Abstract
We explored the pathological changes and the activation of local complement system in COVID-19 pneumonia. Lung paraffin sections of COVID-19 infected patients were analyzed by HE (hematoxylin-eosin) staining. The deposition of complement C3, the deposition of C3b/iC3b/C3d and C5b-9, and the expression of complement regulatory proteins, CD59, CD46 and CD55 were detected by immunohistochemistry. In COVID-19 patients’ lung tissues, fibrin exudation, mixed with erythrocyte, alveolar macrophage and shed pneumocyte are usually observed in the alveoli. The formation of an “alveolar emboli” structure may contribute to thrombosis and consolidation in lung tissue. In addition, we also found that compared to normal tissue, the lung tissues of COVID-19 patients displayed the hyper-activation of complement that is represented by extensive deposition of C3, C3b/iC3b/C3d and C5b-9, and the increased expression level of complement regulatory proteins CD55, and especially CD59 but not CD46. The thrombosis and consolidation in lung tissues may contribute to the pathogenesis of COVID-19. The increased expression of CD55 and CD59 may reflect a feedback of self-protection on the complement hyper-activation. Further, the increased C3 deposition and the strongly activated complement system in lung tissues may suggest the rationale of complement-targeted therapeutics in conquering COVID-19.
Keywords: Pathological changes, Thrombosis, Complement activation, Complement regulatory protein, SARS-CoV-2, COVID-19
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a new member of coronavirus (CoVs) family, is the etiologic agent in the pandemic of COVID-19 (Corona Virus Disease 2019). It is a positive-sense single-stranded RNA virus, coated particles, round or oval, often polymorphic, with a diameter of 60–140 nm [12]. Its genetic characteristics are distantly different from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), and it has 88–89% homology with bat SARS-like coronavirus (bat-SL-CoVZC45 and SL-CoVZXC21) [36]. SARS-CoV-2 infection, like SARS [69] and MERS [70] coronavirus infections, can also cause severe lung damage [65], [72]. Some patients may have acute respiratory distress syndrome (ARDS), multiple organ failure, and the critically ill patients need to be admitted to the intensive care unit (ICU) [72]. Based on the clinical retrospective analysis of nearly 9000 patients, the transmission rate of COVID-19 was 3.77, and the mortality rate is about 3.06% [73]. Although the mortality rate is much lower than SARS and MERS, SARS-CoV-2 infectiousness seems to be stronger.
The spike protein (S protein) of coronavirus helps the virus enter the target cells. Similar to SARS-CoV, SARS-CoV-2 S protein contains a receptor-binding domain (RBD) [73], by which SARS-CoV-2 binds to a receptor called angiotensin-converting enzyme 2 (ACE2) to infect human cells, and enters the target cells in the assistance of the protease TMPRSS2 [30], [75]. Type II alveolar epithelial cells (AT2) become the primary target of COVID-19 invasion due to high expression of ACE2, thus leading to the impaired lung function and pneumonia of the infected individuals [75]. Through the single-cell sequencing, it has been demonstrated that there are more organs such as small intestine, heart, kidney, colon, bladder, and testicles than lung to express ACE2 in varying levels, suggesting that these organs may also be affected by SARS-CoV-2 [11], [16], [71]. Accordingly, SARS-CoV-2 may cause diarrhea[16], liver damage [11], [42], renal damage, and heart attack [22], [29].
The innate immune system plays a first-line defense against virus infection to prevent the virus from invading or replicating. As an important part of innate immunity, complement system is also a bridge connecting innate and adaptive immunity, and plays a key role in removing cellular debris, apoptotic/dead cells and foreign invading pathogens, and further in coordinating the entire immune and inflammatory response [57], [58]. Although complement is mainly synthesized in the liver, a large number of studies have also shown that different components of complement are also expressed in alveolar epithelial cells and pulmonary endothelial cells [1], [15], [25], [62]. The complement system can be activated quickly and strongly upon pathogen invasion via classical, lectin and/or alternative pathways [61], thus causing significant increase of complement activation products such as C3a, C5a, C3d and C5b-9 (MAC, membrane attack complex) in lung tissue [31], [33], [34], [49]. The hyper-activation of complement may subsequently contribute to the lung damage [7], [64] as observed in SARS patients [13], [52]. Besides, C5b-9 staining on tubules and vessels of the kidney, and on the hepatic artery and portal vein of the liver were also observed in COVID-19 patients [45].
In SARS-CoV-2 infection, all of the surface glycoprotein including spike proteins, antiviral antibodies, elevated CRP (C-Reactive Protein), and metabolic acidosis are potential to activate complement via three distinct pathways [8], [9]. The lectin pathway has ever been reported to be activated in COVID-19 patients, resulting in the significant increase of serum C5a [24]. Further, extensive depositions of C4d that is produced in complement activation via classical and lectin pathways and of C5b-C9 that is terminal complement membrane-attack complex (MAC) has been detected in septal capillaries and interalveolar septa of the lungs from COVID-19 autopsies [46]. MASP2 (representative of activation of lectin complement pathway) deposition on microvasculature of skin biopsy was found restricted to severe/critical COVID-19 cases [39]. In addition, SARS-CoV-2 spike proteins are able to directly activate complement alternative pathway [74]. In consistent, serum concentration of C3 decreased in 8 out of 14 (57%) health care workers infected with SARS-CoV-2, indicating the C3 consumption most likely due to the complement activation [68]. Thus, the inhibitors against distinct complement components such as C5a [24], C5 [19], [38], [76] and C3 [48] have achieved a favorable effect in the preliminary clinical trial for COVID-19 treatment. Complement regulatory protein CD55 is known to be over-expressed in peripheral monocytes of COVID-19 patients [35], and the mutations in CD55 have been linked to severity of COVID-19 disease [56]. However, the expression levels of three membrane complement regulatory proteins, CD55, CD46, and CD59 have not been systematically examined in lungs of COVID-19 patients. In the present study, we detect the pathological features, the complement activation and the expression of the above complement regulatory proteins in two COVID-19 patients, which may be helpful for the future therapeutics in COVID-19.
2. Materials and methods
2.1. Patients and clinicopathological information collection
The lung tissues were collected from two female COVID-19 patients at Renmin Hospital of Wuhan University, Wuhan, China, who were 86 (Case 1) and 55 (Case 2) years old, respectively. Laboratory findings, chest CT results and other clinical information of the patients were collected and listed in Table 1. Case 1 has essential hypertension, lacunar infarction and chronic gastritis. CT (Computed Tomography) scan showed ground-glass opacities with consolidation and reticular and/or interlobular septal thickening located on the outside of both lungs upon admission. The nucleic acid testing confirmed that she was infected with SARS-CoV-2. The patient died with oxygen saturation values decreased to 60%. The timelines was 6 h from death to autopsy. The body was stored and autopsy was performed at room temperature. Lung tissue samples were autopsied from the patient with the consent of the patient's family. Lung tissue was fixed with 10% buffered formalin immediately. Case 2 was diagnosed as 10 mm GGO (Ground-glass opacification/opacity) on CT scan, the classical COVID-19 ground-glass type of densities were not seen on the outside of both lungs. Her general condition was good, without fever, dizziness or respiratory symptoms. When she was admitted to the hospital, Wuhan was still in the early stages of the COVID-19 epidemic, so she did not undergo nucleic acid testing before surgery, but the following nucleic acid testing confirmed that she was infected with SARS-CoV-2. The patient underwent right lower lobectomy. The lesion of the GGO was confirmed as pulmonary meningioma-like nodule. On the first day post operation, her blood lymphocyte count decreased to 0.82 × 109/L. She presented with a fever of 38.5 ℃ on the second postoperative day. On the fourth day after the surgery, the patient gradually developed dyspnea and her blood oxygen saturation decreased to 95%. The patient died on the seventh day after the surgery with oxygen saturation values below 55%. In addition, two gender- and age-paired non-small cell lung cancer (NSCLC) patients without SARS-CoV-2 infection at the Zhongshan Hospital of Fudan University, Shanghai, China were included in this study, and their para-cancer lung tissue at least 2 cm away from cancer were collected as control. They were not in any other co-morbid conditions such as diabetes, hypertension, cardiovascular disease and chronic obstructive pulmonary disease, and they did not have symptoms like fever, cough, and dyspnea. They had no history of smoking and they had not received any treatment before the operation. Prior written informed consent was collected from all patients. The study protocol was approved by the ethics board of Zhongshan Hospital of Fudan University and Renmin Hospital of Wuhan University (WDRY2020-K026, 2/14/2020). The diagnosis of pneumonia was confirmed by histology in all cases, though Case 1 and Case 2 were in different stages of pneumonia when the lung tissue samples were collected. Lung paraffin sections were analyzed by HE (hematoxylin-eosin) staining. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Table 1.
