Abstract
Population cardiovascular health, or improving cardiovascular health among patients and the population at large, requires a redoubling of primordial and primary prevention efforts as declines in cardiovascular disease mortality have decelerated over the past decade. Great potential exists for healthcare systems–based approaches to aid in reversing these trends. A learning healthcare system, in which population cardiovascular health metrics are measured, evaluated, intervened on, and re-evaluated, can serve as a model for developing the evidence base for developing, deploying, and disseminating interventions. This scientific statement on optimizing population cardiovascular health summarizes the current evidence for such an approach; reviews contemporary sources for relevant performance and clinical metrics; highlights the role of implementation science strategies; and advocates for an interdisciplinary team approach to enhance the impact of this work.
Keywords: AHA Scientific Statements, cardiovascular diseases, delivery of health care, disease management, interdisciplinary research, learning health system, population health
Worldwide, the population burden of chronic diseases is increasing, amplified by the aging of populations. Additionally, the progress toward improvements in population cardiovascular health (CVH) has been slow. Furthermore, and most important, recent mortality trends observed in the United States generate major concerns. In 2011, the rate of decline in cardiovascular disease (CVD) mortality began decelerating, and the downward trends in deaths attributed to heart disease and stroke reversed course in middle-aged Americans, even among those living in traditionally healthier geographies.1,2 From 2011 to 2017, the magnitude of the decline in annual CVD mortality diminished to <1% per year, and 5-year CVD mortality declined by 4%.3–5
Responses to these concerning trends must involve a redoubling of efforts on primordial and primary prevention of CVD. To emphasize this prevention imperative, the Goals and Metric Committee of the Strategic Planning Task Force of the American Heart Association (AHA) developed the 2020 Impact Goal (Figure 1) “to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular disease and stroke by 20%.”6 There is a need to fully implement what is known and for innovative, integrated approaches to optimize CVH in populations and overcome adverse mortality trends.9
Figure 1.

American Heart Association 2020 Goals—Life’s Simple 7.
This scientific statement on optimizing population CVH summarizes the current evidence for such an approach; reviews contemporary sources for relevant performance and clinical metrics; highlights the role of implementation science strategies; and advocates for an interdisciplinary team approach to enhance the impact of this work. Although many of the initiatives reviewed in this statement support the enhancement of secondary prevention (eg, improving blood pressure control), they can provide a road map to expand efforts to impact primordial and primary prevention to optimize CVH.
A LEARNING HEALTHCARE SYSTEM APPROACH TO ACHIEVING OPTIMAL CVH
If population-level CVH interventions are deployed synergistically with health systems, they can benefit from the operationalization of the learning healthcare system model (Figure 2).10–12 Learning healthcare systems are those in which knowledge is consistently generated and applied in the practice of medicine to yield continuous improvements in healthcare delivery. In realizing this vision, population-level interventions can leverage health information technology and associated data infrastructure to guide evidence-based healthcare delivery in a healthcare ecosystem that in turn promotes high-quality healthcare delivery and whose insights can be used to spur innovation.11 Operationalizing this model calls for a synergistic, integrated, and complementary approach using health system–wide resources. Learning healthcare system models have been deployed and maintained in the Geisinger Health System and Kaiser Permanente, among others. To fully extend the impact of the innovative models in learning healthcare systems, they can be linked with complementary population-health efforts traditionally led by public health practitioners, as described by Auerbach’s 3 buckets of prevention.13
Figure 2.
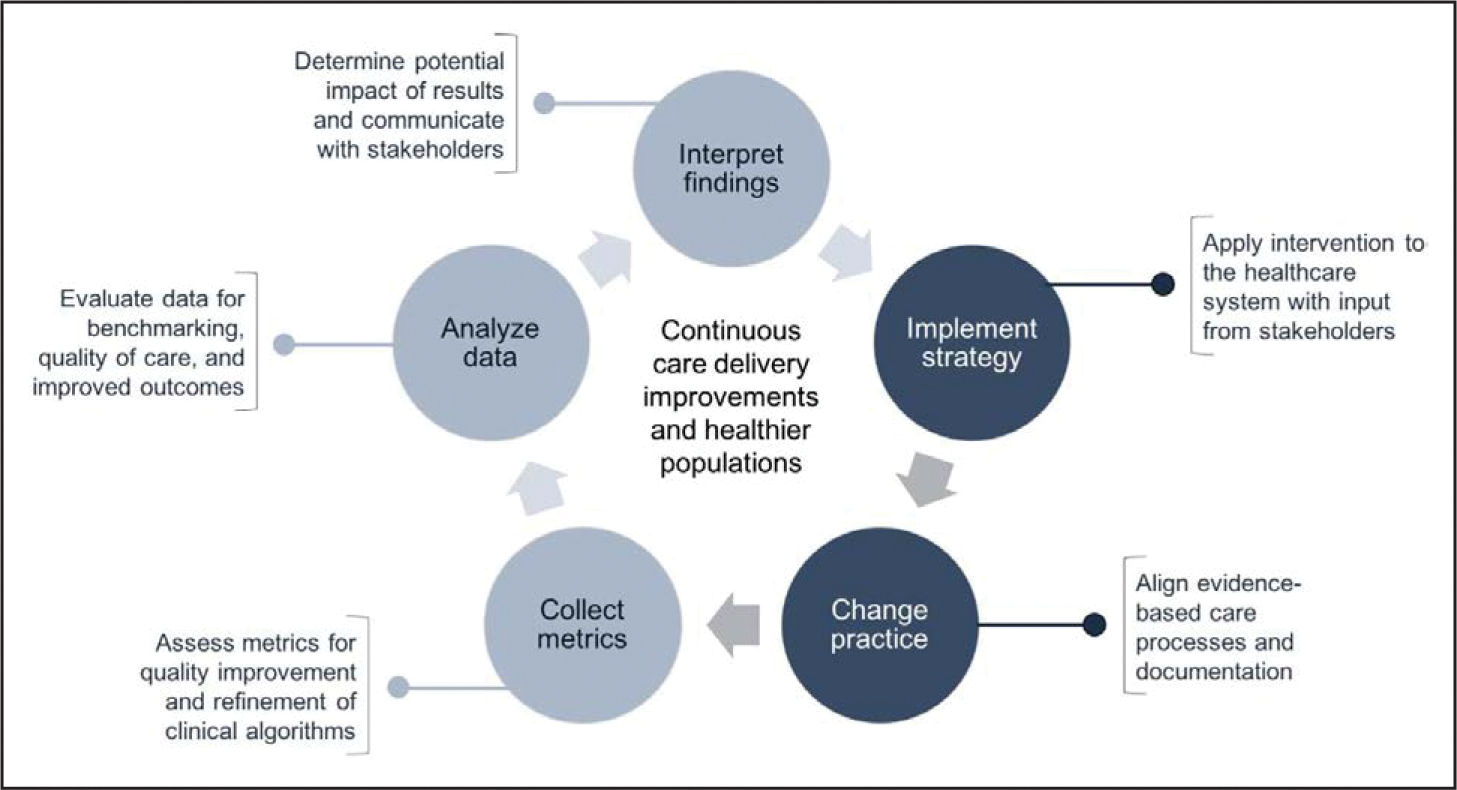
A learning healthcare system context for cardiovascular disease prevention and management.
