Abstract
Immunogenicity and protective efficacy of a DNA vaccine encoding Ag85A from Mycobacterium tuberculosis were compared in BALB/c and C57BL (B6 and B10) mice immunized by intramuscular (i.m.) needle injection or epidermal gene gun (gg) bombardment. In BALB/c mice, gg immunization could induce elevated antibody and cytotoxic T lymphocyte responses with plasmid doses 50-fold lower than those required for i.m. immunization. Interleukin-2 (IL-2) and gamma interferon (IFN-γ) secretion, however, was much lower in gg-immunized than in i.m.-immunized BALB/c mice. On the other hand, C57BL mice reacted only very weakly to gg immunization, whereas elevated Ag85A-specific antibody, IL-2, and IFN-γ responses (significantly higher than in BALB/c mice) were detected following vaccination by the i.m. route. Antibody isotypes were indicative of Th2 activation following gg injection of BALB/c and of Th1 activation following i.m. injection of C57BL mice. Finally, C57BL but not BALB/c mice were protected by i.m. Ag85A DNA immunization against intravenous M. tuberculosis challenge, as measured by reduced numbers of CFU in spleen and lungs, compared to animals vaccinated with control DNA. Gene gun immunization was not effective in either BALB/c or C57BL mice. These results indicate that i.m. DNA vaccination is the method of choice for the induction of protective Th1 type immune responses with the Ag85A tuberculosis DNA vaccine.
Tuberculosis remains a major health problem affecting millions of people worldwide (5). Combination chemotherapy is very effective in curing this disease but, unfortunately, the treatment is long and expensive and requires stringent compliancy to avoid the development of multi-drug-resistant forms of Mycobacterium tuberculosis. The only tuberculosis vaccine currently available is an attenuated strain of M. bovis, termed bacillus Calmette-Guérin (BCG). BCG continues to be widely administered to children in developing countries, yet its efficacy remains controversial, particularly against pulmonary tuberculosis in young adults (4). Clearly, the development of a better vaccine could be an effective solution to the global threat of tuberculosis.
The protective antigens for tuberculosis are still not precisely defined, and this seriously hampers every effort to improve or replace the existing tuberculosis vaccine. It has been hypothesized for more than a decade that extracellular (secreted or cell-wall-associated) proteins rather than intracellular, cytoplasmic proteins are the key antigens recognized by the protective immune response (27). Immunization with whole culture filtrate, which is a rich source of these exported proteins, has been described to protect mice and guinea pigs to some extent against subsequent challenge with the tubercle bacillus (1, 17, 28, 29). A major portion of the secreted proteins in M. tuberculosis and BCG culture filtrate is formed by the Ag85 complex, a 30- to 32-kDa family of proteins (Ag85A, Ag85B, and Ag85C) (39). Ag85 complex induces strong T-cell proliferation and gamma interferon (IFN-γ) production in most healthy individuals infected with M. tuberculosis or M. leprae and in BCG-vaccinated mice and humans (19, 24, 30, 31), making it a promising candidate as a protective antigen. We have previously shown that intramuscular (i.m.) vaccination with plasmid DNA encoding Ag85A induced strong humoral and cell-mediated immune responses and conferred significant protection in C57BL/6 mice challenged by aerosol with live M. tuberculosis H37Rv (20).
Administration of plasmid DNA expression vectors seems broadly applicable for generating protective immune responses against infectious pathogens without the need for live organisms, replicating vectors, or adjuvants (12, 35). Two major inoculation routes have been used so far for DNA vaccination: i.m. needle injection of DNA in saline (40) and epidermal gene gun (gg) bombardment with DNA-coated gold particles (32). For i.m. injections, routine doses of DNA in the mouse range between 10 and 100 μg. gg injections use considerably less DNA, with standard doses of between 0.1 and 1 μg. Because of the low plasmid doses used in gg immunizations, this technique has the potential of lower vaccine cost. Furthermore, mixing of a number of plasmids is possible in gg vaccination, and pools of plasmids can be screened by expression library immunization (3). Finally, gg immunization does not require the use of needles, which makes it an ideal method for use in children and human immunodeficiency virus-infected populations; also, this technique is easier to apply to a large-scale immunization.
In order to analyze whether gg immunization with plasmid DNA would be applicable to tuberculosis, we have compared the two current DNA immunization protocols, i.e., i.m. needle injection and gg bombardment with plasmid DNA encoding Ag85A from M. tuberculosis. Since we have previously shown that C57BL/6 mice demonstrate a stronger Th1-type immune response toward Ag85 following M. bovis BCG vaccination than BALB/c mice (in which this response in partly counterbalanced by Th2 cells) (19), comparative analysis of the gg and i.m. routes was performed on both strains. Whereas gg immunization induced strong antibody and CTL responses, Th1-type cytokine production was disappointingly low compared to i.m. immunization. Furthermore and unexpectedly, gg immunization was effective only in BALB/c mice and not in C57BL mice.
