Abstract
Purpose
Staphylococcus aureus is an important cause of corneal infections (keratitis). To better understand the virulence mechanisms mediating keratitis, a recent comparative genomics study revealed that a set of secreted enterotoxins were found with higher prevalence among ocular versus non-ocular S. aureus clinical infection isolates, suggesting a key role for these toxins in keratitis. Although well known to cause toxic shock syndrome and S. aureus food poisoning, enterotoxins have not yet been shown to mediate virulence in keratitis.
Methods
A set of clinical isolate test strains, including a keratitis isolate that encodes five enterotoxins (sed, sej, sek, seq, ser), its corresponding enterotoxin deletion mutant and complementation strain, a keratitis isolate devoid of enterotoxins, and the non-ocular S. aureus strain USA300 along with its corresponding enterotoxin deletion and complementation strains, were evaluated for cellular adhesion, invasion and cytotoxicity in a primary corneal epithelial model as well as with microscopy. Additionally, strains were evaluated in an in vivo model of keratitis to quantify enterotoxin gene expression and measure disease severity.
Results
We demonstrate that, although enterotoxins do not impact bacterial adhesion or invasion, they do elicit direct cytotoxicity in vitro toward corneal epithelial cells. In an in vivo model, sed, sej, sek, seq, ser were found to have variable gene expression across 72 hours of infection and test strains encoding enterotoxins resulted in increased bacterial burden as well as a reduced host cytokine response.
Conclusions
Our results support a novel role for staphylococcal enterotoxins in promoting virulence in S. aureus keratitis.
Keywords: enterotoxins, staphylococcus aureus, infectious keratitis
Bacterial corneal infection (bacterial keratitis) is a devastating, vision-threatening disease associated with severe ocular tissue damage.1–7 The Gram-positive organism, Staphylococcus aureus, is a major cause of bacterial keratitis, responsible for upwards of 20% of all bacterial keratitis cases worldwide whereas coagulase-negative Staphylococcus (CoNS) species are responsible for nearly 40% of cases.8–13 Importantly, antibiotic resistance among S. aureus and CoNS ocular isolates continues to rise, particularly among methicillin-resistant S. aureus (MRSA) and CoNS (MRCoNS) strains, rendering current therapeutics increasingly ineffective. For example, the widely prescribed fluroquinolone antibiotics such as ciprofloxacin, levofloxacin, and moxifloxacin have been particularly impacted, with resistance rates ranging from 7% to 12% among methicillin-sensitive isolates yet has high as 41% to 96% among MRCoNS and MRSA strains.14–18 Unfortunately, the clinical consequences of resistant infections are significant and include increased disease severity, which manifests as increased infiltrate/scar size, decreased time to re-epithelialization, and worse visual outcomes.19,20 Given the urgent need for new therapies, there is a growing interest in uncovering the key S. aureus drivers of keratitis to provide insight into virulence mechanisms of this important human pathogen, as well as generate attractive targets for future antimicrobial drug development.
Among the extensive arsenal of known S. aureus virulence factors, it is increasingly recognized that although some bacterial virulence determinants may be critical in promoting disease across a variety of infection sites, there may be others that provide a selective advantage in specific physiologic niches. For example, α-toxin (hla), a canonical β-barrel pore-forming toxin has been shown to significantly contribute to virulence in diverse S. aureus infection sites such as pneumonia, skin and soft tissue disease, sepsis, and keratitis.21–26 In contrast, Panton-Valentine leucocidin (PVL), a leukocytic pore-forming toxin has been shown to be important in mediating skin and soft-tissue disease, yet it has a minor or no role in invasive disease.22,27 Additionally, collagen binding adhesion, although commonly identified among diverse S. aureus isolates, is highly conserved among the most virulent osteomyelitis strains,28–30 suggesting a key role for this virulence factor in this specific niche.
Given that the ocular surface is subject to sheer forces generated from blinking, variations in tear film composition and near-constant exposure to the environment, there is likely a unique set of S. aureus virulence factors that promote disease in this distinct environment. Initial studies have shown, for example, that infections with hla(−) strains of S. aureus in a rabbit model of keratitis exhibited decreased bacterial burden and corneal ulceration compared to wildtype strains.26,31 Conversely, β-toxin, a hemolytic sphingomyelinase important in endocarditis and pneumonia,32 appears to have a limited role in driving corneal disease.26 Additional studies have suggested a role for virulence factors such as superantigen-like protein 133 and staphopain A34 in promoting keratitis, whereas PVL may have a strain-specific, variable effect.35
To further identify relevant virulence factors driving S. aureus keratitis, we recently undertook a broad-scale genomics approach to interrogate and compare whole-genome sequences of clinical S. aureus isolates collected from ocular and nonocular sources for the presence/absence of 235 known S. aureus virulence factors.36 This unbiased approach revealed that although both ocular and nonocular pathogenic S. aureus isolates share many overarching genetic similarities such as strain classification types, with regard to virulence factors, a set of 10 staphylococcal secreted enterotoxins (seu, selo, seln, selm, seg, selv, sei, sed, sej, ser), as well as two enterotoxin pseudogenes (Ψ -ent1, Ψ -ent2) were found at a significantly higher prevalence among ocular isolates compared to nonocular isolates. In fact, the majority of these enriched enterotoxins were found at nearly twofold higher rates among ocular versus nonocular strains, suggesting that this class of secreted toxins may provide a selective advantage in mediating ocular disease. Further supporting this conclusion, in a separate yet related study, Afzal et al.37 recently demonstrated a higher prevalence of the enterotoxin, sea, among a set of ocular versus nonocular S. aureus isolates.
Enterotoxins are a class of well-studied S. aureus virulence factors primarily known for their role in producing potent emetic activity in staphylococcal food poisoning38 and functioning as superantigens, capable of promoting widespread T-cell activation to cause fever, sepsis, and end-stage organ failure.39,40 But if and how these secreted toxins may contribute to ocular disease is relatively unknown. Purified staphylococcal enterotoxin B has been used in experimental murine models of corneal transplantation to mitigate host immune rejection,41 and there has been a single report suggesting that the enterotoxins sei, seg, and seb may result in increased corneal ulceration and disease severity in atopic keratoconjunctivitis.42 However, there is yet to be a comprehensive study of how these powerful toxins may promote infectious keratitis.
In the current study, we explore the role of a set of enterotoxins in keratitis using a combination of in vitro corneal epithelial cell models, confocal microscopy, and an in vivo murine model of keratitis. Leveraging contemporary clinical keratitis isolates that encode enterotoxins with corresponding isogenic enterotoxin deletion mutants and complementation strains, we demonstrate that enterotoxins cause direct corneal epithelial cell toxicity, as well as result in increased bacterial burden in murine keratitis. Furthermore, we demonstrate that enterotoxins are expressed throughout the duration of an in vivo infection and can mediate the host response, significantly dampening host cytokine expression. Taken together, our results demonstrate a novel role for enterotoxins in promoting virulence in keratitis.
Material and Methods
Strains and Growth Conditions
All bacterial isolates used in this study are listed in Tables 1A and 1B. IHMA70, an ocular isolate that encodes enterotoxins sed, sej, sek, seq, and ser, and IHMA104, an ocular isolate that is devoid of enterotoxins, were purchased from International Healthcare Management Associates (Schaumburg, IL, USA) and are part of a larger strain collection described previously.17 Comparator non-ocular strains NE1809 (USA300ΔselX) and NE1787 (USA300ΔsrtA) were obtained from the Nebraska Transposon Library.43 Overnight cultures were prepared by inoculation of a single colony into 5 mL brain-heart infusion (BHI) broth (Becton Dickenson, Franklin Lakes, NJ, USA) and incubated overnight (37°C, 200 rpm). For experiments requiring exponential phase cells, overnight cultures were diluted 1:100 in fresh BHI and allowed to grow until an optical density at 600nm (OD600nm) of 0.180 was reached.
Table 1A.
Strains Used in This Study
| Isolate | MLST | Enterotoxin | MRSA/MSSA | Reference |
|---|---|---|---|---|
| IHMA70 | 8 | sed, sej, sek, seq, ser | MSSA | 17 |
| IHMA70Δe1 | 8 | sek, seq | MSSA | This study |
| IHMA70Δe2 | 8 | sed, sej, ser | MSSA | This study |
| IHMA70Δe1Δe2 | 8 | none | MSSA | This study |
| IHMA70Δe1Δe2+pe1 | 8 | sed, sej, ser | MSSA | This study |
| IHMA70Δe1Δe2+pe2 | 8 | sek, seq | MSSA | This study |
| IHMA104 | 8 | None | MSSA | 17 |
| NE1787 (USA300ΔsrtA) | 8 | sek, seq, selX | MRSA | 43 |
| USA300LAC | 8 | sek, seq, selX | MRSA | 87 |
| NE1809 (USA300selX::tn) | 8 | sek, seq | MRSA | 43 |
| USA300selX::tn, Δkq | 8 | None | MRSA | This study |
| USA300selX::tn, Δkq +pKQ | 8 | sek, seq | MRSA | This study |
| USA300selX::tn, Δkq +pX | 8 | selX | MRSA | This study |
MLST, multilocus sequence type.
