Abstract
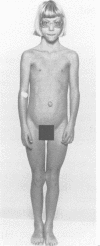
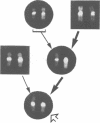
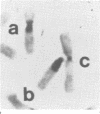
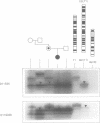
Beckwith-Wiedemann syndrome (BWS), a disorder associated with neonatal hypoglycaemia, increased growth potential, and predisposition to Wilms's tumour (WT) and other malignancies, has been mapped to 11p15. The association with 11p15 duplications of paternal origin, of balanced translocations and inversions with breakpoints within 11p15.4-p15.5 of maternal origin, and the demonstration of uniparental paternal 11p15 isodisomy in some sporadic cases point towards the involvement of genomic imprinting. In agreement with this, we show the paternal origin of a de novo 9;11 translocation in a phenotypically normal mother, whose carrier daughter developed BWS. This supports the fact that BWS associated with balanced chromosome mutations is transmitted in the same sex dependent pattern as non-cytogenetic forms of familial BWS.
Full text
PDF

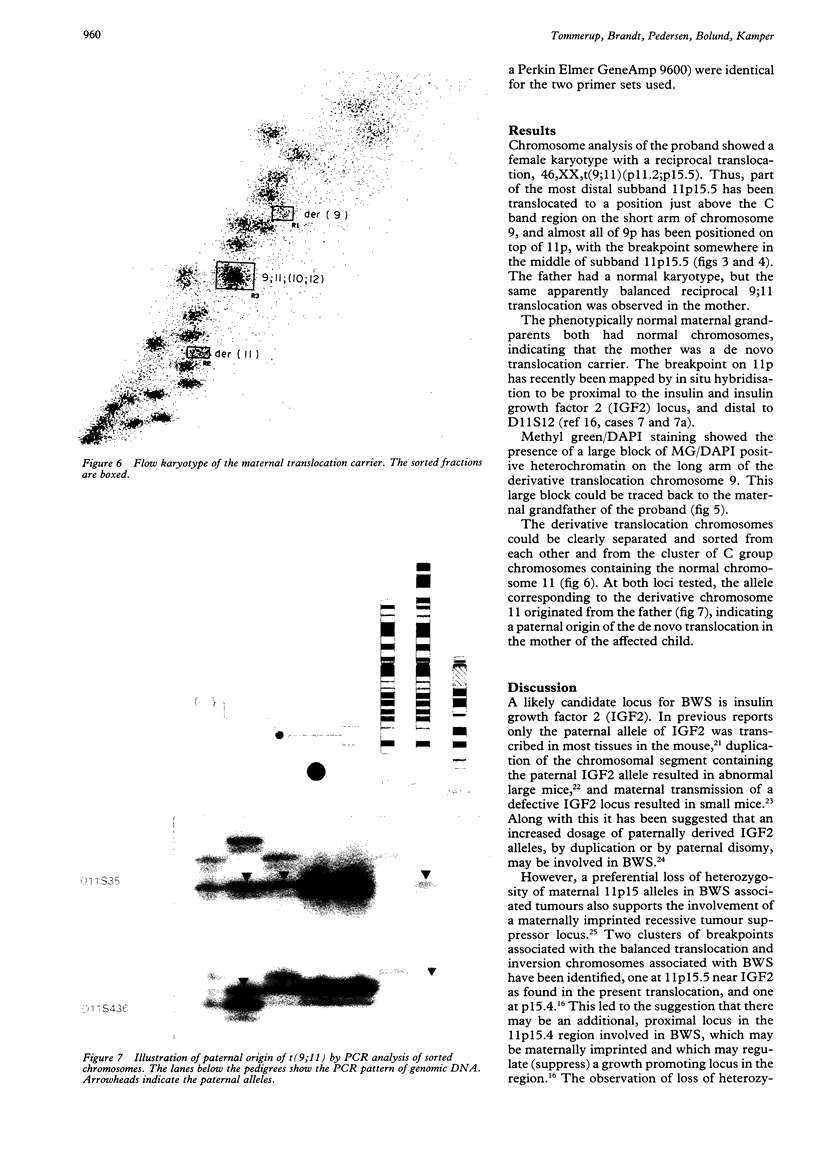

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleck K. A., Hadro T. A. Dominant inheritance of Wiedemann-Beckwith syndrome: further evidence for transmission of "unstable premutation" through carrier women. Am J Med Genet. 1989 Jun;33(2):155–160. doi: 10.1002/ajmg.1320330202. [DOI] [PubMed] [Google Scholar]
- Andersen L. B., Tommerup N., Koch J. Formation of a minichromosome by excision of the proximal region of 17q in a patient with von Recklinghausen neurofibromatosis. Cytogenet Cell Genet. 1990;53(4):206–210. doi: 10.1159/000132931. [DOI] [PubMed] [Google Scholar]
- Best L. G., Hoekstra R. E. Wiedemann-Beckwith syndrome: autosomal-dominant inheritance in a family. Am J Med Genet. 1981;9(4):291–299. doi: 10.1002/ajmg.1320090405. [DOI] [PubMed] [Google Scholar]
- Brown K. W., Williams J. C., Maitland N. J., Mott M. G. Genomic imprinting and the Beckwith-Wiedemann syndrome. Am J Hum Genet. 1990 May;46(5):1000–1001. [PMC free article] [PubMed] [Google Scholar]
- Chamberlin J., Magenis R. E. Parental origin of de novo chromosome rearrangements. Hum Genet. 1980;53(3):343–347. doi: 10.1007/BF00287054. [DOI] [PubMed] [Google Scholar]
- Chandley A. C. On the parental origin of de novo mutation in man. J Med Genet. 1991 Apr;28(4):217–223. doi: 10.1136/jmg.28.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Robertson E. J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991 Feb 22;64(4):849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Donlon T. A., Magenis R. E. Methyl green is a substitute for distamycin A in the formation of distamycin A/DAPI C-bands. Hum Genet. 1983;65(2):144–146. doi: 10.1007/BF00286651. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith A. C., Cattanach B. M., Barton S. C., Beechey C. V., Surani M. A. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature. 1991 Jun 20;351(6328):667–670. doi: 10.1038/351667a0. [DOI] [PubMed] [Google Scholar]
- Haas O. A., Zoubek A., Grümayer E. R., Gadner H. Constitutional interstitial deletion of 11p11 and pericentric inversion of chromosome 9 in a patient with Wiedemann-Beckwith syndrome and hepatoblastoma. Cancer Genet Cytogenet. 1986 Oct;23(2):95–104. doi: 10.1016/0165-4608(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Henry I., Bonaiti-Pellié C., Chehensse V., Beldjord C., Schwartz C., Utermann G., Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991 Jun 20;351(6328):665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Journel H., Lucas J., Allaire C., Le Mée F., Defawe G., Lecornu M., Jouan H., Roussey M., Le Marec B. Trisomy 11p15 and Beckwith-Wiedemann syndrome. Report of two new cases. Ann Genet. 1985;28(2):97–101. [PubMed] [Google Scholar]
