Abstract
Immune responses to exogenous antigens in infant experimental animals display various degrees of Th2 polarization. Preliminary evidence from small human studies suggest a similar age-dependent response pattern to vaccines, but detailed investigations on vaccine immunity during infancy have not yet been undertaken. We report below the results of a comprehensive prospective study on responses to the tetanus component of the diphtheria, tetanus, acellular pertussis (DTaP) vaccine in a cohort of 55 healthy children, employing peripheral blood mononuclear cells (PBMC) collected at the 2-, 4-, and 6-month vaccinations and at 12 months. Antigen-specific production of interleukin-4 (IL-4), IL-5, IL-6, IL-9, IL-10, IL-13, and gamma interferon (IFN-γ) was determined at each sample point, in parallel with polyclonal (phytohemagglutinin PHA-induced) cytokine responses. Our results indicate early and persistent Th2 responses to the vaccine, in contrast to a more delayed and transient pattern of IFN-γ production. This initial disparity between the Th1 and Th2 components of the vaccine response was mirrored by patterns of polyclonally induced cytokine production, suggesting that the delayed maturation of the Th1 component of the vaccine response during infancy is secondary to developmental processes occurring within the overall Th cell system.
The current schedule for vaccination of infants with the diphtheria, tetanus, acellular pertussis (DTaP) vaccine is the subject of increasing debate, in particular the relationship between the timing and frequency of dosing and the subsequent generation of immunological memory. The nature of the response to the initial cycle of three primary vaccinations given during infancy represents the least understood aspect of this question. Although systematic kinetic studies have been conducted on antibody responses, studies of cellular responses in large samples of subjects over this age range have not yet been performed.
Of particular interest in this context are vaccine antigen-specific T-helper (Th)-cell cytokine responses during early infancy. It is evident from a number of clinical efficacy trials focusing on the pertussis component of the vaccine that protection against infection does not correlate consistently with specific serum antibody titer (1, 8, 11, 12, 22, 24). This argues that other aspects of the host response (notably cellular immunity) may also be important in the defense against infection, and this conclusion is reinforced by results from animal model systems which demonstrate a key role for cytokine-secreting CD4+ T cells, in particular T cells secreting Th1 cytokines, in protection against respiratory tract challenge with pertussis (15, 17). Similarly, in terms of adult responses to tetanus toxoid (TT), both Th1 and Th2 cytokines have been implicated in vaccine-induced protection (9, 10).
However, recent studies in mice (6, 20, 23), and also in humans (reviewed in reference 13) suggest that the capacity to generate both acute and persistent Th1 responses to antigen challenge during the early postnatal period is normally compromised, unless selective Th1 stimulants are coadministered with the antigen. In relation to the development of cellular immunity during the early phase of DTaP vaccination in humans, detailed information on the kinetics, range, and magnitude of responses during infancy is lacking, since the only available information is limited to two small studies focusing on pertussis-specific production of a limited range of cytokines 4 weeks after completion of the initial course of three primary vaccinations (3, 28).
The present study focuses on tetanus-specific responses in a cohort of children; it uses blood samples collected at the time of the 2-, 4-, and 6-month primary vaccinations and contrasts these with a further sample collected at 12 months. Specific responses were measured by determining the production of a comprehensive range of cytokines at the protein (interleukin-5 [IL-5], IL-6, IL-10, IL-13, and gamma interferon [IFN-γ]) and mRNA (IL-4 and IL-9) levels. Postnatal maturation of overall Th1 and Th2 functions was monitored in parallel cultures by measurement of cytokine production triggered by the polyclonal stimulant phytohemagglutinin (PHA).
Our results indicate divergent patterns of vaccine antigen-specific Th1 and Th2 cytokine production in human infants which are broadly consistent with recent studies in infant mice (5), i.e., initial polarization towards the Th2 cytokine phenotype and relatively poor persistence of the Th1 component of the response. Moreover, the relative Th2 bias of these early antigen-specific responses is mirrored by cytokine patterns obtained with the polyclonal stimulant PHA, suggesting that the principal rate-limiting determinants of the host response to the vaccine during infancy are factors intrinsic to the postnatal development of the Th cell system.
MATERIALS AND METHODS
DTaP vaccine.