Data-centric steps are shown in light gray; methods-centric steps are in dark gray. Data derived from Moloney et al,10 Maddox et al,11 and Institute of Medicine.12
Available data streams providing information on CVH metrics include the electronic health record, as well as data from mobile devices and wearables. Data streams require preprocessing and, once deemed valid and reliable, can be integrated into current data models using advanced data management techniques. Expertise with mobile device and wearable data is needed to complete this cycle. Findings can then be evaluated to determine the level of evidence to support the intervention, in which clinical contexts, and with which patient population. The aforementioned parts of the learning healthcare system model are shown in Figure 2; next, as also shown in Figure 2, the intervention is deployed and evaluated. A feature of this model is that it is iterative and serves as the foundation for cyclic assessments and clinical practice adaptation.
EVIDENCE-BASED CVH METRICS FOR POPULATION CVH
The data-centric steps (Figure 2) are best enabled by the selection of a prioritized set of performance measures, or clinical quality measures, for ongoing collection, analysis, and interpretation. With the breadth of interventions available to impact CVH, the selection of a prioritized set of performance measures may be a useful first step for health systems seeking to implement and evaluate performance related to CVH. We propose herein a number of evidence-based and actionable population CVH metrics for ongoing monitoring and evaluation of primordial and primary prevention, including maintenance of physical activity, a heart-healthy diet, ideal body mass index, and nonsmoking. Although we do not explicitly state which metrics a healthcare system should monitor, we aim to promote those most commonly measured and evaluated to streamline such efforts. We also acknowledge that there is a health–disease continuum that exists across the life course, and traditional health systems are frequently engaged when disease emerges.14
One example relevant to the evaluation of population-level CVH initiatives is Million Hearts 2022. This national initiative is co-led by the Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services in partnership with numerous public and private partners. The primary aim of the initiative is to prevent 1 million heart attacks and strokes within 5 years. Million Hearts 2022 seeks to align performance measures across federal and partner programs to reduce reporting burden, promote a common target, allow for comparisons across systems, and encourage the use of measures aligned with current guidelines.15 Replication of these activities would be relevant to CVD prevention and management across healthcare settings. The Million Hearts 2022 initiative uses national guidelines to set targets and to link those targets to performance measures. Resources for performance measure alignment are provided by the program.16 Since its inception in 2012, this initiative has focused on a set of common, high-burden risk factors. The 5-year initiative to prevent 1 million CVD events, now in its second cycle, identified the ABCS (aspirin when appropriate, blood pressure control, cholesterol management, and smoking cessation) as optimal areas of focus because of the significant evidence base for CVD primordial and primary prevention and high population burden.17 It was recently reported that numerous clinical quality measures from the Million Hearts initiative are available for population health monitoring efforts from routinely collected electronic health record data elements.18
Acknowledging the need for resources to support the translation of guidelines into modern clinical practice, new guidelines released by the AHA and its partners have been accompanied by recommended performance measures for measuring and evaluating population-level blood pressure.19 The AHA has partnered with industry leaders and other associations, such as the American Medical Association and the American Diabetes Association, to improve the diagnosis and management of blood pressure (Target:BP), cholesterol (Check.Change.Control.Cholesterol), and diabetes mellitus (Know Diabetes by Heart). These quality improvement programs are designed to help healthcare professionals and health systems treat important risk factors for the primary prevention of CVD.
