SUMMARY
Background
The coronavirus disease 2019 (COVID-19) has influenced antimicrobial consumption and incidence of multidrug-resistant organisms (MDROs). We aimed to study the epidemiology of MDROs before and during the COVID-19 pandemic in Hong Kong.
Methods
With the maintenance of infection control measures, we described the trend of MDRO infections, including methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Acinetobacter species (CRA), and extended-spectrum-beta-lactamase-(ESBL)-producing Enterobacterales, in a healthcare region with 3100-bed before (1 January 2016 to 31 December 2019, period 1) and during COVID-19 (1 January 2020 to 30 September 2022, period 2), together with the antimicrobial consumption using piecewise Poisson regression. The epidemiological characteristics of newly diagnosed COVID-19 patients with or without MDRO infections were analyzed.
Results
Between period 1 and 2, we observed a significant increase in the trend of CRA infections (P<0.001), while there was no significant increase in the trend of MRSA (P=0.742) and ESBL-producing Enterobacterales (P=0.061) infections. Meanwhile, a significant increase in the trend of carbapenems (P<0.001), extended-spectrum beta-lactam-beta-lactamase inhibitor combinations (BLBI) (P=0.045), and fluoroquinolones (P=0.009) consumption was observed. The observed opportunity (23,540 ± 3703 vs 26,145 ± 2838, p=0.359) and compliance (81.6% ± 0.5% vs 80.1% ± 0.8%, P=0.209) of hand hygiene per year was maintained. In a multivariable model, older age, male sex, referral from residential care home for the elderly, presence of indwelling device, presence of endotracheal tube, and use of carbapenems, use of BLBI, use of proton pump inhibitors and history of hospitalization in the past 3 months were associated with higher risks of infections by MDROs among COVID-19 patients.
Conclusion
Infection control measures may control the surge of MDROs despite an increasing trend of antimicrobial consumption.
Keywords: Epidemiology, Multidrug-resistant organisms, COVID-19, Methicillin-resistant Staphylococcus aureus, Carbapenem-resistant Acinetobacter species, ESBL-producing Enterobacterales
Introduction
Antimicrobial resistance has long been a threat in the healthcare setting [1]. The control of multidrug-resistant organisms (MDROs) relies on two important tools, antimicrobial stewardship programs and infection control practices. The former minimizes the risk of emergence of MDROs by reducing antimicrobial selection pressure, while the latter reduces the risk of dissemination of MDROs after they have emerged. Queen Mary Hospital has been a pioneer in antimicrobial stewardship [2] and was one of the first to introduce the “World Health Organization guidelines on hand hygiene in health care” in Hong Kong [3]. We have also proposed innovative measures such as electronic monitoring of hand hygiene [4], patient empowerment in hand hygiene [5], and directly observed hand hygiene for patients in hospitals [6,7] and residents in the residential care home for the elderly (RCHE) [8] for controlling the spread of MDROs. All these measures have successfully prevented and controlled nosocomial transmission and outbreaks of epidemiologically important MDROs including methicillin-resistant Staphylococcus aureus (MRSA) [9], vancomycin-resistant enterococci (VRE) [10], carbapenem-resistant and multidrug-resistant Acinetobacter baumannii [11,12], and carbapenem-resistant Enterobacterales [13]. As a result of these efforts, Queen Mary Hospital had the lowest rate of hospital outbreaks per patient discharge or patient days among regional acute care hospitals in Hong Kong [14]. In addition, through the promotion of proactive infection control measures, including directly observed hand hygiene, to all hospitals under the governance of the Hospital Authority, we were able to reverse the rising trend of VRE and prevent territory-wide outbreaks in Hong Kong [15]. This was the first time in the medical history of Hong Kong that the establishment of an MDRO's endemicity was successfully prevented.
However, our effort in MDRO control faced a great challenge when the unprecedented outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Hong Kong in 2020. Healthcare facilities around the world have reported outbreaks of MDROs during the COVID-19 pandemic, which is probably related to the disruption to infection control practices and antimicrobial stewardship programs [16]. In Hong Kong, we learned from our experience during the severe acute respiratory syndrome (SARS) outbreak in 2003 [17], and the pandemic influenza A H1N1 outbreak in 2009 [18]. Infection control preparedness for emerging infectious diseases such as COVID-19 has since been improving [19,20]. Hence, this study aims to investigate changes in the epidemiology of MDROs and antimicrobial use during the COVID-19 pandemic in Hong Kong.
Material and methods
Setting and data source
This is a retrospective study on the epidemiology of MDROs, including MRSA, carbapenem-resistant Acinetobacter species (CRA), and extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales, in a healthcare region in Hong Kong, namely Hong Kong West Cluster (HKWC), under the governance of the Hospital Authority. HKWC comprised a university-affiliated teaching hospital (Queen Mary Hospital) with 1700-bed, together with five extended care hospitals with another 1400 beds. The study period is divided into two parts, i.e., period 1 (1 January 2016 to 31 December 2019), which serves as the baseline before the occurrence of COVID-19, and period 2 (1 January 2020 to 30 September 2022), which is the study period during the COVID-19 pandemic. The study period includes the first 1000 days of COVID-19 in Hong Kong, where day 1 (31 December 2019) was the day of the official announcement of the outbreak of community-acquired pneumonia in Wuhan, Hubei Province, made by the Health Commission of Hubei Province of the People's Republic of China.