Clinical Features and Treatments of Patients.
| Patient No. | Case 1 | Case 2 |
|---|---|---|
| Age | 86 | 55 |
| Gender | Female | Female |
| Duration of hospitalization (days) | 18 | 11 |
| Co-morbidities |
Essential hypertension Coronary heart disease Lacunar infarction Chronic gastritis |
No |
| Symptoms (fever, cough, dyspnea) | Yes (6 days before admission) | Yes (4thday after admission, one day after surgery) |
| Smoking history | No | No |
| Laboratory findings |
2 days before death: White blood cell count: 15.90 × 10^9/L↑; Red blood cell count: 2.89 × 10^12/L↓; Hemoglobin: 82 g/L↓; Platelet count: 76 × 10^9/L↓; Percentage of neutrophils: 93.5%↑; Percentage of lymphocytes: 1.3%↓; Absolute neutrophils count: 14.88 × 10^9/L↑; Absolute lymphocytes count: 0.20 × 10^9/L↓; Plasma prothrombin time: 14.5 s↑; Activated partial thromboplastin time: 46.3 s↑; Thrombin time: 18.2 s; Fibrinogen: 6.1 g/L↑; D-dimer: 1.30 mg/L↑; C-reactive protein: 33.3 mg/L↑ |
2 days before death: White blood cell count: 3.98 × 10^9/L; Hemoglobin: 121 g/L; Platelet count: 121 × 10^9/L↓; Absolute neutrophils count: 2.99 × 10^9/L; Absolute lymphocytes count: 0.68 × 10^9/L↓; Plasma prothrombin time: 14.30 s↑;Activated partial thromboplastin time: 33.40 s↑; Fibrinogen: 6.37 g/L↑; D-dimer: 1.33 mg/L↑; C1q: 271 mg/L↑ |
| Computed tomography (CT) presentation | Ground-glass opacities with consolidation and reticular and/or interlobular septal thickening located on both lungs |
One day before surgery: 10 mm Ground-glass opacification/opacity (confirmed as pulmonary meningioma-like nodule) 4thday of appearance of symptoms: patchy ground-glass opacity and focal consolidation with increasing density in the left and right lungs |
| Antiviral therapy | No | No |
| Steroids therapy | No | No |
| Ventilator support | Mask oxygen inhalation | Mask oxygen inhalation |
| Surgery | No | Right lower lobectomy before the appearance of symptoms |
| Oxygen saturation |
99% (21stday of appearance of symptoms) 98% (23rdday of appearance of symptoms) 60% (24thday of appearance of symptoms) |
99% (1stday of appearance of symptoms) 95% (4thday of appearance of symptoms) 90% (6thday of appearance of symptoms) 55% (7thday of appearance of symptoms) |
| Days from appearance of symptoms to death (days) | 24 | 7 |
| Cause of death |
Respiratory failure Heart failure |
Respiratory failure Heart failure |
| Autopsy | Yes | No |
2.2. HE and Immunohistochemistry (IHC) staining
Tissues were thoroughly fixed in 10% buffered formalin for at least 24 h within 30 min after resection. Then the sectioned paraffin-embedded tissues were stained with HE. Two pathologists reviewed the sections to confirm the diagnosis. IHC assay using C3b/iC3b/C3d (ab136916, Abcam, Cambridge, MA, USA), C3 (ab200999, Abcam, Cambridge, MA, USA), C5b-9 (ab55811, Abcam, Cambridge, MA, USA), CD59 (ab133707, Abcam, Cambridge, MA, USA), CD55 (ab133684, Abcam, Cambridge, MA, USA), CD46 (ab108307, Abcam, Cambridge, MA, USA) rabbit monoclonal antibody was performed with iView DAB Detection Kit (Ventana, AZ, USA) on a BenchMark XT automated staining system (Ventana, AZ, USA). In brief, the tissue sections were deparaffinized and heat pretreated for antigen retrieval at 95 °C. Then tissue sections were incubated with primary antibody for 24 min at 37 °C after inactivation of the endogenous peroxidase. After washing, tissue sections were incubated with a biotinylated secondary antibody for 8 min at 37 °C and then with a streptavidin-HRP conjugate. Primary antibody from the same species was used as the negative control. Same concentration of primary antibody and other IHC conditions were used to detect the COVID-19 group and control group. The results of IHC were assessed by two independent observers. If there was any discrepancy, the result was verified by a discussion panel consists of three observers. All observers were blinded with regard to the clinicopathological characteristics. The IHC images were analyzed with ImageJ software(1.51J8).
2.3. Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
3. Results
3.1. The histologic features in lung tissues of COVID-19 patients
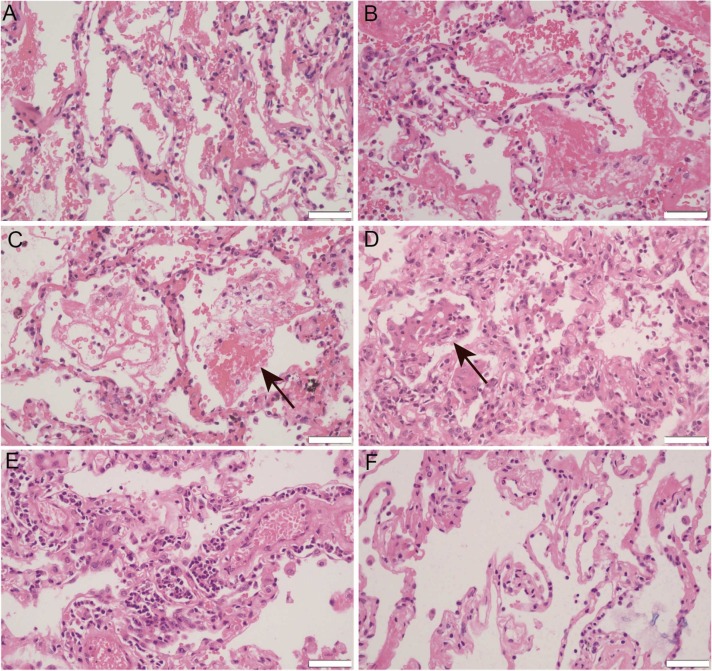
Representative laboratory findings, chest CT and other clinical information of the patients were listed in Table 1. In Case 1, alveolar edema, dilated capillaries in the alveolar wall and vascular congestion were universally observed in the peripheral part of the pneumonia areas ( Fig. 1A). In the center of the lesions, alveolar epithelial detachment along with ruptured capillaries of alveolar wall were noted (Fig. 1B), which led to fibrin exudation, mixed with erythrocyte, alveolar macrophage and shed pneumocyte in the alveoli (Fig. 1C). The formation of an “alveolar emboli” structure resulted in thrombosis and consolidation of lung tissue (Fig. 1D), which may induce dyspnea and decreased oxygen saturation when infection is uncontrollable. The rupture of alveolar wall capillaries could be very sudden, leading to the rapid generation of blood clots in the alveoli, which might cause the sudden death of the COVID-19 patients. There was patchy inflammatory infiltration in the tissue. Medium to large blood vessels were found to be surrounded by some lymphocytes and plasma cells (Fig. 1E). Microthrombi were found within the alveolar capillaries.
