Abstract
Background:
To evaluate the role of cortical amyloid deposition as a factor contributing to memory dysfunction and increased risk of dementia associated with late life depression (LLD).
Methods:
119 older adult participants with current diagnosis of Major Depression (LLD) from the ADNI Depression study and 119 non-depressed (ND) cognitively unimpaired participants matched on age, gender, and APOE genotype obtained from the ADNI database.
Results:
Thirty-three percent of LLD participants met ADNI criteria for MCI. Compared to ND individuals, the LLD group exhibited less global amyloid (Aβ) accumulation (p = 0.05). The proportion of amyloid positivity in the LLD group was 19.3% compared to 31.1% for the ND participants (p =0.02). Among LLD participants, global Aβ was not associated with lifetime number of depressive episodes, lifetime length of depression, length of lifetime SSRI use, or lifetime length of untreated depression (p > 0.21 for all). Global Aβ was associated with worse memory performance (p = 0.05). Similar results were found in secondary analyses restricting comparisons to the cognitively unimpaired LLD participants and also when comparing the LLD group to a ND group which included MCI participants.
Conclusions:
Contrary to expectation, the LLD group showed less Aβ deposition than ND and Aβ deposition was not associated with depression history characteristics. Aβ was associated with memory but this relationship did not differ between LLD and ND. Our results suggest that memory deficits and accelerated cognitive decline reported in previous studies of late life depression is not due to greater cortical Aβ accumulation.
Keywords: Late Life Depression, amyloid, cognition, depressive symptoms, mild cognitive impairment, Alzheimer’s disease
Introduction
Subsyndromal symptoms of depression and major depression in older adults are among the most consistently reported risk factors associated with more rapid rates of future cognitive decline (1–8), more rapid conversion to dementia (9, 10), and higher rates of incident dementia (11). Specifically, subsyndromal symptoms of depression have been linked to a 2.4 fold increase risk of dementia (12), whereas major depression has been linked to a nearly 4.3 fold increase risk for dementia (13). As a result of these collective findings, increased Aβ deposition in LLD has been implicated as one potential pathway to increased risk of dementia in this patient population (14).
The accumulation of Aβ plaques in the brain is widely accepted as being one of the primary factors in the degeneration of neurons and cortical atrophy leading to memory and other cognitive dysfunction in AD (15–17). Initial support for the possible link between LLD and increased Aβ deposition was found in autopsy studies showing an association between amyloid plaques in dementia patients with a history of major depression (18, 19) as well as in studies of plasma Aβ40 and cerebrospinal fluid (CSF) Aβ42 levels that indicate altered metabolism of Aβ in LLD (20–22). Further support for Aβ as a possible mechanism underlying the connection between LLD and dementia comes from PET studies showing elevated cortical amyloid deposition in LLD relative to controls (23, 24) and associations between amyloid binding and clinical features of LLD, including increased treatment resistance (25) and apathy severity (26). Studies using PET imaging have also found positive associations between amyloid burden and subsyndromal depressive symptoms in older adults (27, 28), including a higher risk for incident depression (29) and poorer cognitive functioning over time (30). PET imaging has also been used to examine associations between amyloid deposition and depressive symptoms in MCI samples, showing increased amyloid deposition and an increased risk for incident dementia (31–34).
Evidence for increased Aβ pathology in LLD has been equivocal with some studies showing no association of depression history or current depression with increased Aβ (35). Additionally, many of the published studies showing elevated Aβ in LLD have been limited by small sample sizes. Only two studies (29, 34) evaluated APOE genotype in group comparisons, which is salient given the known association of APOE with AD risk. Further, the majority of previous studies have incomplete depression and treatment histories, which are particularly relevant given studies that have suggested that SSRI treatment may be associated with reductions of Aβ pathology (36). Further, only two (30, 34) of these previous studies evaluated the relationship of Aβ to memory performance and showed mixed results. Evaluating the association of Aβ to memory functioning in LLD is particularly important given that memory dysfunction is central to diagnostic conversion to dementia.
The goal of the current study was to determine if LLD is associated with increased Aβ accumulation and to evaluate the relationship of Aβ deposition to memory performance, and depression history characteristics, including SSRI use. Based on previous studies we predicted that LLD participants would have higher levels of amyloid deposition than ND cognitively unimpaired control participants. Further, we expected that greater Aβ accumulation would be associated with poorer memory performance in both LLD and ND participants. Specific to our LLD study sample, we hypothesized that length of lifetime depression history, untreated depression, and number of discrete depressive episodes would be positively associated with Aβ, and lifetime use of SSRI treatment would be negatively associated with Aβ in the LLD sample.
Methods and Materials
Participants
Participants included in the primary analysis to evaluate the relationship between LLD and Aβ PET SUVR included 119 older adults with Major Depression Disorder enrolled in the Alzheimer’s Disease Neuroimaging Initiative – Depression Project (ADNI-D) and 119 non-depressed (ND) cognitively unimpaired individuals enrolled in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study. In sensitivity analyses, we also compared LLD to ND individuals restricting the sample to cognitively unimpaired participants in each group. Separately, we also compared LLD to ND participants, matching the proportion of MCI participants in each group, as defined by ADNI criteria for determination of MCI. Exclusion of MCI participants from the ND group in the primary analysis was done to provide the most conservative point of comparison for measures of Aβ PET SUVR between groups as major depression has consistently been associated with memory impairments independent of AD (37, 38). All participants provided written informed consent upon their enrollment in the study. The study was conducted in accordance with the Declaration of Helsinki for protection of human subjects, with procedures approved by the institutional review boards of each study site.
For LLD participants, inclusion criteria included current diagnosis of Major Depressive Disorder (MDD), unipolar type, without psychotic features, with reported symptom severity of ≥15 on the 17-item HDRS (39), and current episode lasting at least six weeks. Diagnoses of MDD were made by a licensed clinical psychologist using the Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders – IV (40). Other Axis I disorders and significant current neurologic illness such as epilepsy, Parkinson’s disease, traumatic brain injury or cortical stroke excluded individuals from participation. Those with diagnoses of dementia or evidence of dementia (<25 on the Mini Mental Status Exam [MMSE]) were also excluded from participation. If inclusion criteria for the study were met, and consent was given, participants underwent a blood draw for DNA and RNA banking (with APOE genotyping), MRI imaging, and AV-45 (florbetapir) amyloid PET imaging.
