Abstract
Cancer immunotherapies have unique toxicities. Establishment of grading scales and standardized grade-based treatment algorithms for toxicity syndromes can improve the safety of these treatments, as observed for cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) in patients with B cell malignancies treated with chimeric antigen receptor (CAR) T cell therapy. We have observed a toxicity syndrome, distinct from CRS and ICANS, in patients treated with cell therapies for tumors in the central nervous system (CNS), which we term tumor inflammation-associated neurotoxicity (TIAN). Encompassing the concept of ‘pseudoprogression,’ but broader than inflammation-induced edema alone, TIAN is relevant not only to cellular therapies, but also to other immunotherapies for CNS tumors. To facilitate the safe administration of cell therapies for patients with CNS tumors, we define TIAN, propose a toxicity grading scale for TIAN syndrome and discuss the potential management of this entity, with the goal of standardizing both reporting and management.
Immunotherapy has revolutionized the treatment of cancer. The US Food and Drug Administration (FDA) has approved dozens of immunotherapies spanning immune checkpoint inhibitors, antibody-based therapies, adoptive cell therapies, cytokines, cancer vaccines and oncolytic viruses for various cancer indications1. Preclinical and clinical studies have shown early promise for immune-based therapy in the treatment of brain and spinal cord tumors, including CNS lymphoma2–7. Preclinical studies3,8–14 and early clinical trial reports15–22 also suggest that CAR T cell therapy may hold potential for treatment of CNS tumors, including aggressive ones such as glioblastoma, medulloblastoma, ependymoma and H3K27-altered diffuse midline glioma (DMG, including diffuse intrinsic pontine glioma, DIPG)10,15,17,18,23. In addition to CAR T cell therapy approaches, a recent clinical study reported promising results using an oncolytic virus for diffuse midline gliomas24, and checkpoint inhibitor therapy exerts a modest therapeutic benefit when used in the neoadjuvant setting for recurrent glioblastoma25.
Immunotherapies are often associated with unique toxicity profiles—such as immune checkpoint inhibitor associated autoimmune toxicity, CRS, and ICANS—that differ from those seen with traditional cytotoxic or molecularly targeted therapies. Standardized descriptions of these toxicity syndromes and the institution of grade-based treatment algorithms have helped facilitate the safety and clinical management of patients being treated with immunotherapies26–29. Common Terminology Criteria for Adverse Events (CTCAE) neurotoxicity grading scales are focused on individual toxicities30. However, the toxicity assessment and treatment recommendations for CRS and ICANS are better served by grading the constellation of symptoms in individual patients (Box 1), rather than individual toxicities; accordingly, CRS and ICANS grading scales have been formalized27. Such scales are useful for comparing toxicity rates across studies and products, and can serve as the basis for standardizing management and treatment interventions. Identifying, grading and treating the specific toxicities of immunotherapeutic strategies have been essential for the success of the field.
Box 1. CRS and ICANS.
CRS and ICANS are two systemic toxicities associated with immunotherapies such as CAR T cell therapy. CRS can present with a constellation of symptoms that result from immune cell activation and include persistent fevers, hypotension necessitating the use of vasopressor support, hypoxia requiring non-invasive or invasive respiratory support and, in severe cases, multi-organ failure. Interleukin-6 (IL-6) is believed to be a key mediator of CRS42; accordingly, CRS has been responsive to therapies that neutralize IL-6 and/or corticosteroids. Emerging data suggest improvement of CRS with IL-1 neutralization as well42,43. CAR T cell therapy has also been associated with ICANS, which manifests with a multitude of neurological symptoms, including encephalopathy, tremor, aphasia, dysgraphia, apraxia, seizures and, in severe cases, cerebral edema41,42,44–47. The pathophysiology of ICANS is less well understood than that of CRS, and the effectiveness of specific therapies, beyond supportive care and corticosteroids, has not been clearly demonstrated.
Emerging preclinical and clinical experiences with cell therapies for CNS tumors have demonstrated a syndrome of localized neurotoxicity—which we have termed TIAN—that is distinct from the systemic CRS and ICANS toxicity syndromes. Although CRS and ICANS can occur in patients treated for CNS malignancies, they are agnostic to tumor location, routinely occur in the absence of CNS tumor involvement and are indicative of systemic inflammation. By contrast, we have observed neurotoxicity emerging from localized, rather than systemic, tumor-associated inflammation. The manifestations of TIAN depend on the location of the tumor within a certain neuroanatomical region16; indeed, the potential consequences of on-tumor and on-target inflammation in perilously delicate neuroanatomical locations, like the brainstem or thalamus, was predicted in preclinical models (Box 2)8.
Box 2. TIAN: insights from preclinical models.
In a preclinical study8, mice with H3K27M -altered diffuse intrinsic pontine gliomas (DIPGs) and other H3K27M-altered DMGs in the thalamus and spinal cord were treated with GD2 CAR T cell therapy. Although the treatment cleared tumors in mouse models of DIPG, during the treatment phase, the mice developed evidence of peritumoral brainstem inflammation—leading to compression of the fourth ventricle, obstructive hydrocephalus (buildup of cerebrospinal fluid in the brain) and death in a small subset of the mice. Although CAR T cell treatment of H3K27M altered spinal cord DMGs was well-tolerated, all mice bearing thalamic DMGs experienced life-threatening hydrocephalus due to the compression of the third ventricle and the exceedingly precarious location of the thalami above the tentorial notch, which placed the mice at risk for herniation and consequent death with inflammation at this anatomical site. This study helped illustrate that the specific location of a CNS tumor can confer varying degrees of risk on the basis of the neuroanatomical location of the tumor and the structural consequences of local neuroinflammation. The risk and severity of TIAN may also be related to the specific CAR T target, the kinetics and magnitude of the inflammatory response and use of lymphodepleting chemotherapy, among other possible factors. Additionally, these preclinical data helped inform safety measures for a first-in-human phase I clinical trial using GD2-CAR T cells to treat children and young adults with DIPG or spinal DMG (NCT04196413). Such measures included the placement of an indwelling intraventricular catheter (such as an Ommaya reservoir) to measure intracranial pressure and remove cerebrospinal fluid, thereby treating transient hydrocephalus that may develop secondary to inflammation.
