Abstract
Background
Anterior cervical corpectomy and fusion (ACCF) is often required to adequately decompress the spinal cord in patients with multilevel cervical spondylosis. Unfortunately, multilevel corpectomy constructs have high rates of early failure and frequently require supplemental posterior fixation. First described in 2003, skip ACCF (sACCF) is defined by corpectomies above and below an intervening vertebral body, which serves as an additional fixation point to augment biomechanical stability. Subsequent studies report high fusion rates and low construct failure rates secondary to superior biomechanical stability.
Objective
The goal of this study was to demonstrate the safety and efficacy of sACCF in the largest series published to date.
Methods
This study was a retrospective case series of all patients who underwent sACCF at a single institution over a 10-year period. Standard demographic and perioperative data were collected. Outcome data included immediate postoperative complications, long-term reoperation, and pre- and postoperative radiographic parameters.
Results
Forty-five patients underwent sACCF: 42 at C4-C6 and 3 at C5-C7. Mean age was 57.5 years. More than half (64.4%) of patients were smokers. Almost all patients were discharged home, the vast majority (82.2%) within 3 days of surgery. Five patients (11.1%) developed complications during the index hospitalization: 2 C5 palsies and 3 medical complications. Three patients (6.7%) developed instrumentation failure requiring anterior revision and supplemental posterior fixation. There were statistically significant increases in C1-C7 (47.8 vs 41.1, P < 0.001) and C2-C7 lordosis (11.1 vs 5.0, P < 0.001) on postoperative radiographs compared with preoperative imaging. Average follow-up was 21.1 months.
Conclusion
sACCF can be performed safely with complication rates similar to those reported for multilevel anterior cervical discectomy and fusion or adjacent segment ACCF. It should be considered for patients with multilevel cervical pathology for whom an anterior approach is favored.
Clinical Relevance
sACCF is an effective surgical technique for multilevel cervical decompression and correction of cervical alignment.
Level of Evidence
3.
Keywords: corpectomy, skip, cervical spondylotic myelopathy, multilevel corpectomy, anterior cervical corpectomy and fusion, myelopathy, radiculopathy, ossified posterior longitudinal ligament
Introduction
The anterior approach to the cervical spine was first described by Bailey and Badgley in 1960.1 Widespread adoption led to the development of more aggressive techniques, begetting the first cervical corpectomies in the 1970s.2 Decades later, the literature reports generally good outcomes after single- and 2-level cervical corpectomy.3–9 In contrast, multilevel (3 or more levels) cervical corpectomy has a high rate of early construct failure and frequently requires supplemental posterior fixation.10–15
Numerous alternative techniques have been described to address the deficiencies of multilevel anterior cervical corpectomy and fusion (ACCF).16–21 A hybrid approach in which corpectomies are performed above and below an intervening vertebral body was first proposed by Edwards et al in 2003.16 Ashkenazi and associates subsequently reported outcomes in 13 patients who underwent this novel procedure.20 All patients achieved fusion, and only 1 patient experienced construct failure. Dalbayrak and colleagues coined the term “skip” corpectomy in 2010 to describe this procedure in their report of 29 patients with ossification of the posterior longitudinal ligament (OPLL) who underwent corpectomies at C4 and C6.21 All patients achieved radiographic fusion, and only 1 instance of instrumentation failure was reported. Biomechanical studies have demonstrated superior stability of the skip corpectomy construct compared with 3-level corpectomy.22 Subsequent studies comparing skip ACCF (sACCF) with multilevel anterior cervical discectomy and fusion (ACDF) or posterior decompression and fusion have reported no significant differences in perioperative complication rates, postoperative radiographic outcomes, and clinical outcomes including modified Japanese Orthopedic Association scores and patient-reported outcomes.23,24
In this manuscript, we report our experience with 45 patients who underwent sACCF over a 10-year period. While Dalbayrak et al described a technique involving corpectomies at C4 and C6, our cohort includes a few patients who underwent corpectomy at C5 and C7. This study seeks to further demonstrate the safety and efficacy of sACCF in the largest series published to date.