The audits of hand hygiene opportunity and compliance as well as audit data on the appropriate use of broad-spectrum antimicrobial agents were retrieved from infection control team. The opportunity and compliance of hand hygiene audit is performed by infection control nurses as per the World Health Organization protocol [3]. The appropriate use of broad-spectrum antimicrobial agents by the frontline clinicians is determined by clinical microbiologists based on a locally prepared IMPACT (Interhospital Multi-disciplinary Programme on Antimicrobial ChemoTherapy) guideline [21]. The data on patients infected with MDROs, COVID-19, and antimicrobial consumption were retrieved from the Clinical Data Analysis and Reporting System, which is an electronic database of health records under the governance of the Hospital Authority, as previously described [22].
Incidence of multidrug-resistant organisms before and during COVID-19
In this study, MRSA, CRA, and ESBL-producing Enterobacterales were selected as the representative MDROs. Clinical specimens that tested positive for MRSA, CRA, or ESBL-producing Enterobacterales during hospitalization in HKWC were included in the analysis. New cases of these MDROs were defined as patients who had any clinical specimens positive for these MDROs during hospitalization, without a history of culture positive by the same MRDO in the preceding 12 months. An episode of bacteremia was defined as a blood culture positive for MRSA, CRA, or ESBL-producing Enterobacterales during hospitalization. The length of an infective episode was defined as 14 days for MRSA, CRA, and ESBL-producing Enterobacterales bacteremia, with the date of specimen collection considered as day 1. A new episode of bacteremia was considered when it occurred after an infective episode. The incidence of MDROs was expressed in terms of the number of new cases and bacteremia episodes per 1000 patient days before COVID-19 (period 1) and during COVID-19 (period 2).
Consumption of antimicrobial agents before and during COVID-19
The monthly consumption of broad-spectrum antimicrobial agents including carbapenems, cephalosporins, extended-spectrum beta-lactam-beta-lactamase inhibitor combinations (BLBI), and fluoroquinolones, expressed in defined daily dose (DDD) per 1000 patient days before COVID-19 (period 1) and during COVID-19 (period 2), were analyzed.
COVID-19 patients with or without multidrug-resistant organisms
The epidemiological characteristics of newly diagnosed COVID-19 patients with or without MDROs infection were analyzed, including the demographic data, referral from RCHE, Charlson comorbidity index, history of antimicrobial agent and proton pump inhibitor use, history of hospitalization in the past 3 months, as well as the crude mortality within 90 days of COVID-19 diagnosis.
This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
Statistical analysis
Differences in the incidence rate of MRSA, CRA, ESBL-producing Enterobacterales, and antimicrobial consumption were evaluated between period 1 and period 2 using piecewise Poisson regression (interrupted time series). At the breakpoint of 1 January 2020, the change of slope was investigated by including the corresponding term in the regression. If the change was not significant, the corresponding term was removed from the model. To facilitate results interpretation in the case of significant change in slope, the slopes of the two segments were presented (instead of the change). Chi-Square test and t-test were used to compare characteristics of COVID-19 patients with and without infections by MDROs. Multiple logistic regression was used to identify characteristics of COVID-19 patients associated with infections by MDROs. All statistical analyses were performed using IBM SPSS Statistics (version 28). A two-sided P-value of < 0.05 was considered statistically significant.
Results
Compliance of hand hygiene and use of broad-spectrum antimicrobial agents
Before and during the COVID-19 pandemic, the observed opportunity (23,540 ± 3703 vs 26,145 ± 2838, P=0.359) and compliance (81.6% ± 0.5% vs 80.1% ± 0.8%, P=0.209) of hand hygiene per year was maintained (Supplementary Figure 1). Although the appropriate use of broad-spectrum antimicrobial agents per quarter was maintained before and during the COVID-19 pandemic (82.7% ± 4.7% vs 83.4% ± 2.2%, P=0.695), the number of antimicrobial audits per quarter was significantly lower during the COVID-19 pandemic (988 ± 315 vs 632 ± 225, P=0.016) (Supplementary Figure 2).
Incidence of multidrug-resistant organisms before and during COVID-19
During the study period, the total number of new cases of MRSA, CRA, and ESBL-producing Enterobacterales were 4793, 1649, and 9247, with the corresponding incidence being 1.023, 0.352, and 1.973 per 1000 patient days, respectively. The number of bacteremic episodes due to MRSA, CRA, and ESBL-producing Enterobacterales were 459, 95, and 1422, with the corresponding incidence being 0.098, 0.020, and 0.303, respectively.
