ABSTRACT
A hallmark of all germ cells is the presence of germ granules: assemblies of proteins and RNA that lack a delineating membrane and are proposed to form via condensation. Germ granules across organisms share several conserved components, including factors required for germ cell fate determination and maintenance, and are thought to be linked to germ cell development. The molecular functions of germ granules, however, remain incompletely understood. In this Development at a Glance article, we survey germ granules across organisms and developmental stages, and highlight emerging themes regarding granule regulation, dynamics and proposed functions.
Keywords: RNA, Condensate, Germ granule, Oocyte, Primordial germ cell, Sperm
Summary: A survey of germ granules across organisms and developmental stages, highlighting emerging themes regarding granule regulation, dynamics and proposed functions.
Introduction
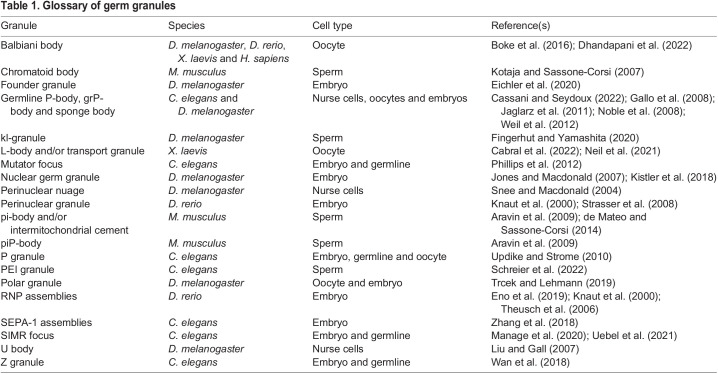
In sexually reproducing organisms, specialized cells called germ cells undergo meiosis to produce gametes, oocytes and sperm, which subsequently fuse to create a zygote. Germ cells contain unique structures known as germ granules that concentrate hundreds of RNAs and RNA-binding proteins, including conserved factors such as small RNA (sRNA) machinery, DEAD-box helicases and mRNAs that are crucial for primordial germ cell (PGC) development (Voronina et al., 2011). PGCs are specified in early development through two different mechanisms: preformation and induction (Hansen and Pelegri, 2021; Strome and Updike, 2015). In organisms that use the preformation mechanism, including C. elegans, D. melanogaster, X. laevis and D. rerio, germ granules assemble in a specialized cytoplasm called germ plasm that is transmitted from oocytes to embryos and asymmetrically partitioned to PCGs. In other organisms, including mammals, PGCs are induced later in development from undifferentiated progenitors, and germ granule components are expressed de novo in newly specified PGCs. Germ granules are therefore a prominent feature of organisms with either inherited germ plasm or induced PGCs. Given the high enrichment of RNA-binding proteins and conservation across diverse organisms, germ granules are proposed to play important roles in RNA regulation to facilitate PGC specification, formation and protection. In this article, we use ‘germ granule’ as a generic, catch-all term for RNA granules (Table 1) unique to germ cells, with the understanding that these comprise different granule types characterized by distinct compositions, including perinuclear granules characteristic of PGCs and immature germ cells, and cytoplasmic granules found in gametes and embryos.
Table 1.
Glossary of germ granules
Potential functions for germ granules in development
Diverse germ granule functions have been proposed based on analysis of granule composition, localization and dynamics, as well as genetic experiments in model organisms. Key proposed functions include: localization of germ cell determinants (Voronina et al., 2011), post-transcriptional RNA regulation (Eichler et al., 2020; Sheth et al., 2010; Updike et al., 2014), epigenetic inheritance and sRNA amplification (Ishidate et al., 2018; Schreier et al., 2022; Wan et al., 2018), sequestration/compartmentalization of RNA degradation, sRNA processing and translation activities (Eichler et al., 2020; Aravin et al., 2009; Wan et al., 2018), storage and protection of translationally repressed mRNAs (Hubstenberger et al., 2013; Jud et al., 2008; Noble et al., 2008), mitochondrial inheritance (Bilinski et al., 2017; Trcek and Lehmann, 2019), assembly and storage of uridine-rich small nuclear ribonucleoproteins (U snRNPs) (Liu and Gall, 2007), and responding to environmental stresses (Buckingham and Liu, 2011; Jud et al., 2008; Lee et al., 2020; Snee and Macdonald, 2009).
However, in most cases, germ granule function is still speculative. A key challenge in assigning function is that germ granule proteins, although concentrated in granules, often also exist at lower concentrations in the cytoplasm, making it challenging to uncouple germ granule-specific functions from the activity of soluble proteins. Methods to visualize biochemical activities in vivo will be needed to demonstrate granule-specific function. In the case of germ granules that form in specific locations in oocytes and embryos, in situ hybridization to visualize granule RNAs has provided strong support for a role in mRNA localization and germ cell fate specification. For example, in D. melanogaster, mislocalization of the granule nucleator Oskar leads to ectopic enrichment of mRNAs encoding germ cell fate regulators and, thus, ectopic induction of PGCs (Ephrussi and Lehmann, 1992).