For the ND comparison group, data for the current study was obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies, and non-profit organizations. The ADNI study is conducted in accordance with the Declaration of Helsinki and procedures were approved by the institutional review boards of all participating sites. All participants provided written informed consent at enrollment. Criteria for cognitively unimpaired participants included a MMSE score >24, Global Clinical Dementia Rating Scale (CDR) score = 0.0, and no evidence of memory impairment on neuropsychological assessment based on WMS-R Logical Memory II raw score adjusted for education. MCI criteria included a Mini-Mental Status Score >24, a Global CDR score of 0.5, and lower score on WMS-R Logical Memory II relative to an education corrected cutscore. Exclusion criteria for the ADNI study at baseline included: 1) the presence of Major Depressive Disorder or significant symptoms of depression (Geriatric Depression Scale [GDS] score >6); 2) modified Hachinski ischemia score>5; 3) significant neurological or psychiatric illness; 4) high dose of neuroleptics or chronic sedatives or hypnotics, antiparkinsonian medication, and use of narcotic analgesics. ND participants for the primary analysis were selected to match the LLD participants on age, sex, APOE ε4-positivity, Aβ PET uptake in the reference region (whole cerebellum), and PET image smoothing parameters (as a proxy for scanner type). ND participants were also restricted to have GDS scores < 3. In one of two sensitivity analyses, both LLD and ND groups were restricted to cognitively unimpaired participants only and were matched on the same variables as the primary analysis. In a second sensitivity analysis, in which MCI participants were included in both groups, LLD participants were matched to ND participants on diagnosis (proportion of MCI), delayed logical memory, education, as well as the variables used to match in the primary analysis. The matching procedure was done using propensity scores (41).
Procedures
After an initial screening phone interview where inclusion criteria were assessed and demographic data obtained, eligible participants were referred to their corresponding Psychiatry Department site, either at the University of Pittsburgh or the University of California San Francisco for clinical psychiatric assessment (Structured Clinical Interview for DSM Disorders; SCID), depression history, and cognitive assessment. Subsequently participants were evaluated at the ADNI site at the University of Pittsburgh Medical Center or the University of California San Francisco where they underwent core ADNI study protocol (including cognitive tests, blood draw for DNA and RNA, and MRI and PET imaging).
Measures
Memory
Verbal learning and memory were assessed using the Wechsler Memory Scale – Revised. The Logical Memory test was used to assess learning and memory for stories, with number of story elements recalled as the outcome measure (42).
Depression severity and history.
Severity of depression symptoms at baseline was assessed using the Geriatric Depression Scale (43) and the 17-item Hamilton Depression Rating Scale (39). Depression history was collected with a self-reported retrospective measure that was verified in clinic with research coordinators. Lengths of individual depressive episodes were coded in months. Additionally, participants recorded all treatment modalities sought and the duration of each method of treatment including use of antidepressants. The depression history retrospective measure was developed utilizing the basic structure of NIMH’s life-chart method (NIMH-LCM) (44).
Amyloid burden.
Four five-minute frames were acquired at 50–70min post-injection and pre-processed as previously described (45). Florbetapir images were coregistered to a contemporaneous structural MRI, which was processed with FreeSurfer v5.3. The cortical summary SUVR was calculated by creating an average of frontal, cingulate, temporal, and parietal regions relative to the whole cerebellum. Aβ positive status in this cortical summary region was defined as ≥ 1.11 which is 2 SD above the mean of cognitively normal individuals (46). Individual cortical regions divided by the whole cerebellum were also examined. Finally, for voxelwise analyses, native-space florbetapir images were intensity normalized at the voxelwise level using the mean of the whole cerebellum. The co-registered structural MRI image was nonlinearly warped into MNI template space, and this transformation was applied to the florbetapir images.
APOE genotype.
DNA was extracted from blood samples using commercial reagents (FlexiGene, Qiagen, Valencia, CA, USA). Two APOE single-nucleotide polymorphisms, specifically rs7412 and rs429358, were typed using allelic discrimination assays with TaqMan reagents (Applied Biosystems, Foster City, CA, USA). The genotyping results were subsequently incorporated into an algorithm, resulting in designation of ε2, ε3, or ε4 genotypes. For the purpose of this study, genotype was analyzed as a dichotomous variable (presence or absence of 3|4 or 4|4 genotypes, referred to as ε4 allele, commonly associated with increased AD risk).
Statistical Analyses
LLD and ND group differences in demographic characteristics were analyzed using Mann-Whitney tests for continuous measures and Fisher’s Exact test for categorical measures. Because LLD participants came from two PET imaging sites and ND participants came from 33 imaging sites with 9 scanner types/models, we utilized measures of cerebellum amyloid and PET image smoothing characteristics as covariates in our statistical models to account for observed group differences in cerebellum amyloid and to limit site variability due to scanner type. We compared models summarizing scanner information by their smoothing characteristics and separately by scanner type. Models covarying for smoothing characteristics were selected as the better fitting and more parsimonious models by the Akaike Information Criterion. Group differences in global and regional Aβ accumulation were tested using robust linear regression with nonparametric bootstrap permutation tests. Independent variables in the model included age, gender, APOE status (at least one APOE ε4 allele versus ε4 non-carrier), Aβ uptake in the whole cerebellum, PET image smoothing characteristics, and depression group (LLD versus ND). Group differences in terms of Aβ positivity were tested using logistic regression. We then evaluated whether memory performance was associated with Aβ deposition. For our LLD specific analyses, the effect of depression history characteristics (lifetime duration of depression, untreated depression, length of SSRI treatment, and lifetime number of depressive episodes) on global Aβ SUVR was also evaluated with robust linear regression and permutation tests. Voxelwise analyses were carried out in SPM12 using the same covariates. P-values for group differences in regional Aβ accumulation were adjusted for multiple comparisons using a false discovery rate (FDR) correction. P-values for the effects of depression history characteristics were adjusted using a Holm correction (47). All p-values were two-tailed and considered significant at the α = 0.05 level. Statistical analyses were conducted with the R Package (v3.6.0, http:/www.r.project.org/).
Results
Participants included 119 LLD and 119 cognitively unimpaired ND individuals with a mean age of 71.4 years (SD = 5.6), a mean of 16.4 years of education (SD = 2.2), 63.9% female, and 26.9% were APOE+. The LLD group and ND group did not differ on these characteristics (Table 1). In the LLD group 33% met criteria for MCI and the mean Hamilton Depression rating score was 18.2 (SD = 2.6). By design, the LLD group reported greater symptoms of depression on the GDS than the ND group (LLD = 7.3, ND = 0.56; p < 0.001) and they exhibited poorer performance on the logical memory delayed recall test (LLD = 11.2, ND = 13.3; p < 0.001). The two groups did not differ with respect to overall measures of mental status (mean MMSE = 29.1 in both groups; p = 0.45). In regard to depression history characteristics, the LLD group had a mean lifetime length of depression of 263.4 months (SD = 223.8), a mean lifetime length of untreated depression of 139.1 months (SD = 190.0), a mean lifetime length of SSRI treatment of 56.8 months (SD = 92.8), and mean lifetime number of depressive episodes of 2.5 (SD = 1.8).