Fig. 1.
Representative images for hematoxylin-eosin (HE) staining of Case 1 COVID-19 pneumonia. A. Alveolar edema, dilated capillaries in the alveolar wall and vascular congestion. B. In the center of the lesion, alveolar epithelial detachment along with ruptured capillaries of alveolar wall. C. Fibrin exudation, mixed with erythrocyte, alveolar macrophage and shed pneumocyte in the alveoli (arrows). D. The formation of an “alveolar emboli” structure led to thrombosis and consolidation of lung tissue (arrows). E. Medium to large blood vessels were found be surrounded by some lymphocytes and plasma cells. F. Normal lung tissue of paired control patient. Microphotography was carried out by Olympus DP74 microscope under a 40 × objective. Scale bar is 50 µm.
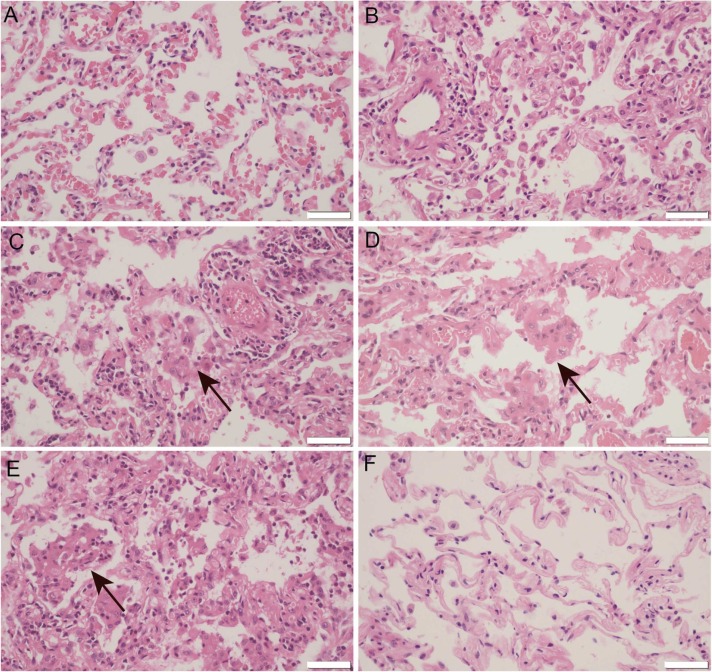
In Case 2, the lung lesion were generally less severe than that in CASE 1, suggesting that pneumonia may be in the early stage. Histologically, the lesions were mainly composed of alveolar edema, dilated capillaries in the alveolar wall and vascular congestion ( Fig. 2A). In the center area of the lesions, patchy shed alveolar epithelia, bleeding of capillaries of alveolar wall and fibrin exudate could be found (Fig. 2B). Type II pneumocyte hyperplasia and alveolar macrophage aggregation were also observed (Fig. 2C and 2D). The “alveolar emboli” structure could also be found in the alveolar cavity (Fig. 2E), though the alveolar injury were less severe than the former patient.
Fig. 2.
Representative images for HE staining of Case 2 COVID-19 pneumonia. A. Alveolar edema, dilated capillaries in the alveolar wall and vascular congestion. B. In the center area of the lesion, patchy shed alveolar epithelia, bleeding of capillaries of alveolar wall and fibrin exudate. C and D. Type II pneumocyte hyperplasia and alveolar macrophage aggregation (arrows). E. An “alveolar emboli” structure in the alveoli (arrows). F. Normal lung tissue of paired control patient. Microphotography was carried out by Olympus DP74 microscope under a 40 × objective. Scale bar is 50 µm.
3.2. The extensive complement activation in lungs of COVID-19 patients
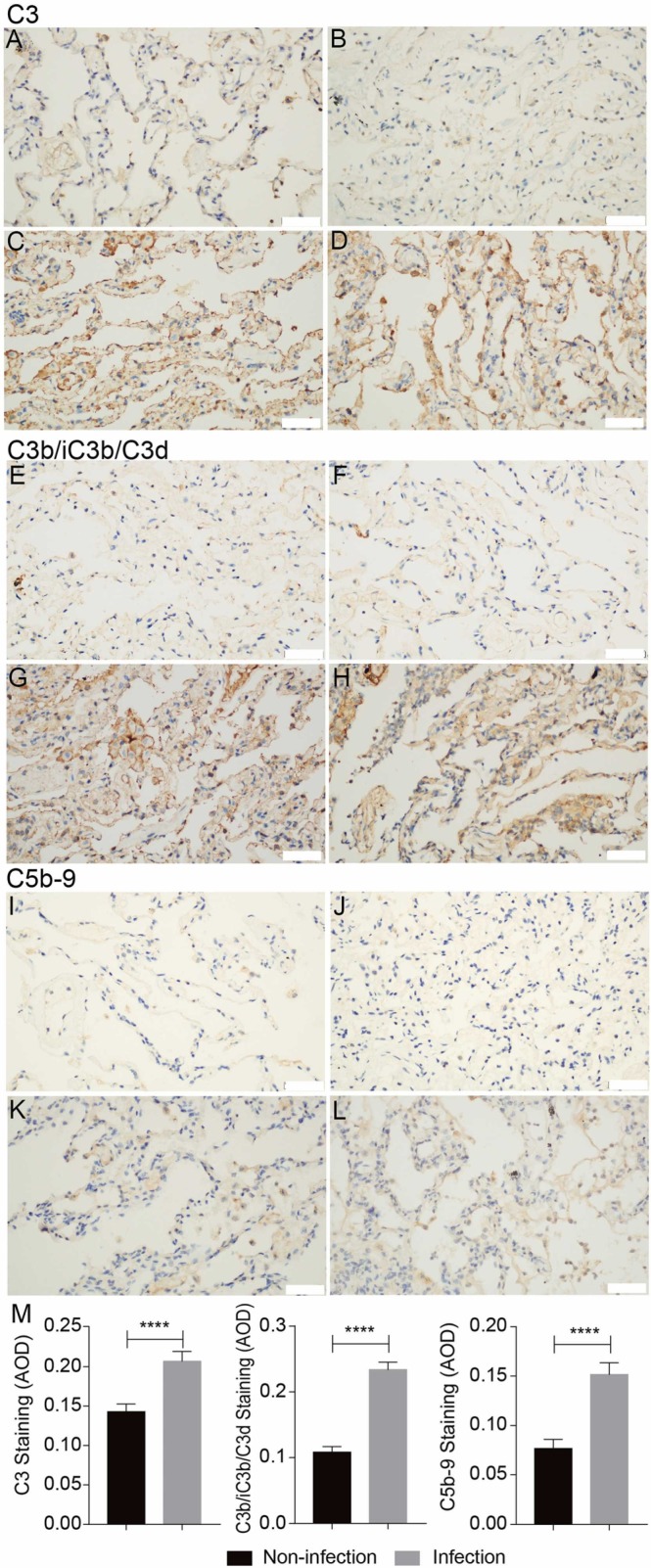
The paraformaldehyde-fixed lung tissues were subjected to IHC staining with C3, C3b/iC3b/C3d and C5b-9 antibodies. We found that compared to control lung tissues, the SARS-CoV-2-infected lung tissues displayed much higher C3 deposition levels, accompanied by much extensive deposition of complement activation products C3b/iC3b/C3d and C5b-9 ( Fig. 3). In addition, C3, C3b/iC3b/C3d and C5b-9 distributed mainly along the alveolar wall and vascular intima, and partially on the membrane of macrophages and alveolar epithelial cells (Fig. 3). These results suggest that the complement system is strongly activated during COVID-19 infection, at least in part, due to the remarkably increased C3 deposition level.