In this Perspective, we define TIAN as a distinct entity and review hypotheses about its pathophysiology. We discuss the clinical symptomatology of TIAN and propose grading and treatment paths for this unique form of localized neuroinflammation, on the basis of our collective clinical experiences of treating patients with CNS cancers, such as DMGs16,19, glioblastomas15 and CNS lymphomas31, with immunotherapy.
Pathophysiology and clinical symptomatology of TIAN
The specific symptoms of TIAN may reflect neuronal dysfunction due to the effects of local inflammation, and/or the effects of transient local inflammation-induced edema (in contrast to generalized and diffuse cerebral edema seen in severe ICANS). The edema associated with TIAN may cause tissue shifts that obstruct flow of cerebrospinal fluid (CSF), increase intracranial pressure, and may even cause a herniation syndrome (Fig. 1). Since TIAN can be associated with inflammation-induced tumoral edema, it encompasses the concept of ‘pseudoprogression’, a known post-treatment effect of immunotherapies associated with tumoral and peritumoral edema32,33. However, it is possible to have TIAN in the absence of edema, as primary local neural dysfunction can occur because of the powerful direct effects of neural–immune interactions and inflammatory signaling molecules, like cytokines and chemokines, on neural cell function34. TIAN is considered secondary to CNS tumor inflammation and on-target effects, and thus can be distinguished from previously reported neurotoxicities that occur with immunotherapies, including autoimmune encephalitis, peripheral sensory neuropathies, myasthenia gravis and inflammatory myopathies35.
Fig. 1 |. Herniation syndromes.
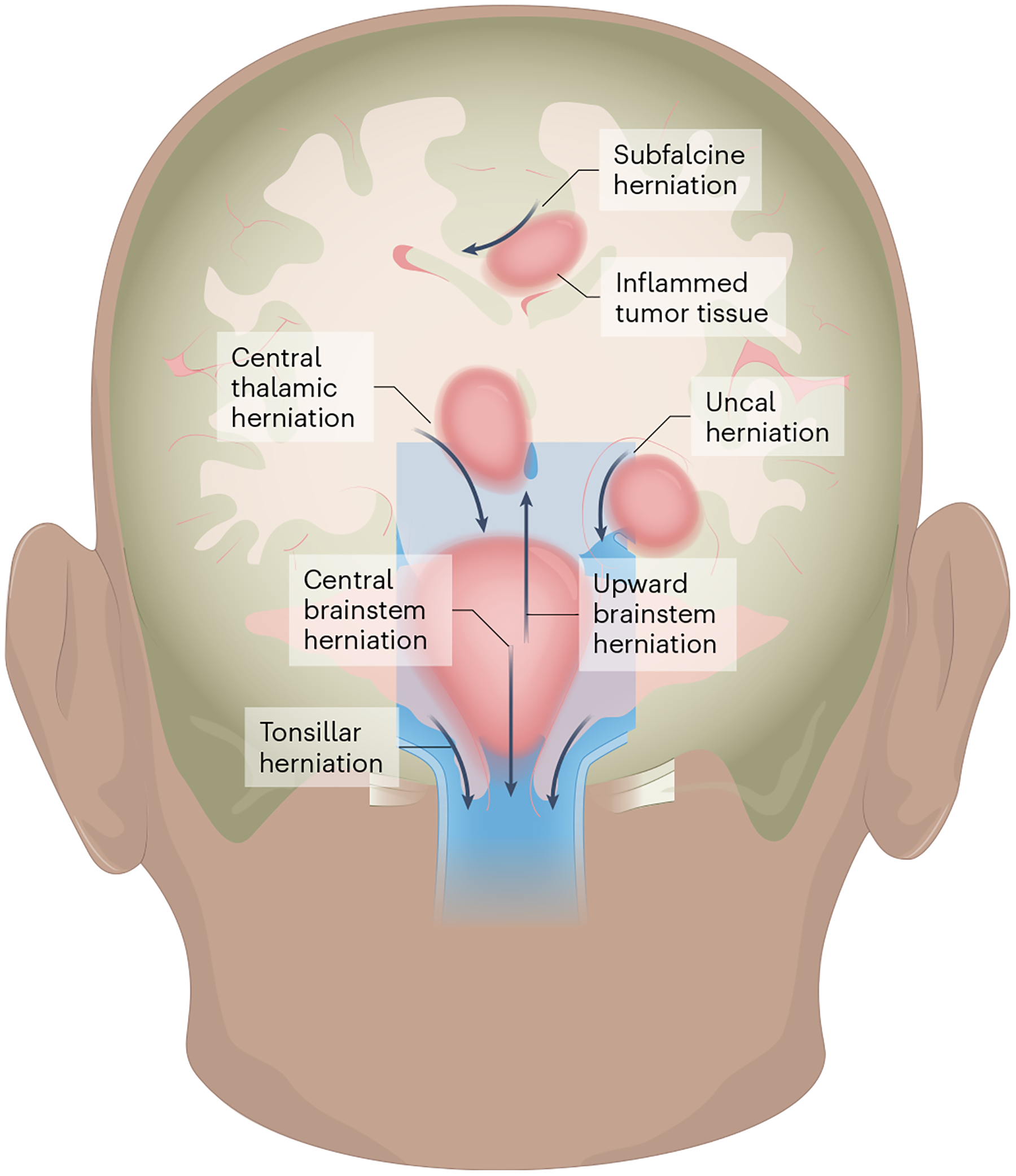
Edema caused by localized therapy-related inflammation depends on the location of the tumor (dark pink) and can cause herniation of brain tissue in the direction indicated by the arrows. Subfalcine herniation: arises when the peri-tumor mass effect compresses and displaces the cingulate gyrus under the falx cerebri and can clinically manifest with altered mental status, aphasia and contralateral leg weakness. Central thalamic herniation: represents downward displacement through the notch of the tentorium cerebelli and resulting compression of the diencephalon, which can lead to decreased arousal and coma. Uncal herniation: occurs when there is downward, transtentorial displacement of the uncus with consequent compression of the midbrain, resulting in a fixed and dilated pupil, a cranial nerve III palsy resulting in a ‘down and out’ eye, and new onset of hemiparesis. Upward brainstem herniation: occurs when a posterior fossa tumor pushes the cerebellar vermis and midbrain upwards, causing herniation through the tentorial notch with impairment of vertical eye movements and decreased level of consciousness. Central brainstem herniation: occurs when there is downward displacement of the brainstem, which can lead to Cushing’s triad (hypertension, bradycardia, abnormal respirations), a decreased level of consciousness (somnolence, with decreased response to noxious stimuli) and pathologic posturing. Tonsillar herniation: occurs because of compression of the cerebellar tonsils against the medulla and through the foramen magnum, compressing the medulla and resulting in Cushing’s triad, a decreased level of consciousness and pathologic extensor posturing36. Dural structures are shown in light blue, CSF in darker blue. The illustration was created by SciStories LLC.
