Abstract
Long-term memory (LTM) formation is dependent on neurochemical changes that guarantee that a recently formed memory (short-term memory [STM]) remains in the specific neural circuitry via the consolidation process. The persistence of recognition memory has been evidenced by using behavioral tagging in young adult rats, but it has not been effective on aging. Here, we investigated the effects of treatment with a standardized extract of Ginkgo biloba (EGb) associated with novelty on the consolidation of object location memory (OLM) and its persistence after weak training of spatial object preference in young adult and aged rats. The object location task used in this study included two habituation sessions, training sessions associated or not associated with EGb treatment and contextual novelty, and short-term or long-term retention testing sessions. Altogether, our data showed that treatment with EGb associated with novelty close to the time of encoding resulted in STM that lasted for 1 h and persisted for 24 h for both young adult and aged rats. In aged rats, the cooperative mechanisms induced robust long-term OLM. Our findings support and extend our knowledge about recognition memory in aged rats and the modulating effects of EGb treatment and contextual novelty on the persistence of memory.
Memory formation guarantees our ability to live in society; learn concepts and meanings; recognize positive or negative properties of the stimulus or context, preparing the organism for appropriate responses (i.e., approach and avoidance behaviors); and acquire and store information about the temporal–spatial relationship of events/episodes (Tulving and Markowitsch 1998; Dere et al. 2005; Dudai et al. 2007). Therefore, it is a fundamental biological process at multiple stages for humans and animals to acquire (encoding/training), store (short term or long term), and retrieve (recall) new information (Tulving and Markowitsch 1997; Abel and Lattal 2001; Daumas et al. 2005; Kesner et al. 2008), which requires integrity and changes in the state of the specific neuronal distributed network. Episodic memory (personal experiences) or episodic-like memory formation depends on the interactions among the medial temporal lobe, prefrontal cortex, prosencephalon basal, and diencephalic structures. It is meant to assess the integration of memories about when (time)/what (item)/where (spatial) information of items (Tulving and Markowitsch 1997; Morris 2001; Daumas et al. 2005; Dere et al. 2005; Kart-Teke et al. 2006; Kesner et al. 2008; Gros et al. 2022) and is more vulnerable to the effects of aging and cognitive disorders (Robitsek et al. 2008; Bishop et al. 2010; Hamezah et al. 2018).
Spontaneous recognition memory can be assessed using a natural preference for object novelty (what), a preference for novel location (where), and temporal order (when) or context (which) in which objects or events are presented. Object location memory (OLM) evaluates object place preference, which requires the dorsal hippocampus for encoding and consolidation processes besides the entorhinal cortex and thalamic (Vishnoi et al. 2016; Chao et al. 2020, 2022; Teratani-Ota and Wiltgen 2022).
Throughout the acquisition/encoding process, intern representations of a given sensorial input may occur by electrochemical changes in specific neural circuitry neurons (Tulving 2002). After encoding, this information might be stored by engram cells, in which the retention interval of information depends on the nature of the engram; that is, the cellular and molecular memory traces (Tonegawa et al. 2015). Long-term memory (LTM) formation is dependent on neurochemical changes that guarantee that a recently formed memory (short-term memory [STM]) remains in the specific neural circuitry via the consolidation process (Dudai 2002; Davis and Zhong 2017). The best-characterized changes for LTM formation include transcription regulation and de novo translation (Bourtchouladze et al. 1998; Silva et al. 1998; Josselyn and Nguyen 2005; Alberini et al. 2006; Dere et al. 2007; Kandel et al. 2014; Alberini and Kandel 2015).
Different factors might affect the encoding process and influence how memory is formed and established. A newly encoded memory initially exists in a labile state, which might be improved or impaired by time-dependent physiological changes, behavioral experience, and/or pharmacological interference (Ballarini et al. 2009; Alberini et al. 2013; Stern and Alberini 2013; Moncada et al. 2015). Evidence suggests that a novelty exposure session, as a novel context, occurring close in time to another event (e.g., weak training session) might modulate the synthesis of plasticity-related proteins and result in long-lasting memory (Moncada and Viola 2007; Alberini and Ledoux 2013; Moncada et al. 2015; Vishnoi et al. 2018). Thus, a weak experience (which usually can produce only STM), when temporally associated with another behavioral relevant experience, can result in an LTM. This up-regulation of protein synthesis induced by a stronger associate behavioral experience was named behavioral tagging (Moncada and Viola 2007; Ballarini et al. 2009; Moncada et al. 2015), and the memory that persists over time suggests that an overlap of the neuronal ensembles is activated by both weak and novelty stimuli. Overlap in the population of neurons activated by conditioned and unconditioned stimuli was observed in Pavlovian conditioning and conditioned taste aversion (Ballarini et al. 2009; Moncada et al. 2015; Nomoto et al. 2016).
The encoding and retention of relevant daily information decline with aging, and age-associated place recognition memory impairment has been associated with altered hippocampal circuits (Rosenzweig and Barnes 2003). However, the persistence of memories might be improved by using behavioral tagging, a general process that takes place to establish a transient short-term memory into long-term memory at a young age but not in early aging (Moncada and Viola 2007; Ballarini et al. 2009; Moncada et al. 2015; Nomoto et al. 2016; Vishnoi et al. 2016, 2018; Gros et al. 2022). Furthermore, age-related impairment in spatial memory is the first form of memory loss in the aging process or early stage of dementia (Morris 2001; Davis et al. 2013).
In addition to behavioral tagging, we postulated that treatment with a standardized extract of Ginkgo biloba (EGb) could modulate the molecular targets involved in the consolidation process and induce LTM formation, since standardized extract of Ginkgo biloba (EGb) has been associated with improvements in memory formation in healthy young and middle-age volunteers (Elsabagh et al. 2005; Kennedy et al. 2007; Tan et al. 2015) and has been used in the treatment and prevention of neurodegenerative disease and memory impairment in the elderly (Tang et al. 2002; Sangiovanni et al. 2017; Zhao et al. 2021). We wondered whether aged rats treated with EGb would have improved the original memory. That is, does EGb affect memory consolidation in aged rats and promote LTM formation in a procedure that usually can produce only STM? This hypothesis was also substantiated in previous data from our laboratory showing that EGb treatment improved conditioned suppression memory formation by modulating neuronal circuits into hippocampal formation in young adult rats (Oliveira et al. 2009, 2013; Gaiardo et al. 2019; Zamberlam et al. 2016, 2019) and middle-aged rats (Ribeiro et al. 2016). Furthermore, we also showed that EGb treatment results in the persistence of fear memory (Zamberlam et al. 2016, 2019) and object recognition memory by up-regulating BDNF into hippocampal formation (Muratori et al. 2021).
Here, we investigated the effects of the administration of EGb and/or novelty on the consolidation of spatial memory and its persistence after a weak training of object location memory (OLM) in young adult and aged rats. In addition, it allowed us to assess hippocampal-dependent memory, which is based on the natural tendency of rats to spend more time investigating the new object or new location object (Sweatt 2010).
Results
Experiment I: effects of novelty on memory in young adult and aged rats
To investigate the effect of novelty on promoting STM and LTM when it was experienced after a weak training task, we first established the weak stimulation paradigm in young adult rats using a novel object location task (exploration for 5 min), which was compared with strong training (exploration for 15 min). Initially, the rats were randomly assigned into two groups: stronger training (ST; n = 16) and weak training (WT; n = 14). One half was tested 1 h (STM; n = 8–7/per group) (Fig. 1A,B), and the other half of each group was tested 24 h after training, where LTM was evaluated (n = 8–7/per group) (Fig. 1C,D). The choice to explore the novel location more than the familiar location reflects short-term or long-term object location memory formation.
