Abstract
The adenylate cyclase (CyaA) of Bordetella pertussis delivers the N-terminal catalytic domain into the cytosol of a large number of eukaryotic cells, in particular, professional antigen-presenting cells. This allows the delivery of CD8+ T-cell epitopes to the major histocompatibility complex class I presentation pathway. We have previously shown that immunization of mice with CyaA carrying a single CD8+ T-cell epitope leads to antiviral protection as well as to protective and therapeutic antitumor immunity associated with the induction of specific cytotoxic T-lymphocyte (CTL) responses. Here, we evaluated the capacity of CyaA carrying one to four copies of the CD8+ CD4+ T-cell epitope from the nucleoprotein of the lymphocytic choriomeningitis virus to induce T-cell responses. Both CTL and Th1-like specific responses were detected in mice immunized with recombinant CyaA with or without adjuvant. Although the insertion of the larger peptides resulted in partial loss of the invasive capacity of recombinant CyaA, insertion of several copies of the same epitope led to a strong enhancement of Th1 responses and, to a lesser degree, CTL responses. These results underscore the potency of CyaA for vaccine design with a new impact on diseases in which the Th1 response has been described to have a beneficial effect.
Cytotoxic T lymphocytes (CTL) are important immune effector cells arising in response to intracellular pathogens such as viruses, parasites, and intracellular bacteria as well as in protective and therapeutic immunity against tumors (11, 13, 16). Indeed, development of efficient and safe CD8+ T-cell vaccines with applications ranging from induction of protective immunity against infectious agents to the development of immunotherapeutic strategies against cancers remains an important challenge.
CD8+ T cells recognize epitopes presented by major histocompatibility complex (MHC) class I molecules at the cell surface of target cells. These epitopes are peptides, seven to nine amino acids long, derived from cytosolic proteins and are generated through proteasome-mediated cleavage. Peptides then associate with MHC class I molecules, and the peptide-MHC class I complexes are transported to the cell membrane, where they can be recognized by CD8+ T cells (17). Indeed, to be presented at the cell surface by an MHC class I molecule, antigenic epitopes must be present in the cytosolic compartment of the presenting cells for processing and association with MHC class I molecules. Various procedures have been developed to allow the cytosolic expression of foreign genes, including the use of live attenuated vectors and DNA vaccination strategies (4). An alternative approach consists in the development of nonreplicative systems to translocate antigenic epitopes across the cell membrane to the interior of the cell, where appropriate processing and MHC class I interaction with the peptide can occur. This approach represents a lower safety risk than live vectors and DNA vaccination because the risk of genetic recombination with other infectious agents or DNA integration into the host genome can be excluded. Indeed, the invasive property of some bacterial toxins has been used for inducing specific CTL responses (2, 8).
The adenylate cyclase toxin (CyaA) of Bordetella pertussis has the capacity to deliver its catalytic domain into the cytosol of eukaryotic cells (12). Delivery of a CD8+ T-cell epitope by CyaA results in intracellular processing and presentation of the epitope by MHC class I molecules at the surface of antigen-presenting cells (10). Immunization of mice with recombinant CyaA toxin bearing a viral epitope leads to the induction of a strong CTL response (8) and to full protection against a lethal viral challenge (19). Moreover, CyaA toxins carrying a single CTL epitope could also stimulate efficient protective and therapeutic antitumor immunity (7). Importantly, genetically detoxified CyaA toxins are able to induce protective antiviral or antitumoral immunity, like CyaA molecules that still express adenylate cyclase activity (7, 19).
In this study, we evaluated the potency of recombinant CyaAs carrying one to four copies of the MHC class I and class II restricted T-cell epitope from the nucleoprotein of the lymphocytic choriomeningitis virus (LCMV) to induce T-cell responses. These CyaA hybrid molecules were able to induce both CTL and Th responses against the LCMV peptide in both the absence and the presence of adjuvant. The T-cell response induced by such molecules was characterized by interleukin-2 (IL-2) and gamma interferon (IFN-γ) production, indicative of a Th1-like cytokine profile. Both CTL and Th responses induced by the recombinant CyaA molecules were enhanced by insertion of multiple copies of the LCMV epitope, but this potentiation was nevertheless limited by the decrease in invasive activity of these proteins when the size of the insert increased.