Fig. 3.
Excessive complement activation in the lung tissues of COVID-19 and control cancer patients detected by Immunohistochemistry (IHC) staining for C3, C3b/iC3b/C3d and C5b-9. Compare to normal tissues (A, B, E, F, I, J), the deposition of C3, C3b/iC3b/C3d and C5b-9 in COVID-19 infected lung tissues (C, D, G, H, K, L) was remarkably elevated. Microphotography was carried out by Olympus DP74 microscope under a 40 × objective. Scale bar is 50 µm. The pooled results were obtained by analyzing the IHC images by ImageJ software (M).
3.3. Elevated expression of complements regulatory proteins in lungs of COVID-19 patients
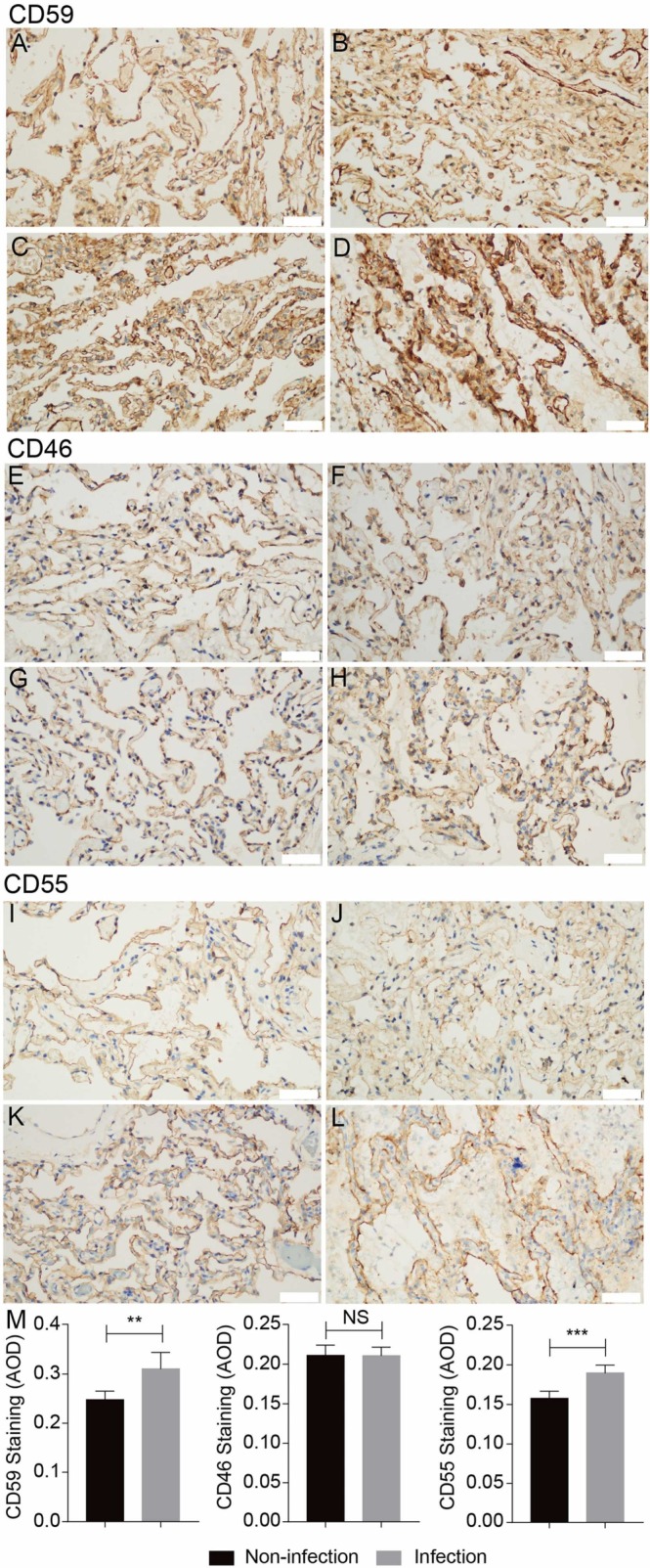
To understand the protective response to the complement hyper-activation, we next detected the expression levels of three membrane complement regulatory proteins, CD46, CD55, and CD59, in the above lung tissues. We found that the expression levels of CD55 and especially CD59 but not CD46 were elevated ( Fig. 4), which suggests the local inflammatory condition may promote the expression of CD55 and CD59 but not CD46. CD46 suppresses C3 activation by functioning as a cofactor for factor I–mediated cleavage of C3b and C4b [43]; while CD55 inactivates C3 and C5 convertases by preventing the formation of new and accelerating the decay of activated convertase via binding to C3b and C4b [44]. In addition, CD59 suppresses the complement activation at terminal stage by preventing MAC assembly on the cell membrane via binding to C8α and C9 [77]. Therefore, the remarkable increase of CD59 expression (upper panel in Fig. 4) may restrain the formation of C5b-9 (bottom panel in Fig. 3) in lung tissues. These feedback upregulation of membrane complement regulatory proteins may provide a protective effect from the complement attack on host cells due to excessive activation.
Fig. 4.
The expression levels of complement regulatory proteins in the lung tissues of COVID-19 and control cancer patients detected by IHC staining for CD46, CD55 and CD59. Compare to normal tissues (A, B, E, F, I, J), the expression of CD59 and CD55 but not CD46 in COVID-19 infected lung tissues (C, D, G, H, K, L)were upregulated. Microphotography was carried out by Olympus DP74 microscope under a 40 × objective. Scale bar is 50 µm. The pooled results were obtained by analyzing the IHC images by ImageJ software (M).
4. Discussion
COVID-19 is still a global public health crisis, thus requiring further study especially on the effective therapeutics. In this study, we found that the thrombosis and consolidation in lung tissues may contribute to the pathogenesis of COVID-19 based on the pathological changes. More importantly, in the lung tissues of COVID-19 patients, there are extensive deposition of complement activation products including C3b/iC3b/C3d and C5b-9, both of which are stably inserted into cell membrane, indicating the excessive complement activation in SARS-CoV-2 infection. These findings provide the rationality of the therapeutic strategy targeting the complement and/or coagulation systems in COVID-19 treatment.