Material properties of germ granules
Germ granules belong to a class of cellular structures known as biomolecular condensates. Condensates are membraneless assemblies that lack a defined stoichiometry and concentrate biomolecules, most commonly proteins and nucleic acids (Banani et al., 2017). Material properties of biomolecular condensates are distinct from the surrounding cytosol, including higher viscosity, which can either enrich or exclude molecules in a size and property-dependent manner (Nott et al., 2016; Updike et al., 2011). Here, we focus on condensates that are specific to the germline; however, germ cells also contain condensates found in somatic cells, including stress granules, P-bodies and nucleoli (reviewed by Banani et al., 2017). The molecular mechanisms of condensate assembly are still under active investigation and studies of germ granules have played crucial roles in advancing this field. Two predominant mechanisms to describe the behavior of condensates were first described for germ granules.
Liquid-liquid phase separation (LLPS), first described for P granules in C. elegans, is proposed to play a role in the formation of many condensates (Brangwynne et al., 2009). LLPS is a process by which a solution of polymeric molecules spontaneously de-mixes into dense and dilute phases when above a critical concentration. The dense phase has liquid-like properties and enriches specific biomolecules, allowing the dense phase to function as a compartment (Alberti et al., 2019). LLPS is driven by dynamic, multivalent interactions between biomolecules, involving specific binding motifs in proteins and RNA (Banani et al., 2017; Li et al., 2012). Unlike membrane-bound structures, biomolecules in the dense phase can dynamically exchange with the dilute phase and are sensitive to environmental and biological changes, including temperature and post-translational modifications (PTMs) (Brangwynne, 2013).
Some germ granules are built around non-liquid scaffolds that resemble amyloid protein aggregates. For example, Balbiani bodies in X. laevis oocytes are scaffolded by Xvelo, a mostly disordered protein that forms a non-dynamic amyloid-like mesh in a reconstituted system (Boke et al., 2016). Amyloids have historically been studied in the context of neurodegenerative disease and Balbiani bodies were the first condensate to be described as ‘physiological amyloids’ that assemble and disassemble as part of normal development (Boke and Mitchison, 2017). Solid, but non-amyloid, condensates have also been described in the context of P granules in C. elegans, where MEG-3, an intrinsically disordered RNA binding protein, forms RNA-rich clusters on the surface of the liquid core of P granules (Putnam et al., 2019).
Key questions for the field involve understanding how the material properties of a condensate arise from the assembly of individual biomolecules and whether these material properties execute specific cellular tasks. Liquid-like material states can dynamically respond to cellular cues, enabling them to respond to environmental changes that occur on short time scales in early embryogenesis (Wang et al., 2014). In contrast, solid-like material properties could be used to suppress activities and protect biomaterials during times of dormancy, as oocytes can exist for months to years before fertilization (Jamieson-Lucy and Mullins, 2019). In vitro studies have highlighted that liquid-like condensates can mature over time to become more solid (Alberti and Hyman, 2016; Jawerth et al., 2020), suggesting that cells may have active processes to prevent maturation. In support of this idea, loss of the helicase CGH-1 results in the transition of the grP-body component CAR-1 into a solid lattice in the C. elegans germline (Hubstenberger et al., 2013). Future studies are needed to explore the contribution of material state to biological function.
The role of RNA in germ granule dynamics
Nearly all identified germ granules contain RNA and RNA likely plays a crucial role in germ granule formation and material properties. In vitro systems have shown that, independently of protein, RNA can condense into liquid, gel-like or solid structures depending on sequence and length (Jain and Vale, 2017; Tauber et al., 2020; Van Treeck et al., 2018). Several perinuclear condensates, including Mutator foci and P granules in C. elegans, and the chromatoid body of mammalian sperm, assemble near nuclear pore complexes and disassemble when transcription is blocked (Lehtiniemi and Kotaja, 2018; Sheth et al., 2010; Uebel et al., 2020), consistent with a role for nascent transcripts in granule assembly. Condensation of germ granule proteins in vitro is often sensitive to RNA concentration. For example, RNA enhances the condensation of the P granule protein PGL-3 (C. elegans) and the L-body protein PTBP3 (X. laevis) (Cabral et al., 2022; Saha et al., 2016), and at high concentrations can also prevent condensation of the P granule protein MEG-3 (C. elegans) (Lee et al., 2020).
Factors that support mRNA production prevent the solidification of grP-bodies in C. elegans oocytes, suggesting a role for RNA in maintaining granule proteins in a liquid state (Hubstenberger et al., 2013). Similarly, in a reconstituted system, short RNAs decrease the viscosity and increase internal dynamics of condensates formed by the P granule helicase LAF-1 (Elbaum-Garfinkle et al., 2015).