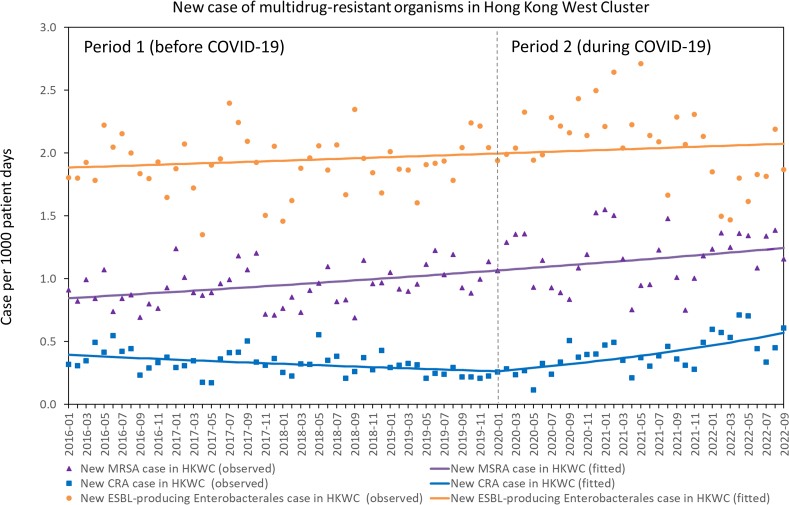
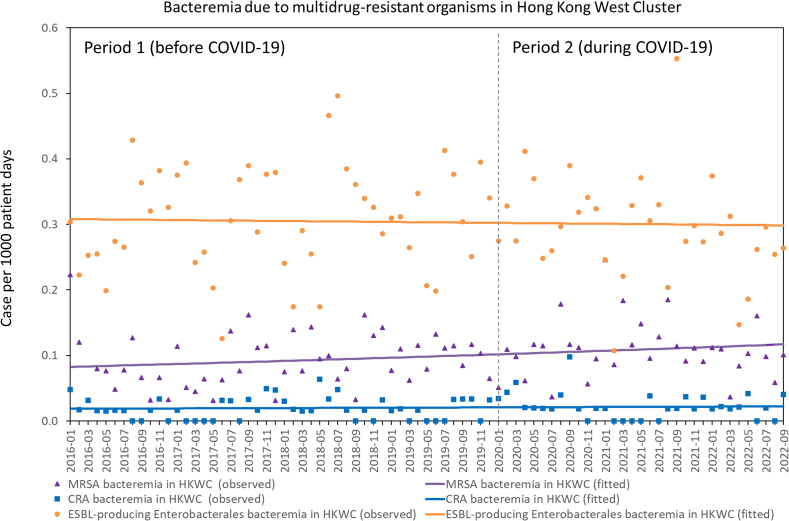
For new case of MDROs infection, there was no significant change in the trend of new cases of MRSA (P=0.742) and ESBL-producing Enterobacterales (P=0.061) infections between period 1 and 2, whereas there was a significant change in the trend of new cases of CRA infections between period 1 and 2 (P<0.001) (Figure 1). The number of new cases of CRA infections per 1000 patient days significantly decreased per month (RR: 0.992, 95% CI: 0.988–0.996, P<0.001) in period 1, but significantly increased (RR: 1.024, 95% CI: 1.017–1.031, P<0.001) in period 2. For bacteremia due to MDROs, there was no significant change in the trend of MRSA (P=0.616), CRA (P=0.338), and ESBL-producing Enterobacterales (P=0.052) bacteremia between period 1 and 2 (Figure 2).
Figure 1.
Trend of new case of MRSA, CRA, and ESBL-producing Enterobacterales infections in Hong Kong West Cluster (HKWC) from January 2016 to September 2022. Note. A new case of MRSA, CRA, or ESBL-producing Enterobacterales was defined as a patient with any clinical specimen positive for these MDROs during hospitalization, without a history of colonization or infection by the same MRDO in the preceding 12 months.
Figure 2.
Trend of bacteremia due to MRSA, CRA, and ESBL-producing Enterobacterales in Hong Kong West Cluster (HKWC) from January 2016 to September 2022.
Consumption of antimicrobial agents before and during COVID-19
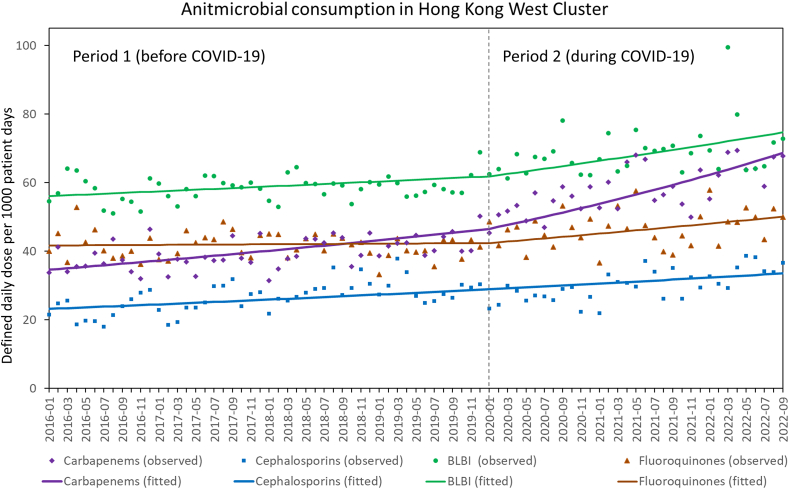
During the study period, the overall usage of antimicrobial agents, expressed in DDD per 1000 patient days, was 46.27 for carbapenems, 27.80 for cephalosporins, 62.21 for BLBI, and 43.50 for fluoroquinolones. The trend of antimicrobial consumption before and during COVID-19 is shown in Figure 3. Comparing the trend of antimicrobial consumption between period 1 and 2, there was a significant increase in carbapenems (P<0.001), BLBI (P=0.045), and fluoroquinolones (P=0.009) consumption, but there was no significant change in the trend of cephalosporins consumption between period 1 and 2 (P=0.920).
Figure 3.