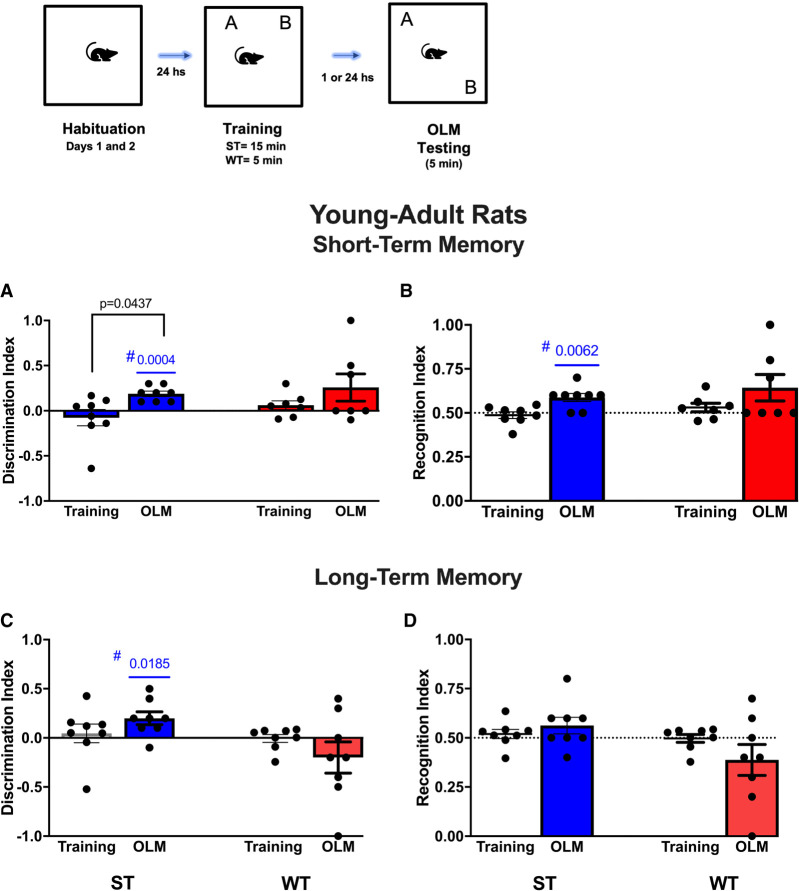
Figure 1.
Schematic representation of the procedure for acquisition and retrieval of the object location memory (OLM) task. Young adult rats were allowed to freely explore the sample objects (A and B) for 15 min (strong training [ST]) or for 5 min (weak training [WT]). (A–D) Memory retention was evaluated 1 h (short-term memory [STM]) or 24 h (long-term memory [LTM]) after the training session. The mean discrimination index (DI) and recognition index (RI) of the sample objects were evaluated on training session for WT and ST groups. Mean DI (A,C) and RI (B,D) of the moved and sample objects were evaluated on STM (A,B) and on LTM (C,D) (n = 8–7 per group/time probe test). All individual values are shown. The values are presented as the means (±SEMs). The horizontal lines represent the chance value for the DI (0.0) and for RI (0.5). Blue significant P-values represent the ratio discriminations from the group above chance level, according to one-sample t-tests. Intragroup comparisons (testing vs. training sessions) used a paired t-test.
Data from the DI (Fig. 1A,C) and RI (Fig. 1B,D) for object location memory retrieval revealed that young adult rats subjected to stronger training spent more time interacting with a novel location object during STM probe testing (DI = 0.1875, P = 0.0437) in relation to the sample session (DI = −0.0769). Further analysis using one-sample t-tests showed that the DI and RI were greater than chance (DI = 0) during the STM testing session for the strong training group (P = 0.0004 and P = 0.0062, respectively) (Fig. 1A,B). Moreover, rats subjected to weaker training spent more time interacting with a novel location object during STM, since the DI (0.2571) and RI (0.6429) were >0.0 and >0.5, respectively, but no significant differences were seen between these values and chance level.
During LTM retrieval testing, the ST group showed a DI >0.0 (DI = 0.200) and a RI >0.5 (RI = 0.5625), indicating that the ST groups showed a preference for the reallocated object. Corroborating these data, the DI was greater compared with the chance exploration level of 0.0 (P = 0.0185) during the LTM testing session. Conversely, the WT group spent a similar amount of time exploring the object in the familiar and novel object locations (DI = 0.0140). Analysis of the recognition index for the WT group (RI = 0.3429) corroborated the data from the DI, since weak training did not result in STM or LTM (Fig. 1C,D; for details, see Table 1).
Table 1.
Discrimination index (DI) and recognition index (RI) from the young adult rats and aged group rats during the sample and test phase
Effect of novelty on young adult animals
To analyze the effect of novel context exploration after training for short-term and long-term object location memory (OLM) retention, we first analyzed the amount of time spent in the spontaneous exploration of moved object versus the familiar location object by using the discrimination index and the recognition index for the WT and WT + novelty groups during testing sessions 1 h (Fig. 2A,B) or 24 h (Fig. 2C,D) after training and compared with the WT group in the sample session.
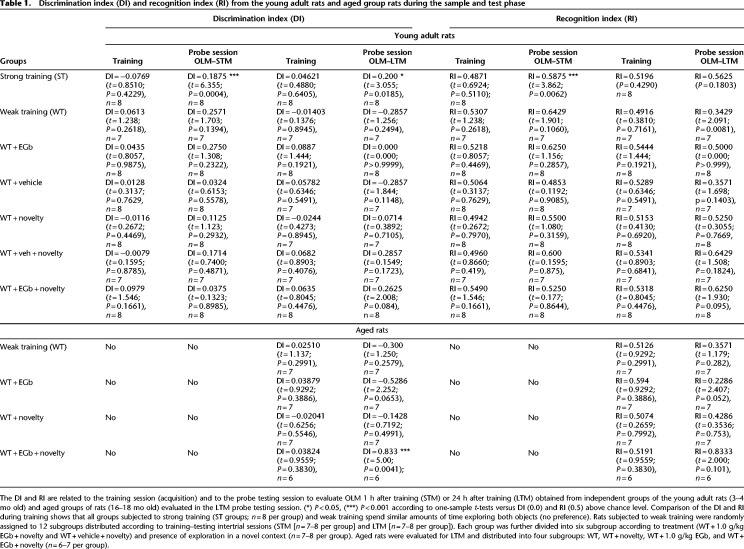
Figure 2.
Schematic time line of the procedure for the acquisition and retrieval of object location memory (OLM). The data show the effect of contextual novelty after weak encoding on short-term memory (STM) and long-term memory (LTM) sessions. In the training session, young adult rats were allowed to freely explore the sample objects (A and B) for 5 min (weak training [WT]), and the mean discrimination index (DI) and recognition index (RI) of the sample objects were evaluated in independent cohort of rats subjected to STM or LTM. The DI (A,C) and RI (B,D) of the moved and sample objects were evaluated for the 5 min in the object location memory testing (OLM) on STM (A,B) and on LTM (C,D) in rats of the WT groups or in rats subjected to contextual novelty 30 min after weak training (WT + novelty; n = 8 per group/time probe test). All individual values are shown. The values are presented as the means (±SEMs). Lines represent the chance value for the DI (0.0) and RI (0.5). Comparison of ratio discriminations from the group above chance level used one-sample t-tests. Intragroup comparisons (testing vs. training sessions) used a paired t-test.
