Abstract
Background
Hemichorea typically results from a contralateral subthalamic nuclei (STN) lesion, although it has been reported in the cortex in a minority of cases. However, to our best knowledge, there are no documented cases in literature of hemichorea occurring as a secondary condition to an isolated temporal stroke.
Case presentation
We present a case of an elderly female who sustained a sudden onset of hemichorea in her right extremities, predominantly in the distal region, lasting over a period of two days. Brain diffuse weighted image (DWI) demonstrated a high signal in the temporal region, while magnetic resonance angiography (MRA) revealed severe stenosis of the middle cerebral artery. During the symptomatic phase, computed tomography perfusion (CTP) revealed delayed perfusion in the left middle cerebral artery territory, characterized by the time-to-peak (TTP) measure. Based on the results of her medical history and laboratory tests, we were able to rule out the possibility of infectious, toxic, or metabolic encephalopathy. Her symptoms gradually improved with antithrombotic and symptomatic treatment.
Conclusions
It is important to recognize and consider acute onset hemichorea as an initial symptom of stroke to avoid misdiagnosis and delays in appropriate treatment. Further research on temporal lesion that lead to hemichorea is warranted to gain a better understanding of the underlying mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-023-03230-6.
Keywords: Hemichorea, Temporal Stroke, Cortical Infarction
Background
Hemichorea is defined as a syndrome characterized by continuous, irregular, and involuntary jerky movements on one side of the body. Its pathogenesis can originate from infections, immune reaction, metabolic abnormalities, malignancy, neurodegeneration, vascular diseases, or drugs [1]. Hemichorea typically results from a focal lesion of the contralateral subthalamic nuclus (STN), which is thought to be an essential node in the complex neuronal network consisting of the basal ganglia and different motor cortical areas producing hyperkinetic movements, while causative lesions in cortex have been documented in a minority. However, to the best of our knowledge, there have been no previous reports in the literature of hemichorea resulting from isolated temporal stroke.
Herein, we present a case of an acute temporal stroke responsible for hemichorea in the right extremities, which have significantly resolved after treatment.
Case presentation
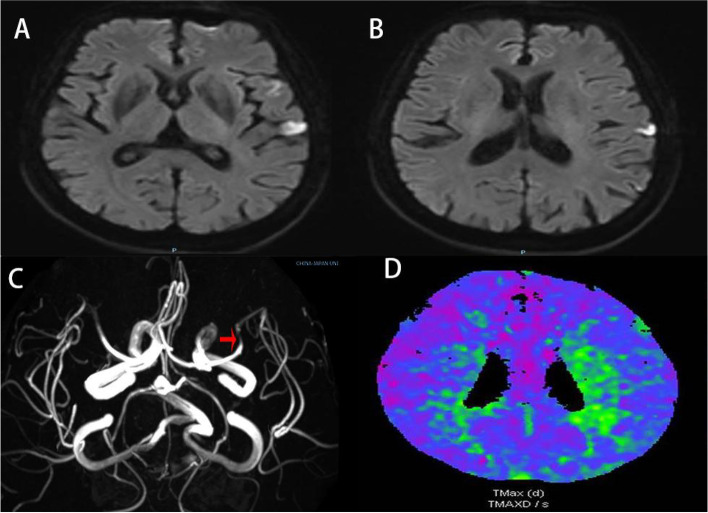
A 71-year-old female with a past medical history of hypertension was admitted to our hospital due to hyperkinesia in her right extremities for two days. She had a sudden onset of involuntary flailing movements of her right upper extremity, unexpected grasping of her right hand, and abrupt kicks with her right lower extremity. These involuntary movements in the right upper and lower extremities were persistent, unsynchronized, variable and worsened when intentionally carrying out tasks (See Video 1 in the online supplementary material). The patient had mild dysarthria throughout the entire course of the illness, which abruptly deteriorated to complete aphasia with a decrease in muscle strength of her right upper extremity to level 3 for a duration of 10 min—during this time, her hemichorea continued. Her speech and muscle strength subsequently returned to normal, and she did not experience any further episodes. The results of a brain computed tomography (CT) scan showed unremarkable findings. Laboratory examinations and her medical history ruled out infectious, toxic, and metabolic causes of encephalopathy. Brain magnetic resonance images (MRI) illustrated an acute ischemic lesion in the left temporal cortex (Fig. 1A and B), and magnetic resonance angiography (MRA) of the head demonstrated severe stenosis in the left M1 branch (Fig. 1C). The computed tomography perfusion (CTP) scan during the symptomatic phase showed delayed perfusion in the left parietal cortex and corona radiata in the time-to-peak (TTP) measure (Fig. 1D).