Post-translational modification of germ granule proteins
PTMs have emerged as a versatile mechanism for the spatiotemporal regulation of both somatic condensates and germ granules (Hofweber and Dormann, 2019; Schisa and Elaswad, 2021). Condensation is exquisitely sensitive to the valency of interactions and PTMs can modify valency by creating or occluding binding sites. Moreover, the combinatorial effects of multiple PTMs may act as a tunable mechanism to regulate condensate dynamics. Phosphorylation is a reversible modification where kinases and opposing phosphatases cooperate to regulate condensates. The kinase DYRK3/MBK-2 and phosphatase PP2A play crucial roles in the asymmetric polarization of P granules during embryonic development (Wang et al., 2014). Phosphorylation of MEG proteins by MBK-2 promotes P granule disassembly; additionally, MBK-2-mediated phosphorylation fluidizes the core protein PGL-3 to enable both efficient growth and regulated dissolution of P granules (Folkmann et al., 2021; Wang et al., 2014).
Many germ granule components contain arginine (R)-glycine (G) repeats (e.g. RGG- or RG-rich motifs) that are targeted for methylation (Anne et al., 2007; Kirino et al., 2010; Roovers et al., 2018). Unlike phosphorylation, arginine methylation is a low-dynamic modification and is thought to promote assembly, rather than dissolution, of germ granules. Methylated arginines are recognized by Tudor-domain proteins (Pek et al., 2012). In D. melanogaster, the methyltransferase Capsuléen promotes condensation of Vasa, Tudor and Maelstrom in the nurse cell nuage and facilitates assembly of the oocyte pole plasm via methylation of Sm proteins (Anne et al., 2007). Similarly, in D. rerio, methylation is suggested to promote condensation of Bucky ball to form the Balbiani body by generating binding sites for the Tudor domain-containing protein Tdrd6 (Roovers et al., 2018).
Germ granule architecture
High-resolution imaging has revealed that many germ granules are multilayered and in fact correspond to a collection of condensates with distinct composition and material properties (Fare et al., 2021). A core/shell structure has been observed for embryonic P granules, Z granules, SEPA-1 assemblies, piP bodies and D. melanogaster Tudor/Aubergine condensates (Aravin et al., 2009; Vo et al., 2019; Wan et al., 2021; Wang et al., 2014; Zhang et al., 2018). For both P granules and Z granules, disruption of the shell-forming protein increases condensate size and decreases condensate number (Folkmann et al., 2021; Wan et al., 2021), and also affects the material properties of the condensates (Folkmann et al., 2021; Wan et al., 2021; Zhang et al., 2018). In the case of P granules, the shell-forming protein MEG-3 forms solid clusters that modulate condensate size by decreasing surface tension and recruits the kinase DYRK3/MBK-2 to fluidize the P granule core (Folkmann et al., 2021). In the examples described above, the substructure likely plays a key role in condensate function and/or regulation. In other cases, however, substructure may be a consequence of assembly, as proposed for the core/shell architecture of stress granules (Jain et al., 2016).
RNAs have non-homogeneous distributions in germline condensates, including polar granules in D. melanogaster and RNP assemblies in D. rerio (Eno et al., 2019; Trcek et al., 2015). RNAs in polar granules are organized in homotypic clusters with distinct spatial positioning relative to Vasa-protein condensates (Trcek et al., 2020). Whereas localization to polar granules requires specific RNA regions, the mechanism driving formation of homotypic clusters appears to involve the entire mRNA in a sequence-independent manner.
Non-homogenous distribution has also been noted for proteins, such as Vasa in polar granule precursors and Xvelo in Balbiani bodies (Boke et al., 2016; Jaglarz et al., 2011; Vo et al., 2019). High-resolution imaging studies of condensate components are likely to reveal additional condensate substructures; however, for most germ granules, the assembly, regulation and function of substructure remain unknown.
Germ granule interactions with other granules and membranous organelles
A recurring feature for many germ granules is their ability to dock with other condensates. A prominent example is highlighted in the perinuclear nuage of C. elegans, where sRNA biogenesis factors form multi-condensate assemblages that include P granules, Mutator foci, Z granules and SIMR foci (Manage et al., 2020; Phillips et al., 2012; Wan et al., 2018). Strikingly, each nuage condensate contains a distinct set of proteins involved in sRNA regulation. Docking of germ granule condensates also occurs in spermatogenesis, where piP-bodies associate with pi-bodies to act in related steps of sRNA processing (Aravin et al., 2009). It is intriguing to speculate that such condensate interactions may partition sRNA processing steps to enable organization of pathway intermediates. Future studies are needed to determine the biological significance of condensate docking and the molecular rules that dictate the formation of these condensate assemblages.