Within the spectrum of TIAN, we propose two categories of neurotoxicity. Type 1 TIAN primarily represents neurological symptoms and signs due to mechanical factors, such as elevated intracranial pressure (related to edema within the space constraints of the cranium). Type 2 TIAN, however, primarily reflects local neural dysfunction. Distinguishing between these two categories is important for appropriate clinical management, but because the two mechanistic categories can be related, the grading system is singular.
Type 1 TIAN: inflammation-induced mechanical mechanisms of neurotoxicity
Type 1 TIAN primarily reflects inflammatory edema causing increased intracranial pressure and mechanical space constraints. The skull is non-expandable and the volume of the intracranial cavity remains constant; according to the Monro–Kellie doctrine36, maintaining a normal intracranial pressure (<20 mmHg) requires equilibrium in the volume of brain tissue, blood and CSF. Immunotherapy-induced brain tumor inflammation disrupts the equilibrium of such parameters in the intracranial cavity and can increase intracranial pressure through increased tissue edema or through mass effect, causing the obstruction of CSF flow. The severity of type 1 TIAN may range from mild (with isolated headaches) to life-threatening—with impending herniation syndromes that arise when peritumoral edema leads to increased intracranial pressure that displaces brain tissue through intracranial compartments, necessitating urgent intervention to preserve life (Fig. 1). It is important to note that even mild type 1 TIAN is a potential harbinger of imminent life-threatening complications and therefore necessitates careful monitoring. Obstruction of CSF flow can rapidly lead to the development of hydrocephalus that may present as new onset of a positional headache, a decreased level of consciousness and new hypertension. If untreated, hydrocephalus can progress to dangerously increased intracranial pressure and possibly a life-threatening herniation syndrome.
The specific type of herniation syndrome depends on the location of the tumor (Fig. 1). For example, patients with tumors in the cerebellum are at increased risk for tonsillar herniation with downward medullary compression, which clinically presents with Cushing’s triad (hypertension, bradycardia, abnormal respirations), decreased level of consciousness, and/or pathological posturing, the latter of which is indicative of disconnection of communication between the brain and the spinal cord. Individuals with tumors causing obstructive hydrocephalus or with tumors in the temporal lobes are at increased risk for uncal herniation, which can clinically be associated with a fixed and dilated pupil, a cranial nerve III palsy resulting in a ‘down and out eye,’ and new onset of hemiparesis36. Neuroimaging can demonstrate evidence of new obstructive hydrocephalus, ventriculomegaly, tumoral edema, and signs of herniation (such as the effacement of basal cisterns or brain tissue shifts). In some cases, imaging can lag behind clinical symptoms or no radiographic changes may be seen. High-grade type 1 TIAN is considered a neurological emergency, as herniation can lead to death. However, timely and appropriate neurocritical interventions (such as CSF diversion, corticosteroids and hyperosmolar therapy) to address obstructive hydrocephalus and/or mitigate peritumoral edema can resolve type 1 TIAN, and neurological injury can be avoided.
Patients with spinal cord tumors may also be at risk for high-grade type 1 TIAN, as there is a theoretical risk of developing inflammation-induced increased intraspinal pressure and consequently impaired blood flow in the spinal cord that could threaten spinal cord function and lead to ischemia or infarction. If the loss of spinal cord function is thought to be due to increased intraspinal pressure, this would be considered high-grade type 1 TIAN, and intervention may be warranted.
Type 2 TIAN: inflammation-induced electrophysiological mechanisms of neurotoxicity
Type 2 TIAN primarily reflects dysfunction of the specific nervous system region in which immunotherapy-related local inflammation is present, and typically manifests as transient worsening of pre-existing neurological symptoms. Inflammatory signaling molecules are well known to influence the function of neurons and other neural cell types37–39, and this typically transient worsening of symptoms may reflect electrophysiological dysfunction within a tumor-infiltrated neural circuit. Neuroimaging may demonstrate evidence of increased T2 signal (which highlights water)40, indicative of increased local edema (Fig. 2), but without obstruction/hydrocephalus or herniation of brain structures. It is also possible that no radiographic changes are seen during the period of peak inflammation, as the neural dysfunction may be unrelated to edema and due only to inflammatory influences at the molecular or cellular level on neural circuit function. In contrast to type 1 TIAN, type 2 TIAN can often be managed conservatively with observation and supportive care, unless the neurological dysfunction involves critical lower brainstem or cervical spinal cord functions such as respiratory drive and phrenic nerve function—in which case intensive care and pharmacological interventions may be required until there is resolution of inflammation and improvement of neurological function. It should be noted that the two types of TIAN are not mutually exclusive, and can occur simultaneously.