MATERIALS AND METHODS
Plasmid construction.
Plasmid DNA encoding Ag85A was prepared as described previously. Briefly, the 85A gene of M. tuberculosis was amplified without its mycobacterial signal sequence from plasmid p85A.tub (7) by PCR and ligated to the dephosphorylated VR1020 (Vical, Inc., San Diego, Calif.) vector. Recombinant plasmid DNA was amplified in Escherichia coli DH5 and purified on two cesium chloride-ethidium bromide gradients. Plasmid DNA was adjusted to a final concentration of 1 mg/ml in saline and stored at −20°C. In this plasmid, the Ag85A gene is expressed under control of the promoter and intron A of the first immediate-early antigen IE1 from cytomegalovirus and followed by a polyadenylation site of the bovine growth hormone. In the VR1020 vector a leader sequence of human tissue plasminogen activator is cloned upstream of the mature Ag85A gene, resulting in increased transcription and translation efficacy and increased immunogenicity (2).
Mice.
BALB/c (H-2d), C57BL/6 (B6, H-2b) and C57BL/10 (B10, H-2b) mice were bred in the Animal Facilities of the Pasteur Institute of Brussels. Only female mice, 6 to 8 weeks old at the start of vaccination, were used.
Coating of gold beads.
Gold beads were coated with plasmid DNA according to the manufacturer's recommendations. In short, 25 mg of gold powder was mixed with 100 μl of spermidine (0.05 M; Sigma) and sonicated. Next, 500 μg (first experiment) or 100 μg (all other experiments) of plasmid DNA encoding Ag85A in a 100-μl volume was added. Finally, 200 μl of 1 M CaCl2 was added dropwise to the mixture with gentle vortexing. After a 10-min precipitation step at room temperature, the pellets were washed three times and then resuspended in 100% ethanol. Finally, the pellets were resuspended in an ethanol solution containing 0.01 mg polyvinylpyrrolidone (Bio-Rad) per ml and coated on special tubing (Gold-Coat tubing; Bio-Rad). Cut cartridges containing nitrogen-dried DNA-coated gold beads were stored at −20°C.
gg immunization.
Mice were vaccinated on the shaved ventral skin using the Helios Gene Gun System (Bio-Rad) at a helium discharge pressure of 400 lb/in2. In the first experiment, three gg immunizations were performed at 3-week intervals, consisting of two nonoverlapping shots of 0.5 mg of either 0.6, 1, or 1.6-μm-diameter gold beads coated with 5 μg of plasmid DNA. In the other experiments, mice were vaccinated three times at 3-week intervals with two nonoverlapping shots of 1-μm gold beads coated with 1 μg of plasmid DNA.
i.m. DNA vaccination.
Mice were anesthetized by an intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg, respectively) and injected i.m. in both quadriceps with plasmid DNA encoding Ag85A in saline, using a 0.3-ml insulin syringe (Becton Dickinson). Mice received three injections at 3-week intervals of 10 μg of Ag85A encoding DNA in the first experiment and of 100 μg of Ag85A DNA in the other experiments.
Antigens.
Antigen 85A was purified from M. bovis BCG culture filtrate as described previously by sequential chromatography on phenyl-Sepharose, DEAE-Sephacel ion exchange, and molecular sieving on Sephadex G75 (10). Pokeweed mitogen (PWM; Gibco-BRL) was used as a T-cell-dependent B-cell mitogen to analyze polyclonal cytokine secretion.
ELISA.
Sera from gene gun and needle injected mice were collected by orbital bleeding 3 weeks after each DNA vaccination. Levels of anti-Ag85 antibodies were determined by enzyme-linked immunosorbent assay (ELISA) in sera from individual mice (three to five/group). The serum titer was converted to antibody concentration (in nanograms/milliliter) by comparison with a standard monoclonal antibody, and the mean antibody concentration was calculated from at least three points of the linear portion of the titration curve. Concentrations were converted to log10 values. For isotype analysis, peroxidase-labeled, rat anti-mouse immunoglobulin G1 (IgG1), IgG2a, and IgG2b (Experimental Immunology Unit, Université Catholique de Louvain, Brussels, Belgium) were used. Titers were expressed in dilution endpoints (last serum dilution with an optical density [OD] value higher than a cutoff OD value calculated from the OD with the secondary antibody only plus three standard deviations (SD). [20]).
Cytokine production.
