Abstract
New diagnostic criteria for myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) have recently been proposed, distinguishing this syndrome from other inflammatory diseases of the central nervous system. Seropositivity status for MOG-IgG autoantibodies is important for diagnosing MOGAD, but only in the context of robust clinical characterization and cautious interpretation of neuroimaging. Over the last several years, access to cell-based assay (CBA) techniques has improved diagnostic accuracy, yet the positive predictive value of serum MOG-IgG values varies with the prevalence of MOGAD in any given patient population. For this reason, possible alternative diagnoses need to be considered, and low MOG-IgG titers need to be carefully weighted. In this review, cardinal clinical features of MOGAD are discussed. Key challenges to the current understanding of MOGAD are also highlighted, including uncertainty regarding the specificity and pathogenicity of MOG autoantibodies, the need to identify immunopathologic targets for future therapies, the quest to validate biomarkers that facilitate diagnosis and detect disease activity, and the importance of deciphering which patients with MOGAD require long-term immunotherapy.
Keywords: Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), Inflammatory demyelinating diseases (IDDs), Neuro-ophthalmology, Ophthalmology, Neurology, MOG-IgG
Introduction
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a relatively new addition to the category of central nervous system (CNS) inflammatory demyelinating diseases [1, 2]. CNS inflammatory demyelinating conditions, including multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD), are differentiated based on severity, clinical phenotype, imaging, laboratory, and pathological findings [2] (Table 1). While patients with MS, MOGAD and NMOSD may present with similar clinical manifestations, such as optic neuritis and myelitis, those with MOGAD lack a clear sex predilection, and more commonly experience a monophasic course [2–5]. MOGAD also has the greatest predilection in children, representing 20–30% of inflammatory CNS syndromes in this population as compared to approximately 5% in adults. The current estimated range of incidence in the pediatric population is 3.1 per 1 million, as compared to 1.6 and 2.39 per 1 million among adults [3]. Notably, these numbers, along with the estimated worldwide prevalence of 20 million [5], are likely to increase with growing recognition of the disease and improved availability of serological testing. Unlike NMOSD, cases of MOGAD are not strongly associated with other systemic autoimmune disorders or circulating autoantibodies [3]. Yet, disease manifestations may occur after prodromal infections, particularly those caused by viral pathogens, such as influenza, Epstein–Barr virus, herpes simplex virus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), to name a few [3]. Occasionally, patients with MOGAD have an overlap syndrome with anti-NMDA receptor encephalitis, characterized by clinical features of demyelination associated with encephalopathy, seizures, dyskinesias, or psychosis [5]. As the clinical spectrum of MOGAD continues to expand, so too does our appreciation for diagnostic and management challenges associated with this enigmatic condition. Key areas of ongoing research include determining the specificity and pathogenicity of MOG autoantibodies, identifying immunopathologic targets for future therapies, discovering and validating biomarkers that detect disease activity, and deciphering which patients with MOGAD require long-term immunotherapy.
Table 1.
| Patient Profile | Clinical Features | Serum autoantibody | Radiology | Long-term Treatment | |
|---|---|---|---|---|---|
| MOGAD | Less race or sex predilection than MS or NMOSD | Recurrent or isolated attacks of ON, myelitis, ADEM, cerebral monofocal or multifocal deficits, brainstem or cerebellar deficits, cerebral cortical encephalitis often with seizures. Disability is driven by attacks without a progressive phase | Serum MOG-IgG targets the MOG protein located on the outermost myelin sheath layers and oligodendrocyte cell surface | MOGAD is often associated with large or longitudinally extensive lesions of the brain, spine, or optic nerve. Cranial lesions are sometimes called “fluffy” because the margins are poorly defined compared to the well circumscribed lesions seen in MS. Longitudinal intra-orbital optic nerve lesions are seen with T2, STIR, and gadolinium enhanced T1-weighted MRI, often with perineural enhancement. Myelitis cases demonstrate more frequent involvement of the lower cord (including the conus) than NMOSD and often involve the central gray matter causing the so-called “H-sign”. Cerebellar peduncle involvement is a helpful distinguishing feature of MOGAD. MOGAD MRI lesions tend to resolve over time | Off label immunotherapies for relapsing cases |
| MS | Mean age of onset is 32 years. More common in patients of white European descent with a female to male ratio of 3:1 | ON, myelitis, brainstem syndromes, cerebellar dysfunction with relapsing and progressive components of disability accrual | There is no serum autoantibody | MS lesions are often hyper-intense on T2 and FLAIR MRI involving the periventricular, juxta-cortical, infra-tentorial and spine regions. Gadolinium enhancement indicates active disease. Relative to MOGAD and NMOSD, ON lesions tend to be shorter and unilateral, often affecting the intra-orbital optic nerve segments. Spinal lesions are also shorter and affect peripheral regions of the cord with nodular enhancement. The so-called “central vein” sign, a brain lesion characterized by a central area (dot or line) of hypo-intensity with T2 hyper-reflective white matter lesions (in 2 planes), is helpful in distinguishing MS cases from MOGAD | Approved MS disease modifying therapies |
| NMOSD | Mean age of onset is 40 years. More common in patients of Asian, Afro-Caribbean descent. Female-to-male ratio is 9:1 for AQP4-IgG seropositive cases | Recurrent ON, myelitis, area postrema syndrome, cerebral syndromes, narcolepsy and diencephalic syndromes, acute brainstem syndromes. Disability is driven by attacks without a progressive phase | AQP4-IgG binds to AQP4 on astrocytic foot processes | Brain MRI often shows normal findings or nonspecific white matter lesions. ON may manifest with a T2-hyperintense lesion or T1-weighted gadolinium-enhancing lesion extending over more than half the optic nerve length, involving the intracranial optic nerve segments and optic chiasm. Acute myelitis may be associated with LETM on MRI (lesions that extend over 3 contiguous segments) or 3 contiguous segments of focal spinal cord atrophy. Area postrema syndrome may show associated dorsal medulla/area postrema lesions. Acute brainstem syndromes may associate with peri-ependymal lesions | Relapses are common and long-term immunosuppression is required. Previously off label use of many agents was the mainstay of therapy, but targeted therapies approved for use in seropositive NMOSD include eculizumab, satralizumab, and inebilizumab |
ON optic neuritis, ADEM acute disseminated encephalomyelitis, STIR short tau inversion recovery, MRI magnetic resonance imaging, FLAIR fluid-attenuated inversion recovery, LETM longitudinal extensive transverse myelitis
MOGAD: the evolving clinical spectrum
Our growing appreciation of the full clinical spectrum of MOGAD will likely mirror the NMOSD experience. Once considered to be a severe form of MS targeting the optic nerves and spinal cord, NMOSD has since been recognized as a distinct autoimmune astrocytopathy with pathognomonic clinical features [2, 6]. Similarly, MOGAD has now been identified as a separate entity from both MS and NMOSD. Recently, diagnostic criteria proposed by an international panel of experts highlight optic neuritis, myelitis, acute disseminated encephalomyelitis (ADEM), cerebral mono-focal or multifocal deficits, brainstem or cerebellar syndromes, and cerebral cortical encephalitis (often with seizures) as cardinal features of MOGAD (Tables 2 and 3) [5]. Unlike MS, neurological deterioration does not typically progress in the absence of relapses [5]. In real-world settings, there will be challenges in diagnosing MOGAD despite having clearly proposed criteria for the disease, because, as mentioned, many clinical manifestations of MOGAD overlap with other CNS inflammatory syndromes, including but not limited to MS and NMOSD. Diagnostic confusion may also arise from the interpretation of radiological and serological findings since these features may all be caused by other etiologies and are not specific to MOGAD. For this reason, it will be important to not overweigh any single finding in isolation. As a rule of thumb, clinical features of MOGAD, particularly acute optic neuritis, may closely resemble those of NMOSD with severe vision loss (often bilateral) at its nadir. Patients with MOGAD, however, often show greater therapeutic response to high-dose corticosteroid treatment and may also demonstrate significant spontaneous improvement [7–10]. Importantly, the phenotypic expression of MOGAD may vary with age, such that ADEM-like lesions are more likely to affect children, whereas optic neuritis and isolated myelitis tend to be more common among adults [5]. In addition, relapse risk in MOGAD patients is often higher in adults than children [3].