MATERIALS AND METHODS
Mice, peptides, antibodies, and recombinant adenylate cyclase toxins.
Six- to eight-week-old female inbred BALB/c mice were used in all experiments and were purchased from Janvier (Le Genest St. Isle, France). The synthetic peptide p118-132 (RPQASGVYMGNLTAQ), corresponding to the H-2d T-cell epitope of the LCMV nucleoprotein (1, 22), was synthesized by Neosystem (Strasbourg, France). Anti-CD4 (GK1.5), anti-CD8 (H35.17.2), and anti-HLA-Bw8 (HB152) monoclonal antibodies (MAbs) were prepared from ascites as previously described (9). The construction, purification, and biochemical characterization of the recombinant adenylate cyclase toxins (CyaAs) carrying one copy (CyaA224LCMV1), two copies (CyaA224LCMV2), three copies (CyaA224LCMV3), and four copies (CyaA224LCMV4) of the H-2d T-cell epitope p118-132 of LCMV nucleoprotein, inserted between residues 224 and 225 of the catalytic domain of CyaA, were described previously (20). The wild-type CyaA (CyaA-wt) and the different mutants carrying the LCMV epitope were produced in the presence of the activating protein CyaC, using the Escherichia coli B strain BL21 (Novagene, Abingdon, United Kingdom) transformed with the appropriate expression plasmid. Exponential-phase 500-ml cultures were induced with IPTG (isopropyl-β-d-thiogalactopyranoside, 1 mM), extracts of insoluble cell debris after sonication were prepared in 8 M urea–50 mM Tris-HCl (pH 8.0)–0.2 mM CaCl2, and the recombinant toxins were further purified by ion exchange chromatography on DEAE-Sepharose and phenyl-Sepharose, as previously described (3).
T-cell proliferation assay and cytokine production.
Mice were immunized intraperitoneally (i.p.) with purified recombinant adenylate cyclase toxins CyaA-wt, CyaA224LCMV1, CyaA224LCMV2, CyaA224LCMV3, and CyaA224LCMV4 in phosphate-buffered saline (PBS) with or without 1 mg of aluminum hydroxide. Seven days later, spleens were removed and single-cell suspensions were prepared and cultured in complete medium (RPMI 1640 medium supplemented with 10% fetal calf serum [FCS], antibiotics, 2 mM l-glutamine, and 5 × 10−5 M beta-2-mercaptoethanol) in the presence of the p118-132 peptide. After 72 h, proliferation of spleen cells and cytokine production were determined. Proliferation was measured by the incorporation of [3H]thymidine (ICN, Orsay, France). In inhibition experiments, 10 μg of MAb per ml was added during the test. Cytokine production was determined in culture supernatants. IL-2 was measured using the CTLL cell line. The levels of IL-4, IL-5, and IFN-γ were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using, respectively, BVD-1D11, TRFK5, and R4-6A2 (Pharmingen, San Diego, Calif.) MAbs as the capture antigen and BVD6-24G2, TRFK4, and XMG1.2 (Pharmingen) as secondary biotinylated MAbs. All assays were standardized with recombinant murine cytokines (Pharmingen).
In vivo induction of CTL responses and cytotoxic assays.
BALB/c mice were immunized i.p. on days 0 and 21 with purified recombinant adenylate cyclase CyaA224LCMV1, CyaA224LCMV2, CyaA224LCMV3, CyaA224LCMV4, or CyaA-wt in the presence or absence of 1 mg of aluminum hydroxide in PBS. Control groups were injected i.p. with PBS with or without 1 mg of aluminum hydroxide. One week after the last injection, spleen cells from immunized mice were stimulated in complete medium containing 1 μg of the p118-132 peptide per ml as previously described (8). Effector cells were then harvested by centrifugation and tested in a chromium release assay using 51Cr-radiolabeled P815 (H-2d) pulsed with 50 μM p118-132 peptide as target cells. Nonspecific killing was evaluated on P815 target cells pulsed with medium only. The released radioactivity was measured in the supernatants, and the percent specific lysis was calculated as [(experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm release)] × 100, as described previously (8). The spontaneous release from target cells was always less than 20% of the maximum release observed.