DNA-vaccinated mice were sacrificed 3 weeks after the third DNA vaccination, and spleens were removed aseptically. Spleens from three mice were analyzed individually in each group. Spleen cells were adjusted to a concentration of 4 × 106 cells/ml and were grown in round-bottom microwell plates (Nunc), in RPMI 1640 (Gibco-BRL) medium supplemented with glutamine, HEPES, 50 mM 2-mercaptoethanol, antibiotics, and 10% heat-inactivated fetal calf serum (Gibco-BRL). A volume of 180 μl of cell suspension was added to a 20-μl volume of purified Ag85A (final concentration, 5 μg/ml) or PWM (dilution of 1:50 of stock solution). Cells were incubated at 37°C in a humidified CO2 incubator, and supernatants were harvested after 24 h (interleukin-2 [IL-2]) and 72 h (IL-4, IL-10, and IFN-γ). Supernatants from three separate wells were pooled and stored frozen at −20°C until assay.
IL-2 assay.
IL-2 activity was measured using a bioassay, as reported before (19). Each sample was tested in duplicate. IL-2 levels are expressed as the mean counts per minute (cpm). The SD was <10%. In this assay, 50,000 cpm correspond to 3.12 IU/ml (19), or about 600 pg/ml, and the detection limit was ca. 10 pg/ml.
IFN-γ assay.
Antiviral IFN-γ activity was quantified in duplicate on 72-h culture supernatants by using a bioassay, as reported before (21). Titers are expressed as mean log2 values obtained in three to five individual mice. The value of log2 = 1 corresponds to 110 pg/ml as measured in the Genzyme Mouse Interferon-γ DuoSet (80-3931-00). The detection limit of the bioassay was about 75 pg/ml.
IL-4 assay.
IL-4 activity was measured on a 72-h culture supernatant by ELISA using the Genzyme IL-4 DuoSet (80-3537-00).
IL-10 assay.
IL-10 activity was measured on a 72-h culture supernatant by ELISA using the Genzyme Mouse IL-10 ELISA kit (80-3749-05).
In vitro stimulation of cytotoxic T lymphocytes (CTLs).
Spleen cells (5 × 106/well) from DNA-vaccinated BALB/c mice were cultured for 6 days in 24-well plates with the immunodominant major histocompatibility complex type I (MHC-I) Kd-restricted peptide (amino acids 144 to 152 (VYAGAMSGL) from Ag85A (11).
Cytolytic assay.
CTL assay was performed as described previously (11). Briefly, lymphocytes from the stimulated cultures were tested for cytotoxicity in a 4-h 51Cr release assay in round-bottom microwell plates with 104 51Cr-labeled P815 cells pulsed with peptide VYAGAMSGL (5 μg/ml) at various effector/target ratios. Data are expressed as the percent specific lysis. Spontaneous release was generally 10 to 15% of the total release.
M. tuberculosis challenge.
BALB/c and C57BL/6 mice were vaccinated three times at 3-week intervals with control plasmid or Ag85A DNA either by gg bombardment (two shots, 1 μg/shot) or by i.m. injection (two injections, 50 μg/injection). Mice were rested for 2 months after the third DNA immunization and challenged intravenously in a lateral tail vein with 106 CFU of M. tuberculosis H37Rv grown as a surface pellicle for 2 weeks on synthetic Sauton medium and stored as a stock solution at −70°C in glycerol. Four weeks after challenge, mice were sacrificed, and serial threefold spleen and lung homogenate dilutions were plated on 7H11 Middlebrook agar supplemented with OADC (33). Petri dishes were incubated for 4 weeks in sealed plastic bags at 37°C, and colonies were counted visually. For statistical analysis (Student's t test), data obtained from two or three dilutions were used to calculate the mean log10 CFU values per spleen or lung. Data are expressed as mean log10 values per experimental group (each consisting of four to six animals).
RESULTS
Antibody production in mice vaccinated with 10 μg of plasmid DNA encoding Ag85A from M. tuberculosis administered i.m. by needle or epidermally with gold particles of three different sizes.