Germ granules also contact membrane-bound organelles, including mitochondria, the nucleus, the endoplasmic reticulum and the Golgi. Although some interactions may be due to the crowded nature of the cytoplasm or to the general affinity of condensates for membranes, several of these interactions have clear functional relevance. For example, germ granules across diverse organisms associate stably with the nucleus, often in regions with highly clustered nuclear pore complexes (Voronina et al., 2011). Association with nuclear pore complexes likely facilitates germ granule surveillance of transcripts as they emerge from the nucleus, as nascent transcripts accumulate in perinuclear P granules and the chromatoid body (Sheth et al., 2010; Söderströmm and Parvinen, 1976).
In some cases, interactions with membranous organelles may mediate germ granule localization. For example, interactions with the endoplasmic reticulum have been speculated to mediate translocation of sponge bodies from nurse cells to the oocyte (Jaglarz et al., 2011), and PEI granules may ‘hitchhike’ on fibrous body-membranous organelles (FB-MOs) to be partitioned during spermatogenesis (Schreier et al., 2022). Germ granules across species and developmental stages commonly associate with mitochondria. As mitochondria are maternally inherited, enrichment of healthy mitochondria in the Balbiani body and D. melanogaster germ plasm may prevent passage of damaged mitochondria (Bilinski et al., 2017). Additionally, interaction with mitochondria has been proposed to mediate nucleation of intermitochondrial cement in sperm (Huang et al., 2011; Watanabe et al., 2011). Association with membranes may lower the threshold for condensation, as membrane surfaces restrict protein diffusion to a two-dimensional surface (Snead and Gladfelter, 2019).
Conclusions and perspectives
Germ granules were originally observed by electron microscopy or cytochemistry as amorphous granulo-fibrillar structures (Eddy, 1976; Guraya, 1979). Recent advances in microscopy have revealed an increasing number of diverse granules and it is likely that many germ granules remain to be discovered. Although condensation of biomolecules is a potentially exciting mechanism for the unique requirements of germ cells, the role of most germ granules is still speculative. Ongoing research to dissect the function of condensates from soluble proteins will be crucial. To address this challenge, it will be necessary to better understand the mechanisms of assembly and regulation of germ granules through PTMs and enzymes, including kinases and RNA helicases.
Studies of germ granules in model systems have revealed a remarkable diversity of granule architecture, dynamics, material properties and interactions with other cellular structures. These findings raise many exciting questions regarding the functional relevance of these features. For example, it is speculated that granule material state corresponds to function, yet this proposal has not been conclusively demonstrated. Similarly, although solid granules such as the Balbiani body represent physiological amyloids, whether misregulated condensation leads to disease remains incompletely understood. For many germ granules, the function of elaborate substructure and granule-granule interactions remains unclear; indeed, there is no clear consensus as to what is considered a distinct granule versus the substructure of the same granule.
Finally, although model systems have significantly advanced our understanding of germ granule regulation, studies in mammals remain largely descriptive. Given the conservation of many granule components across species, an important future goal will be to determine whether similar mechanisms regulate germ granules in mammals and organisms with induced PGCs.
Poster
Acknowledgements
We thank G. Seydoux, T. Trcek, A. Scholl, D. Bodas and all members of the Seydoux lab for discussions and helpful feedback on this article.
Footnotes
Funding
This work was supported by the Howard Hughes Medical Institute and the National Institutes of Health (R37HD037047). L.T. is a postdoctoral fellow of the Life Sciences Research Foundation supported by the Howard Hughes Medical Institute. Open access funding provided by the Howard Hughes Medical Institute. Deposited in PMC for immediate release.
Contributor Information
Laura Thomas, Email: tlaura2@jhmi.edu.
Andrea Putnam, Email: aaputnam@wisc.edu.
Andrew Folkmann, Email: andrew.folkmann@vanderbilt.edu.