Table 1B.
Strains and Plasmids Used in This Study
| Cloning Strains or Plasmids | Genotype/Phenotype | Selection Marker | Reference |
|---|---|---|---|
| DH5α | E. coli cloning strain | — | 45 |
| RN4220 | S. aureus Restriction deficient cloning strain | — | 47 |
| pUC19 | Cloning vector | Amp | 44 |
| pUC19-Δe1 | 600bp flanking regions of e1 | Amp, Erm | This study |
| pUC19-Δe2 | 600bp flanking regions of e2 | Amp, Kan | This study |
| pUC19-USA300Δkq | 600bp flanking regions of seq sek | Amp, Kan | This study |
| pCL52.2 | E. coli S. aureus shuttle vector | Tet | 46 |
| pCL52.2-Δe1 | 600bp flanking regions of e1 | Tet, Erm | This study |
| pCL52.2-Δe2 | 600bp flanking regions of e2 | Tet, Kan | This study |
| pCL52.2- USA300Δkq | 600bp flanking regions of seq sek | Tet, Kan | This study |
| pRMC2 | Expression vector | Cam | 49 |
| pRMC2-e1 | Complementation of IHMA70 e1 enterotoxin cluster | Cam | This study |
| pRMC2-e2 | Complementation of IHMA70 e2 enterotoxin cluster | Cam | This study |
| pRMC2-kq | Complementation of USA300 sek seq enterotoxin cluster | Cam | This study |
| pRMC2-selX | Complementation of USA300 selX | Cam | This study |
Amp, ampicillin; Cam, chloramphenicol; Erm, erythromycin; Kan, kanamycin; Tet, tetracycline
In the clinical keratitis isolate IHMA70, 5 enterotoxins (sed, sej, ser, sek, seq) are genetically located in two distinct clusters. Thus enterotoxin deletion mutants (IHMA70Δe1, IHMA70Δe2, and IHMA70Δe1Δe2) (Table 1) were created by amplifying 600bp flanking regions up- and down-stream of enterotoxin cluster 1 (e1; sed, sej, ser) or enterotoxin cluster 2 (e2; sek, seq) (Primers listed in Table 2) using DreamTaq Master Mix (Thermo Scientific, Waltham, MA, USA). In NE1809 seq and sek are located adjacent to each other, and thus 600bp flanking regions up- and down-stream of these genes were also amplified (Table 2). Amplified flanking sequences were gel purified (PureLink Quick Gel Extraction & PCR Combo Kit; Invitrogen, Waltham, MA, USA), ligated into the cloning vector pUC19,44 and transformed into Escherichia coli DH5α.45 To facilitate detection of deletion mutants, two selection markers, erythromycin and kanamycin, were amplified from the Bursa aurelias (erythromycin) or EZ-Tn5-Kan (kanamycin) transposons, gel purified, and ligated between the respective flanking sequences and cloned into pUC19 to create pUC19-e1:erm, pUC19-e2:kan, and pUC19-USA300kq:kan. Next, flanking regions with their respective selection makers were amplified, gel purified, and ligated into the temperature sensitive tetracycline-resistant shuttle vector pCL52.2.46 The pCL52.2 derivatives were then electroporated into the restriction-deficient cloning strain S. aureus RN422047 using standard electroporation technique48 and grown in BHI broth supplemented with 10 µg mL−1 tetracycline at 30°C. Plasmids were subsequently isolated from RN4220 using the QiaPrep Spin mini kit (Qiagen, Germantown, MD, USA), and electroporated into IHMA70 or NE1809. Allelic replacement was carried out by heat shock at 43°C for 24 hours, followed by incubation in brain-heart infusion broth at 37°C for five days without selection to cure the plasmid. To identify successful clones, cells were plated onto nonselective media, and colonies were replica plated onto brain-heart infusion agar plates containing tetracycline (10 µg mL−1) or the appropriate selection marker (erythromycin, 10 µg mL−1 or kanamycin, 50 µg mL−1). To verify the deletion of enterotoxins, colony PCR was performed from colonies displaying growth on the appropriate selection marker, but not tetracycline, with colonies negative for detection of e1, e2 or sek-seq subsequently confirmed by sequencing the PCR fragment and qPCR using the relevant primers in Table 2.
Table 2.
Primers Used for the Knockout and Complementation of Enterotoxins in IHMA70 and USA300
| Primer | Target | Sequence |
|---|---|---|
| SEJ-REF | 600bp upstream of sej | AATAACTGCAGTACAGAACCAAAGGTAGAC |
| SEJ-RER | AGGTCGACAACAAGTAGATCTATACGG | |
| SER-REF | 600bp downstream of ser | CTGGTACCTGACTGGTGCTATG |
| SER-RER | TTTGAATTCTAACATGAATACACCTC | |
| SEK-REF | 600bp upstream of sek | TTTGACTGCAGTAAATTGGCTACTTACTC |
| SEK-RER | TCAGTCGACTCCTTGAGTATATTGGTTG | |
| SEQ-REF | 600bp downstream of seq | CGAGGTACCAGTACAAAGACCCACTC |
| SEQ-RER | TCAGTCGACTCCTTGAGTATATTGGTTG | |
| e1Comp-F | 150bp upstream of sej | CAACATCGGATCCTATTCTCATAGAATTTGTCTAATTAAGTGTACG |
| e1Comp-R | 150bp downstream of ser | GTCTCTCGAGGATGTTAAAGTATTTGAATTGACTAC |
| e2Comp-F | 150bp upstream of sek | TTACAACTCGAGACTCGGAAGATGATAAAACTAAAAGAGAC |
| e2Comp-R | 3ʹ end of seq | GGCGGGCTAGGATCCCCGAAAAATAATG |
| USA300K-F | 600bp downstream of sek | GCATTGGGAATTCGCCTTTATGATTAGTAAATAC |
| USA300K-R | CGGGTACCGCATGCCTACCC | |
| USA300Q-F | 600bp upstream of seq | ACTCTCAACGGATCCTCAAAT |
| USA300Q-R | GTACCACGTTTACACCTGCAGCTATC | |
| UCompKQ-F | 150bp upstream of seq | CCTCGAATTCGTGTACAAGATAAA |
| UCompKQ-R | 3ʹ end of sek | AGATCACCTCTGGTACCAAA |
| UCompX-F | 150bp upstream of selX | ACGAAAGGATCCAACGCATGACG |
| UCompX-R | 3ʹ end of selX | CATGCATTTAGCTGACTTCTGCAGTTG |
Complementation plasmids were then created for e1, e2, and USA300 sek-seq. Each enterotoxin cluster, including predicted promoter elements, was amplified from genomic DNA, gel purified, ligated into pRMC2,49 and electroporated into RN4220. Plasmids were subsequently isolated and electroporated into IHMA70Δe1Δe2 or NE1809Δkq as appropriate.
Gene Expression of Enterotoxins
Expression of five enterotoxins (sed, sej, sek, seq, and ser) was evaluated by qRT-PCR using the primers listed in Table 3. Cells from exponential or stationary phase cultures were pelleted by centrifugation (3000g, 10 minutes), washed twice with phosphate buffered saline solution (PBS), and lysed by bead mill homogenization in Lysing Matrix B tubes (MP Biomedicals, Irvine, CA, USA). RNA was extracted from the resulting homogenate using the RNeasy mini kit (Qiagen) following the manufacturer's instructions. cDNA synthesis was performed utilizing the qScript cDNA SuperMix kit (QuantaBio, Beverly, MA, USA). Expression analysis was performed on a BioRad CFXConnect real-time PCR system (Bio-Rad Life Science, Hercules, CA, USA) using PerfeCTa SYBR Green FastMix (QuantaBio) following the manufacturer's instructions, with normalization to the bacterial transcription termination factor, rho, expression.50
Table 3.