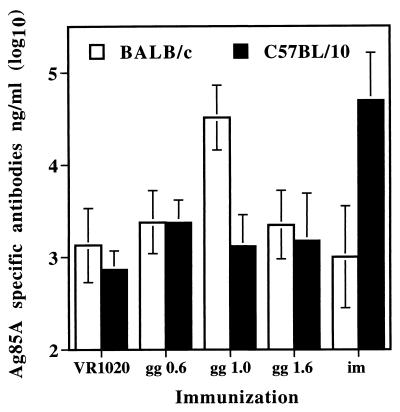
In a preliminary experiment, we compared gg and i.m. administration of a same dose of plasmid DNA, i.e., 10 μg/injection. Although this dose was probably not optimal for either route (too high for gg method and too low for the i.m. method), the idea was to have the same level of immunostimulatory effects linked to the CpG ODN content (23) of the vector backbone for both immunization routes. Ag85A DNA was administered three times at 3-week intervals to BALB/c or C57BL/10 mice, either by i.m. injection in saline in both quadricep muscles (5 μg/hind leg) or epidermally by two gg injections in the abdomen using DNA-coated gold particles 0.6, 1, and 1.6 μm in diameter (5 μg/bead). Mice were bled 3 weeks after the last DNA inoculation. As shown in Fig. 1, significant Ag85-specific antibody production could be detected following gg vaccination in BALB/c mice, whereas antibody levels in gg-injected B10 mice were only slightly above values obtained in mice vaccinated by gg with empty VR1020 vector. A bead size of 1 μm was found to give the best antibody response. i.m. vaccination with 10 μg of plasmid DNA induced elevated antibody levels in B10 mice, but this dose was too low to be effective in BALB/c mice. Mean Ag85A-specific antibody levels in gg-injected BALB/c mice were ca. 40,000 ng/ml, and mean antibody levels in i.m. vaccinated B10 mice were ca. 75,000 ng/ml.
FIG. 1.
Mean Ag85-specific antibodies in sera from BALB/c and B10 mice vaccinated with plasmid DNA encoding Ag85A administered either by gg on gold beads 0.6, 1, or 1.6 μm in diameter or i.m. Three doses of 10 μg of DNA were given at 3-week intervals, and mice were sacrificed 3 weeks after the third DNA injection. Individual antibody levels were calculated in nanograms/milliliter and converted to log10 values. Results are reported as mean log10 values from four individual mice in each group.
As shown in Fig. 2, antibody levels in gg-vaccinated BALB/c mice started to increase after the second shot of DNA and increased further after the third gg administration. After i.m. immunization of B10 mice, immunoglobulin antibodies started to increase already after one injection, and concentrations increased after the second and third immunization. In gg-immunized B10 mice and in i.m.-immunized BALB/c mice, antibody levels were close to the control values found in mice vaccinated with empty vector.
FIG. 2.
Mean Ag85A-specific antibodies in sera from BALB/c and B10 mice vaccinated once, twice, or thrice with plasmid DNA encoding Ag85A by gg or i.m. injection. Results are expressed as in Fig. 1.
Antibody production in mice vaccinated with plasmid DNA encoding Ag85A from M. tuberculosis administered i.m. (100 μg/injection) or by gg (2 μg/injection).
Since plasmid doses used in the first experiment were probably not optimal, we decided to compare the two immunization routes at the more classical doses used, i.e., the low dose of 2 μg by the gg route and the high dose of 100 μg by the i.m. route. As shown in Table 1, gg immunization again induced significant antibody production in BALB/c mice, whereas B10 mice (and B6 mice [data not shown]) again reacted only modestly to this immunization route. In contrast, i.m. injection with the high dose of DNA induced elevated antibody production in both mouse strains.
TABLE 1.
Mean Ag85A-specific antibodies in sera from BALB/c and C57BL/10 mice vaccinated three times with empty vector by gg (2 μg) or with DNA encoding Ag85A by gg (2 μg) or i.m. (100 μg)
| Immunization (route) | Mean log10
antibody levelsa (SD) in:
|
|
|---|---|---|
| BALB/c sera | C57BL/10 sera | |
| Vector (gg) | 3.13 ± 0.4 | 2.87 ± 0.2 |
| 85A DNA (gg) | 4.19 ± 0.34 | 3.57 ± 0.19 |
| 85A DNA (i.m.) | 4.33 ± 0.15 | 4.80 ± 0.26 |
Individual antibody levels were calculated in nanograms/milliliter and converted to log10 values. Results are reported as the mean log10 ± SD values from four individual mice in each group.
Antibody isotype in mice vaccinated by the gg or the i.m. route with plasmid DNA encoding Ag85A from M. tuberculosis.
Antibody responses in gg-vaccinated BALB/c and B10 mice were preferentially of the IgG1 isotype with little IgG2a, a finding indicative of Th2-type helper-T-cell activation (37). In contrast, antibody isotypes in i.m.-vaccinated C57BL/10 mice were strongly indicative of Th1 activation, with IgG2a and IgG2b titers 10- to 20-fold higher than IgG1 titers both at low and high DNA doses. Isotypes in i.m.-vaccinated BALB/c mice were of a mixed phenotype, even at the high DNA dose used, indicating that it is not only the dose of DNA that determines the isotype profile in DNA vaccination, but the genetic background of the mouse strain as well. Antibody isotypes in i.m.-vaccinated B6 mice were also strongly biased towards IgG2 (20).