Table 2.
| Clinical Feature | Distinguishing Characteristics | Key Diagnostic Pearls |
|---|---|---|
| Optic Neuritis | ON is the most frequent manifestation of MOGAD, and recurrent ON occurs in 30–50% of cases. Incident cases may be unilateral or bilateral, but bilateral simultaneous optic nerve involvement is more likely to be seen in MOGAD than MS. Vision loss is usually severe at the nadir and is often heralded by pain or headache. Moderate to severe optic disk edema is more common with MOGAD than MS or NMOSD. MOGAD ON is typically steroid responsive and is associated with good recovery, despite OCT evidence of significant neuro-axonal injury. It is reasonable to test for MOG-IgG in ON cases characterized by bilateral simultaneous optic nerve involvement, recurrent ON events, quick steroid responsivity, and/or significant optic disk swelling. The propensity for MOG-IgG in pediatric cases (up to 50%) makes serological testing a reasonable component of the routine evaluation for ON in this patient population | Optic neuritis may be over-diagnosed if critical features of the history and examination are not interpreted correctly. It is important to clarify whether patient have recurrent patterns of pain that suggest an underlying primary headache disorder, since overweighting pain in the absence of vision loss has been identified as a cause of overdiagnosis in patients referred for ON evaluation. It is also imperative to look for the presence of a RAPD in unilateral or asymmetrically severe ON cases. Mimics for MOGAD ON include ON related to MS, NMOSD, or other etiologies. Anterior ischemic optic neuropathy is far more common than MOGAD ON and presents with similar features of optic disk edema. Cases of non-arteritic AION however are frequently not associated with pain, affect older patients, do not demonstrate MRI enhancement of the optic nerve, and show less robust visual recovery. Leber hereditary optic neuropathy may present sporadically with pseudo-optic disk edema and severe vision loss. Yet, vision loss in LHON is painless and generally not associated with MRI enhancement of the optic nerve. Similarly, drugs including immune checkpoint inhibitors, and TNF alpha blockers may cause optic nerve injury, and must be checked for during history-taking. Inflammatory optic neuropathies caused by sarcoid and other systemic diseases may mimic MOGAD ON. Lymphomatous optic neuropathy may present with optic disk edema and show acute steroid responsivity. Fulminant IIH, particularly in children could be mistaken for MOGAD. To determine MOGAD diagnosis, symptoms derived from history, and careful review of MRI (and venography) imaging features will be critical to avoid delaying appropriate management |
| Acute Myelitis | Cases of MOGAD myelitis may be preceded by a viral illness. Patients may experience weakness, a sensory level, bladder/bowel dysfunction, and (over time) spasticity. Motor recovery tends to be relatively good, but sexual and sphincter dysfunction may persist. In 10% of MOGAD myelitis cases, the MRI may appear normal at symptom onset. Conversely, patients with MOGAD may have LETM lesions like NMOSD; spinal lesions may involve any part of the cord from the medulla to the conus, and shorter segmental lesions may occur. A minority of individuals may manifest features resembling acute flaccid myelitis (with areflexia) instead of the more common upper motor neuron syndrome. Gadolinium enhancement is uncommon and subtle when present. One imaging feature that may help diagnose MOGAD myelitis is disproportionate spinal cord gray matter involvement, visualized as an “H pattern” in the axial plane and a linear central T2 hyper-intensity in the sagittal plane. In contrast to MS, a burden of asymptomatic spinal cord lesions is relatively uncommon. Spinal imaging abnormalities often resolve after clinical recovery which is a distinguishing feature of MOGAD | Acute myelitis may be caused by a myriad of CNS inflammatory syndromes including sarcoidosis, MS, NMOSD and tumors. Cases of imaging negative myelitis have been not only been associated MOGAD, but also with COVID infection. Longitudinal T2 weighted MRI spinal cord lesions have also been described in other etiologies, such as LHON and biotinidase deficiencies. MOGAD myelitis cases differ from enterovirus D68–associated acute flaccid myelitis, because the former tend to respond well to short-term immunotherapy |
| Cerebral Cortical Encephalitis (Often with Seizures) | MOGAD patients may present with focal encephalitis characterized by fever, decreased consciousness, seizures, and focal weakness. Patients may manifest the FLAMES (unilateral cortical FLAIR hyper-intense Lesions in Anti-MOG-associated Encephalitis with Seizures) phenotype. MRI may also reveal enhancement of overlying meninges | In the setting of suspected encephalitis patients should be evaluated for other infectious and inflammatory causes, including anti-NMDA receptor encephalitis given that patients may have an overlap syndrome with dual serum positivity to MOG-IgG and NMDA receptors antibodies |
| ADEM | ADEM is the most common presentation of MOGAD in young children and MOG-IgG may be found in up to half of children presenting with this clinical phenotype. An infectious prodrome may precede onset of ADEM manifestations. Clinical findings include fever, headaches, irritability, fatigue, encephalopathy, seizures, brainstem/cerebellar dysfunction, and other neurologic symptoms. Clinical and radiological features typically resolve in the majority of MOGAD patients, but deficits may persist in ADEM patients who develop MRI features of a leukodystrophy | ADEM is the most common presenting feature of MOGAD in children but represents only 5–6% of adult presentations. Regardless of age, other causes of encephalopathy should be excluded in cases of ADEM. Since children with MOGAD are highly likely to present with ADEM, it is reasonable to test for MOG-IgG in pediatric patients presenting with encephalitic or encephalopathic features, particularly those aged less than 12 years |
| Autoimmune/Atypical Encephalitis | Some MOGAD patients do not fulfill ADEM criteria or exhibit a clear-cut focal encephalitis syndrome (FLAMES). Instead, these patients may present with altered consciousness, seizures, or a brainstem syndrome. Rarely, patients may exhibit minimal brain MRI changes, despite dramatic clinical phenotypes that may include refractory status epilepticus. For this reason, it is reasonable to check for MOG-IgG in cases of suspected autoimmune or atypical encephalitis | The usual suspects for infectious (bacterial, viral, and fungal) encephalitis, paraneoplastic syndromes and other autoimmune encephalitis subtypes should be considered as part of the diagnostic evaluation |
| CLIPPERS | MOGAD may (rarely) show a predilection for pontine involvement. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) has been associated with MOGAD. The diagnosis requires evidence of subacute pontocerebellar dysfunction, without peripheral nerve involvement, that is responsive to steroids. On MRI, small, homogeneous gadolinium-enhancing nodules are visible in the pons and cerebellum with matching T2 lesions. Spinal cord lesions may also be seen. Gadolinium enhancement resolves with steroid therapy | Many cases of CLIPPERS remain idiopathic. Other causes of a CLIPPERS-like phenotype include MS, primary angiitis of the CNS, primary CNS lymphoma, and CNS lymphomatoid granulomatosis |
| Brainstem and Cerebellar Syndromes | Brainstem involvement is seen in up to 30% of MOGAD patients and may be a risk factor for a higher disability over time. Patients may present with weakness, cranial nerve deficits (diplopia in particular), ataxia, hypoventilation syndrome, and impaired consciousness. Area postrema syndrome (APS), while much rarer than in NMOSD, has also been described in MOGAD patients, but is usually not found in isolation. Finally, MOGAD can mimic infective rhomboencephalitis when a patient presents with fever, CSF leukocytosis, brainstem enhancing lesions and leptomeningeal enhancement | Brainstem and cerebellar lesions are seen in numerous CNS inflammatory disorders including MS and NMOSD. It is important to exclude other potential causes. Isolated attacks involving the brainstem, cerebellum, or both may be less frequent in MOGAD than MS, but not different to that seen in NMOSD. The presence of diffuse middle cerebellar peduncle MRI lesions favors MOGAD over other contending etiologies. Involvement of the brainstem, cerebellum, or both is common in MOGAD but usually occurs as a component of a multifocal CNS attack rather than in isolation |
| Cerebral Monofocal or Multifocal Demyelinating Lesions with Clinical Deficits | Examples of mono-focal or multifocal deficits may be accompanied by MRI T2 hyper-intense lesions in the middle cerebellar peduncle, around the fourth ventricle, supratentorial white matter, cortical juxtacortical regions, and deep gray nuclei. Tumefactive lesions may occur | This category of deficits included in the proposed criteria for MOGAD is somewhat nonspecific. For this reason, a high degree of vigilance is needed to exclude alternative diagnoses. Well circumscribed lesions on MRI in a pattern consistent with MS, particularly when accompanied by CSF OCB would be atypical for MOGAD and suggest MS as an alternative diagnosis |
| Papilledema and Raised Intracranial Pressure | Rarely MOGAD has been associated with a syndrome of raised intracranial pressure with vision loss. Raised ICP can occur in the context of ADEM, and tumefactive lesions may rarely lead to subfalcine and tentorial herniation | Mechanisms of raised intracranial pressure include inflammation, cerebral venous sinus thrombosis, mass effect or coincidental IIH. Understanding the mechanism will inform the most suitable therapeutic strategy |