The qPCR Primers Used in This Study
| Name | Gene | Sequence |
|---|---|---|
| rpoB-F | rpoB | GCATTAGGACCTGGTGGTTTA |
| rpoB-R | TTTGGTCCCTCAGGTGTTTC | |
| rho-F | rho | CAACGCGCATCATGGATTTAG |
| rho-R | CGTACTGATTGCATTCGCTATTT | |
| sed-F | sed | GTCACTCCACACGAAGGTAATA |
| sed-R | CCTTGCTTGTGCATCTAATTCT | |
| sej-F | sej | GACGGACATCAAACAGAAATAGAA |
| sej-R | CAATGTCGCCACCTTGTTC | |
| sek-F | selK | CATTTATGGACATAACGGCACTAA |
| sek-R | CCCATCATCTCCTGTGTAGAATAA | |
| seq-F | selQ | CTTTGGAATAAGTTACTCAGGTCTTTG |
| seq-R | GCTTACCATTGACCCAGAGATT | |
| ser-Fwd | ser | GACAAACGGTTAGATGTGTTTGG |
| ser-Rev | AGCTGTGGAGTGCATTGTAA |
Cytotoxicity, Invasion, and Adherence Assays
Human primary corneal epithelial cells (PCS-700-010) were purchased from ATCC (Manassas, VA, USA) and maintained in Corneal Epithelial Cell Basal Medium (PCS-700-030; ATCC) supplemented by the Corneal Epithelial Cell Growth Kit (PCS-700-040, ATCC) and 1000 U mL−1 penicillin with 1 µg mL−1 streptomycin (PenStrep, Gibco, Waltham, MA, USA). Corneal epithelial cells were seeded in 96 well tissue culture treated plates at a density of ∼104 cells mm−1 and allowed to grow until 90% confluency.
S. aureus cellular invasion of corneal epithelial cells was assessed using the gentamicin exclusion assay as described by Edwards et al.51 Briefly, bacterial cells were grown to mid-exponential phase, pelleted by centrifugation (3000g, 10 minutes), and washed twice with PBS. Cells were then re-suspended in PBS to a final OD600nm = 0.300. Bacterial infection was then initiated by adding 10 µL of washed bacterial cells to a confluent corneal epithelial cell monolayer for a final bacterial concentration of ∼107 colony forming units (CFU) mL−1. Cells were incubated for 15, 30, 60, or 90 minutes after which the growth medium was removed, and corneal cells washed three times. Corneal epithelial cells were then treated with 50 µg mL−1 gentamicin for 60 minutes to cure any remaining extracellular bacterial cells. To measure invasion, corneal epithelial cells were lysed using 0.5% Triton ×100 in PBS, and the number of surviving bacterial cells was enumerated by plating onto BHI agar. To assess adherence, the same protocol was used except for the gentamicin wash. To calculate adherence, the number of cells recovered from the gentamicin protection assay (invasion) was subtracted from the total number of cells recovered in the adherence assays. Adherence and invasion assays were conducted in triplicate (biological replicates). All bacterial strains were confirmed to susceptible to gentamicin before conducting studies.
To assess bacterial cytotoxicity of secreted toxins, bacterial supernatant was prepared by pelleting overnight cultures in BHI broth (3000g for 10 minutes), after which the spent culture media was removed and filter sterilized by passage through a 200 nm syringe filter. Filtered supernatant was mixed 1:1 with corneal cell growth media and added to each well, whereas fresh BHI broth at a 1:1 mixture and 50 µg mL−1 mitomycin C were used as controls. Cytotoxicity was first measured at 15, 30, 60, and 90 minutes after challenge using the vital dye trypan blue. Corneal cell death after supernatant challenge was also visualized via confocal microscopy. After 60 minutes of incubation, cells were stained by using the PrimoKine Live/Dead II kit following the manufacturer's instructions for 45 minutes. Stained cells were visualized using green (calcein-AM), red (ethidium homodimer-III), and bright field channels, on a Zeiss PALMBeam at objective ×20, and images were captured using an Axio MRm camera and AxioVision software (version 4.8, Carl Zeiss Microscopy, Jena, Germany).
Murine Model of Keratitis
Corneal infections were performed as described previously,52 with adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Briefly, four- to six-week-old female BALB/c mice were obtained from Charles River Laboratories (Washington, MA, USA) and housed following protocols approved by the University of Rochester Council on Animal Research. To initiate corneal infection, mice were anesthetized by subcutaneous injection of 100 mg kg−1 ketamine (Par Pharmaceutical, Chestnut Ridge, NY, USA) in combination with 10 mg kg−1 xylazine (Akorn, Inc., Lake Forest, IL, USA). After anesthesia, topical 0.5% proparacaine (Akorn) solution was applied to the right eye, and any excess liquid blotted from the corneal surface. Three parallel 1-mm scratches across the central cornea were created using a 27-gauge needle carrying a single S. aureus colony, followed by inoculation with a 5-µL suspension of washed cells resuspended in PBS containing 107 CFU mL−1 of the same bacterial strain.
To assess bacterial burden mice were euthanized 24, 36, or 48 hours after infection, the right eye was removed, homogenized in sterile PBS ×1 using 1.4 mm ceramic beads (Fisher Scientific, Waltham, MA, USA), serial diluted in PBS, and plated onto mannitol salt agar. Uninfected left eyes were collected at each time point as negative controls. To assess the expression of enterotoxins in vivo, RNA was extracted from eye homogenate lysate using the RNeasy mini kit (Qiagen), with cDNA synthesis and quantification conducted following the steps described above with the primers in Table 3.
Cytokine ELISA Assays
Production of interleukin (IL)-6, IL-12 (p70 fragment), IL-13, tumor necrosis factor (TNF)-α, and tumor growth factor (TGF)-β was assessed in whole mouse eye homogenate (see above) by standard ELISA kits (R&D Systems) following the manufacturer's instructions and visualized using streptavidin-conjugated alkaline phosphatase (BioRad).
Statistical Analysis
In vitro invasion and adherence assays were compared using the Student's t-test, whereas percent survival data was analyzed using Fisher's exact test. In vivo bacterial burden was compared across 45 independent mice by 2-way ANOVA, followed by post hoc testing with Tukey's honestly significant difference (Tukey's HSD) test. All analysis was performed using JMP Pro (version 15; SAS Inc, Cary, NC, USA) and R (version 4.2.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
S. aureus Clinical Keratitis Isolate Test Strains
Previous studies have identified over 25 S. aureus enterotoxin or enterotoxin-like proteins based on overarching structural and functional homology. Given that many S. aureus isolates encode an average of five to six enterotoxin genes,53,54 we first screened our clinical keratitis strain set for comparator strains that demonstrated high genetic similarity yet differed in the presence/absence of a set of multiple enterotoxins. IHMA70, a 2015 California isolate collected from a 65-year-old male, and IHMA104, a 2016 isolate collected from a 62-year-old female, also from California, exhibited broad similarities including classification as methicillin-sensitive (MSSA), multilocus sequence type 8 (one of the most common S. aureus sequence types identified among ocular infections),36 and in fact, based on whole genome sequence alignment, shared 99.8% identity across the entire genome (data not shown). However, importantly, IHMA70 encoded five enterotoxins (sed, sej, sek, seq, ser) (Table 1), whereas IHMA104 encoded none. Although three of these enterotoxins (sej, sed, ser) are often found encoded within S. aureus plasmids, in IHM70 they were found adjacent to each other integrated into the chromosome within a S. aureus pathogenicity island (SaPI). SaPIs are large mobile genetic elements frequently identified among S. aureus strains that serve as conduits for horizontal gene transfer of antibiotic resistance markers and virulence factors such as enterotoxins.55,56 Sek and seq were also adjacent to each other but were outside of this SaPI containing sej, sed and ser. Although individual enterotoxin genes in each region were adjacent to each other, sequence analysis identified individual promoter regions for each gene, and qRT-PCR studies revealed unique gene expression patterns (Fig. 1). Although enterotoxins are generally considered to be produced during the post-exponential growth phase,57,58 gene expression of ser, sed, sej, sek, and sej was measured in both exponential and stationary growth phases. Among the ser-sed-sej group, ser gene expression did not increase in stationary compared to exponential phase whereas sej increased 4.31-fold and sed 10.1-fold. In the sek-seq group, seq also did not demonstrate a change in expression in stationary phase versus exponential, whereas sek increased 3.5-fold (Fig. 1).
Figure 1.
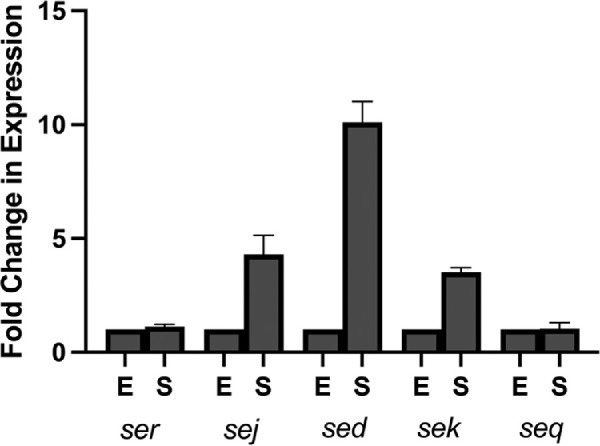
Relative in vitro expression of enterotoxins seq, seq, sed, sej, ser in stationary (S) versus exponential (E) growth phase as measured by qPCR in strain IHMA70.
