Abstract
The geneticist Thomas Dobzhansky famously declared: “Nothing in biology makes sense except in the light of evolution.” A key evolutionary adaptation of Metazoa is directed movement, which has been elaborated into a spectacularly varied number of behaviors in animal clades. The mechanisms by which animal behaviors have evolved, however, remain unresolved. This is due, in part, to the indirect control of behavior by the genome, which provides the components for both building and operating the brain circuits that generate behavior. These brain circuits are adapted to respond flexibly to environmental contingencies and physiological needs and can change as a function of experience. The resulting plasticity of behavioral expression makes it difficult to characterize homologous elements of behavior and to track their evolution. Here, we evaluate progress in identifying the genetic substrates of behavioral evolution and suggest that examining adaptive changes in neuromodulatory signaling may be a particularly productive focus for future studies. We propose that the behavioral sequences used by ecdysozoans to molt are an attractive model for studying the role of neuromodulation in behavioral evolution.
Keywords: Evo-Devo, Ecdysozoa, GPCR, Neuropeptide, motor program
Part I: Introduction
The seventh chapter of Darwin’s Origin of Species is titled simply “Instinct” [1]. In it, Darwin argues that some behaviors may be inherited similarly to morphological traits. This parallel has inspired subsequent generations of behavioral biologists. Ethologists found evidence for heritable units of natural behavior in species-specific “fixed action patterns,” while behavioral ecologists sought such units in “evolutionary stable strategies” [2, 3]. While this research has strengthened the argument that certain behavioral traits evolve under natural selection, the number of established examples remains small and the mechanisms of behavioral evolution are still poorly understood [4]. This slow progress stands in contrast to recent exciting achievements in understanding morphological evolution [5, 6]. The burgeoning discipline of evolutionary development, or Evo-Devo, has shown that many morphological traits vary across species as the result of adaptations in developmental programs. While behavioral evolution is also likely driven, in part, by genetic changes in developmental programs—namely, those that give rise to the brain—identifying such changes has been hampered by the complexity of both animal nervous systems and behavior itself. While analogous to morphological characters, behavioral phenotypes are in some ways quite different.
Behavioral traits differ from morphological traits
One important way in which behavioral phenotypes differ from morphological characters relates to the issue of homology. Whereas the body axes provide a natural spatial coordinate system for comparing the positions and forms of anatomical features across animals, no similar reference frame exists for behavioral patterns. Indeed, multiple spatiotemporal coordinate systems are required to describe behavior, which can play out over milliseconds and millimeters, as in the punch of a baby mantis shrimp, to decades and thousands of kilometers, as in the interactions of migrating barnacle geese pairs. Because behavior in general leaves no fossil record, the study of its evolution is also unusually reliant on comparative studies in extant species. This requires parsing the motor patterns observed in different species over time and space into units that are identifiably heritable and homologous. This typically requires comparing closely related species. While criteria for behavioral homology have been proposed [7], and phylogenetic trees constructed using them often conform well with trees constructed using other criteria [8, 9], the issue of how—and even whether—behavioral homology can be accurately assessed remains contentious [10, 11].
A confound in assessing behavioral homology is the extreme variability of behavioral execution, which often differs both between and within individuals. As Darwin put it, even the simplest instinctive acts are rarely devoid of “a little dose…of judgement or reason.” Importantly, behavior is shaped not only by an animal’s genetic inheritance, but by learning. Behavior is the product of two distinct types of adaptation that operate in parallel —one phylogenetic and the other individual. Both types inform the many behavioral decisions that determine an animal’s actions under a given set of conditions. This produces a phenotypic plasticity that is not normally seen in morphological characters. While this plasticity gives animal behavior its enormous adaptive value, it also complicates the isolation of those components of behavior that are heritable and subject to natural selection.
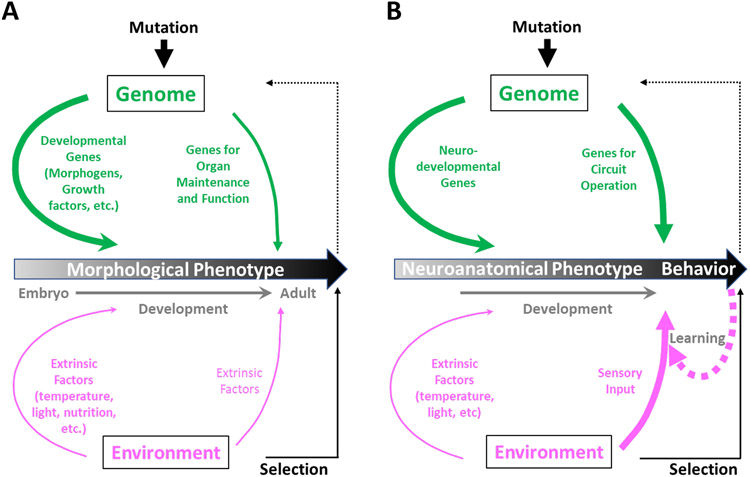
A final important difference between behavioral and morphological phenotypes is in how they are produced. As noted above, most anatomical features are generated during development by the activity of genetic networks. These networks use information from the genome to direct the proliferation and differentiation of specific cell types in response a variety of cues. Behaviors, in contrast, are generated by the activity of neuronal networks. These networks use electrical and chemical signals to translate cues derived from the environment or body into specific actions. The neuronal networks—or brain circuits—are, of course, themselves products of developmental genetic networks. As such, they constitute a more appropriate analogue of morphological traits than behaviors per se (Fig. 1).
Figure 1: Evolution of morphological and behavioral traits.
A) Animal bodies are built during development by genetic programs, which are governed by developmental genes (thick green arrow). Mutations that alter these genes—or where and when they are expressed—give rise to changes in morphological phenotype. Environmental selection of adaptive phenotypes (solid black arrow) fixes the corresponding mutations in the genome (dotted black arrow). Environmental factors (in magenta) can also influence morphological phenotypes both during development and adulthood (e.g. seasonal changes in coat color), but play a secondary role to genetic factors, as indicated by the thinner magenta arrows. Genes for maintaining the function of morphological features, such as organs, are also required post-developmentally.
B) The organ most critical for behavior, the nervous system, is composed of neural circuits, which are anatomical features built during development. They are therefore subject to alterations by mutational change in neurodevelopmental genes. These alterations can cause evolutionary changes in neuroanatomical phenotypes and thus animal behavior, which is the target of selection. However, behavior will also be strongly affected by mutations affecting genes responsible for neural circuit operation. This is because behavior is a product of both the structure and function of neural circuits. Importantly, the behavior of an individual animal can also change in response to its interactions with the environment (i.e. sensory input) by learning (thick dotted arrow). Learning alters neural circuit operation and is ultimately also dependent on the genes expressed by neurons in these circuits. Line thicknesses, in general, represent the relative importance of contributions to phenotype, with learning emphasized for behavior in higher animals.
Like morphological traits, brain circuits are laid down during development and large-scale post-developmental rewiring is rare. This means that behavioral plasticity in individual animals derives not so much from the development of brain circuits as from their operation. Importantly, the operation of brain circuits also requires the genome, which supplies the transcription factors, signaling molecules, receptors, transporters, biosynthetic enzymes, and other components that support neuronal function. The genes encoding these components and the regulatory domains that govern their expression are possible sites of evolutionary adaptation. In principle, natural selection can thus achieve behavioral adaptation by acting on genes that affect either the operation or development of the nervous system, or both.
Genetic determinants of behavior
Until the 1960’s little was known about the genes responsible for nervous system development and operation. If there were genes dedicated to generating specific behaviors, they remained to be identified. This changed in the 1960’s when so-called genetic screens were initiated in the fruit fly, Drosophila melanogaster, and the roundworm, C. elegans [12, 13]. These screens were designed to generate mutant animals with heritable deficits in behavior, neural function, and/or development. Analysis of behavioral mutants revealed a wide range of genes, mostly with pleiotropic effects [14-16]. Some clearly acted to alter developmental processes, while others altered physiological properties, such as membrane conductance and synaptic transmission. However, some genes caused remarkably specific deficits, indicating that some behavior—like the phenotypes of Mendel’s peas—might be particulate. A notable example was the period (per) gene, one of the first genes to be isolated in a behavioral screen. Period mutants exhibited altered circadian activity, and subsequent analysis indicated that per might be a target of natural selection [17]. Natural per variants collected from Bristol, England to Casablanca, Morocco showed systematic differences in particular amino acid sequences that were found to be important in buffering the timing of the circadian clock to differences in temperature at different latitudes.
Although the discovery of per and other genes comprising the circadian clock represents a triumph of behavioral genetic screens and was recognized by the Nobel Prize in 2017, the pleiotropy of most “behavior genes” discovered by this method and the profoundly polygenic nature of most behaviors underscored the complex relationship of behavior to the genome. As noted by one of the pioneers of behavioral screens, Sidney Brenner: “Behaviour is the result of a complex ill-understood set of computations performed by nervous systems and it seems essential to decompose the question into two: one concerned with the question of the genetic specification of nervous systems and the other with the way nervous systems work to produce behaviour” [13].
The genes that specify nervous systems and other metazoan tissues yielded more readily to mutagenesis screens than behavior, again highlighting the more direct relationship of morphological characters to the genome. The swiftness and scope of the discoveries made in developmental screens is most evident in the famous “Heidelberg screen” conducted by Eric Wieschaus and Christiane Nusslein-Vollhard [18]. By inducing random point mutations in the Drosophila genome and examining the resulting defects in the segmentation patterns of the developing epidermis, Wieschaus and Nusslein-Vollhard identified approximately 120 genes that were essential to assembling the body plan of the larval fly. Most of these genes were transcription factors and cell signaling molecules. Some, such as the homeobox-containing Hox genes, were already known, but many others were novel. A large number of the genes proved to be used not only to lay down the basic features of the larval epidermis, but also to form other parts of the fly body. More remarkably, the genes were conserved in mammals where, as Nusslein-Vollhard stated in her Nobel address, “homology is not restricted to amino acid sequence and … biochemical function, but extends to the biological role played in development. This remarkable conservation came as a great surprise. It had been neither predicted nor expected” [19]. By uncovering the basic palette of cell signals and nuclear effectors used in growth and development, the Heidelberg screen revealed an essential genetic “toolkit” used by metazoans to create anatomical structure. It thus laid the foundations for comparative studies of development and spawned the advances that came to define the field of Evo-Devo [20].
Numerous developmental “toolkit” genes play conserved roles in neurodevelopment. Hox and other homeodomain transcription factors partition developing regions of the nervous system and help determine neuronal identities [21]. Notch and its ligands contribute to neuronal cell fate decisions, and conserved secreted factors govern cell survival, axon pathfinding, and other developmental events in a variety of contexts [22, 23]. Research on developmental toolkit genes continues to inform approaches to behavioral evolution [23-25]. Mutations in such genes clearly have potential to alter circuitry—and with it, behavior—across species, but definitive examples of such mutations have been difficult to establish.
Indeed, what types of genes are targeted by natural selection in the evolution of behavior remains largely unknown. As discussed in the next section, mutations that give rise to differences in nervous system function, rather than development, have been the first to yield to investigation. Among the mutations so far identified, those that affect neuromodulatory signaling are particularly well represented and we will argue for the significance of this observation. However, current studies have clearly only scratched the surface. The wealth of genetic mechanisms revealed by mutagenesis screens—as well as emerging evidence from evolutionary studies—suggest that selection has more varied targets for driving behavioral evolution than those currently known.
Part II: Genetic and Neural Substrates of Behavioral Evolution
Within the last two decades, new methods have made it increasingly possible to examine not only the genetic, but also the neurobiological, changes responsible for behavioral variation and to better understand how and where natural selection acts to alter behavior (Fig. 2). The new methods facilitate the mapping of naturally occurring genetic differences to differences in brain function, and differences in brain function to differences in behavior that can be shown to promote an animal’s fitness. Given the complexity of the interactions that must be analyzed across multiple levels—genetic, neurobiological, behavioral, and ecological—it is perhaps not surprising that only a few cases of behavioral evolution have been definitively established. A broad survey of papers and recent reviews on behavioral evolution [25-30] reveals only a handful of cases in which compelling candidate genetic and/or neural substrates were shown to correlate with a behavioral difference, typically between two closely related species (Table 1). Although selective advantages are often mooted, in very few of these cases was it explicitly shown that the behavioral differences were adaptive. In only seven cases (Table 1, Group A) was a specific genetic change shown to be causative for the behavioral difference using functional manipulations [31-37]. These seven cases are worth examining in detail since they bear most directly on the question of what genetic changes drive behavioral evolution.
Figure 2: Emerging methods for identifying genetic and neural substrates of behavioral evolution.
New techniques for characterizing and manipulating both genomes and nervous systems are revolutionizing studies of behavioral evolution. On the side of genomics: whole genome sequencing has enabled genome-wide association studies and quantitative trait loci (QTL) mapping, while transcriptomics has enabled the temporal tracking of gene expression in the brain. On the side of the brain: a range of new genetically encoded tools for monitoring and manipulating neuronal function have enabled the study of brain circuits. Genetic methods now allow many of these methods to be combined so that gene expression, electrical activity, and neuronal function can be selectively characterized or manipulated in targeted neurons. Sophisticated electrophysiological methods, such as the dynamic clamp, also permit manipulation of function. These methods are complemented by a range of new tools for behavioral phenotyping, including computational methods for automating the acquisition and analysis of behavioral.
Table 1. Examples of behavioral evolution.
Of sixty-eight examples culled from the literature on behavioral evolution, we selected 16 for discussion in the text. Because heritable changes in behavior must derive from genetic changes, we prioritized seven studies (A) in which specific genetic differences (“Gene substrate”) were causally linked to behavioral differences observed in two or more closely related species. In most of these cases, plausible sites of gene action in the nervous system (i.e. “Neural Substrate”) were also identified. We selected an additional 9 examples because they causally link specific neural substrates to species differences in behavior without necessarily identifying the underlying genetic differences. In three studies (B), genetic substrates were either implicated without being definitively established (indicated by a “?”) or genetic substrates were identified, but a specific behavioral difference was not resolved. In the remaining six studies (C), only a neural substrate for the behavioral difference was implicated. As indicated in the “Methodology” columns, many of these examples rely on the new classes of tools indicated in Fig. 2. In addition to standard genetic methods (not indicated), most of the selected studies also used classical techniques from electrophysiology and pharmacology (“Ephys/Pharmacology) and some used hybrid crosses and QTL mapping or interspecies allelic exchange (“Hybrids/Allelic exchange”). With the exception of the cavefish studies, we excluded examples of heritable differences in behavior within a single species (as occurs, for example, with balancing selection), and examples in which genetic differences in sensory function have been demonstrated without explicit reference to distinct behaviors. This less restricted set of examples is well covered in previous reviews by Katz and Lillvis [50] and Niepoth and Bendesky [28], to which interested readers are referred. The key indicates the coding of examples by color according to the molecular function of the genetic substrates identified to underlie species differences in behavior: neuromodulatory, orange; sensory reception, green; ion-channel, pink.

|
Genetic changes underlying behavioral evolution
As indicated above, the seven identified mutations underlying behavioral evolution alter not nervous system development, but function. In general, alterations in function may reflect changes in how neurons receive, propagate, or transmit signals. This depends on whether the mutations affect receptors and their transduction mechanisms, intrinsic membrane properties, or synaptic and non-synaptic mechanisms of signaling. In fact, mutations in genes from all of these categories have been identified. Two of the genes affected by mutation are involved in sensory reception (Table 1, green), two in electrical signaling (Table 1, pink), and three in neuromodulatory signaling (Table 1, orange). Consideration of the seven implicated genes also reveals that they operate at different levels of neural processing, ranging from sensory and motor processing at the extremes to central processing of basic behavioral decisions in between.