| Progressive Leukodystrophies | Rarely, patients (usually pediatric) seropositive for MOG-IgG present with cranial MRI features suggestive on an inherited leukodystrophy. Immunotherapy may help stabilize these cases | Leukodystrophies can be caused by a myriad of causes including genetic disorders, infections, drug effects, mitochondrial syndromes, and metabolic dysregulation, and alternative causes need to be ruled out. Patients with a history of immunosuppression need to be evaluated for PML |
| Peripheral Nervous System Syndromes | AIDP, myeloradiculitis, multifocal motor neuropathy, brachial neuritis, migrant sensory neuritis, combined central and peripheral demyelination, and paresthesias with limb pain have been rarely reported in MOGAD patients with CNS findings | The differential for these entities is quite broad. Investigations need to be tailored to exclude more common causes of these syndromes |
| CNS Vasculitis Association | Rare cases of histologically proven CNS vasculitis associated with seropositive MOG-IgG status have been reported. Affected individuals presented with fever, headache, confusion, and focal neurologic deficits | The strength of this association is not entirely clear, yet a strong association with MOG-IgG could help target immunotherapy |
| Uveitis | Rare cases of anterior, intermediate, and posterior uveitis, affecting one or both eyes have been linked to MOGAD | The differential diagnosis for uveitis is broad, and many cases remain idiopathic. A standard uveitic work-up to exclude other causes (TB, sarcoid, syphilis, HSV, CMV, VZV, inflammatory bowel disease, lymphoma, HLA-B27-related disease, toxoplasmosis) should be considered |
| Cranial Neuropathies | Cranial neuropathy is an emerging clinical phenotype in MOGAD, and patients may have features of both central and peripheral nervous system involvement, with the trigeminal nerve being the most affected nerve | Cranial neuropathies are common with ischemic, inflammation, compression, and demyelinating etiologies in the differential diagnosis. MOG-IgG antibody testing in patients with cranial neuropathies is warranted in the appropriate clinical setting. However, because this is a rare MOGAD phenotype, caution in concluding a diagnosis of MOGAD is recommended, especially if the MOG-IgG titer is low. Alternative diagnoses must be ruled out |
| Ocular Flutter/Opsoclonus Myoclonus (OMS) | OMS is a rare autoimmune neurological entity caused by antibodies against specific surface antigens on neuronal cells. Features include rapid multidirectional conjugate eye movement, ataxia, and myoclonus. OMS has been rarely reported in MOGAD | MOGAD has been implicated in OMS and serum MOG-IgG should be included in the diagnostic evaluation, in the appropriate clinical setting. Other causes of OMS including tumors, paraneoplastic conditions, and autoimmune processes – all of which need to be excluded |
|
Non-Optic Nerve Ophthalmic Issues |
Ulcerative keratitis, acute macular neuro-retinopathy, neuro-retinitis, venous stasis retinopathy, large pre-retinal macular hemorrhage, and orbital inflammatory syndromes have been reported in MOGAD | It is possible that more non-optic nerve-related findings may be associated with seropositive MOG-IgG status. Alternatively, a bystander effect of MOG seropositivity also needs to be considered in the evaluation of these conditions |
ON optic neuritis, ADEM acute disseminated encephalomyelitis, OCT Optical coherence tomography, MRI magnetic resonance imaging, FLAIR fluid-attenuated inversion recovery, LETM longitudinal extensive transverse myelitis, TNF alpha Tumor necrosis factor alpha, FLAMES Lesions in Anti-MOG-associated Encephalitis with Seizures, LHON Leber hereditary optic neuropathy, CLIPPERS chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids, APS area postrema syndrome, CNS central nervous System, CSF cerebral spinal fluid, OCB oligo-clonal banding, ICP intracranial pressure, PE plasma exchange, IVIG = intravenous immune globulin, MS multiple sclerosis, PML progressive multifocal leukoencephalopathy, IIH idiopathic intracranial hypertension, PNS peripheral nervous system, TB tuberculosis, HSV herpes simplex virus, CMV cytomegalovirus, VZV varicella zoster virus, HLA-B27 human leukocyte antigen B27, OMS ocular flutter/opsoclonus myoclonus
Table 3.
Proposed MOGAD diagnostic criteria by an international panel of experts. This table is adapted from Banwell et al., 2023 [5]
| MOGAD diagnosis necessitates the fulfillment of 1, 2 and 3 | |||
|---|---|---|---|
| 1.Clinical demyelinating event |
Optic neuritis Myelitis ADEM Cerebral, brainstem or cerebellar deficits Cerebral cortical encephalitis |
||
| 2. Positive MOG-IgG test | Serum cell-based assay | Clear positive [titer ≥ 1:100] | No additional supporting features required |
| Low positive [titer ≥ 1:10 and < 1:100] |
A and B must be true: A) AQP4-IgG seronegative B) one or more supporting clinical or MRI features ψ |
||
| Positive without reported titer | |||
| Serum negative but CSF positive | |||
| 3. Exclusion of better diagnoses, including MS | |||
| Ψ Supporting clinical and MRI features | |||
|---|---|---|---|
| Optic neuritis |
Simultaneous bilateral optic nerve involvement Longitudinal optic nerve involvement [> 50% of the optic nerve length] Optic disk edema/swelling Perineural optic sheath enhancement |
||
| Myelitis |
Longitudinally extensive myelitis Conus lesion H-sign or central cord lesion |
||
| Brain, brain stem, or cerebral syndrome |
Deep gray matter involvement Multiple ill-defined T2 hyper-intense lesions in the supra-tentorial and infra-tentorial white matter Cortical lesion with or without lesional and overlying meningeal enhancement Ill-defined T2-hyperintensity involving medulla, pons, or middle cerebellar peduncle |
||
MOGAD MOG antibody-associated disease, ADEM acute disseminated encephalomyelitis, MRI magnetic resonance imaging, AQP4 aquaporin 4, CSF cerebral spinal fluid, MS multiple sclerosis
MOG autoantibodies: specificity, pathogenicity, and interpreting titers
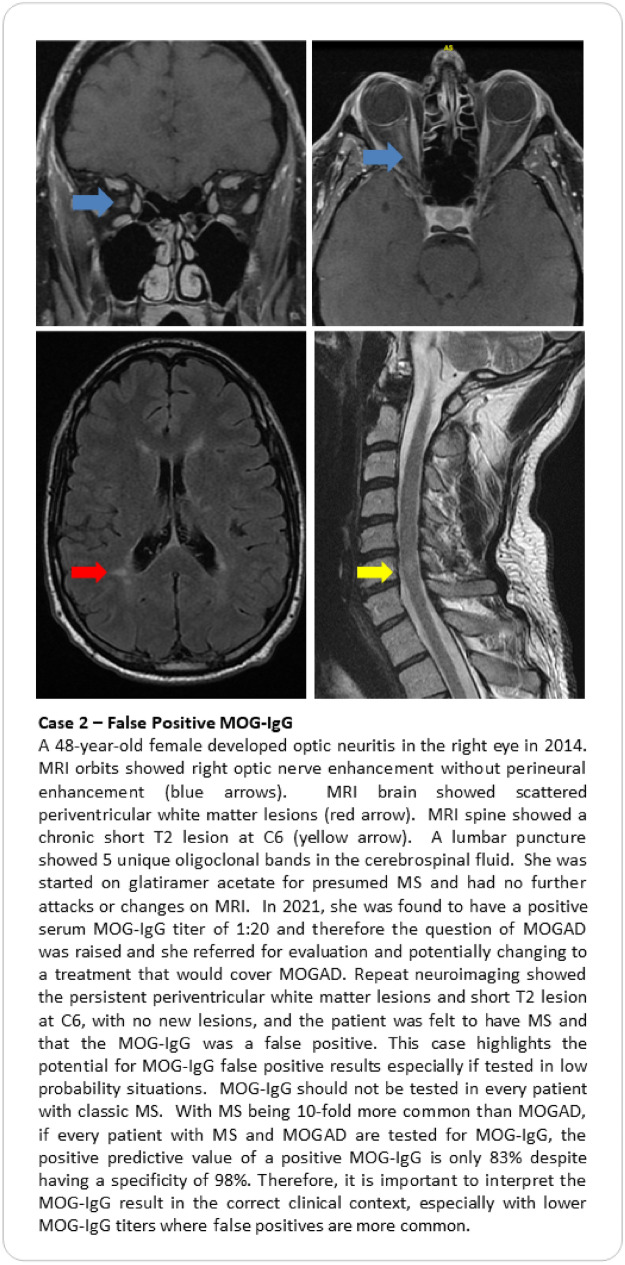
Seropositivity status for MOG-IgG autoantibodies is key to applying the recently proposed criteria for MOGAD (Table 3) [5]. It is also imperative to exclude alternate diagnoses, just as we do for patients with suspected MS or NMOSD. Without rigorous clinical characterization and thoughtful deliberation, individuals with true MOGAD run the risk of being under-treated, whereas those without MOGAD might be inappropriately exposed to long-term immunotherapies or incorrect therapies (Case vignettes here contrasting two scenarios of overlooked MOGAD [Case 1] and false positive [Case 2]). As highlighted by the recently proposed MOGAD criteria [5], there are challenges to relying on a serum MOG-IgG antibody with imperfect specificity and uncertain pathogenicity because causation can be confused with association. Notably, cell-based assays (CBA) are used to establish a MOGAD diagnosis, with live CBA techniques performing slightly better than fixed-based assays. Importantly, enzyme-linked immunosorbent assay (ELISA) testing should not be used to make a diagnosis, as findings are less specific and often discordant with CBA results [32]. While access to CBA techniques has improved diagnostic accuracy, the positive predictive value (the probability that a patient with a positive serum MOG-IgG result harbors MOGAD) still varies with the prevalence of MOGAD in any given patient population. Investigators at the Mayo Clinic have reported a positive predictive value (PPV) of MOG-IgG testing by live CBA of 72% using a cut-off of 1:20 despite having an overall specificity of 98% [33]. Their study found there was a positive correlation between serum MOG-IgG titer cut-off value and PPV, such that the PPV was 100% for 1:1000, 82% for 1:100, 51% for antibody titers 1:20–1:40 [33]. Notably, a ≥ 1:40 titer “cut-off,” (n = 65) yielded a PPV of 93.8%, but lowered sensitivity [34]. Subsequently, Manzano and colleagues also performed an institutional cohort study to determine the PPV of serum MOG-IgG for clinically defined MOGAD, using the same live MOG-IgG CBA [34]. Among 1,877 patients tested, 78 (4.2%) patients tested positive for MOG-IgG with titer ≥ 1:20; of those who were seropositive, 67 had a validated MOGAD diagnosis, yielding a PPV of 85.9%. Undoubtedly, low positive titers need to be interpreted with caution. Yet, it is worth mentioning that excluding patients with MOG IgG titers of 1:20 without proper clinical analysis may lead to a significant number of missed diagnoses of MOGAD [33–35], further reiterating the need for thoughtful clinical judgment. Ideally, patients should be tested for MOG-IgG sero-status at their incident event, and when possible, before administration of corticosteroids, immunoglobulins, or apheresis [5]. When there are concerns regarding a false positive MOG-IgG result, repeat serum testing should be performed after 3 months or at the time of relapse [5]. Finally, like aquaporin 4 (AQP4)-IgG, MOG autoantibodies are induced in the peripheral circulation, though the triggering cause for their production remains unclear [2, 4]. Serological testing with CBA techniques is more sensitive for detecting MOG autoantibodies than CSF sampling. Occasionally, patients with MOGAD may have negative serum MOG-IgG results, but show seropositivity with CSF testing [5, 36, 37]. For this reason, it is reasonable to consider CSF testing for MOG-IgG to support the diagnosis of MOGAD when the serum results are negative, but the clinical and MRI findings are otherwise highly suggestive of the diagnosis [36–40].