Methods
Study Population and Data Collection
This study was approved by the local Institutional Review Board. We performed a retrospective review of consecutive patients who underwent sACCF between 1 January 2010 and 31 December 2020. Data regarding patient demographics, comorbidities, operative details, postoperative complications, instrumentation failure, and need for reoperation were collected. Indications for surgery included degenerative disease, deformity, and trauma. Patients younger than 18 years and those undergoing long-segment posterior cervicothoracic fusions were excluded from the analysis.
Surgical Procedure
The skip corpectomy procedure performed in this cohort was independently proposed and demonstrated in the early 2000s by 2 authors of the present study (F.F. and S.J.C.). Our technique is similar to that reported by Dalbayrak et al, who described a C4 and C6 corpectomy, C5 osteophytectomy, and decompression of the posterior-superior and posterior-inferior aspects of the C5 vertebral body.21 In our cohort, a minority of patients underwent corpectomy at C5 and C7 (Figure 1). Pathologic intervertebral discs were removed at the intervening levels. Preservation of the central vertebral body (C5 or C6), as well as the vertebral endplates, is the essential aspect of this technique. Bone from the corpectomy levels was harvested for autograft. The operative microscope was used during resection of the posterior longitudinal ligament. Choice of cage was left to the discretion of the operating surgeon. Cages were packed with local autograft in addition to allograft and/or iliac crest aspirate. Intraoperative radiographs were obtained to confirm proper cage positioning. A fixed anterior cervical plate was contoured for lordosis and placed from C3 to C7 or C4 to T1. Screws were inserted at the rostral and caudal ends of the plate prior to fixation at the intervening vertebral body (C5 or C6). Long fixation screws were utilized, and bicortical purchase sought where necessary with the screw tips extending through the dorsal vertebral body cortex to improve construct strength and stability.
Figure 1.
Skip corpectomy involving the C5 and C7 vertebral bodies.
Bulb suction drains were left in almost all cases. Patients were placed in a rigid cervical orthosis.
Postoperative Management
Patients were admitted to a standard nursing unit after recovery in the postanesthesia care unit. Routine postoperative care was provided. Drains were removed prior to discharge. Overall length of stay (LOS), incidence of dysphagia requiring steroids or speech evaluation, and discharge disposition were recorded. Routine outpatient follow-up was performed with serial upright cervical radiographs, with additional imaging modalities obtained at the discretion of the operative surgeon.
Radiographic Measurements
Pre- and postoperative radiographs were reviewed. Cervical lordosis was assessed using the Cobb method for C1-C7 and C2-C7. Sagittal balance was measured using the C2-C7 cervical sagittal vertical axis as described by Ames et al.25 Upright preoperative radiographs were not available in some patients due to acute presentation to the emergency or inpatient setting.
Statistical Analysis
Cohort demographics, perioperative data, and postoperative complications were assessed using descriptive statistics. Student’s t test was used to compare pre- and postoperative radiographic measurements. Statistical significance was set at 0.05.
Results
Patient Cohort
A total of 45 patients underwent sACCF during the 10-year study period. Demographic data are shown in Table 1. The average age was 57.5 years, and most patients (55.6%) were women. The majority of patients (64.4%) were current smokers, and the average body mass index was 28.7. The most common indication for surgery was myelopathy (82.2%). A subset of patients (20%) had isolated radiculopathy. Patient-specific factors such as significant malalignment, dynamic instability, retrovertebral stenosis, OPLL, or a combination of these factors supported consideration of skip corpectomy in these cases. Only 3 patients (6.7%) had previous cervical spine surgery. Follow-up data were available for all but 2 trauma patients who did not return for evaluation after discharge (95.6% follow-up rate). Mean length of follow-up was 21.1 months.
Table 1.