References
- Alberti, S. and Hyman, A. A. (2016). Are aberrant phase transitions a driver of cellular aging? BioEssays 38, 959-968. 10.1002/bies.201600042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti, S., Gladfelter, A. and Mittag, T. (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419-434. 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne, J., Ollo, R., Ephrussi, A. and Mechler, B. M. (2007). Arginine methyltransferase Capsuléen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134, 137-146. 10.1242/dev.02687 [DOI] [PubMed] [Google Scholar]
- Aravin, A. A., Van Der Heijden, G. W., Castañeda, J., Vagin, V. V., Hannon, G. J. and Bortvin, A. (2009). Cytoplasmic compartmentalization of the Fetal piRNA pathway in mice. PLoS Genet. 5, e1000764. 10.1371/journal.pgen.1000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani, S. F., Lee, H. O., Hyman, A. A. and Rosen, M. K. (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285-298. 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski, S. M., Kloc, M. and Tworzydlo, W. (2017). Selection of mitochondria in female germline cells: is Balbiani body implicated in this process? J. Assist. Reprod. Genet. 34, 1405-1412. 10.1007/s10815-017-1006-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke, E. and Mitchison, T. J. (2017). The balbiani body and the concept of physiological amyloids. Cell Cycle 16, 153-154. 10.1080/15384101.2016.1241605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke, E., Ruer, M., Wühr, M., Coughlin, M., Lemaitre, R., Gygi, S. P., Alberti, S., Drechsel, D., Hyman, A. A. and Mitchison, T. J. (2016). Amyloid-like self-assembly of a cellular compartment. Cell 166, 637-650. 10.1016/j.cell.2016.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne, C. P. (2013). Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 203, 875-881. 10.1083/jcb.201308087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne, C. P., Eckmann, C. R., Courson, D. S., Rybarska, A., Hoege, C., Gharakhani, J., Jülicher, F. and Hyman, A. A. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729-1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Buckingham, M. and Liu, J.-L. (2011). U bodies respond to nutrient stress in Drosophila. Exp. Cell Res. 317, 2835-2844. 10.1016/j.yexcr.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Cabral, S. E., Otis, J. P. and Mowry, K. L. (2022). Multivalent interactions with RNA drive recruitment and dynamics in biomolecular condensates in Xenopus oocytes. iScience 25, 104811. 10.1016/j.isci.2022.104811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani, M. and Seydoux, G. (2022). Specialized germline P-bodies are required to specify germ cell fate in C. elegans embryos. Development 149, dev200920. 10.1101/2022.08.15.504042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mateo, S. and Sassone-Corsi, P. (2014). Regulation of spermatogenesis by small non-coding RNAs: role of the Germ Granule. Semin. Cell Dev. Biol. 29, 84-92. 10.1016/j.semcdb.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani, L., Salzer, M. C., Duran, J. M., Zaffagnini, G., De Guirior, C., Martínez-Zamora, M. A. and Böke, E. (2022). Comparative analysis of vertebrates reveals that mouse primordial oocytes do not contain a Balbiani body. J. Cell Sci. 135, jcs259394. 10.1242/jcs.259394 [DOI] [PubMed] [Google Scholar]
- Eddy, E. M. (1976). Germ plasm and the differentiation of the germ cell line. In International Review of Cytology (ed. Bourne G. H., Danielli J. F. and Jeon K. W.), pp. 229-280. Academic Press. [DOI] [PubMed] [Google Scholar]
- Eichler, C. E., Hakes, A. C., Hull, B. and Gavis, E. R. (2020). Compartmentalized oskar degradation in the germ plasm safeguards germline development. ELife 9, e49988. 10.7554/eLife.49988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle, S., Kim, Y., Szczepaniak, K., Chen, C. C.-H., Eckmann, C. R., Myong, S. and Brangwynne, C. P. (2015). The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 112, 7189-7194. 10.1073/pnas.1504822112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eno, C., Hansen, C. L. and Pelegri, F. (2019). Aggregation, segregation, and dispersal of homotypic germ plasm RNPs in the early zebrafish embryo. Dev. Dyn. 248, 306-318. 10.1002/dvdy.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi, A. and Lehmann, R. (1992). Induction of germ cell formation by Oskar. Nature 358, 387-392. 10.1038/358387a0 [DOI] [PubMed] [Google Scholar]