Trend of carbapenems, cephalosporins, extended-spectrum beta-lactam-beta-lactamase inhibitor combinations, and fluoroquinolones before and during COVID-19 in Hong Kong West Cluster from January 2016 to September 2022. Note. BLBI, extended-spectrum beta-lactam-beta-lactamase inhibitor combinations. Carbapenems includes meropenem, imipenem-cilastatin, and ertapenem. Cephalosporins (3rd and 4th generation cephalosporins) includes cefpodoxime, ceftibuten, cefotaxime, ceftriaxone, ceftazidime, cefoperazone-sulbactam, and cefepime. Extended-spectrum beta-lactam-beta-lactamase inhibitor combinations includes piperacillin-tazobactam and ticarcillin-clavulanate. Fluoroquinolones includes oral and intravenous formulation of ciprofloxacin, levofloxacin, and moxifloxacin.
COVID-19 patients with or without multidrug-resistant organisms
Among the 4443 newly diagnosed cases of COVID-19, 2372 (53.4%) were male. The median age was 68 years (range: 7 days to 108 years). Co-infections with MRSA, CRA, and ESBL-producing Enterobacterales were observed in 149 (3.4%), 110 (2.5%) and 57 (1.3%) COVID-19 patients, respectively. Co-infections with any MDROs were found in 272 (6.1%) of COVID-19 patients. The epidemiological characteristics of COVID-19 patients with or without co-infections by MDROs are shown in Table 1. In a multivariable model, older age, male sex, referral from RCHE, presence of indwelling device, presence of endotracheal tube, use of carbapenems, use of BLBI, use of proton pump inhibitors and history of hospitalization were associated with higher risks of infections by MDROs among COVID-19 patients in HKWC (Table 2).
Table 1.
Epidemiological characteristics of COVID-19 patients with or without infections by multidrug-resistant organisms in Hong Kong West Cluster
| COVID-19 patients with infections by MDROs (n=272) | COVID-19 patients without infection by MDROs (n=4171) | P Valuea | |
|---|---|---|---|
| Age (mean ± SD) | 77.19 ± 17.54 | 59.76 ± 28.28 | < 0.001 |
| Male sex | 178 (65.4%) | 2194 (52.6%) | < 0.001 |
| Referral from RCHE | 119 (43.8%) | 591 (14.2%) | < 0.001 |
| Charlson comorbidity index (mean ± SD) | 3.83 ± 2.41 | 2.66 ± 2.47 | < 0.001 |
| Presence of indwelling device | 137 (50.4%) | 575 (13.8%) | < 0.001 |
| Presence of endotracheal tube | 25 (9.2%) | 57 (1.4%) | < 0.001 |
| Use of corticosteroids as COVID-19 therapy | 72 (26.5%) | 713 (17.1%) | < 0.001 |
| Use of antimicrobial agents in the past 3 months | |||
| Carbapenems | 71 (26.1%) | 265 (6.4%) | < 0.001 |
| Cephalosporins | 27 (9.9%) | 150 (3.6%) | < 0.001 |
| BLBI | 104 (38.2%) | 539 (12.9%) | < 0.001 |
| Fluoroquinolones | 23 (8.5%) | 135 (3.2%) | < 0.001 |
| Use of proton pump inhibitors in the past 3 months | 172 (63.2%) | 1184 (28.4%) | < 0.001 |
| History of hospitalization in the past 3 months | 170 (62.5%) | 1589 (38.1%) | < 0.001 |
| Mortality within 90 days of COVID-19 diagnosis | 90 (33.1%) | 568 (13.6%) | < 0.001 |
Note. BLBI, extended-spectrum beta-lactam-beta-lactamase inhibitor combinations; MDROs, multidrug-resistant organisms; RCHE, residential care home for the elderly; SD, standard deviation.
Based on Chi-square test, except for age and Charlson comorbidity index which were based on t test.
Table 2.
Multivariable analysis of COVID-19 patients with infections by multidrug-resistant organisms in Hong Kong West Cluster
| Adjusted odds ratio for COVID-19 patients with infections by MDROsa |
P Value | |
|---|---|---|
| Age | 1.015 (1.006–1.025) | 0.001 |
| Male sex | 1.597 (1.205–2.118) | 0.001 |
| Referral from RCHE | 2.891 (2.137–3.910) | < 0.001 |
| Charlson comorbidity index | 1.005 (0.935–1.081) | 0.892 |
| Presence of indwelling device | 3.339 (2.517–4.429) | < 0.001 |
| Presence of endotracheal tube | 3.880 (2.206–6.824) | < 0.001 |
| Use of corticosteroids as COVID-19 therapy | 0.798 (0.580–1.098) | 0.166 |
| Use of antimicrobial agents in the past 3 months | ||
| Carbapenems | 1.926 (1.345–2.8760) | < 0.001 |
| Cephalosporins | 1.128 (0.684–1.861) | 0.637 |
| BLBI | 1.419 (1.037–1.942) | 0.029 |
| Fluoroquinolones | 1.331 (0.787–2.253) | 0.286 |
| Use of proton pump inhibitors in the past 3 months | 1.653 (1.207–2.265) | 0.002 |
| History of hospitalization in the past 3 months | 1.429 (1.058–1.929) | 0.020 |
Note. BLBI, extended-spectrum beta-lactam-beta-lactamase inhibitor combinations; MDROs, multidrug-resistant organisms; RCHE, residential care home for the elderly; SD, standard deviation.
Based on multivariable logistic regression.