Data from the DIs for OLM of both the WT and WT + novelty groups showed DI values >0.0, indicating a preference for the moved object, but no differences were found compared with WT (DI = 0.2571) and WT + novelty (DI = 0.1125) groups in relation to chance levels for the DI (0.0) during STM (for details, see Table 1). Moreover, paired t-test was conducted to compare the object discrimination index for the WT group during the sample session in relation to the WT or WT + novelty groups, for which retrieval was assessed 1 h after training, and revealed no significant difference between groups. The RI values were >0.5 for the WT (RI = 0.6429) and WT + novelty (RI = 0.5500) groups, suggesting that the familiar location object was remembered in STM, but the recognition indices of both groups were similar to chance level (0.5) (Fig. 2B).
The exposition of novel context failed in LTM generation, since no differences were found compared with the mean of discrimination indices for the WT + novelty (DI = −0.0714) and weak training (DI = −0.2875) groups in relation to the sample session (DI = 0.0140 and DI = 0.0244, respectively) and both groups in relation to chance level for the DI (0.0) (Fig. 2C). Nevertheless, during LTM recall, while the RI was <0.5 for the WT group (RI = 0.3428), the WT + novelty group showed RI = 0.5225, suggesting that novelty after weak training might contribute to the recognition of familiar location objects. However, no difference was seen between groups and in relation to chance level (for details, see Table 1).
In summary, our data revealed that contextual novelty did not facilitate short-term retention of weak encoding (30 min later) in young adult rats. On the other hand, they suggest its role in the modulation of LTM formation, since the DI and RI were higher than the chance value, although we did not see a significant difference between groups. Thus, we investigated whether weak training and subsequent novelty would affect long-term object location memory in aged rats.
Long-term memory in aged animals
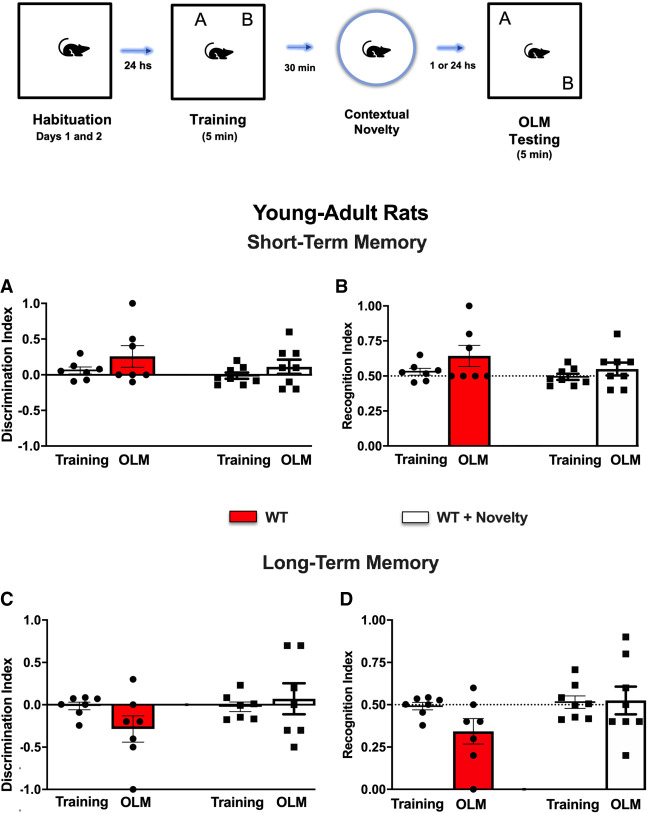
Comparative analysis of time exploring both objects using a paired t-test revealed no significant difference for the WT and WT + novelty groups as compared with the DI during the sample session. Data from the DIs for the WT (DI = −0.300) and WT + novelty (DI = −0.1428) groups on OLM–LTM retrieval showed values <0.0, indicating that the familiar location object was not remembered (Fig. 3A). Similarly, the RI values for the WT (RI = 0.3571) and WT + novelty (RI = 0.4285) groups corroborated the DI data and revealed no recognition of LTM formation for either group (Fig. 3B; for details, see Table 1).
Figure 3.
Schematic time line of the procedure for the acquisition and retrieval of object location memory (OLM). The data show the effect of contextual novelty after weak encoding on long-term memory (LTM) sessions. In the training session, aged rats were allowed to freely explore the sample objects (A and B) for 5 min (weak training [WT]), and the mean discrimination index (DI) and recognition index (RI) of the sample objects were evaluated in rats of the WT groups or in rats subjected to contextual novelty 30 min after weak training (WT + novelty; n = 7 per group). The DI (A) and RI (B) of the moved and sample objects were evaluated for 5 min in the object location memory testing (OLM) 24 h after training (LTM)) in rats of the WT or WT + novelty (n = 7 per group) groups. All individual values are shown. The values are presented as the means (±SEMs). Lines represent the chance value for the DI (0.0) and RI (0.5). Intragroup comparisons (testing vs. training sessions) used a paired t-test. Comparisons between the ratio discriminations from the group above chance level used one-sample t-tests. There was no difference between any experimental group (for details, see Table 1).
Experiment Il: effect of EGb treatment on OLM
Considering previous data from our laboratory that showed enhanced effects of EGb on memory formation, we next investigated whether EGb treatment before novel context experiences might impact weak training and improve long-term object location memory in young adult rats and aged rats.
Effect of EGb treatment on young adult animals
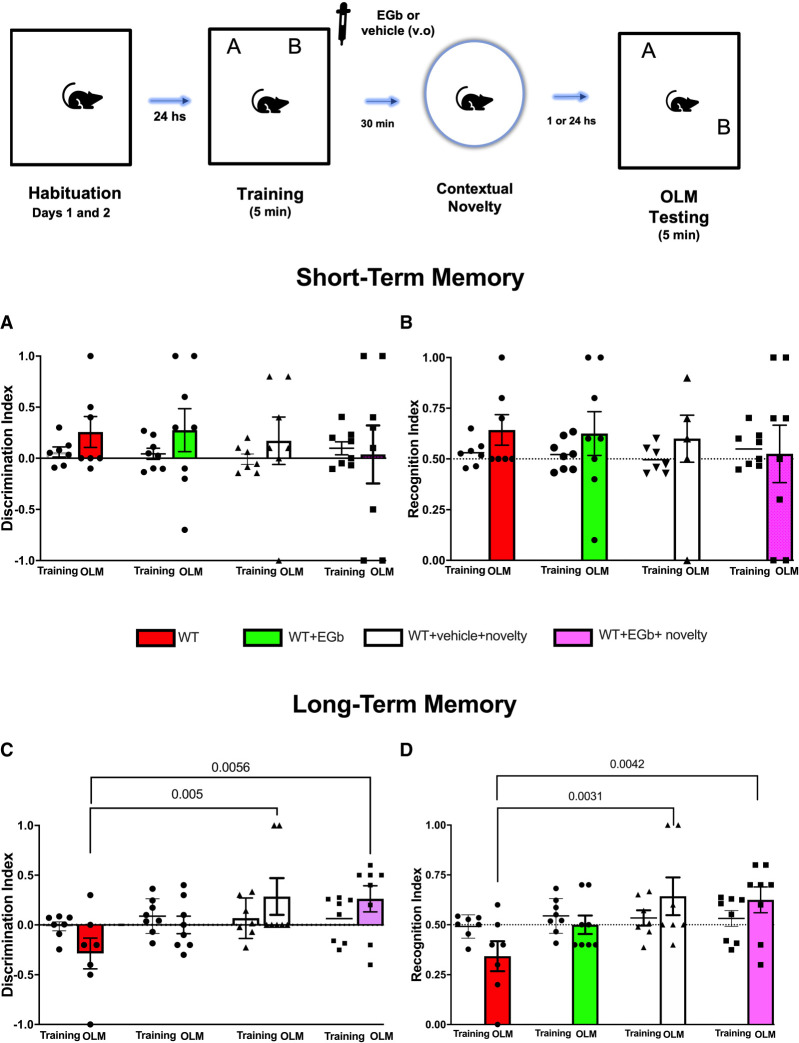
The effects of the EGb treatments on STM and LTM, associated or not with novelty, are depicted in Figure 4. Comparisons between the DI results of the novel location object test by two-way ANOVA revealed no interaction between factors (EGb treatment vs. novelty; F(3,26) = 0.3316, P = 0.8025) during the STM testing session, but rats subjected to EGb treatment or novelty following weak training showed the DI was >0.25, suggesting that the familiar location object was remembered (Fig. 4A). Similarly, no interactions between factors were seen when evaluating the RI during the STM testing session (F(3,26) = 0.3817, P = 0.7670) (Fig. 4B). Here, the WT (RI = 0.6429), WT + EGb (RI = 0.6250), WT + vehicle + novelty (RI = 0.600), and WT + EGb + novelty (RI = 0.5250) groups were able to recognize moved objects. Further analysis using one-sample t-tests revealed that the DI and RI were found to be not significantly higher than 0.0 and 0.5, respectively, during the STM testing session (for details, see Table 1). Moreover, comparisons of the DI and RI during training for WT group versus the STM testing session were examined by paired t-test and revealed no significant difference in percentage of the time spent exploring both objects after the object moved for all groups (for details, see Table 1).