When considering specificity, MOG autoantibodies are uncommon in patients with MS (0.4%) [4]. However, because of the high prevalence of MS compared to MOGAD, many false positive results may occur if all patients with possible MS are tested for MOG-IgG, therefore discretion is required [33]. Co-existing seropositivity for MOG-IgG is also uncommon in cases of AQP4-IgG-positive NMOSD; when this occurs, AQP4 IgG titers are often higher, and the patient usually follows a NMOSD phenotype [5, 41]. Yet the high prevalence of MOG autoantibodies among patients with AQP4-IgG-seronegative NMOSD has created some diagnostic confusion, such that MOGAD patients have been erroneously labeled as seronegative NMOSD. With the newly proposed MOGAD criteria (Table 3), patients with presenting features of NMOSD who are negative for AQP4-IgG should be tested for MOG-IgG, as part of their diagnostic evaluation for MOGAD [5]. In fact, with more accessible use of reliable CBA for MOG-IgG, rigorous classification of clinical phenotypes and improved understanding of pathognomonic radiological findings, many patients previously diagnosed with autoimmune demyelinating diseases including seronegative NMOSD, atypical MS, chronic relapsing inflammatory optic neuropathy (CRION), and ADEM may be re-classified as MOGAD.
Going forward, it will be important to consider whether clinical manifestations in patients with suspected MOGAD reflect pathogenic effects of MOG autoantibodies, as opposed to a scenario in which MOG-IgG seropositivity more likely represents a “bystander” phenomenon [42]. The pathogenicity of MOG autoantibodies is the subject of ongoing debate. In vivo and in vitro studies suggest that MOG autoantibodies may cause primary demyelination in the CNS with loss of microtubule cytoskeleton in oligodendrocytes and altered expression of proteins [3, 4]. Evidence of the pathogenicity of MOG autoantibodies has also been inferred from models of experimental autoimmune encephalitis (EAE) [3, 4]. Specifically, serum from individuals with MOGAD administered in rat models of EAE has been associated with worse clinical disease and increased evidence of demyelination and axonal loss [3, 4]. In other experimental work, serum derived from pediatric patients with MOGAD has coincided with disruption to F-actin and β-tubulin networks in immortalized oligodendrocyte cells [3]. These data support potential pathologic roles for MOG antibodies in the clinical expression of MOGAD, but further research is needed.
Established and emerging biomarkers for the diagnosis and monitoring of MOGAD [3, 4, 33]
The clinical features of MS, MOGAD, and NMOSD significantly overlap. Therefore, biomarkers will be needed to distinguish these diagnoses accurately and reliably, especially in acute care settings where antibody results are often delayed (Table 4). Future research will also need to explore the impact of patient-related factors (biological sex, racial background, and pregnancy status), co-morbidities (infections), and treatment effects (long-term immunotherapies) on relapse risk and severity. In the path toward patient-centered “precision-based medicine”, it would be beneficial to identify biomarkers that not only facilitate diagnosis and predict relapses, but also prognosticate recovery for those with MOGAD.
Table 4.
Established and Emerging Biomarkers to help Diagnose MOGAD, Monitor Disease Activity, and Prognosticate Recovery [2–5, 9, 12, 14, 43–51]
| Biomarker | Key Points | Considerations |
|---|---|---|
| Serology | ||
| MOG-IgG | Live cell-based assays (CBA) using full-length human MOG are optimal for MOGAD evaluation. The sensitivity of fixed CBA is lower when compared to the live CBA, yet fixed CBA is still superior to other non-CBAs, such as ELISA, which cannot reliably identify MOG-IgG. Testing for MOG IgG with either an IgG Fc or IgG1 specific secondary antibody is recommended, as is use of an IgG (heavy and light) secondary antibody. Serum testing for MOG-IgG is preferred over CSF, but CSF sampling may be considered in patients with clinical and MRI features suggestive of MOGAD who have negative results on serum testing. Anti-MOG antibodies titers may fluctuate, and sometimes increase during disease exacerbations. While persistent MOG-IgG seropositivity is associated with an increased risk of relapse, it is difficult to tailor treatment to MOG-IgG titers as patients may remain seropositive while relapse-free for years. Alternatively, patients may become seronegative during follow-up but then seroconvert back to a positive status at the time of relapse. It is uncommon to become seropositive when baseline serology for MOG IgG is negative | False positive MOG IgG results may occur, particularly when patients lack clinical or MRI features of MOGAD. It has been reported that MOG-IgG was detected in approximately 1% of patients with other neurologic diseases and generally at low titers. For borderline or low titer results, consider repeat testing. When MOG IgG co-exists with a variety of other autoantibodies, consider the specificity of the result, as it may represent a bystander effect and not true MOGAD |
| Serum Neurofilament Light (sNFL) Chain | sNFL, a scaffolding protein in the neuronal cytoskeleton, has shown promise in detecting relapses in NMOSD and may be an indicator of disease severity in MOGAD | As a biomarker, sNfL may be useful in quantifying relapse severity, however sNfL levels do not necessarily differ between patients with NMOSD, MS, or MOGAD. sNfL levels may also be elevated in various pathological (CNS inflammatory syndromes) and physiological states (aging, fever) |
| Magnetic Resonance Imaging | ||
| Optic Nerve | Features may include longitudinal optic nerve involvement (> 50% of optic nerve length, involving orbital, canalicular, and intracranial regions), optic disk edema, peri-neural optic nerve sheath enhancement, and simultaneous bilateral involvement | The detection of optic nerve lesions relies upon dedicated orbital imaging, with fat suppressed T2 images and gadolinium enhanced T1W views or short tau inversion recovery sequences. Otherwise, poor visualization of findings may occur particularly when imaging is delayed over a month or more. It is noteworthy that other inflammatory and infiltrative conditions can cause similar MRI findings. Lesion enhancement persisting beyond 6 months would be unusual for MOGAD as would failure to respond to steroids |
| Spinal Cord | Most lesions are T2 hyper-intense and centrally located, often restricted to the gray matter producing the so-called “H” sign on axial views. Involvement of the conus and thickening and enhancement of the dorsal nerve roots have been described in MOGAD. Contrast enhancement is seen in half of patients with transverse myelitis | Most patients with MOGAD have visible T2 lesions on MRI but 10% may be imaging negative (acutely). Patients with MOGAD-related myelitis may have LETM akin to NMOSD, which can cause diagnostic confusion. Spinal lesions related to MOGAD tend to resolve and failure to do so should prompt concern, and reinvestigations for an alternative diagnosis. Different than that seen in MS, is that accumulating spinal cord lesions independent of attacks is uncommon. Note that bilateral vision loss and LETM have been described in LHON |
| Brain | Brain imaging may be normal initially for patients with ON or myelitis. MOGAD lesions are often bilateral poorly defined and appear large with deep gray matter involvement. In the brainstem, involvement of the pons and middle cerebellar peduncles is observed | Area postrema involvement is uncommon in MOGAD. MRI findings of well circumscribed T2 lesions meeting criteria dissemination of space for MS would be a red flag for MOGAD diagnosis, as would lesion enhancement > 6 months. Brain lesions in MOGAD often resolve over time, unlike MS |
| Cerebrospinal Fluid | ||
| Cellular Constituents | CSF pleocytosis may be seen particularly during attacks, and elevations in protein are also observed. During attacks, CSF may show evidence of a pleocytosis particularly in patients with myelitis, brainstem, and multifocal relapses, more so than optic neuritis events | CSF pleocytosis is not specific for any CNS inflammatory condition and can be seen across a variety of disorders. Results need to be interpreted in the appropriate clinical context |
| Oligoclonal Bands (OCBs) | OCBS are detected in up to 20% of MOGAD cases | OCB are common in MS (found in 85% of cases) but less frequently observed in AQP4-IgG seropositive NMOSD and MOGAD. In MOGAD, they may be occasionally found but may also be transient |
| MOG IgG | Occasional CSF MOG IgG can be used to confirm diagnosis when serum MOG IgG is falsely negative | CSF sensitivity for MOG IgG is generally low |
| Optical Coherence Tomography | ||