Fig. 2 |. Radiographic changes seen in TIAN.
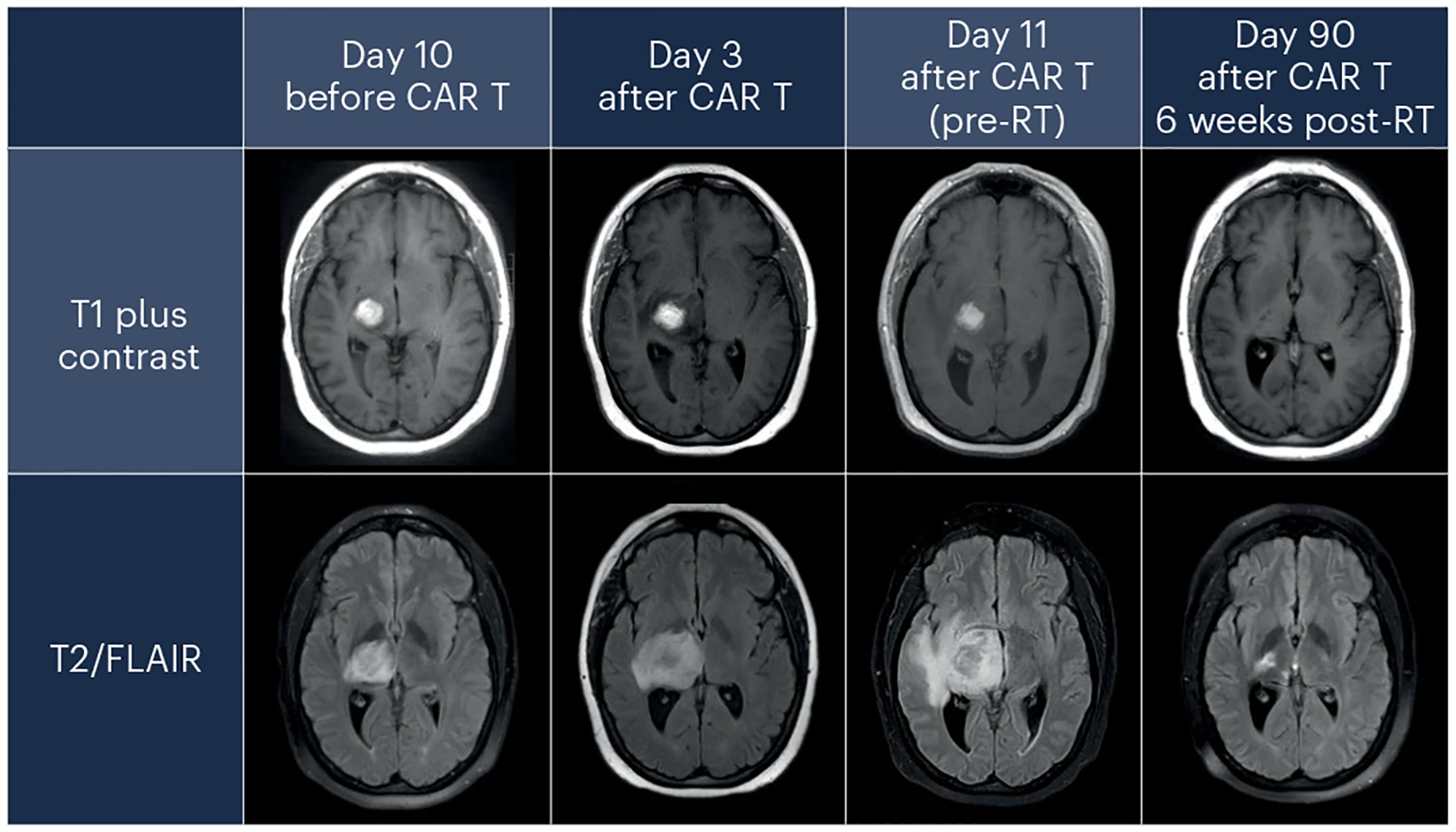
Three days after receiving tisagenlecleucel to treat a primary CNS lymphoma, a patient developed increased T2/fluid-attenuated inversion recovery (FLAIR) signal surrounding the tumor bed, indicative of cerebral edema, that increased by day 11. These radiographic findings correlated with transient clinical worsening on day 11, which was associated with increased headaches, somnolence, left hemiparesis and left-sided, painful dysesthesias. Both the clinical and the radiographic findings then resolved over time. RT, radiation therapy.
Fever
Fever commonly accompanies immunotherapy, and TIAN is often accompanied by fever and headache of variable degree and duration—which likely reflects a general response to inflammation in the CNS, and not necessarily only at the tumor site. Although all fevers are regulated by the hypothalamus in the brain, they may be triggered by either peripheral or central inflammatory signals; therefore, the fevers seen in TIAN are likely distinct from those associated with the systemically driven inflammation seen in CRS, which can be associated with other signs of systemic inflammation, such as hypotension and hypoxia. We thus consider fever and headache following intracranially delivered cellular immunotherapy for a CNS tumor to be a component of TIAN, but not necessarily owing to the effects of localized inflammation restricted to the tumor. Because fever and headache can also result from infection, consideration of infectious causes and appropriate diagnostic studies, and possibly empiric treatment for potential infection, should be conducted when appropriate.