Figure 4.
Schematic time line of the procedure for the acquisition and retrieval of object location memory (OLM). The data show the effect of treatment with standardized extract of Ginkgo biloba (EGb) or vehicle immediately after the weak encoding session associated or not with contextual novelty (30 min after training) in short-term memory (STM) and long-term memory (LTM) sessions. In the training session, young adult rats were allowed to freely explore the sample objects (A and B) for 5 min (weak training [WT]), and the mean discrimination index (DI) and recognition index (RI) of the sample objects were evaluated in independent cohorts of rats subjected to STM or LTM (n = 7-–8 per group/time probe test). The DI (A,C) and RI (B,D) of the moved and sample objects were evaluated for 5 min in the object location memory testing (OLM) 1 h after training (STM) (A,B) or 24 h after training (LTM) (C,D) in rats of the WT, WT + EGb, WT + vehicle + novelty, and WT + EGb + novelty groups (n = 7–8 per group/time probe test). All individual values are shown. The values are presented as the means (±SEMs). Lines represent the chance value for the DI (0.0) and RI (0.5). Comparisons between the ratio discriminations from the group above chance level used one-sample t-tests. Intragroup comparisons (testing vs. training sessions) used a paired t-test and revealed the effect of novelty and/or EGb on LTM.
Analysis of data from the recall LTM session (Fig. 4C) by two-way ANOVA revealed no interactions between factors (EGb treatment vs. novelty; F(3,26) = 1.990, P = 0.1402); however, our finding revealed that novelty associated with EGb modulates LTM formation after weak training (F(3,26) = 4.101, P = 0.0165). Rats subjected to weak training before treatment with EGb + novelty have a DI >0.0 (DI = 0.2625). Further analysis using paired t-tests revealed that the DI during the testing session for WT + vehicle + novelty (DI = 0.2875, P = 0.0291) and WT + EGb + novelty groups was significantly different from the DI during sample for WT (DI = −0.2857, P = 0.0219).
Comparison between the RIs by two-way ANOVA revealed no interaction between factors (EGb treatment vs. novelty; F(3,26) = 2.051, P = 0.1314) during the LTM testing session but did show the effects of treatment (F(3,26) = 4.486, P = 0.0115). Analysis of the RIs for WT + vehicle + novelty (RI = 0.642) and WT + EGb + novelty (RI = 0.625) groups revealed that they were able to recognize the moved object in LTM testing sessions, but no LTM of the location object was seen in the WT + EGb-treated (RI = 0.500) or WT (RI = 0.3428) groups (Fig. 4D). One subject was excluded from the analysis according to the statistical outlier criterion (Prisma 9.0 program) (for details see Table 1).
Effect of EGb treatment on aged animals
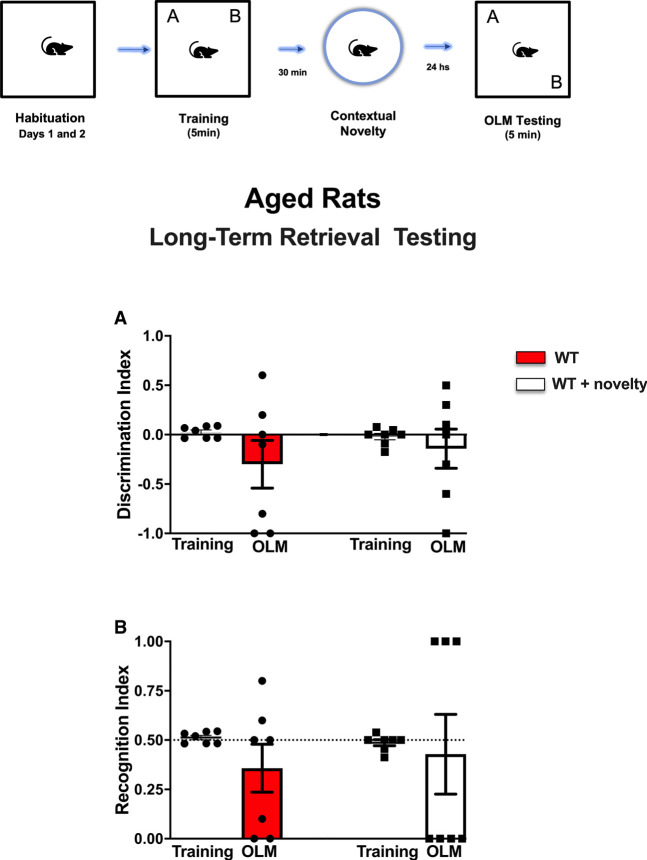
Data from the DI and RI during the training and the recall of LTM sessions for aged rats are shown in Figure 5, A and B, respectively. Comparative analysis of the time spent exploring both objects using two-way ANOVA revealed no interaction between factors (F(1,46) = 0.2519, P = 0.6181) but did show the effects of treatment (F(3,46) = 7,131, P = 0.0005). Rats subjected to EGb treatment and novelty following weak training showed a higher DI (DI = 0.833) in relation to the WT (DI = −0.300), WT + EGb (DI = −0.528), and WT + novelty (DI = −0.1428) groups during the LTM testing session. Moreover, within-group comparisons revealed a significant difference in relation to the training session, indicating that the EGb treatment associated with novelty resulted in an improvement of memory, and the aged rats remembered the familiar location object.
Figure 5.