Based on their overarching similarities apart from the presence/absence of enterotoxins, comparison studies between IHMA70 and IHMA104 could allow for a preliminary evaluation of the role of enterotoxins, as represented by the set of ser, sed, sej, sek, and seq, in mediating keratitis. However, to further appreciate the effects of this set of enterotoxins, isogenic knockout mutants in IHMA70 of either enterotoxin group 1, comprised of ser-sed-sej (IHMA70Δe1), or group 2 comprised of sek-seq (IHMA70Δe2) were constructed, as well as a double knockout of both groups (IHMA70Δe1Δe2). As listed in Table 1, complementation strains were also created with either enterotoxin group 1 or 2 (IHMA70Δe1Δe2:pe1, IHMA70Δe1Δe2:pe2), with a cloning strategy that included each enterotoxin's native promoter. Mutants and corresponding complementation strains did not display any growth defect compared to the wildtype IHMA70 strain (Supplementary Fig. S1). Moreover, to mitigate any IHMA70 strain–specific effects in our results, as well as further study an additional enterotoxin, selX, USA300LAC, a well-studied nonocular S. aureus clinical isolate that encodes three enterotoxins (sek, seq, selX) was selected as an additional comparator strain. Using background strain NE1809 (USA300ΔselX), an isogenic knockout of sek-seq was created, as well as a corresponding complementation strains (USA300Δkqx:pKQ and USA300Δkqx:pX) (Table 1).
Role of Enterotoxins in S. aureus Corneal Epithelial Cell Adherence and Invasion
First, as the corneal epithelium represents a critical anatomic and physiologic barrier to infection,59 the role of enterotoxins ser, sed, sej, sek, and seq in mediating S. aureus corneal cell adhesion and invasion was investigated. Using co-culture and gentamicin protection assays, all S. aureus test strains were directly incubated with a corneal epithelial cell monolayer for 15, 30, 60, or 90 minutes. Gentamicin was used to cure bacteria from the culture media, as well as on the surface of corneal cells allowing for enumeration of bacterial cells that had become internalized (bacterial invasion). To determine adhesion, the number of invading cells was subtracted from bacterial cell counts in which the media was removed, but not treated with gentamicin. A Sortase-deficient mutant (NE1787)43 was selected for a negative control because Sortase plays a key role in cell wall anchoring, and thus this mutant strain is deficient for both adherence and invasion of host epithelial cells.60
As shown in Figure 2, both bacterial adherence and invasion steadily increased over time for test strains IHMA104, IHMA70, IHMA70Δe1, IHMA70Δe2, and IHMA70Δe1Δe2. Importantly, compared to the sortase-deficient mutant, NE1787, the lack of enterotoxins did not diminish the ability of these strains to either adhere or invade corneal epithelial cells, and in fact, there were no significant differences across strains at any measured time point. As a means to further evaluate the role of enterotoxins in bacterial adherence or invasion in an alternative strain background, USA300LAC and its corresponding enterotoxin mutant, USA300Δkqx, were also tested in this corneal cell model. Again, overall levels of adhesion and invasion increased over time, and there were no differences between the wildtype and mutant strains, nor were there significant differences between these strains and IHMA104 or IHMA70 and its derivatives. Taken together, these data suggest that enterotoxins are unlikely to mediate corneal epithelial cell adherence or invasion.
Figure 2.
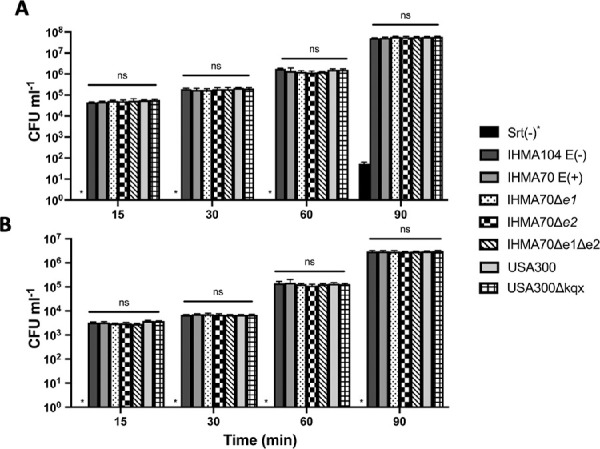
Bacterial cell adhesion (A) or invasion (B) of the indicated test strain following 15, 30, 60, or 90 minutes of co-incubation with corneal epithelial cells. Asterisk indicates none detected
Enterotoxins Promote Corneal Epithelial Cell Cytotoxicity
Although little is yet known regarding the role of enterotoxins in keratitis, enterotoxin-positive S. aureus isolates have been previously linked to corneal ulceration in severe atopic keratoconjunctivitis patients,42 and enterotoxin B (SEB) has been shown to have direct toxic effects on corneal epithelial cells.61 As such, the cytotoxic effect of SER, SED, SEJ, SEK, and SEQ, as measured by exposure to bacterial culture supernatants, was evaluated in an in vitro corneal epithelial model. Because enterotoxins are known secreted toxins, bacterial cell culture supernatant derived from an overnight culture of all test strains was applied to corneal cells for 15, 30, 60, or 90 minutes and resulting cell death evaluated via a trypan blue exclusion assay. As shown in Figure 3A, IHMA70 demonstrated significant toxicity to corneal epithelial cells by 30 minutes, leading to 46% corneal cell death by 90 minutes compared to 14% cell death produced by enterotoxin-negative strain IHMA104 (P = 2e−8). Interestingly, deleting individual enterotoxin groups did not reduce IHMA70’s cytotoxic effect, with supernatant derived from IHMA70Δe1 resulting in 47% corneal cell death and IHMA70Δe2 resulting in 44% corneal cell death, suggesting that neither group is essential for this toxic phenotype. However, deleting both enterotoxin groups (IHMA70Δe1Δe2) led to a significant decrease in cytotoxicity with only 17% corneal cell death at 90 minutes compared to 46% from the parental strain, IHMA70 (P = 8.7e−9). In fact, the cytotoxicity of IHMA70Δe1Δe2 was equivalent to the native enterotoxin-negative IHMA104 isolate at all time points (P > 0.05). Consistent with our findings that only one enterotoxin group was required to elicit toxicity, complementing IHMA70Δe1Δe2 with either enterotoxin group 1 or 2 resulted in full restoration of cytotoxicity. Although at 30 and 60 minutes complemented strains demonstrated slightly less cytotoxicity compared to IHMA70, by 90 minutes there were no differences in corneal cell death between IHMA70 (46% cell death), and the complementation strains IHMA70Δe1Δe2p:e1 (46%) and IHMA70Δe1Δe2p:e2 (47%) (Fig. 3a).
Figure 3.
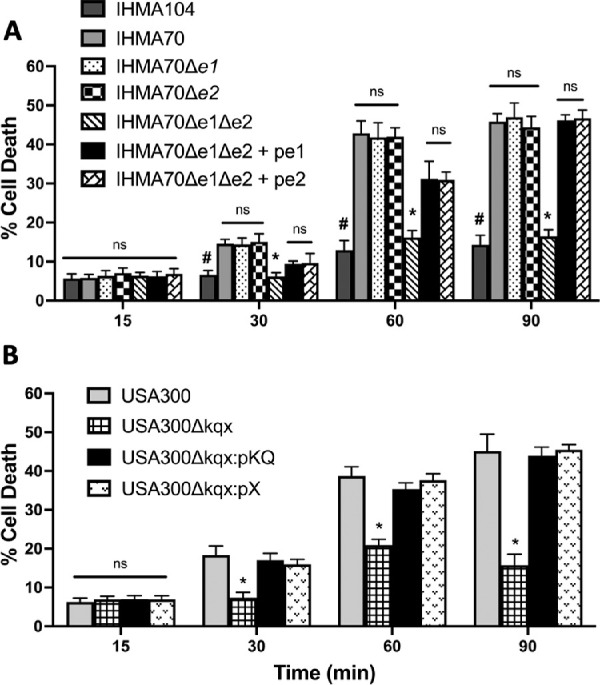
Corneal epithelial cell cytotoxicity after exposure to test strain supernatants over 15, 30, 60, and 90 minutes. (A) IHMA104, IHMA70, as well as the IHMA70 deletion mutants and complementation strains. #P < 3.7e-6 in all instances comparing IHMA104 versus IHMA70. *P < 1.7e-6 in all instances comparing IHMA70Δe1Δe2 versus IHMA70. (B)USA300LAC and corresponding mutant USA300Δkqx and complementation strains. *P < 1.7e-5 for all instances comparing USA300Δkqx versus USA300LAC.