DTaP vaccine (Infanrix; SmithKline Beecham, Rixensart, Belgium) contained 25 Lf of diphtheria toxoid, 10 Lf of TT, 25 μg of pertussis toxoid, 25 μg of filamentous hemagglutinin and 8 μg of pertactin adsorbed onto 0.5 mg of aluminum (aluminum hydroxide).
Subjects.
Fifty-five healthy subjects were recruited into this study at 2 months of age. The infants received DTaP at 2, 4, and 6 months of age, in addition to the oral polio (SmithKline Beecham) and HibTitre (Lederle) vaccines. Prior to immunization, peripheral blood was obtained at these time points, as well as at 12 months; samples were obtained from ≥78% of the group on each occasion. Blood was collected into an equal volume of RPMI 1640 (Cytosystems, Castle Hill, Australia) containing preservative-free heparin. Peripheral blood mononuclear cells (PBMC) were isolated and cryopreserved at collection as previously described (25). This study was carried out with the approval of the Princess Margaret Hospital Ethics Committee (Perth, Australia), and written informed consent was provided by the parents or guardians of all the children.
Cell preparation and culture.
The studies were performed with PBMC which had been cryopreserved at collection; previous studies from our laboratory (14, 25) and elsewhere (2) have demonstrated that this procedure does not distort PBMC cellular immune responses.
PBMC which had been cryopreserved were thawed and resuspended at 106 viable cells/ml in either RPMI 1640 supplemented with 5% pooled human AB serum (for cultures with TT) or AIM-V serum-free medium (Gibco Life Technologies, Waverley, Australia) supplemented with 2-mercaptoethanol (4 × 10−5 M final concentration [Sigma, Castle Hill, Australia]) (for cultures with PHA). Aliquots of 0.5 to 1.0 ml from each individual were cultured at 37°C under 5% CO2 for 48 h as follows: medium alone or medium containing TT (0.5 Lf/ml [CSL, Parkville, Australia]) or PHA (HA16; 1 μg/ml [Murex, Northmead, Australia]). After culture, the cells were collected by centrifugation and used immediately for RNA extraction while the supernatants were stored at −20°C for enzyme-linked immunosorbent assays ELISA.
Semiquantitative reverse transcription-PCR detection of cytokine-specific mRNA.
Total RNA from the cell pellets was obtained using RNAzol B extracting solution as previously described (27). cDNA was transcribed in a total volume of 25 μl at 42°C using oligo (dT)15 (250 ng [Biotech International, Bentley, Australia]) and avian myeloblastosis virus (AMV) reverse transcriptase (4.5 U [Promega, Madison, Wis.]) in the presence of RNase inhibitor (10 U [RNaseOUT; Biotech]). cDNA was amplified for β-actin, IL-4, and IL-9. The PCR mixture contained 1 μl of cDNA, 50 ng of the specific primers (Gibco), 1× PCR buffer (Gibco), 0.2 mM each deoxynucleoside triphosphate (Biotech), 1.5 mM MgCl2 (Gibco), and 0.5 U of Platinum Taq DNA polymerase (Gibco) in a total volume of 12.5 μl overlaid with mineral oil. The PCR was run as follows: an initial denaturation step of 94°C for 3 min, denaturation at 94°C for 1 min, annealing at respective temperatures for 1 min, and extension at 72°C for 1 min. All reactions were performed in a programmable thermocycler (Perkin-Elmer, Melbourne, Australia). A positive cDNA control was always amplified in parallel in all PCRs performed.
Primers.
As described previously (27), the sequences for the primers were as shown in Table 1. Rigorous cycle analyses were performed with each primer set to ensure that we had reached detection levels and that the reaction remained in the linear phase (30 cycles for β-actin, 43 cycles for IL-4, and 40 cycles for IL-9). In each case, PCR products of the expected size were obtained, as verified by analysis in a 1.5% agarose gel and staining with ethidium bromide of a subset of samples and a positive control.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′→3′) | Annealing temp (°C) | Product size (bp) |
|---|---|---|---|
| β-actin F | CGT GAC ATT AAG GAG AAG CTG TGC | 58 | 375 |
| β-actin R | CCT AGG AGG AGC AAT GAT CTT GAT | ||
| IL-4 F | CAA GTG CGA TAT CAC CTT ACA GG | 58 | 306 |
| IL-4 R | CCT TCA CAG GAC AGG AAT TCA AGC | ||
| IL-9 F | GGG ATC CTG GAC ATC AAC TT | 56 | 307 |
| IL-9 R | CAG AAG ACT CTT CAG AAA TG |
Slot-blot analysis, hybridization, and detection.