Demographics of patients undergoing skip anterior cervical corpectomy and fusion.
| Demographics | N = 45 |
| Age, y, mean ± SD | 57.5 ± 7.9 |
| Body mass index, mean ± SD | 28.7 ± 5.4 |
| Men, n (%) | 20 (44.4) |
| Smoker, n (%) | 29 (64.4) |
| Myelopathy, n (%) | 37 (82.2) |
| Radiculopathy, n (%) | 27 (60.0) |
Perioperative Data
The vast majority of patients (93.3%) underwent C4 and C6 corpectomy with C3 to C7 anterior plating (Table 2). The remaining 3 patients underwent C5 and C7 corpectomy. The majority of cases utilized static interbody polyetheretherketone cages (93.3%), while 3 (6.7%) cases implanted expandable titanium cages. An assistant surgeon was used in approximately half of cases. Local autograft and allograft were used in almost all cases. Bone morphogenic protein was not utilized in any patients. Average estimated blood loss was 293 cc. Almost all patients had a surgical drain placed intraoperatively. Mean LOS was 4.4 days, but this number is substantially skewed by significant outliers. The median LOS was 2 days, with the vast majority (82.2%) having an LOS ≤3 days.
Table 2.
Operative details for patients undergoing skip anterior cervical corpectomy and fusion (n = 45).
| Operative Parameters | n (%) a |
| Corpectomy levels | |
| C4, C6 | 42 (93.3) |
| C5, C7 | 3 (6.7) |
| Anterior plating levels | |
| C3-C7 | 42 (93.3) |
| C3-T1 | 1 (2.2) |
| C4-T1 | 2 (4.4) |
| Local autograft | 45 (100) |
| Iliac crest aspirate | 27 (60.0) |
| Allograft | 44 (97.8) |
| Bone morphogenic protein | 0 (0) |
| Drain | 44 (97.8) |
| Estimated blood loss, mean ± SD | 293.2 ± 380.1 |
| Drain duration, d, mean ± SD | 1.6 ± 0.9 |
Data reported as n (%) except where otherwise noted.
Dysphagia
Bedside swallow evaluations were administered by nursing staff to all patients. Scheduled postoperative steroids and formal speech consultations and/or swallow studies were ordered at the discretion of the surgical team in cases with concern for or evidence of dysphagia. Eight patients (17.8%) received postoperative steroids for dysphagia, but only 3 patients (6.7% of all patients) required a formal speech consultation. These 3 patients required nothing by mouth status and alternative enteral access during the index hospitalization. Only 1 patient developed dysphagia as a direct result of surgery. The other 2 patients were unable to safely tolerate an oral diet secondary to encephalopathy and stroke, respectively. These 2 patients ultimately required percutaneous endoscopic gastrostomy (PEG) placement due to prolonged dysphagia and altered mental status. The single patient who developed postoperative dysphagia as a result of surgery had a prolonged hospital course and ultimately required revision surgery and PEG placement.
Postoperative Complications
Five patients (11.1%) developed a complication during the index hospitalization. Two patients (4.4%) had postoperative C5 palsies that were initially treated with steroids. Unfortunately, one of these patients subsequently underwent a posterior laminoforaminotomy 1 month later due to persistent weakness. Three patients (6.7%) developed severe medical complications requiring extended LOS (mean 25.7 days). The first patient developed pneumonia and dysphagia requiring PEG tube placement. She unfortunately also had early instrumentation failure during the initial hospitalization requiring a 2-stage anterior revision and posterior instrumented fusion. The second patient went into alcohol withdrawal. His hospital course was subsequently complicated by seizures, respiratory failure, and Clostridioides difficile infection. The third patient suffered a large-vessel infarction 4 hours after surgery. She ultimately required PEG tube placement for stroke-related dysphagia. Notably, 4 of the 5 complications occurred during the first 5 years of our experience.
Readmission
Three patients (6.7%) were readmitted, 1 electively, within 90 days of surgery. All 3 required secondary surgical interventions as detailed in the following section. The causes of readmission included a ground-level fall, prevertebral fluid collection with dysphagia and dysphonia, and scheduled readmission for refractory C5 palsy.