At the sensory periphery, mutations that alter perception can change basic behavioral responses. Sensory receptors in fact appear to be a common target of behavioral mutations [28, 30], and this observation is well-illustrated by two in-depth studies of Drosophila seychellia. These flies are specialized for existence on the so-called “noni fruit” of their host plant, Morinda citrifolia, and specific odorant receptors of D. seychellia encoded by the OR22a and IR75b genes have mutated to be highly sensitive to noni fruit volatiles [31, 35]. These mutant receptors are expressed in conserved populations of sensory neurons that generally promote approach behavior in Drosophilids. Their altered sensitivity thus renders the otherwise repellent odor of noni fruit attractive and unlike Drosophilids of other species, D. seychellia move instinctively towards this odor.
As we will discuss in greater detail below, the three examples of behavioral evolution in which mechanisms of interneuronal signaling have changed all involve neuromodulation rather than synaptic communication. Two of these examples illustrate how genetic mutations can alter central processing to influence an animal’s behavioral preferences or its motivation to pursue particular activities. For example, some species of rodents form long-lasting pair bonds, exhibiting a preference for a particular reproductive partner, often over the lifespan. In a monogamous vole species, increased partner preference in males has been shown to derive from altered expression of a vasopressin receptor, V1aR [34]. Vasopressin is a hypothalamic peptide that modulates a wide range of affiliative behaviors, and monogamous male voles show increased expression of V1aR in the ventral pallidum. This region of the mammalian brain functions in reward learning, and V1aR expression in this region in males of a polygamous vole species was found to be substantially lower. The difference in expression pattern was traced to the insertion of a microsatellite DNA in the enhancer domain of the V1aR gene [34]. Similarly, changes in vasopressin expression have been shown to account for enhanced post-mating nest building behavior in mice of a monogamous species relative to mice of a closely related promiscuous species [32].
The third example in which a change in neuromodulatory signaling has been implicated in evolutionary behavioral change comes from nudibranch molluscs, where the cellular composition of the central pattern generators (CPGs) governing locomotion has been intensively studied. In a pair of elegant papers from the laboratory of Paul Katz, a conserved, identified neuron (i.e. “A1/C2”) has been shown to express specific receptors for the neuromodulator serotonin (5-HT) in two species that swim, but not in a non-swimming species [36, 38]. In one of the swimming species, swim behavior cycles over periods of days and two specific 5-HT receptors are expressed only on days when swimming ability is present. Although the particular mutation(s) underlying the species-specific differences in A1/C2 expression of 5-HT receptors remains to be determined, these studies indicate how the identification of neuronal substrates of evolutionary change can facilitate the subsequent investigation of the relevant genetic substrates.
The example of nudibranch swimming also indicates how genetic differences can underlie species differences in motor control. Motor output is also altered in two other examples in which genes whose products regulate membrane conductances have been shown to underlie evolved behavioral differences. In male fruit flies, which generate species-specific courtship “songs” by vibrating their wings, a difference in song carrier frequency between two related species (D. simulans and D. mauritiana) has been shown to derive from differences in expression of a calcium-activated K+ channel called Slowpoke [33]. Similarly, in certain species of South American electric fish (i.e. the Apteronotidae), a recently evolved voltage-activated Na+ channel (sn4ab1) supports the unusually high-frequency electrical discharges used for navigation and communication [37]. The sn4ab1 gene arose from the duplication and divergence of a channel expressed in the electric organ of ancestors of the Apteronotidae. Structural mutations in the channel lead to persistent, depolarizing Na+ currents in the “electromotorneurons” (EMNs) of Apteronotidae accounting for their high discharge rates.
In this last example, it is worth noting that the mechanisms of electric discharge that distinguish the Apterotidae from related electric fish go well beyond changes in snab1 structure. The electric organ of most electric fish is derived from muscle, but in Apterotidae this muscle-derived organ degenerates during development and a morphologically unique electric organ forms from the EMN axons [37]. This evolutionary restructuring of the electric organ implies genetic changes in developmental programming at the level of both muscle and nerve, but the nature of these changes remains unknown. Changes in developmental programming that accompany changes in nervous system function also occur in the host specialization of D. seychellia for noni fruit. Structural mutations in specific odorant receptors make flies of this species more receptive to noni fruit volatiles, but these changes in nervous system operation are paralleled by morphological changes: neurons that express the mutated olfactory receptors also increase in number [31, 35]. Downstream projection neurons that convey odor information to the Lateral Horn, an integrative center of the fly brain, have also been found to be altered. Although the behavioral consequences of these morphological changes were not determined, they likely promote the odor-evoked attraction of D. seychellia to noni fruit and presumably originate from as yet to be determined genetic changes in developmental programs that regulate neuron numbers and projection patterns.
Neurobiological Changes Underlying Behavioral Evolution
The concomitant genetic and neuroanatomical changes seen to underlie evolutionary behavioral change in both Apterotidae and Drosophila are likely to be typical. Cave-dwelling populations of the fish A. mexicanus that have been reproductively isolated from surface-dwelling members of their species for some 30,000 years exhibit significantly reduced sleep, which has been linked to both increased expression of hypocretin and increased numbers of the hypothalamic neurons that express this neuromodulator [39, 40]. The genetic mutations underlying both changes remain to be determined, emphasizing the difficulty of identifying the substrates of behavioral evolution even when a particular gene has been implicated. However, this example again illustrates the benefits of leveraging neuroanatomical differences to investigate genetic alterations, a feature of other examples in Groups B and C of Table 1.
The most compelling examples of changes in neural circuit architecture underlying behavioral evolution come from invertebrates, where the stereotypy of nervous system organization often allows the identification of homologous neurons across individual animals in multiple species. Taking advantage of such homology, Bumbarger et al. [41] compared electron microscope reconstructions of the pharyngeal nervous systems of two nematode species to provide what is perhaps the most comprehensive example of evolved differences in nervous system structure. The two species examined differ in pharynx design and in feeding strategies, but in both cases feeding is controlled by a set of 20 homologous neurons. The synaptic connectivity of these neurons is “massively” different, suggesting substantial divergence in developmental wiring mechanisms to support the different feeding strategies. Similarly, studies of swim CPGs carried out on multiple species of nudibranch mollusks, demonstrate that homologous neurons can have different connectivity that promotes different swim patterns across species [42].
Study of molluscan swim CPGs has more generally demonstrated that homologous neurons can function differently in different species [43-45] and often function in multiple behaviors [46]. This multifunctionality of homologous neurons has been frequently noted and has also been shown to underlie differences in male courtship singing between two closely related Drosophilids [47]. In D. melanogaster and D. yakuba, a homologous “command” neuron promotes different types of courtship song depending on its level of activation. In D. yakuba, the threshold for switching from one song type (i.e. “clack”) to another (i.e. “pulse”) is quite high, leading to a prevalence of “clack” song, whereas in D. melanogaster, the threshold is quite low and easily exceeded, leading to the prevalence of “pulse” song. Because the inputs and physiological properties of the homologous command neurons are similar in both species, the difference in courtship singing between species appears to be due to differing thresholds of activation of downstream motor circuits.
While the genetic and neurobiological mechanisms responsible for the threshold differences remain unknown, this example illustrates how the behavioral repertoire of a species can be strongly biased towards the expression of one type of behavior over another by what may be small changes in interneuronal signaling. This phenomenon is likely to be common. The bias to court females in response to a specific pheromone has been shown to be determined by the sign of interneuronal signaling in two Drosophila species [48]. Another example in which a relatively minor change in neuromodulatory signaling causes a major switch in behavior is provided by the study described above by Tamvacakis et al. [36]. In that case, the presence or absence of 5-HTR expression in particular neurons determined the presence or absence of swimming behavior. The authors of that study note that neuromodulatory “switches” may be a relatively common mechanism of behavioral evolution, a point also made by Lim et al. [34] in their study of vole mating strategies.
The idea that mutations that alter neuromodulatory signaling represent a common driver of behavioral evolution has been repeatedly proposed [30, 49-52]. The fact that three out of the seven demonstrated genetic determinants of behavioral evolution affect neuromodulatory signaling strongly supports this idea as do the numerous other examples listed in Table 1 (orange highlight) in which neuromodulatory signaling is altered, but the underlying genetic changes have not been determined [39, 43, 53-57] or the behavioral effects have not been firmly established [58]. In addition, genes affecting neuromodulatory pathways have been extensively implicated in natural behavioral variation within species, and thus provide plausible substrates for selection in the course of evolution [30].
The importance of neuromodulatory mechanisms in behavior
Altering neuromodulatory signaling represents a particularly attractive mechanism for behavioral evolution for reasons articulated in several previous reviews, particularly those of Paul Katz and his colleagues [30, 50-52]. First, neuromodulators play important roles in ensuring that an animal’s behavioral priorities are aligned with its physiological needs and environmental circumstances [59]. Neuromodulators thus regulate a wide range of behaviors. As their name implies, they do so by modulating the electrical and synaptic properties of neurons in sensorimotor circuits, often inducing slow and long-lasting changes in activity [60] (Fig. 3A, B). By acting on many, often broadly distributed, targets neuromodulators can up- and downregulate the activity of multiple components of neuronal circuits, producing persistent changes in activity and in some cases completely altering, or “reconfiguring,” circuit output [61] (Fig. 3C). By altering the gain or function of neural circuits, neuromodulators tend to bias rather than dictate behavioral decisions. They thus exercise considerable influence over an animal’s activities, but loss of function does not usually result in overt circuit failure. It is therefore little surprise that mutations that alter when, where, how, or how potently, a given neuromodulator acts within the nervous system might serve as levers for evolutionary change in behavior.
Figure 3: Neuromodulators and their cellular and behavioral actions.
A) Neuromodulators represent a diverse group of molecules. These include simple biogenic amines such as dopamine, serotonin, and adrenaline, a broad range of neuropeptides and small peptide hormones, and several gaseous molecules, such as nitric oxide. Most of these molecules act by binding to GPCRs as indicated by the highlight.
B) At the cellular level, neuromodulator binding to GPCRs leads to the release of heterotrimeric G-protein subunits, which typically induce the generation of “second messengers,” such as cAMP, Ca++, and IP3. These, in turn, alter the activity of ion channels, enzymes, or GPCRs to change the electrical and synaptic properties of neurons.
C) Neuromodulators often act on many cells and cell types within neural circuits to induce changes in cellular and synaptic activity. These circuit-level changes can alter the function of sensorimotor processing and decision-making to alter behavioral output.
D) While most molecular components of GPCR signaling are quite conserved in opisthokont lineages (i.e. eukaryotes most closely related to animals), the number of GPCRs themselves has dramatically expanded specifically in animals. Shown is the median number of signaling components of three types (heterotrimeric G-proteins, regulators of G-protein signaling, and GPCRs), calculated for taxa of each clade shown (Figure adapted from de Mendoza et al. [196]; Creative Commons License: http://creativecommons.org/licenses/by-nc/3.0/)
A point less frequently emphasized is that the smaller number of components involved in neuromodulator, as opposed to synaptic, signaling may also facilitate behavioral evolution. This is because fewer mutations may be required to produce an adaptive change in behavior. Synaptic circuits rely on the concerted action of many genes to establish and maintain precise point-to-point contacts between pre- and postsynaptic cells and to synthesize and transport appropriate neurotransmitters or their receptors. Coordinated changes in multiple genes are likely to be required to repurpose a given circuit. Because the genes involved in neurotransmission also are used in many circuits, their mutation is likely to have pleiotropic effects. In contrast, neuromodulator signaling can require the participation of only a few genes, and mutation is less likely to be disruptive. For the large class of neuromodulators comprised of peptides, only the genes encoding the neuromodulator and its receptor may be critical. Cell-cell contacts are not required for signaling, and the mechanisms of peptide synthesis, transport, and secretion in the neuromodulator-releasing cells are relatively generic. The same is true of the mechanisms of signal transduction in cells that respond to neuromodulators. Most neuromodulators act through G-protein coupled receptors (GPCRs), which use a small number of highly conserved G-proteins and other cytoplasmic effectors to mediate their downstream effects on cellular function [62, 63]. The ubiquitous expression of these effectors ensures that cells expressing a GPCR, will likely be able to respond to its activation. As evident from the examples above, mutations that alter the spatiotemporal expression of neuromodulator receptors can thus produce novel behavioral phenotypes.
GPCRs have diversified extensively in metazoans (Fig. 3D), and those for monoamines and peptides— two principal neuromodulator types—were present in the common bilaterian ancestor of protostomes and deuterostomes [64, 65]. The genomes of extant bilaterians typically contain genes encoding some 30 homologous GPCRs and their cognate ligands. Whether these conserved neuromodulatory signaling systems have retained conserved functions over evolution remains unclear. Most ligand-receptor pairs function within a variety of physiological and behavioral contexts, even within single species, and comparisons of function between distantly related species can be problematic. An extensive comparison of neuromodulatory signaling systems in flies (protostomes) and mammals (deuterostomes) points to only modest similarities in function between homologous systems [66]. However, other observations suggest that certain ligand-receptor pairs may have maintained an association with particular types of behavior over the course of evolution. For example, vasopressin, mentioned above, and oxytocin belong to a conserved family of neuromodulators that are implicated in a variety of affiliative behaviors [67]. Similarly, the family of Neuropeptide Y homologs appears to modulate feeding-related behaviors in several species across the phylogenetic spectrum [68]. Characterization of the behavioral roles of specific neuromodulatory systems across a broader range of animal species will help determine whether such similarities are indeed indicative of ancestral functions or are merely examples of coincidence or convergence.
In general, however, the finding that genes encoding neuromodulators and their receptors are frequently implicated in behavioral evolution is consistent with the known roles of neuromodulators in regulating survival behaviors [69, 70]. Strategies for both survival and reproduction must necessarily adapt in the course of speciation, as environments and conspecific interactions change. Determining what adaptations in neuromodulatory systems accompany, and possibly, underlie adaptations in behavior thus provides an important entry point for the mechanistic study of behavioral evolution.
The rich variety of behaviors in which neuromodulation plays a role offers abundant opportunities for research, but in general progress will be facilitated by selecting behaviors that meet certain stringent criteria. Apart from a strong dependence on neuromodulatory signaling, a behavior of interest should: 1) be important for survival and/or reproduction for all animals within a clade [29]; 2) exhibit clear similarities in motor programming that reflect behavioral homologies, but also clear differences that likely reflect fitness-enhancing adaptations; 3) be readily observable—preferably in quantifiable terms. In addition, it will be useful if some or all of the members of a clade being compared are readily accessible to genetic manipulation. As described more fully in the following section, the behavioral strategies used in molting by arthropods and other Ecdysozoa satisfy these criteria. In addition, the molting-related behaviors of Ecdysozoa offer several unique advantages that make them an excellent model system for evolutionary studies.
Part III: Ecdysis as a model for studying behavioral evolution
Advantages of ecdysis as a model
Behavior and morphology are linked phenotypes in ecdysis
The Ecdysozoa are a major monophyletic clade of animals (Fig. 4A, orange), characterized by the presence of an epidermally-derived cuticle that must be periodically shed [71]. The act of shedding, called “ecdysis,” is typically performed to permit growth and gives the clade its name. Extant ecdysozoans group into at least two major subdivisions one of which includes nematodes and the other arthropods (Fig. 4B). Although the behaviors used in ecdysis are quite varied, characterization of ecdysis in species spanning multiple ecdysozoan taxa, especially arthropods, indicates that certain features are shared. These include locomotor quiescence and feeding cessation prior to ecdysis, swallowing of air or water to increase internal volume, and anteriorly-directed peristaltic movements to propel the animal out of its old exoskeleton. The ecdysis behavior of extinct arthropods, such as trilobites, has been deduced from the fossilized remains of their cast-off exoskeletons [72, 73] and indicate that behavioral strategies that promote anterior ecdysis have ancient evolutionary antecedents [74-76]. Ecdysis thus represents one of the few exceptions to the rule that behavior does not leave a fossil record.