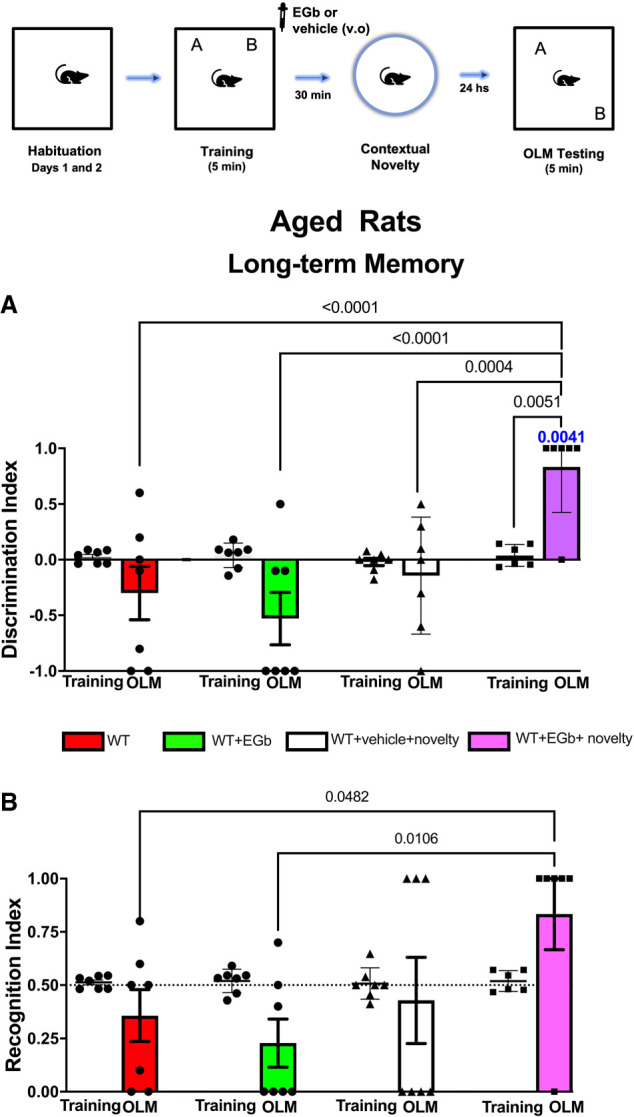
Schematic time line of the procedure for the acquisition and retrieval of object location memory (OLM). The data show the effect of treatment with standardized extract of Ginkgo biloba (EGb) immediately after a weak encoding session associated or not with contextual novelty (30 min after training) on long-term memory (LTM) sessions. In the training session, aged rats were allowed to freely explore the sample objects (A and B) for 5 min (weak training [WT]), and the mean discrimination index (DI) and recognition index (RI) of the sample objects were evaluated in an independent cohort of rats subjected to STM or LTM (n = 7–8 per group/time probe test). The DI (A) and RI (B) of the moved and sample objects were evaluated for 5 min in the object location memory testing (OLM) 24 h after training (LTM) for WT, WT + EGb, WT + vehicle + novelty, and WT + EGb + novelty (n = 7–8 per group/time probe test). All individual values are shown. The values are presented as the means (±SEMs). Lines represent the chance value for the DI (0.0) and RI (0.5). Comparisons between the ratio discriminations from the group above chance level used one-sample t-tests (blue value). Intragroup comparisons (testing vs. training sessions) used a paired t-test and revealed the effects of EGb associated with novelty in LTM.
Corroborating the previous findings, two-way analysis showed an interaction between factors (novelty vs. treatment; F(1,23) = 7.106, P = 0.0138) (Fig. 5B). Rats subjected to weak training and treatment with EGb and novelty showed a higher RI (RI = 0.833). Conversely, rats of the WT (RI = 0.428), WT + EGb (RI = 0.228), and WT + novelty (RI = 0.428) groups showed a lower RI in LTM testing sessions.
Additional data from the frequency of contact with the familiarly located object versus the moved location object are shown in Supplemental Figure 1 for young adult rats during training (Supplemental Fig. S1A,C) and on STM (Supplemental Fig. S1B) and LTM (Supplemental Fig. S1D), as well as for aged rats during training (Supplemental Fig. S1E) and on LTM testing sessions (Supplemental Fig. S1F). Statistical analysis revealed that all animals had similar frequency of contact with objects (paired t-test, P > 0.05) (see details in the Supplemental Material), except the ST group, for which retrieval of OLM was assessed 1 h after training (t = 2.959, P = 0.0211). However, data from the DI and RI for the ST groups revealed that time spent at each object (A and B) was similar (Fig. 1).
Discussion
In summary, our present results substantiate the following conclusions: (1) Weak training of object location (explored for 5 min) resulted in STM formation that lasted for 1 h but did not induce LTM formation. (2) Novelty (novel context exploration for 5 min) was effective in generating LTM in young adult rats subjected to weak training but not in aged rats, which showed impaired long-term spatial memory. (3) Treatment with EGb associated with novelty close to the time of the encoding resulted in LTM for both young adult and aged rats. In aged rats, the cooperative mechanisms induced robust long-term spatial memory.
Altogether, our data suggest that EGb treatment immediately after weak training and associated in a short time interval with a novel context might have improved the molecular mechanisms correlated with the consolidation process, leading to the persistence of memory. Furthermore, EGb might modulate molecular changes involved in the early phase of long-term potentiation (LTP), induced by weak stimulation, which can persist as a result of the converging pathways within a critical time window around the weak stimulation (Nomoto et al. 2016; Vishnoi et al. 2018; Gros et al. 2022).
LTM formation of object place preference is a highly dynamic process that involves cellular and molecular changes required for consolidation processes, which occur in the hippocampal formation (Morris 2001; Armentia et al. 2007; Tonegawa et al. 2015). Supporting data in animal studies highlight that de novo expression of cAMP response element binding protein (CREB) is a long-term memory formation marker in hippocampus-dependent consolidation and plasticity-related proteins involved in synaptic changes necessary for the maintenance of memory (Yin et al. 1995; Guzowiski and Mcgaugh 1997; Silva et al. 1998; Rosenzweig and Barnes 2003; Zhou et al. 2009; Josselyn et al. 2015; Lisman et al. 2018).
Supporting the effect of EGb on the persistence of memory, we have previously shown that acute treatment with 1 g/kg EGb resulted in long-term persistence of fear memory through increased levels of cAMP response element binding protein (CREB), serotonin type 1A receptor, and both the GluN2B and GluN2A subunits of the N-methyl-D-aspartate (NMDA)-type glutamate receptor mRNA and the protein in the dorsal hippocampal formation (dHF) that are involved in the consolidation process of lick suppression (Oliveira et al. 2009, 2013; Zamberlam et al. 2019) and differential protein expression in the dHF involved in established morphological changes in neurons, which are required for the persistence of memory (Gaiardo et al. 2019). Similarly, EGb modulated BDNF expression in the dHF of rats subjected to object recognition memory (Muratori et al. 2021). The role of the dHF on behavioral tagging and capture was described by other groups (Armentia et al. 2007; Nomoto et al. 2016; Gros et al. 2022).
Additionally, age-related structural and physiological changes in the neural system involved in spatial recognition memory, such as hippocampal formation (Akkerman et al. 2012; Maasberg et al. 2012), can be a main target of EGb through modulating molecular mechanisms related to memory loss. We have demonstrated that 1.0 g/kg EGb improved short-term spatial memory and preventive effects in the prefrontal cortex in middle-aged rats (Ribeiro et al. 2016). Furthermore, data suggest that the anatomical correlation of CA1 with other subfields of the hippocampus or extrahippocampal structures might substantiate the encoding of some aspects of memory function during temporary inactivation.
Regarding age-related changes in the detection of object location and novelty, our findings are in accordance with prior studies from Maasberg et al. (2012). Aged rats might require longer exposition to novelty for the behavioral tagging and persistence of memory to take place as observed for young adult rats, since no significant age-related factor was observed at the time of contact with objects (familiar and moved) between groups. Furthermore, our data demonstrated that STM lasted for 1 h, distinct from recent works that evaluated STM as lasting for 30 min (Nomoto et al. 2016).
Conclusion
Our data revealed for the first time the effects of the cooperative mechanism of behavioral tagging and EGb treatment in young and aged animals, which might provide remarkable insights into the functional recovery; notably, that it is involved in long-term spatial memory formation that is lost in normal aging and Alzheimer's disease. In this sense, the present findings add to the growing literature demonstrating age-related memory impairments and provide further evidence of the role of EGb as a cognitive enhancer.
