anti-OVA IgA, and the extremely high anti-CT IgA responses did not disturb the correlations of the anti-OVA titers for the low OVA dose.
We therefore favor a different explanation based on a possible clonal homing behavior of mucosal IgA plasma cells. In two recent publications, Quiding-Järbrink et al. (25, 26) demonstrated compartmentalization of mucosal IgA responses due to differences in the homing receptor equipment of the B-cell clones expanded. Consequently, the smaller the number of B-cell clones, the more compartmentalization should arise. This raises the question of how different types and doses of antigen could affect the clonality of a B-cell response. One conceivable but still speculative explanation might be found in the stability of the antigens. The more resistant an antigen is against intestinal digestion, the more homogeneous will be the material that is delivered to the gut-associated lymphoid tissue. Consequently, fewer B-cell clones are necessary to clear this antigen. On the other hand, the more fragments are generated in the gut and taken up, the more clones must be activated to eliminate the antigen. Thus, more-stable antigens like CT may generate less clonal diversity and therefore a lower number of site-to-site correlations, as we observed. High antigen doses could mimic a similar situation, since the higher the antigen concentration, the smaller a fraction of antigen will be broken down by the digestive enzymes in the time window between application and uptake. Experiments to test this hypothesis are under way in our laboratory.
ACKNOWLEDGMENTS
This work was supported by a personal grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie and research grant FR 958/2-1 from the Deutsche Forschungsgemeinschaft to A.F. Support for D.E. was provided in part by project D4 of the Interdisziplinäres Zentrum für Klinische Forschung of the Westfälische Wilhelms-Universität Münster. B.M. is a recipient of a personal stipend from the State of Nordrhein-Westfalen.
Appendix
Supplementary information for the calculation of plasma leakage into mucosal secretions. The determination of plasma leakage into feces or filter wick-sampled secretions is based on the assumption that plasma leaks are caused by physical damage to the mucosal epithelium and the underlying blood vessels, resulting in an unhindered flow of blood plasma into the sample. Since no specific retardation of a certain plasma molecule can occur under these conditions, any soluble plasma- or serum-specific protein may serve as a marker for the amount of plasma transudated. We decided on IgG as plasma-serum marker. Since the final algorithms contain both IgA and IgG titers, the mutual cross-reactivity of the anti-mouse IgG and IgA detection reagents (secondary antibodies) should be taken into consideration.
The percentage of mucosal IgA which is due to transudation, can be described by a mass balance equation (all terms are defined at the end of the Appendix):
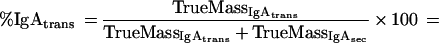 |
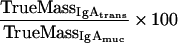 |
while the percentage of fluid volume transudated can be described by a volume balance equation:
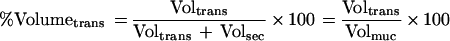 |
With the help of the following relationships, which take the antibody cross-reactivity into account:
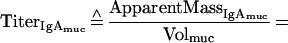 |
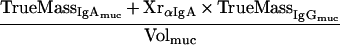 |
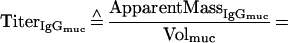 |
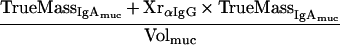 |
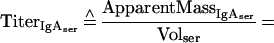 |
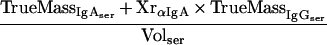 |
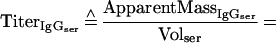 |
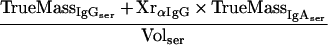 |
and two terms which describe the unhindered plasma flow (i.e., serum IgG and IgA transudate equally well) and the plasma specificity of IgG (i.e., no active or passive transport mechanisms for IgG except physical damage of the epithelium exist):
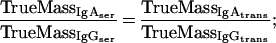 |
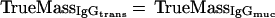 |
the mass balance equation can be resolved to equation 1:
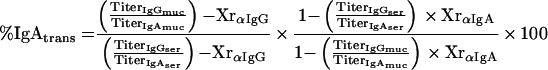 |
and the volume balance equation to equation 2:
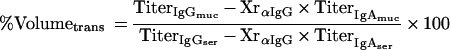 |
Definitions. ApparentMass, amount of analyte detected; IgAmuc or IgGmuc, mucosal IgA or IgG; IgAser or IgGser, serum IgA or IgG; IgAtrans or IgGtrans, transudated IgA or IgG; IgAsec, secreted IgA; Titer, dimensionless or volume (weight)-normalized measure of analyte content, e.g., endpoint titer, antibody units, or gravimetric units; TrueMass, actual amount of analyte in the sample; Volsec, Volser, Volmuc, Voltrans, volume of secreted, serum, mucosal, or transudated sample, respectively; XrαIgA, cross-reactivity of anti-mouse IgA with mouse IgG; XrαIgG, cross-reactivity of anti-mouse IgG with mouse IgA.