- Fare, C. M., Villani, A., Drake, L. E. and Shorter, J. (2021). Higher-order organization of biomolecular condensates. Open Biol. 11, 210137. 10.1098/rsob.210137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkmann, A. W., Putnam, A., Lee, C. F. and Seydoux, G. (2021). Regulation of biomolecular condensates by interfacial protein clusters. Science 373, 1218-1224. 10.1126/science.abg7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerhut, J. M. and Yamashita, Y. M. (2020). mRNA localization mediates maturation of cytoplasmic cilia in Drosophila spermatogenesis. J. Cell Biol. 219, e202003084. 10.1083/jcb.202003084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, C. M., Munro, E., Rasoloson, D., Merritt, C. and Seydoux, G. (2008). Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev. Biol. 323, 76-87. 10.1016/j.ydbio.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Guraya, S. S. (1979). Recent advances in the morphology, cytochemistry, and function of Balbiani's vitelline body in animal Oocytes11This article is dedicated to the memory of Professor Vishwa Nath who made outstanding contributions to the cytology of animal gametes. In International Review of Cytology (ed. Bourne G. H., Danielli J. F. and Jeon K. W.), pp. 249-321. Academic Press. [DOI] [PubMed] [Google Scholar]
- Hansen, C. L. and Pelegri, F. (2021). Primordial germ cell specification in vertebrate embryos: phylogenetic distribution and conserved molecular features of preformation and induction. Front. Cell Dev. Biol. 9, 730332. 10.3389/fcell.2021.730332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber, M. and Dormann, D. (2019). Friend or foe—Post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 294, 7137-7150. 10.1074/jbc.TM118.001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., Gao, Q., Peng, X., Choi, S.-Y., Sarma, K., Ren, H., Morris, A. J. and Frohman, M. A. (2011). piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 20, 376-387. 10.1016/j.devcel.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger, A., Noble, S. L., Cameron, C. and Evans, T. C. (2013). Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 27, 161-173. 10.1016/j.devcel.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate, T., Ozturk, A. R., Durning, D. J., Sharma, R., Shen, E., Chen, H., Seth, M., Shirayama, M. and Mello, C. C. (2018). ZNFX-1 functions within perinuclear nuage to balance epigenetic signals. Mol. Cell 70, 639-649.e6. 10.1016/j.molcel.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglarz, M. K., Kloc, M., Jankowska, W., Szymanska, B. and Bilinski, S. M. (2011). Nuage morphogenesis becomes more complex: two translocation pathways and two forms of nuage coexist in Drosophila germline syncytia. Cell Tissue Res. 344, 169-181. 10.1007/s00441-011-1145-2 [DOI] [PubMed] [Google Scholar]
- Jain, A. and Vale, R. D. (2017). RNA phase transitions in repeat expansion disorders. Nature 546, 243-247. 10.1038/nature22386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, S., Wheeler, J. R., Walters, R. W., Agrawal, A., Barsic, A. and Parker, R. (2016). ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487-498. 10.1016/j.cell.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson-Lucy, A. and Mullins, M. C. (2019). The vertebrate Balbiani body, germ plasm, and oocyte polarity. Curr. Top. Dev. Biol. 135, 1-34. 10.1016/bs.ctdb.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Jawerth, L., Fischer-Friedrich, E., Saha, S., Wang, J., Franzmann, T., Zhang, X., Sachweh, J., Ruer, M., Ijavi, M., Saha, S.et al. (2020). Protein condensates as aging Maxwell fluids. Science 370, 1317-1323. 10.1126/science.aaw4951 [DOI] [PubMed] [Google Scholar]
- Jones, J. R. and Macdonald, P. M. (2007). Oskar controls morphology of polar granules and nuclear bodies in Drosophila. Development 134, 233-236. 10.1242/dev.02729 [DOI] [PubMed] [Google Scholar]
- Jud, M. C., Czerwinski, M. J., Wood, M. P., Young, R. A., Gallo, C. M., Bickel, J. S., Petty, E. L., Mason, J. M., Little, B. A., Padilla, P. A.et al. (2008). Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev. Biol. 318, 38-51. 10.1016/j.ydbio.2008.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino, Y., Vourekas, A., Kim, N., De Lima Alves, F., Rappsilber, J., Klein, P. S., Jongens, T. A. and Mourelatos, Z. (2010). Arginine methylation of vasa protein is conserved across Phyla*. J. Biol. Chem. 285, 8148-8154. 10.1074/jbc.M109.089821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler, K. E., Trcek, T., Hurd, T. R., Chen, R., Liang, F.-X., Sall, J., Kato, M. and Lehmann, R. (2018). Phase transitioned nuclear Oskar promotes cell division of Drosophila primordial germ cells. Elife 7, e37949. 10.7554/eLife.37949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut, H., Pelegri, F., Bohmann, K., Schwarz, H. and Nüsslein-Volhard, C. (2000). Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 149, 875-888. 10.1083/jcb.149.4.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja, N. and Sassone-Corsi, P. (2007). The chromatoid body: a germ-cell-specific RNA-processing centre. Nat. Rev. Mol. Cell Biol. 8, 85-90. 10.1038/nrm2081 [DOI] [PubMed] [Google Scholar]
- Lee, C.-Y. S., Putnam, A., Lu, T., He, S., Ouyang, J. P. T. and Seydoux, G. (2020). Recruitment of mRNAs to P granules by condensation with intrinsically-disordered proteins. Elife 9, e52896. 10.7554/eLife.52896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtiniemi, T. and Kotaja, N. (2018). Germ granule-mediated RNA regulation in male germ cells. Reproduction 155, R77-R91. 10.1530/REP-17-0356 [DOI] [PubMed] [Google Scholar]