Single IFN-γ-producing cell ELISPOT assay.
Ninety-six-well multiscreen filtration plates (Millipore, Molsheim, France) were coated with anti-IFN-γ MAb (clone R4-6A2; Pharmingen) and then blocked with RPMI supplemented with 10% FCS. Seven days after the last injection, spleen cells from immunized mice were stimulated with syngenic feeder cells and 10 U of recombinant murine IL-2 (Pharmingen) per ml in the presence or absence of p118-132 peptide at 1 μg/ml for 36 h. After extensive washes, the plates were revealed by incubation with a biotinylated anti-IFN-γ MAb (clone XMG 1.2; Pharmingen) followed by streptavidin-alkaline phosphatase (Pharmingen). Finally, spots were developed using 5-bromo-4-chloro-3-indolylphosphate–nitro blue tetrazolium (Sigma, St. Louis, Mo.) as the substrate. The frequency of IFN-γ-producing cells was determined by counting the number of spot-forming cells (SFC) in each well, and the results were expressed as the number of SFC per spleen. No SFC were obtained if the stimulation of spleen cells was done in the absence of p118-132 or with spleen cells from mice immunized with CyaA-wt and stimulated in the presence of p118-132.
RESULTS AND DISCUSSION
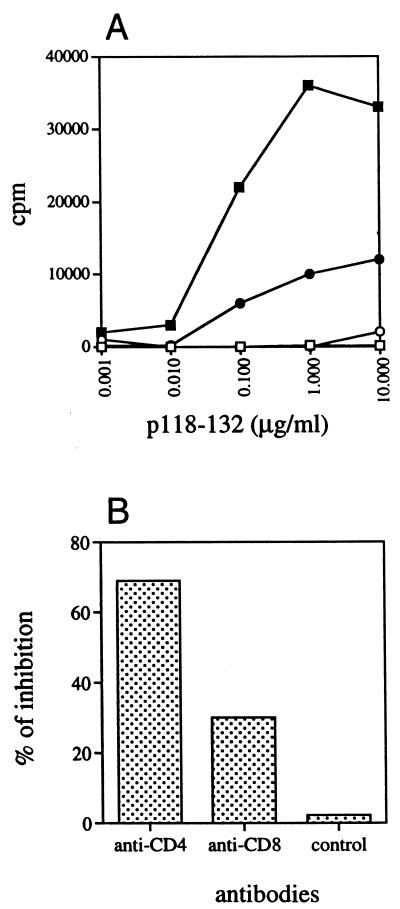
We have previously demonstrated that recombinant CyaA toxin carrying one copy of the 118-132 LCMV peptide (CyaA224LCMV1) is able to induce in vivo a strong specific cytotoxic T-cell response when injected in the presence of alum (8). The 118 to 132 sequence of the LCMV nucleoprotein carries both MHC class I and class II H-2d-restricted T-cell epitopes (6). To determine the ability of the recombinant toxin to induce an MHC class II-restricted response to the LCMV peptide, we first analyzed the LCMV-specific proliferative response of spleen cells from mice immunized with a single dose of 10 μg of CyaA224LCMV1 or control CyaA-wt either in the presence or in the absence of alum. As shown in Fig. 1, immunization with CyaA224LCMV1 leads to a p118-132-specific proliferative response, whereas injection of control CyaA-wt does not. It is noteworthy that the best response was obtained for CyaA224LCMV1 injected in the absence of adjuvant. To characterize the cells which proliferate in response to the p118-132 peptide, spleen cells from mice immunized once with 10 μg of CyaA224LCMV1 in PBS were stimulated in vitro with the peptide in the presence of anti-CD4, anti-CD8, or control MAb (Fig. 1B); 70% of the proliferative response was inhibited by anti-CD4 and 30% was inhibited by anti-CD8 MAb. This result indicates that the observed LCMV-specific proliferative response is mainly mediated by CD4+ T cells, although CD8+ T cells account for 30% of this response.