The complement system can be activated through three pathways: Classic, Lectin and Alternative [57], all of which converge on C3 level to produce the activation products including C3a, C3b, iC3b, C3f, C3d, C3g and C3c [66]. Among them, C3d may stably insert into cell membrane via its thioester bond, thus being an indicative of C3 activation. Pulmonary C3d depositions were also found in the lungs of patients without COVID-19, independent of the diagnosis of ARDS (acute respiratory distress syndrome) [17]. At the terminal stage, C5 is cleaved into C5a and C5b, the latter of which assembles C5b-9 (MAC) in cell membrane by sequentially binding to C6, C7, C8 and C9. Among the complement cleavage products, C3a, especially C5a, may recruit a variety of immune cells and secrete a large number of proinflammatory cytokines (TNF-α, IL −1, IL-6, etc.) and chemokines (MCP-1, MIP-2, KC, etc.) [59] by interacting with their receptors C3aR, or C5aR1 and C5aR2 / C5L2, respectively. Indeed, some cytokines such as IL-6, IL-10, and TNF-α were found to be significantly increased in the serum of severe COVID-19 patients [18]. Moreover, C3b and its more stable degradation product iC3b can bind to a variety of complement receptors (CR) such as CR1, CR2, CR3, CR4 and CRIg that are expressed in immune cells, by which the immune cells may engulf or lyse these invaders. In addition to the direct cytolysis, C5b-9 can also activate NLRP3 inflammatory bodies, which in turn induces the maturation and secretion of IL-1β [37], [67]. In this study, we found that the extensive deposition of C3b/iC3b/C3d and C5b-9 in the lung tissue of patients with COVID-19, indicating the excessive local complement activation, which may eventually lead to a severe immune attack on host cells and the resultant pneumonia. In animal models of viral infections such as SARS or MERS, the deletion of complement C3 gene, the pre-depletion of complement, or the use of complement inhibitors against different components can significantly reduce the infiltration of immune cells in the lungs and the level of related cytokines, thereby significantly reducing lung tissue damage [28], [32], [33], [63], [64], indicating that after virus infection, complement activation plays an important role in immune cell recruitment and cytokine release. In similar, anti-C5a antibody BDB-001 [24] or IFX-1, anti-C5 antibody eculizumab and LFG316 [19], [38], [76], and C3 antagonist AMY-101 [48] have shown some favorable effect based on their preliminary data in a few COVID-19 patients.
In order to avoid the "by-stander injury" effect on host cells after complement activation, there are more than 10 kinds of complement regulatory proteins, including membrane-bound complement regulatory proteins CD46, CD55 and CD59, and circulating complement inhibitors C1-INH, C4BP, FH, which play a complement inhibitory role at different stages of complement activation. It has been reported that CD55 [2], [14], [60] and CD59 [21], [27], [60] together with C3 [6], [20], [23], [41], [50] can be upregulated by the pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, TGF-β and IFN-γ, acute-phase proteins, lipopolysaccharide (LPS), and the inflammation-associated transcription factor NF-κB. In the SARS-CoV-2-infected lung tissues, we observed the upregulation of CD55, CD59 and C3, which may result from the stimulation of the pro-inflammatory factors in the infectious milieu. The increased deposition of C3 may enhance the complement activation; In contrast, the upregulation of CD55 and CD59 may restrict the complement activation to tip the balance, thus protecting the host cells from the complement attack in COVID-19.
Microvascular injury and thrombosis are the important characters of COVID-19 pneumonia, which displays a pauci-inflammatory septal capillary injury with significant septal capillary mural and luminal fibrin deposition [46]. The tight interaction between the coagulation and complement system has been widely appreciated [4], [5], [51], [55]. Thrombotic microangiopathy (TMA) can occur in many different clinical scenarios including pathogenic complement activation [10]. In atypical hemolytic uremic syndrome (aHUS) patients with severe COVID-19, C5a induced the exocytosis of von Willebrand factor (vWF) and P-selectin from Weibel-Palade bodies, thus promoting microthrombosis by favoring vWF binding on the endothelium and platelet [3]. The characteristics of aHUS is microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure, this kind of disorder is result of uncontrolled complement activation [10]. Herein, we found that fibrin exudation mixed with erythrocyte, alveolar macrophage and shed pneumocyte in the alveoli, the formation of an “alveolar emboli” structure. The strong deposition of C3b/iC3b/C3d and C5b-9 in the lung tissues of COVID-19 pneumonia patients may further indicate the connection between capillary injuries, thrombosis and complement activation.
Therefore, similar to the usage of complement inhibitors, our findings also imply the rationality of anti-coagulation drugs in treating COVID-19. The systemic anticoagulation might be associated with improved outcomes among patients hospitalized with COVID-19 [53]. Currently, LMWH (low molecular weight heparin), UFH (unfractionated heparin), or fondaparinux indicated for prophylaxis of venous thromboembolism (VTE) was advised in all COVID-19 hospitalized patients by the Italian Society on Thrombosis and Hemostasis [47]. Though anticoagulation has no benefit in hospitalized, critically ill COVID-19 patients [26] but has a clear benefit in hospitalized non-critically ill COVID-19 patients [40]. Therefore, the complement inhibitors alone [54], the anti-coagulants alone, or their combination are worthy of clinical trials in COVID-19 treatment.
5. Conclusions
There is a tight interaction between coagulation and complement systems. In the lung tissues of COVID-19 patient, both of thrombosis and excessive complement activation were simultaneously observed, which may severely damage host cells, eventually lead to an irrecoverable lung injury and death.
Ethical Approval
The study protocol was approved by the ethics board of Zhongshan Hospital of Fudan University and Renmin Hospital of Wuhan University (WDRY2020-K026, 2/14/2020).
Funding
This work was supported by Research Fund from Shanghai Municipal Key Clinical Specialty (shslczdzk01302) and the Fundamental Research Funds for the Central Universities (2042020kf1012).
CRediT authorship contribution statement
Xiaowen Ge, Zhui Yu, Jingping Yuan, Weiguo Hu, Chouwen Zhu, and Yingyong Hou undertook study design. Zhui Yu, Xinxin Guo, Ling Li, Ling Ye, Maosong Ye, Yuan Ji, and Jingping Yuan enrolled patients and acquired data. Xiaowen Ge, Jingping Yuan, Weiguo Hu, Chouwen Zhu, and Yingyong Hou drafted the manuscript and revised it critically. All authors reviewed the manuscript and approved the final version.
Declaration of Competing Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ackerman S.K., Friend P.S., Hoidal J.R., Douglas S.D. Production of C2 by human alveolar macrophages. Immunology. 1978;35:369–372. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad S.R., Lidington E.A., Ohta R., Okada N., Robson M.G., Davies K.A., Leitges M., Harris C.L., Haskard D.O., Mason J.C. Decay-accelerating factor induction by tumour necrosis factor-alpha, through a phosphatidylinositol-3 kinase and protein kinase C-dependent pathway, protects murine vascular endothelial cells against complement deposition. Immunology. 2003;110:258–268. doi: 10.1046/j.1365-2567.2003.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S. Aiello, S. Gastoldi, M. Galbusera, P. Ruggenenti, V. Portalupi, S. Rota, N. Rubis, L. Liguori, S. Conti, M. Tironi, S. Gamba, D. Santarsiero, A. Benigni, G. Remuzzi, M. Noris, C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood advances 6 (2022) 866–881. [DOI] [PMC free article] [PubMed]
- 4.Amara U., Flierl M.A., Rittirsch D., Klos A., Chen H., Acker B., Brückner U.B., Nilsson B., Gebhard F., Lambris J.D., Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., Lambris J.D., Huber-Lang M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.S.R. Barnum, G. Fey, B.F. Tack, Biosynthesis and genetics of C3. Curr Top Microbiol Immunol 153 (1990) 23–43. [DOI] [PubMed]
- 7.Berdal J.E., Mollnes T.E., Waehre T., Olstad O.K., Halvorsen B., Ueland T., Laake J.H., Furuseth M.T., Maagaard A., Kjekshus H., Aukrust P., Jonassen C.M. Excessive innate immune response and mutant D222G/N in severe A (H1N1) pandemic influenza. J. Infect. 2011;63:308–316. doi: 10.1016/j.jinf.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Bosmann M. Complement activation during critical illness: current findings and an outlook in the era of COVID-19. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202005-1926ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating covid-19-related systemic thrombosis? Circulation. 2020;141:1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 10.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 11.X. Chai, L. Hu, Y. Zhang, W. Han, Z. Lu, A. Ke, J. Zhou, G. Shi, N. Fang, J. Fan, J. Cai, J. Fan, F. Lan, Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv (2020) 2020.2002.2003.931766.