Table 1:
Demographic and Clinical Characteristics of LLD and ND Groups (n = 238)
| ND (n = 119) | LLD (n = 119) | p-value | |||
|---|---|---|---|---|---|
|
|
|||||
| Mean ± SD | Range | Mean ± SD | Range | ||
|
| |||||
| Age, years | 71.9 ± 5.8 | 60 – 89 | 70.9 ± 5.3 | 65 – 91 | 0.15 |
| Education, years | 16.5 ± 2.5 | 12 – 20 | 16.3 ± 2.0 | 12 – 20 | 0.39 |
| Gender, No. female (%) | 72 (61%) | 80 (67%) | 0.35 | ||
| APOE Status, No. ε4+ (%) | 32 (27%) | 32 (27%) | >0.99 | ||
| Cognitive Status, No. MCI (%) | 0 (0%) | 39 (33%) | <0.01 | ||
| MMSE | 29.1± 1.2 | 24 – 30 | 29.1 ± 1.0 | 26 – 30 | 0.45 |
| CDR (0:0.5) | 118:1 | 43:75 | <0.01 | ||
| GDS | 0.6 ± 0.7 | 0 – 2 | 7.3 ± 3.2 | 1 – 15 | <0.01 |
| LM Delayed Recall | 13.3 ± 3.1 | 5 – 23 | 11.2 ± 4.4 | 1 – 20 | <0.01 |
| Amyloid SUVR | 1.10 ± 0.16 | 0.85 – 1.70 | 1.06 ± 0.14 | 0.87 – 1.61 | 0.02 |
| Amyloid Positivity Rate (Aβ+:Aβ−) | 37:82 | 23:96 | 0.05 | ||
Note: Group differences were analyzed using Mann-Whitney tests for continuous measures and Fisher’s Exact test for categorical measures. All values represent mean ± standard deviation (SD) unless otherwise noted. MMSE= Mini Mental Status Exam, LM = WAIS-IIIR Logical Memory Test, GDS = Geriatric Depression Scale total score; CDR = Clinical Dementia Rating Scale
Aβ deposition
Compared to the cognitively unimpaired ND group, the LLD group displayed significantly lower global Aβ deposition (β= −0.04, p= 0.05) and a lower rate of Aβ positivity (19.3% compared to 31.1%; log OR = −0.97, p= 0.02, Figure 1). Regional Aβ SUVR and whole brain differences are shown in Figures 2 and 3. Other significant predictors of Aβ positivity included increasing age (log OR = 0.09, p < 0.01), female gender (log OR = 1.08, p = 0.01), and APOE positivity (log OR = 1.47, p < 0.01) (Table 2). Individual regions of interest (ROI) were compared between groups and adjusted for multiple comparisons (Supplemental Table 1). Aβ deposition was significantly lower in the LLD group in the frontal pole (β= −0.063, p <.001), lingual gyrus (β = −0.34, p <.001), caudal (β = −0.06, p = 0.02) and rostral anterior cingulate (β = −0.06, p = 0.02) as well as the fusiform gyrus (β = −0.04, p = 0.02) medial orbitofrontal cortex (β = −0.05, p = 0.02, lateral orbitofrontal (β = −0.04, p = 0.05, supramarginal (β = −0.04, p = 0.02, middle temporal (β = −0.04, p = 0.04, parahippocampal (β = −0.03, p = 0.04), pericalcarine (β = −0.04, p = 0.0), hippocampus (β = −0.03, p = 0.04), isthmus cingulate (β = −0.04, p = 0.04), temporal pole (β = −0.03, p = 0.04), and inferior parietal regions (β = −0.05, p = 0.05). Within the LLD group there was no difference in amyloid positivity or SUVR associated with MCI diagnosis (p > 0.23).
Figure 1.
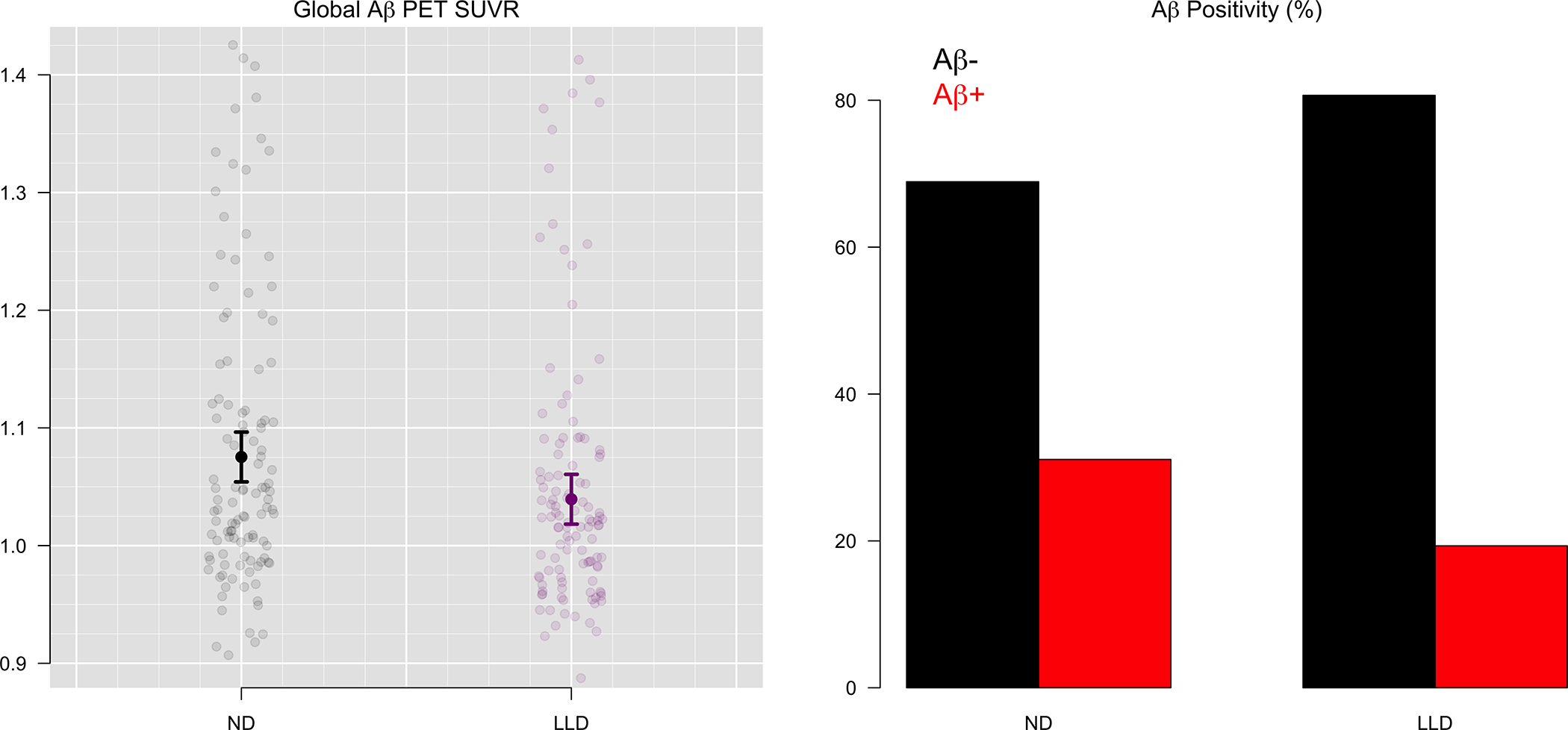
Global Aβ PET Uptake and Amyloid Positivity for LLD and ND groups (n=238)
Figure 2.