Discussion
We summarized the incidence rates of MRSA, CRA, and ESBL-producing Enterobacterales before (period 1) and during COVID-19 (period 2) in Hong Kong. In period 2, we observed a significant change in the trend of new cases of CRA when compared with period 1 using piecewise Poisson regression (interrupted time series). The significant change in trend observed in period 2 may be related to the reversion of the decreasing incidence of CRA infections in period 1, which was achieved through enhanced infection control interventions against carbapenem-resistant Acinetobacter baummanii, including the implementation of directly observed hand hygiene practice and priority use of single room isolation [11]. During the COVID-19 pandemic, the single rooms were allocated to COVID-19 patients. This may have led to an increase in incidence of CRA in period 2. However, we did not observe a corresponding significant trend of increase in the new cases of MRSA and ESBL-producing Enterobacterales infections in period 2. This is in contrast to the observation made in many parts of the world [[23], [24], [25], [26]]. The lack of a significant increase in the trend of MRSA and ESBL-producing Enterobacterales infections was likely due to the fact that these patients were managed in open cubicles with no changes in infection control practices before and during COVID-19. In fact, the number of hand hygiene observations and overall compliance in our hospitals per year were maintained during the COVID-19 pandemic in our healthcare region, although 100% compliance with hand hygiene was achieved in our clinical unit [27]. Further investigation is warranted to better understand the varying impact of hand hygiene and single room isolation on different types of MDRO.
However, the trend of antimicrobial consumption significantly increased in period 2 when compared with period 1 in our healthcare region. Our finding was consistent with the hospital-based studies in Lebanon, Spain, Italy, India, and the UK, where an increase in antimicrobial consumption was observed in 2020 [28]. The increase in antimicrobial consumption in our healthcare region may be related to a significantly decrease in the number of antimicrobial audits during the COVID-19 pandemic. The increase in CRA infections may also be related to the disruption of the antimicrobial stewardship program.
The COVID-19 pandemic had a significant impact on healthcare systems and patient outcomes worldwide [29,30], including in the areas of cardiovascular diseases [31,32], cerebrovascular diseases [33,34], cancer prevention and treatment [[35], [36], [37]], surgical operations [38,39], and blood transfusion [40]. Antimicrobial stewardship program, as one of the interventions to control the emergence of MDROs, might also have been affected [41,42], which may have contributed to the rapid increase in MDROs during the COVID-19 pandemic [43]. In the meantime, enhancement of infection control measures may also result in unintended consequences including the reduction of MRSA acquisition rate and healthcare-associated respiratory virus infection in Singapore [44]. In Hong Kong, we also demonstrated the reduction in bacteremia caused by bacterial species with respiratory transmission potential and respiratory viruses in the community and healthcare setting, respectively [45,46], as a result of enforcement of hand hygiene and universal masking during the early phase of COVID-19 [27,47]. However, there are numerous factors that may influence the levels of antimicrobial resistance. Antimicrobial use in hospitals as well as infection prevention and control measures may favor an increase or a decrease in antimicrobial resistance during the COVID-19 pandemic [48]. Every healthcare center may have unique characteristics that could contribute to changes in the epidemiology of MDROs during the COVID-19 pandemic. Our findings illustrate the maintenance of infection control measures prevented the surge of MDROs despite the increasing consumption of antimicrobial agents.
The incidence of MDRO infection among our COVID-19 patients was 6%, which was lower than that reported previously in the United States (52%) and in the Republic of Korea (28%) [49,50]. The risk factors for MDRO infection among COVID-19 patients, as shown in the multivariable analysis, were similar to our local population with MDRO colonization, including the prior use of antimicrobial agents and proton pump inhibitors, and recent history of hospitalization [22,51,52]. In addition, patients referred from RCHE were particularly associated with MDRO infection in this study, given that the prevalence of MDROs among our elderly, especially MRSA and CRA, were on a rising trend during our active surveillance studies conducted in the RCHE in the past decade [[53], [54], [55]]. It is interesting to note that the use of corticosteroids was not a significant risk factor for MDRO infection among COVID-19 patients in the multivariable analysis, which is in contrast to the finding of a previous study that corticosteroids use in COVID-19 patients may increase the risk of bacterial co-infection [50]. This difference in findings could be due to the use of a corticosteroid-sparing triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of COVID-19 patients admitted to our hospitals [56].
There are several limitations in this study. First, this study ended early in September 2022 rather than in December 2022 because we needed to cover the first 1000 days of COVID-19 combat in Hong Kong, as hospital services gradually resume normalcy after day 1000 [57]. Second, we only included MRSA, CRA, and ESBL-producing Enterobacterales as the representative MDROs, excluding VRE and carbapenemase-producing Enterobacterales (CPE). With the territory-wide implementation of directly observed hand hygiene-based infection control measures in our public hospitals since 2013, we had successfully controlled the spread of VRE in Hong Kong. VRE was no longer endemic in our healthcare setting before the COVID-19 pandemic [15]. While the incidence of gastrointestinal colonization with CPE has been increasing in recent years [58], the number of patients with CPE infection remained low both before and during the COVID-19 pandemic in our locality. Third, we only included MDROs identified from clinical specimens but not screening specimens in the analysis, as the protocol of MDROs screening was evolving during the study period. Finally, the case mix of patient admission may be altered, especially when there was overwhelming number of COVID-19 patients during the peak of Omicron subvariant BA.2 in February and March 2022. Therefore the incidence of MDRO may be interpreted with caution. However, our finding can provide an overview of the epidemiology of MDRO infection before and during the COVID-19 pandemic in Hong Kong. Given the observational nature of the study, it could not establish a causal relationship.