| Peripapillary RNFL (pRNFL) | Approximately 3–6 months after acute ON, there is progressive thinning of the peri-papillary RNFL thickness, but this does not distinguish ON cases caused by MOGAD, MS, or NMOSD. One distinguishing feature of MOGAD ON is the extent of optic disk swelling seen acutely, sometimes with associated hemorrhage. Accordingly, mean pRNFL measures for MOGAD ON patients tend to be higher than MS ON patients, such that a baseline measure of 118 microns may prove to be a useful cut-off to distinguish MOGAD ON patients from MS ON. Moreover, MOGAD ON patients often have at least 10 microns of inter-eye asymmetry (with higher values in the ON eyes) compared to MS ON patients | Many acute optic neuropathies including inflammatory and ischemic (non-arteritic anterior ischemic optic neuropathy) insults may present with significant optic disk swelling and pRNFL thickening at symptom onset. It is therefore important to ensure that MOGAD patients have a classical clinical phenotype, ideally with good radiological and corresponding serological evidence of disease |
| Macular GCIPL | In acute ON, the first sign of neuro-axonal injury tends to be thinning of the macular GCIPL, but this is not specific for MOGAD | Due to floor effects of the OCT technology, it is challenging to detect new evidence of ganglion layer injury in the setting of prior injury, even with recurrent ON attacks |
| OCT-Angiography | MOGAD may be associated with significant decreases in vascular density. It is thought that peripheral vessel density might predict the visual outcomes in MOGAD patients [52, 53] | OCT-A changes may not be specific for MOGAD and need to be interpreted in the appropriate clinical context |
Management of MOGAD patients
Acute treatment
The Optic Neuritis Treatment Trial (ONTT) has informed our understanding regarding the relative risks and benefits of high-dose corticosteroid therapy for patients presenting with acute optic neuritis [15]. This seminal study reported that administering IVMP (1 g/day for 3 days, followed by 1 mg/kg for 11 days) to patients presenting with acute ON expedited visual recovery, but it did not improve long-term visual outcomes [15]. Yet, the lessons of the ONTT may not be extendable to patients with MOGAD, due to study design (bilateral ON was a basis of exclusion). Notably, an examination of serum samples obtained from 177 out of the 448 ONTT participants revealed that only 3 (1.7%) were positive for MOG-IgG antibody [8]. Thus, the conclusions drawn from the ONTT regarding the role of corticosteroids in optic neuritis cannot be extrapolated to predict long-term visual outcomes in MOGAD patients. MOGAD attacks are often very steroid responsive and therefore most experts recommend high-dose corticosteroids as first-line treatment for patients presenting with acute MOGAD attacks [54–57]. Like the established NMOSD treatment approach, there is observational evidence suggesting that early management of MOGAD-related ON with IVMP is crucial for optimal visual recovery, as it has been shown that early steroid treatment is associated with better visual outcomes and less thinning of the peri-papillary retinal nerve fiber layer [9, 57–59]. This signifies a shift in the treatment paradigm of ONTT for ON, as it is felt that in NMOSD and MOGAD, time is of the essence to preserve vision [60]. However, it is important to note that there are cases of MOGAD ON with spontaneous improvement without steroid treatment [9, 10].
Despite the lack of a universally accepted high-dose steroid treatment regimen, the use of IVMP at a dosage of 1 g/day for 5 days is the most prevalent strategy [61, 62]. While no study has compared the efficacy of IVMP to oral corticosteroid in treating acute MOGAD attacks, Morrow et al. have reported that bioequivalent doses of oral corticosteroids are noninferior to IVMP in treating acute ON [63], and therefore 1250 mg of oral prednisone is sometimes offered as an alternative to IVMP. Further research is needed to establish the role of oral corticosteroids in the acute management of MOGAD manifestations. Patients suffering from a recent MOGAD attack are commonly considered to be at risk for relapse upon cessation or reduction of corticosteroid therapy. Therefore, considering multiple observational studies, to mitigate relapse risk, it is recommended that corticosteroids are tapered gradually over a period of 1–2 months [57, 64–67].
Most MOGAD attacks are responsive to corticosteroids, yet a subset of MOGAD patients do not respond well to IVMP, necessitating further interventions. Both plasmapheresis (PLEX) and immuno-adsorption (IA) have been proposed as therapeutic options for those failing IVMP treatment. PLEX and IA have been successfully utilized to treat immune-mediated neurological diseases, including acute NMOSD attacks [68–75]. An international multicenter retrospective study of 92 MOGAD ON attacks treated with PLEX demonstrated significant improvement in all attacks except for one eye that was treated over 6 months after the initial attack [76]. While preliminary reports are encouraging, prospective studies are required to confirm the efficacy of PLEX for acute MOGAD attacks [35]. Intravenous immunoglobulin (IVIG) is another therapeutic option that has been employed in other demyelinating conditions, such as NMOSD. While no published studies have shown IVIG efficacy in treating acute MOGAD [69, 77], a multicenter retrospective study looking at IVIG for the treatment of acute MOGAD suggests it may be associated with improved outcomes (unpublished data). Further research is required to uncover the utility and role of PLEX, IA, and IVIG in the acute management of MOGAD patients.
Maintenance therapy
The best maintenance therapy for MOGAD patients remains a topic of ongoing debate, particularly considering that approximately 50% of MOGAD patients exhibit monophasic disease, and may not require long-term immunosuppression [9, 14]. Unfortunately, it is difficult to predict if a newly diagnosed patient is destined to develop a relapsing course. Longitudinal MOG-IgG seropositivity was found to be associated with relapsing MOGAD, but many of the patients who remain seropositive do not relapse. Roughly 25% of MOGAD patients will become seronegative and are more likely to have monophasic disease [78–81]. Furthermore, findings by Cobo-Calvo et al., suggest that adults are at a higher risk of relapse and may experience worse clinical outcomes than children [80]. Long-term immunotherapy is typically initiated after a second MOGAD attack [61]. However, the type of maintenance treatment for recurrent MOGAD remains disputed, and has been extrapolated from NMOSD studies [60]. This is not ideal due to the well-described differences in pathophysiology, demographics, severity, and prognosis between the two disease entities. Common maintenance therapies employed may include oral steroids, azathioprine (AZA), mycophenolate mofetil (MMF), and B cell-targeting biologics, such as rituximab (RTX), and tocilizumab, all of which have been employed in NMOSD, in addition to maintenance IVIG, which is not a common treatment for NMOSD [60, 62].
Multiple studies have shown that maintenance therapy with oral corticosteroids has been associated with a reduction in relapse rate [64, 82, 83]. Bridging immunosuppressive agents with oral steroids is linked with better therapeutic outcomes [56, 64]. However, the potential benefits of chronic corticosteroids use must be weighed against the health risks associated with their long-term use.
Broad spectrum immunosuppressants, such as AZA and MMF, are well tolerated and have been proposed to be effective maintenance therapies for MOGAD patients [84–86]. Chen and colleagues reported that MOGAD patients maintained on AZA had a significant reduction in the annualized relapse rate (ARR) from 1.6 (range: 0–9.7) to 0.2 (range: 0–3.2) [84]. A modest reduction in relapse rates was noted for those treated with MMF [84]. Additionally, a prospective observational cohort study provided Class IV evidence that MMF therapy reduces relapse in MOGAD patients [87]. When initiating AZA and MMF, it is recommended to bridge these patients with prednisolone for the first few months until a decrease in lymphocyte counts is observed [56, 62]. While observational studies have shown that AZA and MMF are associated with a reduction in relapses, it is important to note that relapses can still occur and there is no randomized clinical trial data in MOGAD patients.
The efficacy of RTX in reducing relapses and EDSS scores in NMOSD patients has been well-established through RCTs [88, 89]. However, RTX’s efficacy in MOGAD patients remains ambiguous as there are no randomized clinical trials evaluating its effectiveness in this patient population. Meta-analyses have suggested that RTX reduces relapses and EDSS scores in MOGAD, though to a lesser extent than in NMOSD [90, 91]. Additionally, retrospective studies have also reported varying degrees of ARR reduction for MOGAD patients treated with RTX [61, 84, 92]. In addition, MOGAD relapses can occur despite depletion of memory B cells [93].