FIG. 1.
A single immunization of mice with CyaA carrying one copy of the LCMV epitope, in the absence of adjuvant, induces a specific T-cell proliferative response. (A) BALB/c mice were immunized i.p. with 10 μg of control CyaA-wt (□, ○) or with 10 μg of CyaA224LCMV1 (■, ●) in PBS (□, ■) or with 1 mg of alum (○, ●). Seven days later, spleen cells were stimulated in vitro with serial doses of the LCMV peptide and then monitored for the proliferative response. Results are expressed as mean [3H]thymidine incorporation in triplicate wells and are representative of three experiments. Standard deviations were always <2,000 cpm. (B) Assay for inhibition of proliferative response performed in the same conditions except that 10 μg of anti-CD4, anti-CD8, or control anti-HLA-Bw8 MAb per ml was added to the culture medium. Results are expressed as the mean percent inhibition calculated from triplicate wells, with standard deviations always <5%.
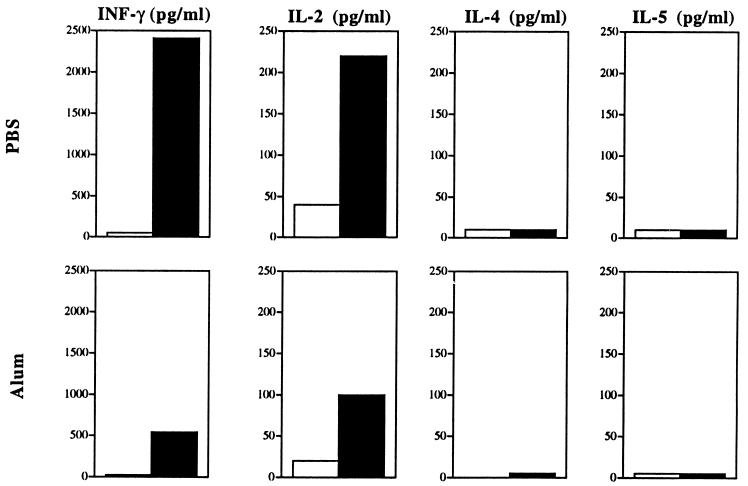
We have previously shown that a particulate vector such as parvovirus-like particles induce a Th1 response, whereas protein antigens linked to 1-μm beads induce a nonpolarized Th0 cell response when both vectors are administered in mice without adjuvant (14, 21). Furthermore, the use of adjuvant can drive the cytokine profile of the T-cell response towards a Th1 or Th2 response (5). It was therefore of interest to characterize the type of T-cell response induced by the CyaA toxin when injected with and without adjuvant. Thus, BALB/c mice were immunized once with 10 μg of CyaA224LCMV1, either with or without 1 mg of alum, and cytokine synthesis by T cells was determined after in vitro LCMV p118-132 peptide stimulation of spleen cells from immunized mice. In both cases, a Th1-like profile was obtained, which is characterized by the production of IL-2 and IFN-γ and by the lack of detectable levels of IL-4 and IL-5 (Fig. 2). The specificity of the induced cytokine production was assessed by the lack of response after injection of the control CyaA-wt. As observed for proliferative response, the higher response is obtained with spleen cells from mice immunized without alum, indicating that the use of adjuvant has a negative effect on this type of response. Th1 responses play an important role in protection against intracellular pathogens and tumor development, whereas Th2 responses exacerbate such diseases (15, 18). Therefore, induction of a polarized Th1 response after CyaA immunization is an important observation in view of the development of CyaA as an antigen delivery system.
FIG. 2.