- Lubinsky M., Herrmann J., Kosseff A. L., Opitz J. M. Letter: Autosomal-dominant sex-dependent transmission of the Wiedemann-Beckwith syndrome. Lancet. 1974 May 11;1(7863):932–932. doi: 10.1016/s0140-6736(74)90383-3. [DOI] [PubMed] [Google Scholar]
- Mannens M., Devilee P., Bliek J., Mandjes I., de Kraker J., Heyting C., Slater R. M., Westerveld A. Loss of heterozygosity in Wilms' tumors, studied for six putative tumor suppressor regions, is limited to chromosome 11. Cancer Res. 1990 Jun 1;50(11):3279–3283. [PubMed] [Google Scholar]
- Moore T., Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991 Feb;7(2):45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Niikawa N., Ishikiriyama S., Takahashi S., Inagawa A., Tonoki H., Ohta Y., Hase N., Kamei T., Kajii T. The Wiedemann-Beckwith syndrome: pedigree studies on five families with evidence for autosomal dominant inheritance with variable expressivity. Am J Med Genet. 1986 May;24(1):41–55. doi: 10.1002/ajmg.1320240107. [DOI] [PubMed] [Google Scholar]
- Okano Y., Osasa Y., Yamamoto H., Hase Y., Tsuruhara T., Fujita H. An infant with Beckwith-Wiedemann syndrome and chromosomal duplication 11p13----pter.: correlation of symptoms between 11p trisomy and Beckwith-Wiedemann syndrome. Jinrui Idengaku Zasshi. 1986 Dec;31(4):365–372. doi: 10.1007/BF01907937. [DOI] [PubMed] [Google Scholar]
- Pettenati M. J., Haines J. L., Higgins R. R., Wappner R. S., Palmer C. G., Weaver D. D. Wiedemann-Beckwith syndrome: presentation of clinical and cytogenetic data on 22 new cases and review of the literature. Hum Genet. 1986 Oct;74(2):143–154. doi: 10.1007/BF00282078. [DOI] [PubMed] [Google Scholar]
- Schmutz S. M. Deletion of chromosome 11(p11p13) in a patient with Beckwith-Wiedemann syndrome. Clin Genet. 1986 Sep;30(3):154–156. doi: 10.1111/j.1399-0004.1986.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Tommerup N. Mendelian cytogenetics. Chromosome rearrangements associated with mendelian disorders. J Med Genet. 1993 Sep;30(9):713–727. doi: 10.1136/jmg.30.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerup N., Schempp W., Meinecke P., Pedersen S., Bolund L., Brandt C., Goodpasture C., Guldberg P., Held K. R., Reinwein H. Assignment of an autosomal sex reversal locus (SRA1) and campomelic dysplasia (CMPD1) to 17q24.3-q25.1. Nat Genet. 1993 Jun;4(2):170–174. doi: 10.1038/ng0693-170. [DOI] [PubMed] [Google Scholar]
- Tonoki H., Narahara K., Matsumoto T., Niikawa N. Regional mapping of the parathyroid hormone gene (PTH) by cytogenetic and molecular studies. Cytogenet Cell Genet. 1991;56(2):103–104. doi: 10.1159/000133059. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J., Chavin-Colin F., Martelli H., Voyer M., Charlas R. Trisomy 11p15 and Beckwith-Wiedemann syndrome. A report of two cases. Hum Genet. 1984;67(2):219–221. doi: 10.1007/BF00273006. [DOI] [PubMed] [Google Scholar]
- Wales J. K., Walker V., Moore I. E., Clayton P. T. Bronze baby syndrome, biliary hypoplasia, incomplete Beckwith-Wiedemann syndrome and partial trisomy 11. Eur J Pediatr. 1986 Apr;145(1-2):141–143. doi: 10.1007/BF00441878. [DOI] [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]