Materials and Methods
Animals
One-hundred-four experimental young adult (age range 3–4 mo) and 28 aged (age range 16–18 mo) male Wistar rats were obtained from the Center for the Development of Experimental Medicine and Biology (University of São Paulo [USP]). The animals were group-housed (three or four per cage) with ad libitum access to food and water during all experimental procedures. The animals were kept at a controlled temperature (21°C ± 3°C) and relative humidity (55 ± 10) on a 12-h light/dark cycle, with light onset at 6 a.m., and treatments and behavioral analysis were performed during the light cycle (between 7:00 a.m. and 1:00 p.m.). All procedures were carried out in accordance with the Ethics Committee on the Use of Animals (CEUA) of the Federal University of São Paulo (project number license CEUA UNIFESP 4035281119) and were conducted following the Brazilian Federal law (11794/2008) on the care and procedure of animal experimentations.
Adult young rats were randomly assigned into 14 subgroups (n = 7–8 per group) as follows: groups 1 and 2: strength of training (strong [ST] or weak [WT]), groups 3 and 4: treatment (WT + 1.0 g/kg EGb or WT + vehicle [0.9% saline]), group 5: the presence of exploration in a novel context (WT + novelty), and groups 6 and 7: EGb treatment plus novelty (WT + 1.0 g/kg EGb + novelty and WT + vehicle + novelty), which were subjected to short-term probe testing (1 h) to evaluate object location memory (OLM) in rats. Another seven independent groups of rats were evaluated on long-term (24 h, n = 52) training–testing intertrial sessions. Aged rats were distributed into four subgroups: WT (n = 7), WT + novelty (n = 7), WT + 1.0 g/kg EGb (n = 7), and WT + EGb + novelty (n = 6) (see Table 1).
Drug administration
As previously described, the standardized extract obtained from the green leaves of Ginkgo biloba (EGb) containing a flavonoid-rich fraction (∼24% ginkgo flavoglycosides) was administered at a dose of 1.0 g/kg, which was chosen according to previous studies conducted in our laboratory that showed improvement in fear memory (Oliveira et al. 2013; Ribeiro et al. 2016; Zamberlam et al. 2016; Gaiardo et al. 2019) and nonaversive memory (Muratori et al. 2021). The extract was resuspended in 0.9% saline (vehicle) and administered orally via a gastric tube (IG) immediately after the acquisition phase (training session).
Object location memory task
Apparatus and objects
The object location task (OLT) was conducted using a black wooden square arena (70 cm long × 70 cm wide × 70 cm high). For OLT, two different objects with a similar degree of complexity were used (Lueptow 2017) that were previously validated in our laboratory to ensure there was no innate preference to any object or discrimination. Furthermore, the two objects were similar in their pattern (e.g., by size and no odor cues) (Muratori et al. 2021). The objects were placed in the same corner of the open field for all of the animals and according to the phase of the OL (training or test). We counterbalanced the use of each object serving as A and B (e.g., half of the rats had a bottle as object A and a statuette as object B, and the remaining rats received the inverse arrangement). The pattern was reversed for the remaining rats to minimize any potential induced object preference. Exploration of an object was considered directing the nose at a distance of ∼2 cm to the object and/or sniffing or touching it with the nose (Ennaceur and Delacour 1988; Ennaceur et al. 2005). As a relative novelty, a circular arena of transparent acrylic walls was used. Rats are very sensitive to their environment, since they have an innate preference for novelty and exploration (Rangel-Gomez et al. 2015). The novelty consisted of a circular arena (90 cm in diameter and 50 cm high) that was presented 30 min after the training session or training + treatment (EGb or vehicle).
Prior to behavioral testing, rats were transferred to a room adjacent to the behavioral space that was kept on low-intensity light and were single-housed for 5 min before the experimental sessions. The experimental sessions occurred in a testing room in which a 60-W incandescent bulb was angled at the ceiling over the experimental arena. On each day of the experiment, the arena and objects were cleaned with 70% ethanol, and fresh bedding was placed to limit olfactory cues. The experimental sessions were recorded using a digital camera (Samsung ES68) fixed above the apparatus. Due to the number of animals and time requirements, the experiment was developed in six sets (three aged/three young adults). In each set, all experimental sessions were conducted using one rat from each group (control or treated) undergoing the same protocol. After each rat was tested, the apparatus and objects were carefully cleaned with 20% ethanol solution to remove odor cues, and the animals were returned to their home cages. After each session, the animals were returned to a clean home cage but in the same conditions as before to avoid stress and discomfort. After the animals were placed in the square (training and testing sessions) or circular arena (contextual novelty), the experimenter left the room to avoid interfering with the animal's behavior.
Prior to the beginning of the experimental sessions, all animals were handled for a period of 10 min/d over 2 d before the experiment to avoid the effects of experimenter handling (Shimoda et al. 2021). Before each experimental session, the rats were individually transferred to a room adjacent to the behavioral space. The room was kept on low-intensity light, and rats were single-housed for 10 min before the experimental sessions.
Experimental procedure
The OLT procedure used in this study included two habituation sessions: a familiarization session (training session) that was followed or not for contextual novelty and short-term or long-term retention testing session, as described below.
Habituation
On the first and second days of habituation, each rat was allowed to freely explore the empty arena (square) for 10 min, and there were no objects in the arena. The rats were placed against the center of the opposite wall. It was important to allow the habituation to minimize the interest of the animal in the novel environment and avoid interference in the acquisition and retention phase.
Acquisition of OLM
On day 3, during the training session (i.e., sample/encoding session), the rats were allowed to freely explore the objects (objects A and B) for 15 min (strong training [ST[) or for 5 min (weak training [WT]). The rationale for choosing the familiarization procedure used in this study was based on previous data from the literature, such as the study by Shimoda and colleagues (Ozawa et al. 2011; Shimoda et al. 2021), which showed differences between the length of familiarization in the object recognition training session and long-term recognition memory (24 h). Complementary data from the study by Ozawa and colleagues (Ozawa et al. 2011; Akkerman et al. 2012; Nomoto et al. 2016; Shimoda et al. 2021) suggest that longer exploration of the objects during the familiarization session may allow the animals to make associations between each element of information, such as objects. Data from our laboratory corroborate these studies (Muratori et al. 2021). In addition, we considered that we also used aged rats, which might show a larger range of individual differences in the acquisition and retention of OLM (Robitsek et al. 2008; Akkerman et al. 2012). Objects used had similar patterns (e.g., similar size, texture, and color and no odor cues) to avoid bias resulting from the individual preference for specific objects (Bevins and Besheer 2006; Antunes and Biala 2012; Muratori et al. 2021).
Novelty
The novelty exposure procedure involved placing a rat in a novel context (that is, a novel arena), and the rats were left to explore freely for 5 min. To examine the effects of novelty on short-term and long-term OLM retention, the animals were exposed to a novelty session 30 min after weak training or weak training + treatment (EGb or vehicle) and then returned to a holding cage.
Memory retention test
Memory retention was evaluated 1 h (short-term memory [STM]) or 24 h (long-term memory [LTM]) after the training session. The OLM requires the identification and discrimination of object location, which were evaluated by the differences in the exploration time of objects that were in novel or familiar locations. During the probe session, the animals were returned to the same arena of training with the same objects (object A and object B); however, one of the objects was moved to a novel object location. To counterbalance the preference for objects across groups, half of the animals in each group received one of each object with the location of the novel object. For all groups, we considered the reallocated object as object B.
Object location analysis
For the analysis of the OLM, we used the mean discrimination index (DI = time spent exploring object B − time spent in object A/time spent in object B + time spent in object A). The DI values range from −1 to 1. A DI = 0 indicates no preference, exploring both objects for the same time. Values >0 indicate that the rat preferred to explore the reallocated object (object B), and values <0 (i.e., negative values) indicate a preference for the familiar object (object A). In addition, we evaluated the recognition index (RI = time spent exploring object B/total object exploration time [novel location vs. familiar]). Values equal to 0.5 indicate no recognition that the object is in a novel location. Values >0.5 indicate that the rat preferred to explore the reallocated object. These analyses allow us to examine differences between groups regarding the effects of novelty and treatment and each individual group's ability to discriminate between the novel and familiar objects.