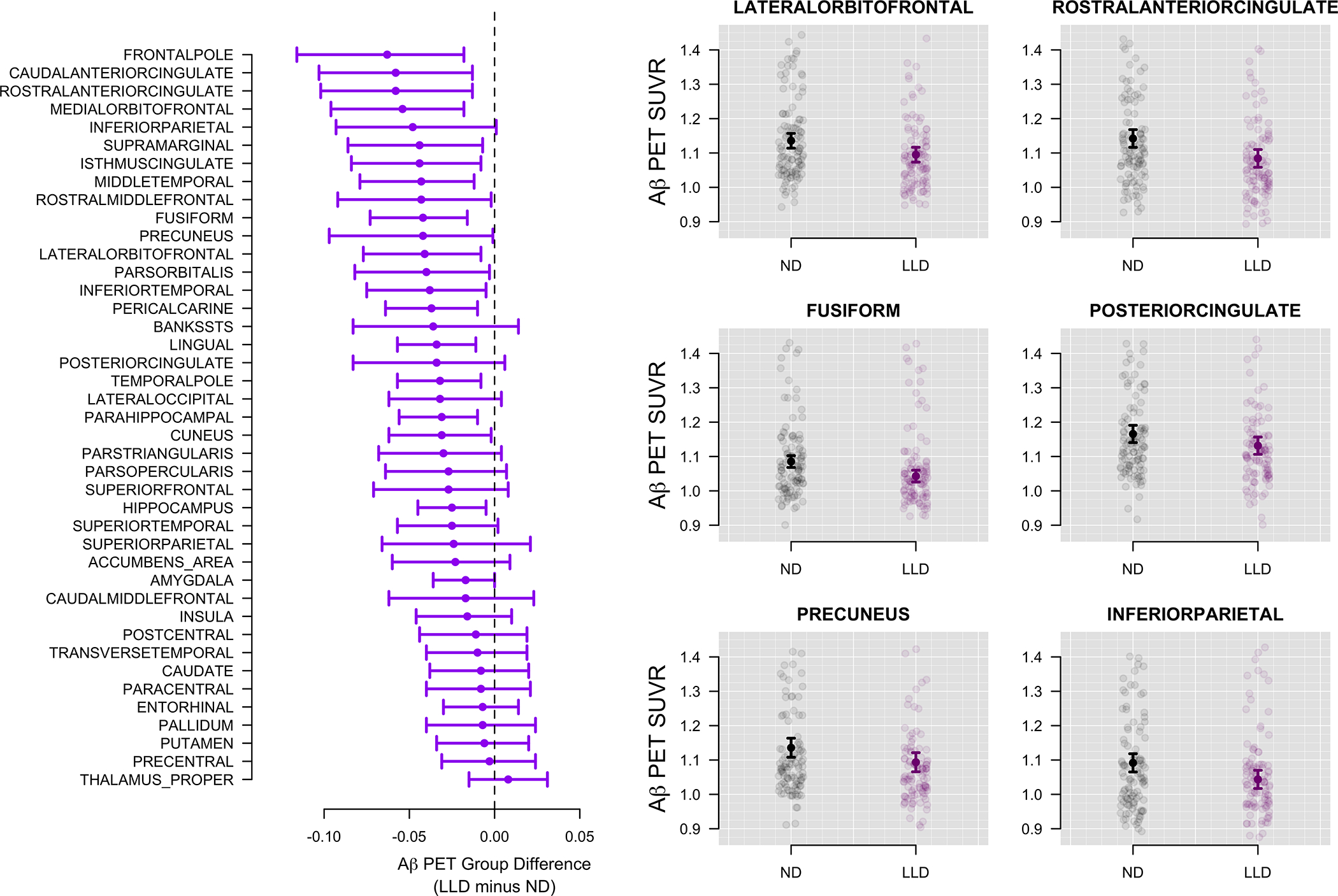
Regional differences in Aβ for LLD and ND groups (n=238)
Estimated Aβ SUVR differences between LLD and ND groups for all regions are shown on the left. A value of −0.05, for example, indicates that the LLD group had 0.05 lower average SUVR compared to the ND group.
Figure 3:
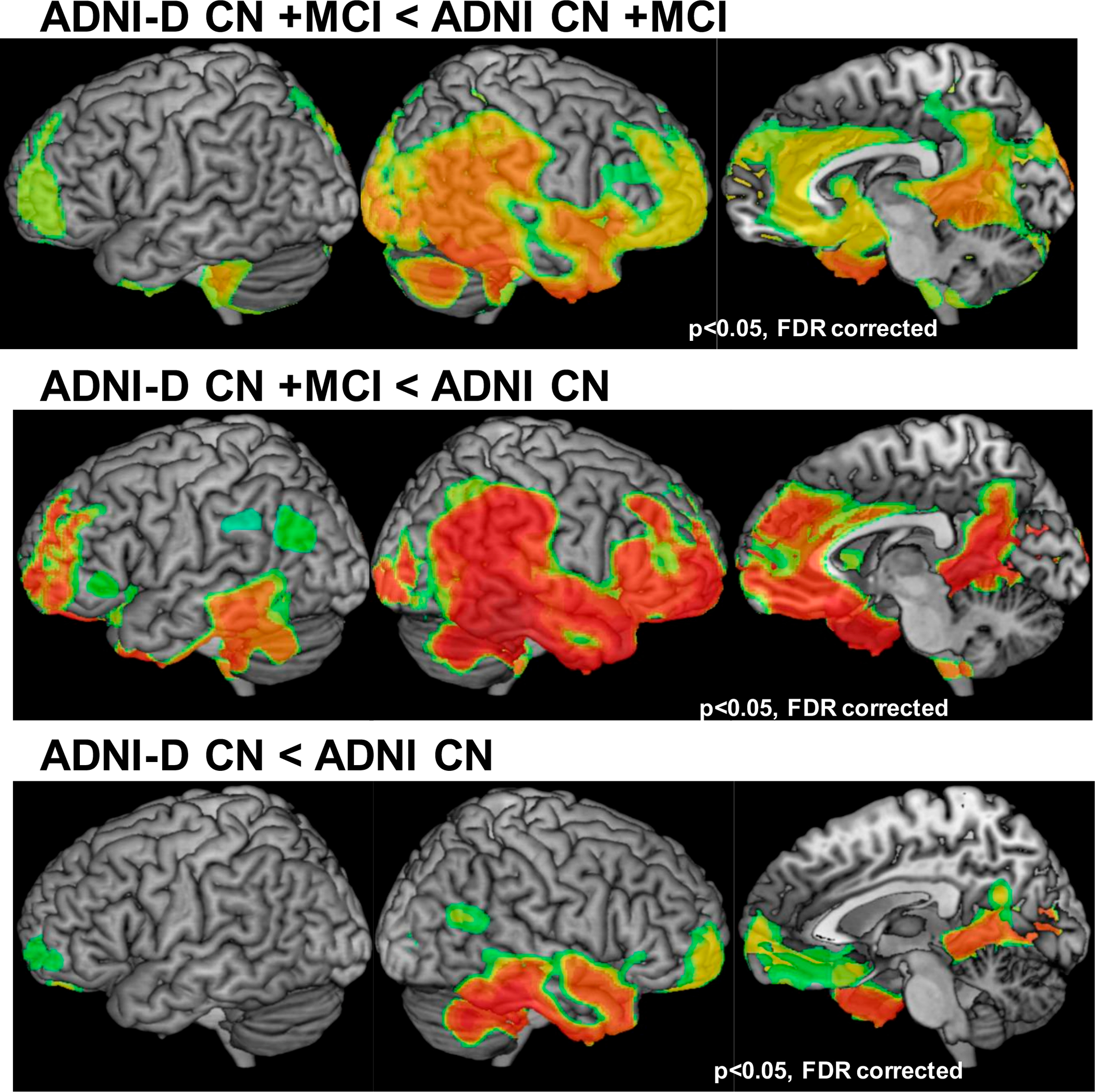
Depression group differences in amyloid beta. Top Row: LLD n = 44, ND n = 119; Middle Row: LLD n = 44, ND n = 119; Bottom Row: LLD n = 80, ND n = 80. Not all LLD participants were included in all comparisons. CN, cognitively normal; FDR, false discovery rate; LLD, late life depression; MCI, mild cognitive impairment; ND, nondepressed
Table 2:
Regression Results of the Relationship between LLD and Amyloid Positivity
| log OR | SE | Z value | P value | |
|---|---|---|---|---|
|
| ||||
| (Intercept) | −6.36 | 5.81 | −1.10 | 0.27 |
| Whole Cerebellum | 7.00 | 3.04 | 2.31 | 0.02 |
| Age | 0.09 | 0.03 | 3.09 | <0.01 |
| Gender | 1.08 | 0.40 | 2.72 | 0.01 |
| APOE E4+ | 1.47 | 0.35 | 4.16 | <0.01 |
| XY.smoothing | −0.67 | 0.85 | −0.79 | 0.43 |
| Z.smoothing | −1.28 | 1.05 | −1.23 | 0.22 |
| Depression Group | −0.97 | 0.41 | −2.35 | 0.02 |
Sensitivity Analyses