More recently, IVIG has emerged as a promising maintenance therapy for MOGAD patients [55]. A multicenter retrospective cohort study conducted by Chen et al. reported that maintenance IVIG therapy was associated with a reduced relapse rate in adult MOGAD patients, especially when administered at a dose of 1–2 g/kg every 4 weeks [84, 94]. Other retrospective studies have suggested maintenance IVIG therapy is effective in pediatric MOGAD patients [55]. Looking forward, IL-6 inhibitors may play a role in MOGAD maintenance therapy with growing evidence suggesting that tocilizumab might be effective for highly relapsing MOGAD patients, and satralizumab is being investigated in a multicenter randomized clinical trials [95, 96]. Additionally, rozanolixizumab, an anti-neonatal Fc receptor inhibitor, is also a promising therapy that is currently in randomized clinical trials for the treatment of relapsing MOGAD patients [14]. As evidences for therapeutic approaches continue to accumulate, randomized clinical trials with sufficient follow-up are necessary to uncover the most efficacious and well-tolerated maintenance therapy for patients with recurrent MOGAD.
Prognosis
The duration of immunosuppressive treatment for MOGAD remains uncertain, as the field currently lacks robust biomarkers to inform therapy. Specifically, it is challenging to identify patients who will remain monophasic after their first attack. Several indicators of disease activity have been proposed, including attack severity and frequency, MOG-IgG sero-status and titers, patient age, relapse-free interval, spinal cord involvement, and resultant neurological disability [61, 97–99]. The identification of prognostic factors indicating the likelihood of relapse and disability in a patient would catalyze a personalized treatment approach for MOGAD patients.
Adult MOGAD patients tend to have more recurrent episodes and poorer functional recovery compared to pediatric patients [82, 97, 100, 101]. MOG-IgG sero-status, particularly in adult patients, is proposed to be a predictor of disease course, with ‘seroreversion’ (from positive to negative) being indicative of a low relapse risk [67, 79, 82]. Yet, the utility of MOG-IgG titers for treatment planning remains debatable [102]. Nevertheless, recurrent disease has been associated with elevated initial MOG-IgG titers, while transiently low titers have been shown to be associated with a monophasic course [80, 82, 103].
Long-term outcomes and disability are quite challenging to predict as studies with long follow-up are sparse for patients with MOGAD. While it is believed that, on average, even frequently relapsing MOGAD results in less disability than NMOSD, inter-patient variability has been documented [16, 104, 105]. Indeed, the clinical heterogeneity of MOGAD is an increasingly recognized phenomenon [97]. Sufficiently powered prospective studies with extended follow-up would enable a more comprehensive understanding of the natural history of MOGAD, identify predictors of its course and establish guidelines for its treatment.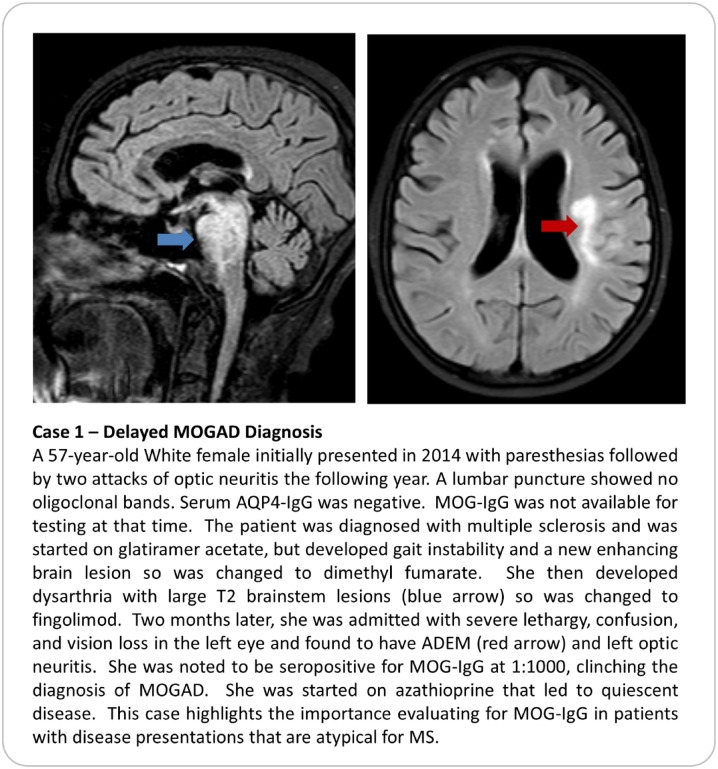
Data availability
Data Availaiblity statement is not applicable as this review article is based exclusively on published work.
Declarations
Conflict of interest
Dr. Abdullah Al-Ani has no conflict of interest. Dr. John J Chen served as a consultant to UCB and Horizon. Dr. Fiona Costello has received advisory board or speaker fees from Alexion, Novartis, Accure Therapeutics, Frequency Therapeutics, and Sanofi.
Ethical standard
An ethics statement is not applicable because this article is based on published literature.
References
- 1.Cañellas AR, Gols AR, Izquierdo JR, et al. Idiopathic inflammatory-demyelinating diseases of the central nervous system. Neuroradiology. 2007;49:393–409. doi: 10.1007/s00234-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 2.Costello F. Neuromyelitis optica spectrum disorders. Contin Lifelong Learn Neurol. 2022;28:1131–1170. doi: 10.1212/CON.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 3.Longbrake E. Myelin oligodendrocyte glycoprotein-associated disorders. Contin Lifelong Learn Neurol. 2022;28:1171–1193. doi: 10.1212/CON.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20:762–772. doi: 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 5.Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023 doi: 10.1016/S1474-4422(22)00431-8. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett JL, Costello F, Chen JJ, et al. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2023;22:89–100. doi: 10.1016/S1474-4422(22)00187-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen JJ, Tobin WO, Majed M, et al. Prevalence of myelin oligodendrocyte glycoprotein and aquaporin-4–IgG in patients in the optic neuritis treatment trial. JAMA Ophthalmol. 2018;136:419. doi: 10.1001/jamaophthalmol.2017.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JJ, Flanagan EP, Bhatti MT, et al (2022) Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord 68:104237. 10.1016/j.msard.2022.104237 [DOI] [PubMed]
- 10.Vosoughi AR, Muccilli A, Schneider R, et al (2022) Recovery of Vision in Myelin Oligodendrocyte Glycoprotein–IgG Optic Neuritis Without Treatment: A Case Series. J Neuro-Ophthalmology Publish Ah:3–5. 10.1097/WNO.0000000000001583 [DOI] [PubMed]
- 11.Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. 2021;20:60–67. doi: 10.1016/S1474-4422(20)30392-6. [DOI] [PubMed] [Google Scholar]
- 12.Sechi E, Krecke KN, Messina SA, et al. Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology. 2021;97:e1097–e1109. doi: 10.1212/WNL.0000000000012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 14.Sechi E, Cacciaguerra L, Chen JJ, et al (2022) Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front Neurol 13:885218. 10.3389/fneur.2022.885218 [DOI] [PMC free article] [PubMed]
- 15.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–588. doi: 10.1056/nejm199202273260901. [DOI] [PubMed] [Google Scholar]
- 16.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8–15. doi: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaitán MI, Paday Formenti ME, Calandri I, et al (2022) The central vein sign is present in most infratentorial multiple sclerosis plaques. Mult Scler Relat Disord 58:103484. 10.1016/j.msard.2021.103484 [DOI] [PubMed]
- 18.Costello F, Scott JN. Imaging in Neuro-ophthalmology. Contin Lifelong Learn Neurol. 2019;25:1438–1490. doi: 10.1212/CON.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 19.Myers KA, Nikolic A, Romanchuk K, et al. Optic neuropathy in the context of leukemia or lymphoma: diagnostic approach to a neuro-oncologic emergency. Neuro-Oncology Pract. 2017;4:60–66. doi: 10.1093/nop/npw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrams RMC, Safavi F, Tuhrim S, et al. MRI negative myelopathy post mild SARS-CoV-2 infection: vasculopathy or inflammatory myelitis? J Neurovirol. 2021;27:650–655. doi: 10.1007/s13365-021-00986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobin WO, Guo Y, Krecke KN, et al. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2017;140:2415–2425. doi: 10.1093/brain/awx200. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Bu Y, Liu W (2022) Case Report: A Case With MOGAD and Anti-NMDAR Encephalitis Overlapping Syndrome Mimicing Radiological Characteristics of CLIPPERS. Front Immunol 13:832084. 10.3389/fimmu.2022.832084 [DOI] [PMC free article] [PubMed]
- 23.Banks SA, Morris PP, Chen JJ, et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. 2021;92:384–390. doi: 10.1136/jnnp-2020-325121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike M (2013) Opsoclonus–myoclonus syndrome. In: Handbook of Clinical Neurology. Elsevier, pp 1209–1211 [DOI] [PubMed]
- 25.Stunkel L, Kung NH, Wilson B, et al. Incidence and causes of overdiagnosis of optic neuritis. JAMA Ophthalmol. 2018;136:76. doi: 10.1001/jamaophthalmol.2017.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunchok A, Krecke KN, Flanagan EP, et al. Does area postrema syndrome occur in myelin oligodendrocyte glycoprotein-IgG–associated disorders (MOGAD)? Neurology. 2020;94:85–88. doi: 10.1212/WNL.0000000000008786. [DOI] [PubMed] [Google Scholar]
- 27.Jonathan Maran J, Sharpe C, Carroll S. Journal Pre-proof Paediatric MOG-antibody disease presenting with intracranial hypertension and unilateral vision loss without radiological evidence of optic neuritis. J Neuroimmunol. 2023 doi: 10.1016/j.jneuroim.2023.578083. [DOI] [PubMed] [Google Scholar]