Secondary Surgical Procedures
Eight patients (17.8%) underwent a secondary surgical procedure during the follow-up period, of which 7 (15.6%) occurred within the first year after surgery (see Table 3). Three patients (6.7%) developed instrumentation failure at an average of 2.9 months postoperatively. All 3 required anterior revision with supplemental posterior fixation as a 2-stage procedure (Figure 2). Two patients (4.4%) developed adjacent segment disease that required operative intervention. The first patient developed stenosis above the fusion construct 4 years postoperatively. His skip corpectomy construct was intact with solid bony fusion, so he was treated with an adjacent C2-C3 ACDF. The second patient developed stenosis below the fusion construct 12 months postoperatively and was treated with a posterior foraminotomy and C3-T3 posterior instrumented fusion. One patient (2.2%) had a ground-level fall 1 month after surgery leading to a C7 vertebral body fracture at the caudal end of his construct that required posterior fixation. As discussed above, a patient with a persistent postoperative C5 palsy and radiographic evidence of residual foraminal stenosis underwent a posterior laminoforaminotomy 1 month after the index surgery. Finally, 1 patient (2.2%) developed dysphagia and dysphonia secondary to a prevertebral fluid collection 1 week after surgery. A subsequent wound exploration revealed a sterile fluid collection without hematoma. She was discharged 2 days later without need for antibiotics.
Table 3.
Secondary surgical interventions required after skip anterior cervical corpectomy and fusion.
| Patient No. | Reason | Mo Postoperative | Management |
| Patient 1 | Hardware failure | 0.5 | Anterior revision + posterior fixation |
| Patient 2 | Hardware failure | 4 | Anterior revision + posterior fixation |
| Patient 3 | Hardware failure | 5 | Anterior revision + posterior fixation |
| Patient 4 | Adjacent segment disease | 48 | C2-C3 anterior cervical discectomy and fusion |
| Patient 5 | Adjacent segment disease | 12 | Posterior foraminotomy and fusion |
| Patient 6 | Fall leading to C7 fracture | 1 | Posterior fixation |
| Patient 7 | Refractory C5 palsy | 1 | Posterior foraminotomy |
| Patient 8 | Prevertebral fluid collection | 0.5 | Exploration and washout |
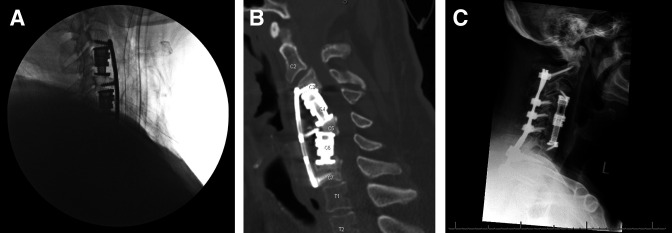
Figure 2.
Early hardware failure requiring anterior revision and posterior fixation. The final intraoperative lateral fluoroscopic image after the index procedure (A), upright lateral radiograph obtained 2 weeks postoperatively (B), and an upright lateral radiograph following anterior and posterior revision (C) in a single patient with early hardware failure.
Radiographic Alignment
Preoperative imaging was available for 31 patients. Postoperative imaging was obtained for all patients. Changes in radiographic alignment are reported in Table 4. There was a statistically significant increase in C1-C7 lordosis (46.8° ± 10.3° vs 41.1° ± 11.0°, P = 0.001) and C2-C7 lordosis (11.10° ± 9.0° vs 4.96° ± 10.5°, P < 0.001) postoperatively. There was no significant difference between pre- and postoperative C2-C7 sagittal vertical axis.
Table 4.
Alignment correction in degrees after skip anterior cervical corpectomy and fusion (n = 31).
| Radiographic Alignment | Preoperative | Postoperative | Change (P) |
| C2-C7 lordosis | 4.96 ± 10.5 | 11.10 ± 9.0 | 5.83 (<0.001) a |
| C1-C7 lordosis | 41.13 ± 11.0 | 46.75 ± 10.3 | 3.83 (0.001) a |
| C2-C7 sagittal vertical axis | 30.60 ± 18.0 | 33.51 ± 10.7 | 1.17 (0.250) |
Significant at P < 0.05.