Additionally, since 2015, Million Hearts has released 3 quality improvement change packages that provide evidence-based strategies for driving improvement on blood pressure control, tobacco cessation, and cardiac rehabilitation utilization. Using a quality improvement framework, these packages provide validated tools and resources from national organizations and healthcare provider organizations in the field who have demonstrated improvement. However, evidence-based interventions supporting enhanced CVH and CVD management are frequently underutilized because of the complexity of translating guidelines and recommendations into practice. Consistency in performance measures (developed according to guidelines) that are used by health systems proves useful for achieving the vision of a learning healthcare system, as well as reporting performance to health insurers and other key partners.
The prioritization of select clinical measures to drive performance improvement has already been effectively demonstrated across a variety of health systems in the United States. For example, the Kaiser Permanente Northern California hypertension program, although multidimensional in implementation, prioritized a hypertension control performance measure to demonstrate population health management and program effectiveness.20 Lessons learned and common attributes from programs similar to Kaiser Permanente Northern California have been highlighted through the Million Hearts Hypertension Control Challenge and are complementary to the World Health Organization’s HEARTS technical package.21 The HEARTS technical package serves as a comprehensive tool kit for improving CVH at the country level that includes pragmatic recommendations for the prioritization of performance measures for evaluating program effectiveness.22 Additional core metrics that can be considered as performance measures for population-level CVH program development and evaluation of intervention effectiveness are listed in Table 1.
Table 1.
Selected Performance Measures for Evaluating CVH Intervention Effectiveness
| 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease23 |
| Minnesota Statewide Quality Reporting and Measurement System24 |
| Robert Wood Johnson Foundation Culture of Health Framework25 |
| Institute of Medicine Vital Signs: Core Metrics for Health and Health Care Progress26 |
| Centers for Medicare & Medicaid Services Meaningful Measures27 |
ACC indicates American College of Cardiology; AHA, American Heart Association; and CVH, cardiovascular health.
EVIDENCE-BASED METHODS FOR IMPLEMENTATION OF POPULATION CVH INTERVENTIONS
The methods-centric steps shown in Figure 2 comprise implementation and evaluation of strategies to improve CVH and to prevent chronic diseases. Although evidence-based medicine continues to be a cornerstone in modern medicine, some evidence-generation activities are more mature than others. Epidemiologists and other researchers have the skills to help define which outcomes of medical interventions are relevant and how to measure them effectively and efficiently. They can assist healthcare systems in designing, monitoring, and evaluating efforts to improve population CVH.
Healthcare systems often use quality improvement (QI) as an approach for furthering the field of healthcare delivery by evaluating care processes. QI projects allow for real-life variations in the environment and often rely on adaptive and rapid-cycle methodologies, such as the plan–do–study–act cycle28 represented by the learning healthcare system model (Figure 2). QI projects rarely undergo rigorous evaluation or provide a systematic reporting of results; therefore, the reasons why they fail to achieve their anticipated outcomes often remain unknown. This is a missed opportunity for on-the-ground learning, and the inclusion of the evaluation and dissemination of lessons learned from QI projects in the planning phase could allow for new and innovative interventions.
Implementation science, on the other hand, offers rigorous metrics to design and evaluate clinical research questions. Implementation science research seeks to understand what is required, in terms of feasibility, time, and cost, to facilitate the adoption, uptake, and sustainability of an intervention in clinical settings. It can help us understand “for whom” and “under what conditions” an intervention works best so as to ensure equitable access to evidence-based care. Learning healthcare systems are informed by QI efforts and are implemented, or deimplemented, according to best practices in implementation science.
According to the Institute of Medicine, implementation science is central to addressing the “quality chasm and is a key component of learning healthcare systems designed to iteratively develop and evaluate innovations to deliver high-quality patient-centered care and to evaluate the effectiveness of this care.”29 Implementation science methods can be applied in synergy with QI methodologies. Clinical research questions can be first tested as QI interventions. This will aid the refinement of an idea and pilot testing of an intervention before a research study is designed, which would be a longer-term project with less flexibility in modifying data collection, primary outcomes, or elements of the intervention once the research was under way.
Epidemiologists and health services researchers are well positioned to work alongside implementation scientists and clinicians to support successful implementation of clinical interventions for several reasons. They are trained in the use of relevant theories, models, and frameworks and thereby are well poised to design and test strategies for deploying effective interventions and evidence-based practices into a variety of settings, including healthcare delivery systems.30 Although training programs vary in their integration of program evaluation or QI, many include rigorous evaluation methods such as learning evaluation. Learning evaluation is a methodological approach that blends QI and implementation science research methods to study healthcare innovations.31 In learning evaluation, qualitative and quantitative data are collected to conduct real-time assessments of implementation processes while also assessing changes in context, facilitating QI, and generating transportable lessons. This approach is designed to balance the flexibility needed for within-system innovation and the structure needed to support rigorous evaluation and cross-organization learning. The blending of these methodologies can help researchers define best practices for scaling population CVH interventions across healthcare delivery systems.