Figure 4: Phylogenetic classification of metazoan and ecdysozoan lineages.
A) The Ecdysozoa represent a major clade of Metazoans. They are distinguished by an exoskeleton composed at least partly of chitin that is periodically shed. Together with Lophotrochazoans, the Ecdysozoa make up the Protostomes, one of the two major subdivisions of bilaterian animals. Deuterostomes, which includes vertebrate animals, make up the second major subdivision. (Adapted from Semmens et al. [197]; Creative Commons License: http://creativecommons.org/licenses/by/4.0/. Vertebrate and urochordate images obtained directly from http://phylopic.org.)
B) The Ecdysozoa comprise two major groups when classified based on morphology: the Panarthropods and the Cycloneuralians [71]. The former group comprises such segmented animals as insects, crustaceans, myriapods (centipedes, millipedes) and chelicerates (spiders, scorpions, ticks), while the latter group includes nematodes and a variety of other animals distinguished by a central nerve ring. Red circles indicate those taxa for which the endocrinological basis of molting has been at least partially characterized (see text). Descriptions of ecdysis across Ecdysozoan species reveal common recurring themes—behaviors and related features that may represent conserved characteristics within certain groups. Several such features (represented by colored dots) are indicated, together with the groups in which they have been observed and their possible origins in Ecdysozoan evolution. These assignments are necessarily speculative and are intended as suggestive rather than definitive. For many taxa, the number of species studied is small and the behavioral descriptions fragmentary. The absence of a particular feature in the table may thus simply reflect a gap in the published record. Future efforts to categorize ecdysis behaviors for comparative purposes will benefit not only from more extensive and careful observations, but from development of a controlled vocabulary for characterizing ecdysial events. (Phylogenetic tree adapted from Schumann et al. [154]; Creative Commons license: http://creativecommons.org/licenses/by/4.0/.)
The intimate link between behavior and exoskeleton morphology revealed by ecdysozoan fossils is not coincidental. To be efficiently removed, the exoskeleton must rupture along predetermined lines, called ecdysial “seams.” These seams are developmentally determined weak points and breaking them is accomplished by executing motor patterns that selectively apply pressure to them. Executing anterior peristaltic movements is a common mechanism for doing this, as is ingesting the external medium (air or water) to increase internal pressure. The latter mechanism can also be recruited in arthropods to expand the new exoskeleton during hardening. At the time of shedding, the animal remains mostly soft and transiently pressurizing the new exoskeleton until it hardens ensures that it is large enough to accommodate growth. In diverse species, the old exoskeleton is eaten, presumably to recover nutrients.
While ecdysis motor patterns are relatively simple, they are organized into sequences, and individual steps can be sensitive to environmental input. As for more complex behaviors, ecdysis sequences thus rely not only on neural mechanisms that generate the movements themselves, such as CPGs, but also on decision-making mechanisms that prioritize the timing of individual motor programs for execution. Recent studies in ecdysis indicate that neuromodulators act both in the higher-level ordering of motor programs in the sequence and in the lower-level specification of motor rhythms and muscle contractions that generate the behavior [77, 78]. Ecdysis thus serves as a useful model to study evolutionary changes in the genetic mechanisms that act to assemble behavior at all levels of neural hierarchy, from the generation of simple motor programs to the broader organization of such programs into goal-directed strategies. It is perhaps at the latter level that studies of ecdysis evolution will have their greatest impact. This is because the mechanisms responsible for organizing ecdysis sequences may be more conserved than the specific motor components themselves.
For example, ecdysis sequences usually begin with animals entering a state of relative quiescence, but the motor programs supporting this transition are quite varied. In a dragonfly larva the actions involve crawling from the water and firmly grasping a stem to anchor the exoskeleton [79]; in a tarantula, they involve spinning a bed of silk and lying down on it backwards [80]; and in a nematode, like C. elegans, they involve simply ceasing locomotion, albeit interrupting quiescence with occasional 180° body flips [81]. Non-homologous motor patterns thus appear to support a homologous phase in the ecdysis sequence. Even when homologous motor programs occur, it may be at different phases to accomplish different goals. For example, swallowing (of water) in a shore crab commences with behavioral quiescence and slowly increases pressure to break open the old carapace [82]. In contrast, swallowing (of air) in adult flies is initiated only after escape from the old exoskeleton to pressurize the new one [83]. Ecdysis sequences thus exhibit a modularity, with motor programs recruited into different phases as needed to serve phase-specific purposes. The motor programs are often not specific to ecdysis, but may be adaptations of existing motor programs for feeding, locomotion, predation, escape, etc. These patterns are recruited into the novel behavioral context of ecdysis and assembled into species-specific sequences by the nervous system. As will be described in the following section, the execution and assembly of ecdysis sequences is profoundly dependent on conserved hormones and neuromodulators. These factors presumably alter the biases in sensorimotor circuits, shifting an animal’s behavioral priorities to favor the execution of one motor program over others. Phylogenetic comparison of how conserved neuromodulators act to assemble ecdysis sequences should shed light on the role of neuromodulation in behavioral evolution generally.
Ecdysis is under hormonal and neuromodulatory control
In insects, where the molecular and behavioral details of ecdysis have been extensively studied, the coupling of behavior and morphology is achieved by placing the synthesis of the new cuticle and the generation of ecdysis motor patterns under the joint control of steroid hormones collectively called ecdysteroids [83-85]. The most important of these closely related hormones is ecdysone, which is derivatized in peripheral tissues to its active form, 20-hydroxyecdysone (20E). As has been demonstrated in a variety of insect species, rising titers of ecdysone activate growth of the epidermis, which secretes a new cuticle as well as a “molting fluid” containing digestive enzymes that partially dissolve the old cuticle. Rising titers of ecdysone stimulate the production of a peptide hormone called ecdysis triggering hormone (ETH) and falling titers of ecdysone facilitate ETH release (Fig 5A). Once released, ETH orchestrates the physiological and behavioral processes that support ecdysis by working in concert with a range of other factors expressed by neurons targeted by ETH (Fig. 5B). The large majority of these factors are, like ETH, peptides that act as neuromodulators within the brain and signal using GPCRs (Fig. 5C).
Figure 5: Endocrinology of insect molting behavior.
A) Timeline of Drosophila development showing the fluctuating titers of the steroid hormone 20-hydroxyecdysone (20E), which regulates molting [198]. Peaks in 20E are followed by key developmental transitions, four of them molting events at which the animal sheds its old cuticle and secretes a new one (red lines, “molt”). Molting behaviors (i.e. ecdysis) at each developmental stage are governed by the peptide hormone ETH, which is secreted by Inka cells located on the trachea. Non-molting transitions (hatching, “h,” and puparium formation, “pf”) are also indicated.
B) The working model for the endocrinological control of ecdysis in Manduca (see [85]). Low-level of ETH secretion drives “pre-ecdysis” motor patterns by activating abdominal neurons (ABLK) expressing the neuropeptides Leucokinin (Lk) and DH44. ETH also initiates release of the Eclosion Hormone (EH) from ventromedial (Vm) neurons. EH feeds back on the Inka cells to further promote ETH release and surging ETH and EH levels evoke release of Crustacean Cardioactive Peptide (CCAP), which drives the motor programs responsible for “ecdysis” proper and the actual removal of the cuticle. By analogy to its action in flies, bursicon has been proposed to mediate the “postecdysis” motor patterns. In Drosophila, ETH production in the Inka cells has also been shown to be controlled by ecdysone, which initiates a transcriptional cascade in which early-, mid-, and late-acting transcription factors are sequentially activated. Expression of the ETH gene is under the direct control of EcR at the top of the cascade, but ETH release is dependent upon the action of a late-acting transcription factor known as FTZ-f1—a “competence factor” for secretion. In Manduca, the neuropeptide corazonin (Crz) acts as a proximal signal for the release of ETH.
C) The principal hormones implicated in the control of insect ecdysis are listed together with the types of receptor they act on. Most receptors of ecdysis hormones are GPCRs.
The known dependence of ecdysis behaviors in insects on a conserved suite of neuromodulators makes ecdysis an attractive model for studying how neuromodulatory signaling has been adapted during behavioral evolution. As a model, ecdysis offers the additional advantage that the motor sequences governing it lack many of the confounds present in other behaviors. Because ecdysis is essential for survival and must be performed successfully from the first time it is needed, ecdysis motor programs are specified genetically and have little obvious dependence on learning. The relative stereotypy of motor program execution is further enhanced by the fact that the surge of released ETH completely commits the animal to the all-or-nothing act of ecdysis [84, 86, 87], which is performed free of competition with other motor programs (see, for example, [88]). There is, in fact, a limited amount the vulnerable animal can undertake while it breaks the muscular contacts to the old exoskeleton, reinforces those to its new exoskeleton and replaces the cuticular linings of its digestive and respiratory tracts. As a consequence, the ecdysis motor programs generated by the nervous system run sufficiently autonomously that they can be studied as fictive behavioral output in reduced preparations, sometimes even in excised nervous systems. Finally, many of the motor patterns evoked, such as swallowing and abdominal peristalsis, are simple. This should facilitate recognizing those that are similar across species and identifying their conserved components.
Experimental advantages of ecdysis as a model for behavioral evolution
From an experimental standpoint, it is also useful that ecdysis generally depends on only simple physiological and environmental cues (e.g. circadian cycles, light conditions, availability of perch sites, etc.). Appropriate conditions are therefore often easily replicated in the laboratory. Observation of ecdysis is additionally facilitated by the fact that it is performed at predictable developmental time points and locomotion is usually limited, in part due to the need to re-form musculo-skeletal attachments. These advantages have enabled not only close behavioral observation, but also highly precise measurements of single muscle activity by both classical electrophysiological and modern imaging methods [77, 89, 90]. Increasingly, genetic methods of physiological manipulation are also being recruited to analyze the neural circuitry and neuromodulatory control of ecdysis [78, 91, 92].
The endocrinological and neurobiological analysis of ecdysis is most advanced in Drosophila, which offers an armamentarium of sophisticated genetic tools. Curiously, the traditional strengths of Drosophila for identifying genes underlying basic biological processes by performing unbiased genetic screens have not yet been exploited in the study of ecdysis. However, such screens have been the primary focus of ecdysis studies in another widely used ecdysozoan model organism: the nematode, Caenorhabditis elegans [93]. Thus far, however, genes that regulate cuticle structure or ecdysis timing have received more attention in C. elegans than those that regulate behavior. An exception is the quiescent phase that precedes worm ecdysis known as “lethargus” which has been equated with sleep [94].
The ability to leverage the technical advantages of two “super model” organisms, C. elegans and Drosophila melanogaster, is a powerful argument for a more generalized study of ecdysis as a model for behavioral evolution. Not only can sophisticated molecular and cellular manipulations be carried out in these organisms, but for both comprehensive “connectomes” exist that map the synaptic connectivity of their nervous systems [95]. Nematodes and fruit flies represent distantly related phyla within the Ecdysozoa and their molecular and behavioral substrates of ecdysis appear to be quite different. By studying such differences, together with similarities, across Ecdysozoan species, researchers have an auspicious opportunity to investigate how behavioral strategies have been maintained during evolution and of how behavioral novelty has arisen. Preliminary behavioral descriptions of ecdysis already exist for species in all major Ecdysozoan taxa and whole genome sequencing is increasingly revealing which genes are held in common and which ones differ. Analyzing gene function using methods such as RNA interference and CRISPR/Cas, it should be possible to systematically examine common and divergent mechanisms of neuromodulatory control underlying ecdysis across the ecdysozoans using results from Drosophila and C. elegans as starting points.
Insect ecdysis as an entry point for studying the evolution of ecdysis
Insects provide rich material for phylogenetic comparisons
Insects have diversified into a large number of orders that exhibit widely different life histories and body plans (Fig. 6) [96]. This diversity implies that the ecdysis sequences used to molt were repeatedly modified during evolution. This is evident even in single species, such as Drosophila, in which animals undergo complete metamorphosis. The worm-like larvae lack appendages, but change into adults with legs, wings, and extensible, tubular mouthparts (Fig. 5A). These very different body plans necessitate the use of distinct ecdysis motor sequences. The evolutionary elaboration of developmentally distinct ecdysis programs suggests that the mechanisms that govern ecdysis are highly “evolvable.”
Figure 6: Insect evolution, metamorphosis, and ecdysis.
A) The evolution of insects gave rise to three major phylogenetic subdivisions, each characterized by a distinct developmental strategy. The basal Ametabola (blue, a group that also includes non-insect hexapods, the Entognatha) do not undergo metamorphosis and molt throughout their lives; the Hemimetabola (green), undergo partial metamorphosis, typically deploying wings at the final, adult molt; and the most derived orders comprising the Holometabola (yellow) undergo complete metamorphosis, with molts frequently punctuating changes in body plan and habitat.
How insect ecdysis sequences evolved is currently an open question that is closely tied to the evolution of metamorphosis. The most basal insect orders (i.e. the “Ametabola”) consist of direct developers in which the postembryonic nymph approximates a miniature adult. These insects are wingless and molt throughout the lifecycle to accommodate growth, but not changes in either morphology or habitat. Although limited analysis of the ecdysis behavior of ametabolous insects has been carried out, it is likely that the same basic behavioral pattern serves to remove the exoskeleton at each molt [97].
Insects that undergo partial metamorphosis belong to the Hemimetabola. Hemimetabolous insects arose with the evolution of wings, which are typically a feature of only the adult stage. Molting terminates when this stage is achieved, and the wings are deployed as part of the adult ecdysis sequence. This necessarily makes adult ecdysis unique, although wing expansion is closely coupled to the more general expansion and hardening of the cuticle and may require few new mechanisms. In cases where multiple molts have been examined, such as in the kissing bug, Rhodnius [98-100], and the stick insect [101], the patterns of behavior used at all molts appear similar. Behavioral characterization of adult ecdysis in species from all major superorders of hemimetabolous insects has been carried out and despite numerous differences, many behavioral patterns are shared (Fig. 4B).
Knowledge of the endocrinological basis of ecdysis comes mainly from study of insects that undergo complete metamorphosis. These insects, which include moths, beetles, and flies, comprise the “Holometabola,” and arose most recently in evolution (Fig. 6). As noted above, holometabolous species have developmentally distinct ecdysis sequences and the study of both larval and adult ecdysis has provided considerable insight into the molecular and cellular basis of molting throughout the Insecta. Evidence of both conserved mechanisms and distinct adaptations suggests that the evolutionary trajectory of insect ecdysis will provide a productive model for studying neuromodulatory mechanisms of behavioral evolution.
Conserved and divergent hormone functions in insect ecdysis
While the model presented in Fig. 5B is based on results from the hawkmoth, Manduca sexta, evidence from other insect species suggests that the hormonal control of ecdysis is broadly conserved. This is based not only on the action of individual hormones, but also on their association with specific cell types. For example, in all orders of insect examined, including the most primitive, ETH is expressed in endocrine cells located on the trachea [102]. Eclosion Hormone (EH) is prominently expressed in a pair of central brain cells in numerous insects [103], and Crustacean Cardioactive Peptide (CCAP) is observed in one-to-two pairs of homologous neurons in the subesophageal, thoracic, and abdominal ganglia of insects of all major orders [104]. In Drosophila and Manduca, a subset of CCAP-expressing neurons co-expresses bursicon, a pattern that appears to be ancient as it has also been observed in the descending ganglia of crustaceans [105, 106]. While comparative functional analysis suggests important roles for each of these hormones in ecdysis, they also indicate that the precise roles may vary.