Similar results were observed when the ND comparison group was matched for MCI status (Supplemental Table 2) with LLD showing decreased global Aβ SUVR (β = −0.05, p = 0.02) and reduced amyloid positivity (19.3% vs 39.5%; log OR = −1.27, p = 0.002). When only cognitively unimpaired LLD participants were compared to cognitively unimpaired ND participants (Supplemental Table 3) SUVR comparison between groups was not significant (β = −0.03, p = 0.39), however the rate of amyloid positivity was significantly lower (16.2% vs 30.0%; log OR = −1.12, p = 0.03).
Relation between Memory Performance and Amyloid Deposition
After adjusting for age, sex, education and depression group, Aβ SUVR was associated with poorer memory performance (β = −3.29, p =0.05; Figure 4) and this relationship did not differ by depression group (p=0.25). Depression was significantly associated with poorer memory performance after accounting for age, gender, education, Aβ SUVR and MCI diagnosis (β = −1.32, p = 0.01)
Figure 4.
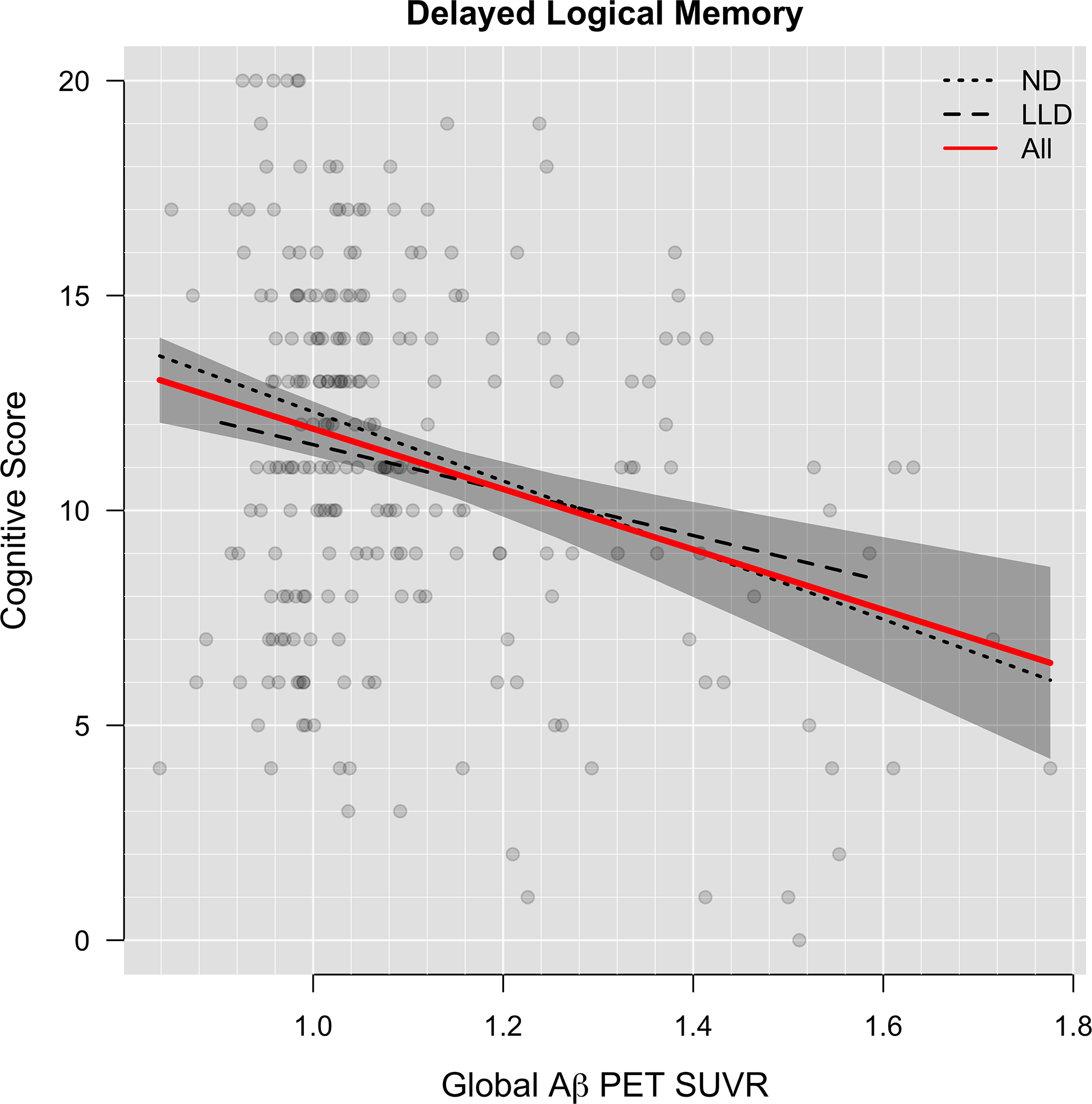
Association of Aβ with Memory Performance (n=238)
Delayed logical memory is plotted against global Aβ SUVR. Dashed lines show LLD and ND groups separately. The red curve shows both groups combined.