REFERENCES
- 1.Åhren C, Andersson K, Wiklund G, Wennerås C, Svennerholm A-M. Optimization of the intestinal lavage procedure for determination of intestinal immune responses. Vaccine. 1995;13:1754–1758. doi: 10.1016/0264-410x(95)00153-r. [DOI] [PubMed] [Google Scholar]
- 2.Barton J R, O'Mahoney S, Ferguson A. Regulation of antibodies to food proteins within the common mucosal immune system: lack of correlation between antibody titers in saliva and intestinal fluid. Adv Mucosal Immunol. 1990;1990:495–496. [Google Scholar]
- 3.Cancellieri V, Fara G M. Demonstration of specific IgA in human feces after immunization with live Ty21a Salmonella typhi vaccine. J Infect Dis. 1985;151:482–484. doi: 10.1093/infdis/151.3.482. [DOI] [PubMed] [Google Scholar]
- 4.Elson C O, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 5.Elson C O, Ealding W, Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- 6.Faria A M C, Garcia G, Rios M J C, Michalaros C L, Vaz N M. Decrease in susceptibility to oral tolerance induction and occurrence of oral immunization to ovalbumin in 20–38-week-old mice: the effect of interval between oral exposures and rate of antigen intake in the oral immunization. Immunology. 1993;78:147–151. [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson A, Humphreys K A, Croft N M. Technical report: results of immunological tests on faecal extracts are likely to be extremely misleading. Clin Exp Immunol. 1995;99:70–75. doi: 10.1111/j.1365-2249.1995.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest B D. Effects of sample processing on the measurement of specific intestinal IgA immune responses. Vaccine. 1992;10:802–805. doi: 10.1016/0264-410x(92)90517-n. [DOI] [PubMed] [Google Scholar]
- 9.Frey A, Meckelein B, Externest D, Schmidt M A. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J Immunol Methods. 2000;233:47–56. doi: 10.1016/s0022-1759(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 10.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 11.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haneberg B, Aarskog D. Human faecal immunoglobulins in healthy infants and children, and in some with diseases affecting the intestinal tract or the immune system. Clin Exp Immunol. 1975;22:210–222. [PMC free article] [PubMed] [Google Scholar]
- 13.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A-M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184:157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 15.Jackson R J, Fujihashi K, Kiyono H, McGhee J R. Luminometry: a novel bioluminescent immunoassay enhances the quantitation of mucosal and systemic antibody responses. J Immunol Methods. 1996;190:189–197. doi: 10.1016/0022-1759(95)00276-6. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z D, Nelson A C, Mathewson J J, Ericsson C D, DuPont H L. Intestinal secretory immune response to infection with Aeromonas species and Plesiomonas shigelloides among students from the United States in Mexico. J Infect Dis. 1991;164:979–982. doi: 10.1093/infdis/164.5.979. [DOI] [PubMed] [Google Scholar]
- 17.Lagergård T, Trollfors B, Claesson B A, Schneerson R, Robbins J B. Comparison between radioimmunoassay and direct and indirect enzyme-linked immunosorbent assays for determination of antibodies against Haemophilus influenzae type b capsular polysaccharide. J Clin Microbiol. 1988;26:2554–2557. doi: 10.1128/jcm.26.12.2554-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane E M, Batchelor R A, Bourgeois A L, Burr D H, Olson J G. Urine and faecal IgA response during naturally acquired infection with Campylobacter jejuni. Lancet. 1987;8542:1141. doi: 10.1016/s0140-6736(87)91694-1. [DOI] [PubMed] [Google Scholar]
- 19.McDermott M R, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 20.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 21.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neutra M R, Frey A, Kraehenbuhl J-P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 23.Nimmo G R, Lew A M, Stanley C M, Steward M W. Influence of antibody affinity on the performance of different antibody assays. J Immunol Methods. 1984;72:177–187. doi: 10.1016/0022-1759(84)90446-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Mahony S, Barton J R, Crichton S, Ferguson A. Appraisal of gut lavage in the study of intestinal humoral immunity. Gut. 1990;31:1341–1344. doi: 10.1136/gut.31.12.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quiding-Järbrink M, Nordström I, Granström G, Kilander A, Jertborn M, Butcher E C, Lazarovitz A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 28.Ruggeri F M, Johansen K, Basile G, Kraehenbuhl J-P, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol. 1998;72:2708–2714. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano T, Smith C L, Cantor C R. Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA conjugates. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 30.Sukhacheva E, Novikov V, Plaksin D, Pavlova I, Ambrosova S. Highly sensitive immunoassays for detection of barley stripe mosaic virus and beet necrotic yellow vein virus. J Virol Methods. 1996;56:199–207. doi: 10.1016/0166-0934(95)01962-6. [DOI] [PubMed] [Google Scholar]
- 31.Weisz-Carrington P, Roux M E, McWilliams M, Phillips-Quagliata J M, Lamm M E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979;123:1705–1708. [PubMed] [Google Scholar]
- 32.Weltzin R, Traina-Dorge V, Soike K, Zhang J Y, Mack P, Soman G, Drabik G, Monath T P. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis. 1996;174:256–261. doi: 10.1093/infdis/174.2.256. [DOI] [PubMed] [Google Scholar]
- 33.Winner L, 3rd, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J-P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]