Fig. 1.
Brain images. DWI demonstrated high signal in the temporal region (A and B); MRA illustrated severe left M1 stenosis (arrow) (C); CTP during the symptomatic phase showed delayed perfusion in the left parietal cortex and corona radiata in the TTP measure (D). DWI = diffuse weighted image; MRA = magnetic resonance angiography; CTP = computed tomography perfusion; TTP = time-to-peak
The patient was given aspirin and atorvastatin separately, with a dosage of 100 mg and 20 mg, respectively, once a day and received 100 ml of butylphthalide injection twice daily. Despite being prescribed oral haloperidol at a dose of 2 mg twice daily for 3 days, she did not experience any relief of symptoms. Given no improvement, the dose of haloperidol was up-titrated to three times daily—4 mg in the morning, 2 mg in the afternoon, and 2 mg in the evening. Ten days after the initiation of antithrombotic therapy and pharmacological management of movement disorders mentioned above, she was discharged with significant improvement in hemichorea. The patient provided informed consent and has agreed to the publication of this case report.
Discussion
Hemichorea is a rare initial or sole sign of acute ischemic stroke that originates purely from the cortex, especially in the temporal lobe, which is not typically associated with motor function. In this case report, we demonstrated a patient who exhibited hemichorea in the right extremities as the initial symptom of an isolated temporal stroke with severe middle cerebral artery stenosis, and no other intracranial pathology was found to account for the symptoms. This was confirmed by DWI and CTP imaging performed during the symptomatic phase. Furthermore, hemichorea was diagnosed, as opposed to stereotypy, due to the patient’s presentation of ongoing random-appearing sequences of one or more discrete involuntary movements or movement fragments. In contrast, stereotypies are repetitive, simple, rhythmic movements that can be voluntarily suppressed (e.g., simple back-and-forth movements such as waving or flapping the hands or arms) and they do not typically involve more complex sequences or movement fragments. There is probably no premonitory urge to move, and the movements tend to occur when the patient is stressed, excited, distracted or engrossed. Stereotypies can be stopped by distraction or initiation of another activity. In conclusion, stereotypy is distinguished from chorea by the predictability of the phenomenology and triggers of the movement [2].
By using search terms with Boolean operators—i.e., (“hemichorea” [Title/Abstract] OR “hemiballism” [Title/Abstract]) AND (“cortex” [Title/Abstract] OR “cortical” [Title/Abstract]), we searched the PubMed database up until March 2022 and identified 60 articles. Among those, 10 articles were selected which reported on hemichorea following a cortical lesion [3-12] (see Table 1). Of the 10 articles, only 5 reported on hemichorea after lesions that included the temporal lobe [3, 4, 7, 11 and 12], but none of which were purely secondary to a temporal lesion. Notably, symptoms arising from the cortex were less severe and had good long-term prognosis, as the symptoms subsided with treatment or resolved completely over time.
Table 1.