Credit author statement
Shuk-Ching Wong: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - original draft, Writing - review and editing. Pui-Hing Chau: Data curation, Formal analysis, and Statistical analysis. Simon Yung-Chun So: Data curation, Formal analysis. Kelvin Hei-Yeung Chiu: Formal analysis, Writing - review and editing. Lithia Lai-Ha Yuen: Investigation. Christine Ho-Yan AuYeung: Investigation. Germaine Kit-Ming Lam: Investigation. Veronica Wing-Man Chan: Investigation. Jonathan Hon-Kwan Chen: Resources. Hong Chen: Resources. Xin Li: Writing - review and editing. Pak-Leung Ho: Writing - review and editing. Sophia Siu-Chee Chan: Writing - review and editing. Kwok-Yung Yuen: Funding acquisition, Supervision. Vincent Chi-Chung Cheng: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing - original draft, Writing - review and editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We are grateful to the contribution of our frontline staff and laboratory staff in enforcing the infection control measures and performing the laboratory work in the Queen Mary Hospital.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.infpip.2023.100286.
Funding sources
This study was partially supported by the Health and Medical Research Fund (HMRF) Commissioned Research on Control of Infectious Disease (Phase IV), CID-HKU1-16, Health Bureau, Hong Kong SAR Government.
Conflict of interest
All authors report no conflicts of interest relevant to this article.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Leung E., Weil D.E., Raviglione M., Nakatani H., World Health Organization World Health Day Antimicrobial Resistance Technical Working Group The WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011 May 1;89(5):390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng V.C., To K.K., Li I.W., Tang B.S., Chan J.F., Kwan S., et al. Antimicrobial stewardship program directed at broad-spectrum intravenous antibiotics prescription in a tertiary hospital. Eur J Clin Microbiol Infect Dis. 2009 Dec;28(12):1447–1456. doi: 10.1007/s10096-009-0803-8. [DOI] [PubMed] [Google Scholar]
- 3.WHO guidelines on hand hygiene in health care. https://www.who.int/publications/i/item/9789241597906 (Accessed 5 March 2023).
- 4.Cheng V.C., Tai J.W., Ho S.K., Chan J.F., Hung K.N., Ho P.L., et al. Introduction of an electronic monitoring system for monitoring compliance with Moments 1 and 4 of the WHO "My 5 Moments for Hand Hygiene" methodology. BMC Infect Dis. 2011 May 26;11:151. doi: 10.1186/1471-2334-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng V.C.C., Wong S.C., Wong I.W.Y., Chau P.H., So S.Y.C., Wong S.C.Y., et al. The challenge of patient empowerment in hand hygiene promotion in health care facilities in Hong Kong. Am J Infect Control. 2017 May 1;45(5):562–565. doi: 10.1016/j.ajic.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Cheng V.C., Tai J.W., Li W.S., Chau P.H., So S.Y., Wong L.M., et al. Implementation of directly observed patient hand hygiene for hospitalized patients by hand hygiene ambassadors in Hong Kong. Am J Infect Control. 2016 Jun 1;44(6):621–624. doi: 10.1016/j.ajic.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Cheng V.C.C., Wong S.C., Wong S.C.Y., Yuen K.Y. Directly observed hand hygiene - from healthcare workers to patients. J Hosp Infect. 2019 Apr;101(4):380–382. doi: 10.1016/j.jhin.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Cheng V.C.C., Chen H., Wong S.C., Chen J.H.K., Ng W.C., So S.Y.C., et al. Role of Hand Hygiene Ambassador and Implementation of Directly Observed Hand Hygiene Among Residents in Residential Care Homes for the Elderly in Hong Kong. Infect Control Hosp Epidemiol. 2018 May;39(5):571–577. doi: 10.1017/ice.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng V.C., Tai J.W., Chau P.H., Chen J.H., Yan M.K., So S.Y., et al. Minimal intervention for controlling nosocomial transmission of methicillin-resistant staphylococcus aureus in resource limited setting with high endemicity. PLoS One. 2014 Jun 19;9(6) doi: 10.1371/journal.pone.0100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng V.C., Tai J.W., Chen J.H., So S.Y., Ng W.C., Hung I.F., et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc. 2014 Oct;113(10):734–741. doi: 10.1016/j.jfma.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Wong S.C., Chau P.H., So S.Y., Lam G.K., Chan V.W., Yuen L.L., et al. Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumanniiby Enhancement of Infection Control Measures. Antibiotics (Basel) 2022 Aug 8;11(8):1076. doi: 10.3390/antibiotics11081076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng V.C., Chen J.H., Poon R.W., Lee W.M., So S.Y., Wong S.C., et al. Control of hospital endemicity of multiple-drug-resistant Acinetobacter baumannii ST457 with directly observed hand hygiene. Eur J Clin Microbiol Infect Dis. 2015 Apr;34(4):713–718. doi: 10.1007/s10096-014-2281-x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng V.C., Chan J.F., Wong S.C., Chen J.H., Tai J.W., Yan M.K., et al. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J (Engl). 2013 Dec;126(23):4504–4509. [PubMed] [Google Scholar]
- 14.Cheng V.C., Tai J.W., Wong L.M., Ching R.H., Ng M.M., Ho S.K., et al. Effect of proactive infection control measures on benchmarked rate of hospital outbreaks: An analysis of public hospitals in Hong Kong over 5 years. Am J Infect Control. 2015 Sep 1;43(9):965–970. doi: 10.1016/j.ajic.2015.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng V.C., Tai J.W., Chau P.H., Lai C.K., Chuang V.W., So S.Y., et al. Successful control of emerging vancomycin-resistant enterococci by territory-wide implementation of directly observed hand hygiene in patients in Hong Kong. Am J Infect Control. 2016 Oct 1;44(10):1168–1171. doi: 10.1016/j.ajic.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Sun Jin L., Fisher D. MDRO transmission in acute hospitals during the COVID-19 pandemic. Curr Opin Infect Dis. 2021 Aug 1;34(4):365–371. doi: 10.1097/QCO.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 17.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007 Oct;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng V.C., To K.K., Tse H., Hung I.F., Yuen K.Y. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev. 2012 Apr;25(2):223–263. doi: 10.1128/CMR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng V.C.C., Wong S.C., To K.K.W., Ho P.L., Yuen K.Y. Preparedness and proactive infection control measures against the emerging novel coronavirus in China. J Hosp Infect. 2020 Mar;104(3):254–255. doi: 10.1016/j.jhin.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng V.C.C., Wong S.C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020 May;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reducing antimicrobial resistance with IMPACT. https://www.chp.gov.hk/files/pdf/reducing_bacterial_resistance_with_impact.pdf (Accessed 5 March 2023).