The PCR products were analyzed by slot-blot analysis (Hoefer Scientific Instruments, San Francisco, Calif.), a modification of previously described methods (27). Briefly, double-stranded probes were produced by PCRs using biotin-16-dUTP (Boehringer Mannheim, Perth, Australia) at a ratio of 5:1. The template for probe synthesis was cDNA obtained from adult PBMC stimulated with PHA (1 μg/ml) for 24 h at 37°C. Following overnight hybridization with biotinylated probes, the binding was visualized by chemiluminescence using a commercial kit (ECL; Amersham, Little Chalfont, United Kingdom) as specified by the manufacturer. The membranes were exposed to hyperfilm (Amersham), and the intensity of each dot was determined using a densitometer (Scan Analysis 2.02; Biosoft, Cambridge, United Kingdom). The results were then expressed as a ratio of cytokine to β-actin density.
ELISAs for detection of cytokine protein.
The level of IL-6, IL-13, and IFN-γ in the supernatants were determined by using commercially available ELISA kits (PeliKine CompactTM CLB, Amsterdam, The Netherlands). The sensitivity of the assay was 5 pg/ml for IL-6, 3 pg/ml for IL-13, and 4 pg/ml for IFN-γ. IL-5 protein was measured by an in-house ELISA, using rat immunoglobulin G1 (IgG1) anti-human IL-5 monoclonal antibody (clone TRFK5; Pharmingen, San Diego, Calif.) for capture and biotinylated rat IgG2a anti-IL-5 monoclonal antibody (clone JES1-5 A10; Pharmingen) for detection. The standard curve was generated using serial dilutions of recombinant human IL-5 (Pharmingen); the limit of detection was 6 pg/ml. IL-10 protein was also measured by an in-house ELISA, using rat IgG1 anti-human IL-10 monoclonal antibody (clone JES3-9D7; Pharmingen) for capture and biotinylated rat IgG2a anti-human IL-10 monoclonal antibody (clone JES3-12G8; Pharmingen) for detection. For the standard curve, we used recombinant human IL-10 (Pharmingen); the limit of detection was 4 pg/ml.
Statistical analysis.
Cytokine responses induced by TT and PHA were analyzed by the Wilcoxon matched-pairs signed rank test for paired responses. The statistical package StatView 5.0.1 was used.
RESULTS
Vaccine antigen-specific cytokine responses.
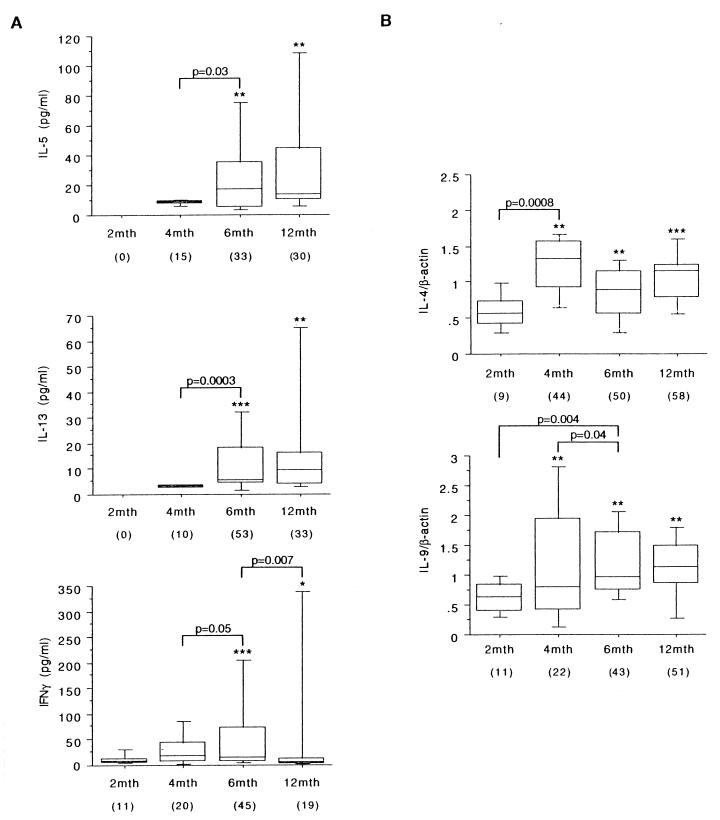
The vaccine antigen-specific cytokine responses are illustrated in Fig. 1. At the 2-month bleed, prior to vaccination, TT antigen-induced IL-5, IL-13, IFN-γ, IL-4, and IL-9 responses were infrequent and extremely low. Cytokine protein responses remained low at the 4-month bleed, but IL-4 and IL-9 were detectable at the mRNA level. At 6 and 12 months, the Th2 cytokine responses were increased (IL-5 and IL-13 protein) or sustained (IL-4 and IL-9 mRNA) compared to the responses seen at 4 months. In contrast, IFN-γ responses peaked in frequency (45%) and intensity at 6 months but waned significantly by 12 months. At the population level, antigen-induced IL-6 and IL-10 production was not significant but low-level responses were observed in approximately 15% of children for IL-6, including at the prevaccination bleed (data not shown); indirect evidence from other studies (18) suggests that the principal source of IL-6 here consists of monocytes armed with transplacentally transferred maternal antibody.