IL-2 and IFN-γ production in mice vaccinated by the gg or the i.m. route with plasmid DNA encoding Ag85A from M. tuberculosis.
As shown in Fig. 3A, spleen cells from BALB/c mice vaccinated with plasmid DNA encoding Ag85A produced weak IL-2 levels in response to purified Ag85A following gg or low-dose i.m. injection. High-dose i.m. DNA induced significant IL-2 production in BALB/c mice. In B10 mice, elevated IL-2 levels could be induced following i.m. vaccination with both low and high doses of DNA, whereas gg vaccination was completely ineffective for IL-2 induction in B10 mice. As for IL-2, IFN-γ production in spleen cell cultures from DNA-vaccinated mice restimulated in vitro with purified Ag85A was highest in B10 mice injected i.m. (about 6 log2 U, corresponding to ca. 3,200 pg of IFN-γ per ml (Fig. 3B). Spleen cell cultures from BALB/c mice produced considerably lower titers (between 400 and 800 pg/ml) following either gg or low-dose i.m. injection, whereas significantly better IFN-γ production, albeit still at least twofold lower than in B10 mice, was observed upon high-dose i.m. immunization. As for IL-2, gg vaccination elicited only a marginal Ag85-specific IFN-γ response (ca. 100 pg/ml) in spleen cell cultures from B10 (or B6 [data not shown]) mice.
FIG. 3.
Mean IL-2 (A) and IFN-γ (B) levels in spleen cell culture supernatant from BALB/c and B10 mice vaccinated with plasmid DNA encoding Ag85A by gg (2 or 10 μg) or i.m. (10 or 100 μg) and restimulated in vitro with purified Ag85A protein. IL-2 was measured in a 24-h culture supernatant using a IL-2-dependent CTLL-2 line. Results are expressed as the mean cpm ± the SD from four individual mice/group. IFN-γ was measured in a 72-h culture supernatant using ELISA. Data are expressed as the mean picograms/milliliter ± the SD from four individual mice/group.
CTL responses in BALB/c mice vaccinated by the gg or the i.m. route with plasmid DNA encoding Ag85A from M. tuberculosis.
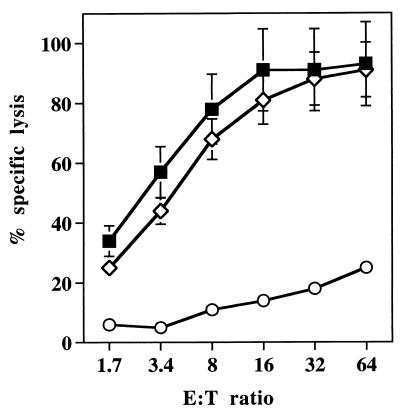
So far we have only been able to detect Ag85A-specific CD8+-mediated CTL activity (as measured in a 51Cr release assay) following i.m. immunization in BALB/c mice but not in B10 or B6 mice (11). Whether this lack of detectable CTL activity in H-2b mice vaccinated with Ag85A DNA is a technical problem or related to an absence of immunodominant MHC-I-restricted epitopes for the Kb and Db alleles on the protein is not yet clear. Therefore, we could only analyze BALB/c mice for the generation of CTL activity. Both gg and i.m. immunization were capable of generating strong CTL responses to the MHC-I Kd-restricted peptide from Ag85A (amino acids 144 to 152), which we have defined previously (11) (Fig. 4). As for antibody responses, gg immunization was effective for the induction of CTL responses at plasmid doses 50-fold lower than those required for optimal CTL induction by i.m. immunization. i.m. immunization with doses of 2 or 10 μg of plasmid DNA induced only suboptimal CTL responses (data not shown).
FIG. 4.
Ag85A specific cytolytic activity in spleen cell cultures from naive BALB/c mice (○) or BALB/c mice vaccinated with Ag85A DNA by gene gun (2 μg; ■) or i.m. (100 μg; ▵) and amplified for 1 week with peptide VYAGAMSGL. Spleens from five mice were pooled in each group. Results are expressed as the percent specific lysis at increasing effector/target (E:T) ratios.
IL-4 and interleukin-10 production in mice vaccinated with plasmid DNA encoding Ag85A from M. tuberculosis, administered i.m. or by gg with 1-μm gold particles.
As shown in Table 2 and as already described following i.m. immunization (20), IL-4 and IL-10 production in spleen cells from gg-vaccinated mice was very low following in vitro restimulation with the purified Ag85A. However, production of these two Th2-type cytokines following polyclonal PWM stimulation showed that the basal production level was about fivefold higher in BALB/c mice than in B10 mice and that i.m. but not gg immunization could reduce the PWM-induced stimulation of IL-4 more than 10-fold. However, PWM-induced IL-4 levels in i.m.-vaccinated BALB/c mice were still considerable (ca. 1,000 pg/ml). PWM-induced IL-10 levels did not seem to be affected by either immunization route and remained at least five times higher in BALB/c than in B10 mice.