Depression Characteristics for the LLD Group
After accounting for age, gender, education, and APOE status, and correcting for multiple comparisons, lifetime duration of depression (β = 0.00, p > 0.99), duration of untreated depression (β = 0.00, p > 0.99); lifetime depressive episodes (β = 0.0001, p > 0.99), and length of lifetime SSRI treatment (β = 0.0002, p = 0.22) were not associated with Aβ PET SUVR (Figure 5).
Figure 5:
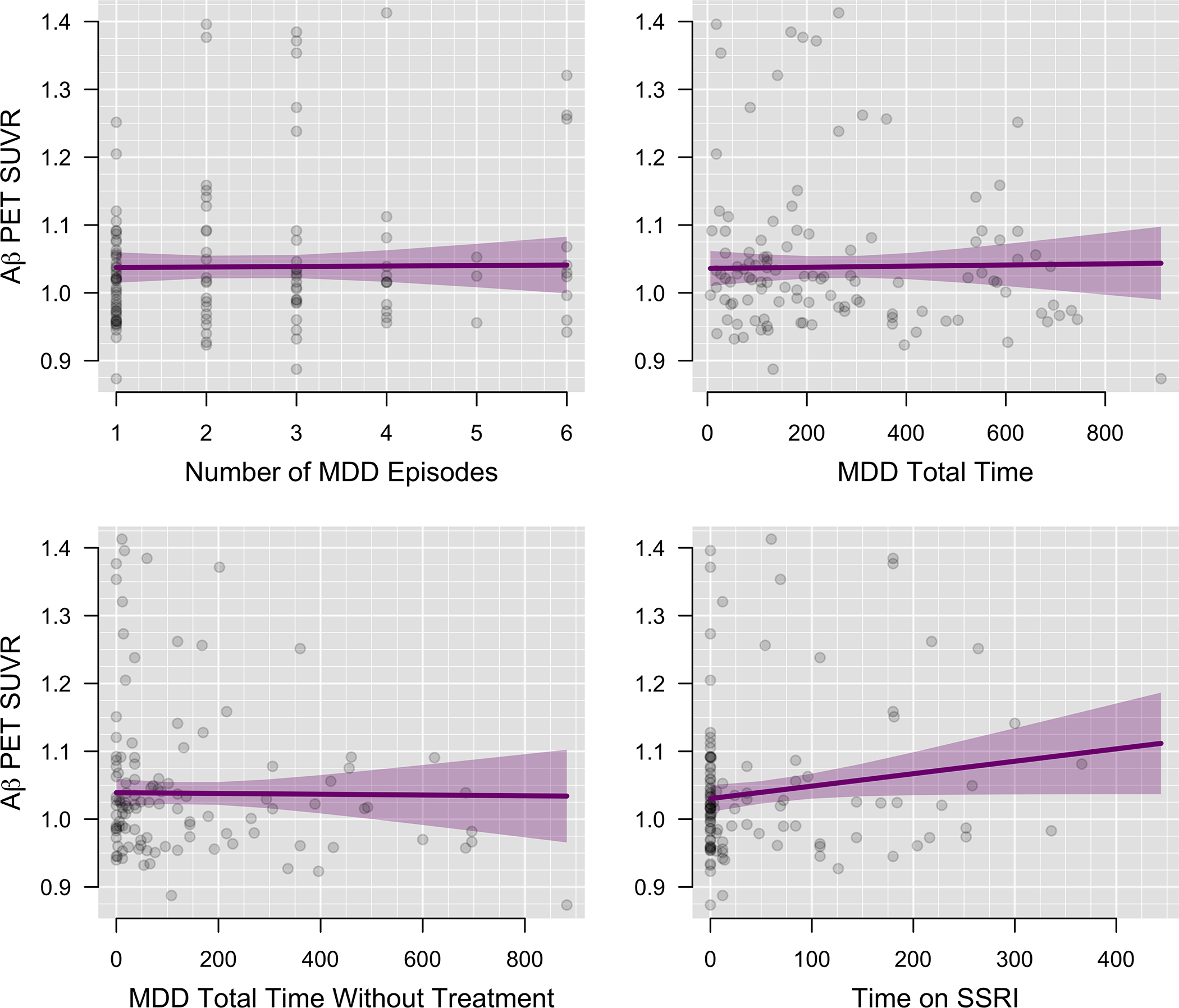
Association of Aβ with Depression Characteristics and Treatment History (n=238)
Global Aβ SUVR is plotted against depression characteristics. Time variables are depicted in days.
Discussion
This study evaluated group differences in amyloid deposition in a large, well-characterized sample of older adults with and without major depression. Contrary to expectations that the LLD group would exhibit higher rates of amyloid deposition based on literature of accelerated cognitive decline (9) and neurodegeneration in older depressed adults (24) the LLD group had significantly lower total amyloid deposition and lower rates of amyloid positivity than ND participants. When looking at regional deposition of amyloid, the differences were particularly pronounced in the frontal pole, rostral and anterior cingulate, medial orbitofrontal and middle temporal cortex. Our results also showed that amyloid burden was associated with memory performance but was not associated with depression history characteristics in this sample. Prior to discussing our results, it is worth noting that our decision to compare a mixed cognitive status LLD group (cognitively unimpaired, MCI) to cognitively unimpaired controls in primary analyses was in order to place the most stringent constraints on identifying group differences which might indicate increased amyloid accumulation in the sample because MDD has been shown to impact memory performance and functional status independent of AD (37, 48). Therefore, relying solely on a cognitively matched sample may have likely resulted in a higher incidence of AD pathology in the comparison group. Each of our findings will be discussed below.
In our primary analysis we compared the LLD sample (of whom 33% had MCI) with the non-depressed controls without MCI. Our results showed that amyloid burden was reduced in the LLD group. Comparison of the LLD group with a non-depressed control group matched for the proportion with MCI produced similar results. Our finding of reduced amyloid in our depression sample was not expected given prior literature stating that LLD is a risk factor for accelerated cognitive decline and the development of both MCI and dementia (1, 9, 49), the comorbidity of LLD and AD (50), and CSF studies of Aβ (19–21). As might be expected, when the comparison subjects matched for cognitive status (MCI) were added into the analysis, these group differences (lower amyloid deposition and positivity in LLD group) became even more pronounced. However, when restricting the analyses to only cognitively normal LLD and ND participants, SUVR comparisons were no longer significant whereas differences in amyloid positivity persisted. Collectively these results suggest other non-amyloid mediated pathways are likely associated with risk for accelerated cognitive decline and dementia reported in LLD. It is worth noting that most previous work supporting the link between LLD and increased amyloid had not been done with in vivo amyloid PET imaging, nor included a large number of clinically depressed participants with controls matched for demographic, genetic and clinical features for comparison purposes.