CyaA224LCMV1 recombinant toxin administered with or without adjuvant induces a Th1-like response. BALB/c mice were primed i.p. with 10 μg of CyaA-wt (open bars) or 10 μg of CyaA224LCMV1 (solid bars) in PBS (top row) or in the presence of 1 mg of alum (bottom row). Seven days later, spleen cells were stimulated in vitro with the LCMV peptide, and the supernatants were tested for cytokine content. Results are expressed as the mean concentration of cytokines released in the supernatant from triplicate wells and are representative of five experiments. Standard deviations were always <10% of the maximum response.
Recently, we have shown that CyaA toxin carrying one to four concatenated copies of the LCMV epitope can induce specific CTL responses in vivo in mice, irrespective of the number of epitope copies (20). However, in this preliminary study, any quantitative analysis was performed to evaluate the efficiency with which these toxins induce a specific immune response. Thus, it was of interest to determine if an additional number of copies could influence the capacity of CyaA to induce the T-cell proliferative response and/or cytokine production. Since better responses were obtained after immunization without adjuvant, we analyzed the spleen cell proliferative response and IFN-γ production in response to p118-132 in vitro stimulation, following a single i.p. administration of 10 μg of CyaA-wt or CyaA carrying one copy (CyaA224LCMV1), two copies (CyaA224LCMV2), three copies (CyaA224LCMV3), or four copies (CyaA224LCMV4) of the LCMV epitope in PBS without alum. As illustrated in Fig. 3, both proliferative response and IFN-γ production were detected for all four groups of mice immunized with CyaA carrying the LCMV epitope, while spleen cells from mice immunized with CyaA-wt did not respond to in vitro LCMV peptide stimulation. All CyaA toxins induced a Th1-like response characterized by a lack of production of IL-4 and IL-5 (data not shown). However, a great discrepancy in the ability of the different recombinant toxins to induce these responses was observed. Indeed, a fivefold increase in the proliferative response and a 10-fold increase in the production of IFN-γ were obtained after immunization with CyaA224LCMV2 compared with CyaA224LCMV1. CyaA224LCMV3 gave an intermediate response characterized by a twofold and a sevenfold increase in proliferative response and IFN-γ production, respectively, compared with CyaA224LCMV1. Finally, comparable responses were obtained in mice immunized with CyaA224LCMV4 and CyaA224LCMV1. Hence, insertion of two copies of the same epitope highly enhanced the capacity of recombinant CyaA to stimulate Th1 cell responses. However, a progressive loss of this enhancement was seen after insertion of additional copies of the T-cell epitope.
FIG. 3.
Proliferative response and IFN-γ production induced by recombinant CyaA are greatly increased by the insertion of two or three copies of the LCMV epitope. BALB/c mice were injected i.p. with 10 μg of recombinant CyaA in PBS carrying, as indicated, no copies (CyaA-wt), one copy, two copies, three copies, or four copies of the LCMV epitope. Seven days later, spleen cells were stimulated in vitro with the LCMV peptide. Four days later, stimulated splenocytes were monitored for the proliferative response (A), and the supernatants were tested for cytokine content (B). Results are expressed as mean [3H]thymidine incorporation or as the mean concentration of cytokines released in the supernatant from triplicate wells and are representative of five experiments. Standard deviations were always <10% of the maximum response.