TABLE 2.
IL-4 and IL-10 production in BALB/c and B10 mice vaccinated with plasmid DNA encoding Ag85A by gg (2 μg) or i.m. (100 μg) injectionsa
| Immunization (route) | IL-4 (pg/ml)
stimulated with:
|
IL-10 (pg/ml) stimulated
with:
|
||||
|---|---|---|---|---|---|---|
| Control | Ag85A | PWM | Control | Ag85A | PWM | |
| BALB/c | ||||||
| Control | 223 | 113 | >10,000 | <40 | <40 | >10,000 |
| Ag85A (gg) | 337 | 267 | >10,000 | <40 | 272 | >10,000 |
| Ag85A (i.m.) | 145 | 41 | 1,140 | <40 | 285 | 8,990 |
| C57BL/10 | ||||||
| Control | <5 | <5 | 2,562 | <40 | <40 | 1,179 |
| Ag85A (gg) | 87 | 15 | 2,026 | <40 | 104 | 1,350 |
| Ag85A (i.m.) | <5 | 42 | 119 | <40 | 245 | 2,042 |
IL-4 and IL-10 levels in 72-h spleen cell culture supernatants from control or DNA-vaccinated mice (four mice pooled/group) stimulated in vitro with purified Ag85A or PMW are shown.
Protection against M. tuberculosis H37Rv replication in the spleen and lungs of mice vaccinated with Ag85A DNA by the i.m. and not the gg route.
As shown in Fig. 5, only i.m. vaccination and not gg vaccination with Ag85A DNA was capable of reducing significantly the number of CFU in the spleen and lungs compared to the number of CFU in animals vaccinated with empty vector. Furthermore, this protection was only observed in B6 mice and not in BALB/c mice. C57BL/10 mice were also protected by i.m. Ag85A DNA vaccination (data not shown). BALB/c mice were more susceptible to a same intravenous inoculum (106 CFU) of M. tuberculosis than B6 mice, as reflected by higher CFU counts, in spleen and lungs, in control DNA vaccinated animals.
FIG. 5.
Replication of M. tuberculosis H37Rv in spleen (A and B) and lungs (C and D) from mice vaccinated by gg (2 μg) or i.m. (100 μg) with control DNA or plasmid DNA encoding Ag85A. Data represent the mean log10 CFU counts ± the SD of four to six animals per experimental group. Bars: □, BALB/c control; , BALB/c85A; ░⃞, B6 control; ■, B6 85A.
DISCUSSION
Over the last 4 years, we and others have reported that vaccination of mice with plasmid DNA encoding the 65-kDa heat shock protein (34), the 38-kDa phosphate-binding PstS-1 homolog (41), the 40-kDa phosphate-binding PstS-3 homolog (33), and the 30- to 32-kDa trehalose-mycolyl-transferase antigens 85A (20) and 85B (22) from M. tuberculosis is a powerful means for inducing strong humoral and cell-mediated immune responses and protective immunity against tuberculosis challenge (18). All of these observations used the i.m. immunization route and, to our knowledge, no data on the immunogenicity or protective efficacy of tuberculosis DNA vaccines administered by gg have been published thus far.
Using plasmid DNA encoding the nucleoprotein and hemagglutinin from influenza. Feltquate et al. have reported that i.m. injection of BALB/c mice with saline solutions of DNA induces preferentially a Th1-type T-helper response, whereas epidermal gg injection biases the immune response toward a strong Th2-type profile (13, 36). In order to find out whether this dichotomy could also be observed with plasmid DNA encoding mycobacterial genes, which are all characterized by a high GC content (ca. 70%) and therefore probably a high inherent CpG linked Th1-type immunostimulatory activity (23), we vaccinated BALB/c and C57BL mice with various doses of plasmid DNA encoding Ag85A from M. tuberculosis by epidermal gg bombardment or i.m. needle injection.