It is also possible that there are mechanisms of major depression that reduce amyloid deposition. In particular, reduced cerebral blood flow or hypometabolism may limit amyloid uptake in the regions most affected in depression – specifically, regions such as the cingulate and orbitofrontal cortex. However, in our data we did not see any association of depression history that was significantly associated with amyloid which would have supported this hypothesis. Similarly, we did not see any association of depression treatments, most notably length of SSRI treatments, being linked to decreased amyloid accumulation in the sample. We also note that the majority of LLD participants in this sample were early onset, with long histories of depression, and there is also the potential for late onset of depression being more strongly linked to increased amyloid burden.
In the entire sample of LLD and ND we also reported an association between amyloid burden and decreased memory performance. These results are notable in that the association between cognition and amyloid is often weak in cognitively normal individuals (51) and the majority of our sample was cognitively unimpaired. Further, we also reported the expected association between memory performance and depression status, with LLD participants performing worse on memory tests. We did not, however, see an interaction between amyloid positivity and depression status with regards to memory performance. Taken together, we would conclude the impact of depression on memory function, independent of amyloid, is likely a significant contributor to risk of future cognitive decline. This relationship could be a direct consequence of depressed mood on cognition and functional status, or stem from other factors such as cortical atrophy associated with depressive symptoms (52). Further, for individuals with other concurrent neurodegenerative processes, including amyloid deposition, this additional effect of depression could contribute to accelerated rates of cognitive decline and faster conversion to dementia.
Though our results are robust and have several possible explanations to consider in terms of etiology, there are some limitations to state. Particularly, the cross-sectional nature of the data presented does not allow for evaluation of longitudinal cognitive function or future amyloid status in either group. That is, it is possible that the LLD group will still experience accelerated cognitive decline relative to ND participants when followed over the course of years, or convert more rapidly to amyloid positivity, when measured over time. Further, our results suggest that there is potential for sampling bias in that individuals with MCI due to AD that also have concurrent LLD may have two distinct contributors to cognitive dysfunction. As a result, these individuals with greater amyloid burden and associated impact on memory performance, may be more likely to receive a dementia diagnosis earlier and would have been excluded from this study. Additionally, despite protocols designed to limited site specific effects of PET image acquisition and our approach to minimize these potential effects in our analyses there is the potential that site specific imaging acquisition influenced our results given that our imaging data was obtained from many sites. To our knowledge this study is the first to investigate in vivo amyloid differences in large samples of clinically depressed and ND older adults that has matched participants on APOE status. Given known associations between APOE and amyloid accumulation we believe this is a critical factor to consider when evaluating links between amyloid and depression. Our sample also met criteria for current major depression and reported long lifetime histories of depression and various depression treatments, which also represent critical considerations when evaluating group differences in amyloid.
While we did not evaluate longitudinal outcomes in this study, our results suggest that increased cortical amyloid burden is not a primary causal factor in reported increased risk for dementia associated with major depression in older adults (13). This conclusion is strengthened by a lack of association between depression characteristics and history of treatment with amyloid burden. In contrast, our findings that depression was strongly linked to memory dysfunction, independent of amyloid, suggests that LLD may accelerate progression to dementia by contributing to cognitive and functional burden among individuals with concurrent MCI or incipient neurodegenerative disease.
Supplementary Material
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Depression project (ADNI D) (National Institute of Mental Health Grant R01098062 and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). We acknowledge Ray and Dagmar Dolby Family Fund for research support and Avid Radiopharmaceuticals for providing Florbetapir for this study. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative-Depression project (ADNI-D) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) databases (www.loni.usc.edu). As such, the investigators within the ADNI D and ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI D investigators and ADNI investigators include http://adni.loni.usc.edu/study-design/ongoing-investigations/ ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of California, Los Angeles.
Footnotes
Disclosures
During the past 2 years, Dr. Mackin has received research support from The National Institute of Mental Health, and Johnson and Johnson.
During the past 2 years, Dr. Nelson has been an advisor or consultant to Assurex, Eiasi, FVS-7, and Janssen.
Mr. Insel, Dr. Morin, Dr. Rhodes, Dr. Tosun, Dr. Toga, Dr. Saykin, Dr. Jack, Dr. Rosen, Dr. Aisen, Dr. Koeppe, Dr Butters and Mr. Bickford reported no biomedical financial interests or potential conflicts of interest.
Dr. Raman has received research support from the National Institute on Aging, Eli Lilly and Janssen.
Dr Landau has received research support from the National Institutes on Aging and has consulted for NeuroVision and Cortexyme.
Dr. Weiner has served on the Scientific Advisory Boards for Pfizer, BOLT International, Neurotrope Bioscience, Alzheon, Inc., Alzheimer’s Therapeutic Research Institute (ATRI), Eli Lilly, U. of Penn’s Neuroscience of Behavior Initiative, National Brain Research Centre (NBRC), India, Dolby Family Ventures, LP, and ADNI.