- 12.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes. Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J.H., Chang Y.W., Yao C.W., Chiueh T.S., Huang S.C., Chien K.Y., Chen A., Chang F.Y., Wong C.H., Chen Y.J. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc. Natl. Acad. Sci. 2004;101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocuzzi E.T., Bardenstein D.S., Stavitsky A., Sundarraj N., Medof M.E. Upregulation of DAF (CD55) on orbital fibroblasts by cytokines. differential effects of TNF-beta and TNF-alpha. Curr. Eye Res. 2001;23:86–92. doi: 10.1076/ceyr.23.2.86.5478. [DOI] [PubMed] [Google Scholar]
- 15.Cole F.S., Matthews W.J., Jr., Rossing T.H., Gash D.J., Lichtenberg N.A., Pennington J.E. Complement biosynthesis by human bronchoalveolar macrophages. Clin. Immunol. Immunopathol. 1983;27:153–159. doi: 10.1016/0090-1229(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 16.F. D'Amico, D.C. Baumgart, S. Danese, L. Peyrin-Biroulet, Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association (2020). [DOI] [PMC free article] [PubMed]
- 17.de Beer F.M., Begieneman M.P.V., Roelofs J., Horn J., Niessen H.W.M., Schultz M.J., Lagrand W.K. Pulmonary complement depositions in autopsy of critically ill patients have no relation with ARDS. Intensive care Med. Exp. 2019;7:35. doi: 10.1186/s40635-019-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.B. Diao, C. Wang, Y. Tan, X. Chen, Y. Liu, L. Ning, L. Chen, M. Li, Y. Liu, G. Wang, Z. Yuan, Z. Feng, Y. Wu, Y. Chen, Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). (2020) 2020.2002.2018.20024364. [DOI] [PMC free article] [PubMed]
- 19.Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., De Negri P., Di Gennaro C., Pagano A., Allegorico E., Bressy L., Bosso G., Ferrara A., Serra C., Montisci A., D'Amico M., Schiano Lo Morello S., Di Costanzo G., Tucci A.G., Marchetti P., Di Vincenzo U., Sorrentino I., Casciotta A., Fusco M., Buonerba C., Berretta M., Ceccarelli M., Nunnari G., Diessa Y., Cicala S., Facchini G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharm. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 20.Drouin S.M., Kiley S.C., Carlino J.A., Barnum S.R. Transforming growth factor-beta2 regulates C3 secretion in monocytes through a protein kinase C-dependent pathway. Mol. Immunol. 1998;35:1–11. doi: 10.1016/s0161-5890(98)00014-5. [DOI] [PubMed] [Google Scholar]
- 21.Du Y., Teng X., Wang N., Zhang X., Chen J., Ding P., Qiao Q., Wang Q., Zhang L., Yang C., Yang Z., Chu Y., Du X., Zhou X., Hu W. NF-κB and enhancer-binding CREB protein scaffolded by CREB-binding protein (CBP)/p300 proteins regulate CD59 protein expression to protect cells from complement attack. J. Biol. Chem. 2014;289:2711–2724. doi: 10.1074/jbc.M113.525501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C. Fan, K. Li, Y. Ding, W.L. Lu, J. Wang, ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. (2020) 2020.2002.2012.20022418.
- 23.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 24.T. Gao, M. Hu, X. Zhang, H. Li, L. Zhu, H. Liu, Q. Dong, Z. Zhang, Z. Wang, Y. Hu, Y. Fu, Y. Jin, K. Li, S. Zhao, Y. Xiao, S. Luo, L. Li, L. Zhao, J. Liu, H. Zhao, Y. Liu, W. Yang, J. Peng, X. Chen, P. Li, Y. Liu, Y. Xie, J. Song, L. Zhang, Q. Ma, X. Bian, W. Chen, X. Liu, Q. Mao, C. Cao, Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv (2020) 2020.2003.2029.20041962.
- 25.P. Georg, R. Astaburuaga-García, L. Bonaguro, S. Brumhard, L. Michalick, L.J. Lippert, T. Kostevc, C. Gäbel, M. Schneider, M. Streitz, V. Demichev, I. Gemünd, M. Barone, P. Tober-Lau, E.T. Helbig, D. Hillus, L. Petrov, J. Stein, H.P. Dey, D. Paclik, C. Iwert, M. Mülleder, S.K. Aulakh, S. Djudjaj, R.D. Bülow, H.E. Mei, A.R. Schulz, A. Thiel, S. Hippenstiel, A.E. Saliba, R. Eils, I. Lehmann, M.A. Mall, S. Stricker, J. Röhmel, V.M. Corman, D. Beule, E. Wyler, M. Landthaler, B. Obermayer, S. von Stillfried, P. Boor, M. Demir, H. Wesselmann, N. Suttorp, A. Uhrig, H. Müller-Redetzky, J. Nattermann, W.M. Kuebler, C. Meisel, M. Ralser, J.L. Schultze, A.C. Aschenbrenner, C. Thibeault, F. Kurth, L.E. Sander, N. Blüthgen, B. Sawitzki, Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell 185 (2022) 493–512 e425. [DOI] [PMC free article] [PubMed]
- 26.E.C. Goligher, C.A. Bradbury, B.J. McVerry, P.R. Lawler, J.S. Berger, M.N. Gong, M. Carrier, H.R. Reynolds, A. Kumar, A.F. Turgeon, L.Z. Kornblith, S.R. Kahn, J.C. Marshall, K.S. Kim, B.L. Houston, L.P.G. Derde, M. Cushman, T. Tritschler, D.C. Angus, L.C. Godoy, Z. McQuilten, B.A. Kirwan, M.E. Farkouh, M.M. Brooks, R.J. Lewis, L.R. Berry, E. Lorenzi, A.C. Gordon, T. Ahuja, F. Al-Beidh, D. Annane, Y.M. Arabi, D. Aryal, L. Baumann Kreuziger, A. Beane, Z. Bhimani, S. Bihari, H.H. Billett, L. Bond, M. Bonten, F. Brunkhorst, M. Buxton, A. Buzgau, L.A. Castellucci, S. Chekuri, J.T. Chen, A.C. Cheng, T. Chkhikvadze, B. Coiffard, A. Contreras, T.W. Costantini, S. de Brouwer, M.A. Detry, A. Duggal, V. Džavík, M.B. Effron, H.F. Eng, J. Escobedo, L.J. Estcourt, B.M. Everett, D.A. Fergusson, M. Fitzgerald, R.A. Fowler, J.D. Froess, Z. Fu, J.P. Galanaud, B.T. Galen, S. Gandotra, T.D. Girard, A.L. Goodman, H. Goossens, C. Green, Y.Y. Greenstein, P.L. Gross, R. Haniffa, S.M. Hegde, C.M. Hendrickson, A.M. Higgins, A.A. Hindenburg, A.A. Hope, J.M. Horowitz, C.M. Horvat, D.T. Huang, K. Hudock, B.J. Hunt, M. Husain, R.C. Hyzy, J.R. Jacobson, D. Jayakumar, N.M. Keller, A. Khan, Y. Kim, A. Kindzelski, A.J. King, M.M. Knudson, A.E. Kornblith, M.E. Kutcher, M.A. Laffan, F. Lamontagne, G. Le Gal, C.M. Leeper, E.S. Leifer, G. Lim, F. Gallego Lima, K. Linstrum, E. Litton, J. Lopez-Sendon, S.A. Lother, N. Marten, A. Saud Marinez, M. Martinez, E. Mateos Garcia, S. Mavromichalis, D.F. McAuley, E.G. McDonald, A. McGlothlin, S.P. McGuinness, S. Middeldorp, S.K. Montgomery, P.R. Mouncey, S. Murthy, G.B. Nair, R. Nair, A.D. Nichol, J.C. Nicolau, B. Nunez-Garcia, J.J. Park, P.K. Park, R.L. Parke, J.C. Parker, S. Parnia, J.D. Paul, M. Pompilio, J.G. Quigley, R.S. Rosenson, N.S. Rost, K. Rowan, F.O. Santos, M. Santos, M.O. Santos, L. Satterwhite, C.T. Saunders, J. Schreiber, R.E.G. Schutgens, C.W. Seymour, D.M. Siegal, D.G. Silva, Jr, A.B. Singhal, A.S. Slutsky, D. Solvason, S.J. Stanworth, A.M. Turner, W. van Bentum-Puijk, F.L. van de Veerdonk, S. van Diepen, G. Vazquez-Grande, L. Wahid, V. Wareham, R.J. Widmer, J.G. Wilson, E. Yuriditsky, Y. Zhong, S.M. Berry, C.J. McArthur, M.D. Neal, J.S. Hochman, S.A. Webb, R. Zarychanski, Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med 385 (2021) 777–789.