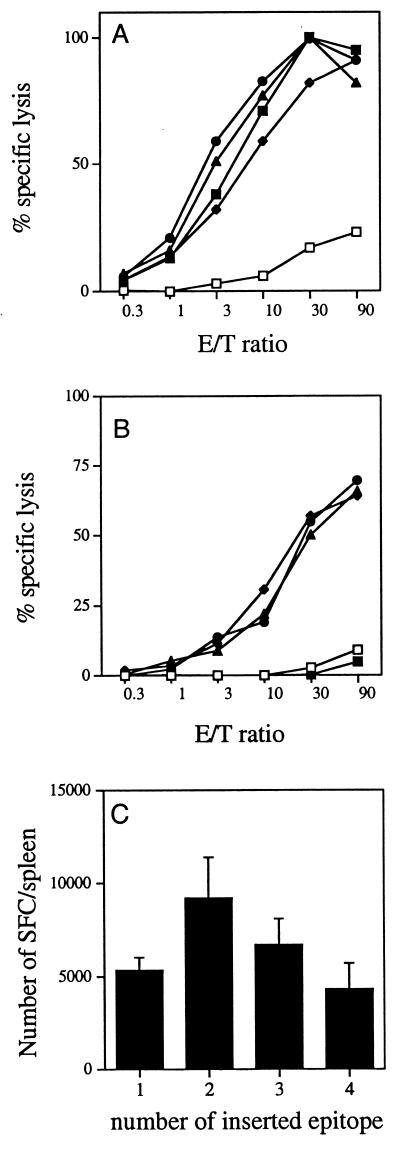
We next analyzed the capacity of the recombinant CyaA toxins carrying multiple copies of the LCMV epitope to induce in vivo a specific CTL response following different immunization protocols. BALB/c mice were immunized on days 0 and 21 by the i.p. route with either 50 or 10 μg of recombinant CyaA or Cya-wt and 1 mg of alum. After in vitro stimulation with the p118-132 LCMV peptide, effector cells were tested in a chromium release assay using the P815 target loaded with the same peptide as the target cells. When injected at 10 μg in the presence of alum, all four recombinant CyaA toxins carrying different numbers of copies of the LCMV epitope induced a high specific cytotoxic response (Fig. 4A). Similar results were obtained when mice were injected with 50 μg of recombinant toxin in presence of alum (data not shown). Since both CD4+ and CD8+ T-cell proliferative responses were obtained after immunization with recombinant CyaA in the absence of adjuvant, it was of interest to determine if specific cytotoxic T-cell responses could also be induced in these experimental conditions. Immunization of BALB/c mice by two i.p. injections of 10 μg of CyaA224LCMV1, CyaA224LCMV2, or CyaA224LCMV3 in PBS resulted in the activation of LCMV-specific cytotoxic activities (Fig. 4B). No difference in the level of CTL responses was observed in mice immunized with these three recombinant molecules. However, it should be noted that no CTL activity was observed with splenocytes from mice immunized with CyaA224LCMV4. This observation could be explained by the weaker invasiness of this recombinant toxin (50% of the in vitro invasive activity of CyaA224LCMV1), which might be an in vivo limiting factor when the toxin is injected without adjuvant. These results show that alum is not strictly required to induce good CTL responses. However, an enhancement of CTL response was observed when CyaA was injected in the presence of alum (Fig. 4A and B). The beneficial effect of alum might be linked to the adsorption of CyaA to alum and to particle formation, as previously discussed (8). Interestingly, the recombinant CyaA molecules used in this study induced stronger CTL responses than in our previous study (8), in which five-times-higher doses of CyaA-LCVM1 administered with alum as the adjuvant were needed to stimulate the same level of CTL response. This increased immunogenicity of recombinant CyaA could be due to the use of toxin production of the E. coli BL21 strain (which lacks the OmpT protease) instead of the XL1 strain used previously in order to reduce CyaA degradation. Most importantly, the use of this E. coli strain for recombinant CyaA production allows us to induce strong specific CTL responses in the absence of adjuvant.
FIG. 4.
Induction of LCMV-specific CTL responses by CyaA carrying one, two, three, or four copies of the LCMV epitope. (A and B) On days 0 and 21, BALB/c mice were injected i.p. with control wild-type CyaA (□) or recombinant toxin CyaA224LCMV1 (⧫), CyaA224LCMV2 (●), CyaA224LCMV3 (▴), or CyaA224LCMV4 (■). Seven days after the last injection, spleen cells were stimulated in vitro with the LCMV peptide and then tested for CTL activity on 51Cr-labeled P815 target cells incubated with the same peptide. No specific lysis was obtained on P815 target cells incubated with medium alone. (A) Mice injected twice with 10 μg of toxin in the presence of 1 mg of alum; (B) mice injected twice with 10 μg of toxin without adjuvant. Standard deviations were always <10% of the maximum response. (C) Quantitation of the LCMV-specific T-cell responses. BALB/c mice were immunized i.p. on days 0 and 21 with 50 μg of CyaA-wt or CyaA224LCMV1, CyaA224LCMV2, CyaA224LCMV3, CyaA224LCMV4 toxins (bars 1 through 4, respectively) in the presence of 1 mg of alum. Seven days after the last injection, the frequency of LCMV-specific T cells in the spleen was measured by the single-cell IFN-γ ELISPOT assay in the presence of p118-132 as described in Materials and Methods. Data are expressed as the number of SFC per spleen and represent the mean for four mice in each group plus standard deviation. No SFC were obtained in the absence of p118-132 or with spleen cells from mice immunized with CyaA-wt and stimulated in the presence of p118-132.