For example, in Manduca, ETH peptides release the larval pre-ecdysis motor program, but this role is played by EH in larval Drosophila [107]. Also, a neuropeptide called corazonin has been shown to stimulate the initial secretion of ETH in Manduca, but not in Drosophila [108]. Additionally, CCAP potently stimulates ecdysis in Manduca and has been shown to be essential for this function in the red flour beetle, Tribolium [109]. However, in another beetle, L. decemlineata, RNAi experiments indicate that CCAP is dispensable for adult ecdysis [110], and in Drosophila knockout of the CCAP gene causes no overt ecdysis deficits [111]. In the fly, CCAP’s role seems to be subsidiary to bursicon. Interestingly, bursicon’s role in Drosophila ecdysis appears to vary in importance over development [83]. Bursicon is not required for larval molting, but is essential for wing expansion, which concludes the adult ecdysis sequence. Bursicon also plays an essential role at a cryptic molt that initiates morphological change at the onset of metamorphosis during the pupal stage (Fig. 5A), but the ecdysis behaviors it governs differ from those that occur at the adult stage [78]. At wing expansion, bursicon has been implicated in air swallowing and tonic abdominal flexion, while at pupal ecdysis it is implicated in rhythmic abdominal “swinging.” These results suggest that the hormonal regulation of an ecdysis sequence may differ both within and across species, a conclusion that is underscored by findings with other neuropeptides. For example, knockdown of the gene encoding Orcokinin peptides in the kissing bug, Rhodnius prolixus potently blocks ecdysis at both the larval and adult molts, evidently inhibiting behavioral initiation [112]. However, Orcokinin loss-of-function in neither Drosophila nor Tribolium has an effect on ecdysis [113, 114].
Evolutionary accretion of neuromodulators at cellular nodes to promote behavioral coordination
The co-expression of neuropeptides with related functions, such as CCAP and bursicon, is not uncommon in animal nervous systems [115-117]. In mammals, for example, hypothalamic neurons in the arcuate nucleus co-express Agouti-related peptide and Neuropeptide Y, two factors involved in neuromodulation of feeding [118]. Likewise, in the lobster stomatogastric ganglion, Red pigment-concentrating hormone and Cancer borealis tachykinin-related peptide released from the same neurons jointly control the generation of the pyloric rhythm [119]. From the standpoint of evolution, the coordinate control of a biological process by multiple factors released from the same neurons may reflect one of the advantages of using neuromodulatory signals to regulate behavior. Once a neuroendocrine cell type acquires a regulatory function by broadcasting such a signal, co-expression of any additional neuromodulator whose action tends to support that function may offer selective advantages during subsequent evolution. Over time, neuroendocrine cells that control a behavioral transition might accumulate the expression of multiple neuromodulators whose co-release promotes the transition (Fig. 7A). Interestingly, there is evidence of extensive neuromodulator accumulation in the neurons that co-express CCAP and bursicon. In both Manduca and Drosophila, these neurons also express myoinhibitory peptides (MIPs) and Allatostatin DoubleC (AlstCC), two neuromodulators that have also been implicated in ecdysis regulation [91, 120]. Future comparative studies will reveal whether similar—or different—co-expression of neuromodulators occurs in these neurons outside the Holometabola.
Figure 7: Possible neuromodulatory mechanisms of behavioral evolution.
A) Cell types (“Source Neurons”) that express a neuromodulator (NM1) that promotes a particular behavior at an early point in evolution may come to express additional neuromodulators (NM2, NM3) that synergistically promote the same behavior as evolution proceeds. The additional neuromodulators may act on the same or different target cells to refine or augment the original behavior to make it more effective. A’) A neuromodulator (NM) that originally targets a particular set of target cells or circuits (T1) may accrue additional targets (T2, T3) over the course of evolution if the pattern of expression of its receptor broadens. This may happen if it results in the recruitment of new targets that selectively improve behavioral performance by adding to or altering the original behavior(s) evoked by NM.
B) A model for the sequential execution of ecdysis motor programs in response to ETH. Neuronal targets of ETH express one of two receptor isoforms: ETHRA or ETHRB. ETHRB has substantially higher affinity for ETH than ETHRA and neurons that express it are proposed to prime the ecdysis network, activating that part governing the first behavioral phase as well as inhibitory nodes that suppress neurons that express ETHRA. The latter neurons release neuromodulators (NM) that act on downstream targets to activate motor networks that generate specific behavioral phases. ETHRB thus regulates delay circuits that determine when the nodes regulated by ETHRA become active. Sequential inactivation of ETHRB-mediated delay circuits (perhaps by sensory feedback signals reporting the successful conclusion of a step in the sequence) serially activates the networks driving the phasic motor patterns of the ecdysis sequence, which are critically modulated by neurons expressing ETHRA. Mutations that alter the patterns of expression of ETHRB, ETHRA, or the receptors of neuromodulators such as CCAP and bursicon are predicted to alter the character and progression of the ecdysis sequence. For example: 1) ETHRA expression patterns identify which neuromodulatory neurons are important for ecdysis behavior and changes in these patterns should change behavioral output. 2) ETHRB expression patterns identify nodes in inhibitory circuits and changes in these patterns should change the order in which distinct motor patterns occur in the ecdysis sequence. 3) Receptor expression patterns of hormones acting downstream of ETH (e.g. CCAP, MIPs, etc) identify neurons that generate distinct motor outputs and changes in these patterns may change the character of these outputs. 4) Changing the pattern of neuromodulator expression at any given ETHRA node (e.g. by adding expression of a new neuromodulator) will alter (e.g. facilitate) the motor output associated with that node.
Evolutionary expansion of neuromodulator receptor expression to promote behavioral diversification
In addition to mutations that alter the expression patterns of neuromodulators and lead to neuromodulator accretion in particular cell types, mutations can also occur that alter the expression pattern of neuromodulator receptors (Fig. 7A’). The latter can spawn new behaviors, as seen from the example of vole pair-bonding discussed above [34]. Is there any evidence that species-specific differences in ecdysis behaviors derive from differences in ecdysis hormone receptor expression? While there is currently only limited data to address this question, the evolution of ecdysis provides a useful paradigm for investigating this possible mechanism.
To the extent that the distributions of ecdysis hormone receptors have been compared across species, the focus has been primarily on similarities rather than differences. However, emerging information about the distribution of ETH receptors in Drosophila, Manduca, and the silkmoth, Bombyx mori, has suggested a general model for the neuroendocrine control of insect ecdysis that makes testable predictions about where and how evolutionary change might occur [120-123] (Fig. 7B). The ETHR gene in most insect species examined has two splice isoforms, ETHRA and ETHRB. Within the nervous system, these isoforms are expressed in distinct populations of neurons. Interestingly, neurons that express ETHRA are overwhelmingly peptidergic in function and include the known neuroendocrine neurons expressing EH, CCAP, Bursicon, MIPs, and AlstCC. Based on this and other evidence, it has been proposed that ETH primes the activity of multiple nodes in a neuromodulatory network, each of which regulates (or co-regulates) part of the ecdysis sequence. These nodes are peptidergic neurons that express ETHRA. In addition, ETH has been posited to activate inhibitory delay circuits characterized by their expression of ETHRB [122]. These delay circuits determine when the nodes regulated by ETHRA become active. In the model, all inhibitory circuits are activated with the initial release of ETH, and their sequential inactivation leads to the serial activation of the ETHRA-expressing nodes and hence to the serial production of the motor patterns of the ecdysis sequence.
Although there is too little comparative data at present to test the predictions of the model in Fig. 7B, expression of both the bursicon and CCAP receptors in Drosophila at the pupal stage conforms with the prediction that downstream ecdysis hormone receptors identify neurons involved in motor output. CCAP-R expresses in behaviorally important subsets of motor neurons and muscles, and the bursicon receptor, which is encoded by the rk gene, expresses in central neurons involved in motor pattern generation [77, 78]. It is also worth noting that although interspecies comparisons are sparse, detailed comparison of ETHRA and ETHRB expression patterns at different developmental stages in Bombyx have shown considerable variation [120]. Such variation is consistent with the idea that changing receptor expression may underlie developmental changes in ecdysis motor programming in this holometabolous insect. It will be interesting to see if further work on Bombyx, Drosophila, and other holometabolous insects in which ETHRA and ETHRB expression can be readily manipulated, bears out this conjecture.
More generally, the model presented here provides a ground plan for studying the contribution of altered neuromodulatory signaling to the evolution of ecdysis. A promising start has been made in several holometabolous species that should provide traction for further comparative studies of more basal insects. Establishing the links between specific behavioral patterns and the action of neuromodulatory factors in multiple species should reveal where similarities in neuromodulatory signaling underlie conserved behavioral strategies and, more importantly, where differences in such signaling underlie behavioral adaptation. For the hemimetabola, both Schistocerca and Rhodnius offer experimental advantages, including detailed behavioral descriptions of adult ecdysis, RNAi technology, and fully sequenced genomes [124, 125]. Among the ametabolous Zygentoma, emerging genomic data for the firebrat, Thermobia domestica, make it a promising preparation [126]. Studies of hemimetabolous and ametabolous insects will not only inform our understanding how ecdysis evolved in insects, but connect such understanding to our existing knowledge of the molecular mechanisms of ecdysis control in crustaceans.
Beyond the Insects: Control of molting in other Ecdysozoa
Although the hormonal and neural mechanisms of ecdysis are best understood in insects, analysis of molting in crustacea, chelicerates, and nematodes has also provided promising points for comparative study and permits a broader investigation of the evolutionary roots of ecdysis (Fig. 4B). Molting in decapod crustaceans shares many similarities with insect molting [127], but its endocrinological control also exhibits notable differences. Greater differences in both behavior and hormonal control are seen in ecdysis of the even more distantly related chelicerates and nematodes. The rapid progress that has been made in identifying the genetic substrates of molting in C. elegans exemplifies the power of studying ecdysis in this model organism. Here we summarize salient findings from ecdysozoans outside of the insects and touch briefly upon the function of ecdysis-like pathways in non-ecdysozoan taxa.
Hormonal determinants of ecdysis behavior in decapods vs insects: similarities and differences
Detailed descriptions of the ecdysis sequences performed by several crustacean species include many elements recognized in insects (Fig. 4B) [82, 128]. Generally, feeding and locomotor activity cease as the animal finds a place to undergo ecdysis. Water swallowing is initiated to expand the stomach and detach the carapace and to produce high internal hydrostatic pressure that can be used to expand the new exoskeleton [129]. Peristaltic movements of the abdomen are also executed, typically to shed the abdominal exuvia. As in insects, specific motor patterns support appendage extrication, and exuvia consumption occurs in some species. A variety of species-specific adaptations have also been described, such as the escape-like tailflips that conclude ecdysis in the crayfish, O. limosus, but not the shore crab, C. maenas [82].
As in insects, the master regulator of the molt in decapod crustaceans is ecdysone. Production of this hormone across the molt cycle has been extensively characterized [130-132], and crustacean ecdysis occurs, as in insects, in response to falling ecdysone titers. How this decline is translated into nervous system activity and ecdysis behavior is less clear. However, the recent introduction of whole-genome sequencing and transcriptomics, have allowed protein expression profiles across the molt cycle to be characterized in a number of species [132-134]. These studies reveal that neuromodulatory factors and their G-protein coupled receptors are highly conserved between insects and crustaceans [135] and many of the signaling molecules familiar from studies of insect ecdysis have been identified as candidate regulators of ecdysis behavior.
In the green shore crab, C. maenas, CCAP, bursicon, and AlstCC expression in the nervous system peaks just prior to ecdysis [132], as one would expect for colocalized factors [105]. CCAP and bursicon secretion also surges at the time of ecdysis in both C. maenas and the crayfish, O. limosus [82, 136] and recent RNAi knock-down data from the mud crab, Scylla paramamosain, demonstrates an essential role for CCAP in ecdysis [137]. RNAi knock-down data similarly demonstrate essential roles in ecdysis for EH and ETH in the mud crab, with expression of the latter hormone strongly upregulated at ecdysis. Such upregulation is also observed in C. maenas at adult ecdysis. It is, however, doubtful that ETH acts generally as a “triggering hormone” for ecdysis in crustaceans as it does in insects. In larvae of the ornate spiny lobster, Panulirus ornatus, ETH upregulation is observed only prior to a metamorphic molt, but not at a subsequent molt [138]. In addition, ETH injection into the crayfish, Cherax quadricarinatus, during the intermolt period significantly delays rather than triggers molting [139].
Ambiguity also exists around the role of EH peptides, which are encoded by two paralogous genes in most crustaceans [135]. In C. maenas, one gene is expressed at very low levels in the nervous system and the other does not exhibit differential expression over the molt cycle. However, in S. paramamosain, high levels of EH expression are observed in the nervous system, with pronounced elevation just prior to ecdysis. Similar EH elevation is observed in the shrimp, Exopalaemon carinicauda, albeit in non-neural tissues, namely the eyestalks, gills, and epidermis [140]. Interestingly, recent evidence from Drosophila also supports a role in ecdysis for EH expressed in non-neural tissues [103]. While some evidence thus supports an essential role for both ETH and EH signaling in decapod ecdysis, it remains unclear how much homology there is in the action of these signaling pathways between crustaceans and insects.
A signaling pathway implicated in decapod, but not insect, ecdysis involves Crustacean Hyperglycemic Hormone (CHH). CHH belongs to a diverse family of hormones, several of which critically regulate crustacean molting [141]. One of these is molt-inhibiting hormone, which is expressed in the eyestalks and suppresses ecdysone production during the intermolt. CHH also contributes to this suppression in some species, but in C. maenas it also positively regulates ecdysis. Expression of a particular CHH peptide, CHH-2, is strongly upregulated in the nervous system just prior to ecdysis [132], and ecdysis onset is accompanied by massive release of CHH from gut endocrine cells to induce molting and swallowing of seawater [142]. Since water consumption is accompanied by uptake of water from the gut, CHH has been proposed to additionally facilitate water transport across the gut epithelia. This would be consistent with the known role of the insect homolog of CHH, which is called Ion Transport Peptide (ITP) because it facilitates chloride transport out of the hindgut to promote isosmotic water re-uptake [143]. While major increases in hemolymph volume are known to occur at ecdysis in some insects, it is not known if ITP is involved in mediating this effect.
Promising points for comparative study in crustaceans and insects
This brief survey establishes likely homologies in ecdysis behavior in decapod crustaceans and insects and indicates both commonalities and differences in the mechanisms of hormonal control. One promising focus for future investigations might be control of swallowing (of water or air), which serves to increase internal pressure. Rhythmic swallowing appears to engage a feeding CPG that has been recruited to serve the purposes of molting in both crustaceans and insects. Work from C. maenas suggests that CHH may initiate swallowing, whereas circumstantial evidence from insects suggests a role for CCAP [144] and possibly bursicon [145]. It should be possible to determine whether these hormones indeed initiate central pattern generation, whether they do so directly or indirectly, and whether they act in concert with other—possibly conserved—neuromodulators. In addition, it should be possible to determine where and how the relevant hormones act on the underlying circuitry. The historical strengths of C. maenas in electrophysiological studies of CPG neuromodulation may offer unique advantages.