- 28.Marignier R. Unusual presentations of MOG antibody-associated central nervous system demyelination: expanding the spectrum. Mult Scler J. 2019;25:128–129. doi: 10.1177/1352458518804127. [DOI] [PubMed] [Google Scholar]
- 29.Valdrighi A, Russ J, Waubant E, et al (2021) Atypical myelin oligodendrocyte glycoprotein antibody disease presenting with isolated elevated intracranial pressure. Neuroimmunol Reports 1:100028. 10.1016/j.nerep.2021.100028
- 30.Vosoughi AR, Ling J, Tam KT, et al. Ophthalmic manifestations of myelin oligodendrocyte glycoprotein-IgG-associated disorder other than optic neuritis: a systematic review. Br J Ophthalmol. 2021;105:1591–1598. doi: 10.1136/bjophthalmol-2020-317267. [DOI] [PubMed] [Google Scholar]
- 31.Costello F (2014) Inflammatory optic neuropathies. Continuum (Minneap Minn) 20:816–37. 10.1212/01.CON.0000453316.60013.52 [DOI] [PMC free article] [PubMed]
- 32.Reindl M, Schanda K, Woodhall M, et al (2020) International multicenter examination of MOG antibody assays. Neurol—Neuroimmunol Neuroinflammation 7:e674. 10.1212/NXI.0000000000000674 [DOI] [PMC free article] [PubMed]
- 33.Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. 2021;78:741. doi: 10.1001/jamaneurol.2021.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzano GS, Salky R, Mateen FJ, et al. Positive predictive value of MOG-IgG for clinically defined MOG-AD within a real-world cohort. Front Neurol. 2022;13:1–4. doi: 10.3389/fneur.2022.947630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benard-seguin E, Costello F (2023) Optic neuritis : current challenges in diagnosis and management. 36:10–18. 10.1097/WCO.0000000000001128 [DOI] [PubMed]
- 36.Carta S, Cobo Calvo Á, Armangué T, et al. Significance of myelin oligodendrocyte glycoprotein antibodies in CSF. Neurology. 2023;100:e1095–e1108. doi: 10.1212/WNL.0000000000201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinoto A, Sechi E, Flanagan EP, et al. Serum and cerebrospinal fluid biomarkers in neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disease. Front Neurol. 2022;13:1–13. doi: 10.3389/fneur.2022.866824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pace S, Orrell M, Woodhall M, et al. Frequency of MOG-IgG in cerebrospinal fluid versus serum. J Neurol Neurosurg Psychiatry. 2022;93:334–335. doi: 10.1136/jnnp-2021-326779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon YN, Kim B, Kim J-S, et al (2022) Myelin Oligodendrocyte glycoprotein-immunoglobulin G in the CSF. Neurol—Neuroimmunol Neuroinflammation 9:e1095. 10.1212/NXI.0000000000001095 [DOI] [PMC free article] [PubMed]
- 40.Mariotto S, Gajofatto A, Batzu L, et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology. 2019;93:e1867–e1872. doi: 10.1212/WNL.0000000000008479. [DOI] [PubMed] [Google Scholar]
- 41.Kunchok A, Chen JJ, McKeon A, et al. Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurol. 2020;77:257. doi: 10.1001/jamaneurol.2019.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerch M, Bauer A, Reindl M. The Potential pathogenicity of myelin oligodendrocyte glycoprotein antibodies in the optic pathway. J Neuro-Ophthalmology. 2023;43:5–16. doi: 10.1097/WNO.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. Am J Neuroradiol. 2016;37:394–401. doi: 10.3174/ajnr.A4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacioglu M, Maia LF, Preische O, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91:56–66. doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 46.Luo W, Chen Y, Mao S, et al. Serum neurofilament light chain in adult and pediatric patients with myelin oligodendrocyte glycoprotein antibody-associated disease: Correlation with relapses and seizures. J Neurochem. 2022;160:568–577. doi: 10.1111/jnc.15549. [DOI] [PubMed] [Google Scholar]
- 47.Chang X, Huang W, Wang L, et al (2021) Serum Neurofilament Light and GFAP Are Associated With Disease Severity in Inflammatory Disorders With Aquaporin-4 or Myelin Oligodendrocyte Glycoprotein Antibodies. Front Immunol 12:647618. 10.3389/fimmu.2021.647618 [DOI] [PMC free article] [PubMed]
- 48.Kim H, Lee E-J, Kim S, et al (2020) Serum biomarkers in myelin oligodendrocyte glycoprotein antibody–associated disease. Neurol—Neuroimmunol Neuroinflammation 7:e708. 10.1212/NXI.0000000000000708 [DOI] [PMC free article] [PubMed]
- 49.Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7:2508–2523. doi: 10.1002/acn3.51234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Lott LB, Bennett JL, Costello F. The changing landscape of optic neuritis: a narrative review. J Neurol. 2022;269:111–124. doi: 10.1007/s00415-020-10352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oertel FC, Sotirchos ES, Zimmermann HG, et al. Longitudinal retinal changes in <scp>MOGAD</scp>. Ann Neurol. 2022;92:476–485. doi: 10.1002/ana.26440. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Huang Y, Quan C, et al. Alterations in the retinal vascular network and structure in MOG antibody-associated disease: an optical coherence tomography angiography study. J Neuro-Ophthalmology. 2021;41:e424–e432. doi: 10.1097/WNO.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 53.Yao Y, Li X, Xu Y, et al. The difference of the retinal structural and microvascular characteristics in patients with MOGAD-ON and AQP4-ON. BMC Neurol. 2022;22:323. doi: 10.1186/s12883-022-02848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. 2019;266:1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hacohen Y, Banwell B. Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol. 2019;21:2. doi: 10.1007/s11940-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarius S, Ruprecht K, Kleiter I, et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 13:280. 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed]
- 57.Stiebel-Kalish H, Hellmann MA, Mimouni M, et al (2019) Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol—Neuroimmunol Neuroinflammation 6:e572. 10.1212/NXI.0000000000000572 [DOI] [PMC free article] [PubMed]
- 58.Soelberg K, Specovius S, Zimmermann HG, et al. Optical coherence tomography in acute optic neuritis: a population-based study. Acta Neurol Scand. 2018;138:566–573. doi: 10.1111/ane.13004. [DOI] [PubMed] [Google Scholar]
- 59.Rode J, Pique J, Maarouf A, et al (2022) Time to steroids impacts visual outcome of optic neuritis in MOGAD. J Neurol Neurosurg Psychiatry jnnp-2022–330360. 10.1136/jnnp-2022-330360 [DOI] [PubMed]
- 60.Chwalisz BK, Levy M. The treatment of myelin oligodendrocyte glycoprotein antibody disease: a state-of-the-art review. J Neuro-Ophthalmology. 2022;42:292–296. doi: 10.1097/WNO.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 61.Whittam DH, Karthikeayan V, Gibbons E, et al. Treatment of MOG antibody associated disorders: results of an international survey. J Neurol. 2020;267:3565–3577. doi: 10.1007/s00415-020-10026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Healy S, Elhadd KT, Gibbons E, et al. Treatment of myelin oligodendrocyte glycoprotein immunoglobulin G–associated disease. Clin Exp Neuroimmunol. 2021;12:22–41. doi: 10.1111/cen3.12630. [DOI] [Google Scholar]
- 63.Morrow SA, Fraser JA, Day C, et al. Effect of treating acute optic neuritis with bioequivalent oral vs intravenous corticosteroids. JAMA Neurol. 2018;75:690. doi: 10.1001/jamaneurol.2018.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramanathan sudarshini, Mohammad S, Tantsis E, et al (2018) Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 89:127–137. 10.1136/jnnp-2017-316880 [DOI] [PMC free article] [PubMed]
- 65.Song H, Zhou H, Yang M, et al. Different characteristics of aquaporin-4 and myelin oligodendrocyte glycoprotein antibody-seropositive male optic neuritis in China. J Ophthalmol. 2019;2019:1–7. doi: 10.1155/2019/4015075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tajfirouz DA, Bhatti MT, Chen JJ. Clinical characteristics and treatment of MOG-IgG–associated optic neuritis. Curr Neurol Neurosci Rep. 2019;19:100. doi: 10.1007/s11910-019-1014-z. [DOI] [PubMed] [Google Scholar]
- 67.Huda S, Whittam D, Jackson R, et al (2021) Predictors of relapse in MOG antibody associated disease: a cohort study. BMJ Open 11:e055392. 10.1136/bmjopen-2021-055392 [DOI] [PMC free article] [PubMed]
- 68.Osman C, Jennings R, El-Ghariani K, Pinto A. Plasma exchange in neurological disease. Pract Neurol. 2020;20:92–99. doi: 10.1136/practneurol-2019-002336. [DOI] [PubMed] [Google Scholar]