Finally, we quantified the specific T-cell responses induced by these toxins carrying multiple copies of the LCMV epitope by the ELISPOT assay. We determined the number of IFN-γ-producing cells in response to in vitro stimulation with the LCMV peptide of spleen cells from mice immunized with two i.p. injections of 50 μg of the recombinant toxins in the presence of alum (Fig. 4C). Despite small differences observed among the four toxins, the pattern of T-cell frequencies was similar to the one obtained for proliferative response and cytokine production. Indeed, CyaA224LCMV2 gave the highest response, followed by CyaA224LCMV3. A smaller response was obtained after immunization of mice with CyaA224LCMV4, which induces levels of IFN-γ-producing cells comparable to those with CyaA224LCMV1. These results therefore demonstrate that the capacity of CyaA to induce T-cell responses (proliferative response, cytokine production, and specific T-cell frequency) can be optimized by insertion of several copies of the same epitope. However, this enhancing effect is counteracted by a parallel decrease in the invasiveness of the CyaA toxin (70% of the activity of CyaA224LCMV1 for CyaA224LCMV2, 62% for CyaA224LCMV3, and 50% for CyaA224LCMV4 [20]). Thus, the efficiency of induction of specific T-cell responses by recombinant CyaA molecules is probably driven by the number of peptide-MHC complexes expressed at the surface of antigen-presenting cells, which could be related to the quantity of epitopes delivered to the cytosol. The efficiency of CTL induction is therefore controlled both by the number of inserted epitope copies and by the capacity of the recombinant CyaA molecule to translocate its catalytic domain into the target cells.
In conclusion, we demonstrate here that recombinant CyaA molecules bearing CD8+ CD4+ T-cell epitopes are able to induce both CTL and Th1-like responses in both the absence and the presence of adjuvant. Although we previously demonstrated that the protective antiviral immunity induced in vivo by CyaA carrying an LCMV epitope was independent of the presence of CD4+ T cells (8), the induction of a Th1 response could be very important to induce protective immunity against infectious diseases and tumor cells, where Th1 response is an essential factor. This response can be greatly enhanced by the addition of a second or third copy of the T-cell epitope to the CyaA224LCMV hybrid protein, while insertion of several copies of this epitope has only a small effect on the efficacy of CTL induction. However, the CD8+ T-cell epitope 118 to 132 from the LCMV nucleoprotein is highly immunogenic in BALB/c mice, and insertion of multiple copies of epitopes with weak immunogenicity, such as tumoral epitopes, might enhance the induction of specific CTL responses against these epitopes. The fact that peptide inserts of more than 60 amino acids into the catalytic domain of CyaA did not affect the efficiency of CTL induction should allow us to insert CTL polyepitopes into the same site, leading to the induction of CTL responses with large specifities and/or broad MHC restriction. Indeed, insertion of polyepitopes might permit us to overcome the variability of viruses such as human immunodeficiency virus and/or cover the diversity of HLA alleles in the human population. Taken together, these results indicate that CyaA is a very powerful vector for induction of T-cell immunity, which might potentially be used as a vaccine candidate for a number of pathologies, such as cancer and infectious diseases.
ACKNOWLEDGMENTS
This work was supported by grant 310/98/0432 from the Grant Agency of the Czech Republic, grants VS96149 and ME167 from the Ministry of Education Youth and Sports of the Czech Republic and the French-Czech research collaboration program Barrande (97054) to P.S., by grants from the Agence Nationale de Recherche sur le SIDA and Association pour la Recherche sur le Cancer to C.L., and by grant VACAT QLRT-PL1999-00556 from the EEC.