More recently, the implementation science field has championed the concept of deimplementation, or the discontinuation of practices known to be ineffective.32 Deimplementation (reducing or stopping the use of ineffective, harmful, low-value, or unproven interventions, practices, and programs) is particularly important for population CVH interventions that may occur during already time-strapped clinical encounters. To advance population CVH management, the development and testing of frameworks, methods, measures, outcomes, and strategies that address issues specific to deimplementation is important.
ROLE OF AN INTERDISCIPLINARY TEAM
Expertise of diverse team members is needed in such efforts to ensure that population CVH interventions are acceptable to patients, healthcare professionals, health system administrators, and information technology staff before implementation. Researchers can adapt by working on interdisciplinary teams to enable unique insights into healthcare delivery, epidemiology, implementation science, health information technology, and informatics.33 Examples of the expertise needed for improving population CVH in this context are shown in Table 2.
Table 2.
Interdisciplinary Team Members and Their Relevance for the Study of Learning Healthcare System Interventions Designed to Improve Population-Level CVH
| Team Member | Expertise |
|---|---|
| Epidemiologist | Study design |
| Biostatistician/data scientist | Statistical analysis and evaluation |
| Implementation scientist | Best practices for implementation and dissemination |
| Healthcare professionals | Healthcare context and workflow analysis |
| Informatician | Usability and workflow analysis |
| Information technology | Database resources and implementation considerations |
| Data abstractor | Reporting and monitoring outcomes |
| Systems scientist | Contextual approaches to problem solving |
| Marketing and communications | Communicate with stakeholders |
| Ethics | Patient safety and algorithmic bias detection |
| Individuals, families, and communities | Engagement with population health interventions |
The experts listed in Table 2 can contribute significantly to CVH-promoting activities that occur in a learning healthcare system (Figure 2) at many stages of the effort. Researchers and healthcare professionals are essential components of a comprehensive strategy to improve CVH. Through their complementary expertise, they can provide direct patient care, implement new guidelines into management, track metrics and evaluate them over time, test novel strategies with the potential to improve CVH, and implement strategies to prevent the onset of chronic disease. Researchers in healthcare organizations and academic medical centers play an increasingly important role in systems-level approaches to preventing and managing CVD.
Developing, deploying, and evaluating interventions to improve population CVH requires a system-wide, interdisciplinary approach; however, researchers are rarely trained in how to work with stakeholder groups to successfully implement and evaluate healthcare delivery interventions. Healthcare professionals and researchers in training would benefit from participating on interdisciplinary teams to observe how interventions are designed, implemented, and evaluated within the constraints of busy healthcare workflows. Structured opportunities to shadow health service delivery, epidemiology, implementation science, and informatics researchers would provide invaluable experience in designing and implementing healthcare delivery interventions to improve population-level CVH.
Although the healthcare team does not typically include an epidemiologist, epidemiologists are uniquely positioned to contribute to health and the deployment of learning healthcare system models. Epidemiologists serve as experts in study design and in the measurement and interpretation of results, yet they can also serve as connectors to other fields of expertise. In addition, epidemiologists are well trained to help navigate the transformations that are taking place in modern medicine, from strengthening primary care to better managing chronic diseases to building integrated health systems, and from facilitating implementation of appropriate payment schemes that support value to enabling health information technology and data-driven programs to improve care.
At the same time, biostatisticians and data scientists play a key role in data management and analysis, given recent and ongoing expansion of data.34 As data streams themselves become more vast and diverse, from electronic health records to wearable devices, there is also a considerable increase in data complexity. If the data are to be presented electronically as clinical decision support (CDS), informaticians and health information technology experts need to be consulted, not only to evaluate the extant information technology landscape in the healthcare ecosystem, but also to determine how best to present the information to the end user. Incorporating every recommendation and guideline into CDS prompts would likely overload the end user; thus, prioritization, staging, and a team-based approach are needed to disperse the demands and streamline implementation. End users themselves, whether patients, healthcare professionals, or caregivers, can be consulted with respect to acceptability and usability of a CVH intervention to ensure its feasibility and utility.
Healthcare professionals can help evaluate the research evidence for a particular intervention, serve as clinical champions for projects to encourage their uptake, and, alongside implementation scientists, provide insight into clinical workflows and how population CVH interventions can be integrated into usual care processes. It is also important to engage patients and their advocates early and throughout the process, because they can provide information on patient preferences for messaging and the delivery of the CVH intervention. It is important to keep in mind that institutional support for interventions from the broader healthcare system and data governance committees is often necessary before a project can proceed.