- Li, P., Banjade, S., Cheng, H.-C., Kim, S., Chen, B., Guo, L., Llaguno, M., Hollingsworth, J. V., King, D. S., Banani, S. F.et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336-340. 10.1038/nature10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.-L. and Gall, J. G. (2007). U bodies are cytoplasmic structures that contain uridine-rich small nuclear ribonucleoproteins and associate with P bodies. Proc. Natl. Acad. Sci. USA 104, 11655-11659. 10.1073/pnas.0704977104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manage, K. I., Rogers, A. K., Wallis, D. C., Uebel, C. J., Anderson, D. C., Nguyen, D. A. H., Arca, K., Brown, K. C., Cordeiro Rodrigues, R. J., De Albuquerque, B. F.et al. (2020). A tudor domain protein, SIMR-1, promotes siRNA production at piRNA-targeted mRNAs in C. elegans. Elife 9, e56731. 10.7554/eLife.56731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil, C. R., Jeschonek, S. P., Cabral, S. E., O'connell, L. C., Powrie, E. A., Otis, J. P., Wood, T. R. and Mowry, K. L. (2021). L-bodies are RNA–protein condensates driving RNA localization in Xenopus oocytes. Mol. Biol. Cell 32, ar37. 10.1091/mbc.E21-03-0146-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, S. L., Allen, B. L., Goh, L. K., Nordick, K. and Evans, T. C. (2008). Maternal mRNAs are regulated by diverse P body–related mRNP granules during early Caenorhabditis elegans development. J. Cell Biol. 182, 559-572. 10.1083/jcb.200802128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott, T. J., Craggs, T. D. and Baldwin, A. J. (2016). Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat. Chem. 8, 569-575. 10.1038/nchem.2519 [DOI] [PubMed] [Google Scholar]
- Pek, J. W., Anand, A. and Kai, T. (2012). Tudor domain proteins in development. Development 139, 2255-2266. 10.1242/dev.073304 [DOI] [PubMed] [Google Scholar]
- Phillips, C. M., Montgomery, T. A., Breen, P. C. and Ruvkun, G. (2012). MUT-16 promotes formation of perinuclear Mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 26, 1433-1444. 10.1101/gad.193904.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, A., Cassani, M., Smith, J. and Seydoux, G. (2019). A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos. Nat. Struct. Mol. Biol. 26, 220-226. 10.1038/s41594-019-0193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers, E. F., Kaaij, L. J. T., Redl, S., Bronkhorst, A. W., Wiebrands, K., De Jesus Domingues, A. M., Huang, H.-Y., Han, C.-T., Riemer, S., Dosch, R.et al. (2018). Tdrd6a regulates the aggregation of Buc into functional subcellular compartments that drive germ cell specification. Dev. Cell 46, 285-301.e9. 10.1016/j.devcel.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S., Weber, C. A., Nousch, M., Adame-Arana, O., Hoege, C., Hein, M. Y., Osborne-Nishimura, E., Mahamid, J., Jahnel, M., Jawerth, L.et al. (2016). Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572-1584.e16. 10.1016/j.cell.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa, J. A. and Elaswad, M. T. (2021). An emerging role for post-translational modifications in regulating RNP condensates in the germ line. Front. Mol. Biosci. 8, 658020. 10.3389/fmolb.2021.658020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier, J., Dietz, S., Boermel, M., Oorschot, V., Seistrup, A.-S., De Jesus Domingues, A. M., Bronkhorst, A. W., Nguyen, D. A. H., Phillis, S., Gleason, E. J.et al. (2022). Membrane-associated cytoplasmic granules carrying the Argonaute protein WAGO-3 enable paternal epigenetic inheritance in Caenorhabditis elegans. Nat. Cell Biol. 24, 217-229. 10.1038/s41556-021-00827-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, U., Pitt, J., Dennis, S. and Priess, J. R. (2010). Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development 137, 1305-1314. 10.1242/dev.044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead, W. T. and Gladfelter, A. S. (2019). The control centers of biomolecular phase separation: how membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 76, 295-305. 10.1016/j.molcel.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snee, M. J. and Macdonald, P. M. (2004). Live imaging of nuage and polar granules: evidence against a precursor-product relationship and a novel role for Oskar in stabilization of polar granule components. J. Cell Sci. 117, 2109-2120. 10.1242/jcs.01059 [DOI] [PubMed] [Google Scholar]
- Snee, M. J. and Macdonald, P. M. (2009). Dynamic organization and plasticity of sponge bodies. Dev. Dyn. 238, 918-930. 10.1002/dvdy.21914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, M. J., Mackenzie, N. C., Dumstrei, K., Nakkrasae, L.-I., Stebler, J. and Raz, E. (2008). Control over the morphology and segregation of Zebrafish germ cell granules during embryonic development. BMC Dev. Biol. 8, 58. 10.1186/1471-213X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S. and Updike, D. (2015). Specifying and protecting germ cell fate. Nat. Rev. Mol. Cell Biol. 16, 406-416. 10.1038/nrm4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderströmm, K.-O. and Parvinen, M. (1976). Transport of material between the nucleus, the chromatoid body and the golgi complex in the early spermatids of the rat. Cell Tissue Res. 168, 335-342. 10.1007/BF00215311 [DOI] [PubMed] [Google Scholar]