FIG. 1.
TT-specific cytokine production by PBMC. The data shown illustrate TT-specific cytokine responses in infants undergoing DTaP vaccination, as detailed in Materials and Methods. The results are presented as box plots. The limits of the boxes represent the 25th and 75th percentiles of the results. The enclosed line represents the median (50th percentile), and the bars represent the 10th and 90th percentiles. (A) The cytokine protein content of culture supernatants was assayed by ELISA and expressed as picograms per milliliter. (B) Cytokine-specific mRNA was determined by semiquantitative reverse transcription-PCR and is presented as a ratio relative to β-actin. The data are expressed as delta values (treatment minus control) from those positively responding to TT (a twofold increase above control production was defined as a positive cytokine response; percentages are shown in parentheses). The significance of differences between test and control cultures within the overall study population, and between the various sampling points, was determined using the Wilcoxon matched-pairs signed rank test for paired responses (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.0001).
Age-related changes in cytokine production capacity.
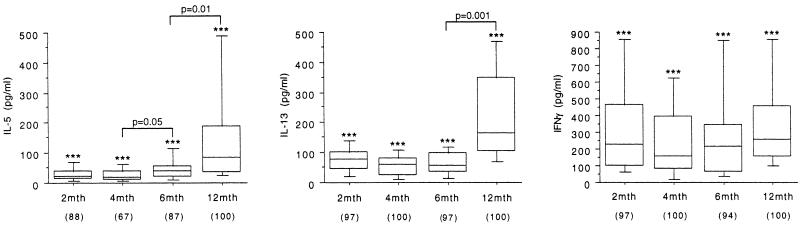
The experiments in Fig. 2 sought to document developmental changes in cytokine production capacity over the first 12 months of life, employing polyclonal PHA stimulation.
FIG. 2.
PHA induction of cytokine production by PBMC. The data shown illustrate parallel PHA-induced cytokine responses in the PBMC samples used in the experiment in Fig. 1, as detailed in Materials and Methods. The results are presented for positive responders (the percentage of each age group that was positive is shown in parentheses) as box plots, as in Fig. 1. Differences between groups were analyzed as in Fig. 1.
As an example of the Th2 cytokine compartment, production of IL-5 and IL-13 was compared at the four time points shown. IL-5 production capacity began to rise after 4 months, and production levels of both IL-5 and IL-13 at 12 months were significantly elevated over those observed at earlier sampling points. In contrast, IFN-γ production levels did not rise over the same period; IFN-γ responses in PBMC from age 12 months were approximately sixfold lower than those observed in 6-year-old children stimulated under identical conditions (data not shown).
DISCUSSION
The range of vaccines used in pediatric practice is increasing, and further increases can be expected in the medium-term future. However, our level of understanding of the nature of vaccine-induced immune responses in human infants has remained relatively static. The present study addresses this important issue at a very basic level, by assessment of time-dependent changes in Th-cell responses to TT antigen in a cohort of 55 infants undergoing DTaP vaccination.