More generally, it may be possible to exploit the traditional power of electrophysiological approaches—and their application to the identified cells of the crustacean nervous system—to study ecdysis. Extracellular recordings have been effectively used to study fictive ecdysis sequences in reduced preparations of large insects and should be possible in crustaceans [146-148]. In addition, transcriptomic approaches can be extended to less studied crustacean species to establish the temporal expression patterns of ecdysis-related genes. In some species, such as the parthenogenic marbled crayfish, genetic manipulations are now possible [149]. Given the complex life histories and diverse habitats of crustaceans, both crustacean ecdysis sequences and their neuroendocrine determinants can be expected to be quite varied, which should provide ample material for comparative studies. Among the species that will be particularly interesting to investigate are the freshwater remipede crustaceans, whose ancestors likely made the evolutionary transition from crustaceans to insects [150].
Control of Molting and Ecdysis in Nematodes
Separating insects and crustaceans from nematodes are other arthropod taxa, including chelicerates and myriapods (Fig. 4B). While detailed behavioral descriptions of ecdysis have been given for multiple chelicerates, including scorpions [151], spiders [152], and even one extinct species [153], little is known about the genetic determinants of ecdysis in these arthropods. Genomic data alone, however, indicate that the molecular mechanisms of molting must differ from those of insects and crustaceans. The genes encoding several enzymes for synthesis of 20E in insects are absent in myriapods and chelicerates, and in chelicerates the ecdysteroid ponasterone A (i.e. 25-deoxy-20E) rather than 20E is known to be used as the molting hormone [154]. In yet more distantly related Ecdysozoans, such as tardigrades, even the neverland gene, which encodes the initial enzyme in the biosynthetic pathway for ecdysteroid synthesis, is absent. Surprisingly, the genome of the nematode C. elegans, lacks all of the major genes for insect ecdysis hormones [155]. Although this is not generally true for nematodes, it suggests that fundamental differences exist in the regulation of ecdysis across ecdysozoan taxa.
In nematodes, molting and its genetic specification have been best characterized in C. elegans for the specialized molt known as a dauer molt [156]. This molt marks the entry into disapause and occurs in free-living nematodes, when environmental conditions are unfavorable for growth. Entry into the dauer molt is governed by sterol-derived dafachronic acids that bind the nuclear receptor, DAF-12. DAF-12 belongs to the same subfamily 1 of nuclear receptors as the ecdysone receptor (EcR), but neither it nor the nuclear receptors implicated in normal molting are orthologous to EcR. The latter receptors—Nhr-23 and Nhr-25—are homologous to two Drosophila nuclear receptors—Hr3 and Ftz-f1—which act downstream of EcR in flies [94]. Their ligands in the worm are unknown.
Molting behavior in C. elegans has been described by Singh and Sulston [81] and is typically divided into two phases. The first, relatively quiescent, phase is called lethargus, while the second, more active phase, is ecdysis. During lethargus, pharyngeal pumping ceases and locomotion is generally suppressed as the cuticle attachments are dissolved. As this period ends, distinctive “flips” or rapid rotations about the longitudinal axis of 180° are performed, perhaps to further loosen the cuticle. Spasmodic movements of the pharynx and foregut follow, which break and then expel the pharyngeal cuticle, while the animal repeatedly pulls back to facilitate this process. Once the pharynx has been successfully expelled, the animal ruptures the old cuticle by forward thrusts of the head and then crawls out of it. While the neural and hormonal basis of these active behavioral components have not been well-characterized, the lethargus phase is likely under neuromodulatory control and has received considerable attention because of its possible relationship to sleep in other animals [157].
Genetic screens have specifically implicated in lethargus several neuropeptides and their G-protein-coupled receptors [158-161] in addition to two particular G-proteins [162]. Among the neuropeptides implicated is the nematode ortholog of orcokinin, which is required for ecdysis in the kissing bug [163]. Further characterization of orcokinin’s role in ecdysis in both nematodes and insects will be required to know if this peptide has an ancestral function in this process. As indicated in Fig. 4B, quiescent states preceding ecdysis in which the animal stops feeding and perches are a common feature of arthropod molting. In Manduca this state has been called “molt sleep” [164], and its molecular basis has recently begun to be investigated [165]. Several similarities of lethargus to the circadian-regulated, sleep-like states of Drosophila have also been noted. Both, for example are under the control of the period gene [166].
Growth and the Conservation of Ecdysis Hormones outside of Ecdysozoa
Findings from C. elegans, a free-living nematode, do not readily generalize to parasitic nematode species. Ecdysis behavior in the mammalian parasite, B. malayi, lacks both the lethargus phase and the “flipping” movements used to loosen the cuticle in C. elegans [167]. Instead, coiling and uncoiling movements substitute for flipping. Interestingly, Brugia malayi and another parasitic nematode, Dirofilaria immitis, have homologs for the ecdysone receptor that respond to ecdysteroids [168-171]. The loss in C. elegans of the ecdysis-related genes known from insects thus may be an anomaly, even among nematodes. Underscoring this is the observation that orthologs of these genes are found outside of the Ecdysozoans in Lophotrochozoan species and even basal deuterostomes [155]. This broad conservation among the Bilateria indicates that these intercellular signaling systems functioned originally in processes other than ecdysis. Transcriptomics analysis of the ecdysis-related genes in 19 invertebrate species including 13 non-Ecdysozoans, suggests that they are upregulated at key developmental transitions, such as metamorphosis [172].
In ecdysozoans, metamorphosis typically occurs in conjunction with molting as in larval crustaceans and holometabolous insects. Ecdysis can thus be viewed as a developmental transition to promote growth and changes in form. Consistent with this view, epidermal growth is activated at the time of the molt and the generation and regeneration of other tissues often occurs in conjunction with molting. For example, molting and tissue growth are directly coupled in the development of myriapods, such as centipedes and millipedes. In these animals, as in some larval crustaceans, new posterior body segments are added at each molt so that the animal incrementally lengthens over development [173, 174]. In crustaceans and ametabolous insects, the regeneration of appendages is also coupled to the molt with lost limbs regrown over one or more successive molts (Fig. 4B) [175]. Conversely, loss of legs in these animals potently induces molting. Coordination of limb replacement with molting is also observed in hemimetabolous insects and in some holometabolous insects, such as beetles, that have legs in the larval form.
Even in larval Drosophila, in which the adult limb primordia grow inside the body as “imaginal discs,” repair of damaged leg discs is accommodated by extending the timing of the molt. Replacement of the appendages as a general rule is thus coordinated with replacement of the cuticle. In Drosophila, incorporation of the growing appendages into the body plan is also coordinated with the pupal molt, which occurs at the onset of metamorphosis. Indeed, the behaviors of pupal ecdysis are used not to shed the exoskeleton, but instead to deploy the adult legs and wings. As for other ecdysis events, pupal ecdysis follows a pulse of 20E (Fig. 5A). A subsequent surge of 20E governs the further histological transformations of metamorphosis. Ecdysis and metamorphosis thus both depend on ecdysone signaling in holometabolous insects.
Interestingly, metamorphosis in many deuterostomes, depends on thyroid hormone (TH), which is also implicated in the cyclic bouts of “molting” (i.e. the shedding of scales, feathers, or hair) seen in a variety of vertebrates [176]. Striking parallels between ecdysone and TH signaling in the control of insect and amphibian metamorphosis have been previously noted [177] and intriguing similarities can also be seen in the signaling pathways governing insect ecdysis and mammalian metabolic homeostasis by TH (Fig. 8). The ETHR of insects, for example, is closely related to the thyrotropin releasing hormone receptor (TRHR) governing thyroid stimulating hormone (TSH) release in the mammalian hypothalamic-pituitary-thyroid axis [64]. TSH and its receptor likewise share ancestral precursors with bursicon and its receptor. Both TSH and bursicon signaling pathways derive from the ancient family of cystine knot hormones and their leucine-rich repeat GPCRs, which play a variety of roles in development [178]. The ecdysone receptor itself belongs to the same subfamily of nuclear receptors as the TH receptor, and both receptors form functional heterodimers with receptors encoded by orthologous genes—USP and RXR, respectively [179]. It is tempting to speculate that these similarities in the intercellular circuits governing ecdysis and TH action may be the consequence of cell type evolution of the kind postulated by Arendt to give rise to hypothalamic-pituitary-gonadal axis [180]. At present, the evolutionary trajectories that might account for the extant pathways can only be conjectured, but a better understanding of the evolution of both ecdysis and TH signaling are likely to illuminate this point.
Figure 8: Molecular signaling in ecdysis and the mammalian hypothalamic-pituitary-thyroid axis.
The mammalian H-P-T axis regulates growth and metabolic homeostasis via a canonical signaling pathway. Thryotropin Releasing Hormone (TRH) from hypothalamic neurons acts via its receptor (TRHR) on pituitary thyrotropes to cause the release of Thyroid Stimulating Hormone (TSH). TSH (a heterodimeric glycoprotein with α and β subunits) binds to receptors (TSHR) in the thyroid gland to stimulate thyroid hormone (TH) production. TH acts via a heterodimeric nuclear receptor consisting of TR and RXR subunits, which are expressed in many tissues of the body including the in the hypothalamic neurons that make TRH. TH thus provides negative feedback to maintain metabolic homeostasis. In insects, such as Drosophila, ecdysis also involves the coordination of metabolism and growth. While not a homeostatic process, it involves alternating cycles of ecdysone production (by the prothoracic gland) corresponding to periods of growth, which are briefly interrupted by shedding of the exoskeleton and renewed growth. Shedding is mediated by ETH and bursicon and the organization of signaling in the ecdysis pathway is similar to that of the H-P-T axis. Evolutionarily related signaling molecules act at analogous points in the two pathways. For example, the subunits of bursicon are paralogs of ancestral α and β subunits that gave rise to the TSH subunits; the closest homolog of Drosophila ETHR is human TRHR; DLGR2 (the bursicon receptor) belongs to the same family of long-leucine-rich repeat GPCRs as TSHR; and EcR and TR belong to subfamily 1 of the nuclear receptors, while their binding partners (USP and RXR) are orthologous. Whether this similarity in organization reflects variations on the organization of an ancestral signaling pathway involved in coordinating growth and/or metabolism remains to be determined. (Images were obtained from http://phylopic.org.)
Part IV: Conclusion
Summary: toolkit genes for behavioral evolution
Some 160 years after Darwin suggested that animal behaviors evolve by natural selection, the nature of the genetic changes that drive behavioral evolution remain obscure. Fortunately, advances in our understanding of how nervous systems generate behavior—and the role genes play in nervous system development and operation—have set the stage for rapid progress. New methods in genetics, genomics, neuroscience, and behavioral analysis are beginning to reveal the kinds of mutations that govern adaptive behavioral change. A major target of mutation revealed by recent studies is genes involved in neuromodulatory signaling. This is unsurprising in that neuromodulators endow neurons and neural circuits with flexibility of function. Individual variation in the degree or timing of neuromodulatory signaling can thus serve as a target for selection. Alternatively, mutations in neuromodulatory signaling that alter which circuits are active under given circumstances can introduce new behavioral variants upon which natural selection can act. In the latter case, behavioral programs can be altered in a manner reminiscent of how developmental programs are altered to generate novelty and diversity in morphology, as revealed by studies in Evo-Devo.
The potential of altered neuromodulatory signaling to drive evolutionary behavioral change—even in the absence of developmental changes—has been previously emphasized by Katz and others [50, 52]. We suggest that genes encoding components of neuromodulatory signaling—principally GPCRs and their ligands (or their biosynthetic enzymes)—constitute a possible set of operational “toolkit genes” for behavioral evolution, analogous to the developmental toolkit genes responsible for morphological evolution. The gene products of both toolkits mediate intercellular signaling and evolved to serve the demands of multicellularity in metazoans, but while those of the Evo-Devo toolkit regulate gene transcription to control developmental programs [181], those involved in GPCR signaling typically regulate cellular physiology to control behavioral programs.
Ecdysis as a model for behavioral evolution
While a variety of behaviors are likely to provide good entry points to the study of behavioral evolution [25, 29, 182, 183], the behavioral programs used by ecdysozoans to shed their exoskeletons are uniquely suited for examining the role of neuromodulatory signaling in this process. Ecdysis behaviors also permit comparative analysis at multiple phylogenetic scales within a clade of diverse animals. Using tools such as those described above, a systematic program for analyzing the roles of neuromodulators in ecdysis can be pursued. Importantly, the simple presence or absence of hormonal signaling systems underlying ecdysis can be determined by genomic analysis alone. This strategy has already been widely exploited to examine ecdysozoan genomes for known signaling molecules in ecdysis [154, 155, 184-186]. Transcriptomics analysis of the genes encoding such molecules has been further used to reveal temporal patterns of expression of relevant signaling genes, again providing evidence that supports (or refutes) conclusions about whether these genes are used in ecdysis [130-132, 134, 187]. In principle, further candidate neuromodulatory genes can be identified by conducting comprehensive transcriptomics analyses across the molt cycle(s) of an animal. Single cell transcriptomics and in situ hybridization can then be used to localize expression of signaling genes to particular cells in the brain [106, 120]. RNAi, CRISPR and more refined genetic tools for targeted manipulation of function can be used conduct loss- and gain-of-function experiments to probe the roles of specific genes and classes of neurons in ecdysis behaviors. Peptide-specific antibodies can be used to examine profiles of release to identify correlations with ecdysis behaviors. Emerging methods for behavioral analysis should facilitate scoring the relatively stereotyped motor patterns that comprise most ecdysis sequences and identifying differences in behavioral execution between different species, hybrid offspring, and/or mutant animals [188, 189]. Whenever behavioral differences—particularly between closely related species—are found to be linked to specific neuromolutatory genes, the relevant structural or regulatory changes in genomic sequence can be sought.
Neuromodulators can act from both inside and outside the neural circuits whose activity they influence (Fig. 9A) [190, 191]. These circuits, in turn, may be responsible for organizing behavioral output at a variety of levels, from muscle contractions to organized motor sequences (Fig. 9B) [189]. Examples from the previous section make it clear that neuromodulation acts at all levels of the behavioral hierarchy to broadly shape ecdysis behavior. Some of the types of change in ecdysis behavior that might be anticipated in response to evolutionary changes in neuromodulatory signaling are summarized in Fig. 9. Insofar as neuromodulators exercise control over which movements are incorporated into ecdysis motor programs, and which motor programs are recruited to different phases of an ecdysis sequence, it should be possible to gain insight into how these sequences are assembled by nervous systems. From this analysis, broader insights into the mechanisms of behavioral evolution are likely to emerge. For example, neuromodulation has been proposed to favor the independent emergence of the same behavior in phylogenetically related taxa by enabling the repeated recruitment of homologous motor circuits [52]. Such parallel evolution might be expected in ecdysis networks, given the modular control neuromodulators appear to exercise over the composition and occurrence of different ecdysis motor programs. Like the extensively studied swimming behavior of opisthobranch mollusks [44], ecdysis may thus offer a possible test of the hypothesis that neuromodulation facilitates the evolution of homoplasious behaviors. As suggested in Fig. 8, analysis of ecdysis evolution may also reveal deep homologies at the level of metamodulatory circuits. Neuromodulatory networks, such as those underlying ecdysis, may share an ancestry with other networks that regulate basic physiological and reproductive processes in other taxa.
Figure 9: Possible mechanisms of ecdysis evolution based on hierarchies of neuromodulator action and behavior.