To ensure that the cytotoxic effects observed from IHMA70 supernatant were not strain-specific, trypan blue exclusion assays were also performed with USA300LAC, its corresponding enterotoxin mutant (USA300Δkqx), as well as a complementation strains USA300Δkqx:pKQ and USA300Δkqx:pX). As shown in Figure 3B, the supernatant collected from USA300LAC elicited an equivalent cytotoxic effect on corneal epithelial cells as IHMA70 at all time points (45% vs. 46% cell death). However, deleting all three enterotoxins abrogated this effect, reducing cytotoxicity to levels equivalent IHMA104. For example, by 90 minutes, similar to IHMA104 (14% corneal cell death), USA300Δkqx supernatant caused 16% corneal cell death compared to 45% cell death seen in USA300LAC wild type treated cells (P = 1.6e−6). Again, partial complementation of either sek-seq or just selX in USA300Δkqx, fully restored the cytotoxic phenotype, resulting in 44% and 46% corneal cell death at 90 minutes, respectively. Both IHMA70 and USA300LAC studies reveal that even a single enterotoxin may be sufficient to elicit host cell toxicity, and these effects may be interchangeable across multiple enterotoxin genes. Additionally, the presence of multiple enterotoxins do not appear to produce an additive effect (i.e., deletion of either enterotoxin group 1 or group 2 in IHMA70 did not diminish its cytotoxic effects compared to the parent strain).
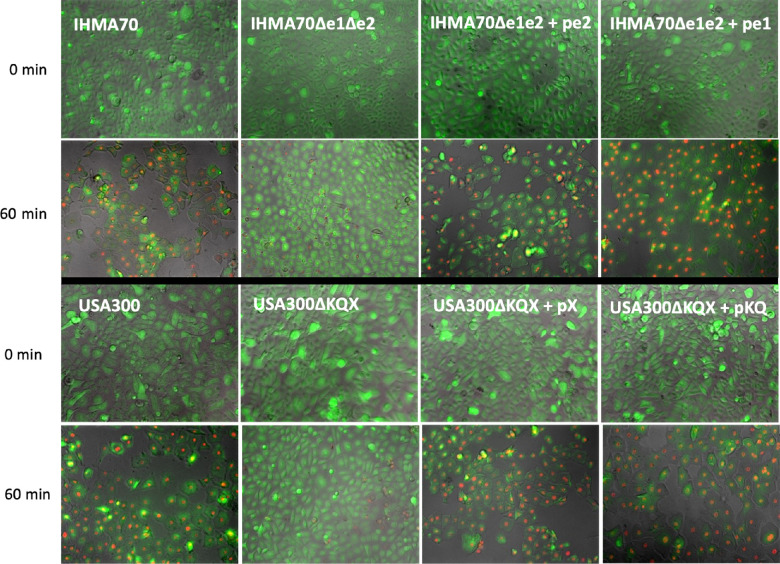
We next visualized the impact of SER, SED, SEJ, SEK, and SEQ toxicity on corneal epithelial cells using fluorescence microscopy and the vital dyes calcein and ethidium-homodimer III. Consistent with our cytotoxicity assays, strains lacking enterotoxins (IHMA104, IHMA70Δe1Δe2, USA300Δkqx) demonstrated little disruption to the corneal epithelial monolayer (Fig. 4). In contrast, corneal cells exposed to strains expressing enterotoxins (IHMA70, IHMA70Δe1Δe2:pe1, IHMA70Δe1Δe2:pe2, USA300:pX, USA300pKQ) demonstrated significant cell dropout after 60 minutes of exposure to the test strain supernatant, indicating substantial cell death. The remaining surviving corneal cells also displayed gross morphologic abnormalities and increased cell membrane permeability as indicated by increased dye uptake (Fig. 4).
Figure 4.
Microscopy demonstrating the cytotoxic effects of test strain supernatants on corneal epithelial cell monolayers at zero and 60 minutes. Fluorescent calcein is produced by live-cell esterase activity resulting in green fluorescence. Conversely, ethidium bromide enters cells with damaged membranes to bind DNA, resulting in red fluorescence of dead or dying cells.
Enterotoxins Promote Virulence in an In Vivo Model Of Keratitis
To further understand whether the observed in in vitro effects of SER, SED, SEJ, SEK, and SEQ in mediating corneal disease correlates with in vivo outcomes, IHMA104, IHMA70 and the corresponding IHMA70Δe1Δe2 enterotoxin mutant strain were evaluated in an in vivo murine model of keratitis. IHMA104 was selected as a comparator strain because although it is a known keratitis isolate, based on in vitro data, this strain may result in less-severe disease. Using a standard corneal scratch model,52,62 groups of five mice were infected with either IHMA104, IHMA70 or IHMA70Δe1Δe2 and bacterial burden measured at 24, 36, and 48h from whole-eye homogenates. The enterotoxin-positive strain IHMA70 demonstrated high bacterial loads at all time points with an average 2 × 106 CFU mL−1 at 24 hours, 1.41 × 107 CFU mL−1 at 36 hours and 7.2 × 106 CFU mL−1 at 48 hours after infection (Fig. 5). However, compared to IHMA70, IHMA70Δe1Δe2 displayed significantly decreased bacterial counts displaying a consistent 3-log decrease at 24 (P = 3.36e−8), 36 (P = 4.39e−8), and 48 hours (P = 6.48e−13) (Fig. 5). In fact, the reduction of bacterial load of IHMA70Δe1Δe2 was equivalent to the enterotoxin-negative strain IHMA104 at all time points (P > 0.5 at all time points). These data demonstrate that enterotoxins are sufficient to increase S. aureus replication in the cornea, thereby promoting keratitis virulence.
Figure 5.
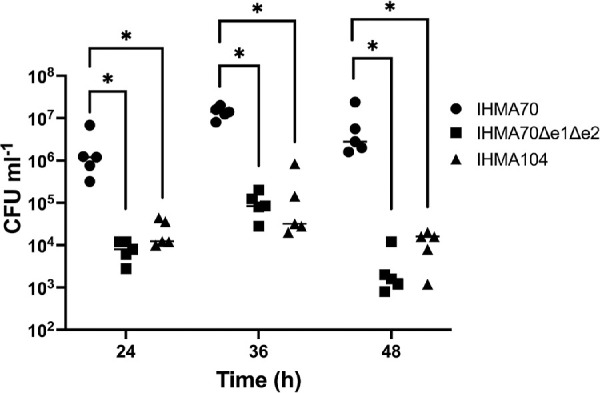
Bacterial burden (CFUs) of murine whole eye homogenates at 24, 36, and 48 hours after infection with the indicated strain.
Given that the set of five enterotoxins found in IHMA70 displayed variable in vitro gene expression, in a separate experiment, enterotoxin expression of sed, ser, sej, seq and sek was determined in vivo via qRT-PCR at 24, 36, 48, and 72 hours after infection. Despite the inherent variability in isolating bacterial RNA from eukaryotic RNA, our experiments suggest that each enterotoxin has a unique expression pattern over the course of 72 hours (Fig. 6). For example, seq displayed a slight increase in gene expression over time, resulting in a 1.6-fold increase by 72 hours, whereas both sej and sek demonstrated a more substantial increase over time of 2.7- and 3.2-fold by 72 hours, respectively. In contrast, sed displayed a more variable pattern with an initial 2.5-fold increase in expression at 36 hours that decreased at 48 hours and ultimately increased again 4.2-fold at 72 hours. Additionally, ser showed minimal change in expression at both 36 and 48 hours, ultimately decreasing to 0.18-fold expression at 72 compared to 24 hours. Taken together, these data suggest that each enterotoxin may be uniquely regulated and may have variable roles in initiating or maintaining infection.
Figure 6.
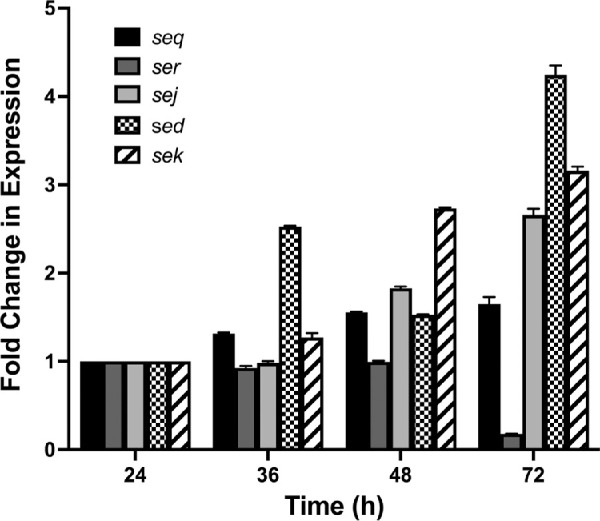
Gene expression levels of five enterotoxins found in IHMA70 over 72 hours in a murine model of keratitis. Fold change in expression is normalized to each individual's gene expression at 24 hours.