- 22.Wong S.C., Chen J.H., Chau P.H., So S.Y., AuYeung C.H., Yuen L.L., et al. Gastrointestinal Colonization of Carbapenem-Resistant Acinetobacter baumannii: What Is the Implication for Infection Control? Antibiotics (Basel) 2022 Sep 22;11(10):1297. doi: 10.3390/antibiotics11101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez S., Innes G.K., Walters M.S., Mehr J., Arias J., Greeley R., et al. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions - New Jersey, February-July 2020. MMWR Morb Mortal Wkly Rep. 2020 Dec 4;69(48):1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Toole R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect. 2021 Dec;27(12):1772–1776. doi: 10.1016/j.cmi.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polly M., de Almeida B.L., Lennon R.P., Cortês M.F., Costa S.F., Guimarães T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. 2022 Feb;50(2):238–239. doi: 10.1016/j.ajic.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bongiovanni M., Barilaro G., Zanini U., Giuliani G. Impact of the COVID-19 pandemic on multidrug-resistant hospital-acquired bacterial infections. J Hosp Infect. 2022 May;123:191–192. doi: 10.1016/j.jhin.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong S.C., AuYeung C.H., Lam G.K., Leung E.Y., Chan V.W., Yuen K.Y., et al. Is it possible to achieve 100 percent hand hygiene compliance during the coronavirus disease 2019 (COVID-19) pandemic? J Hosp Infect. 2020 Aug;105(4):779–781. doi: 10.1016/j.jhin.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukushige M., Ngo N.H., Lukmanto D., Fukuda S., Ohneda O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front Public Health. 2022 Oct 18;10 doi: 10.3389/fpubh.2022.946077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemos D.R.Q., D'Angelo S.M., Farias L.A.B.G., Almeida M.M., Gomes R.G., Pinto G.P., et al. Health system collapse 45 days after the detection of COVID-19 in Ceará, Northeast Brazil: a preliminary analysis. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0354-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva S.J.R.D., Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021 Dec;13 doi: 10.1016/j.onehlt.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rattka M., Dreyhaupt J., Winsauer C., Stuhler L., Baumhardt M., Thiessen K., et al. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart. 2020 Dec 17 doi: 10.1136/heartjnl-2020-318360. [DOI] [PubMed] [Google Scholar]
- 32.Teoh S.E., Masuda Y., Tan D.J.H., Liu N., Morrison L.J., Ong M.E.H., et al. Impact of the COVID-19 pandemic on the epidemiology of out-of-hospital cardiac arrest: a systematic review and meta-analysis. Ann Intensive Care. 2021 Dec 7;11(1):169. doi: 10.1186/s13613-021-00957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You Y., Niu Y., Sun F., Zhang J., Huang S., Ding P., et al. Impact of COVID-19 pandemic on haemorrhagic stroke admissions: a systematic review and meta-analysis. BMJ Open. 2021 Dec 14;11(12) doi: 10.1136/bmjopen-2021-050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsanos A.H., Palaiodimou L., Zand R., Yaghi S., Kamel H., Navi B.B., et al. Changes in Stroke Hospital Care During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Stroke. 2021 Nov;52(11):3651–3660. doi: 10.1161/STROKEAHA.121.034601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayo M., Potugari B., Bzeih R., Scheidel C., Carrera C., Shellenberger R.A. Cancer Screening During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1109–1117. doi: 10.1016/j.mayocpiqo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teglia F., Angelini M., Casolari G., Astolfi L., Boffetta P. Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis. Cancers (Basel) 2022 Nov 8;14(22):5490. doi: 10.3390/cancers14225490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson B.A., Waddimba A.C., Ogola G.O., Fleshman J.W., Jr., Preskitt J.T. A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: Implication for surgical triage during the COVID-19 pandemic. Am J Surg. 2021 Aug;222(2):311–318. doi: 10.1016/j.amjsurg.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate S.M., Mantefardo B., Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020 Oct 12;14:37. doi: 10.1186/s13037-020-00262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K.C., Xiao R., Cheung Z.B., Barbera J.P., Forsh D.A. Early mortality after hip fracture surgery in COVID-19 patients: A systematic review and meta-analysis. J Orthop. 2020 Nov-Dec;22:584–591. doi: 10.1016/j.jor.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiem C., Alghamdi K., Nguyen T., Han J.H., Huo H., Jackson D. The Impact of COVID-19 on Blood Transfusion Services: A Systematic Review and Meta-Analysis. Transfus Med Hemother. 