- 69.Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206–216. doi: 10.1002/ana.24554. [DOI] [PubMed] [Google Scholar]
- 70.Sokolov AA, Solovyev AG. Russian pioneers of therapeutic hemapheresis and extracorporeal hemocorrection: 100-year anniversary of the world’s first successful plasmapheresis. Ther Apher Dial. 2014;18:117–121. doi: 10.1111/1744-9987.12067. [DOI] [PubMed] [Google Scholar]
- 71.Levy M (2018) Plasmapheresis for acute attacks in neuromyelitis optica spectrum disorders. Neurol - Neuroimmunol Neuroinflammation 5:e510. 10.1212/NXI.0000000000000510 [DOI] [PMC free article] [PubMed]
- 72.Pinching AJ, Peters DK. Remission of myasthenia gravis following plasma-exchange. Lancet (London, England) 1976;2:1373–1376. doi: 10.1016/s0140-6736(76)91917-6. [DOI] [PubMed] [Google Scholar]
- 73.Oji S, Nomura K. Immunoadsorption in neurological disorders. Transfus Apher Sci. 2017;56:671–676. doi: 10.1016/j.transci.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi M, Nanri K, Taguchi T, et al. Immunoadsorption therapy for neuromyelitis optica spectrum disorders long after the acute phase. J Clin Apher. 2015;30:43–45. doi: 10.1002/jca.21324. [DOI] [PubMed] [Google Scholar]
- 75.Faissner S, Nikolayczik J, Chan A, et al. Immunoadsorption in patients with neuromyelitis optica spectrum disorder. Ther Adv Neurol Disord. 2016;9:281–286. doi: 10.1177/1756285616646332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen JJ, Flanagan EP, Pittock SJ, et al. Visual outcomes following plasma exchange for optic neuritis: an international multicenter retrospective analysis of 395 optic neuritis attacks. Am J Ophthalmol. 2023 doi: 10.1016/j.ajo.2023.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsone L, Panicker J, Mutch K, et al. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: experience in 10 patients. Mult Scler J. 2014;20:501–504. doi: 10.1177/1352458513495938. [DOI] [PubMed] [Google Scholar]
- 78.Oliveira LM, Apóstolos-Pereira SL, Pitombeira MS, et al. Persistent MOG-IgG positivity is a predictor of recurrence in MOG-IgG-associated optic neuritis, encephalitis and myelitis. Mult Scler J. 2019;25:1907–1914. doi: 10.1177/1352458518811597. [DOI] [PubMed] [Google Scholar]
- 79.Hyun JW, Woodhall MR, Kim SH, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88:811–817. doi: 10.1136/JNNP-2017-315998. [DOI] [PubMed] [Google Scholar]
- 80.Cobo-Calvo A, Ruiz A, Rollot F, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2021;89:30–41. doi: 10.1002/ana.25909. [DOI] [PubMed] [Google Scholar]
- 81.López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG–associated disorders. JAMA Neurol. 2018;75:1355. doi: 10.1001/jamaneurol.2018.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–3138. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 83.Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75:478–487. doi: 10.1001/JAMANEUROL.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen JJ, Flanagan EP, Bhatti MT, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95:e111–e120. doi: 10.1212/WNL.0000000000009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, et al (2020) Treatment of MOG-IgG-associated disorder with rituximab: An international study of 121 patients. Mult Scler Relat Disord 44:102251. 10.1016/j.msard.2020.102251 [DOI] [PMC free article] [PubMed]
- 86.Lu Q, Luo J, Hao H, et al. Efficacy and safety of long-term immunotherapy in adult patients with MOG antibody disease: a systematic analysis. J Neurol. 2021;268:4537–4548. doi: 10.1007/s00415-020-10236-4. [DOI] [PubMed] [Google Scholar]
- 87.Li S, Ren H, Xu Y, et al (2020) Long-term efficacy of mycophenolate mofetil in myelin oligodendrocyte glycoprotein antibody-associated disorders. Neurol - Neuroimmunol Neuroinflammation 7:e705. 10.1212/NXI.0000000000000705 [DOI] [PMC free article] [PubMed]
- 88.Tahara M, Oeda T, Okada K, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:298–306. doi: 10.1016/S1474-4422(20)30066-1. [DOI] [PubMed] [Google Scholar]
- 89.Nikoo Z, Badihian S, Shaygannejad V, et al. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: a randomized clinical trial. J Neurol. 2017;264:2003–2009. doi: 10.1007/s00415-017-8590-0. [DOI] [PubMed] [Google Scholar]
- 90.Spagni G, Sun B, Monte G, et al. Efficacy and safety of rituximab in myelin oligodendrocyte glycoprotein antibody-associated disorders compared with neuromyelitis optica spectrum disorder: a systematic review and meta-analysis Neuro-inflammation. J Neurol Neurosurg Psychiatry. 2023;94:62–69. doi: 10.1136/jnnp-2022-330086. [DOI] [PubMed] [Google Scholar]
- 91.Nepal G, Kharel S, Coghlan MA, et al (2022) Safety and efficacy of rituximab for relapse prevention in myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG)-associated disorders (MOGAD): A systematic review and meta-analysis. J Neuroimmunol 364:577812. 10.1016/j.jneuroim.2022.577812 [DOI] [PubMed]
- 92.Barreras P, Vasileiou ES, Filippatou AG, et al (2022) Long-term Effectiveness and Safety of Rituximab in Neuromyelitis Optica Spectrum Disorder and MOG Antibody Disease. Neurology 99:10.1212/WNL.0000000000201260. 10.1212/WNL.0000000000201260 [DOI] [PMC free article] [PubMed]
- 93.Durozard P, Rico A, Boutiere C, et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol. 2020;87:256–266. doi: 10.1002/ana.25648. [DOI] [PubMed] [Google Scholar]
- 94.Chen JJ, Huda S, Hacohen Y, et al. Association of maintenance intravenous immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2022;79:518. doi: 10.1001/jamaneurol.2022.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ringelstein M, Ayzenberg I, Lindenblatt G, et al (2022) Interleukin-6 Receptor Blockade in Treatment-Refractory MOG-IgG–Associated Disease and Neuromyelitis Optica Spectrum Disorders. Neurol - Neuroimmunol Neuroinflammation 9:e1100. 10.1212/NXI.0000000000001100 [DOI] [PMC free article] [PubMed]
- 96.Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM (2021) Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): A case-series and review. Mult Scler Relat Disord 48:102696. 10.1016/j.msard.2020.102696 [DOI] [PubMed]
- 97.Havla J, Pakeerathan T, Schwake C, et al. Age-dependent favorable visual recovery despite significant retinal atrophy in pediatric MOGAD: how much retina do you really need to see well? J Neuroinflammation. 2021;18:121. doi: 10.1186/s12974-021-02160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satukijchai C, Mariano R, Messina S, et al (2022) Factors Associated With Relapse and Treatment of Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disease in the United Kingdom. JAMA Netw Open 5:e2142780. 10.1001/jamanetworkopen.2021.42780 [DOI] [PMC free article] [PubMed]
- 99.Bruijstens AL, Breu M, Wendel E-M, et al. E.U. paediatric MOG consortium consensus: Part 4—outcome of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:32–40. doi: 10.1016/j.ejpn.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 100.Cobo-Calvo A, Sepúlveda M, D’Indy H, et al. Usefulness of MOG-antibody titres at first episode to predict the future clinical course in adults. J Neurol. 2019;266:806–815. doi: 10.1007/s00415-018-9160-9. [DOI] [PubMed] [Google Scholar]
- 101.Pineles SL, Repka MX, Liu GT, et al. Assessment of pediatric optic neuritis visual acuity outcomes at 6 months. JAMA Ophthalmol. 2020;138:1253–1261. doi: 10.1001/JAMAOPHTHALMOL.2020.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carnero Contentti E, Marrodan M, Correale J. Emerging drugs for the treatment of adult MOG-IgG-associated diseases. Expert Opin Emerg Drugs. 2021;26:75–78. doi: 10.1080/14728214.2021.1919082. [DOI] [PubMed] [Google Scholar]
- 103.Gastaldi M, Foiadelli T, Greco G, et al (2022) Prognostic relevance of quantitative and longitudinal MOG antibody testing in patients with MOGAD: a multicentre retrospective study. J Neurol Neurosurg Psychiatry 0:jnnp-2022–330237. 10.1136/jnnp-2022-330237 [DOI] [PubMed]
- 104.Lopez-Chiriboga AS, Sechi E, Buciuc M, et al. Long-term outcomes in patients with myelin oligodendrocyte glycoprotein immunoglobulin g-associated disorder. JAMA Neurol. 2020;77:1575. doi: 10.1001/jamaneurol.2020.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deschamps R, Pique J, Ayrignac X, et al. The long-term outcome of MOGAD: An observational national cohort study of 61 patients. Eur J Neurol. 2021;28:1659–1664. doi: 10.1111/ene.14746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Availaiblity statement is not applicable as this review article is based exclusively on published work.