A) Neuromodulation of synaptically-connected circuits has been divided into three types based on whether the source of neuromodulator (red cell) lies within the circuit (“Intrinsic neuromodulation”), outside the circuit (“Extrinsic neuromodulation”), or upstream of another neuromodulatory cell (blue circle) that acts on the circuit (“metamodulation). Circles; synaptically connected neurons. Sticks with flat and ball endings; inhibitory and excitatory synapses, respectively.
B) Left column: Hierarchy of motor components in behavior, increasing in complexity from muscle contractions to goal-directed sequences. At each level, the motor components are composites of components found in levels above. Middle column: examples of ecdysis motor components and their neuromodulatory control, showing that neuromodulators shape ecdysis behavior at all levels. Right column: examples of how behavior at each level might change with evolutionary changes in neuromodulator action of different types.
Ecdysis: behavioral evolution at the intersection of Evo-Devo
There has been a long-standing hope that the field of Evo-Devo will help illuminate the mechanisms of behavioral evolution [11, 25, 182, 192, 193]. Examples are no doubt forthcoming as the attractive idea that behavior evolves via changes in the developmental programs that build neural circuits continues to be elaborated in productive ways [27]. We have proposed that the evolution of ecdysis likely owes more to changes in the operation than the development of the nervous system, but ecdysis is behavior in the service of development and it is intimately coupled to the removal of a morphological feature—the cuticle—which is the product of a developmental program. Ecdysis thus naturally integrates behavioral evolution with morphological evolution as traditionally studied in the discipline of Evo-Devo. Underlying links between behavioral and morphological evolution are indeed evident in the molecular mechanisms that govern cuticle development and ecdysis behaviors. Ecdysone, for example, plays the dual role of reactivating epidermal growth and cuticle secretion, while also preparing cells that make and respond to ETH for their behavioral functions. It has been postulated that ancestrally ecdysone alone may have coordinated insect ecdysis, with ETH and other hormones added later in evolution to augment flexibility of control and robustness of execution [84]. The pairing of nuclear receptor signaling—an element of the Evo-Devo toolkit—with GPCR-mediated signaling—an element of what we propose to be the toolkit for behavioral evolution—is consistent with the hypothesis that behavior itself was added on to a purely physiological process of shedding the integument.
It is worth pointing out in closing that Darwin conceived of behavioral evolution, like morphological evolution, as a gradual process, changing only in increments. With the advent of Evo-Devo came the recognition that mutations in developmental programs could, in principle, generate large-scale morphological variants—so-called "hopeful monsters.” If, as seems likely, changes in neuromodulatory signaling similarly can alter behavioral programs to drive behavioral evolution, it is possible that behavioral change in some instances has been saltatory rather than incremental. One thing is certain: the evolution of mechanisms for wresting behavioral production from the genome has endowed behavior with a degree of plasticity not seen in other biological phenotypes and has accelerated the emergence of novelty—as evinced most spectacularly in the behavioral repertoire of humans—to a degree not seen anywhere else. Gaining a better understanding of the mechanisms underlying behavioral evolution will likely provide us with tools for better understanding ourselves.
Acknowledgements
The authors thank the Intramural Research Program of NIMH for funding (Project ZIA MH002800-20 to BHW). We would also like to thank the FEBS Journal editors for their kind invitation to write this review and their patience on what proved to be a very extended timeline for completion. We are also grateful to the two anonymous referees for their thoughtful comments on the manuscript and constructive suggestions for improving it.
Abbreviations:
- GPCR
G-protein coupled receptor
- CPG
Central Pattern Generator
- AVP
Arginine Vasopressin
- 5-HT
Serotonin
- per
period gene
- 20E
20-hydroxyecdysone
- ETH
Ecdysis Triggering Hormone
- EH
Eclosion Hormone
- EcR
Ecdysis Receptor
- CCAP
Crustacean Cardioactive Peptide
- MIPs
Myoinhibitory Peptides
- AlstCC
Allatostatin DoubleC
- CHH
Crustacean Hyperglycemic Hormone
- ITP
Ion Transport Peptide
- TH
Thyroid Hormone
- TSH
Thyroid Stimulating Hormone
- TRH
Thryotropin-releasing Hormone
- LK
Leucokinin
- Crz
Corazonin
- QTL
Quantitative Trait Loci
- Vm
Ventromedial neurons
- EMN
Electromotor neurons
Footnotes
Conflicts of Interest: the authors cite no conflicts of interest
References
- 1.Darwin C (1859) On the origin of species by means of natural selection, or, The preservation of favoured races in the struggle for life, Murray J, London. [PMC free article] [PubMed] [Google Scholar]
- 2.Maynard Smith J (1982) Evolution and the theory of games, Cambridge University Press, Cambridge ; New York. [Google Scholar]
- 3.Tinbergen N (1951) The Study of Instinct, Oxford University Press, London. [Google Scholar]
- 4.Arguello JR & Benton R (2017) Open questions: Tackling Darwin's "instincts": the genetic basis of behavioral evolution, Bmc Biology. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shubin N, Tabin C & Carroll S (2009) Deep homology and the origins of evolutionary novelty, Nature. 457, 818–823. [DOI] [PubMed] [Google Scholar]
- 6.Erwin DH (2020) Evolutionary dynamics of gene regulation, Gene Regulatory Networks. 139, 407–431. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel JW (1992) Behavioral Homology and Phylogeny, Annu Rev Ecol Syst. 23, 361–381. [Google Scholar]
- 8.de Queiroz A & Wimberger PH (1993) The Usefulness of Behavior for Phylogeny Estimation: Levels of Homoplasy in Behavioral and Morphological Characters, Evolution. 47, 46–60. [DOI] [PubMed] [Google Scholar]
- 9.Price JJ, Clapp MK & Omland KE (2011) Where have all the trees gone? The declining use of phylogenies in animal behaviour journals, Animal Behaviour. 81, 667–670. [Google Scholar]
- 10.Atz JW (1970) The application of the idea of homology to behavior in Development and the evolution of behavior Essays in memory of T C Schneirla (Aronson LR, Tobach E, Lehrman DS & Rosenblatt JS, eds) pp. 53–74, Freeman, San Francisco. [Google Scholar]
- 11.Brown RL (2014) Identifying Behavioral Novelty, Biological Theory. 9, 135–148. [Google Scholar]
- 12.Benzer S (1971) From Gene to Behavior, Journal of the American Medical Association. 218, 1015-&.4942064 [Google Scholar]
- 13.Brenner S (1974) Genetics of Caenorhabditis elegans, Genetics. 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan RJ (2008) The origins of behavioral genetics, Current Biology. 18, R192–R198. [DOI] [PubMed] [Google Scholar]
- 15.Hall JC (1982) Genetics of the Nervous-System in Drosophila, Q Rev Biophys. 15, 223–479. [DOI] [PubMed] [Google Scholar]
- 16.Fischbach KF & Heisenberg M (1984) Neurogenetics and Behavior in Insects, Journal of Experimental Biology. 112, 65–93. [Google Scholar]
- 17.Greenspan RJ (1997) A kinder, gentler genetic analysis of behavior: dissection gives way to modulation, Current Opinion in Neurobiology. 7, 805–811. [DOI] [PubMed] [Google Scholar]
- 18.Wieschaus E & Nusslein-Volhard C (2016) The Heidelberg Screen for Pattern Mutants of Drosophila: A Personal Account, Annual Review of Cell and Developmental Biology, Vol 32. 32, 1–46. [DOI] [PubMed] [Google Scholar]
- 19.Nusslein-Volhard C (1995) Christiane Nüsslein-Volhard – Nobel Lecture. in Nobel Lectures, Physiology or Medicine, https://www.nobelprize.org/prizes/medicine/1995/nusslein-volhard/lecture/, [Google Scholar]
- 20.Carroll SB (2008) Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution, Cell. 134, 25–36. [DOI] [PubMed] [Google Scholar]
- 21.Reilly MB, Cros C, Varol E, Yemini E & Hobert O (2020) Unique homeobox codes delineate all the neuron classes ofC. elegans, Nature. 584, 595-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connell LA (2013) Evolutionary Development of Neural Systems in Vertebrates and Beyond, Journal of Neurogenetics. 27, 69–85. [DOI] [PubMed] [Google Scholar]
- 23.Tosches MA (2017) Developmental and genetic mechanisms of neural circuit evolution, Developmental Biology. 431, 16–25. [DOI] [PubMed] [Google Scholar]
- 24.Hoke KL, Adkins-Regan E, Bass AH, McCune AR & Wolfner MF (2019) Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity, Journal of Experimental Biology. 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo DH, De-Miguel FF, Heath-Heckman EAC, Szczupak L, Todd K, Weisblat DA & Winchell CJ (2020) A tale of two leeches: Toward the understanding of the evolution and development of behavioral neural circuits, Evol Dev. 22, 471–493. [DOI] [PubMed] [Google Scholar]
- 26.Stern DL & Orgogozo V (2008) The loci of evolution: How predictable is genetic evolution ?, Evolution. 62, 2155–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson GE & Barron AB (2017) Epigenetics and the evolution of instincts, Science. 356, 26–27. [DOI] [PubMed] [Google Scholar]
- 28.Niepoth N & Bendesky A (2020) How Natural Genetic Variation Shapes Behavior, Annu Rev Genom Hum G. 21, 437–463. [DOI] [PubMed] [Google Scholar]
- 29.Jourjine N & Hoekstra HE (2021) Expanding evolutionary neuroscience : insights from comparing variation in behavior & nbsp;, Neuron. 109, 1084–1099. [DOI] [PubMed] [Google Scholar]
- 30.Bendesky A & Bargmann CI (2011) Genetic contributions to behavioural diversity at the gene-environment interface, Nat Rev Genet. 12, 809–820. [DOI] [PubMed] [Google Scholar]
- 31.Auer TO, Khallaf MA, Silbering AF, Zappia G, Ellis K, Alvarez-Ocana R, Arguello JR, Hansson BS, Jefferis GSXE, Caron SJC, Knaden M & Benton R (2020) Olfactory receptor and circuit evolution promote host specialization, Nature. 579, 402-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao SQ, Peterson BK, He MX, Dulac C & Hoekstra HE (2017) The genetic basis of parental care evolution in monogamous mice, Nature. 544, 434-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Y, Berrocal A, Morita T, Longden KD & Stern DL (2016) Natural courtship song variation caused by an intronic retroelement in an ion channel gene, Nature. 536, 329-+. [DOI] [PubMed] [Google Scholar]
- 34.Lim MM, Wang ZX, Olazabal DE, Ren XH, Terwilliger EF & Young LJ (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene, Nature. 429, 754–757. [DOI] [PubMed] [Google Scholar]
- 35.Prieto-Godino LL, Rytz R, Cruchet S, Bargeton B, Abuin L, Silbering AF, Ruta V, Dal Peraro M & Benton R (2017) Evolution of Acid-Sensing Olfactory Circuits in Drosophilids, Neuron. 93, 661-+. [DOI] [PubMed] [Google Scholar]
- 36.Tamvacakis AN, Senatore A & Katz PS (2018) Single neuron serotonin receptor subtype gene expression correlates with behaviour within and across three molluscan species, Proceedings of the Royal Society B-Biological Sciences. 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson A, Infield DT, Smith AR, Smith GT, Ahern CA & Zakon HH (2018) Rapid evolution of a voltage-gated sodium channel gene in a lineage of electric fish leads to a persistent sodium current, Plos Biology. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillvis JL & Katz PS (2013) Parallel Evolution of Serotonergic Neuromodulation Underlies Independent Evolution of Rhythmic Motor Behavior, Journal of Neuroscience. 33, 2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaggard JB, Stahl BA, Lloyd E, Prober DA, Duboue ER & Keene AC (2018) Hypocretin underlies the evolution of sleep loss in the Mexican cavefish, Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alie A, Devos L, Torres-Paz J, Prunier L, Boulet F, Blin M, Elipot Y & Rataux S (2018) Developmental evolution of the forebrain in cavefish, from natural variations in neuropeptides to behavior, Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bumbarger DJ, Riebesell M, Rodelsperger C & Sommer RJ (2013) System-wide Rewiring Underlies Behavioral Differences in Predatory and Bacterial-Feeding Nematodes, Cell. 152, 109–119. [DOI] [PubMed] [Google Scholar]
- 42.Gunaratne CA, Sakurai A & Katz PS (2017) Variations on a theme: species differences in synaptic connectivity do not predict central pattern generator activity, Journal of Neurophysiology. 118, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newcomb JM & Katz PS (2009) Different functions for homologous serotonergic interneurons and serotonin in species-specific rhythmic behaviours, Proceedings of the Royal Society B-Biological Sciences. 276, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newcomb JM, Sakurai A, Lillvis JL, Gunaratne CA & Katz PS (2012) Homology and homoplasy of swimming behaviors and neural circuits in the Nudipleura (Mollusca, Gastropoda, Opisthobranchia), Proceedings of the National Academy of Sciences of the United States of America. 109, 10669–10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai A & Katz PS (2017) Artificial Synaptic Rewiring Demonstrates that Distinct Neural Circuit Configurations Underlie Homologous Behaviors, Current Biology. 27, 1721-+. [DOI] [PubMed] [Google Scholar]
- 46.Newcomb JM & Katz PS (2007) Homologues of serotonergic central pattern generator neurons in related nudibranch molluscs with divergent behaviors, Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 193, 425–443. [DOI] [PubMed] [Google Scholar]
- 47.Ding Y, Lilivis JL, Cande J, Berman GJ, Arthur BJ, Long X, Xu M, Dickson BJ & Stern DL (2019) Neural Evolution of Context-Dependent Fly Song, Current Biology. 29, 1089-+. [DOI] [PubMed] [Google Scholar]
- 48.Seeholzer LF, Seppo M, Stern DL & Ruta V (2018) Evolution of a central neural circuit underlies Drosophila mate preferences, Nature. 559, 564-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tierney AJ (1995) Evolutionary implications of neural circuit structure and function, Behav Processes. 35, 173–82. [DOI] [PubMed] [Google Scholar]
- 50.Katz PS & Lillvis JL (2014) Reconciling the deep homology of neuromodulation with the evolution of behavior, Current Opinion in Neurobiology. 29, 39–47. [DOI] [PubMed] [Google Scholar]
- 51.Katz PS & Harris-Warrick RM (1999) The evolution of neuronal circuits underlying species-specific behavior, Current Opinion In Neurobiology. 9, 628–633. [DOI] [PubMed] [Google Scholar]
- 52.Katz PS (2011) Neural mechanisms underlying the evolvability of behaviour, Philosophical Transactions of the Royal Society B-Biological Sciences. 366, 2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandra V, Fetter-Pruneda I, Oxley PR, Ritger AL, McKenzie SK, Libbrecht R & Kronauer DJC (2018) Social regulation of insulin signaling and the evolution of eusociality in ants, Science. 361, 398-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer EK, Roland AB, Moskowitz NA, Vidoudez C, Ranaivorazo N, Tapia EE, Trauger SA, Vences M, Coloma LA & O'Connell LA (2019) Mechanisms of Convergent Egg Provisioning in Poison Frogs, Curr Biol. 29, 4145–4151 e3. [DOI] [PubMed] [Google Scholar]