Animals that did not explore for >15 sec for each object during stronger training and 5 sec for each object during weaker training were excluded from our data analysis (Eacott and Norman 2004; Vogel-Ciernia and Wood 2014; Cinalli et al. 2020).
Statistical analysis
The frequency of contact and time spent exploring the objects were manually scored at all phases (sampling and test phases) (see Supplemental Fig. 1). t-tests were used for comparisons of the mean discrimination index (DI) and mean recognition index (RI) between stronger and weaker training groups as well as for the comparisons between data from training and probe testing sessions (STM or LTM). To evaluate whether the mean DI or mean RI of each group was greater than chance (value 0.0 and 0.5, respectively) under each condition, we performed the one-sample t-test (see Table 1). Two analyses of variance (ANOVA) with a post hoc Tukey's HSD test were used to evaluate the effects of treatment or novelty and the interaction between these factors on the DI and RI on short-term and long-term memory, since these measures are more robust against different levels of exploration between groups, considering the age and treatment of animals (Cinalli et al. 2020). Data are reported as the mean ± SEM. The null hypothesis was rejected when the two-tailed probability value was <5% (P ≤ 0.05). Data analyses were performed using GraphPad Prism 9.0.
All procedures were approved by the Local Committee Governing the Ethics on the Use of Animal Experimentation of the Federal University of São Paulo (CEUA UNIFESP 4035281119) and were conducted in accordance with the Brazilian law for the use of animals in scientific research (N°11.794) as suggested by the APA Guidelines for Ethical Conduct in the Care.
Competing interest statement
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This study was supported by the São Paulo State Research Foundation (FAPESP; grant 2019/24614-4 to S.M.C.). C.V.d.A. is a scholar from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Authors contributions: C.V.d.A. conceived the study and performed the in vivo experiments. S.M.C. supervised the experiments and analyzed the results, reviewed the manuscript, and was responsible for funding acquisition. All authors have read and approved the manuscript.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053755.123.
References
- Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11: 180–187. 10.1016/S0959-4388(00)00194-X [DOI] [PubMed] [Google Scholar]
- Akkerman S, Prickaerts J, Steinbusch HWM, Blokland A. 2012. Object recognition testing: statistical considerations. Behav Brain Res 232: 317–322. 10.1016/j.bbr.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Alberini CM, Johnson SA, Ye X. 2013. Memory reconsolidation: lingering consolidation and the dynamic memory trace. In Memory reconsolidation (ed. Alberini CM), pp. 81–117. Academic Press, London, UK. 10.1016/B978-0-12-386892-3.00005-6 [DOI] [Google Scholar]
- Alberini CM, Kandel ER. 2015. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol 7: a021741. 10.1101/cshperspect.a021741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ledoux JE. 2013. Memory reconsolidation. Curr Biol 23: R746–R750. 10.1016/j.cub.2013.06.046 [DOI] [PubMed] [Google Scholar]
- Alberini CM, Milekic MH, Tronel S. 2006. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci 63: 999–1008. 10.1007/s00018-006-6025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110. 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentia ML, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. 2007. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci 27: 13909–13918. 10.1523/JNEUROSCI.3850-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. 2009. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci 106: 14599–14604. 10.1073/pnas.0907078106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. 2006. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1: 1306–1311. 10.1038/nprot.2006.205 [DOI] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. 2010. Neural mechanisms of ageing and cognitive decline. Nature 464: 529–535. 10.1038/nature08983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. 1998. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 5: 365–374. 10.1101/lm.5.4.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, de Souza Silva MA, Yang YM, Huston JP. 2020. The medial prefrontal cortex: hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: behavioral, anatomical and neurochemical properties. Neurosci Biobehav Rev 113: 373–407. 10.1016/J.NEUBIOREV.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, Nikolaus S, Yang YM, Huston JP. 2022. Neuronal circuitry for recognition memory of object and place in rodent models. Neurosci Biobehav Rev 141: 104855. 10.1016/J.NEUBIOREV.2022.104855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinalli DA, Cohen SJ, Guthrie K, Stackman RW. 2020. Object recognition memory: distinct yet complementary roles of the mouse CA1 and perirhinal cortex. Front Mol Neurosci 13: 527543. 10.3389/fnmol.2020.527543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, Lassalle J. 2005. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem 12: 375–382. 10.1101/lm.81905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Zhong Y. 2017. The biology of forgetting—a perspective. Neuron 95: 490–503. 10.1016/j.neuron.2017.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KE, Eacott MJ, Easton A, Gigg J. 2013. Episodic-like memory is sensitive to both Alzheimer's-like pathological accumulation and normal ageing processes in mice. Behav Brain Res 254: 73–82. 10.1016/j.bbr.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. 2005. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res Protoc 16: 10–19. 10.1016/j.brainresprot.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31: 673–704. 10.1016/j.neubiorev.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Dudai Y. 2002. Molecular bases of long-term memories: a question of persistence. Curr Opin Neurobiol 12: 211–216. 10.1016/S0959-4388(02)00305-7 [DOI] [PubMed] [Google Scholar]
- Dudai Y, Roediger HL, Tulving E. 2007. Memory concepts. In Science of memory: concepts (ed. Roediger HL III et al. ), pp. 1–10. Oxford University Press, New York. 10.1093/acprof:oso/9780195310443.003.0001 [DOI] [Google Scholar]
- Eacott MJ, Norman G. 2004. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci 24: 1948–1953. 10.1523/JNEUROSCI.2975-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabagh S, Hartley DE, Ali O, Williamson EM, File SE. 2005. Differential cognitive effects of Ginkgo biloba after acute and chronic treatment in healthy young volunteers. Psychopharmacology 179: 437–446. 10.1007/s00213-005-2206-6 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. 1988. A new one: trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31: 47–59. 10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. 2005. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res 159: 247–266. 10.1016/j.bbr.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Gaiardo RB, Abreu TF, Tashima AK, Telles MM, Cerutti SM. 2019. Target proteins in the dorsal hippocampal formation sustain the memory-enhancing and neuroprotective effects of Ginkgo biloba. Front Pharmacol 9: 1533. 10.3389/fphar.2018.01533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Lim AWH, Hohendorf V, White N, Eckert M, McHugh TJ, Wang SH. 2022. Behavioral and cellular tagging in young and in early cognitive aging. Front Aging Neurosci 22: 809879. 10.3389/fnagi.2022.809879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowiski JF, Mcgaugh JL. 1997. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci 94: 2693–2698. 10.1073/pnas.94.6.2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamezah HS, Durani LW, Yanagisawa D, Ibrahim NF, Aizat WM, Bellier JP, Makpol S, Ngah WZW, Damanhuri HA, Tooyama I. 2018. Proteome profiling in the hippocampus, medial prefrontal cortex, and striatum of aging rat. Exp Gerontol 111: 53–64. 10.1016/j.exger.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. 2005. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord 4: 481–497. 10.2174/156800705774322058 [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW. 2015. Finding the engram. Nat Rev Neurosci 16: 521–534. 10.1038/nrn4000 [DOI] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. 2014. The molecular and systems biology of memory. Cell 157: 163–186. 10.1016/j.cell.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. 2006. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem 85: 173–182. 10.1016/j.nlm.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Haskell CF, Mauri PL, Scholey AB. 2007. Acute cognitive effects of standardised Ginkgo biloba extract complexed with phosphatidylserine. Hum Psychopharmacol 22: 199–210. 10.1002/hup.837 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. 2008. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci 122: 1217–1225. 10.1037/a0013592 [DOI] [PubMed] [Google Scholar]