Summary of cases of hemichorea following cortical lesions
| Ref | Author | Year | HC/HB | lesions | pathogenesis |
|---|---|---|---|---|---|
| 1 | Carbayo á[3] | 2020 | L | R parietal and posterior frontal cortices | R M3 occlusion |
| R | L insular and parietal cortex | L M2 branch occlusion | |||
| L | R parietal and insular cortex | AF | |||
| R | L insular, temporal, and parietal cortex | L M2 occlusion | |||
| 2 | Hernandez Fustes OJ[4] | 2020 | L | R temporo−parietal cortex | AF |
| 3 | Cotroneo M[5] | 2019 | L | R frontoparietal region | R carotid stenosis |
| L | R frontoparietal and insular cortex | AF | |||
| R | R frontal−parietal−insular cortex | AF | |||
| 4 | Strauss S[6] | 2019 | R | R parieto−occipital region | Paroxysmal AF |
| 5 | Jacob S[7] | 2016 | L | R mesial−temporal and hippocampal cortical regions, and minimally the occipital cortex | P2 focal occlusion |
| 6 | Shrestha P[8] | 2015 | R | L posterior parietal lobe | stenosis of the R−P1 segment |
| 7 | Hao M[9] | 2015 | L | both sides of the corona radiate and parietal cortex | stenosis on both of middle and posterior occlusion of ACA |
| 8 | Pichierri A[10] | 2012 | L | R premotor cortex area | tumor |
| 9 | Chung SJ[11] | 2004 | R | L parietal cortex | L M1 stenosis |
| L | R insular cortex | NA | |||
| L | R frontal, and insular cortex | NA | |||
| L | R frontal, and parietal cortex | NA | |||
| L | R parietal and temporal cortex | R ICA occlusion | |||
| R | L temporal, insular cortex | L M1 occlusion | |||
| 10 | Krauss JK[12] | 1999 | R | L temporooccipital cortex | AVM |
HC/HB Hemichorea/hemiballism, R Right; L Left; AF Atrial fibrillation, ACA Anterior cerebral artery, ICA Internal carotid artery, NA Not available, AVM Arteriovenous malformation
The mechanism accounting for this phenomenon may be attributed to the complexity and wide distribution of motor pathways involved in hemichorea, beyond the classic model of basal ganglia circuitry. Notably, the temporal lobe is likely to play a significant node in these pathways. When the metabolism of the temporal lobe is disrupted, it may weaken the inhibitory effect of the cortex, alter sensorimotor integration and spatial firing patterns, and ultimately result in subthalamic hyperactivity that manifests as hemichorea [13]. The hypothesis is strongly supported by the effective relief of symptoms observed in our case after an increased dosage of haloperidol.
During the symptomatic phase in this case, CTP showed hypoperfusion in the left parietal cortex and corona radiata, primarily due to severe MCA stenosis [14], which could have disturbed the metabolism of the basal ganglia, particularly in the striatum. The striatum, which is a part of the basal ganglia along with the thalamus, lentiform nucleus, and sensorimotor cortex, plays a crucial role in mediating muscle tone. The sudden onset of a transient ischemic attack, likely caused by hemodynamic compromise in MCA area, strongly supports this theory.
Most patients with hemichorea associated with a pure cortical infarct show a good prognosis as symptoms tend to disappear spontaneously or with medication, possibly owing to the structural integrity of the STN. Our patient’s hemichorea also demonstrated substantial improvement after 10 days of pharmacological treatment.
Conclusion
Our case suggests that isolated temporal stroke caused by severe MCA stenosis can be associated with hemichorea in the absence of any other lesions. Therefore, clinicians should be aware that acute onset hemichorea could be an initial manifestation of acute ischemic stroke, to prevent misdiagnosis and delay in administering appropriate treatment. Further research on temporal stoke leading to hemichorea is necessary to better elucidate the underlying mechanisms.
Supplementary Information
Acknowledgements
We thank the patient and her daughter for their consents to publish the case report.
Abbreviations
- STN
Subthalamic nucleus
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- CTP
Computed tomography perfusion
- TTP
Time-to-peak
- DWI
Diffuse weighted imaging
- MRA
Magnetic resonance angiography
Authors’ contributions
GS and HD examined the patient and drafted the manuscript. JZ evaluated the neuroimaging findings and provided critical clinical opinions. KYL helped with English editing of the manuscript. GS conceived the case and is accountable for the integrity of the entire work. All authors read and approved the final manuscript.
Funding
We receive no funding support.
Availability of data and materials
All data and material supporting our findings are contained within the manuscript.
Declarations
Ethics approval and consent to participate
The patient has provided consent for the publication of this case report.