Cytokine Expression
In addition to bacterial burden, the severity and extent of tissue damage observed in bacterial keratitis is a consequence of host cytokine production and immune cell infiltration.63–65 Moreover, enterotoxins are known to be powerful immune modulators, thus, a panel of host cytokines was measured over 48 hours in an in vivo keratitis infection. As seen in Figure 7, infection with the enterotoxin proficient IHMA70 strain resulted in significantly reduced cytokine levels of IL-6, IL-12, IL-13 and TNFα at both 24 and 48 hours of infection compared to both IHMA70Δe1Δe2 and IHMA104. For example, IL-6 levels were 1.7 times lower in IHMA70 compared to its corresponding enterotoxin knock out strain IHMA70Δe1Δe2 at 24 (P = 5.45e-7), and 48 hours after infection (P = 2.14e-6), respectively. Similarly, IL-12 levels were 1.5 (P = 3.15e-7) and 2.7 times (P = 7.4e-8) lower in IHMA70 versus IHMA70Δe1Δe2 at 24 and 48 hours, respectively. IL13 levels were 1.4 (P = 1.01e-6) and 1.7 times (P = 2.75e-6) lower and TNFα levels were 1.5 (P = 5.21e-7) and 1.8 times (P = 1.99e-2) lower in IHMA70 versus IHMA70Δe1Δe2 at 24 and 48 hours, respectively. Of note, although there were slight differences between IHMA70Δe1Δe2 and IHMA104, these were not statistically significant at any time point. Thus these experiments combined with corresponding bacterial burdens suggest that enterotoxins may have a role in dampening the host immune response to promote bacterial survival.
Figure 7.
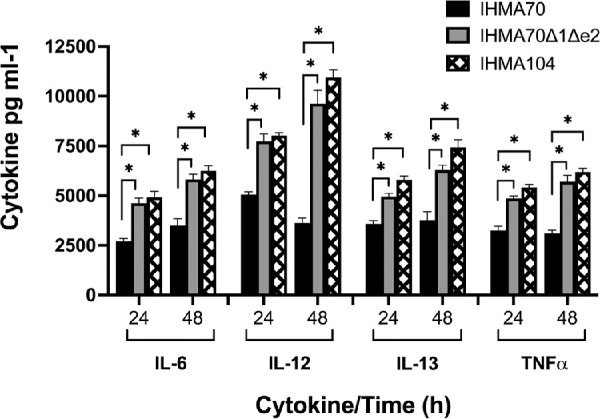
Murine cytokine levels in response to corneal infections with indicated test strain at 24 and 48 hours after infection. *P < 1e-6.
Discussion
Staphylococcus aureus is one of the most important human pathogens, capable of causing disease in every major organ system including severe, vision-threatening, corneal infections.12 Although several important studies have established a role for virulence factors such as α-toxin,26,31 superantigen-like protein 1,33 collagen-binding adhesin,66 and staphopain A34 in mediating keratitis, much is still unknown regarding other mechanisms of S. aureus virulence in ocular infections. As such, to further identify additional virulence factors mediating keratitis we recently undertook a large-scale, whole genomic sequencing approach to compare the prevalence of 235 known S. aureus virulence factors among ocular versus nonocular isolates. This study revealed that a set 10 enterotoxins (seu, selo, seln, selm, seg, selv, sei, sed, sej, ser), as well as two enterotoxin pseudogenes (Ψ -ent1, Ψ -ent2) were found at nearly twofold higher rates among ocular strains, suggesting that these toxins may provide a selective advantage in corneal infections.36
In the current study we evaluated a subset of enterotoxins (sed, sej, sek, seq, and ser) found in IHMA70, a S. aureus clinical keratitis isolate, as well as sek, seq, and selX, found in the nonocular clinical strain USA300LAC. We demonstrate that these enterotoxins do not likely contribute to corneal epithelial cell adhesion or invasion in an in vitro cell culture model, they are directly toxic to corneal cells, causing widespread host cell death over the course of 90 minutes. To demonstrate the effect of this set of enterotoxins in mediating keratitis more directly, we show that an in vivo infection with an enterotoxin-positive strain (IHMA70) led to increased bacterial burden at 24, 36, and 48 hours after infection, compared to the enterotoxin knockout strain (IHMA70Δe1Δe2) and IHMA104, an enterotoxin-negative strain. Correspondingly, S. aureus gene expression of each of the five enterotoxins found in IHMA70 revealed that, with the exception of ser, enterotoxin expression of sek, seq, sej, and sed increased over the course of an infection, albeit to varying degrees. Strikingly, the presence of enterotoxins appeared to play a significant role in altering the host immune response to keratitis, dampening the expression of key host cytokines such as IL-6, IL-12, IL-13, and TNFα. Although this host immune response may be specific to the mouse strain background, taken together, our data suggests a novel role for enterotoxins in direct corneal cell toxicity, as well as modulating the host immune response to promote bacterial survival in the cornea.
Staphylococcal enterotoxins include a diverse family of at least 26 secreted toxins that range in size from 19 to 30 kDa, share structural and functional homology, and are remarkably resistant to heat, proteolysis, desiccation, and acids.38,67–69 Most S. aureus strains, particularly pathogenic isolates, express these toxins, yet the distribution and the number of encoded enterotoxins can vary widely. Within the genome, enterotoxins are commonly associated with S. aureus Pathogenicity Islands (SaPIs),12-18kb phage-derived, mobile genetic elements that are well known conduits for virulence factors and antibiotic resistance markers.55,56,67,70 Virtually all clinical strains of S. aureus carry at least one SaPI, with many harboring multiple islands, and although some SaPIs may be devoid of any known virulence factors, others have been identified as common carriers of virulence determinants including several enterotoxins such as sek and seq.55 For example, in a recent survey of 163 clinical ocular S. aureus isolates, 160 isolates were found to have at least one enterotoxin, and of those, 122 were found to be associated with either SaPI1 (12%), SaPI2 (50%), or SaPI3 (38%).36 The high prevalence of enterotoxins found among ocular S. aureus isolates suggests that they may provide a selective advantage in ocular infections with SaPIs providing a convenient mechanism for the horizontal transfer and acquisition of these toxins.
Enterotoxins are considered superantigens capable of eliciting widespread, non-specific host T-cell activation through directly cross-linking major histocompatibility complex (MHC) class II molecules and T-cell receptors.67,68,71,72 If ingested, as in cases of S. aureus food poisoning, enterotoxins can lead to potent emetic activity; however, in the blood stream, massive T-cell activation can lead to a cytokine storm, ultimately eliciting fever, hypotension, and eventually end-stage organ failure as seen in toxic shock syndrome.40,73–75 As such, these superantigens are associated with the potent release of a variety of pro-inflammatory cytokines including IL-1, IL-2, IL-6, TNFα and IFNγ from T-cells, as well as antigen-presenting cells.67,68 Our current study examined host production of a subset of cytokines, IL-6, IL-12, IL-13, and TNFα, because previous work has demonstrated a crucial role for IL-6 and TNFα in the pathogenesis of S. aureus keratitis,76 and although, to our knowledge, involvement of IL-12 and IL-13 in S. aureus keratitis has not been yet investigated, these cytokines have been implicated in Pseudomonas aeruginosa and fungal causes of keratitis.77–80
Interestingly, however, in our in vivo model of infectious keratitis, enterotoxins appeared to mitigate host cytokine production leading to lower levels of IL-6, IL-12, IL-13, and TNFα in infections caused by IHMA70 versus IHMA70De1De2. Although superantigens have been shown to deplete immature T-cells, the significance of our findings requires further investigation. IL-12 promotes the differentiation of T-helper 1 (Th1) cells and increases IFNγ production, which promotes a host response of increased macrophage recruitment and activity.81 Therefore reduced IL-12 levels may suggest a suppression of the host immune response allowing further infection. Moreover, IL-13 is thought to promote Th2 inflammatory responses and tissue repair, including repair of epithelial cells in the skin.82 Thus it is possible that reduced levels of IL-13 in S. aureus keratitis prevent repair of the corneal epithelium leading to increased pathologic tissue remodeling and fibrosis. Importantly, although S. aureus infection altered these cytokines in the BALB/c mice, which do not generate a robust immune responses to bacterial infection,63 additional investigations with human keratitis patients (i.e., tear cytokine levels) are needed to translate these findings into human disease.