Grading system
Guided by the grading systems for CRS and ICANS developed by the American Society for Transplantation and Cellular Therapy (ASTCT) and the grading system for neurotoxicities outlined by the CTCAE27,30,41, we suggest a grading system for TIAN (Table 1). For practical purposes, this grading system does not distinguish between type 1 and type 2 TIAN, although type 1 TIAN tends to manifest as higher-grade toxicity, and type 2 TIAN tends to manifest as lower-grade toxicity, with the important exception that type 2 TIAN may occur in the brainstem and affect vital functions, such as respiratory drive. Given that patients with CNS tumors often have neurological deficits at baseline as a direct result of their tumors or previous therapy, a critical principle of grading TIAN is that the severity must focus on the comparison to a patient’s baseline neurological examination before immunotherapy, rather than quantifying the absolute level of deficit.
Table 1 |.
TIAN grading scale
| Definition | |
|---|---|
| Grade 1 | Headaches associated with fevers OR mild worsening of existing neurological clinical signs and symptoms from baseline, resulting in minor functional deficits for which only observation or symptomatic management is needed |
| Grade 2 | Moderate changes in the neurological exam from baseline that substantially affect function |
| Grade 3 | Severe neurological clinical signs and symptoms that may affect critical cardiorespiratory functions OR clinical signs and symptoms of increased intracranial pressure (>20 mmHg) that are responsive to intervention* |
| Grade 4 | Life-threatening, clinically significant elevated ICP (>20 mmHg) refractory to CSF drainage with no improvement in clinical symptoms in response to CSF drainage, possibly warranting urgent escalation of neurosurgical intervention (such as with emergent EVD or VPS placement)** OR concerning clinical signs and symptoms of impending/early herniation OR severe medullary dysfunction requiring endotracheal intubation for airway protection and/or mechanical ventilation |
ICP (intracranial pressure), CSF (cerebrospinal fluid), EVD (external ventricular drain), VPS (ventriculoperitoneal shunt).
In patients with spinal cord tumors, grade 3 TIAN can occur when there is risk of debilitating loss of cord function.
Emergently accessing an existing device for CSF drainage does not necessarily qualify as grade 4 TIAN, if such drainage successfully manages ICP.
Grade 1 TIAN is defined as mild worsening of existing neurological signs and symptoms from baseline, resulting in minor functional deficits for which only observation or symptomatic management is needed. This may occur with or without fever. Examples include headaches associated with fevers, worsening sensory loss of an extremity, or the mild worsening of a facial droop that does not substantially affect speech function.
Grade 2 TIAN is characterized as moderate changes in the neurological exam from baseline that significantly affect function. These changes can include hemiparesis or ataxia limiting the ability to ambulate, or cranial neuropathies that limit eating.
Grade 3 TIAN is defined as severe neurological symptoms that may include abnormal breathing, difficulty protecting the airway and cardiovascular instability requiring escalation of care with pressor support or positive pressure airway support (bilevel positive airway pressure (BiPAP) or continuous positive airway pressure support (CPAP)). Grade 3 TIAN may also encompass early signs of symptomatic increased intracranial pressure or cerebral edema, which may manifest clinically as new hypertension or a positional headache with nausea or vomiting, and is responsive to intervention, such as CSF drainage or steroid administration. In patients with spinal cord tumors, grade 3 TIAN can occur when there is risk of lasting and debilitating loss of spinal cord function due to increased intraspinal pressure.
Grade 4 TIAN occurs when patients develop clinically significant elevated intracranial pressure, with no improvement of clinical symptoms in response to CSF drainage, possibly warranting urgent escalation of neurosurgical intervention—such as with emergent external ventricular drain or ventriculoperitoneal shunt placement. Of note, emergently accessing an existing device for CSF drainage does not necessarily qualify as grade 4 TIAN, if such drainage successfully manages intracranial pressure. Grade 4 TIAN can also occur when there are concerning clinical signs and symptoms of impending/early herniation (Fig. 1). In patients with significant medulla disease, grade 4 TIAN can present with severe medullary dysfunction requiring endotracheal intubation for airway protection and/or mechanical ventilation. Grade 4 TIAN is considered a life-threatening neurological emergency.
Grade 5 TIAN is defined as death secondary to TIAN.
Clinical management
Our experiences have taught us that the clinical and radiographic markers of TIAN can be subtle and unique to each patient, and can change rapidly; accordingly, patients undergoing immunotherapy must be closely monitored by a multidisciplinary team of oncologists, neurologists, neurosurgeons and critical-care physicians. Vigilant neuro-monitoring is particularly crucial when patients with posterior fossa tumors that involve the medulla or that compress the fourth ventricle develop early signs of increased intracranial pressure, as these patients can tolerate inflammation-induced changes only to a certain threshold before they can no longer compensate for further intracranial pressure increases. Once patients cross this threshold, they are at risk for herniation syndromes and death. Therefore, we recommend considering placement of an Ommaya reservoir (a catheter system placed beneath the scalp to facilitate removal of CSF) or a similar device for patients with CNS tumors in high-risk locations prior to immunotherapy, in order to monitor intracranial pressure and to serve as a safety valve that allows for the swift removal of CSF when clinically necessary. Although it is sometimes possible to see early signs of ventriculomegaly and herniation on neuroimaging, radiographic changes are often not conspicuous and lag behind the clinical exam in the context of TIAN. Further studies are needed to elucidate whether other radiographic changes could serve as markers for TIAN. Similarly, additional studies are necessary to determine which laboratory inflammatory biomarkers may correspond with the clinical course of TIAN. At this time, the neurological exam remains the most sensitive diagnostic tool for the assessment and treatment of TIAN.
A grading system that encompasses the tumor inflammation-associated neurotoxicity syndrome will facilitate immunotherapy research for CNS tumors and the identification of biomarkers. The delineation of neurotoxicities conceptually into type 1 TIAN and type 2 TIAN may help clinicians to recognize when close monitoring and treatment are necessary. For example, some individuals following CAR T cell therapy who present with transient worsening of neurological deficits consistent with type 2 TIAN may only require observation through serial examinations—while others may need pharmacological interventions to decrease localized edema. Comparatively, patients who exhibit early clinical signs of increased intracranial pressure that are indicative of type 1 TIAN require urgent intervention to remove CSF and reduce intracranial pressure. Although patients can experience both type 1 and type 2 TIAN concurrently, understanding which type of TIAN (primarily mechanical or primarily electrophysiological) that a patient is manifesting is instructive to clinical management. It is also possible that, as with ICANS in the treatment of systemic malignancies, the rate and severity of TIAN may be variable depending on the CAR T product, administration route and dosing.