- 55.Insel TR & Shapiro LE (1992) Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles, Proc Natl Acad Sci U S A. 89, 5981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly AM & Goodson JL (2013) Behavioral relevance of species-specific vasotocin anatomy in gregarious finches, Front Neurosci. 7, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakurai A & Katz PS (2019) Command or Obey? Homologous Neurons Differ in Hierarchical Position for the Generation of Homologous Behaviors, Journal of Neuroscience. 39, 6460–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA & Bargmann CI (2011) Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes, Nature. 477, 321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bargmann CI (2012) Beyond the connectome: How neuromodulators shape neural circuits, Bioessays. 34, 458–465. [DOI] [PubMed] [Google Scholar]
- 60.Harris-Warrick RM & Marder E (1991) Modulation of neural networks for behavior, Annu Rev Neurosci. 14, 39–57. [DOI] [PubMed] [Google Scholar]
- 61.Marder E (2012) Neuromodulation of Neuronal Circuits: Back to the Future, Neuron. 76, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnan A & Schioth HB (2015) The role of G protein-coupled receptors in the early evolution of neurotransmission and the nervous system, Journal of Experimental Biology. 218, 562–571. [DOI] [PubMed] [Google Scholar]
- 63.Staubert C, Le Duc D & Schoneberg T (2014) Examining the Dynamic Evolution of G Protein-Coupled Receptors, Method Pharmacol Tox, 23–43. [Google Scholar]
- 64.Elphick MR, Mirabeau O & Larhammar D (2018) Evolution of neuropeptide signalling systems, Journal of Experimental Biology. 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heger P, Zheng W, Rottmann A, Panfilio KA & Wiehe T (2020) The genetic factors of bilaterian evolution, Elife. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nassel DR & Zandawala M (2020) Hormonal axes inDrosophila:regulation of hormone release and multiplicity of actions, Cell and Tissue Research. 382, 233–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoop R (2012) Neuromodulation by Oxytocin and Vasopressin, Neuron. 76, 142–159. [DOI] [PubMed] [Google Scholar]
- 68.Taghert PH & Nitabach MN (2012) Peptide neuromodulation in invertebrate model systems, Neuron. 76, 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Pol AN (2012) Neuropeptide Transmission in Brain Circuits, Neuron. 76, 98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nassel DR & Zandawala M (2022) Endocrine cybernetics: neuropeptides as molecular switches in behavioural decisions, Open Biol. 12, 220174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giribet G & Edgecombe GD (2017) Current Understanding of Ecdysozoa and its Internal Phylogenetic Relationships, Integrative and Comparative Biology. 57, 455–466. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Vannier J, Schumann I, Wang X, Yang XG, Komiya T, Uesugi K, Sun J & Han J (2019) Origin of ecdysis: fossil evidence from 535-million-year-old scalidophoran worms, Proceedings of the Royal Society B-Biological Sciences. 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang YF, Peng J, Wang DZ, Zhang H, Luo XC, Shao YB, Sun QY, Ling CC & Wang QJ (2021) Ontogenetic moulting behavior of the Cambrian oryctocephalid trilobite Arthricocephalites xinzhaiheensis, Peerj. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daley AC & Drage HB (2016) The fossil record of ecdysis, and trends in the moulting behaviour of trilobites, Arthropod Struct Dev. 45, 71–96. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Bellido DC & Collins DH (2004) Moulting arthropod caught in the act, Nature. 429, 40–40. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Ortega-Hernandez J, Drage HB, Du KS & Zhang XG (2019) Ecdysis in a stem-group euarthropod from the early Cambrian of China, Sci Rep-Uk. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elliott AD, Berndt A, Houpert M, Roy S, Scott RL, Chow CC, Shroff H & White BH (2021) Pupal behavior emerges from unstructured muscle activity in response to neuromodulation in Drosophila, Elife. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diao FC, Elliott AD, Diao FQ, Shah S & White BH (2017) Neuromodulatory connectivity defines the structure of a behavioral neural network, Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frantsevich L & Frantsevich L (2018) Leg deformation during imaginal ecdysis in the downy emerald, Cordulia aenea (Odonata, Corduliidae), Zoology. 127, 106–113. [DOI] [PubMed] [Google Scholar]
- 80.Pizzi R (2012) Spiders in Invertebrate Medicine (Lewbart GA, ed) pp. 187–221, John Wiley and Sons, Inc., New York. [Google Scholar]
- 81.Singh RN & Sulston JE (1978) Some Observations on Molting in Caenorhabditis-Elegans, Nematologica. 24, 63-&. [Google Scholar]
- 82.Phlippen MK, Webster SG, Chung JS & Dircksen H (2000) Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph, Journal Of Experimental Biology. 203, 521–536. [DOI] [PubMed] [Google Scholar]
- 83.White BH & Ewer J (2014) Neural and Hormonal Control of Postecdysial Behaviors in Insects, Annual Review of Entomology, Vol 59, 2014. 59, 363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Truman JW (2005) Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology, Vitam Horm. 73, 1–30. [DOI] [PubMed] [Google Scholar]
- 85.Zitnan D & Adams ME (2012) Neuroendocrine regulation of ecdysis in Insect Endocrinology (Gilbert LI, ed) pp. 253–309, Elsevier. [Google Scholar]
- 86.Zitnan D, Kim YJ, Zitnanova I, Roller L & Adams ME (2007) Complex steroid-peptide-receptor cascade controls insect ecdysis, Gen Comp Endocrinol. 153, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ewer J & Reynolds S (2002) Neuropeptide Control of Molting in Insects in Hormones, Brain, and Behavior (Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM & Rubin RT, eds) pp. 1–92, Elsevier Science (USA), San Diego. [Google Scholar]
- 88.Hughes TD (1980) Imaginal Ecdysis Of The Desert Locust, Schistocerca-Gregaria .1. Description Of The Behavior, Physiological Entomology. 5, 47–54. [Google Scholar]
- 89.Carlson JR & Bentley D (1977) Ecdysis - Neural Orchestration Of A Complex Behavioral Performance, Science. 195, 1006–1008. [DOI] [PubMed] [Google Scholar]
- 90.Hughes TD (1980) Imaginal Ecdysis Of The Desert Locust, Schistocerca-Gregaria .2. Motor-Activity Underlying The Pre-Emergence And Emergence Behavior, Physiological Entomology. 5, 55–71. [Google Scholar]
- 91.Kim DH, Han MR, Lee G, Lee SS, Kim YJ & Adams ME (2015) Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling, PLoS Genet. 11, e1005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mena W, Diegeimann S, Wegener C & Ewer J (2016) Stereotyped responses of Drosophila peptidergic neuronal ensemble depend on down stream neuromodulators, Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frand AR, Russel S & Ruvkun G (2005) Functional genomic analysis of C-elegans molting, Plos Biology. 3, 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazetic V & Fay DS (2017) Molting in C. elegans, Worm. 6, e1330246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barsotti E, Correia A & Cardona A (2021) Neural architectures in the light of comparative connectomics, Curr Opin Neurobiol. 71, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tihelka E, Cai CY, Giacomelli M, Lozano-Fernandez J, Rota-Stabelli O, Huang DY, Engel MS, Donoghue PCJ & Pisani D (2021) The evolution of insect biodiversity, Current Biology. 31, R1299–R1311. [DOI] [PubMed] [Google Scholar]
- 97.Watson JAL (1964) Moulting and Reproduction in the Adult Firebrat, Thermobia-Domestica (Packard) (Thysanura, Lepismatidae) .1. The Moulting Cycle and Its Control, Journal of Insect Physiology. 10, 305–317. [Google Scholar]
- 98.Ampleford EJ & Steel CGH (1982) The Behavior of Rhodnius prolixus (Stal) during the Imaginal Ecdysis, Canadian Journal of Zoology-Revue Canadienne De Zoologie. 60, 168–174. [Google Scholar]
- 99.Lee D, Orchard I & Lange AB (2013) Evidence for a conserved CCAP-signaling pathway controlling ecdysis in a hemimetabolous insect, Rhodnius prolixus, Front Neurosci. 7, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee D, Vanden Broeck J & Lange AB (2013) Identification and expression of the CCAP receptor in the Chagas' disease vector, Rhodnius prolixus, and its involvement in cardiac control, PLoS One. 8, e68897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wadsworth T, Carriman A, Gutierrez AA, Moffatt C & Fuse M (2014) Ecdysis behaviors and circadian rhythm of ecdysis in the stick insect, Carausius morosus, Journal of Insect Physiology. 71, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roller L, Zitnanova I, Dai L, Simo L, Park Y, Satake H, Tanaka Y, Adams ME & Zitnan D (2010) Ecdysis triggering hormone signaling in arthropods, Peptides. 31, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scott RL, Diao FQ, Silva V, Park S, Luan HJ, Ewer J & White BH (2020) Non-canonical Eclosion Hormone-Expressing Cells Regulate Drosophila Ecdysis, Iscience. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ewer J & Truman JW (1996) Increases in cyclic 3', 5'-guanosine monophosphate (cGMP) occur at ecdysis in an evolutionarily conserved crustacean cardioactive peptide-immunoreactive insect neuronal network, J Comp Neurol. 370, 330–41. [DOI] [PubMed] [Google Scholar]
- 105.Sharp JH, Wilcockson DC & Webster SG (2010) Identification and expression of mRNAs encoding bursicon in the plesiomorphic central nervous system of Homarus gammarus, General and Comparative Endocrinology. 169, 65–74. [DOI] [PubMed] [Google Scholar]
- 106.Wilcockson DC & Webster SG (2008) Identification and developmental expression of mRNAs encoding putative insect cuticle hardening hormone, bursicon in the green shore crab Carcinus maenas, General and Comparative Endocrinology. 156, 113–125. [DOI] [PubMed] [Google Scholar]
- 107.Kruger E, Mena W, Lahr EC, Johnson EC & Ewer J (2015) Genetic analysis of Eclosion hormone action during Drosophila larval ecdysis, Development. 142, 4279–4287. [DOI] [PubMed] [Google Scholar]
- 108.Kim YJ, Spalovska-Valachova I, Cho KH, Zitnanova I, Park Y, Adams ME & Zitnan D (2004) Corazonin receptor signaling in ecdysis initiation, Proc Natl Acad Sci U S A. 101, 6704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ & Park Y (2008) Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum, Mechanisms of Development. 125, 984–995. [DOI] [PubMed] [Google Scholar]
- 110.Shen CH, Jin L, Fu KY, Guo WC & Li GQ (2021) Crustacean cardioactive peptide as a stimulator of feeding and a regulator of ecdysis in Leptinotarsa decemlineata, Pestic Biochem Phys. 175. [DOI] [PubMed] [Google Scholar]
- 111.Lahr EC, Dean D & Ewer J (2012) Genetic Analysis of Ecdysis Behavior in Drosophila Reveals Partially Overlapping Functions of Two Unrelated Neuropeptides, Journal of Neuroscience. 32, 6819–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wulff JP, Sierra I, Sterkel M, Holtof M, Van Wielendaele P, Francini F, Vanden Broeck J & Ons S (2017) Orcokinin neuropeptides regulate ecdysis in the hemimetabolous insect Rhodnius prolixus, Insect Biochem Molec. 81, 91–102. [DOI] [PubMed] [Google Scholar]
- 113.Jiang HB, Kim HG & Park Y (2015) Alternatively spliced orcokinin isoforms and their functions in Tribolium castaneum, Insect Biochem Molec. 65, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silva V, Palacios-Munoz A, Volonte M, Frenkel L, Ewer J & Ons S (2021) Orcokinin neuropeptides regulate reproduction in the fruit fly, Drosophila melanogaster, Insect Biochem Molec. 139. [DOI] [PubMed] [Google Scholar]
- 115.Svensson E, Apergis-Schoute J, Burnstock G, Nusbaum MP, Parker D & Schioth HB (2019) General Principles of Neuronal Co-transmission: Insights From Multiple Model Systems, Front Neural Circuit. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jekely G, Melzer S, Beets I, Kadow ICG, Koene J, Haddad S & Holden-Dye L (2018) The long and the short of it - a perspective on peptidergic regulation of circuits and behaviour, Journal of Experimental Biology. 221. [DOI] [PubMed] [Google Scholar]
- 117.Dudas B & Merchenthaler I (2021) Morphology and distribution of hypothalamic peptidergic systems, Handb Clin Neurol. 179, 67–85. [DOI] [PubMed] [Google Scholar]
- 118.Sternson SM & Eiselt AK (2017) Three Pillars for the Neural Control of Appetite, Annual Review of Physiology, Vol 79. 79, 401–423. [DOI] [PubMed] [Google Scholar]
- 119.Thirumalai V & Marder E (2002) Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets, Journal of Neuroscience. 22, 1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Daubnerova I, Roller L, Satake H, Zhang C, Kim YJ & Zitnan D (2021) Identification and function of ETH receptor networks in the silkworm Bombyx mori, Sci Rep-Uk. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diao FC, Mena W, Shi J, Park D, Diao FQ, Taghert P, Ewer J & White BH (2016) The Splice Isoforms of the Drosophila Ecdysis Triggering Hormone Receptor Have Developmentally Distinct Roles, Genetics. 202, 175-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim YJ, Zitnan D, Cho KH, Schooley DA, Mizoguchi A & Adams ME (2006) Central peptidergic ensembles associated with organization of an innate behavior, Proc Natl Acad Sci U S A. 103, 14211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim YJ, Zitnan D, Galizia CG, Cho KH & Adams ME (2006) A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles, Curr Biol. 16, 1395–407. [DOI] [PubMed] [Google Scholar]
- 124.Mesquita RD Vionette-Amaral RJ Lowenberger C Rivera-Pomar R Monteiro FA Minx P Spieth J Carvalho AB Panzera F Lawson D Torres AQ Ribeiro JM Sorgine MH Waterhouse RM Montague MJ Abad-Franch F Alves-Bezerra M Amaral LR Araujo HM Araujo RN Aravind L Atella GC Azambuja P Berni M Bittencourt-Cunha PR Braz GR Calderon-Fernandez G Carareto CM Christensen MB Costa IR Costa SG Dansa M Daumas-Filho CR De-Paula IF Dias FA Dimopoulos G Emrich SJ Esponda-Behrens N Fampa P Fernandez-Medina RD da Fonseca RN Fontenele M Fronick C Fulton LA Gandara AC Garcia ES Genta FA Giraldo-Calderon GI Gomes B Gondim KC Granzotto A Guarneri AA Guigo R Harry M Hughes DS Jablonka W Jacquin-Joly E Juarez MP Koerich LB Lange AB Latorre-Estivalis JM Lavore A Lawrence GG Lazoski C Lazzari CR Lopes RR Lorenzo MG Lugon MD Majerowicz D Marcet PL Mariotti M Masuda H Megy K Melo AC Missirlis F Mota T Noriega FG Nouzova M Nunes RD Oliveira RL Oliveira-Silveira G Ons S Orchard I Pagola L Paiva-Silva GO Pascual A Pavan MG Pedrini N Peixoto AA Pereira MH Pike A Polycarpo C Prosdocimi F Ribeiro-Rodrigues R Robertson HM Salerno AP Salmon D Santesmasses D Schama R Seabra-Junior ES, et al. (2015) Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection, Proc Natl Acad Sci U S A. 112, 14936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Verlinden H, Sterck L, Li J, Li Z, Yssel A, Gansemans Y, Verdonck R, Holtof M, Song H, Behmer ST, Sword GA, Matheson T, Ott SR, Deforce D, Van Nieuwerburgh F, Van de Peer Y & Vanden Broeck J (2020) First draft genome assembly of the desert locust, Schistocerca gregaria, F1000Res. 9, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brand P, Robertson HM, Lin W, Pothula R, Klingeman WE, Jurat-Fuentes JL & Johnson BR (2018) The origin of the odorant receptor gene family in insects, Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Webster SG (2015) Endocrinology of Molting in Physiology (Chang ES & Thiel M, eds), Oxford University Press. [Google Scholar]