- 27.Goswami M.T., Reka A.K., Kurapati H., Kaza V., Chen J., Standiford T.J., Keshamouni V.G. Regulation of complement-dependent cytotoxicity by TGF-β-induced epithelial-mesenchymal transition. Oncogene. 2016;35:1888–1898. doi: 10.1038/onc.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M. Hoffmann, H. Kleine-Weber, N. Krüger, M. Müller, C. Drosten, S. Pöhlmann, The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv (2020) 2020.2001.2031.929042.
- 31.Huber-Lang M., Sarma V.J., Lu K.T., McGuire S.R., Padgaonkar V.A., Guo R.F., Younkin E.M., Kunkel R.G., Ding J., Erickson R., Curnutte J.T., Ward P.A. Role of C5a in multiorgan failure during sepsis. J. Immunol. 2001;166:1193–1199. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y., Li J., Teng Y., Sun H., Tian G., He L., Li P., Chen Y., Guo Y., Li J., Zhao G., Zhou Y., Sun S. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 2019;11 doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y., Li J., Du L., Jiang S., Guo R., Sun S., Zhou Y. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanmura S., Uto H., Sato Y., Kumagai K., Sasaki F., Moriuchi A., Oketani M., Ido A., Nagata K., Hayashi K., Stuver S.O., Tsubouchi H. The complement component C3a fragment is a potential biomarker for hepatitis C virus-related hepatocellular carcinoma. J. Gastroenterol. 2010;45:459–467. doi: 10.1007/s00535-009-0160-5. [DOI] [PubMed] [Google Scholar]
- 35.Lage S.L., Rocco J.M., Laidlaw E., Rupert A., Galindo F., Kellogg A., Kumar P., Poon R., Wortmann G.W., Lisco A., Manion M., Sereti I. Activation of complement components on circulating blood monocytes from COVID-19 patients. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.815833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laudisi F., Spreafico R., Evrard M., Hughes T.R., Mandriani B., Kandasamy M., Morgan B.P., Sivasankar B., Mortellaro A. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J. Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurence J., Mulvey J.J., Seshadri M., Racanelli A., Harp J., Schenck E.J., Zappetti D., Horn E.M., Magro C.M. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin. Immunol. 2020;219 doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurence J., Nuovo G., Racine-Brzostek S.E., Seshadri M., Elhadad S., Crowson A.N., Mulvey J.J., Harp J., Ahamed J., Magro C. Premortem skin biopsy assessing microthrombi, interferon type I antiviral and regulatory proteins, and complement deposition correlates with coronavirus disease 2019 clinical stage. Am. J. Pathol. 2022;192:1282–1294. doi: 10.1016/j.ajpath.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.P.R. Lawler, E.C. Goligher, J.S. Berger, M.D. Neal, B.J. McVerry, J.C. Nicolau, M.N. Gong, M. Carrier, R.S. Rosenson, H.R. Reynolds, A.F. Turgeon, J. Escobedo, D.T. Huang, C.A. Bradbury, B.L. Houston, L.Z. Kornblith, A. Kumar, S.R. Kahn, M. Cushman, Z. McQuilten, A.S. Slutsky, K.S. Kim, A.C. Gordon, B.A. Kirwan, M.M. Brooks, A.M. Higgins, R.J. Lewis, E. Lorenzi, S.M. Berry, L.R. Berry, A.W. Aday, F. Al-Beidh, D. Annane, Y.M. Arabi, D. Aryal, L. Baumann Kreuziger, A. Beane, Z. Bhimani, S. Bihari, H.H. Billett, L. Bond, M. Bonten, F. Brunkhorst, M. Buxton, A. Buzgau, L.A. Castellucci, S. Chekuri, J.T. Chen, A.C. Cheng, T. Chkhikvadze, B. Coiffard, T.W. Costantini, S. de Brouwer, L.P.G. Derde, M.A. Detry, A. Duggal, V. Džavík, M.B. Effron, L.J. Estcourt, B.M. Everett, D.A. Fergusson, M. Fitzgerald, R.A. Fowler, J.P. Galanaud, B.T. Galen, S. Gandotra, S. García-Madrona, T.D. Girard, L.C. Godoy, A.L. Goodman, H. Goossens, C. Green, Y.Y. Greenstein, P.L. Gross, N.M. Hamburg, R. Haniffa, G. Hanna, N. Hanna, S.M. Hegde, C.M. Hendrickson, R.D. Hite, A.A. Hindenburg, A.A. Hope, J.M. Horowitz, C.M. Horvat, K. Hudock, B.J. Hunt, M. Husain, R.C. Hyzy, V.N. Iyer, J.R. Jacobson, D. Jayakumar, N.M. Keller, A. Khan, Y. Kim, A.L. Kindzelski, A.J. King, M.M. Knudson, A.E. Kornblith, V. Krishnan, M.E. Kutcher, M.A. Laffan, F. Lamontagne, G. Le Gal, C.M. Leeper, E.S. Leifer, G. Lim, F.G. Lima, K. Linstrum, E. Litton, J. Lopez-Sendon, J.L. Lopez-Sendon Moreno, S.A. Lother, S. Malhotra, M. Marcos, A. Saud Marinez, J.C. Marshall, N. Marten, M.A. Matthay, D.F. McAuley, E.G. McDonald, A. McGlothlin, S.P. McGuinness, S. Middeldorp, S.K. Montgomery, S.C. Moore, R. Morillo Guerrero, P.R. Mouncey, S. Murthy, G.B. Nair, R. Nair, A.D. Nichol, B. Nunez-Garcia, A. Pandey, P.K. Park, R.L. Parke, J.C. Parker, S. Parnia, J.D. Paul, Y.S. Pérez González, M. Pompilio, M.E. Prekker, J.G. Quigley, N.S. Rost, K. Rowan, F.O. Santos, M. Santos, M. Olombrada Santos, L. Satterwhite, C.T. Saunders, R.E.G. Schutgens, C.W. Seymour, D.M. Siegal, D.G. Silva, Jr, M. Shankar-Hari, J.P. Sheehan, A.B. Singhal, D. Solvason, S.J. Stanworth, T. Tritschler, A.M. Turner, W. van Bentum-Puijk, F.L. van de Veerdonk, S. van Diepen, G. Vazquez-Grande, L. Wahid, V. Wareham, B.J. Wells, R.J. Widmer, J.G. Wilson, E. Yuriditsky, F.G. Zampieri, D.C. Angus, C.J. McArthur, S.A. Webb, M.E. Farkouh, J.S. Hochman, R. Zarychanski, Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med 385 (2021) 790–802. [DOI] [PMC free article] [PubMed]
- 41.Li Y., Song D., Song Y., Zhao L., Wolkow N., Tobias J.W., Song W., Dunaief J.L. Iron-induced local complement component 3 (C3) Up-regulation via non-canonical transforming growth factor (TGF)-β Signaling in the retinal pigment epithelium. J. Biol. Chem. 2015;290:11918–11934. doi: 10.1074/jbc.M115.645903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Z. Li, M. Wu, J. Guo, J. Yao, X. Liao, S. Song, M. Han, J. Li, G. Duan, Y. Zhou, X. Wu, Z. Zhou, T. Wang, M. Hu, X. Chen, Y. Fu, C. Lei, H. Dong, Y. Zhou, H. Jia, X. Chen, J. Yan, Caution on Kidney Dysfunctions of 2019-nCoV Patients. (2020) 2020.2002.2008.20021212.