Case examples
Case 1
A 5-year-old girl with a DIPG and baseline symptoms of restricted horizontal eye movements, an asymmetric smile, right hemiparesis and right upper and lower extremity ataxia received an intracerebroventricular (ICV) infusion of CAR T cells. Two days after her infusion, during the period of peak inflammation (as evidenced by fever and increased levels of CSF cytokines), she developed increased ataxia from her baseline that did not limit ambulation and resolved within 2 days and without medical intervention16. This worsening of a pre-existing symptom was thought to be due to local tumor-inflammation-induced worsening of neural function, and would be considered grade 1 TIAN because it resulted in a mild functional deficit, was self-limiting and did not require medical intervention. This case demonstrates how patients with mild TIAN can be safely observed without intervention.
Case 2
A 47-year-old man with baseline mild impairment of executive functioning and slow processing—following standard surgical resection and chemo-radiotherapy for a left frontal glioblastoma—had evidence of tumor recurrence on brain magnetic resonance imaging (MRI) and began combination therapy with pembrolizumab (anti-PD-1) and bevacizumab (anti-VEGF). After receiving five treatment cycles, his brain MRI showed the development of increased enhancement and edema surrounding the tumor. These radiographic changes were clinically associated with fatigue, headaches and mildly worsened cognitive slowing (relative to baseline). At the time, he met criteria for radiographic disease progression, so pembrolizumab was discontinued, bevacizumab was continued and he was started on lomustine (CCNU). He received only three cycles of CCNU, which was then discontinued because of persistent thrombocytopenia. He did not have clinical progression during this period, and the imaging abnormalities and worsened mild cognitive function slowly resolved. The headaches and mild worsening cognitive function that he developed following pembrolizumab treatment were most consistent with grade 1 TIAN, rather than recurrent glioblastoma. This case illustrates how transient clinical worsening and increased tumor edema on MRI after immunotherapy can be consistent with TIAN, also conceptualized as ‘pseudoprogression,’ and mistaken for actual tumor progression.
Case 3
One day after receiving an ICV infusion of CAR T cells, a 59-year-old woman with a glioblastoma in her left frontal lobe—which was associated with impaired short-term recall at baseline—had worsening of her short-term memory recall and developed new expressive aphasia with word-finding difficulties and difficulty performing simple calculations (acalculia). Her symptoms were suggestive of TIAN as a result of frontal-lobe dysfunction. She had a head computed tomography (CT) scan that showed evidence of peritumoral edema, and she was started on anakinra (interleukin-1-receptor antagonist) with subsequent resolution of her symptoms within 2 days. Although she had expressive aphasia and acalculia, which can be seen with ICANS, there was no concern for ICANS given the timing and duration of her symptoms, the absence of other clinical signs and symptoms of systemic inflammation and localization of her symptoms to functions associated with a tumor of the left frontal lobe. This would be considered grade 2 TIAN because the symptoms resulted in functionally limiting cognitive deficits and aphasia; the case also highlights how ICANS and TIAN can clinically mimic each other, but pathophysiologically represent distinct clinical entities.
Case 4
A 52-year-old woman with primary CNS lymphoma, including a lesion in the superior pons associated with baseline impaired balance and coordination and minor urinary incontinence, received an intravenous infusion of CAR T cells. On day 1 after the infusion, the patient reported worsening urinary incontinence and impaired balance and developed a new, mild left-sided facial asymmetry—consistent with local effects of pontine inflammation (TIAN). On day 3, the patient became febrile (consistent with grade 1 CRS) and had an immune effector cell encephalopathy (ICE) score of 2/10. The ICE score quantifies deficits in orientation, naming, following commands, writing and attention—all hallmarks of ICANS—and incorporates automatic upgrading for severe symptoms, including depressed consciousness, seizures, severe motor deficits or cerebral edema. A score of 2/10 was consistent with a grade 3 ICANS. The patient was subsequently treated with dexamethasone. On day 4, the fever resolved and the ICE score normalized, but ongoing urinary incontinence, somnolence, facial weakness, impaired balance and new dysautonomia characterized by sinus bradycardia (heart rate of 47 beats per minute (bpm)) was reported. This was attributed to ongoing pontine inflammation, in keeping with evolving TIAN, and dexamethasone was continued. Brain imaging confirmed the presence of ventriculomegaly that was stable from the preinfusion baseline with no concerns for herniation. On day 5, the patient’s heart rate dropped to 36 bpm, and she was admitted to the intensive-care unit for cardiac monitoring and was treated with glycopyrronium bromide. On day 7, the bradycardia resolved off glycopyrronium bromide (64 bpm); the patient was transferred from intensive care to the ward and was subsequently weaned off dexamethasone. This transient worsening of existing neurological symptoms and development of new (also transient) neurological symptoms—secondary to local tumor-inflammation-induced neural circuit dysfunction—would be considered grade 3 TIAN owing to the escalation of care and urgent intervention that was needed to address her serious autonomic dysregulation. The case demonstrates how grade 3 TIAN can occur in the absence of increased intracranial pressure if the inflammation affects critical neurological functions.