In addition to modulation of the host immune response, staphylococcal enterotoxins also mediate host cell death via stimulating T-cell apoptosis, as well as causing direct cytotoxicity.83 Consistent with our findings that growth media derived from enterotoxin producing strains caused significant corneal epithelial cell death, enterotoxins have also been shown to result in direct cytotoxicity of renal proximal tubule epithelial cells, pulmonary endothelial cells and bovine mammary epithelial cells even in the absence of activated lymphocytes.67,84–86 Corneal ulceration, a hallmark of keratitis in human patients, may be driven in part by enterotoxin-mediated epithelial cell death and subsequent sloughing.
Interestingly, we noted that it was necessary to delete all five IHMA70 enterotoxins or all three USA300LAC enterotoxins to observe significant reductions in cytotoxicity or infectivity. In fact, in the case of USA300LAC, the presence of just one enterotoxin (selX) in a USA300Δsek-seq mutant strain was sufficient to cause significant corneal cell death in our in vitro model. Conversely, the presence of multiple enterotoxins were not additive, that is, there appeared to be a maximum level of cytotoxicity observed regardless of the number of enterotoxins expressed in each strain. Importantly, our data also suggest that despite varying gene expression levels, the enterotoxins explored in this study may be interchangeable with regards to corneal cytotoxicity. For example, USA300LAC which encodes sek, seq and selX, resulted in similar cytotoxicity as IHMA70Δe2, which contains sed, sej and ser. Thus there may be a selective advantage to a range of enterotoxins. This aligns with previous work that has shown a genetic enrichment of sea in ocular versus nonocular strains, as well as the finding of increased corneal ulceration in S. aureus strains carrying sei, seg, and seb in atopic keratoconjunctivitis patients.42
In conclusion, although enterotoxins are not required for corneal infection, we have demonstrated that strains carrying enterotoxins sed, sej, sek, seq, ser, or selX are significantly more cytotoxic to corneal epithelial cells and promote increased bacterial burden in a murine model of infection. We demonstrate that in addition to promoting bacterial survival, enterotoxin-positive strains also dampen the ocular immune response, a finding that sheds light on the complex interplay between host-pathogen interactions. Taken together, our results highlight the important role this class of toxins play in keratitis, providing new insight into S. aureus pathogenesis.
Supplementary Material
Acknowledgments
The authors thank Paul Dunman for the critical reading of this manuscript.
Supported by a Research to Prevent Blindness unrestricted departmental award (R.A.F.W. and W.L.J.), a Research to Prevent Blindness career development award (R.A.F.W.), and NIH EY031398 (C.F.W.).
Disclosure: W.L. Johnson, None; M. Sohn, None; C.F. Woeller, None; R.A.F. Wozniak, None
References
- 1. Han DP, Wisniewski SR, Wilson LA, et al.. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996; 122: 1–17. [DOI] [PubMed] [Google Scholar]
- 2. Hsu HY, Ernst B, Schmidt EJ, Parihar R, Horwood C, Edelstein SL.. Laboratory results, epidemiologic features, and outcome analyses of microbial keratitis: a 15-year review from St. Louis. Am J Ophthalmol. 2019; 198: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang YC, Hsiao CH, Yeh LK, et al.. Methicillin-resistant Staphylococcus aureus ocular infection in Taiwan: clinical features, genotyping, and antibiotic susceptibility. Medicine (Baltimore). 2015; 94: e1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klos M, Pomorska-Wesolowska M, Romaniszyn D, Chmielarczyk A, Wojkowska-Mach J. Epidemiology, drug resistance, and virulence of Staphylococcus aureus isolated from ocular infections in Polish patients. Pol J Microbiol. 2019; 68: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson JC, Durkee H, Miller D, et al.. Molecular epidemiology and resistance profiles among healthcare- and community-associated Staphylococcus aureus keratitis isolates. Infect Drug Resist. 2019; 12: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimizu Y, Toshida H, Honda R, et al.. Prevalence of drug resistance and culture-positive rate among microorganisms isolated from patients with ocular infections over a 4-year period. Clin Ophthalmol. 2013; 7: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flaxman SR, Bourne RRA, Resnikoff S, et al.. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017; 5: e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 8. Alexandrakis G, Alfonso EC, Miller D.. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000; 107: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 9. Cariello AJ, Passos RM, Yu MC, Hofling-Lima AL.. Microbial keratitis at a referral center in Brazil. Int Ophthalmol. 2011; 31: 197–204. [DOI] [PubMed] [Google Scholar]
- 10. Pandita A, Murphy C.. Microbial keratitis in Waikato, New Zealand. Clin Exp Ophthalmol. 2011; 39: 393–397. [DOI] [PubMed] [Google Scholar]
- 11. Gopinathan U, Sharma S, Garg P, Rao GN.. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009; 57: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J.. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019; 64: 255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tam ALC, Cote E, Saldanha M, Lichtinger A, Slomovic AR.. Bacterial keratitis in Toronto: a 16-year review of the microorganisms isolated and the resistance patterns observed. Cornea. 2017; 36: 1528–1534. [DOI] [PubMed] [Google Scholar]
- 14. Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH.. Trends in antibiotic resistance among ocular microorganisms in the United States From 2009 to 2018. JAMA Ophthalmol. 2020; 138: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW.. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015; 133: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 16. Thomas RK, Melton R, Asbell PA.. Antibiotic resistance among ocular pathogens: current trends from the ARMOR surveillance study (2009–2016). Clin Optom (Auckl). 2019; 11: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laskey E, Chen Y, Sohn MB, Gruber E, Chojnacki M, Wozniak RAF.. Efficacy of a novel ophthalmic antimicrobial drug combination toward a large panel of Staphylococcus aureus clinical ocular isolates from around the world. Cornea. 2020; 39: 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marangon FB, Miller D, Muallem MS, Romano AC, Alfonso EC.. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am J Ophthalmol. 2004; 137: 453–458. [DOI] [PubMed] [Google Scholar]
- 19. Lalitha P, Srinivasan M, Manikandan P, et al.. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. 2012; 54: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oldenburg CE, Lalitha P, Srinivasan M, et al.. Moxifloxacin susceptibility mediates the relationship between causative organism and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2013; 54: 1522–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel). 2013; 5: 1140–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seilie ES, Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol. 2017; 72: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tkaczyk C, Hamilton MM, Datta V, et al.. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One. 2013; 8: e75103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McElroy MC, Harty HR, Hosford GE, Boylan GM, Pittet JF, Foster TJ.. Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect Immun. 1999; 67: 5541–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012; 206: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Callaghan RJ, Callegan MC, Moreau JM, et al.. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997; 65: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC.. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2013; 13: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buxton TB, Rissing JP, Horner JA, et al.. Binding of a Staphylococcus aureus bone pathogen to type I collagen. Microb Pathog. 1990; 8: 441–448. [DOI] [PubMed] [Google Scholar]
- 29. Smeltzer MS, Gillaspy AF.. Molecular pathogenesis of staphylcoccal osteomyelitis. Poult Sci. 2000; 79: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 30. Elasri MO, Thomas JR, Skinner RA, et al.. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone. 2002; 30: 275–280. [DOI] [PubMed] [Google Scholar]
- 31. Callegan MC, Engel LS, Hill JM, O'Callaghan RJ. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect Immun. 1994; 62: 2478–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salgado-Pabon W, Herrera A, Vu BG, et al.. Staphylococcus aureus beta-toxin production is common in strains with the beta-toxin gene inactivated by bacteriophage. J Infect Dis. 2014; 210: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang A, Caballero AR, Bierdeman MA, et al.. Staphylococcus aureus superantigen-like protein SSL1: a toxic protease. Pathogens. 2019; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hume EB, Cole N, Khan S, Walsh BJ, Willcox MD.. The role of staphopain a in Staphylococcus aureus keratitis. Exp Eye Res. 2020; 193: 107994. [DOI] [PubMed] [Google Scholar]