The aforementioned experts can also help prevent biases in algorithm development and deployment. Analytics conducted with data from the learning healthcare system may not result in internally valid CVH profiles given that not all patients are seen by healthcare professionals regularly, nor do they all take the same tests or have the same laboratory values measured. Efforts to improve CVH can be deployed to enhance health outcomes for all, and the proposed interdisciplinary approach should include consideration of the equitable and ethical aspects of each project. Figure 3 shows a multilevel approach to preventing chronic disease, with consideration of affordable health care and public health policies that support healthy decisions. Careful consideration of these issues can protect against unintended consequences of population CVH interventions. Thus, we believe that early and frequent consultation between interdisciplinary teams and members of the healthcare ecosystem bode success for population CVH management strategies.
Figure 3.
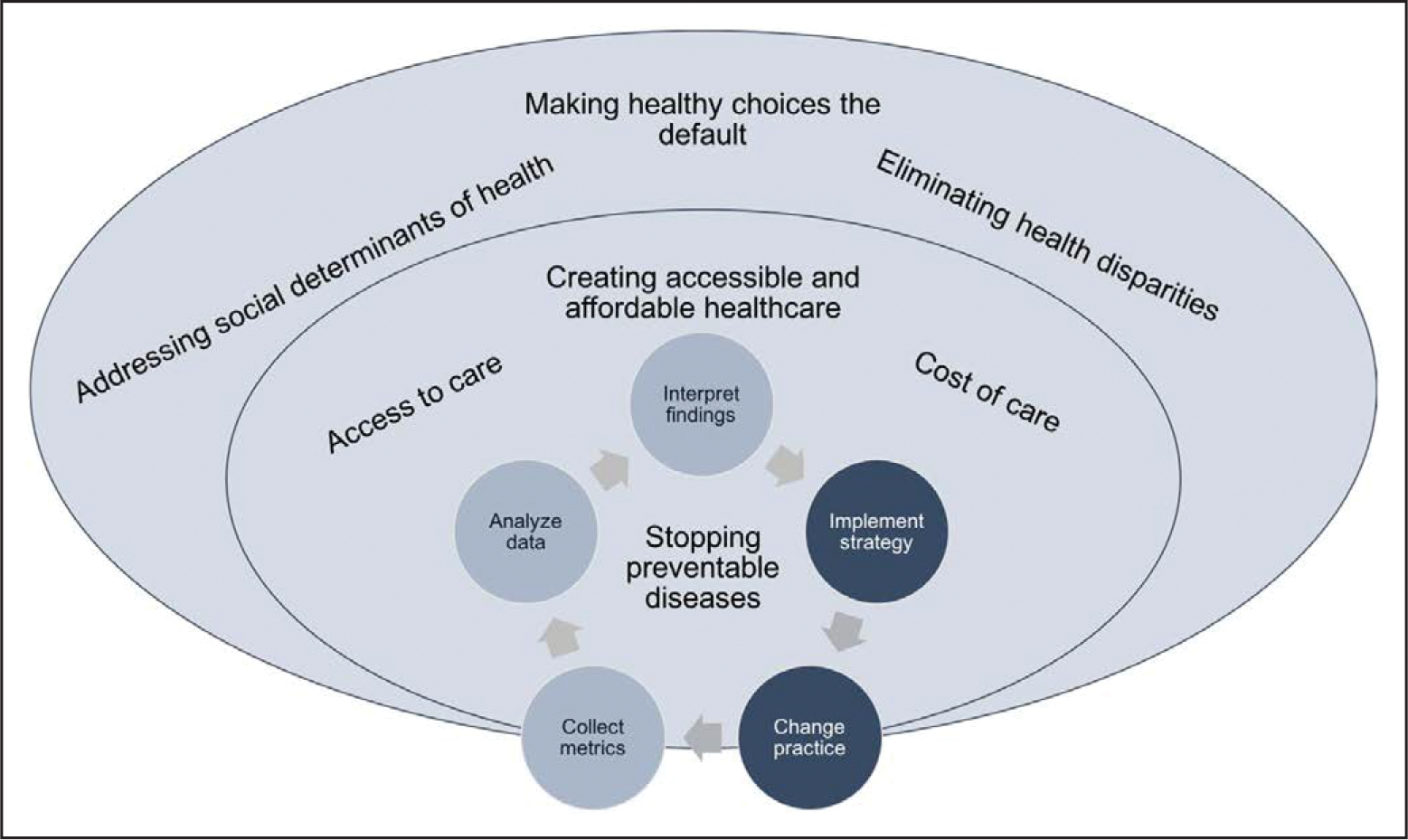
Health equity–based approaches in the context of a learning healthcare system.
TOWARD OPTIMAL CVH: EXAMPLES OF A LEARNING HEALTHCARE SYSTEM APPROACH
Since the establishment of the AHA’s 2020 Impact Goal and the development of CVH metrics, there have been various examples of successful CVH and prevention programs implemented in healthcare systems throughout the United States. Two examples of population-level CVH intervention programs include the SPHERE (Stroke Prevention in Healthcare Delivery Environments) study35 and Priorities Wizard,36 both of which are CDS tools. These examples were chosen because they use evidence-based metrics for CVH, including maintenance of physical activity, a heart-healthy diet, ideal body mass index, and nonsmoking, and can be easily evaluated and disseminated across healthcare delivery systems.