Case 5
Four days after receiving intravenous infusion of CAR T cells and in the context of grade 1 CRS, a 6-year-old girl with a DIPG developed axial weakness, with difficulty holding her head up, bilateral hand weakness, increased slurred speech from baseline and somnolence. Her Ommaya reservoir was accessed, demonstrating elevated intracranial pressure of 24 mmHg. CSF was removed, with immediate improvement in her neurological symptoms, and her Ommaya reservoir was left accessed to allow for continuous CSF drainage and pressure monitoring for 1 day. Head CT illustrated enlarged lateral ventricles and effacement of the fourth ventricle due to increased mass effect within the pons. Her hydrocephalus was thought to result from tumor inflammation-induced edema in her already expanded pons, resulting in obstruction of CSF flow at the level of the fourth ventricle. She was given corticosteroids, from which she was weaned over the course of 7 days, and started on anakinra, from which she was weaned after 9 days. Her hydrocephalus would be considered grade 3 TIAN because it resolved with CSF drainage.
Case 6
Two days after receiving an ICV infusion of CAR T cells through an Ommaya catheter, a 22-year-old man with a DIPG developed fever, became somnolent and developed a new, right third nerve palsy (eye ‘down and out’, with intermittent dilation of his pupil). His Ommaya reservoir was accessed, demonstrating an elevated intracranial pressure of 36 mmHg. CSF was removed, with subsequent clinical improvement within minutes, and the Ommaya reservoir was left accessed for continued CSF drainage and intracranial pressure monitoring. Head CT demonstrated pontine expansion with compression of the fourth ventricle, mildly enlarged lateral ventricles and effacement of bilateral ambient cisterns, indicative of obstructive hydrocephalus. This clinical picture was consistent with inflammation of the pontine tumor causing obstructive hydrocephalus and consequent impending uncal herniation. He was treated with hypertonic saline (a form of osmolar therapy that decreases intracranial pressure), systemic corticosteroids and anakinra. He returned to his neurological baseline within 1 day of these neurocritical care interventions. Monitoring of intracranial pressure and CSF removal through the Ommaya catheter was discontinued within 2 days, and he was weaned off steroids within 4 days as intracranial pressure improved. Over the course of the following weeks, the patient’s neurological function improved substantially above his preinfusion functional baseline, and the tumor decreased in size on MRI16. This would be considered grade 4 TIAN owing to the clinical and radiographic signs of impending uncal herniation.
Challenges and future directions
Defining TIAN as a distinct on-tumor, on-target toxicity provides a framework to better understand the local immunotherapy-related neurotoxicities that can arise in pediatric and adult patients with malignancies involving the CNS—and serves as a guide to help clinicians recognize when intervention is urgent. That inflammation of the nervous system can cause local neural dysfunction is well demonstrated, but the mechanisms underpinning this dysfunction remain to be fully understood. Neurons express receptors for cytokines and chemokines, and local immune responses can directly influence neuronal function (for review, see ref. 34). Other neural cell types that are required for normal neural circuit function (such as astrocytes, microglia and neurovascular cells) also respond to immune signaling; thus, the influence of local inflammation on neurological functions (type 2 TIAN) can be mediated by indirect effects on neurons mediated by dysregulated support cells. Effects of inflammatory signaling on the neurovascular unit (glia and vascular cells) can also contribute to the edema responsible for tissue shifts and the space constraints that define type 1 TIAN. Additional studies are needed to identify the histopathological biomarkers associated with TIAN and the precise mechanistic underpinnings of this toxicity syndrome. Prospective multicenter studies will help to further elucidate the natural history of TIAN and validate the TIAN grading scale.
It is imperative to distinguish TIAN from both ICANS and tumor progression given their unique pathophysiological mechanisms and clinical implications. ICANS is characterized by global cerebral dysfunction and manifests as alterations in consciousness level, seizure activity, motor weakness and features of diffuse cerebral edema, rather than regional symptoms and signs secondary to inflammatory changes at the specific tumor site. If inflammation-induced neurological changes are mischaracterized as being attributed to ICANS secondary to systemic inflammation, rather than to localized tumor-specific dysfunction (TIAN) (or TIAN instead of ICANS), appropriate intervention may not be delivered in a timely manner. Similarly, distinguishing TIAN from tumor progression can inform clinical decision-making and prognostication. TIAN typically occurs after CAR T cell therapy within the first days to weeks after a patient receives immunotherapy (often within days), can be associated with other signs of inflammation such as concurrent CRS and elevated inflammatory markers, and is transient. TIAN after checkpoint inhibitors may occur relatively later than with CAR T cell therapy, sometimes months after therapy begins. Comparatively, the radiographic and clinical signs of tumor progression persist beyond the acute inflammatory window.
As the promise of immunotherapy is realized and becomes an integral treatment modality for CNS tumors, the swift identification and treatment of TIAN will be fundamental to ensuring the safe and efficacious administration of this treatment in the high-risk patient population with CNS tumors.
Acknowledgements
Figure 1 was designed by SciStories LLC. This work was supported by California Institute for Regenerative Medicine CLIN2-12595 (C.L.M., M.M., R.G.M.) and CLIN2-12153 (L.D.W., T.B.D.); National Institutes of Health R01CA263500-01 (C.L.M., M.M., R.G.M.), R01NS092597 (M.M.), DP1NS111132 (M.M.), P50CA165962 (M.M.), R01CA258384 (M.M.), U19CA264504 (M.M.), K08CA201491 (L.D.W), 5K08NS118138-02 (J.G.) and 5U01TR002487-05 (J.P.); the Parker Institute for Cancer Immunotherapy (C.L.M., R.G.M.); CureSearch (C.L.M., M.M., R.G.M.); St Baldrick’s/Stand Up 2 Cancer–Pediatric Cancer Dream Team Translational Research Grant (SU2CAACR-DT1113, C.L.M., M.M.; SU2C-AACR-DT-27-17, N.A.V.); ChadTough Defeat DIPG Foundation (J.M., M.M.); Alex’s Lemonade Stand Foundation (M.M.); Cancer Research UK (M.M.); Virginia and D.K. Ludwig Fund for Cancer Research (M.M., C.L.M.); V Foundation Translational Research Grant (L.D.W); Shurl and Kay Curci Foundation Award, Stanford Maternal & Child Health Research Institute (L.M.P); Cookies for Kid’s Cancer Young Investigator Grant (N.A.V.); DIPG All-In (N.A.V.); Matthew Larson Research Grant (N.A.V.); We Love You Connie Foundation (N.A.V.); Cancer Research UK City of London Centre (K.S., C.R); and Wellcome Trust (C.R.).