We thank Agnès Ullman and Daniel Ladant for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Aichele P, Hengartner H, Zinkernagel R M, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard J D, Collier R J, Starnbach M N. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. Proc Natl Acad Sci USA. 1996;93:12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basar T, Havlièek V, Bezouskova S, Halada P, Hackett M, Sebo P. The conserved lysine 860 in the additional fatty-acylation site of Bordetella pertussis adenylate cyclase toxin is crucial for toxin function independently of its acylation status. J Biol Chem. 1999;274:10777–10784. doi: 10.1074/jbc.274.16.10777. [DOI] [PubMed] [Google Scholar]
- 4.Bona C A, Casares S, Brumeanu T D. Towards development of T-cell vaccines. Immunol Today. 1998;19:126–133. doi: 10.1016/s0167-5699(97)01218-8. [DOI] [PubMed] [Google Scholar]
- 5.Comoy E E, Capron A, Thyphronitis G. In vivo induction of type 1 and 2 immune responses against protein antigens. Int Immunol. 1997;9:523–531. doi: 10.1093/intimm/9.4.523. [DOI] [PubMed] [Google Scholar]
- 6.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991;147:4069–4073. [PubMed] [Google Scholar]
- 7.Fayolle C, Ladant D, Karimova G, Ullmann A, Leclerc C. Therapy of murine tumor with recombinant Bortella pertussis adenylate cyclase carrying a cytotoxic T cell epitope. J Immunol. 1999;162:4157–4162. [PubMed] [Google Scholar]
- 8.Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J Immunol. 1996;156:4697–4706. [PubMed] [Google Scholar]
- 9.Gengoux C, Leclerc C. In vivo induction of CD4+ T cell responses by antigens covalently linked to synthetic microspheres does not require adjuvant. Int Immunol. 1995;7:45–53. doi: 10.1093/intimm/7.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Guermonprez P, Ladant D, Karimova G, Ullmann A, Leclerc C. Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway. J Immunol. 1999;162:1910–1916. [PubMed] [Google Scholar]
- 11.Harty J T, Bevan M J. Responses of CD8+ T cells to intracellular bacteria. Curr Opin Immunol. 1999;11:89–93. doi: 10.1016/s0952-7915(99)80016-8. [DOI] [PubMed] [Google Scholar]
- 12.Ladant D, Ullmann A. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 1999;7:172–176. doi: 10.1016/s0966-842x(99)01468-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y L, Askonas B A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo-Man R, Rueda P, Sedlik C, Deriaud E, Casal I, Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur J Immunol. 1998;28:1401–1407. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Matsui S, Ahlers J D, Vortmeyer A O, Terabe M, Tsukui T, Carbone D P, Liotta L A, Berzofsky J A. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol. 1999;163:184–193. [PubMed] [Google Scholar]
- 16.Melief C J. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 17.Rock K L, Goldberg A L. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 19.Saron M F, Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc Natl Acad Sci USA. 1997;94:3314–3319. doi: 10.1073/pnas.94.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebo P, Moukrim Z, Kalhous M, Schaft N, Dadaglio G, Sheshko V, Fayolle C, Leclerc C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying multiple copies of a viral CD8+ T-cell epitope. FEMS Immunol Med Microbiol. 1999;26:167–173. doi: 10.1111/j.1574-695X.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Sedlik C, Deriaud E, Leclerc C. Lack of Th1 or Th2 polarization of CD4+ T cell response induced by particulate antigen targeted to phagocytic cells. Int Immunol. 1997;9:91–103. doi: 10.1093/intimm/9.1.91. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Motal U M A, Berg L, Jondal M. In vivo priming of cytotoxic T lymphocyte responses in relation to in vitro up-regulation of major histocompatibility complex class I molecules by short synthetic peptides. Eur J Immunol. 1992;22:3085–3090. doi: 10.1002/eji.1830221209. [DOI] [PubMed] [Google Scholar]