The relevant information already available in the literature relating to infants is restricted to two recent reports on a limited range of specific cytokine responses to pertussis antigens, studied at 1 month after completion of the three-step “primary vaccination” schedule (3, 28), at which time the responses may be expected to approximate peak levels. In contrast, the present study examined the production of seven cytokines in response to TT at four time points up to age 12 months, an age midway between the final “primary” dose at 6 months and the first booster (due at age 18 months).
The salient findings from these studies are as follows. First, as reported previously (3, 28), levels of cytokine production exhibit large variations between individual infants. However, clear statistically significant population responses were observed in this cohort, especially after the two initial vaccine doses, by which time approximately half of the group exhibited positive IL-4, IL-9, IL-13, and IFN-γ responses and around one-third were positive for IL-5. The detection of IL-9 and IL-4 mRNA in the early phase of these responses is interesting, since, together with the parallel findings on the presence of IL-5 and IL-13 protein, this emphasizes the strong contribution of the Th2 cytokine compartment to these early vaccine responses. We have reported similar findings recently with respect to responses in infants to nonvaccine antigens from the normal environment, which are encountered at mucosal surfaces (13, 19, 27).
These early responses to the TT component of the DTaP vaccine are not restricted exclusively to Th2 cytokines, since significant production of IFN-γ was noted at the 6-month time point in response to TT. The presence of this mixed response is consistent with an earlier report (10) on a small number of adults boosted with TT.
The key difference between the Th1 and Th2 arms of these responses is not evident until the 12-month bleed. As noted in Fig. 1, unlike the Th2 cytokine responses, which are relatively stable between 6 and 12 months, the IFN-γ component significantly declines during this period, suggesting that Th memory development in the Th1 compartment is poor at this age. These findings are consistent with recent findings with infant mice, which are capable of initiating significant primary Th1 and Th2 responses but in which the subsequent Th memory generation is largely restricted to the Th2 component (4, 5, 7, 23).
The overall Th2 polarity of immune responses during infancy reflects the situation in the fetal compartment, in which Th1 responses are actively suppressed via a variety of control mechanisms in order to protect the placenta against the toxic effects of IFN-γ (26). It is clear, however, that this Th1 deficiency is not absolute, since our current findings and those from earlier studies on infant responses to the DTP vaccine (21) demonstrate moderate IFN-γ production in a proportion of subjects; additionally, strong Th1 responses can be readily stimulated in early infancy with more powerful stimulants such as BCG (16), as has been observed in mice (4). However, this is equally clearly not the case with less potent antigens, which lack intrinsic Th1-stimulatory properties such as environmental allergens (13, 19, 27), and the relatively low capacity to express Th1 immunity during infancy has been suggested to be an important factor in the development of Th1- versus Th2-biased immunity to these agents during early life (13).
Figure 1 suggests that this general paradigm may also be applicable to DTaP vaccine-specific immune responses during infancy. It can be seen that after an initial lag during the immediate postnatal period, the capacity of PBMC from infants in this cohort to produce the archetypal Th2 cytokines IL-5 and IL-13 following polyclonal stimulation increases markedly (Fig. 2). This increase broadly parallels the age-related contribution of these two cytokines to the respective TT-specific responses. In contrast, IFN-γ responses to TT were transient and usually waned between the last inoculation at 6 months and the final PBMC collection at 12 months. The failure of this component of the response to persist after primary vaccination is paralleled by the apparent failure of overall IFN-γ production capacity to expand beyond the initial neonatal range (Fig. 2).
These results suggest that during the period between primary vaccination and boosting (due at 18 months), the level of DTaP vaccine-specific cell-mediated immunity may be relatively low. If recent suggestions that protective immunity against agents covered by the DTP vaccine relies in part upon a cellular (Th1) response component (21) prove to be correct, it may be hypothesized that the phase between primary vaccination and first boost represents a potential “window of increased risk” for infection, due to failure of maturation of the Th1 component of the vaccine-driven response. Further research is required to clarify this important issue. It is also possible that aspects of the initial cytokine responses to the vaccine may to some degree be predictive of quantitative and/or qualitative aspects of ensuing memory, and this possibility will also be examined in longer-term follow-up studies.