Discussion
The optimal surgical approach for multilevel cervical stenosis and myelopathy remains controversial. For patients in which an anterior approach is deemed favorable, standard options for decompression across 4 disc space levels include 4-level ACDF or 3-level ACCF. sACCF represents an alternative approach that may allow for improved decompression compared with 4-level ACDF and enhanced biomechanical stability compared with 3-level ACCF. This report supplements prior studies in demonstrating the safety and efficacy of sACCF in the treatment of multilevel cervical stenosis and myelopathy.
Ashkenazi and colleagues were the first to formally describe the sACCF technique in 2005.20 In what they termed as “hybrid” decompression and fixation technique, 25 patients underwent a combination of corpectomies and discectomies utilizing titanium mesh cages and dynamic plates. Thirteen patients underwent a true “skip” corpectomy in which corpectomies were performed above and below an intervening intact vertebral body. The other 12 patients underwent single-level corpectomy and adjacent discectomy. Of the 13 patients who underwent a true “skip” corpectomy, 12 were fused at final follow-up (mean follow-up, 29 months). Eight patients reported improved subjective neurologic status, along with 4 who were unchanged and 1 who experienced neurologic deterioration. One patient from the entire cohort developed mechanical failure treated with revision anterior surgery, but the authors did not report which initial surgery this patient received.
Dalbayrak et al formally coined the term “skip” corpectomy in 2010.21 In their report, all 29 patients underwent corpectomy at C4 and C6, with preservation of the C5 vertebral body. Iliac crest autograft was used in 15 patients, and fibular allograft was used in 14 patients. All patients underwent anterior cervical plating with a rigid plate that included fixation at C5. All patients were noted to be fused at final follow-up (mean follow-up 22.3 months). One patient (3.4%) experienced mechanical failure requiring revision surgery (type of revision surgery was not reported). Hoarseness and/or dysphagia was reported in 4 patients (13.8%). Postoperative C5 palsy was reported in 1 patient (3.4%).
The current study represents the largest and most robust study of sACCF to date. It importantly adds to the existing literature as no series have been published since the 2 initial reports by Ashkenazi and Dalbayrak. The mechanical failure rate reported in this study (6.7%) is double that of the only reported rate in the literature.21 It should be noted, however, that if the sole mechanical failure in the study by Ashkenazi et al was from the skip corpectomy cohort, this would represent a failure rate in line with the present study (7.7%).
All 3 patients who experienced instrumentation failure did so within 6 months of surgery. All patients were current smokers. Upon review of their imaging studies, there are common alignment features that likely predisposed them to such early failure. Two patients had focal cervical kyphosis with the apex at the level of the intervening vertebral body (C5). The third patient had grade I anterolisthesis at C3-C4 and grade II anterolisthesis at C4-C5. The biomechanical result in all cases was positioning of the cephalad vertebral body ventral to the intervening vertebral body in the sagittal plane. We believe such alignment predisposes patients to early instrumentation failure for 2 reasons. First, it forces the cage, spanning this corpectomy defect, to be tilted anteriorly, which limits lordosis correction and may accelerate screw pullout and graft migration. Second, it positions the intervening vertebral body away from the plate, preventing the screw from “pulling” the intervening body to the plate as described by Dalbayrak et al.21 Surgeons should be aware of these alignment challenges and take measures to overcome them, including aggressive bony and ligamentous decompression to allow for spinal mobility, ventral cage positioning, and bicortical screw purchase (especially in the intervening vertebral body).