SPHERE Study
The SPHERE study aimed to enhance patient-healthcare professional communication around CVH and to improve the delivery of preventive cardiovascular care in the primary care setting.35 SPHERE leveraged the functionality of the electronic health record to deliver a web application, embedded in the electronic health record interface, at the point of care so as to make the data an actionable part of the healthcare encounter. Its interactive interface enabled healthcare professionals to show patients how changes in their CVH behaviors and factors could result in improved CVH. SPHERE was developed in collaboration with clinic champions and informaticians so that it would be responsive to the workflow of usual care and would automatically populate with electronic health record data, to address traditional barriers to usability.
The SPHERE algorithm was programmed according to the AHA’s 2020 Impact Goal with a focus on modifiable risk factors for CVD.6 Five of the 7 risk factors were stored in the electronic health record for most patients, and the remaining 2 (diet and physical activity) were collected by a survey distributed via the personal health record. In this study, the use of the SPHERE tool resulted in 1-year improvements in body mass index and diabetes mellitus relative to the control clinic.35 The tool was also deemed acceptable by healthcare professionals and did not take a significant amount of clinical time to administer at the point of care.
SPHERE investigators are currently implementing and studying this tool in the context of cancer survivorship care, with the ultimate goal of improving survivors’ CVH and ameliorating cardiovascular events and cancer recurrence. Of note, there remains significant opportunity to address CVH, particularly in the areas of diet and physical activity, among children and adolescents, because interventions such as SPHERE can be easily adapted to established cut points relevant to these populations.37,38
Priorities Wizard
Another example of an effective electronic health record–embedded intervention is the Priorities Wizard.36 Similar to SPHERE, it is a web-based CDS tool. The integration of Priorities Wizard in 3 large integrated health systems was associated with significant improvements in high-burden cardiometabolic metrics. Implemented at the patient visit in primary care clinics through the Health Care Systems Research Network, this tool (1) identifies patients who could substantially benefit from evidence-based actions; (2) presents prioritized evidence-based treatment options to both patient and clinician at the point of care; and (3) facilitates efficient ordering of recommended medications, referrals, or procedures. It allows for the rapid dissemination of new knowledge into clinical practice (updated AHA cholesterol, hypertension, or diabetes mellitus treatment guidelines, for example) while being cost-effective and having high clinician satisfaction.
Results from cluster-randomized trials showed that Priorities Wizard significantly improved glucose and blood pressure control in patients with diabetes mellitus, reduced 10-year CVD risk in high-risk adults without diabetes mellitus, improved management of smoking in dental patients, and improved high blood pressure identification and management in adolescents.36 The CDS tool includes a button that allows clinicians to provide immediate feedback if the CDS recommendations seem incomplete or implausible, which has helped debug some algorithms in the initial design phase36; however, it is often the clinician who needs debugging, because clinicians are often unfamiliar with changes in evidence-based guidelines. Interdisciplinary researchers based in healthcare systems are critical for the design and success of these electronic health record–based CDS programs, as well as for ongoing monitoring and evaluation.
OPTIMIZING POPULATION CVH: LESSONS LEARNED AND FUTURE DIRECTIONS
Healthcare systems must continue to meet the growing demand for data-driven intervention strategies and risk algorithms to optimize population CVH. Interdisciplinary teams have the tools needed to effectively integrate population CVH interventions into the healthcare ecosystem and ensure that meaningful metrics and outcomes are being evaluated with the end goal of primordial and primary prevention of CVD, with an emphasis on CVH behaviors such as physical activity, heart-healthy diet, ideal body mass index, and nonsmoking. Researchers on the team who are trained in rigorous study design and evaluation methodologies are complemented by healthcare professionals, patients, and those with expert knowledge of healthcare data systems. Implementation science expertise can add perspective to the team approach for developing and deploying interventions to ensure both their effectiveness and sustainability.39
To make the best use of the learning healthcare system model, interdisciplinary teams can develop a strategy for prioritizing a set of clinical measures to evaluate before, during, and after the intervention. This will ensure that relevant data are being used to evaluate the impact of the intervention, drive clinical and administrative decision making, and enable clinical benchmarking and performance metric reporting. Tracking a common set of metrics over time can also assist healthcare systems in identifying population CVH strategies that have become ineffective over time and could be either deimplemented or revised with updated evidence.
Consistent with the 2020 goals,6 care must be taken to apply CVH interventions equitably across various settings and populations, so as not to skew the application of best practices toward communities and organizations with high capacity and the most resources.40 Application of evidence-based implementation science tools and techniques can facilitate the uptake and effective use of evidence-based interventions to not only improve population CVH but also enhance and ultimately preserve health equity.41,42 To achieve that end, a prioritized set of CVH performance measures, particularly those commonly collected in the electronic health record and consistent with other national initiatives such as Million Hearts, will facilitate dissemination of successful interventions across healthcare systems. Scaling evidence-based interventions across healthcare systems has the potential to maximize the population CVH impact of such approaches.