2021 Nov 16;30(2):1–12. doi: 10.1159/000519245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashiru-Oredope D., Kerr F., Hughes S., Urch J., Lanzman M., Yau T., et al. Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics (Basel) 2021 Jan 23;10(2):110. doi: 10.3390/antibiotics10020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomczyk S., Taylor A., Brown A., de Kraker M.E.A., El-Saed A., Alshamrani M., et al. WHO AMR Surveillance and Quality Assessment Collaborating Centres Network. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J Antimicrob Chemother. 2021 Oct 11;76(11):3045–3058. doi: 10.1093/jac/dkab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai C.C., Chen S.Y., Ko W.C., Hsueh P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021 Apr;57(4) doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee L.E.I., Conceicao E.P., Tan J.Y., Magesparan K.D., Amin I.B.M., Ismail B.B.S., et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am J Infect Control. 2021 Apr;49(4):469–477. doi: 10.1016/j.ajic.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng V.C., Wong S.C., So S.Y., Chen J.H., Chau P.H., Au A.K., et al. Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic. Antibiotics (Basel) 2022 May 31;11(6):746. doi: 10.3390/antibiotics11060746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong S.C., Lam G.K., AuYeung C.H., Chan V.W., Wong N.L., So S.Y., et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: Implication of universal masking in hospitals. Infect Control Hosp Epidemiol. 2021 Feb;42(2):218–221. doi: 10.1017/ice.2020.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng V.C., Wong S.C., Chuang V.W., So S.Y., Chen J.H., Sridhar S., et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020 Jul;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monnet D.L., Harbarth S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill. 2020 Nov;25(45) doi: 10.2807/1560-7917.ES.2020.25.45.2001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhargava A., Riederer K., Sharma M., Fukushima E.A., Johnson L., Saravolatz L. High rate of Multidrug-Resistant Organisms (MDROs) among COVID-19 patients presenting with bacteremia upon hospital admission. Am J Infect Control. 2021 Nov;49(11):1441–1442. doi: 10.1016/j.ajic.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Son H.J., Kim T., Lee E., Park S.Y., Yu S., Hong H.L., et al. Risk factors for isolation of multi-drug resistant organisms in coronavirus disease 2019 pneumonia: A multicenter study. Am J Infect Control. 2021 Oct;49(10):1256–1261. doi: 10.1016/j.ajic.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng V.C., Chen J.H., So S.Y., Wong S.C., Chau P.H., Wong L.M., et al. A Novel Risk Factor Associated With Colonization by Carbapenemase-Producing Enterobacteriaceae: Use of Proton Pump Inhibitors in Addition to Antimicrobial Treatment. Infect Control Hosp Epidemiol. 2016 Dec;37(12):1418–1425. doi: 10.1017/ice.2016.202. [DOI] [PubMed] [Google Scholar]
- 52.Wong S.C., Chen J.H., So S.Y., Ho P.L., Yuen K.Y., Cheng V.C. Gastrointestinal colonization of meticillin-resistant Staphylococcus aureus: an unrecognized burden upon hospital infection control. J Hosp Infect. 2022 Mar;121:65–74. doi: 10.1016/j.jhin.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Cheng V.C., Tai J.W., Wong Z.S., Chen J.H., Pan K.B., Hai Y., et al. Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect Dis. 2013 May 6;13:205. doi: 10.1186/1471-2334-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng V.C.C., Chen J.H.K., Ng W.C., Wong J.Y.H., Chow D.M.K., Law T.C., et al. Emergence of Carbapenem-Resistant Acinetobacter baumannii in Nursing Homes With High Background Rates of MRSA Colonization. Infect Control Hosp Epidemiol. 2016 Aug;37(8):983–986. doi: 10.1017/ice.2016.84. [DOI] [PubMed] [Google Scholar]
- 55.Wong S.C., Chen J.H., Yuen L.L., Chan V.W., AuYeung C.H., Leung S.S., et al. Air dispersal of meticillin-resistant Staphylococcus aureus in residential care homes for the elderly: implications for transmission during the COVID-19 pandemic. J Hosp Infect. 2022 May;123:52–60. doi: 10.1016/j.jhin.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 May 30;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong S.C., Au A.K., Lo J.Y., Ho P.L., Hung I.F., To K.K., et al. Evolution and Control of COVID-19 Epidemic in Hong Kong. Viruses. 2022 Nov 14;14(11):2519. doi: 10.3390/v14112519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong S.C., Chan V.W.M., Lam G.K., AuYeung C.H., Leung E.Y., So S.Y., et al. The use of multi-pronged screening strategy to understand the epidemiology of carbapenemase-producing Enterobacteriaceae in Hong Kong: transition from epidemic to endemic setting. Eur J Clin Microbiol Infect Dis. 2021 Sep;40(9):2017–2022. doi: 10.1007/s10096-021-04173-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.