Two patients (4.4%) developed a postoperative C5 palsy, which is in line with the rate reported by Dalbayrak et al (3.4%).21 The first patient had severe bilateral foraminal stenosis at C4-C5 on preoperative imaging, with residual bony stenosis on postoperative computed tomography image. Additionally, the lordosis correction achieved for this patient was about twice the mean for the study population (change in C2-C7 and C1-C7 lordosis of 12.6° and 7.4°, respectively). This patient ultimately required a posterior foraminotomy to adequately decompress the left C5 nerve root. The second patient also had severe right foraminal stenosis at C4-C5 on preoperative imaging. Motor-evoked potentials decreased in amplitude during the procedure without a concomitant change in somatosensory-evoked potentials. There were no preoperative x-rays available for this patient. Fortunately, his strength returned to baseline within 6 weeks of surgery.
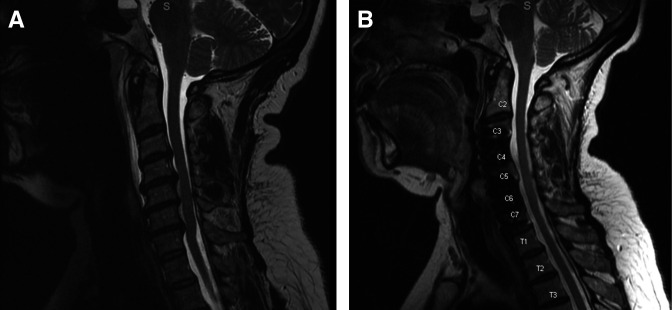
Our experience reinforces the benefit of performing an sACCF when spinal cord compression extends across 4 interspaces. Performing corpectomies at the cephalad and caudal aspects of the construct allows for improved visualization and working angle, thereby facilitating removal of posterior osteophytes for central and foraminal decompression in a manner that may be superior to 4-level ACDF (Figure 3). This procedure may also be particularly useful in the setting of OPLL. It should be noted, however, that this procedure is limited in its ability to decompress the spinal cord directly posterior to the skipped vertebral body (C5 or C6). In cases where there is significant cord compression at this level, alternative procedures should be considered.
Figure 3.
Extent of spinal cord decompression achieved with skip corpectomy (A), (B).
Conclusion
sACCF can be performed safely and effectively for the management of multilevel cervical stenosis. It may allow for improved decompression of neural elements compared with multilevel ACDF with similar complication rates and enhanced biomechanical stability compared with 3-level corpectomy. This procedure should be considered in patients with significant ventral cervical pathology across interspaces for whom an anterior cervical approach is favored.
References
- 1. Bailey RW, Badgley CE. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am. 1960;42-A:565–594. 10.2106/00004623-196042040-00001 [DOI] [PubMed] [Google Scholar]
- 2. Dalbayrak S, Yilmaz M, Naderi S. Cervical SKIP corpectomy. Benzel EC, Benzel’s Spine Surgery. 4th ed. Elsevier; 2017:83–94. [Google Scholar]
- 3. Daubs MD. Early failures following cervical corpectomy reconstruction with titanium mesh cages and anterior plating. Spine (Phila Pa 1976). 2005;30(12):1402–1406. 10.1097/01.brs.0000166526.78058.3c [DOI] [PubMed] [Google Scholar]
- 4. Eleraky MA, Llanos C, Sonntag VK. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg. 1999;90(1 Suppl):35–41. 10.3171/spi.1999.90.1.0035 [DOI] [PubMed] [Google Scholar]
- 5. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990–997. 10.3171/jns.1997.86.6.0990 [DOI] [PubMed] [Google Scholar]
- 6. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 Suppl):10–16. 10.3171/spi.2002.96.1.0010 [DOI] [PubMed] [Google Scholar]
- 7. Naderi S, Alberstone CD, Rupp FW, Benzel EC, Baldwin NG. Cervical spondylotic myelopathy treated with corpectomy: technique and results in 44 patients. FOC. 1996;1(6):E7. 10.3171/foc.1996.1.6.8 [DOI] [PubMed] [Google Scholar]