REFERENCES
- 1.Ad Hoc Study Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet. 1988;31:955–960. [PubMed] [Google Scholar]
- 2.Ausiello C M, Lande R, Urbani F, La Sala A, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect Immun. 1999;67:4064–4071. doi: 10.1128/iai.67.8.4064-4071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausiello C M, Urbani F, La Sala A, Lande R, Cassone A. Vaccine and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios C, Brandt C, Berney M, Lambert P-H, Siegrist C-A. Partial correction of the Th2/Th1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur J Immunol. 1996;26:2666–2670. doi: 10.1002/eji.1830261118. [DOI] [PubMed] [Google Scholar]
- 5.Barrios C, Brawand P, Berney M, Brandt C, Lambert P-H, Siegrist C-A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Field E H. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–941. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chen N, Gao Q, Field E H. Expansion of memory Th2 cells over Th1 cells in neonatal primed mice. Transplantation. 1995;60:1187–1193. [PubMed] [Google Scholar]
- 8.Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illness. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 9.Cooper P J, Espinel I, Paredes W, Guderian R H, Nutman T B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 10.ElGhazali G E B, Paulie S, Andersson G, Hansson Y, Holmquist G, Sun J-B, Olsson T, Ekre H P, Troye-Blomberg M. Number of interleukin-4- and interferon-γ-secreting human T cells reactive with tetanus toxoid and the mycobacterial antigen PPD or phytohemagglutinin: distinct response profiles depending on the type of antigen used for activation. Eur J Immunol. 1993;23:2740–2745. doi: 10.1002/eji.1830231103. [DOI] [PubMed] [Google Scholar]
- 11.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi A, Anemona A, Ciofo Degli Atti M L, Giammanco A, Panei P, Blackwelder W C, Klein D L, Wassilak S G F the Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334:341–348. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson L, Hallander H O, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 13.Holt P G, Macaubas C. Development of long term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr Opin Immunol. 1997;9:782–787. doi: 10.1016/s0952-7915(97)80178-1. [DOI] [PubMed] [Google Scholar]
- 14.Macaubas C, Sly P D, Burton P, Tiller K, Yabuhara A, Holt B J, Smallacombe T B, Kendall G, Jenmalm M, Holt P G. Regulation of Th-cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29:1223–1231. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahon B P, Ryan M S, Griffin F, Mills K H G. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchant A, Goetghebuer T, Ota M, Wolfe I, Ceesay S J, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, Aaby P, McAdam K P, Newport M J. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 17.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott S L, Macaubas C, Holt B J, Smallacombe T, Loh R, Sly P D, Holt P G. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards the Th-2 cytokine profile. J Immunol. 1998;160:4730–4737. [PubMed] [Google Scholar]
- 19.Prescott S L, Macaubas C, Smallacombe T, Holt B J, Sly P D, Holt P G. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 20.Ridge J P, Fuchs E J, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 21.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Øymar K, Miller E, Storsaeter J, Mills K H G. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt H J, Wirsing von König C H, Neiss A, Bogaerts H, Bock H L, Schulte-Wissermann H, Gahr M, Schult R, Folkens J U, Rauh W, Clemens R. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA. 1996;275:37–41. [PubMed] [Google Scholar]
- 23.Singh R R, Hahn B H, Sercarz E E. Neonatal peptide exposure can prime T cells, and upon subsequent immunization induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–1622. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 25.Upham J W, Holt B J, Baron-Hay M J, Yabuhara A, Hales B J, Thomas W R, Loh R K S, O'Keeffe P, Palmer P, Le Souef P, Sly P D, Burton P R, Robinson B W S, Holt P G. Inhalant allergen-specific T-cell reactivity is detectable in close to 100% of atopic and normal individuals: covert responses are unmasked by serum-free medium. Clin Exp Allergy. 1995;25:634–642. doi: 10.1111/j.1365-2222.1995.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 26.Wegmann T G, Lin H, Guilbert L, Mosmann T R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 27.Yabuhara A, Macaubas C, Prescott S L, Venaille T, Holt B J, Habre W, Sly P D, Holt P G. Th-2-polarised immunological memory to inhalant allergens in atopics is established during infancy and early childhood. Clin Exp Allergy. 1997;27:1261–1269. [PubMed] [Google Scholar]
- 28.Zepp F, Knuf M, Habermehl P, Schmitt H J, Rebsch C, Schmidtke P, Clemens R, Slaoui M. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]