- 128.Travis DF (1954) The Molting Cycle of the Spiny Lobster, Panulirus-Argus Latreille .1. Molting and Growth in Laboratory Maintained Individuals, Biol Bull. 107, 433–450. [Google Scholar]
- 129.Taylor JRA & Kier WM (2003) Switching skeletons: Hydrostatic support in molting crabs, Science. 301, 209–210. [DOI] [PubMed] [Google Scholar]
- 130.Mykles DL (2021) Signaling Pathways That Regulate the Crustacean Molting Gland, Front Endocrinol. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mykles DL & Chang ES (2020) Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics, General and Comparative Endocrinology. 294. [DOI] [PubMed] [Google Scholar]
- 132.Oliphant A, Alexander JL, Swain MT, Webster SG & Wilcockson DC (2018) Transcriptomic analysis of crustacean neuropeptide signaling during the moult cycle in the green shore crab, Carcinus maenas, Bmc Genomics. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Das S, Vraspir L, Zhou W, Durica DS & Mykles DL (2018) Transcriptomic analysis of differentially expressed genes in the molting gland (Y-organ) of the blackback land crab, Gecarcinus lateralis, during molt-cycle stage transitions, Comp Biochem Phys D. 28, 37–53. [DOI] [PubMed] [Google Scholar]
- 134.Tran NM, Mykles DL, Elizur A & Ventura T (2019) Characterization of G-protein coupled receptors from the blackback land crab Gecarcinus lateralis Y organ transcriptome over the molt cycle, Bmc Genomics. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veenstra JA (2016) Similarities between decapod and insect neuropeptidomes, Peerj. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Webster SG, Wilcockson DC, Mrinalini & Sharp JH (2013) Bursicon and neuropeptide cascades during the ecdysis program of the shore crab, Carcinus maenas, General and Comparative Endocrinology. 182, 54–64. [DOI] [PubMed] [Google Scholar]
- 137.Zhao YF, Wen QQ, Ao CM, Wang W, Shi LL, Wang CG & Chan SF (2022) Ecdysis Triggering Hormone, Eclosion Hormone, and Crustacean Cardioactive Peptide Play Essential but Different Roles in the Molting Process of Mud Crab, Scylla paramamosain, Front Mar Sci. 9. [Google Scholar]
- 138.Hyde CJ, Nguyen T, Fitzgibbon QP, Elizur A, Smith GG & Ventura T (2020) Neural remodelling in spiny lobster larvae is characterized by broad neuropeptide suppression, General and Comparative Endocrinology. 294. [DOI] [PubMed] [Google Scholar]
- 139.Nhut TM, Mykles DL, Elizur A & Ventura T (2020) Ecdysis triggering hormone modulates molt behaviour in the redclaw crayfish Cherax quadricarinatus, providing a mechanistic evidence for conserved function in molt regulation across Pancrustacea, General and Comparative Endocrinology. 298. [DOI] [PubMed] [Google Scholar]
- 140.Zhou LH, Li SH, Wang ZW, Li FH & Xiang JH (2017) An eclosion hormone-like gene participates in the molting process of Palaemonid shrimp Exopalaemon carinicauda, Development Genes and Evolution. 227, 189–199. [DOI] [PubMed] [Google Scholar]
- 141.Chen HY, Toullec JY & Lee CY (2020) The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade, Front Endocrinol. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chung JS, Dircksen H & Webster SG (1999) A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas, Proceedings of the National Academy of Sciences of the United States of America. 96, 13103–13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Galikova M, Dircksen H & Nassel DR (2018) The thirsty fly: Ion transport peptide (ITP) is a novel endocrine regulator of water homeostasis in Drosophila, Plos Genetics. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zilberstein Y, Ewer J & Ayali A (2006) Neuromodulation of the locust frontal ganglion during the moult: a novel role for insect ecdysis peptides, Journal Of Experimental Biology. 209, 2911–2919. [DOI] [PubMed] [Google Scholar]
- 145.Peabody NC, Diao F, Luan H, Wang H, Dewey EM, Honegger HW & White BH (2008) Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death, J Neurosci. 28, 14379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zitnan D, Ross LS, Zitnanova I, Hermesman JL, Gill SS & Adams ME (1999) Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence, Neuron. 23, 523–35. [DOI] [PubMed] [Google Scholar]
- 147.Zitnan D, Hollar L, Spalovska I, Takac P, Zitnanova I, Gill SS & Adams ME (2002) Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori, J Exp Biol. 205, 3459–73. [DOI] [PubMed] [Google Scholar]
- 148.Weeks JC & Truman JW (1984) Neural Organization of Peptide-activated Ecdysis Behaviors During the Metamorphosis of Manduca Sexta.1. Conservation of the Peristalsis Motor Pattern at the Larval-Pupal Transformation, Journal Of Comparative Physiology. 155, 407–422. [Google Scholar]
- 149.Vogt G (2018) Investigating the genetic and epigenetic basis of big biological questions with the parthenogenetic marbled crayfish: A review and perspectives, J Biosciences. 43, 189–223. [PubMed] [Google Scholar]
- 150.Schwentner M, Combosch DJ, Nelson JP & Giribet G (2017) A Phylogenomic Solution to the Origin of Insects by Resolving Crustacean-Hexapod Relationships, Current Biology. 27, 1818-+. [DOI] [PubMed] [Google Scholar]
- 151.Gaban RD & Farley RD (2002) Ecdysis in scorpions: supine behavior and exuvial ultrastructure, Invertebr Biol. 121, 136–147. [Google Scholar]
- 152.Hagstrum DW (1968) Molting Behavior of Black Widow Spider Latrodectus Mactans, Ann Entomol Soc Am. 61, 591-&. [Google Scholar]
- 153.Tetlie OE, Brandt DS & Briggs DEG (2008) Ecdysis in sea scorpions (Chelicerata : Eurypterida), Palaeogeogr Palaeocl. 265, 182–194. [Google Scholar]
- 154.Schumann I, Kenny N, Hui J, Hering L & Mayer G (2018) Halloween genes in panarthropods and the evolution of the early moulting pathway in Ecdysozoa, R Soc Open Sci. 5, 180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Oliveira APL, Calcino A & Wanninger A (2019) Ancient origins of arthropod moulting pathway components, Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu PJ (2007) Dauer, WormBook, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flavell SW, Raizen DM & You YJ (2020) Behavioral States, Genetics. 216, 315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van der Auwera P, Frooninckx L, Buscemi K, Vance RT, Watteyne J, Mirabeau O, Temmerman L, De Haes W, Fancsalszky L, Gottschalk A, Raizen DM, Nelson MD, Schoofs L & Beets I (2020) RPamide neuropeptides NLP-22 and NLP-2 act through GnRH-like receptors to promote sleep and wakefulness in C. elegans, Sci Rep-Uk. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Turek M, Besseling J, Spies JP, Konig S & Bringmann H (2016) Sleep-active neuron specification and sleep induction require FLP-11 neuropeptides to systemically induce sleep, Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Niu LG, Li Y, Zong PY, Liu P, Shui Y, Chen BJ & Wang ZW (2020) Melatonin promotes sleep by activating the BK channel in C. elegans, Proceedings of the National Academy of Sciences of the United States of America. 117, 25128–25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nelson MD, Trojanowski NF, George-Raizen JB, Smith CJ, Yu CC, Fang-Yen C & Raizen DM (2013) The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans, Nature Communications. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang HY, Zhu CT, Skuja LL, Hayden DJ & Hart AC (2017) Genome-Wide Screen for Genes Involved in Caenorhabditis elegans Developmentally Timed Sleep, G3-Genes Genom Genet. 7, 2907–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Honer M, Buscemi K, Barrett N, Riazati N, Orlando G & Nelson MD (2020) Orcokinin neuropeptides regulate sleep inCaenorhabditis elegans, Journal of Neurogenetics. 34, 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reinecke JP, Buckner JS & Grugel SR (1980) Life-Cycle of Laboratory-Reared Tobacco Hornworms, Manduca-Sexta - Study of Development and Behavior, Using Time-Lapse Cinematography, Biol Bull. 158, 129–140. [Google Scholar]
- 165.MacWilliam D, Arensburger P, Higa J, Cui XP & Adams ME (2015) Behavioral and genomic characterization of molt-sleep in the tobacco hornworm, Manduca sexta, Insect Biochem Molec. 62, 154–167. [DOI] [PubMed] [Google Scholar]
- 166.Jeon M, Gardner HF, Miller EA, Deshler J & Rougvie AE (1999) Similarity of the C-elegans developmental timing protein LIN-42 to circadian rhythm proteins, Science. 286, 1141–1146. [DOI] [PubMed] [Google Scholar]
- 167.Ramesh M, McGuiness C & Rajan TV (2005) The L3 to L4 molt of Brugia malayi: Real time visualization by video microscopy, J Parasitol. 91, 1028–1033. [DOI] [PubMed] [Google Scholar]
- 168.Warbrick EV, Barker GC, Rees HH & Howells RE (1993) The Effect of Invertebrate Hormones and Potential Hormone Inhibitors on the 3rd Larval Molt of the Filarial Nematode, Dirofilaria-Immitis, in-Vitro, Parasitology. 107, 459–463. [DOI] [PubMed] [Google Scholar]
- 169.Tzertzinis G, Egana AL, Palli SR, Robinson-Rechavi M, Gissendanner CR, Liu CH, Unnasch TR & Maina CV (2010) Molecular Evidence for a Functional Ecdysone Signaling System in Brugia malayi, Plos Neglect Trop D. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shea C, Richer J, Tzertzinis G & Maina CV (2010) An EcR homolog from the filarial parasite, Dirofilaria immitis requires a ligand-activated partner for transactivation, Mol Biochem Parasit. 171, 55–63. [DOI] [PubMed] [Google Scholar]
- 171.Shea C, Hough D, Xiao JP, Tzertzinis G & Maina CV (2004) An rxr/usp homolog from the parasitic nematode, Dirofilaria immitis, Gene. 324, 171–182. [DOI] [PubMed] [Google Scholar]
- 172.Zieger E, Robert NSM, Calcino A & Wanninger A (2021) Ancestral Role of Ecdysis-Related Neuropeptides in Animal Life Cycle Transitions, Current Biology. 31, 207-+. [DOI] [PubMed] [Google Scholar]
- 173.Chitty JR (2012) Myriapods (Centipedes and Millipedes) in Invertebrate Medicine, Second Edition (Lewbart GA, ed) pp. 255–265, John Wiley & Sons, Inc. [Google Scholar]
- 174.Bortolin F, Benna C & Fusco G (2011) Gene expression during postembryonic segmentation in the centipede Lithobius peregrinus (Chilopoda, Lithobiomorpha), Development Genes and Evolution. 221, 105–111. [DOI] [PubMed] [Google Scholar]
- 175.Khan SJ, Schuster KJ & Smith-Bolton RK (2016) Regeneration in Crustaceans and Insects in Encyclopedia of the Life Sciences (eLS), John Wiley & Sons, Ltd., Chichester. [Google Scholar]
- 176.Wingfield JC & Silverin B (2010) Molt in Birds and Mammals: Hormones and Behavior in Encyclopedia of Animal Behavior (Breed MD & Moore J, eds) pp. 462–467, Academic Press, [Google Scholar]
- 177.Truman JW (2019) The Evolution of Insect Metamorphosis, Current Biology. 29, R1252–R1268. [DOI] [PubMed] [Google Scholar]
- 178.Roch GJ & Sherwood NM (2014) Glycoprotein Hormones and Their Receptors Emerged at the Origin of Metazoans, Genome Biol Evol. 6, 1466–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.King-Jones K & Thummel CS (2005) Nuclear receptors - A perspective from Drosophila, Nat Rev Genet. 6, 311–323. [DOI] [PubMed] [Google Scholar]
- 180.Arendt D (2008) The evolution of cell types in animals: emerging principles from molecular studies, Nat Rev Genet. 9, 868–882. [DOI] [PubMed] [Google Scholar]
- 181.Pires-daSilva A & Sommer RJ (2003) The evolution of signalling pathways in animal development, Nat Rev Genet. 4, 39–49. [DOI] [PubMed] [Google Scholar]
- 182.Rittschof CC & Robinson GE (2016) Behavioral Genetic Toolkits: Toward the Evolutionary Origins of Complex Phenotypes, Genes and Evolution. 119, 157–204. [DOI] [PubMed] [Google Scholar]
- 183.Auer TO, Shahandeh MP & Benton R (2021) Drosophila sechellia: A Genetic Model for Behavioral Evolution and Neuroecology, Annu Rev Genet. 55, 527–554. [DOI] [PubMed] [Google Scholar]
- 184.Veenstra JA (2016) Neuropeptide evolution: Chelicerate neurohormone and neuropeptide genes may reflect one or more whole genome duplications, General and Comparative Endocrinology. 229, 41–55. [DOI] [PubMed] [Google Scholar]
- 185.Veenstra JA (2015) The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii, General and Comparative Endocrinology. 224, 84–95. [DOI] [PubMed] [Google Scholar]
- 186.Li G, Niu JZ, Zotti M, Sun QZ, Zhu L, Zhang J, Liao CY, Dou W, Wei DD, Wang JJ & Smagghe G (2017) Characterization and expression patterns of key ecdysteroid biosynthesis and signaling genes in a spider mite (Panonychus citri), Insect Biochem Molec. 87, 136–146. [DOI] [PubMed] [Google Scholar]
- 187.Ventura T, Cummins SF, Fitzgibbon Q, Battaglene S & Elizur A (2014) Analysis of the Central Nervous System Transcriptome of the Eastern Rock Lobster Sagmariasus verreauxi Reveals Its Putative Neuropeptidome, Plos One. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Datta SR, Anderson DJ, Branson K, Perona P & Leifer A (2019) Computational Neuroethology: A Call to Action, Neuron. 104, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Anderson DJ & Perona P (2014) Toward a Science of Computational Ethology, Neuron. 84, 18–31. [DOI] [PubMed] [Google Scholar]
- 190.Kiehn O & Katz PS (1999) Making circuits dance: neuromodulation of motor systems in Beyond Neurotransmission: Neuromodulation and its importance for information processing (Katz PS, ed), Oxford University Press, New York, NY. [Google Scholar]
- 191.Katz PS & Edwards DH (1999) Metamodulation: the control and modulation of neuromodulation in Beyond Neurotransmission: Neuromodulation and its importance for information processing (Katz PS, ed) pp. 349–381, Oxford University Press, New York. [Google Scholar]
- 192.Toth AL & Robinson GE (2007) Evo-devo and the evolution of social behavior, Trends in Genetics. 23, 334–341. [DOI] [PubMed] [Google Scholar]
- 193.Katz PS (2007) Evolution and development of neural circuits in invertebrates, Current Opinion in Neurobiology. 17, 59–64. [DOI] [PubMed] [Google Scholar]
- 194.Young LJ, Nilsen R, Waymire KG, MacGregor GR & Insel TR (1999) Increased affiliative response to vasopressin in mice expressing the V-1a receptor from a monogamous vole, Nature. 400, 766–768. [DOI] [PubMed] [Google Scholar]
- 195.Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR & Goodson JL (2011) Vasotocin neurons and septal V-1a-like receptors potently modulate songbird flocking and responses to novelty, Hormones and Behavior. 60, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Mendoza A, Sebe-Pedros A & Ruiz-Trillo I (2014) The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity, Genome Biol Evol. 6, 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Semmens DC, Mirabeau O, Moghul I, Pancholi MR, Wurm Y & Elphick MR (2016) Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution, Open Biol. 6, 150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Riddiford LM (1993) Hormones and Drosophila Development in The Development of Drosophila melanogaster (Bate M & Martinez-Arias A, eds) pp. 899–939, Cold Spring Harbor Press. [Google Scholar]