Footnotes
ARTICLE INFORMATION
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on June 29, 2020, and the American Heart Association Executive Committee on October 26, 2020. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or Meredith.Edelman@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Foraker RE, Benziger CP, DeBarmore BM, Cené CW, Loustalot F, Khan Y, Anderson CAM, Roger VL; on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Lifestyle and Cardiometabolic Health. Achieving optimal population cardiovascular health requires an interdisciplinary team and a learning healthcare system: a scientific statement from the American Heart Association. Circulation. 2021;143:e9–e18. doi: 10.1161/CIR.0000000000000913
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Randi E. Foraker | Washington University, St. Louis, School of Medicine | NCI* | None | None | None | None | None | None |
| Catherine P. Benziger | Essentia Health | None | None | None | None | None | None | None |
| Cheryl A.M. Anderson | University of California, San Diego | None | None | None | None | None | None | None |
| Crystal W. Cené | University of North Carolina, Chapel Hill | None | None | None | None | None | None | None |
| Bailey M. DeBarmore | University of North Carolina, Chapel Hill | None | None | None | None | None | None | None |
| Yosef Khan | American Heart Association/American Stroke Association | None | None | None | None | None | None | None |
| Fleetwood Loustalot | Centers for Disease Control and Prevention | None | None | None | None | None | None | None |
| Véronique L. Roger | Mayo Clinic | None | None | None | None | None | None | None |
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Norrina B. Allen | Northwestern University | None | None | None | None | None | None | None |
| Gerald Jerome | Towson University | NIH (Investigator in the Center to Accelerate Translation of Interventions to Decrease Premature Mortality in Adults with SMI)* | None | None | None | None | None | None |
| Elisabeth L.P. Sattler | University of Georgia | None | None | None | None | None | None | None |
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Hall EW, Vaughan AS, Ritchey MD, Schieb L, Casper M. Stagnating national declines in stroke mortality mask widespread county-level increases, 2010–2016. Stroke. 2019;50:3355–3359. doi: 10.1161/STROKEAHA.119.026695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan AS, Ritchey MD, Hannan J, Kramer MR, Casper M. Widespread recent increases in county-level heart disease mortality across age groups. Ann Epidemiol. 2017;27:796–800. doi: 10.1016/j.annepidem.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtin SC. Trends in cancer and heart disease death rates among adults aged 45–64: United States, 1999–2017. Natl Vital Stat Rep. 2019;68:1–9. [PubMed] [Google Scholar]
- 4.Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS, Rana JS. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. doi: 10.1001/jamacardio.2016.1326 [DOI] [PubMed] [Google Scholar]
- 5.Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiology. 2019;4:1280–1286. doi: 10.1001/jamacardio.2019.4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. ; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 7. Deleted in proof.
- 8. Deleted in proof.
- 9.Mehta NK, Abrams LR, Myrskylä M. US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc Natl Acad Sci U S A. 2020;117:6998–7000. doi: 10.1073/pnas.1920391117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moloney RM, Tambor ES, Tunis SR. Patient and clinician support for the learning healthcare system: recommendations for enhancing value. J Comp Eff Res. 2016;5:123–128. doi: 10.2217/cer.15.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddox TM, Albert NM, Borden WB, Curtis LH, Ferguson TB Jr, Kao DP, Marcus GM, Peterson ED, Redberg R, Rumsfeld JS, et al. ; on behalf of the American Heart Association Council on Quality of Care and Outcomes Research; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. The learning healthcare system and cardiovascular care: a scientific statement from the American Heart Association. Circulation. 2017;135:e826–e857. doi: 10.1161/CIR.0000000000000480 [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. Washington, DC: National Academies Press; 2015. doi: 10.17226/18945 [DOI] [PubMed] [Google Scholar]
- 13.The Auerbach J. 3 buckets of prevention. J Public Health Manag Pract. 2016;22:215–218. doi: 10.1097/PHH.0000000000000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapper JT, Ghasemzadeh N, Khayata M, Patel SP, Quyyumi AA, Mendis S, Mensah GA, Taubert K, Sperling LS. Time to change our focus: defining, promoting, and impacting cardiovascular population health [published correction appears in J Am Coll Cardiol. 2015;66:1641]. J Am Coll Cardiol. 2015;66:960–971. doi: 10.1016/j.jacc.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 15.Frieden TR, Wright JS, Conway PH. Is rapid health improvement possible? Lessons from the Million Hearts initiative. Circulation. 2017;135:1677–1680. doi: 10.1161/CIRCULATIONAHA.117.027461 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services. Clinical Quality Measure Alignment. Million Hearts website. 2019. https://millionhearts.hhs.gov/data-reports/measures.html. Accessed November 8, 2019. [Google Scholar]
- 17.Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for Million Hearts 2022: United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2018;67:983–991. doi: 10.15585/mmwr.mm6735a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heisey-Grove D, Wall HK, Helwig A, Wright JS; Centers for Disease Control and Prevention (CDC). Using electronic clinical quality measure reporting for public health surveillance. MMWR Morb Mortal Wkly Rep. 2015;64:439–442. [PMC free article] [PubMed] [Google Scholar]