- 35. Zaidi T, Zaidi T, Yoong P, Pier GB.. Staphylococcus aureus corneal infections: effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest Ophthalmol Vis Sci. 2013; 54: 4430–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson WL, Sohn MB, Taffner S, et al.. Genomics of Staphylococcus aureus ocular isolates. PLoS One. 2021; 16: e0250975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Afzal M, Vijay AK, Stapleton F, Willcox MDP.. Genomics of Staphylococcus aureus strains isolated from infectious and non-infectious ocular conditions. Antibiotics (Basel). 2022; 11: 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher EL, Otto M, Cheung GYC.. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front Microbiol. 2018; 9: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferry T, Thomas D, Genestier AL, et al.. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis. 2005; 41: 771–777. [DOI] [PubMed] [Google Scholar]
- 40. Lappin E, Ferguson AJ.. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009; 9: 281–290. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Pan Z, Chen Y, Jie Y, He Y.. Specific immunosuppression by mixed chimerism with bone marrow transplantation after Staphylococcal Enterotoxin B pretreatment could prolong corneal allograft survival in mice. Mol Vis. 2012; 18: 974–982. [PMC free article] [PubMed] [Google Scholar]
- 42. Fujishima H, Okada N, Dogru M, et al.. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. 2012; 67: 799–803. [DOI] [PubMed] [Google Scholar]
- 43. Fey PD, Endres JL, Yajjala VK, et al.. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 2013; 4: e00537–00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Norrander J, Kempe T, Messing J.. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983; 26: 101–106. [DOI] [PubMed] [Google Scholar]
- 45. Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983; 166: 557–580. [DOI] [PubMed] [Google Scholar]
- 46. Sau S, Sun J, Lee CY.. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J Bacteriol. 1997; 179: 1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kreiswirth BN, Lofdahl S, Betley MJ, et al.. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983; 305: 709–712. [DOI] [PubMed] [Google Scholar]
- 48. Grosser MR, Richardson AR.. Method for preparation and electroporation of S. aureus and S. epidermidis. Methods Mol Biol. 2016; 1373: 51–57. [DOI] [PubMed] [Google Scholar]
- 49. Corrigan RM, Foster TJ.. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid. 2009; 61: 126–129. [DOI] [PubMed] [Google Scholar]
- 50. Sihto HM, Tasara T, Stephan R, Johler S.. Validation of reference genes for normalization of qPCR mRNA expression levels in Staphylococcus aureus exposed to osmotic and lactic acid stress conditions encountered during food production and preservation. FEMS Microbiol Lett. 2014; 356: 134–140. [DOI] [PubMed] [Google Scholar]
- 51. Edwards AM, Massey RC.. Invasion of human cells by a bacterial pathogen. J Vis Exp. 2011;(49): 2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chojnacki M, Philbrick A, Wucher B, et al.. Development of a broad-spectrum antimicrobial combination for the treatment of Staphylococcus aureus and Pseudomonas aeruginosa corneal infections. Antimicrob Agents Chemother. 2018; 63(1): e01929–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jarraud S, Peyrat MA, Lim A, et al.. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol. 2001; 166: 669–677. [DOI] [PubMed] [Google Scholar]
- 54. Becker K, Friedrich AW, Peters G, von Eiff C.. Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Mol Nutr Food Res. 2004; 48: 488–495. [DOI] [PubMed] [Google Scholar]
- 55. Novick RP, Ram G.. Staphylococcal pathogenicity islands-movers and shakers in the genomic firmament. Curr Opin Microbiol. 2017; 38: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Novick RP. Pathogenicity islands and their role in staphylococcal biology. Microbiol Spectr. 2019; 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stach CS, Herrera A, Schlievert PM.. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 2014; 59: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dinges MM, Orwin PM, Schlievert PM.. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000; 13: 16–34, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mantelli F, Mauris J, Argueso P.. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013; 13: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mazmanian SK, Liu G, Ton-That H, Schneewind O.. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999; 285: 760–763. [DOI] [PubMed] [Google Scholar]
- 61. Thakur A, Clegg A, Chauhan A, Willcox MD.. Modulation of cytokine production from an EpiOcular corneal cell culture model in response to Staphylococcus aureus superantigen. Aust N Z J Ophthalmol. 1997; 25(Suppl 1): S43–S45. [DOI] [PubMed] [Google Scholar]
- 62. Marquart ME. Animal models of bacterial keratitis. J Biomed Biotechnol. 2011; 2011: 680642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hume EB, Cole N, Khan S, et al.. A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunol Cell Biol. 2005; 83: 294–300. [DOI] [PubMed] [Google Scholar]
- 64. Jett BD, Gilmore MS.. Host-parasite interactions in Staphylococcus aureus keratitis. DNA Cell Biol. 2002; 21: 397–404. [DOI] [PubMed] [Google Scholar]
- 65. Kenyon KR. Inflammatory mechanisms in corneal ulceration. Trans Am Ophthalmol Soc. 1985; 83: 610–663. [PMC free article] [PubMed] [Google Scholar]
- 66. Rhem MN, Lech EM, Patti JM, et al.. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect Immun. 2000; 68: 3776–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM.. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013; 26: 422–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tuffs SW, Haeryfar SMM, McCormick JK.. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens. 2018; 7(2): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li SJ, Hu DL, Maina EK, Shinagawa K, Omoe K, Nakane A.. Superantigenic activity of toxic shock syndrome toxin-1 is resistant to heating and digestive enzymes. J Appl Microbiol. 2011; 110: 729–736. [DOI] [PubMed] [Google Scholar]
- 70. McCormick JK, Yarwood JM, Schlievert PM.. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001; 55: 77–104. [DOI] [PubMed] [Google Scholar]
- 71. Johnson HM, Russell JK, Pontzer CH.. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991; 5: 2706–2712. [DOI] [PubMed] [Google Scholar]
- 72. Miethke T, Bendigs S, Bader P, Wagner H, Heeg K.. Murine CD4+ and CD8+ T cells are activated by the superantigen (SA) staphylococcal enterotoxin B (SEB) and exhibit MHC-unrestricted cytotoxicity. Zentralbl Bakteriol. 1991; 275: 264–268. [DOI] [PubMed] [Google Scholar]
- 73. Argudin MA, Mendoza MC, Rodicio MR.. Food poisoning and Staphylococcus aureus enterotoxins. Toxins (Basel). 2010; 2: 1751–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fraser JD. Clarifying the mechanism of superantigen toxicity. PLoS Biol. 2011; 9: e1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chesney PJ, Bergdoll MS, Davis JP, Vergeront JM.. The disease spectrum, epidemiology, and etiology of toxic-shock syndrome. Annu Rev Microbiol. 1984; 38: 315–338. [DOI] [PubMed] [Google Scholar]
- 76. O'Callaghan RJ. The pathogenesis of Staphylococcus aureus eye infections. Pathogens. 2018; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhong J, Peng L, Wang B, et al.. Tacrolimus interacts with voriconazole to reduce the severity of fungal keratitis by suppressing IFN-related inflammatory responses and concomitant FK506 and voriconazole treatment suppresses fungal keratitis. Mol Vis. 2018; 24: 187–200. [PMC free article] [PubMed] [Google Scholar]
- 78. Sun L, Chen C, Wu J, Dai C, Wu X.. TSLP-activated dendritic cells induce T helper type 2 inflammation in Aspergillus fumigatus keratitis. Exp Eye Res. 2018; 171: 120–130. [DOI] [PubMed] [Google Scholar]
- 79. Hazlett LD, Huang X, McClellan SA, Barrett RP.. Further studies on the role of IL-12 in Pseudomonas aeruginosa corneal infection. Eye (Lond). 2003; 17: 863–871. [DOI] [PubMed] [Google Scholar]
- 80. Hazlett LD, Rudner XL, McClellan SA, Barrett RP, Lighvani S.. Role of IL-12 and IFN-gamma in Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci. 2002; 43: 419–424. [PubMed] [Google Scholar]
- 81. Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009; 8: 40–52. [DOI] [PubMed] [Google Scholar]
- 82. Dalessandri T, Crawford G, Hayes M, Castro Seoane R, Strid J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat Commun. 2016; 7: 12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Deacy AM, Gan SK, Derrick JP.. Superantigen recognition and interactions: functions, mechanisms and applications. Front Immunol. 2021; 12: 731845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ionin B, Hammamieh R, Shupp JW, Das R, Pontzer CH, Jett M.. Staphylococcal enterotoxin B causes differential expression of Rnd3 and RhoA in renal proximal tubule epithelial cells while inducing actin stress fiber assembly and apoptosis. Microb Pathog. 2008; 45: 303–309. [DOI] [PubMed] [Google Scholar]
- 85. Liu Y, Chen W, Ali T, et al.. Staphylococcal enterotoxin H induced apoptosis of bovine mammary epithelial cells in vitro. Toxins (Basel). 2014; 6: 3552–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao Y, Tang J, Yang D, Tang C, Chen J.. Staphylococcal enterotoxin M induced inflammation and impairment of bovine mammary epithelial cells. J Dairy Sci. 2020; 103: 8350–8359. [DOI] [PubMed] [Google Scholar]
- 87. Diep BA, Gill SR, Chang RF, et al.. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet. 2006; 367: 731–739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.