Footnotes
Competing interests
C.L.M. holds multiple patents in the arena of CAR T cell therapeutics, is a cofounder and holds equity in Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, CARGO, Link, NeoImmune Tech, Apricity, Nektar, Immatics, Mammoth and Ensoma. R.G.M. holds patents for CAR T cell therapeutics and is a cofounder of and holds equity in CARGO Therapeutics and Link Cell Therapies. R.G.M. has served as a consultant for Lyell Immunopharma, CARGO Therapeutics, Link Cell Therapies, NKarta, Arovella Pharmaceuticals, ImmunAI, Aptorum Group, Zai Labs, Innervate Radiopharmaceuticals, GaDeta and GammaDelta Therapeutics. J.G. is a consultant for Johnson & Johnson. J.D. has been a consultant for Amgen and Unum Therapeutics. M.M. holds patents for CAR T cell therapeutics and holds equity in MapLight Therapeutics. M.L. receives research support from Arbor, BMS, Accuray, Biohaven and Urogen; serves as a consultant to VBI, InCephalo Therapeutics, Merck, Pyramid Bio, Insightec, Biohaven, Sanianoia, Hemispherian, Novocure, Noxxon, InCando, Century Therapeutics, CraniUs, MediFlix and XSense; and is a shareholder in Egret Therapeutics.
References
- 1.Waldman AD, Fritz JM & Lenardo MJ A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol 20, 651–668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medikonda R, Dunn G, Rahman M, Fecci P & Lim M A review of glioblastoma immunotherapy. J. Neurooncol 151, 41–53 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Huang B et al. Current immunotherapies for glioblastoma multiforme. Front. Immunol 11, 603911 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desjardins A et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med 379, 150–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Choi J & Lim M Advances in immunotherapies for gliomas. Curr. Neurol. Neurosci. Rep 22, 1–10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CE et al. The future of cancer immunotherapy for brain tumors: a collaborative workshop. J. Transl. Med 20, 236 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim M, Xia Y, Bettegowda C & Weller M Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol 15, 422–442 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Mount CW et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med 24, 572–579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theruvath J et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med 26, 712–719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan LK et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med 26, 720–731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao H et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS ONE 9, e94281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha D, Martuza RL & Rabkin SD Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32, 253–267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanpay AC et al. EGFR806-CAR T cells selectively target a tumor-restricted EGFR epitope in glioblastoma. Oncotarget 10, 7080–7095 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agliardi G et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun 12, 444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CE et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med 375, 2561–2569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majzner RG et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitanza NA et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med 27, 1544–1552 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 3, 1094–1101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitanza NA et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 13, 114–131 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson JD, Henson JC, Breese RO, Bielamowicz KJ & Rodriguez A CAR T cell therapy for pediatric brain tumors. Front. Oncol 10, 1582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frigault MJ et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 134, 860–866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frigault MJ et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood 139, 2306–2315 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majzner RG et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res 25, 2560–2574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gállego Pérez-Larraya J et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med 386, 2471–2481 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Cloughesy TF et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med 25, 477–486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougan M, Wang Y, Rubio-Tapia A & Lim JK AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology 160, 1384–1393 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Lee DW et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant 25, 625–638 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Dutcher JP et al. High dose interleukin-2 (Aldesleukin)—expert consensus on best management practices-2014. J. Immunother. Cancer 2, 26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maus MV et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, 155 (US Department of Health and Human Services, 2017). [Google Scholar]
- 31.Siddiqi T et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 5, 4059–4063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandsma D, Stalpers L, Taal W, Sminia P & van den Bent MJ Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 9, 453–461 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Okada H et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 16, e534–e542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvador AF, de Lima KA & Kipnis J Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol 21, 526–541 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Perrinjaquet C, Desbaillets N & Hottinger AF Neurotoxicity associated with cancer immunotherapy: immune checkpoint inhibitors and chimeric antigen receptor T-cell therapy. Curr. Opin. Neurol 32, 500–510 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Posner JB, Saper CB, Schiff ND & Claassen J Plum and Posner’s Diagnosis and Treatment of Stupor and Coma 5th edn (Oxford University Press, 2019). [Google Scholar]
- 37.Sm A, Pj T & Nj R Interleukin-1 and neuronal injury. Nat. Rev. Immunol 5, 629–640 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Orhan A, Oliver U, Carmen I-D, Robert N & Frauke Z Neuronal damage in brain inflammation. Arch. Neurol 64, 185–189 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Yirmiya R & Goshen I Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun 25, 181–213 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Gust J & Ishak GE Chimeric antigen receptor T-cell neurotoxicity neuroimaging: more than meets the eye. AJNR Am. J. Neuroradiol 40, E50–E51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DW et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegler EL & Kenderian SS Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front. Immunol 11, 1973 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strati P et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brudno JN & Kochenderfer JN Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown BD et al. Immune effector cell associated neurotoxicity (ICANS) in pediatric and young adult patients following chimeric antigen receptor (CAR) T-cell therapy: can we optimize early diagnosis? Front. Oncol 11, 634445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalabi H et al. Systematic evaluation of neurotoxicity in children and young adults undergoing CD22 chimeric antigen receptor T-cell therapy. J. Immunother 41, 350–358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DW & Shah N Chimeric Antigen Receptor T-Cell Therapies for Cancer: a Practical Guide 1st edn (Elsevier, 2019). [Google Scholar]