- 43.Liszewski M.K., Post T.W., Atkinson J.P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 44.Lublin D.M., Atkinson J.P. Decay-accelerating factor: biochemistry, molecular biology and function. Annu Rev. Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 45.Macor P., Durigutto P., Mangogna A., Bussani R., De Maso L., D'Errico S., Zanon M., Pozzi N., Meroni P.L., Tedesco F. Multiple-organ complement deposition on vascular endothelium in COVID-19 patients. Biomedicines. 2021;9 doi: 10.3390/biomedicines9081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.C. Magro, J.J. Mulvey, D. Berlin, G. Nuovo, S. Salvatore, J. Harp, A. Baxter-Stoltzfus, J. Laurence, Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational research: the journal of laboratory and clinical medicine (2020). [DOI] [PMC free article] [PubMed]
- 47.M. Marietta, W. Ageno, A. Artoni, E. De Candia, P. Gresele, M. Marchetti, R. Marcucci, A. Tripodi, COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood transfusion = Trasfusione del sangue (2020). [DOI] [PMC free article] [PubMed]
- 48.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C., Lambris J.D., Ciceri F. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nascimento E.J., Silva A.M., Cordeiro M.T., Brito C.A., Gil L.H., Braga-Neto U., Marques E.T. Alternative complement pathway deregulation is correlated with dengue severity. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols W.K. LPS stimulation of complement (C3) synthesis by a human monocyte cell line. Complement. 1984;1:108–115. doi: 10.1159/000467823. [DOI] [PubMed] [Google Scholar]
- 51.Oikonomopoulou K., Ricklin D., Ward P.A., Lambris J.D. Interactions between coagulation and complement-their role in inflammation. Semin. Immunopathol. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pang R.T., Poon T.C., Chan K.C., Lee N.L., Chiu R.W., Tong Y.K., Wong R.M., Chim S.S., Ngai S.M., Sung J.J., Lo Y.M. Serum proteomic fingerprints of adult patients with severe acute respiratory syndrome. Clin. Chem. 2006;52:421–429. doi: 10.1373/clinchem.2005.061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polycarpou A., Howard M., Farrar C.A., Greenlaw R., Fanelli G., Wallis R., Klavinskis L.S., Sacks S. Rationale for targeting complement in COVID-19. EMBO Mol. Med. 2020 doi: 10.15252/emmm.202012642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin X., Hu W., Song W., Blair P., Wu G., Hu X., Song Y., Bauer S., Feelisch M., Leopold J.A., Loscalzo J., Halperin J.A. Balancing role of nitric oxide in complement-mediated activation of platelets from mCd59a and mCd59b double-knockout mice. Am. J. Hematol. 2009;84:221–227. doi: 10.1002/ajh.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J., May B., De Freitas J.K., Glicksberg B.S., Mason C.E., Tatonetti N.P., Shapira S.D. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26:1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricklin D., Lambris J.D. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riedemann N.C., Guo R.F., Ward P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 60.Spiller O.B., Criado-García O., Rodríguez De Córdoba S., Morgan B.P. Cytokine-mediated up-regulation of CD55 and CD59 protects human hepatoma cells from complement attack. Clin. Exp. Immunol. 2000;121:234–241. doi: 10.1046/j.1365-2249.2000.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoermer K.A., Morrison T.E. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strunk R.C., Eidlen D.M., Mason R.J. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J. Clin. Investig. 1988;81:1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun S., Zhao G., Liu C., Fan W., Zhou X., Zeng L., Guo Y., Kou Z., Yu H., Li J., Wang R., Li Y., Schneider C., Habel M., Riedemann N.C., Du L., Jiang S., Guo R., Zhou Y. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015;60:586–595. doi: 10.1093/cid/ciu887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun S., Zhao G., Liu C., Wu X., Guo Y., Yu H., Song H., Du L., Jiang S., Guo R., Tomlinson S., Zhou Y. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am. J. Respir. Cell Mol. Biol. 2013;49:221–230. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- 65.S. Tian, W. Hu, L. Niu, H. Liu, H. Xu, S.Y. Xiao, Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 15 (2020) 700–704. [DOI] [PMC free article] [PubMed]
- 66.Toapanta F.R., Ross T.M. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol. Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 67.Triantafilou K., Hughes T.R., Triantafilou M., Morgan B.P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 68.Wei X.S., Wang X.R., Zhang J.C., Yang W.B., Ma W.L., Yang B.H., Jiang N.C., Gao Z.C., Shi H.Z., Zhou Q. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO, Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. (2003).
- 70.WHO, Middle East respiratory syndrome coronavirus (MERS-CoV). (2019).
- 71.Xin Zou K.C., Zou Jiawei, Han Peiyi, Hao Jie, Han Zeguang. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Y. Yang, Q.-B. Lu, M.-J. Liu, Y.-X. Wang, A.-R. Zhang, N. Jalali, N.E. Dean, I. Longini, M.E. Halloran, B. Xu, X.-A. Zhang, L.-P. Wang, W. Liu, L.-Q. Fang, Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv (2020) 2020.2002.2010.20021675.
- 74.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Z.Z. Yu Zhao, Yujia Wang, Yueqing Zhou, Yu Ma, Wei Zuo, Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 76.Zelek W.M., Cole J., Ponsford M.J., Harrison R.A., Schroeder B.E., Webb N., Jolles S., Fegan C., Morgan M., Wise M.P., Morgan B.P. Complement inhibition with the C5 Blocker LFG316 in severe COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:1304–1308. doi: 10.1164/rccm.202007-2778LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X., Hu W., Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]