Confirming Feltquate's data, the gg immunization was a very effective technique for inducing strong antibody responses, with an isotypic profile suggestive of Th2 activation. Plasmid doses needed for an optimal antibody response were at least 50-fold lower with the gg immunization than with i.m. immunization. A new finding, however, was that only BALB/c mice produced strong antibody responses following gg immunization, whereas C57BL mice were only very weakly stimulated by this immunization route. This discrepancy between the two mouse strains could not be attributed directly to MHC-linked differences, since MHC congenic BALB.B10 mice also demonstrated strong antibody responses following gg immunization (data not shown). BALB/c mice have been reported to be Th2 “prone” in a number of experimental situations, such as experimental leishmaniasis (16, 25), BCG vaccination (19), and tuberculosis infection (8). It is tempting to speculate that this Th2 “proneness” of the genetic BALB/c background is the major factor determining their strong antibody reactivity following gg immunizations. Furthermore, the capacity to produce the Th2-type cytokines IL-4 and IL-10 following polyclonal stimulation with PWM was clearly higher in BALB/c mice than in B10 mice. In line with these findings, Virelizier previously reported that BALB/c mice produce significantly lower IFN-γ titers in response to PWM than did B10 mice (38). Finally, antibody isotypes were of a clearcut Th1 type in B10 mice vaccinated i.m. with DNA, but in BALB/c mice even the high i.m. dose of 100 μg of plasmid DNA induced a mixed isotype profile with elevated IgG1 and IgG2 titers, indicating that it is very difficult to overcome this Th2 proneness of BALB/c mice with the Ag85A DNA vaccine. An in vivo dominance of the Th2 over the Th1 response might explain why the Ag85A DNA vaccine only conferred protection against an intravenous M. tuberculosis challenge in C57BL mice and not in BALB/c mice, although i.m. immunization clearly induced a strong IL-2 and IFN-γ response in both mouse strains (although the B10 response was consistently higher than the BALB/c response). Interestingly, only i.m. vaccination of B10 mice completely blocked the PWM-induced IL-4 response, whereas in i.m.-vaccinated BALB/c mice this PWM-induced IL-4 response was still quite elevated, albeit somewhat reduced compared to the response of naive or gg-immunized BALB/c mice. With the exception of the 65-kDa heat shock protein, the new experimental tuberculosis vaccines, whether protein or DNA based, have all been tested in C57BL mice (1, 17, 20, 22, 26, 33, 41). Whether they are also effective in BALB/c mice remains an open question in the light of the results presented here.
A new and unexpected finding was that C57BL mice reacted only weakly to the gg immunization protocol, even at a plasmid dose of 10 μg, at which i.m. immunization induced elevated immune responses comparable to those induced by 100 μg given i.m. As DNA vaccines prime for immune response through the action of professional antigen-presenting cells (APC) (9), our findings could indicate that the Langerhans cells from the skin (the probable APC population involved in gg immunization) would be more effective in some mouse strains, such as BALB/c and BALB.B10, than in others, such as B10 and B6.
Protective immunity with the Ag85A DNA vaccine could only be induced in B6 (and B10) mice and not in BALB/c mice and only by i.m. and not by gg immunization, thus demonstrating that high Th1-type IFN-γ responses rather than strong CTL or antibody responses are critical immune parameters. Indeed, CD8+ cell-mediated CTL activity was very efficiently induced in gg-vaccinated BALB/c mice, but no protection against intravenous M. tuberculosis challenge could be observed. As CD8+-mediated CTL responses require endogenous processing of the antigen, this generation of strong CTL responses despite low Th1 cytokine titers was not completely surprising, and this finding also highlights the potential of DNA vaccines with respect to the generation of MHC-I-restricted CD8+ cell responses. This was corroborated recently by Fensterle et al., who reported on effective DNA vaccination by gg against listeriosis, an intracellular pathogen for which protection primarily depends on type 1 CD8+ T cells (14).
However, the relevance of CD8+ T-cell responses for protection against tuberculosis remains a matter of debate. Studies in mice genetically deleted for β2-microglobulin have indicated that CD8+ T cells play a role in the control of tuberculosis infection, particularly at later time points (15). Thus far we have been unable to demonstrate Ag85A-specific CD8+ responses in +/+ B6 mice vaccinated with Ag85A DNA (probably because the Ag85A protein lacks the necessary immunodominant Kb- and Db-restricted epitopes), although this vaccination can reduce significantly the early replication of M. tuberculosis in the spleen and lungs (33). Moreover, challenge experiments on β2-microglobulin knockout mice vaccinated with plasmid DNA encoding Ag85A also indicate that CD8+ responses are not required for early protection against tuberculosis (C. D. D'Souza et al., submitted for publication).
In conclusion, strong Ag85A-specific and polarized Th1-type immune responses and protection against M. tuberculosis challenge could only be detected in C57BL mice following i.m. and not gg immunization with plasmid DNA encoding Ag85A. To what extent these findings can be extrapolated to other mycobacterial genes remains to be examined, but preliminary results indicate that the immunization of C57BL mice with plasmid DNA encoding another protective antigen of tuberculosis, i.e., the 40-kDa PstS-3 protein, is also ineffective when administered by gg (A. Tanghe et al., unpublished data).