- Tauber, D., Tauber, G., Khong, A., Van Treeck, B., Pelletier, J. and Parker, R. (2020). Modulation of RNA condensation by the DEAD-Box protein eIF4A. Cell 180, 411-426.e16. 10.1016/j.cell.2019.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theusch, E. V., Brown, K. J. and Pelegri, F. (2006). Separate pathways of RNA recruitment lead to the compartmentalization of the zebrafish germ plasm. Dev. Biol. 292, 129-141. 10.1016/j.ydbio.2005.12.045 [DOI] [PubMed] [Google Scholar]
- Trcek, T. and Lehmann, R. (2019). Germ granules in Drosophila. Traffic 20, 650-660. 10.1111/tra.12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek, T., Grosch, M., York, A., Shroff, H., Lionnet, T. and Lehmann, R. (2015). Drosophila germ granules are structured and contain homotypic mRNA clusters. Nat. Commun. 6, 7962. 10.1038/ncomms8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek, T., Douglas, T. E., Grosch, M., Yin, Y., Eagle, W. V. I., Gavis, E. R., Shroff, H., Rothenberg, E. and Lehmann, R. (2020). Sequence-independent self-assembly of germ granule mRNAs into homotypic clusters. Mol. Cell 78, 941-950.e12. 10.1016/j.molcel.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebel, C. J., Agbede, D., Wallis, D. C. and Phillips, C. M. (2020). Mutator foci are regulated by developmental stage, RNA, and the germline cell cycle in Caenorhabditis elegans. G3 10, 3719-3728. 10.1534/g3.120.401514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebel, C. J., Manage, K. I. and Phillips, C. M. (2021). SIMR foci are found in the progenitor germ cells of C. elegans embryos. MicroPubl Biol. 10.17912/micropub.biology.000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike, D. and Strome, S. (2010). P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31, 53-60. 10.2164/jandrol.109.008292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike, D. L., Hachey, S. J., Kreher, J. and Strome, S. (2011). P granules extend the nuclear pore complex environment in the C. elegans germ line. J. Cell Biol. 192, 939. 10.1083/jcb.201010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike, D. L., Knutson, A. K., Egelhofer, T. A., Campbell, A. C. and Strome, S. (2014). Germ-granule components prevent somatic development in the C. elegans Germline. Curr. Biol. 24, 970-975. 10.1016/j.cub.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck, B., Protter, D. S. W., Matheny, T., Khong, A., Link, C. D. and Parker, R. (2018). RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. USA 115, 2734-2739. 10.1073/pnas.1800038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo, H. D. L., Wahiduzzaman, Tindell, S. J., Zheng, J., Gao, M. and Arkov, A. L. (2019). Protein components of ribonucleoprotein granules from Drosophila germ cells oligomerize and show distinct spatial organization during germline development. Sci. Rep. 9, 19190. 10.1038/s41598-019-55747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina, E., Seydoux, G., Sassone-Corsi, P. and Nagamori, I. (2011). RNA granules in germ cells. Cold Spring Harb. Perspect. Biol. 3, a002774-a002774. 10.1101/cshperspect.a002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, G., Fields, B. D., Spracklin, G., Shukla, A., Phillips, C. M. and Kennedy, S. (2018). Spatiotemporal regulation of liquid-like condensates in epigenetic inheritance. Nature 557, 679-683. 10.1038/s41586-018-0132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, G., Bajaj, L., Fields, B., Dodson, A. E., Pagano, D., Fei, Y. and Kennedy, S. (2021). ZSP–1 is a Z granule surface protein required for Z granule fluidity and germline immortality in Caenorhabditis elegans. EMBO J. 40, e105612. 10.15252/embj.2020105612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T., Chuma, S., Yamamoto, Y., Kuramochi-Miyagawa, S., Totoki, Y., Toyoda, A., Hoki, Y., Fujiyama, A., Shibata, T., Sado, T.et al. (2011). MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 20, 364-375. 10.1016/j.devcel.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. T., Smith, J., Chen, B.-C., Schmidt, H., Rasoloson, D., Paix, A., Lambrus, B. G., Calidas, D., Betzig, E. and Seydoux, G. (2014). Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. eLife 3, e04591. 10.7554/eLife.04591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil, T. T., Parton, R. M., Herpers, B., Soetaert, J., Veenendaal, T., Xanthakis, D., Dobbie, I. M., Halstead, J. M., Hayashi, R., Rabouille, C.et al. (2012). Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 14, 1305-1313. 10.1038/ncb2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G., Wang, Z., Du, Z. and Zhang, H. (2018). mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174, 1492-1506.e22. 10.1016/j.cell.2018.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.