- Lisman J, Cooper K, Sehgal M, Silva AJ. 2018. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci 21: 309–314. 10.1038/s41593-018-0076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow LM. 2017. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 126: 55718. 10.3791/55718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maasberg DW, Shelley LE, Gilbert PE. 2012. Age-related changes in detection of spatial novelty. Behav Brain Res 228: 447–451. 10.1016/j.bbr.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Viola H. 2007. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci 27: 7476–7481. 10.1523/JNEUROSCI.1083-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Ballarini F, Viola H. 2015. Behavioral tagging: a translation of the synaptic tagging and capture hypothesis. Neural Plast 2015: 650780. 10.1155/2015/650780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. 2001. Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci 356: 1453–1465. 10.1098/rstb.2001.0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori BG, Zamberlam CR, Mendes TB, Nozima BHN, Cerutti JM, Cerutti SM. 2021. Bdnf as a putative target for standardized extract of Ginkgo biloba-induced persistence of object recognition memory. Molecules 26: 3326. 10.3390/molecules26113326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto M, Ohkawa N, Nishizono H, Yokose J, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, et al. 2016. Cellular tagging as a neural network mechanism for behavioural tagging. Nat Commun 7: 12319. 10.1038/ncomms12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DR, Sanada PF, Saragossa FAC, Innocenti LR, Oler G, Cerutti JM, Cerutti SM. 2009. Neuromodulatory property of standardized extract Ginkgo biloba L. (EGb 761) on memory: behavioral and molecular evidence. Brain Res 1269: 68–89. 10.1016/j.brainres.2008.11.105 [DOI] [PubMed] [Google Scholar]
- Oliveira DR, Sanada PF, Filho ACS, Conceição GMS, Cerutti JM, Cerutti SM. 2013. Long-term treatment with standardized extract of Ginkgo biloba L. enhances the conditioned suppression of licking in rats by the modulation of neuronal and glial cell function in the dorsal hippocampus and central amygdala. Neuroscience 235: 70–86. 10.1016/j.neuroscience.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Ozawa T, Yamada K, Ichitani Y. 2011. Long-term object location memory in rats: effects of sample phase and delay length in spontaneous place recognition test. Neurosci Lett 497: 37–41. 10.1016/j.neulet.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Rangel-Gomez M, Janenaite S, Meeter M. 2015. Novelty's effect on memory encoding. Acta Psychol 159: 14–21. 10.1016/j.actpsy.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Ribeiro ML, Moreira LM, Arçari DP, dos Santos LF, Marques AC, Pedrazzoli J, Cerutti SM. 2016. Protective effects of chronic treatment with a standardized extract of Ginkgo biloba L. in the prefrontal cortex and dorsal hippocampus of middle-aged rats. Behav Brain Res 313: 144–150. 10.1016/j.bbr.2016.06.029 [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Ming TK, Gallagher M, Eichenbaum H. 2008. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci 28: 8945–8954. 10.1523/JNEUROSCI.1893-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. 2003. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69: 143–179. 10.1016/S0301-0082(02)00126-0 [DOI] [PubMed] [Google Scholar]
- Sangiovanni E, Brivio P, Dell'Agli M, Calabrese F. 2017. Botanicals as modulators of neuroplasticity: focus on BDNF. Neural Plast 2017: 5965371. 10.1155/2017/5965371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda S, Ozawa T, Ichitani Y, Yamada K. 2021. Long-term associative memory in rats: effects of familiarization period in object–place–context recognition test. PLoS One 16: e0254570. 10.1371/journal.pone.0254570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. 1998. CREB and memory. Annu Rev Neurosci 21: 127–148. 10.1146/annurev.neuro.21.1.127 [DOI] [PubMed] [Google Scholar]
- Stern SA, Alberini CM. 2013. Mechanisms of memory enhancement. Wiley Interdiscip Rev Syst Biol Med 5: 37–53. 10.1002/wsbm.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. 2010. Mechanisms of memory. Elsevier, Oxford, UK. [Google Scholar]
- Tan MS, Yu JT, Tan CC, Wang HF, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. 2015. Efficacy and adverse effects of Ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis 43: 589–603. 10.3233/JAD-140837 [DOI] [PubMed] [Google Scholar]
- Tang F, Nag S, Shiu SYW, Pang SF. 2002. The effects of melatonin and Ginkgo biloba extract on memory loss and choline acetyltransferase activities in the brain of rats infused intracerebroventricularly with β-amyloid 1-40. Life Sci 71: 2625–2631. 10.1016/S0024-3205(02)02105-7 [DOI] [PubMed] [Google Scholar]
- Teratani-Ota Y, Wiltgen BJ. 2022. Encoding object-location memories along the proximodistal axis of CA1. bioRxiv 10.1101/2022.10.17.512601 [DOI] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. 2015. Memory engram storage and retrieval. Curr Opin Neurobiol 35: 101–109. 10.1016/j.conb.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Tulving E. 2002. Episodic memory: from mind to brain. Annu Rev Psychol 53: 1–25. 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. 1997. Memory beyond the hippocampus. Curr Opin Neurobiol 7: 209–216. 10.1016/S0959-4388(97)80009-8 [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. 1998. Episodic and declarative memory: role of the hippocampus. Hippocampus 8: 198–204. [DOI] [PubMed] [Google Scholar]
- Vishnoi S, Raisuddin S, Parvez S. 2016. Behavioral tagging: a novel model for studying long-term memory. Neurosci Biobehav Rev 68: 361–369. 10.1016/j.neubiorev.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Vishnoi S, Naseem M, Raisuddin S, Parvez S. 2018. Behavioral tagging: plausible involvement of PKMζ arc and role of neurotransmitter receptor systems. Neurosci Biobehav Rev 94: 210–218. 10.1016/j.neubiorev.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Wood MA. 2014. Examining object location and object recognition memory in mice. Curr Protoc Neurosci 69: 8.31.1–8.31.17. 10.1002/0471142301.ns0831s69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JCP, Del Vecchio M, Zhou H, Tully T. 1995. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81: 107–115. 10.1016/0092-8674(95)90375-5 [DOI] [PubMed] [Google Scholar]
- Zamberlam CR, Vendrasco NC, Oliveira DR, Gaiardo RB, Cerutti SM. 2016. Effects of standardized Ginkgo biloba extract on the acquisition, retrieval and extinction of conditioned suppression: evidence that short-term memory and long-term memory are differentially modulated. Physiol Behav 165: 55–68. 10.1016/j.physbeh.2016.06.036 [DOI] [PubMed] [Google Scholar]
- Zamberlam CR, Tilger MAS, Moraes L, Cerutti JM, Cerutti SM. 2019. Ginkgo biloba treatments reverse the impairment of conditioned suppression acquisition induced by GluN2B-NMDA and 5-HT1A receptor blockade: modulatory effects of the circuitry of the dorsal hippocampal formation. Physiol Behav 209: 112534. 10.1016/j.physbeh.2019.04.023 [DOI] [PubMed] [Google Scholar]
- Zhao J, Li K, Wang Y, Li D, Wang Q, Xie S, Wang J, Zuo Z. 2021. Enhanced anti-amnestic effect of donepezil by Ginkgo biloba extract (EGb 761) via further improvement in pro-cholinergic and antioxidative activities. J Ethnopharmacol 269: 113711. 10.1016/j.jep.2020.113711 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Balaji J, Neve R, Poirazi P, Silva AJ. 2009. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 12: 1438–1443. 10.1038/nn.2405.CREB [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.