It is not known at present whether similar genetic variations will be found in the immunogenicity of tuberculosis DNA vaccines in the highly polymorphic human population. However, it is clear that at least for an Ag85A DNA-based DNA vaccine a robust IFN-γ rather than a CTL response seems to be needed for protective efficacy. Therefore, strong induction of this macrophage-activating cytokine without Th2-type counterbalancing cytokines appears to be the key element, and the magnitude of the IFN-γ response rather than the origin (CD4+ or CD8+, as reported by Bonato et al. (6) in the H-2d haplotype mice vaccinated with DNA encoding the hsp65 antigen) may offer the best correlate of protection to be targeted in a new tuberculosis vaccine.
ACKNOWLEDGMENTS
We are very grateful to R. Zaugg (Vical, Inc., San Diego, Calif.) for giving us the VR1020 plasmid. We also thank K. Palfliet, F. Jurion, D. Morales, and A. Vanonckelen for excellent technical assistance.
A.T. holds a grant from the Damiaanaktie Belgium. D.L. holds a grant from the Service du Premier Ministre (Affaires Scientifiques, Techniques et Culturelles). Part of this work was supported by grant G.0355.97 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosisinfection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin S L, D'Souza C D, Orme I M, Liu M A, Huygen K, Denis O, Tang A, Zhu L, Montgomery D, Ulmer J B. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosisAg85A. Tubercle Lung Dis. 1999;79:251–259. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 3.Barry M A, Lai W C, Johnston S A. Protection against mycoplasma infection using expression-library immunization. Nature. 1995;377:632–635. doi: 10.1038/377632a0. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 531–557. [Google Scholar]
- 5.Bloom B R C, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 6.Bonato V L D, Lima V M F, Tascon R E, Lowrie D B, Silva C L. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borremans M, De Wit L, Volckaert G, Ooms J, De Bruyn J, Huygen K, Van Vooren J P, Stelandre M, Verhofstadt R, Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989;57:3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brett S J, Ivanyi J. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology. 1990;7:113–119. [PMC free article] [PubMed] [Google Scholar]
- 9.Corr M, Lee D J, Carson D A, Tighe H. Gene vaccination with naked plasmid DNA: mechanisms of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J-P, Falmagne P, Weckx M, Wiker H G, Harboe M, Turneer M. Purification, characterization and identification of a 32-kDa protein antigen of Mycobacterium bovisBCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 11.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosisH37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 13.Feltquate D M, Heany S, Wabster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 14.Fensterle J, Grode L, Hess J, Kaufmann S H E. Effective DNA vaccination against listeriosis by prime/boost inoculation with the gene gun. J Immunol. 1999;163:4510–4518. [PubMed] [Google Scholar]
- 15.Flynn J L, Goldstein M A, Treibold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-γ or interleukin-4 during resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K. DNA vaccines: application to tuberculosis. Int J Tuberc Lung Dis. 1998;2:971–978. [PubMed] [Google Scholar]
- 19.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J-P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovisBCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 21.Huygen K, Palfliet K, Jurion F, Hilgers J, ten Berg R, Van Vooren J-P, De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovisbacillus Calmette-Guerin. Infect Immun. 1988;56:3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of M. tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinman D M, Yi A-K, Beaucage S L, Konover J, Krieg A M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFN-γ. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launois P, DeLeys R, N'Diaye Niang M, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T cell epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3697–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by V beta 4 V alpha 8 CD8+ T cells instructs Th2 development and susceptibility to Leishmania majorin BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 26.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosisinduces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts A D, Sonnenberg M J, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 30.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver R F, Wallis R S, Ellner J J. Mapping of T cell epitopes of the 30-kDa α antigen of Mycobacterium bovis strain bacillus Calmette-Guérin in purified protein derivative (PPD)-positive individuals. J Immunol. 1995;154:4665–4674. [PubMed] [Google Scholar]
- 32.Tang D, Devit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 33.Tanghe A, Lefèvre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 34.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 35.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 36.Torres C A T, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 37.Vercammen M, Scorza T, Huygen K, De Braekeleer J, Diet R, Jacobs D, Saman E, Verschueren H. DNA vaccination with genes encoding Toxoplasma gondiiantigens GRA1, GRA7 and ROP2 induces partially protective immunity against lethal challenge in mice. Infect Immun. 2000;68:38–45. doi: 10.1128/iai.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virelizier J L. Murine genotype influences the in vitroproduction of immune interferon. Eur J Immunol. 1982;12:988–990. doi: 10.1002/eji.1830121119. [DOI] [PubMed] [Google Scholar]
- 39.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff J A, Malone R W, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X J, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosisinfection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]