- 8. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138–143. 10.1007/BF01358747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thalgott JS, Xiongsheng C, Giuffre JM. Single stage anterior cervical reconstruction with titanium mesh cages, local bone graft, and anterior plating. Spine J. 2003;3(4):294–300. 10.1016/s1529-9430(02)00588-0 [DOI] [PubMed] [Google Scholar]
- 10. Hee HT, Majd ME, Holt RT, Whitecloud TS, Pienkowski D. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16(1):1–8. 10.1097/00024720-200302000-00001 [DOI] [PubMed] [Google Scholar]
- 11. Jones J, Yoo J, Hart R. Delayed fracture of fibular strut allograft following multilevel anterior cervical spine corpectomy and fusion. Spine (Phila Pa 1976). 2006;31(17):E595–E599. 10.1097/01.brs.0000229253.17108.03 [DOI] [PubMed] [Google Scholar]
- 12. Sasso RC, Ruggiero RA, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976). 2003;28(2):140–142. 10.1097/00007632-200301150-00009 [DOI] [PubMed] [Google Scholar]
- 13. Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11(5):410–415. [PubMed] [Google Scholar]
- 14. Foley KT, DiAngelo DJ, Rampersaud YR, Vossel KA, Jansen TH. The in vitro effects of instrumentation on multilevel cervical strut-graft mechanics. Spine (Phila Pa 1976). 1999;24(22):2366–2376. 10.1097/00007632-199911150-00014 [DOI] [PubMed] [Google Scholar]
- 15. Wang JC, Hart RA, Emery SE, Bohlman HH. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine (Phila Pa 1976). 2003;28(10):1016–1021. 10.1097/01.BRS.0000061998.62204.D7 [DOI] [PubMed] [Google Scholar]
- 16. Edwards CC, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. current diagnostic and treatment strategies. Spine J. 2003;3(1):68–81. 10.1016/s1529-9430(02)00566-1 [DOI] [PubMed] [Google Scholar]
- 17. Stewart TJ, Steinmetz MP, Benzel EC. Techniques for the ventral correction of postsurgical cervical kyphotic deformity. Neurosurgery. 2005;56(1 Suppl):191–195; . 10.1227/01.neu.0000144496.36844.7b [DOI] [PubMed] [Google Scholar]
- 18. Rhee JM, Riew KD. Surgical management of cervical myelopathy. J Neurol Sci (Turkish). 2005;22:359–373. [Google Scholar]
- 19. Ozer AF, Oktenoğlu BT, Sarioğlu AC. A new surgical technique: open-window corpectomy in the treatment of ossification of the posterior longitudinal ligament and advanced cervical spondylosis: technical note. Neurosurgery. 1999;45(6):1481–1485. 10.1097/00006123-199912000-00046 [DOI] [PubMed] [Google Scholar]
- 20. Ashkenazi E, Smorgick Y, Rand N, Millgram MA, Mirovsky Y, Floman Y. Anterior decompression combined with corpectomies and discectomies in the management of multilevel cervical myelopathy: a hybrid decompression and fixation technique. J Neurosurg Spine. 2005;3(3):205–209. 10.3171/spi.2005.3.3.0205 [DOI] [PubMed] [Google Scholar]
- 21. Dalbayrak S, Yilmaz M, Naderi S. “ SKIP ” corpectomy in the treatment of multilevel cervical spondylotic myelopathy and ossified posterior longitudinal ligament. J Neurosurg Spine. 2010;12(1):33–38. 10.3171/2009.7.SPINE08965 [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz M, Yüksel KZ, Baek S, et al. Biomechanics of cervical “ SKIP ” corpectomy versus standard multilevel corpectomy. Clin Spine Surg. 2017;30(3):E152–E161. 10.1097/BSD.0b013e318268d30a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Q, Zhou X, Wang X, Cao P, Tsai N, Yuan W. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J. 2012;21(3):474–481. 10.1007/s00586-011-1961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian L, Shao J, Liu Z, et al. Comparison of the safety and efficacy of anterior “ SKIP” corpectomy versus posterior decompression in the treatment of cervical spondylotic myelopathy. J Orthop Surg Res. 2014;9:63. 10.1186/s13018-014-0063-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S149–60. 10.1097/BRS.0b013e3182a7f449 [DOI] [PubMed] [Google Scholar]