Consent for publication
Written informed consent was obtained from the patient and patient’s daughter for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The author(s) declared no conflicts of interest with respect to the research, authorship, funding, and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Postuma RB, Lang AE. Hemiballism: revisiting a classic disorder. Lancet Neurol. 2003;2(11):661–668. doi: 10.1016/S1474-4422(03)00554-4. [DOI] [PubMed] [Google Scholar]
- 2.Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, Singer HS, Alter K, Ben-Pazi H, Butler EE, Chen R, Collins A, Dayanidhi S, Forssberg H, Fowler E, Gilbert DL, Gorman SL, Gormley ME, Jr, Jinnah HA, Kornblau B, Krosschell KJ, Lehman RK, MacKinnon C, Malanga CJ, Mesterman R, Michaels MB, Pearson TS, Rose J, Russman BS, Sternad D, Swoboda KJ, Valero-Cuevas F. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbayo Á, Sarto J, Santana D, Compta Y, Urra X. Hemichorea as presentation of acute cortical ischemic stroke. case series and review of the literature. J Stroke Cerebrovasc Dis. 2020;29(10):105150. doi: 10.1016/j.jstrokecerebrovasdis.2020.105150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez Fustes OJ, Puppi Munhoz R, Arteaga Rodriguez C, Hernandez Fustes OJ. Chorea as the first manifestation of cerebral infarction. Cureus. 2020;12(3):e7384. doi: 10.7759/cureus.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotroneo M, Ciacciarelli A, Cosenza D, Casella C, Dell'Aera C, Grillo F, Fazio MC, La Spina P, Musolino RF. Hemiballism: Unusual clinical manifestation in three patients with frontoparietal infarct. Clin Neurol Neurosurg. 2020;188:105612. doi: 10.1016/j.clineuro.2019.105612. [DOI] [PubMed] [Google Scholar]
- 6.Strauss S, Rafie D, Nimma A, Romero R, Hanna PA. Pure cortical stroke causing hemichorea-hemiballismus. J Stroke Cerebrovasc Dis. 2019;28(10):104287. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Jacob S, Gupta HV. Delayed hemichorea following temporal-occipital lobe infarction. Tremor Other Hyperkinet Mov (N Y) 2016;19(6):414. doi: 10.5334/tohm.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha P, Adhikari J, Poudel D, Pathak R, Karmacharya P. Cortical Hemiballism: A Case of Hemiballismus Associated with Parietal Lobe Infarct. N AmJ Med Sci. 2015;7(12):572–574. doi: 10.4103/1947-2714.172850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao M, Qin X, Gao H. A case of Hemichorea-Hemiballism Induced by Acute Infarction of Bilateral Corona Radiata and Cortex. Cell Biochem Biophys. 2015;73(1):171–174. doi: 10.1007/s12013-015-0608-6. [DOI] [PubMed] [Google Scholar]
- 10.Pichierri A, Cappelletti M, Ruggeri A, Pedace F, Tarantino R, Delfini R. Hemiballism caused by a premotor cortex glioma. Clin Neurol Neurosurg. 2013;115(4):464–467. doi: 10.1016/j.clineuro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol. 2004;251(6):725–729. doi: 10.1007/s00415-004-0412-5. [DOI] [PubMed] [Google Scholar]
- 12.Krauss JK, Kiriyanthan GD, Borremans JJ. Cerebral arteriovenous malformations and movement disorders. Clin Neurol Neurosurg. 1999;101(2):92–99. doi: 10.1016/S0303-8467(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 13.Laganiere Simon, Boes Aaron D, Fox Michael D. Network localization of hemichorea-hemiballismus Neurology. 2016;86(23):2187–2195. doi: 10.1212/WNL.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oya S, Fujisawa N, Matsui T. Hemichorea-hemiballismus caused by postoperative hyperperfusion after clipping of a giant unruptured middle cerebral artery aneurysm. Surg Neurol Int. 2015;21(6):84. doi: 10.4103/2152-7806.157444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material supporting our findings are contained within the manuscript.