References
- 1.Yaffe K, Blackwell T, Gore R, et al. : Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 1999; 56:425–430 [DOI] [PubMed] [Google Scholar]
- 2.Sachs-Ericsson N, Joiner T, Plant EA, et al. : The influence of depression on cognitive decline in community-dwelling elderly persons. Am J Geriatr Psychiatry 2005; 13:402–408 [DOI] [PubMed] [Google Scholar]
- 3.Chodosh J, Kado DM, Seeman TE, et al. : Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry 2007; 15:406–415 [DOI] [PubMed] [Google Scholar]
- 4.Bielak AA, Gerstorf D, Kiely KM, et al. : Depressive symptoms predict decline in perceptual speed in older adulthood. Psychol Aging 26:576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comijs HC, Jonker C, Beekman AT, et al. : The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. Int J Geriatr Psychiatry 2001; 16:361–367 [DOI] [PubMed] [Google Scholar]
- 6.Grabovich A, Lu N, Tang W, et al. : Outcomes of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry 18:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Lyness JM, Tu X, et al. : Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am J Psychiatry 2007; 164:1221–1228 [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Barnes LL, Mendes de Leon CF, et al. : Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002; 59:364–370 [DOI] [PubMed] [Google Scholar]
- 9.Gabryelewicz T, Styczynska M, Luczywek E, et al. : The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry 2007; 22:563–567 [DOI] [PubMed] [Google Scholar]
- 10.Modrego PJ,Ferrandez J: Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol 2004; 61:1290–1293 [DOI] [PubMed] [Google Scholar]
- 11.Saczynski JS, Beiser A, Seshadri S, et al. : Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 2010; 75:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan WC, Lam LC, Tam CW, et al. : Prevalence of neuropsychiatric symptoms in chinese older persons with mild cognitive impairment-a population-based study. Am J Geriatr Psychiatry 2010; 18:948–954 [DOI] [PubMed] [Google Scholar]
- 13.Richard E, Reitz C, Honig LH, et al. : Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol 2013; 70:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kita Y, Baba H, Maeshima H, et al. : Serum amyloid beta protein in young and elderly depression: a pilot study. Psychogeriatrics 2009; 9:180–185 [DOI] [PubMed] [Google Scholar]
- 15.Braak H,Braak E: [Morphological changes in the human cerebral cortex in dementia]. J Hirnforsch 1991; 32:277–282 [PubMed] [Google Scholar]
- 16.Oh H, Madison C, Villeneuve S, et al. : Association of gray matter atrophy with age, beta-amyloid, and cognition in aging. Cerebral cortex 2014; 24:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawas CH, Greenia DE, Bullain SS, et al. : Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2013; 9:199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. : Increased Hippocampal Plaques and Tangles in Patients With Alzheimer Disease With a Lifetime History of Major Depression. JAMA Psychiatry 2006; 63:161–167 [DOI] [PubMed] [Google Scholar]
- 19.Sweet RA, Hamilton RL, Butters MA, et al. : Neuropathologic Correlates of Late-Onset Major Depression. Neuropsychopharmacology 2004; 29:2242–2250 [DOI] [PubMed] [Google Scholar]
- 20.Pomara N, Doraiswamy PM, Willoughby LM, et al. : Elevation in plasma Abeta42 in geriatric depression: a pilot study. Neurochem Res 2006; 31:341–349 [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Steffens DC, Au R, et al. : Amyloid-Associated Depression: A Prodromal Depression of Alzheimer Disease? JAMA Psychiatry 2008; 65:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kita Y, Baba H, Maeshima H, et al. : Serum amyloid β protein in young and elderly depression: a pilot study. Psychogeriatrics 2009; 9:180–185 [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Kepe V, Barrio JR, et al. : Protein binding in patients with late-life depression. Arch Gen Psychiatry 2011; 68:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu KY, Hsiao IT, Chen CS, et al. : Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur J Nucl Med Mol Imaging 2014; 41:714–722 [DOI] [PubMed] [Google Scholar]
- 25.Li P, Hsiao I-T, Liu C-Y, et al. : Beta-amyloid deposition in patients with major depressive disorder with differing levels of treatment resistance: a pilot study. EJNMMI Res 2017; 7:24–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyre HA, Siddarth P, van Dyk K, et al. : Neural correlates of apathy in late-life depression: a pilot [(18) F]FDDNP positron emission tomography study. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society 2017; 17:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krell-Roesch J, Lowe VJ, Neureiter J, et al. : Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the Mayo Clinic Study of Aging. Int Psychogeriatr 2018; 30:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donovan NJ, Locascio JJ, Marshall GA, et al. : Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am J Psychiatry 2018; 175:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington KD, Gould E, Lim YY, et al. : Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry 2017; 32:455–463 [DOI] [PubMed] [Google Scholar]
- 30.Gatchel JR, Rabin JS, Buckley RF, et al. : Longitudinal Association of Depression Symptoms With Cognition and Cortical Amyloid Among Community-Dwelling Older Adults. JAMA Netw Open 2019; 2:e198964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brendel M, Pogarell O, Xiong G, et al. : Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur J Nucl Med Mol Imaging 2015; 42:716–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon B, Kim S, Park YH, et al. : Depressive Symptoms are Associated with Progression to Dementia in Patients with Amyloid-Positive Mild Cognitive Impairment. J Alzheimers Dis 2017; 58:1255–1264 [DOI] [PubMed] [Google Scholar]
- 33.Chung JK, Plitman E, Nakajima S, et al. : Lifetime History of Depression Predicts Increased Amyloid-beta Accumulation in Patients with Mild Cognitive Impairment. J Alzheimers Dis 2016; 49:1189–1190 [DOI] [PubMed] [Google Scholar]
- 34.Wu KY, Liu CY, Chen CS, et al. : Beta-amyloid deposition and cognitive function in patients with major depressive disorder with different subtypes of mild cognitive impairment: (18)F-florbetapir (AV-45/Amyvid) PET study. Eur J Nucl Med Mol Imaging 2016; 43:1067–1076 [DOI] [PubMed] [Google Scholar]
- 35.Madsen K, Hasselbalch BJ, Frederiksen KS, et al. : Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging 2012; 33:2334–2342 [DOI] [PubMed] [Google Scholar]
- 36.Sheline YI, West T, Yarasheski K, et al. : An antidepressant decreases CSF Abeta production in healthy individuals and in transgenic AD mice. Sci Transl Med 2014; 6:236re234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter RJ, Gallagher P, Thompson JM, et al. : Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 2003; 182:214–220 [DOI] [PubMed] [Google Scholar]
- 38.Butters MA, Whyte EM, Nebes RD, et al. : The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004; 61:587–595 [DOI] [PubMed] [Google Scholar]
- 39.Hamilton M: A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 1960; 23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frances A: Diagnostic and statistical manual of mental disorders: DSM-IV, American Psychiatric Association, 1994 [Google Scholar]
- 41.Ho DE, Imai K, King G, et al. : Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 2007; 15:199–236 [Google Scholar]
- 42.Wechsler D: Wechsler memory scale-revised (WMS-R), Psychological Corporation, 1987 [Google Scholar]
- 43.Yesavage JA: Geriatric Depression Scale. Psychopharmacol Bull 1988; 24:709–711 [PubMed] [Google Scholar]
- 44.Born C, Amann BL, Grunze H, et al. : Saving time and money: a validation of the self ratings on the prospective NIMH Life-Chart Method (NIMH-LCM). BMC Psychiatry 2014; 14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagust WJ, Landau SM, Koeppe RA, et al. : The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimer’s & Dementia 2015; 11:757–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landau SM, Mintun MA, Joshi AD, et al. : Amyloid Deposition, Hypometabolism, and Longitudinal Cognitive Decline. Annals of neurology 2012; 72:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm S: A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics 1979; 6:65–70 [Google Scholar]
- 48.Jaeger J, Berns S, Uzelac S, et al. : Neurocognitive deficits and disability in major depressive disorder. Psychiatry Research 2006; 145:39–48 [DOI] [PubMed] [Google Scholar]
- 49.Byers AL,Yaffe K: Depression and risk of developing dementia. Nat Rev Neurol 2011; 7:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi S, Wang C, Jiang T, et al. : The prevalence of depression in Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res 2015; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 51.Hedden T, Oh H, Younger AP, et al. : Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013; 80:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzales MM, Insel PS, Nelson C, et al. : Cortical Atrophy is Associated with Accelerated Cognitive Decline in Mild Cognitive Impairment with Subsyndromal Depression. Am J Geriatr Psychiatry 2017; 25:980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