- 19.Casey DE Jr, Thomas RJ, Bhalla V, Commodore-Mensah Y, Heidenreich PA, Kolte D, Muntner P, Smith SC Jr, Spertus JA, Windle JR, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2019;12:e000057. doi: 10.1161/HCQ.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young A, Ritchey MD, George MG, Hannan J, Wright J. Characteristics of health care practices and systems that excel in hypertension control. Prev Chronic Dis. 2018;15:E73. doi: 10.5888/pcd15.170497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HEARTS: Technical Package for Cardiovascular Disease Management in Primary Health Care. Geneva, Switzerland: World Health Organization; 2016. https://www.who.int/cardiovascular_diseases/hearts/Hearts_package.pdf. Accessed November 8, 2019. [Google Scholar]
- 23.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Circulation. 2019140:e649–e650; Circulation. 2020;141:e60; and Circulation. 2020;141:e774]. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foti K, Auerbach J, Magnan S. Improving hypertension control population-wide in Minnesota. J Public Health Manag Pract. 2018;24:432–439. doi: 10.1097/PHH.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 25.Plough A, Miller C, Tait M. Moving Forward: An Update on Building and Measuring a Culture of Health. Princeton, NJ: Robert Wood Johnson Foundation; 2018. https://www.rwjf.org/en/library/research/2018/05/movingfoward-together–an-update-on-building-and-measuring-a-culture-of-health.html. Accessed November 10, 2019. [Google Scholar]
- 26.IOM (Institute of Medicine). Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: The National Academies Press; 2015. doi: 10.17226/19402 [DOI] [PubMed] [Google Scholar]
- 27.Overview of the CMS Meaningful Measures Initiative. Washington, DC: Centers for Medicare & Medicaid Services; 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Meaningful-Measures_Overview-FactSheet_508_2018-02-28.pdf. Accessed November 10, 2019. [Google Scholar]
- 28.Crowfoot D, Prasad V. Using the plan–do–study–act (PDSA) cycle to make change in general practice. InnovAiT. 2017;10:425–430. doi: 10.1177/1755738017704472 [DOI] [Google Scholar]
- 29.IOM (Institute of Medicine). Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. doi: 10.17226/13444 [DOI] [PubMed] [Google Scholar]
- 30.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. doi: 10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balasubramanian BA, Cohen DJ, Davis MM, Gunn R, Dickinson LM, Miller WL, Crabtree BF, Stange KC. Learning evaluation: blending quality improvement and implementation research methods to study healthcare innovations. Implement Sci. 2015;10:31. doi: 10.1186/s13012-015-0219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bodegom-Vos L, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf. 2017;26:495–501. doi: 10.1136/bmjqs-2016-005473 [DOI] [PubMed] [Google Scholar]
- 33.Brownson RC, Samet JM, Chavez GF, Davies MM, Galea S, Hiatt RA, Hornung CA, Khoury MJ, Koo D, Mays VM, et al. Charting a future for epidemiologic training. Ann Epidemiol. 2015;25:458–465. doi: 10.1016/j.annepidem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury MJ. Planning for the future of epidemiology in the era of big data and precision medicine. Am J Epidemiol. 2015;182:977–979. doi: 10.1093/aje/kwv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foraker RE, Shoben AB, Kelley MM, Lai AM, Lopetegui MA, Jackson RD, Langan MA, Payne PR. Electronic health record-based assessment of cardiovascular health: the Stroke Prevention in Healthcare Delivery Environments (SPHERE) study. Prev Med Rep. 2016;4:303–308. doi: 10.1016/j.pmedr.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sperl-Hillen JM, Rossom RC, Kharbanda EO, Gold R, Geissal ED, Elliott TE, Desai JR, Rindal DB, Saman DM, Waring SC, et al. Priorities Wizard: multisite web-based primary care clinical decision support improved chronic care outcomes with high use rates and high clinician satisfaction rates. EGEMS (Wash DC). 2019;7:9. doi: 10.5334/egems.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ning H, Labarthe DR, Shay CM, Daniels SR, Hou L, Van Horn L, Lloyd-Jones DM. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. 2015;8:164–171. doi: 10.1161/CIRCOUTCOMES.114.001274 [DOI] [PubMed] [Google Scholar]
- 38.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–1376. doi: 10.1161/CIRCULATIONAHA.113.001559 [DOI] [PubMed] [Google Scholar]
- 39.Johnson AM, Moore JE, Chambers DA, Rup J, Dinyarian C, Straus SE. How do researchers conceptualize and plan for the sustainability of their NIH R01 implementation projects? Implement Sci. 2019;14:50. doi: 10.1186/s13012-019-0895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens. 2015;28:699–716. doi: 10.1093/ajh/hpu233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinman M, Woodward EN, Curran GM, Hausmann LRM. Harnessing implementation science to increase the impact of health equity research. Med Care. 2017;55 (suppl 2):S16–S23. doi: 10.1097/MLR.0000000000000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyce CA, Barfield W, Curry J, Shero S, Green Parker M, Cox H, Bustillo J, Price LN. Building the next generation of implementation science careers to advance health equity. Ethn Dis. 2019;29(suppl 1):77–82. doi: 10.18865/ed.29.S1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]


